Key Points
Question
Does MC1R contribute differently to melanoma risk in males and females?
Findings
In this case-control study of 1791 participants, MC1R variants were identified as significant risk factors for melanoma in females but not in males.
Meaning
This finding could help to identify different melanoma risk factors in males and females and should be considered in genetic counseling.
Abstract
Importance
Recently, the red hair variants of MC1R were found to contribute differently to pigmentation phenotype in males and females.
Objective
To investigate the role of these variants in melanoma risk in males and females separately because carriers of the red hair variants of MC1R are at increased risk of melanoma.
Design, Setting, and Participants
In this hospital-based, case-control study, we evaluated the effect of MC1R and melanoma risk for males and females separately by performing multivariate logistic regression analyses.
Main Outcomes and Measures
Association of MC1R variants and melanoma risk in males and females.
Results
A total of 905 females (473 melanoma cases, 432 controls) and 886 males (518 melanoma cases, 368 controls) were included in the analyses. The mean (SD) age of the study population was 59.2 (15.6). In females, carrying any MC1R red hair variants remained an independent risk factor of melanoma in a multivariable analysis (adjusted odds ratio [OR], 2.19 [95% CI, 1.60-2.99]), whereas in males, only signs of actinic skin damage (lentigines on the back [OR, 2.56; 95% CI, 1.47-4.45; P = .001] and the hands [OR, 2.31; 95% CI, 1.24-4.29; P = .008] and wrinkling on the neck [OR, 2.17; 95% CI, 1.23-3.82; P = .007]) and sunburns (OR, 1.65; 95% CI, 1.12-2.42; P = .01) remained significant risk factors.
Conclusions and Relevance
MC1R variants contribute differently to melanoma risk in males and females. This could be helpful to better classify melanoma risk factors between the sexes.
This case-control study evaluates the association of melanocortin-1-receptor (MC1R) with melanoma risk in males vs females, adjusting for risk factors such as age, pigmentation, phenotype, signs of skin damage, and sun exposure.
Introduction
Previous studies have shown population-specific differences in pigmentation phenotype between males and females regarding phototype, tanning ability, and eye color.1,2,3,4,5 Recently, a Spanish group described a stronger effect of melanocortin-1-receptor (MC1R) variants on skin phototype in females compared with males6: in females, MC1R variants were associated with lower phototypes compared with male carriers of the same variants. This finding is of interest because MC1R is known to be highly polymorphic—with more than 80 variants described—and is the main regulator of pigmentation phenotype, including hair color, skin pigmentation, and sun sensitivity at the same time.7,8,9,10 Activation and subsequent signaling of this G-protein coupled receptor result in 2 different types of melanin: the lighter, yellow-reddish pheomelanin and the darker, brown to black eumelanin. The ratio of eumelanin and pheomelanin is determined by the variants an individual carries and results in particular phenotypes. Variants that were found to be associated with red hair and fair skin were classified as “R” (high-risk) and “r” (low-risk) variants. These variants also have been well described as being associated with melanoma risk, which is plausible owing to less effective protection of pheomelanin against UV radiation7,8,10,11,12 as well as pro-oxidant properties. Only recently, MC1R red hair variants were associated with melanoma, even independent of sun exposure.13
Differences between the sexes do not affect only skin pigmentation. There are also differences in age at onset, incidence rates, site of tumor occurrence, and survival between male and female patients with melanoma.14,15,16,17,18,19 Despite these well-acknowledged differences, an explanation for those differences is still missing. It thus seems of particular interest to elucidate the role of genetic risk factors (eg, MC1R) and melanoma risk in males and females separately to better understand possible underlying sex-dependent differences in melanoma development. We therefore aimed to investigate the association of pigmentation phenotype, MC1R variants, and melanoma risk in males and females in our study population to characterize divergent categories of risk factors between the sexes.
Methods
Study Population
Males and females who were recruited within the M3 Study program (Molecular Markers of Melanoma) were included in the analyses. This program was established in 2008 at the Department of Dermatology at the Medical University of Vienna to study risk factors for melanoma in Austria.13,20,21,22 Melanoma cases and (control) patients who visited the outdoor clinic for general dermatological problems (without history of melanoma) were recruited to the M3 Study. The diagnoses of the controls have been reported previously.20 Only participants with European ancestry were included in the current study. More than 90% of the participants were born in Austria. The remaining participants or their ancestors were born in other central European countries, such as Belgium, Czech Republic, Germany, Hungary, the Netherlands, Poland, Romania, Slovakia, and Switzerland.
Written informed consent was obtained from all participants, and they were not compensated. This study was approved by the ethics committee of the Medical University of Vienna.
Assessment of Pigmentation Phenotype, Signs of Actinic Skin Damage, and Sun Exposure
For all participants, a detailed examination of pigmentation phenotype (skin phototype, tanning ability, burning tendency, hair color, freckles in childhood) and actinic skin damage on different body sites (freckling, wrinkling, and solar lentigines) was performed and then categorized as described in detail herein.13,20,21 In addition, variables, such as the number of holiday weeks in life, the number of sunburns in life, outdoor occupation, and recreational time, were recorded as described previously13,20,21 (Figure 1).
Figure 1. The M3 Study Population.
Short description of the M3 study population: Data and genomic data from patients with melanoma and control patients were collected in the M3 database. Information of phenotype, actinic skin type, sun exposure and MC1R status were included in our current analysis. M3 indicates Molecular Markers of Melanoma.
MC1R-Sequencing
MC1R sequencing was performed for 1791 participants of the M3 Study population. After extraction of genomic DNA from whole blood (DNA Purification kit “Wizard Genomic,” Promega Corporation), the 951-bp coding sequence of the MC1R gene was amplified by polymerase chain reaction as described previously.23
Statistical Analyses
Variables that reflect pigmentation phenotype and signs of actinic skin damage were classified into categories as described earlier.13,20,21 The number of holiday weeks and sunburns in life was not normally distributed (Kolmogorov-Smirnov test; P < .001) and therefore categorized into tertiles based on the distribution of all participants (0-1, 2-19, or ≥20 for sunburns and <9, 9-17, or ≥18 for holiday weeks). Outdoor occupation anytime in life was recorded as dichotomous variable (yes or no), and recreational time was recorded as categorical variable (<1, 1-3, or >3 hours per day spent outdoors).
MC1R variants were classified as “R” (high-risk; ie, Ins86_87A, R142H, R151C, R160W, D294H, D84E) and “r” (low-risk; V60L, V92M, I155T, R163Q) variants as described elsewhere.10,24,25,26,27 Participants who did not carry any of these 10 most common variants were pooled in the reference group (0 of 0).
First, the association of different variables (MC1R variants, signs of actinic skin damage, holiday weeks, sunburns, outdoor occupation, and recreational time) and melanoma risk was calculated separately for males and females by using logistic regression procedures to obtain age-adjusted odds ratios (ORs) and their 95% CIs. Second, only variables that remained significant in this prior analysis were included in the multivariate analyses (separately for males and females).
Two different models were constructed (different types of actinic skin damage + age and different types of actinic skin damage + age +MC1R variants) separately for males and females, and receiver operating characteristic (ROC) curves were calculated to assess the predictive ability for each model.
A 2-sided P value <.05 was considered statistically significant. All statistical analyses were performed using SPSS Statistics software (version 19.0; SPSS IBM Inc).
Results
In total, 905 women (50.5%) and 886 men (49.5%) with a mean (SD) age of 59.17 (15.59) years were included in the analyses (n = 1791). The women were younger (mean age, 57.14 [15.48] years) than the men (mean age, 61.25 [15.44] years).
Pigmentation Phenotype in Males and Females
After adjustment for age and melanoma status, female sex was significantly associated with phototype I /II (OR, 1.44; 95% CI, 1.09-1-89; P = .01), a high burning tendency (OR, 1.36; 95% CI, 1.02-1.82; P = .04), and a low tanning ability (OR, 2.33; 95% CI, 1.56-3.50; P < .001) compared with males. In addition, red hair color was significantly associated with female sex (OR, 2.37; 95% CI, 1.60-3.52; P < .001), as was the presence of freckles in childhood (OR, 1.46; 95% CI, 1.18-1.80; P < .001) (Table 1). Stratification for cases and controls showed similar results (eTable 1 in the Supplement).
Table 1. Pigmentation Phenotype in Males and Females.
| Variablea | No. (%) | AORb (95% CI) | P Value | |
|---|---|---|---|---|
| Females (n = 905) |
Males (n = 886) |
|||
| Skin phototype | ||||
| I/II | 625 (71.8) | 554 (64.8) | 1.44 (1.09-1.89) | .01 |
| III | 131 (15.0) | 149 (17.4) | 1.06 (0.75-1.50) | .73 |
| IV/V | 115 (13.2) | 152 (17.8) | 1 [Reference] | |
| Burning tendency | ||||
| High | 167 (18.5) | 134 (15.1) | 1.36 (1.02-1.82) | .04 |
| Intermediate | 483 (53.5) | 488 (55.1) | 0.99 (0.80-1.24) | .95 |
| Low | 253 (28.0) | 263 (29.7) | 1 [Reference] | |
| Tanning ability | ||||
| Low | 96 (10.6) | 65 (7.3) | 2.33 (1.56-3.50) | <.001 |
| Intermediate | 699 (77.4) | 664 (75.0) | 1.53 (1.16-2.00) | .002 |
| High | 108 (12.0) | 156 (17.6) | 1 [Reference] | |
| Hair color | ||||
| Red | 80 (8.8) | 45 (5.1) | 2.37 (1.60-3.52) | <.001 |
| Blond | 208 (23) | 173 (19.5) | 1.65 (1.29-2.11) | <.001 |
| Light brown | 246 (27.2) | 201 (22.7) | 1.55 (1.22-1.95) | <.001 |
| Brown/black | 370 (40.9) | 467 (52.7) | 1 [Reference] | |
| Freckles | ||||
| Yes | 323 (36.9) | 231 (28.1) | 1.46 (1.18-1.80) | <.001 |
| No | 552 (63.1) | 591 (71.9) | 1 [Reference] | |
Abbreviations: AOR, adjusted odds ratio.
The single variables do not sum up to the total number of females and males because of missing data.
Adjusted for age and melanoma status.
MC1R Variants and Melanoma Risk in Males and Females
Although there were no differences between females and males in number of MC1R variants (eTable 2 in the Supplement), carrying a MC1R red hair variant was significantly associated with melanoma risk only in females (Table 2). This association correlated even with the number of the variants: Carrying 2 or more MC1R variants was associated with increased melanoma risk (OR, 2.65; 95% CI, 1.86-3.79; P < .001) compared with carrying only 1 variant. For males, only carrying of 2 or more variants (OR, 1.65; 95% CI, 1.14-2.38; P = .007) (Table 2) was associated with increased melanoma risk. When we analyzed combinations of variants (R/R, R/r, R/0, r/r, and r/0) and melanoma risk, we identified all combinations to be significantly associated with melanoma risk in females (OR, 2.29 for R/R; 95% CI, 1.23-4.30; OR, 3.07 for R/r; 95% CI, 1.92-4.92; OR, 2.05 for R/0; 95% CI, 95% CI, 1.36-3.10; OR, 1.83 for r/r; 95% CI, 1.07-3.15; and OR, 1.73 for r/0; 95% CI, 1.22-2.45), while in males only carriers of R/r (OR, 2.27; 95% CI, 1.39-3.72; P = .001) and R/0 (OR, 1.70; 95% CI, 1.14-2.53; P = .01) were at increased risk.
Table 2. MC1R Variants and Melanoma Risk.
| Variablea | Males, No. (%) | AORb (95% CI) | P Value | Females, No. (%) | AORb (95% CI) | P Value | ||
|---|---|---|---|---|---|---|---|---|
| Cases (n = 518) |
Controls (n = 368) |
Cases (n = 473) |
Controls (n = 432) |
|||||
| MC1R | ||||||||
| Variant | 417 (80.5) | 277 (75.3) | 1.34 (0.97-1.85) | .08 | 391 (82.7) | 295 (68.3) | 2.19 (1.60-2.99) | <.001 |
| Wt | 101 (19.5) | 91 (24.7) | 1 [Reference] | 82 (17.3) | 137 (31.7) | 1 [Reference] | ||
| MC1R variants, No. | ||||||||
| ≥2 | 205 (39.6) | 111 (30.2) | 1.65 (1.14-2.38) | .007 | 200 (42.3) | 124 (28.7) | 2.65 (1.86-3.79) | <.001 |
| 1 | 212 (40.9) | 166 (45.1) | 1.13 (0.80-1.61) | .49 | 191 (40.4) | 171 (39.6) | 1.85 (1.31-2.61) | <.001 |
| 0 (wt) | 101 (19.5) | 91 (24.7) | 1 [Reference] | 82 (17.3) | 137 (31.7) | 1 [Reference] | ||
| Combination of MC1R variantsc | ||||||||
| R/R | 30 (5.8) | 18 (4.9) | 1.49 (0.79-2.84) | .22 | 29 (6.1) | 20 (4.6) | 2.29 (1.23-4.30) | .01 |
| R/r | 76 (14.7) | 31 (8.4) | 2.27 (1.39-3.72) | .001 | 76 (16.1) | 37 (8.6) | 3.07 (1.92-4.92) | <.001 |
| R/0 | 121 (23.4) | 66 (17.9) | 1.70 (1.14-2.53) | .01 | 88 (18.6) | 64 (14.8) | 2.05 (1.36-3.10) | .001 |
| r/r | 46 (8.9) | 25 (6.8) | 1.65 (0.95-2.88) | .08 | 38 (8.0) | 31 (7.2) | 1.83 (1.07-3.15) | .03 |
| r/0 | 127 (24.5) | 119 (32.3) | 0.96 (0.66-1.38) | .81 | 144 (30.4) | 129 (29.9) | 1.73 (1.22-2.45) | .002 |
| 0/0d | 118 (22.8) | 109 (29.6) | 1 [Reference] | 98 (20.7) | 151 (35.0) | 1 [Reference] | ||
Abbreviations: AOR, adjusted odds ratio; wt, wild type.
The single variants do not sum up to the total number of sequenced cases and controls because of missing data
Adjusted for age.
R = 86_87insA, R142H, R151C, R160W, D294H, D84E; r = V60L, V92M, I155T, R163Q.
Participants who did not carry any “r” or “R.”
Sun Exposure Variables and Melanoma Risk in Males and Females
We next tested the association of sun exposure variables and melanoma risk in males and females separately. While more males had reported a higher number of sunburns in the past, the association of melanoma risk and sunburns was similar between the sexes (OR, 2.41; 95% CI, 1.70-3.42; P < .001 for females and OR, 2.24; 95% CI, 1.58-3.17; P < .001 for males) (eTable 3A and 3B in the Supplement). Outdoor occupancy and the number of hours spent outdoors for recreational purposes seemed to be protective for both sexes; however, this effect was statistically significant only in females (eTable 3B in the Supplement).
With regard to moderate to severe signs of actinic skin damage (++/+++), the highest ORs were calculated for the back and the neck in both sexes (OR, 3.68; 95% CI, 2.31-5.85 for wrinkling; OR, 3.25; 95% CI, 2.14-4.93 for solar lentigines and OR, 3.02; 95% CI, 1.98-4.62 for freckling in females, and OR, 4.20; 95% CI, 2.60-6.78 for lentigines; OR, 3.65; 95% CI, 2.22-6.02 for wrinkling; and OR, 2.82; 95% CI, 1.73-4.60 for freckling in males; P < .001 for all comparisons), followed by the hands and the face. While all severe signs of actinic damage were significantly associated with melanoma risk in males, actinic damage on the face did not remain significant after adjustment for age in females; except for solar lentigines with a significant P value of 0.046 (eTable 3A and 3B in the Supplement).
Risk Factors of Melanoma in Males and Females in Multivariate Analyses
For females, we identified wrinkling on the neck (OR, 2.75; 95% CI, 1.61-4.70; P < .001), lentigines on the back (OR, 1.90; 95% CI, 1.14-3.15; P = .01) and the face (OR, 1.56; 95% CI, 1.06-2.28; P = .02) and 20 or more sunburns in life (OR, 1.89; 95% CI, 1.29-2.76; P = .001) as significant melanoma risk factors. With regard to MC1R variants, carriers of combinations of R/r (OR, 2.56; 95% CI, 1.52-4.32; P < .001), R/0 (OR, 1.67; 95% CI, 1.07-2.60; P = .02), and r/0 (OR, 1.54; 95% CI, 1.05-2.25; P = .02) were found to be at increased melanoma risk (Table 3).
Table 3. Risk Factors for Melanoma in a Multivariable Analysis.
| Variablea | Males | Females | ||
|---|---|---|---|---|
| AORb (95% CI) | P Value | AORb (95%CI) | P Value | |
| Combination of MC1R Variantsc | ||||
| R/R | 0.92 (0.45-1.89) | .82 | 1.45 (0.72-2.93) | .40 |
| R/r | 1.57 (0.91-2.71) | .11 | 2.56 (1.52-4.32) | <.001 |
| R/0 | 1.19 (0.77-1.85) | .43 | 1.67 (1.07-2.60) | .02 |
| r/r | 1.28 (0.71-2.33) | .41 | 1.53 (0.85-2.76) | .15 |
| r/0 | 0.74 (0.50-1.10) | .14 | 1.54 (1.05-2.25) | .02 |
| 0/0d | 1 [Reference] | 1 [Reference] | ||
| Freckling face | ||||
| Moderate to severe | 0.94 (0.53-1.68) | .85 | NA | |
| Mild | 1.01 (0.61-1.68) | .97 | NA | |
| None | 1 [Reference] | NA | ||
| Freckling back | ||||
| Moderate to severe | 1.11 (0.61-2.02) | .73 | 1.24 (0.73-2.13) | .43 |
| Mild | 0.85 (0.50-1.44) | .54 | 1.48 (0.94-2.32) | .09 |
| None | 1 [Reference] | 1 [Reference] | ||
| Freckling hands | ||||
| Moderate to severe | 0.87 (0.49-1.55) | .63 | 1.22 (0.67-2.23) | .52 |
| Mild | 0.69 (0.46-1.03) | .07 | 1.37 (0.95-1.99) | .09 |
| None | 1 [Reference] | 1 [Reference] | ||
| Wrinkling face | ||||
| Moderate to severe | 1.13 (0.57-2.23) | .72 | 0.44 (0.24-0.80) | .007 |
| Mild | 1.14 (0.64-2.02) | .66 | 0.88 (0.54-1.42) | .59 |
| None | 1 [Reference] | 1 [Reference] | ||
| Wrinkling neck | ||||
| Moderate to severe | 2.17 (1.23-3.82) | .007 | 2.75 (1.61-4.70) | <.001 |
| Mild | 1.53 (0.93-2.50) | .09 | 1.89 (1.27-2.81) | .002 |
| None | 1 [Reference] | 1 [Reference] | ||
| Solar lentigines face | ||||
| Moderate to severe | 1.36 (0.78-2.36) | .28 | 1.01 (0.60-1.71) | .96 |
| Mild | 2.39 (1.57-3.64) | <.001 | 1.56 (1.06-2.28) | .02 |
| None | 1 [Reference] | 1 [Reference] | ||
| Solar lentigines back | ||||
| Moderate to severe | 2.56 (1.47-4.45) | .001 | 1.90 (1.14-3.15) | .01 |
| Mild | 1.92 (1.18-3.11) | .008 | 1.46 (0.96-2.22) | .08 |
| None | 1 [Reference] | 1 [Reference] | ||
| Solar lentigines hand | ||||
| Moderate to severe | 2.31 (1.24-4.29) | .008 | 1.27 (0.71-2.26) | .41 |
| Mild | 1.32 (0.83-2.11) | .24 | 1.03 (0.66-1.61) | .88 |
| None | 1 [Reference] | 1 [Reference] | ||
| Sunburns in life | ||||
| ≥20 | 1.65 (1.12-2.42) | .01 | 1.89 (1.29-2.76) | .001 |
| 2-19 | 1.23 (0.84-1.79) | .29 | 0.96 (0.68-1.36) | .83 |
| 0-1 | 1 [Reference] | 1 [Reference] | ||
Abbreviations: AOR, adjusted odds ratio; NA, not applicable.
Only variables that remained significant in the univariate analysis were included in the multivariate analysis.
Adjusted for age.
R = 86_87insA, R142H, R151C, R160W, D294H, D84E; r = V60L, V92M, I155T, R163Q.
Participants who did not carry any “r” or “R.”
A multivariable analysis for the subgroup of males showed again an association of severe signs of actinic skin damage (lentigines on the back [OR, 2.56; 95% CI, 1.47-4.45; P = .001] and the hands [OR, 2.31; 95% CI, 1.24-4.29; P = .008] and wrinkling on the neck [OR, 2.17; 95% CI, 1.23-3.82; P = .007]) and melanoma. Similar to the female subgroup, in men, having 20 or more sunburns in life could be identified as a melanoma risk factor (OR, 1.65; 95% CI, 1.12.-2.42; P = .01). In contrast with females, carrying MC1R variants did not remain significant in the male subgroup in a multivariate analysis (Table 3).
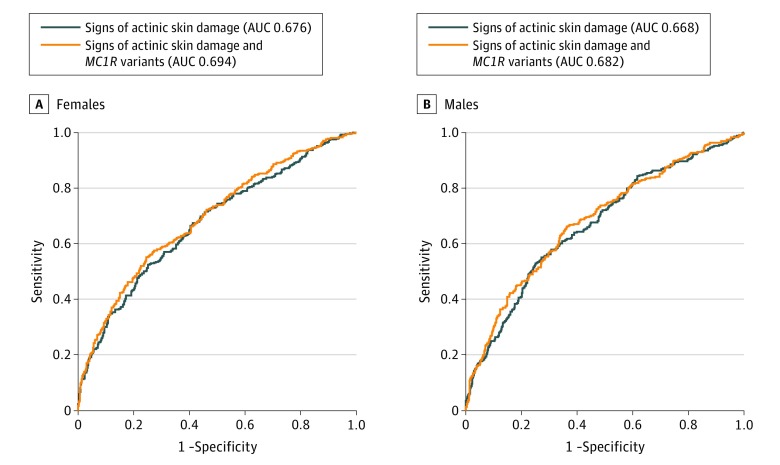
Receiver operating characteristic curves based on a model with age and different signs of actinic skin damage showed an area under the receiver operating characteristic (AUC) of for males of 0.668 (P < .001) and an AUC of 0.676 (P < .001) for females. Adding MC1R variants led to an improvement of the AUC, particularly in females (0.694 vs 0.682 in males; P < .001) (Figure 2).
Figure 2. Receiver Operating Characteristic Curve for Actinic Skin Damage and Actinic Skin Damage + MC1R in Females.
A, Black indicates signs of actinic skin damage (area under the receiver operating characteristic [AUC], 0.676); orange, signs of actinic skin damage and MC1R variants (AUC, 0.694). B, Black indicates signs of actinic skin damage (AUC, 0.668); orange, signs of actinic skin damage and MC1R variants (AUC, 0.682).
Discussion
Despite various efforts to prevent melanoma worldwide, its incidence rate is still increasing, especially in young adults, and it ranks in the top 5 most frequent types of cancer. Differences between females and males have been evident for decades, such as the fact that for females, melanoma is the most frequent type of malignant neoplasm, even more than breast cancer, when they are young.28,29 Other differences include localization of the primary lesions and disease-specific survival even after correction against invasion thickness.28,30,31 Until now, there were only assumptions for this phenomenon, such as differences in lifestyle, fashion, and sun exposure habits; however, differences in pigmentation between female and male patients are not well described.2,4,5
Sun exposure is accepted as the most important external risk factor. Therefore, it is plausible to assume that fair and sun-sensitive skin is more prone to develop melanoma. This assumption is supported by the finding that MC1R variants associated with fair skin complexion and red hair owing to the production of the lighter and less protective pheomelanin compared with eumelanin are associated with an increased risk. A previous study demonstrated even a UV-independent pathway to melanoma in red haired mice,32 and only recently we have shown that red hair variants increase risk independently of sun exposure in humans.13
In agreement with previous reports,33 female sex was more strongly associated with sun sensitivity, fair skin types (I/II), red hair color, and freckles in childhood than with male sex in our study population. However, although females have been described as lighter pigmented than males in some populations—which was hypothesized to be an evolutionary advantage with regard to vitamin D synthesis and sexual selection1—there are also reports of populations where males were identified to have a lighter pigmentation than females, possibly owing to hormonal difference.2,5,33 Differences between the sexes with regard to eye color and freckles have also been described as population specific.3,4 Although these findings indicate unignorable differences between the sexes with regard to pigmentation phenotype, the underlying causes seem to be quite complex; for example, the difference in pigmentation phenotype was not due to different distribution of MC1R red hair variants because they were distributed equally between the sexes in our study population.
However, according to previous findings,6 MC1R red hair variants were found to have a stronger effect on pigmentation phenotype in females compared with males in our study population (eTable 4A and 4B in the Supplement). When we analyzed the association of melanoma risk and MC1R red hair variants in males and females separately in multivariate analyses, we found a significant association of MC1R variants and melanoma in females, but the association failed to remain statistically significant in males (Table 3).
Limitations
There are obvious differences between both sexes with regard to anatomy, physiology, lifestyle, and behavior, and it is difficult to consider all possible sex-dependent determinants as potential effectors of our observation. Although humoral factors have not been the focus of melanoma research so far, sex hormones have been suggested to play a role in pigmentation and even melanoma development.28 Functionally, estrogens have been shown to alter pigmentation of the skin. In mice, estrogen does not only increase the expression of MC1R but was also found to increase the content of pheomelanin in females.34 Pigmentation disorders that are linked to estrogen, such as the ones observed during pregnancy, menstrual cycle, or after intake of contraceptive pills,35 clearly demonstrate the effect of estrogen on pigmentation in humans.36,37,38 Beside the steeper increase in incidence among young women, several studies revealed a link between the use of oral contraceptive or hormonal replacement and melanoma risk.39,40 Together with our previous report13 of MC1R red hair variants being a risk factor of melanoma even without sun exposure in humans and an earlier study32 demonstrating red-haired mice being prone to melanoma even without UV irradiation, it seems plausible that humoral factors alter the effect of MC1R red hair variants on skin phenotype as well as cancer risk by supporting the production of pheomelanin. It is therefore plausible to speculate that the striking incidence of melanoma in young women could be related to the enhanced production of female sexual hormones and their effect on MC1R expression.
Conclusions
In general, sexual hormones, and estrogen in particular, are known to have an effect far beyond effects in reproductive organs. The diverse effect on MC1R expression might be a consequence of evolution in terms of selection advantage.41 In the context of cancer, it is interesting to note that until the age of 49 years, the incidence of any cancer type is higher among females than for males, which might indicate a general effect of female sexual hormones in oncogenesis. In skin, these effects are undeniable because practically all compartments of the skin from the epidermis to the dermis and even below (ie, subcutaneous tissue) respond to estrogen.42 The observation of sex-dependent variation in pigmentation phenotype and melanoma risk indicate the need for further studies elucidating the effect of sexual hormones on skin pigmentation and skin cancer. To our knowledge, we present herein for the first time a detailed analysis of risk factors for melanoma in males and females separately in a central European population. The obvious differences of the properties of male and female patients with melanoma emphasize the importance of future studies that might eventually lead to sex-dependent prevention strategies as well as therapy.
eTable 1. Pigmentation phenotype in males and females stratified for Cases and Controls
eTable 2. Frequency of MC1R variants in males and females
eTable 3a. Sun exposure variables and melanoma risk in males
eTable 3b. Sun exposure variables and melanoma risk in females
eTable 4a. MC1R variants and pigmentation phenotype in males
eTable 4b. MC1R variants and pigmentation phenotype in females
eTable 5a. MC1R variants and pigmentation phenotype in Cases
eTable 5b. MC1R variants and pigmentation phenotype in Controls
References
- 1.Jablonski NG, Chaplin G. The evolution of human skin coloration. J Hum Evol. 2000;39(1):57-106. [DOI] [PubMed] [Google Scholar]
- 2.Candille SI, Absher DM, Beleza S, et al. . Genome-wide association studies of quantitatively measured skin, hair, and eye pigmentation in four European populations. PLoS One. 2012;7(10):e48294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pietroni C, Andersen JD, Johansen P, et al. . The effect of gender on eye colour variation in European populations and an evaluation of the IrisPlex prediction model. Forensic Sci Int Genet. 2014;11:1-6. [DOI] [PubMed] [Google Scholar]
- 4.Sulem P, Gudbjartsson DF, Stacey SN, et al. . Genetic determinants of hair, eye and skin pigmentation in Europeans. Nat Genet. 2007;39(12):1443-1452. [DOI] [PubMed] [Google Scholar]
- 5.Martinez-Cadenas C, Peña-Chilet M, Ibarrola-Villava M, Ribas G. Gender is a major factor explaining discrepancies in eye colour prediction based on HERC2/OCA2 genotype and the IrisPlex model. Forensic Sci Int Genet. 2013;7(4):453-460. [DOI] [PubMed] [Google Scholar]
- 6.Hernando B, Ibarrola-Villava M, Peña-Chilet M, Alonso S, Ribas G, Martinez-Cadenas C. Sex and MC1R variants in human pigmentation: differences in tanning ability and sensitivity to sunlight between sexes. J Dermatol Sci. 2016;84(3):346-348. [DOI] [PubMed] [Google Scholar]
- 7.Valverde P, Healy E, Jackson I, Rees JL, Thody AJ. Variants of the melanocyte-stimulating hormone receptor gene are associated with red hair and fair skin in humans. Nat Genet. 1995;11(3):328-330. [DOI] [PubMed] [Google Scholar]
- 8.Valverde P, Healy E, Sikkink S, et al. . The Asp84Glu variant of the melanocortin 1 receptor (MC1R) is associated with melanoma. Hum Mol Genet. 1996;5(10):1663-1666. [DOI] [PubMed] [Google Scholar]
- 9.Box NF, Wyeth JR, O’Gorman LE, Martin NG, Sturm RA. Characterization of melanocyte stimulating hormone receptor variant alleles in twins with red hair. Hum Mol Genet. 1997;6(11):1891-1897. [DOI] [PubMed] [Google Scholar]
- 10.Kanetsky PA, Rebbeck TR, Hummer AJ, et al. . Population-based study of natural variation in the melanocortin-1 receptor gene and melanoma. Cancer Res. 2006;66(18):9330-9337. [DOI] [PubMed] [Google Scholar]
- 11.Duffy DL, Box NF, Chen W, et al. . Interactive effects of MC1R and OCA2 on melanoma risk phenotypes. Hum Mol Genet. 2004;13(4):447-461. [DOI] [PubMed] [Google Scholar]
- 12.Sturm RA, Duffy DL, Box NF, et al. . The role of melanocortin-1 receptor polymorphism in skin cancer risk phenotypes. Pigment Cell Res. 2003;16(3):266-272. [DOI] [PubMed] [Google Scholar]
- 13.Wendt J, Rauscher S, Burgstaller-Muehlbacher S, et al. . Human determinants and the role of melanocortin-1 receptor variants in melanoma risk independent of UV radiation exposure. JAMA Dermatol. 2016;152(7):776-782. [DOI] [PubMed] [Google Scholar]
- 14.Pruthi DK, Guilfoyle R, Nugent Z, Wiseman MC, Demers AA. Incidence and anatomic presentation of cutaneous malignant melanoma in central Canada during a 50-year period: 1956 to 2005. J Am Acad Dermatol. 2009;61(1):44-50. [DOI] [PubMed] [Google Scholar]
- 15.Joosse A, de Vries E, Eckel R, et al. ; Munich Melanoma Group . Gender differences in melanoma survival: female patients have a decreased risk of metastasis. J Invest Dermatol. 2011;131(3):719-726. [DOI] [PubMed] [Google Scholar]
- 16.Joosse A, Collette S, Suciu S, et al. . Sex is an independent prognostic indicator for survival and relapse/progression-free survival in metastasized stage III to IV melanoma: a pooled analysis of five European Organisation for Research and Treatment of Cancer randomized controlled trials. J Clin Oncol. 2013;31(18):2337-2346. [DOI] [PubMed] [Google Scholar]
- 17.Cho E, Rosner BA, Colditz GA. Risk factors for melanoma by body site. Cancer Epidemiol Biomarkers Prev. 2005;14(5):1241-1244. [DOI] [PubMed] [Google Scholar]
- 18.Pérez-Gómez B, Aragonés N, Gustavsson P, Lope V, López-Abente G, Pollán M. Do sex and site matter? different age distribution in melanoma of the trunk among Swedish men and women. Br J Dermatol. 2008;158(4):766-772. [DOI] [PubMed] [Google Scholar]
- 19.de Vries E, Nijsten TE, Visser O, et al. . Superior survival of females among 10,538 Dutch melanoma patients is independent of Breslow thickness, histologic type and tumor site. Ann Oncol. 2008;19(3):583-589. [DOI] [PubMed] [Google Scholar]
- 20.Wendt J, Schanab O, Binder M, Pehamberger H, Okamoto I. Site-dependent actinic skin damage as risk factor for melanoma in a central European population. Pigment Cell Melanoma Res. 2012;25(2):234-242. [DOI] [PubMed] [Google Scholar]
- 21.Wendt J, Rauscher S, Burgstaller-Mühlbacher S, et al. . Actinic damage on the back is significantly determined by MC1R variants and previous sun exposure compared with other body sites in a multivariate analysis. Br J Dermatol. 2014;171(3):622-630. [DOI] [PubMed] [Google Scholar]
- 22.Müller C, Wendt J, Rauscher S, et al. . Characterization of patients at high risk of melanoma in Austria. Br J Dermatol. 2016;174(6):1308-1317. [DOI] [PubMed] [Google Scholar]
- 23.Landi MT, Bauer J, Pfeiffer RM, et al. . MC1R germline variants confer risk for BRAF-mutant melanoma. Science. 2006;313(5786):521-522. [DOI] [PubMed] [Google Scholar]
- 24.Smith R, Healy E, Siddiqui S, et al. . Melanocortin 1 receptor variants in an Irish population. J Invest Dermatol. 1998;111(1):119-122. [DOI] [PubMed] [Google Scholar]
- 25.Kanetsky PA, Panossian S, Elder DE, et al. . Does MC1R genotype convey information about melanoma risk beyond risk phenotypes? Cancer. 2010;116(10):2416-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmer JS, Duffy DL, Box NF, et al. . Melanocortin-1 receptor polymorphisms and risk of melanoma: is the association explained solely by pigmentation phenotype? Am J Hum Genet. 2000;66(1):176-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beaumont KA, Shekar SN, Cook AL, Duffy DL, Sturm RA. Red hair is the null phenotype of MC1R. Hum Mutat. 2008;29(8):E88-E94. [DOI] [PubMed] [Google Scholar]
- 28.Weir HK, Marrett LD, Cokkinides V, et al. . Melanoma in adolescents and young adults (ages 15-39 years): United States, 1999-2006. J Am Acad Dermatol. 2011;65(5)(suppl 1):S38-S49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reed KB, Brewer JD, Lohse CM, Bringe KE, Pruitt CN, Gibson LE. Increasing incidence of melanoma among young adults: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2012;87(4):328-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chevalier V, Barbe C, Le Clainche A, et al. . Comparison of anatomical locations of cutaneous melanoma in men and women: a population-based study in France. Br J Dermatol. 2014;171(3):595-601. [DOI] [PubMed] [Google Scholar]
- 31.Nosrati A, Wei ML. Sex disparities in melanoma outcomes: the role of biology. Arch Biochem Biophys. 2014;563:42-50. [DOI] [PubMed] [Google Scholar]
- 32.Mitra D, Luo X, Morgan A, et al. . An ultraviolet-radiation-independent pathway to melanoma carcinogenesis in the red hair/fair skin background. Nature. 2012;491(7424):449-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hernando B, Ibarrola-Villava M, Fernandez LP, et al. . Sex-specific genetic effects associated with pigmentation, sensitivity to sunlight, and melanoma in a population of Spanish origin. Biol Sex Differ. 2016;7:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirobe T, Kiuchi M, Wakamatsu K, Ito S. Estrogen increases hair pigmentation in female recessive yellow mice. Zoolog Sci. 2010;27(6):470-476. [DOI] [PubMed] [Google Scholar]
- 35.Thornton MJ. The biological actions of estrogens on skin. Exp Dermatol. 2002;11(6):487-502. [DOI] [PubMed] [Google Scholar]
- 36.Handel AC, Miot LD, Miot HA. Melasma: a clinical and epidemiological review. An Bras Dermatol. 2014;89(5):771-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scott MC, Suzuki I, Abdel-Malek ZA. Regulation of the human melanocortin 1 receptor expression in epidermal melanocytes by paracrine and endocrine factors and by ultraviolet radiation. Pigment Cell Res. 2002;15(6):433-439. [DOI] [PubMed] [Google Scholar]
- 38.Kim NH, Cheong KA, Lee TR, Lee AY. PDZK1 upregulation in estrogen-related hyperpigmentation in melasma. J Invest Dermatol. 2012;132(11):2622-2631. [DOI] [PubMed] [Google Scholar]
- 39.Jemal A, Saraiya M, Patel P, et al. . Recent trends in cutaneous melanoma incidence and death rates in the United States, 1992-2006. J Am Acad Dermatol. 2011;65(5)(suppl 1):S17-25.e1, 3. [DOI] [PubMed] [Google Scholar]
- 40.Saraiya M, Hall HI, Thompson T, et al. . Skin cancer screening among U.S. adults from 1992, 1998, and 2000 National Health Interview Surveys. Prev Med. 2004;39(2):308-314. [DOI] [PubMed] [Google Scholar]
- 41.Frost P. Sexual selection and human geographic variation. J Soc Evol Cult Psychol. 2008;2(4):169-191. [Google Scholar]
- 42.Thornton MJ. Human skin: a mirror for estrogen action? Menopause. 2016;23(2):119-120. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Pigmentation phenotype in males and females stratified for Cases and Controls
eTable 2. Frequency of MC1R variants in males and females
eTable 3a. Sun exposure variables and melanoma risk in males
eTable 3b. Sun exposure variables and melanoma risk in females
eTable 4a. MC1R variants and pigmentation phenotype in males
eTable 4b. MC1R variants and pigmentation phenotype in females
eTable 5a. MC1R variants and pigmentation phenotype in Cases
eTable 5b. MC1R variants and pigmentation phenotype in Controls