Key Points
Question
Is the retinoic acid receptor β (RARβ) tumor-suppressor gene involved in the onset and/or progression of lichen sclerosus-associated vulvar squamous cell carcinoma (LS-VSCC)?
Findings
In this case-control study of 80 tissue specimens, RARβ expression was strongly downregulated by promoter methylation in LS-VSCC. The degree of promoter methylation correlated with the severity of LS-VSCC: full promoter methylation of RARβ was associated with LS-VSCC among patients with lymph nodes metastasis at diagnosis who subsequently experienced recurrence.
Meaning
RARβ dysregulation may play a role in the tumorigenic process of LS-VSCC and promoter methylation of RARβ may be used as a clinical prognostic marker in patients with LS-VSCC.
This case-control study examines the association of retinoic acid receptor β tumor-suppressor gene with the onset and progression of lichen sclerosus–associated vulvar squamous cell carcinoma.
Abstract
Importance
Molecular alterations in lichen sclerosus–associated vulvar squamous cell carcinoma (LS-VSCC) are largely unknown.
Objective
To determine whether the retinoic acid receptor β (RARβ) tumor-suppressor gene is involved in the onset and/or progression of LS-VSCC.
Design, Setting, and Participants
The case-control study, conducted at University-Hospital of Ferrara, Italy, included 20 LS-VSCC (mean [SD] age, 75 [3] years) and 20 cancer-associated vulvar LS (caVLS; mean [SD] age, 62 [11] years) formalin-fixed embedded tissue specimens, 20 cancer-free vulvar LS (cfVLS), and 20 normal skin fresh specimens from diagnostic biopsies and women surgically treated for nonmalignant skin lesions, respectively. RARβ gene expression and promoter methylation were investigated in LS-VSCC and caVLS adjacent to VSCC specimens, and in cfVLS and normal skin specimens, as controls, by RT-Q real-time polymerase chain reaction (PCR) analysis, and sequencing of PCR-amplified bisulfite-treated DNA. c-Jun expression, an RARβ pathway–related gene, was also investigated.
Main Outcomes and Measures
RARβ expression, correlation with its promoter methylation and c-Jun expression, and association with onset or progression of LS-VSCC.
Results
In LS-VSCC, RARβ messenger RNA was 3.4-, 3.6-, and 4.8-fold lower than in caVLS (P = .001), cfVLS (P = .005), and normal skin (P < .001), respectively. The RARβ mRNA levels were similar in caVLS, cfVLS, and normal skin. The RARβ promoter was hypermethylated in 18 (90%) of 20 LS-VSCC, 11 (55%) of 20 cfVLS, 10 (50%) of 20 caVLS, and 5 (25%) of 20 in the normal skin group. The degree of methylation of RARβ promoter was higher in LS-VSCC, ranging from 5 to 9 (full promoter methylation) CpGs methylated, than in caVLS (P = .02), cfVLS (P = .03), or normal skin (P < .001), which was up to 5 CpGs methylated. Importantly, 0 of 8 LS-VSCC with 5 to 6 CpGs methylated and 5 (63%) of 8 LS-VSCC with 7 to 8 CpGs methylated were from patients with lymph node metastasis at diagnosis, respectively, whereas there were 2 of 2 (100%) LS-VSCC samples with 9 CpG methylated from patients with lymph node metastasis at diagnosis and subsequent recurrence. In LS-VSCC c-Jun mRNA was 4.3-, 1.4-, and 2.6-fold higher than in caVLS (P < .001), cfVLS (P = .001), and normal skin (P < .001), respectively. The expression of c-Jun was similar in caVLS, cfVLS, and normal skin.
Conclusions and Relevance
Hypermethylation-induced RARβ down-expression was associated with LS-VSCC and correlates with the upregulation of c-Jun. The degree of methylation of RARβ promoter increased with the malignancy of LS-VSCC. Therefore, RARβ gene dysregulation may play a role in progression of LS-VSCC, and RARβ promoter methylation status may be used as a prognostic marker in clinical treatment of patients with LS-VSCC.
Introduction
Vulvar squamous cell carcinoma (VSCC) represents 5% of all female genital cancers.1 Up to 80% of all VSCCs arises in a background of inflammatory vulvar dermatosis, typically lichen sclerosus (LS).2,3 The molecular alterations involved in onset and/or progression of LS-associated VSCC (LS-VSCC) are largely unknown.
The RARβ gene is a tumor suppressor gene encoding retinoic acid receptor β (RARβ), a member of nuclear receptor superfamily.4 The RARβ protein binds retinoic acid, the biologically active form of vitamin A, which mediates cellular signaling in embryonic morphogenesis, cell growth, and differentiation.4 Loss of RARβ activity, especially through gene promoter methylation, has been observed in different types of cancers.5,6,7 The tumor suppressor function of RARβ depends on down-regulatory activity of activator protein-1 (AP-1), a transcription factor composed of proto-oncoproteins c-Jun and c-Fos, which is a positive regulator of cell proliferation.8
The aim of this study was to investigate the role of the RARβ gene in onset/progression of LS-VSCC. Therefore, levels of mRNA expression and promoter methylation of RARβ were assessed in pathologic specimens including LS-VSCC, cancer-associated vulvar LS, cancer-free vulvar LS, and in normal skin specimens. In addition, the expression level of the c-Jun gene and its relationship with RARβ expression were investigated.
Methods
Tissue samples were from University-Hospital of Ferrara, Ferrara, Italy. They included: (1) 20 formalin-fixed and paraffin-embedded VSCC specimens adjacent to LS (LS-VSCC) (HPV-negative); (2) 20 cancer-associated VLS (caVLS) formalin-fixed and paraffin-embedded specimens adjacent to VSCC; (3) 20 cancer-free VLS (cfVLS) fresh specimens obtained from diagnostic biopsies; (4) 20 normal fresh skin specimens obtained from women surgically treated for nonmalignant skin lesions. The study was approved by the University-Hospital of Ferrara institutional review board. Patients provided their written informed consent. DNA/RNA from formalin-fixed and paraffin-embedded and fresh tissue specimens were extracted as described.9,10
Participant DNA was treated with sodium bisulfite using Epitect Bisulfite kit (Qiagen).9 Methylation was assayed at the RARβ promoter (OMIM: 180220) by sequencing the PCR-amplified bisulfite-treated DNA using the automated ABI-Prism-3130X DNA sequencer (Applied).9 The RARβ promoter region studied contained 9 CpGs within a polymerase chain reaction (PCR) amplicon of 86 bp.11
The RNA was reverse transcribed with random primers using RNA-to-cDNA Kit (Roche). RARβ and c-Jun messenger RNA (mRNA) expressions were analyzed with the ABI7500 fast real-time PCR system (Thermofisher) using Power SYBR Green PCR Master Mix (Sigma-Aldrich).8,12,13 The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was used as an internal control. A series of quantitative reverse transcription PCR (RT-qPCR) assays were performed 3 times, each with 3 repeated measures. Data analysis was performed with the 2−ΔΔCt method.13
The χ2 trend test with Yate’s correction was used to compare the observed RARβ epigenotypes among LS-VSCC, caVLS, cfVLS, and normal skin specimens. Statistical analyses were carried out with GraphPad Prism for Windows (version 5.0, GraphPad). P < .05 was considered statistically significant.
Results
Analysis of RARβ mRNA Expression
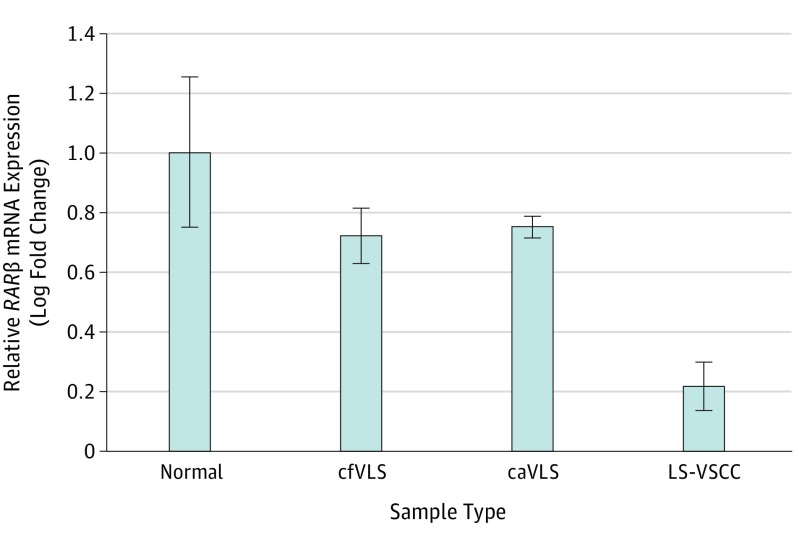
In LS-VSCC specimens, RARβ mRNA results were 3.4-fold and 3.6-fold downregulated compared with caVLS (P = .001) and cfVLS (P = .005), respectively, and 4.8-fold downregulated compared with normal skin specimens (P < .001) (Figure 1). In caVLS and cfVLS specimens, the RARβ mRNA expression results were statistically similar to those in normal skin specimens.
Figure 1. Relative RARβ messenger RNA (mRNA) Expression in Normal Skin, cfVLS, caVLS, and LS-VSCC Tissues Measured by RT-qPCRa.
caVLS indicates cancer-associated vulvar lichen sclerosus; cfVLS, cancer-free vulvar lichen sclerosus; LS-VSCC, lichen sclerosus-associated vulvar squamous cell carcinoma; RARβ, retinoic acid receptor β; RT-qPCR, quantitative reverse transcription polymerase chain reaction. Relative expression was calculated with the ΔΔCt method. Data are expressed as the ratio of the number of copies of the target gene relative to GAPDH. The results are expressed as a relative fold change (2-ΔΔCt) over the value of normal skin specimens. The bar graphs represent relative fold change (mean [standard error of the mean], for n = 20).
aLS-VSCC vs normal skin (P < .001), vs cfVLS (P = .005), and vs caVLS (P = .001), respectively.
RARβ Promoter Methylation Analysis
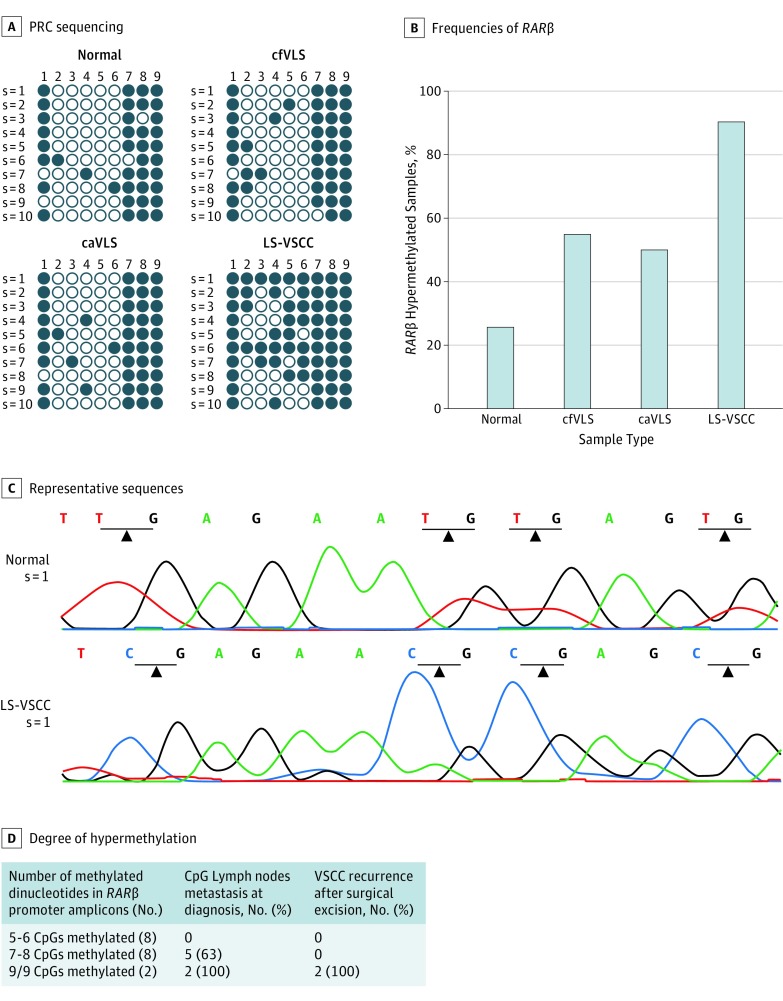
To investigate whether RARβ gene expression might be modified by its promoter methylation, the methylation status of the RARβ promoter region was investigated by sequencing analysis of the RARβ-PCR amplicons in specimens. RARβ hypermethylated amplicons, ie, amplicons showing 50% or more methylated CpG dinucleotides, were taken into account in this analysis.
Overall, RARβ hypermethylation was detected in 18 of 20 (90%) LS-VSCC specimens, 10 of 20 (50%) caVLS, 11 of 20 (55%) cfVLS, and 5 of 20 (25%) normal skin specimens. The differences in RARβ hypermethylation between LS-VSCC (P = .02) and caVLS (P = .03), cfVLS, and normal skin specimens were statistically significant (P < .001) (Figure 2). The degree of methylation of RARβ promoter was higher in LS-VSCC, ranging from 5 to 9 (full promoter methylation) CpGs methylated, than in caVLS, cfVLS and normal skin specimens, which was up to 5 CpGs methylated. No differences in RARβ promoter hypermethylation frequencies were assessed among caVLS, cfVLS, and normal skin groups (P > .05). Figure 2D shows the clinical course of LS-VSCC patients according to their degree of hypermethylation.
Figure 2. RARβ Promoter Methylation Analysis in Normal Skin, cfVLS, caVLS, and LS-VSCC Tissues.
caVLS indicates cancer-associated vulvar lichen sclerosus; cfVLS, cancer-free vulvar lichen sclerosus; LS-VSCC, lichen sclerosus-associated vulvar squamous cell carcinoma. A, Bisulfite–polymerase chain reaction (PCR) sequencing of the RARβ promoter region in representative specimens of normal skin, cfVLS, caVLS, and LS-VSCC tissues. The filled-in and clear circles represent methylated and unmethylated CpG dinucleotides, respectively. The CpGs in the RARβ locus are numbered across the top of the grid. Each row represents 1 PCR product. B, Frequencies of RARβ hypermethylation in normal skin, cfVLS, caVLS, and VSCC tissues. Only clones showing 50% or more methylated CpG islands were considered hypermethylated in this analysis. LS-VSCC vs normal skin (P < .001), vs cfVLS (P = .03), and vs caVLS (P = .02), respectively. C, Two representative sequences showing 4 CpGs (2-5) (unmethylated and methylated in normal and LS-VSCC specimens, respectively) in the RARβ PCR product. D, Degree of RARβ promoter region hypermethylation in relation with clinical course of LS-VSCC, namely lymph node metastasis at diagnosis and recurrence after complete surgical excision.
Analysis of c-Jun mRNA Expression
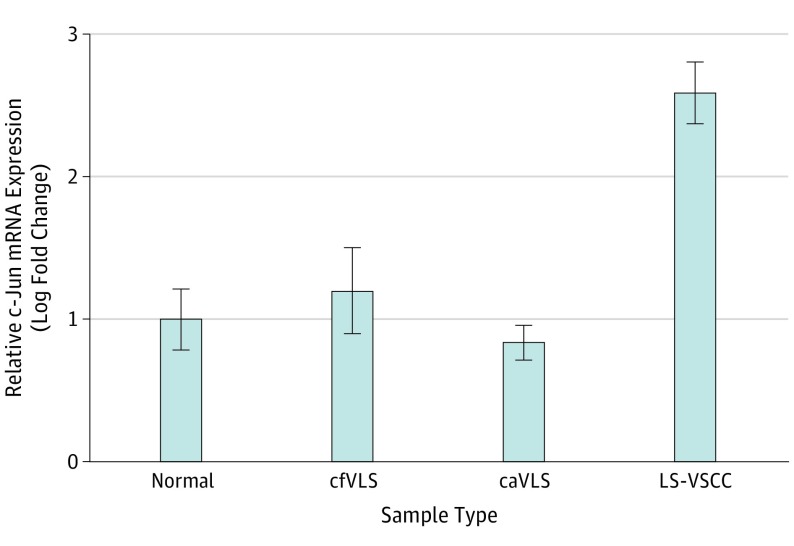
Expression of c-Jun mRNA was measured by RT-qPCR in the same specimens analyzed for RARβ mRNA expression. In LS-VSCC c-Jun mRNA was increased by 4.3-fold and 1.4-fold compared with caVLS (P < .001) and cfVLS (P = .001), respectively, and by 2.6-fold compared with normal skin specimens (P < .001) (Figure 3). In cfVLS and caVLS specimens, the c-Jun mRNA expression was statistically similar to that in normal skin specimens.
Figure 3. Relative c-Jun mRNA Expression in Normal Skin, cfVLS, caVLS, and LS-VSCC Tissues Measured by RT-qPCR.
caVLS indicates cancer-associated vulvar lichen sclerosus; cfVLS, cancer-free vulvar lichen sclerosus; LS-VSCC, lichen sclerosus-associated vulvar squamous cell carcinoma; RARβ, retinoic acid receptor β; RT-qPCR, quantitative reverse transcription polymerase chain reaction. Relative expression was calculated with the ΔΔCt method. Data are expressed as the ratio of the number of copies of the target gene relative to GAPDH. The results are expressed as a relative fold change (2-ΔΔCt) over the value of normal skin specimens. The bar graphs represent relative fold change (mean [standard error of the mean (SEM)], for n = 20). LS-VSCC vs normal skin (P < .001), vs cfVLS (P = .001), and vs caVLS (P < .001), respectively.
Discussion
To date, few reports have addressed the epigenetic alterations involved in VSCC. Hypermethylation of some genes has been described in VSCC, associated or not with LS, indicating that aberrant promoter methylation is a common event in VSCC.2,10,14 Moreover, some gene promoters have been found to be methylated in progression from VLS to VSCC, suggesting that such epigenetic deregulations are early events affecting VLS.2,10,14
In this study, RARβ gene promoter was found to be strongly methylated and consequently downexpressed in LS-VSCC compared with caVLS, cfVLS, and normal skin, indicating that RARβ dysregulation may play a role in the etiopathogenesis of LS-VSCC. In contrast, RARβ promoter methylation and gene expression were found to be at similar levels in caVLS, cfVLS, and normal skin, indicating that RARβ methylation is a specific risk factor in development of LS-VSCC. These data are in agreement with other studies reporting methylation-induced RARβ downexpression in different carcinomas.5,6 To our knowledge, this is the first report on the hypermethylation of RARβ gene in LS-VSCC.
We found that RARβ promoter showed different degrees of methylation, ranging from 5 to 9 CpGs methylated, in LS-VSCC. importantly, none of the LS-VSCC with 5 to 6 CpGs methylated, and 5 of 8 (63%) LS-VSCC with 7 to 8 CpGs methylated were from patients with lymph node metastases at diagnosis, respectively. Of note, 2 of 2 (100%) LS-VSCC with 9 of 9 CpGs methylated were from patients with lymph node metastases at diagnosis and subsequent recurrence. These data indicate an association between degree of methylation of RARβ and clinical course, and suggest that promoter methylation status may be a prognostic factor in patients with LS-VSCC.
It is well known that cancer develops in a stepwise manner through inactivation of tumor suppressor genes and activation of oncogenes. In this study, mRNA levels of c-Jun tested higher in LS-VSCC than in caVLS, cfVLS, and normal skin, underscoring a cross-link between the RARβ tumor suppressor gene and the c-Jun oncogene. Overexpression of c-Jun has been found in several human cancers,15 but there is no report of its dysregulation in LS-VSCC. As RARβ counteracts cell proliferation through c-Jun degradation,8 our results indicate that loss of RARβ gene function may be linked to c-Jun dysregulation in LS-VSCC.
Limitations
The limitation of this study is the small sample size of specimens analyzed.
Conclusions
Results of this study indicate that the RARβ gene is hypermethylated and consequently downexpressed in LS-VSCC, and that there is an inverse correlation between RARβ expression and c-Jun expression, indicating that RARβ gene dysregulation may play a role in the tumorigenic multiphase process of LS-VSCC. We also show that the degree of methylation of RARβ promoter correlates with the clinical course in patients with LS-VSCC. Therefore, RARβ promoter methylation status may be used as a prognostic factor in clinical treatment of patients with LS-VSCC.
References
- 1.Clancy AA, Spaans JN, Weberpals JI. The forgotten woman’s cancer: vulvar squamous cell carcinoma (VSCC) and a targeted approach to therapy. Ann Oncol. 2016;27(9):1696-1705. [DOI] [PubMed] [Google Scholar]
- 2.Trietsch MD, Nooij LS, Gaarenstroom KN, van Poelgeest MI. Genetic and epigenetic changes in vulvar squamous cell carcinoma and its precursor lesions: a review of the current literature. Gynecol Oncol. 2015;136(1):143-157. [DOI] [PubMed] [Google Scholar]
- 3.Virgili A, Borghi A, Toni G, Minghetti S, Corazza M. Prospective clinical and epidemiologic study of vulvar lichen sclerosus: analysis of prevalence and severity of clinical features, together with historical and demographic associations. Dermatology. 2014;228(2):145-151. [DOI] [PubMed] [Google Scholar]
- 4.di Masi A, Leboffe L, De Marinis E, et al. . Retinoic acid receptors: from molecular mechanisms to cancer therapy. Mol Aspects Med. 2015;41:1-115. [DOI] [PubMed] [Google Scholar]
- 5.Ivanova T, Petrenko A, Gritsko T, et al. . Methylation and silencing of the retinoic acid receptor-beta 2 gene in cervical cancer. BMC Cancer. 2002;2(2):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Youssef EM, Lotan D, Issa JP, et al. . Hypermethylation of the retinoic acid receptor-beta(2) gene in head and neck carcinogenesis. Clin Cancer Res. 2004;10(5):1733-1742. [DOI] [PubMed] [Google Scholar]
- 7.Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991;1072(2-3):129-157. [DOI] [PubMed] [Google Scholar]
- 8.De-Castro Arce J, Soto U, van Riggelen J, Schwarz E, zur Hausen H, Rösl F. Ectopic expression of nonliganded retinoic acid receptor beta abrogates AP-1 activity by selective degradation of c-Jun in cervical carcinoma cells. J Biol Chem. 2004;279(44):45408-45416. [DOI] [PubMed] [Google Scholar]
- 9.Rotondo JC, Selvatici R, Di Domenico M, et al. . Methylation loss at H19 imprinted gene correlates with methylenetetrahydrofolate reductase gene promoter hypermethylation in semen samples from infertile males. Epigenetics. 2013;8(9):990-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rotondo JC, Borghi A, Selvatici R, et al. . Hypermethylation-induced inactivation of the IRF6 gene as a possible early event in progression of vulvar squamous cell carcinoma associated with lichen sclerosus. JAMA Dermatol. 2016;152(8):928-933. [DOI] [PubMed] [Google Scholar]
- 11.Park SY, Kwon HJ, Lee HE, et al. . Promoter CpG island hypermethylation during breast cancer progression. Virchows Arch. 2011;458(1):73-84. [DOI] [PubMed] [Google Scholar]
- 12.Bohlken A, Cheung BB, Bell JL, et al. . ATP7A is a novel target of retinoic acid receptor beta2 in neuroblastoma cells. Br J Cancer. 2009;100(1):96-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rotondo JC, Bosi S, Bassi C, et al. . Gene expression changes in progression of cervical neoplasia revealed by microarray analysis of cervical neoplastic keratinocytes. J Cell Physiol. 2015;230(4):806-812. [DOI] [PubMed] [Google Scholar]
- 14.Guerrero D, Guarch R, Ojer A, et al. . Differential hypermethylation of genes in vulvar cancer and lichen sclerosus coexisting or not with vulvar cancer. Int J Cancer. 2011;128(12):2853-2864. [DOI] [PubMed] [Google Scholar]
- 15.Blau L, Knirsh R, Ben-Dror I, et al. . Aberrant expression of c-Jun in glioblastoma by internal ribosome entry site (IRES)-mediated translational activation. Proc Natl Acad Sci U S A. 2012;109(42):E2875-E2884. [DOI] [PMC free article] [PubMed] [Google Scholar]