Key Points
Question
What is the role of the type 1/type 17 immune response in hidradenitis suppurativa (HS)?
Findings
In this semantic map analysis study of a series of 24 patients with HS, the map shows a convincing clustering of all T helper 1/T helper 17–associated cytokines (interleukin 17 [IL-17], interferon γ, IL-12, IL-23, IL-32, IL-1β, tumor necrosis factor) around overall lesional inflammation, highlighting the importance of the T helper 1/T helper 17 cytokines in HS pathogenesis.
Meaning
These findings suggest that HS is a T helper 1/T helper 17–driven inflammatory skin disease.
This case series uses semantic map analysis to assess the association of hidradenitis suppurativa with T helper 1/T helper 17 phenotypes.
Abstract
Importance
In spite of progress in understanding the mechanisms underlying hidradenitis suppurativa (HS) as an inflammatory skin disease, there is still a demand for an overview on immunopathogenesis of HS.
Objective
To demonstrate the importance of the type 1/type 17 immune response in lesional HS skin by drawing a semantic connectivity map.
Design, Setting, and Participants
Single-center case series of 24 patients with HS. Association of HS with T helper 1/T helper 17 (TH1/TH17) phenotype was assessed using semantic map analysis.
Main Outcomes and Measures
Association of HS with TH1/TH17 phenotype.
Results
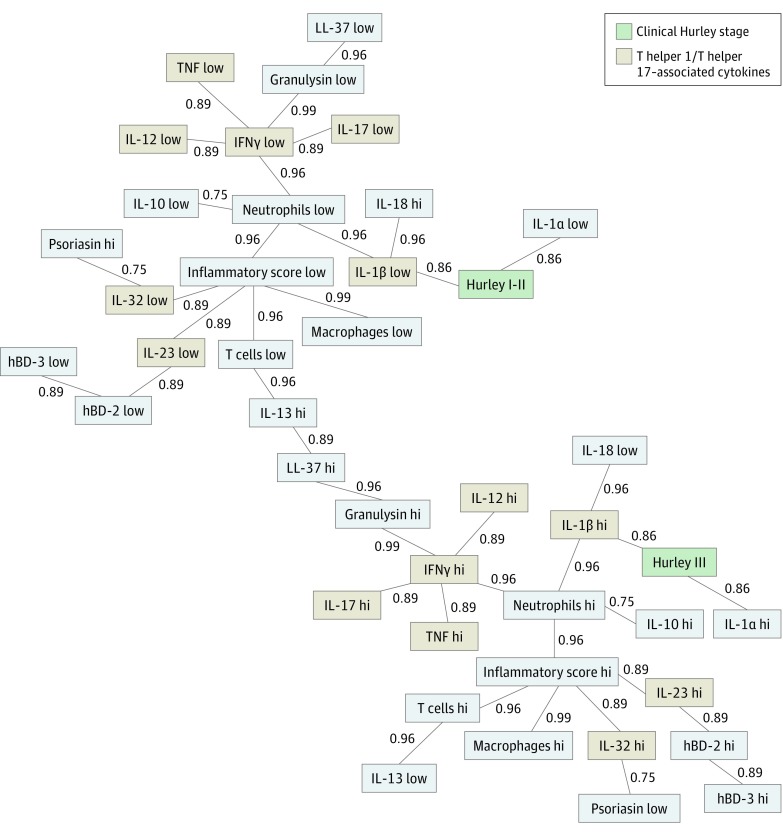
The analysis was performed on 24 lesional HS biopsy samples from untreated patients with HS (16 [67%] female; median age, 36.5 years [range, 21-51 years]) with a mean (SD) Hurley stage of 2.29 (0.62) and 9 punch biopsy samples from healthy controls (6 [67%] female; median age, 43 years [range, 23-66 years]). The map shows a clustering of all TH1/TH17-associated cytokines (interleukin 17 [IL-17], interferon γ, IL-12, IL-23, IL-32, IL-1β, tumor necrosis factor) around overall lesional inflammation. Tumor necrosis factor, IL-12, and IL-17 are even directly connected via interferon γ. In contrast, IL-13, a TH2-associated cytokine, was inversely correlated with the presence of TH1/TH17-associated cytokines, further highlighting the importance of the TH1/TH17 cytokines in HS pathogenesis.
Conclusions and Relevance
These findings suggest that HS may be a TH1/TH17-driven inflammatory skin disease.
Introduction
Hidradenitis suppurativa (HS) is one of the most distressing conditions in dermatology, with a remarkable negative effect on the quality of life of affected patients.1 The pathogenesis of HS remains unclear. However, according to previous histologic studies, it is a multifocal inflammatory skin disease, in which atrophy of the sebaceous glands is followed by an early lymphocytic inflammation and hyperkeratosis of the pilosebaceous unit and, later, by granuloma formation and hair follicle destruction.1,2,3 Previous studies reported the expression of various cytokines in the lesional HS skin.4,5,6,7,8,9 Nevertheless, there is still a large demand for an overview to clarify the role of type 1/type 17 immune response in HS. To shed more light on immunopathogenesis of HS, the present study aimed to demonstrate the importance of these important axes in lesional HS skin by drawing a semantic connectivity map.
Methods
For this study, 24 lesional HS biopsy samples from untreated patients with HS (16 women and 8 men; median age, 36.5 years) with a mean Hurley stage of 2.25 and 9 punch biopsy samples from healthy controls (6 women and 3 men; median age, 41 years) were obtained (Table). Informed consent (oral and written) was obtained from the study participants. Furthermore, institutional review board approval was obtained and all procedures were carried out in accordance with the standards of the ethics committee of the Canton of Bern, Switzerland, on human experimentation and with the Helsinki Declaration.
Table. Participant Characteristics.
| Characteristic | Patients With Hidradenitis Suppurativa (n = 24) | Healthy Controls (n = 9) |
|---|---|---|
| Sex, No. (%) | ||
| Female | 16 (67) | 6 (67) |
| Male | 8 (33) | 3 (33) |
| White race, No. (%) | 24 (100) | 9 (100) |
| Age, median (range), y | 36.5 (21-51) | 43 (23-66) |
| Clinical Hurley stage, mean (SD) | 2.29 (0.62) | NA |
Abbreviation: NA, not applicable.
Sampling and Testing
After the biopsy samples were snap-frozen, messenger RNA (mRNA) was extracted, processed into complementary DNA, and analyzed by semiquantitative real-time polymerase chain reaction. Lesional mRNA expression of tumor necrosis factor (TNF), interferon γ (IFNγ), interleukin 10 (IL-10), IL-12, IL-13, IL-17, IL-23, IL-32, LL-37, psoriasin, human β-defensin 2 (hBD-2) and hBD-3, as well as IL-1α, IL-18, and granulysin was normalized to healthy skin. At the same time, tissue sections were cut from the same 24 HS biopsies and analyzed by immunohistochemical staining for the presence of lesional inflammation (semiquantitative score taking into consideration the spread of inflammation over the whole biopsy of the presence of neutrophils [CD66b], T cells [CD3], and macrophages [CD68] and the mean of these 3 scores was entitled as inflammatory score). All these methods were performed as previously described.9 For analysis purposes, continuous variables (normalized mRNA levels and semiquantitative immunohistochemistry scoring) were categorized into high vs low values by using their medians as cutoff points.
Semantic Map Analysis
Associations among categorized factors were analyzed by means of an algorithm that is able to put variables in reciprocal correlation and display the best connections among them.10 Briefly, sequential univariate logistic regression models were fitted by taking each time one variable as the predictor and the other as the dependent factor. This process is reiterated until all variables in the model are processed. Due to the relatively low numbers of patients in the study, only univariate analysis was conducted. Finally, a matrix of normalized weights is produced from the regression coefficients. A mathematical filter, the maximum spanning tree,10 is then applied to the matrix of weights and a semantic connectivity map is generated. In the resulting map, direct lines between variables show the strongest positive associations; these are quantified with a number between 0 (no correlation) and 1 (perfect correlation). In addition, spatial proximity between variables indicates patterns of direct correlations. The analysis was carried out using MATLAB, version 7.8 (MathWorks).
Results
Our map shows a clustering of all TH1/TH17-associated cytokines (IL-17, IFNγ, IL-12, IL-23, IL-32, IL-1β, TNF) around overall lesional inflammation. Tumor necrosis factor, IL-12, and IL-17 are even directly connected via IFNγ. In contrast, IL-13, a TH2-associated cytokine, correlates inversely with the presence of TH1/TH17-associated cytokines, further highlighting the importance of the TH1/TH17 cytokines in HS pathogenesis. Interestingly, patients with a clinical Hurley stage III are not only closely associated with high levels of IL-1β, but also with acute inflammation (high levels of neutrophils) and the anti-inflammatory IL-10. Meanwhile, Hurley stages I and II are more closely associated with low amounts of macrophages and also low levels of IL-1β (Figure).
Figure. Semantic Connectivity Map of Important Players in Hidradenitis Suppurativa (HS) Pathogenesis.
The map shows the best associations (in a normalized range between 0 and 1) among variables in lesional HS skin. Continuous variables were categorized as high (hi) vs low values based on their medians. Direct lines show the strongest positive associations, while spatial proximity between variables also indicates patterns of direct correlations. hBD-2 indicates human β-defensin 2; IFNγ, interferon γ; IL-1α, interleukin 1α; inflammatory score, mean of the scores of T cells, macrophages, and neutrophils; TNF, tumor necrosis factor.
Discussion
The present study presents an interesting clustering of all TH1/TH17-associated cytokines around the lesional inflammation using the semantic map analysis. Furthermore, to our knowledge, we report for the first time that IL-1α and granulysin are upregulated in lesional HS skin compared with healthy skin. Similar to the present study, previous studies reported that the expression of cytokines TNF, IFNγ, IL-10, IL-12, IL-17, IL-23, and IL-32 and antimicrobial peptides LL-37, psoriasin, and β-defensins (hBD) 2 and 3 is elevated in lesional HS skin.4,5,6,7,8,9
In early stages of HS, neutrophilic abscess formation and influx of monocytes and macrophages and dendritic cells are prominent, whereas in the chronic state, the infiltrate expands with increased frequencies of plasma cells and B cells.11,12 Furthermore, the proinflammatory cytokines IL-23 and IL-12 are expressed by macrophages infiltrating papillary and reticular dermis,5,11 which are believed to be important mediators in autoimmune tissue destruction.11,13 Especially, IL-23 has been shown to be involved in the induction of a T helper cell subset producing IL-17, which were found to infiltrate the dermis in the chronic HS lesions.11,14 Additionally, IL-12 produced in macrophages is important for the differentiation of TH1 cells.15
Limitations
One possible limitation of this study is that the analysis is mainly exploratory and specific hypotheses emerging from the map should be tested in further ad hoc studies. Another limitation is the relatively low number of patients included in the analysis, which does not allow generalization of results.
Conclusions
The results of the present study suggest that HS is a TH1/TH17-driven inflammatory skin disease, which could raise the idea that patients with HS might benefit from anti-TH1/TH17 treatment strategies. However, the relevance of the single cytokines in HS pathogenesis remains to be established.
References
- 1.Jemec GBE. Hidradenitis suppurativa. N Engl J Med. 2012;366(2):158-164. [DOI] [PubMed] [Google Scholar]
- 2.Kamp S, Fiehn AM, Stenderup K, et al. . Hidradenitis suppurativa: a disease of the absent sebaceous gland? sebaceous gland number and volume are significantly reduced in uninvolved hair follicles from patients with hidradenitis suppurativa. Br J Dermatol. 2011;164(5):1017-1022. [DOI] [PubMed] [Google Scholar]
- 3.Houriet C, Seyed Jafari SM, Thomi R, et al. . Canakinumab for severe hidradenitis suppurativa: preliminary experience in 2 cases. JAMA Dermatol. 2017;153(11):1195-1197. [DOI] [PubMed] [Google Scholar]
- 4.Wolk K, Warszawska K, Hoeflich C, et al. . Deficiency of IL-22 contributes to a chronic inflammatory disease: pathogenetic mechanisms in acne inversa. J Immunol. 2011;186(2):1228-1239. [DOI] [PubMed] [Google Scholar]
- 5.Schlapbach C, Hänni T, Yawalkar N, Hunger RE. Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. J Am Acad Dermatol. 2011;65(4):790-798. [DOI] [PubMed] [Google Scholar]
- 6.van der Zee HH, de Ruiter L, van den Broecke DG, Dik WA, Laman JD, Prens EP. Elevated levels of tumour necrosis factor (TNF)-α, interleukin (IL)-1β and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-α and IL-1β. Br J Dermatol. 2011;164(6):1292-1298. [DOI] [PubMed] [Google Scholar]
- 7.Bechara FG, Sand M, Skrygan M, Kreuter A, Altmeyer P, Gambichler T. Acne inversa: evaluating antimicrobial peptides and proteins. Ann Dermatol. 2012;24(4):393-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hotz C, Boniotto M, Guguin A, et al. . Intrinsic defect in keratinocyte function leads to inflammation in hidradenitis suppurativa. J Invest Dermatol. 2016;136(9):1768-1780. [DOI] [PubMed] [Google Scholar]
- 9.Thomi R, Yerly D, Yawalkar N, Simon D, Schlapbach C, Hunger RE. Interleukin-32 is highly expressed in lesions of hidradenitis suppurativa. Br J Dermatol. 2017;177(5):1358-1366. [DOI] [PubMed] [Google Scholar]
- 10.Pemmaraju S, Skiena S. Computational Discrete Mathematics: Combinatorics and Graph Theory with Mathematica. New York, NY: Cambridge University Press; 2003. [Google Scholar]
- 11.Zouboulis CC, Desai N, Emtestam L, et al. . European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol. 2015;29(4):619-644. [DOI] [PubMed] [Google Scholar]
- 12.van der Zee HH, de Ruiter L, Boer J, et al. . Alterations in leucocyte subsets and histomorphology in normal-appearing perilesional skin and early and chronic hidradenitis suppurativa lesions. Br J Dermatol. 2012;166(1):98-106. [DOI] [PubMed] [Google Scholar]
- 13.Griffiths CE, Strober BE, van de Kerkhof P, et al. ; ACCEPT Study Group . Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362(2):118-128. [DOI] [PubMed] [Google Scholar]
- 14.van der Zee HH, Laman JD, de Ruiter L, Dik WA, Prens EP. Adalimumab (antitumour necrosis factor-α) treatment of hidradenitis suppurativa ameliorates skin inflammation: an in situ and ex vivo study. Br J Dermatol. 2012;166(2):298-305. [DOI] [PubMed] [Google Scholar]
- 15.Liu J, Chen M, Wang X. Calcitonin gene-related peptide inhibits lipopolysaccharide-induced interleukin-12 release from mouse peritoneal macrophages, mediated by the cAMP pathway. Immunology. 2000;101(1):61-67. [DOI] [PMC free article] [PubMed] [Google Scholar]