Key Points
Question
Does sirolimus gel, 0.2%, demonstrate efficacy, safety, and tolerability for treatment of facial angiofibromas in pediatric and adult patients with tuberous sclerosis complex?
Findings
In this multicenter, randomized clinical trial that included 62 pediatric and adult patients, sirolimus gel, 0.2%, significantly improved facial angiofibromas compared with placebo without requiring treatment discontinuation due to adverse events during the 12-week treatment.
Meaning
Sirolimus gel, 0.2%, was clinically beneficial for pediatric and adult patients with tuberous sclerosis complex involving facial angiofibromas.
This randomized clinical trial assesses the efficacy and safety of sirolimus gel, 0.2% vs placebo for treatment of angiofibromas and skin lesions in adult and pediatric patients with tuberous sclerosis complex.
Abstract
Importance
Most patients with tuberous sclerosis complex (TSC), an autosomal-dominant disorder that is caused by the constitutive activation of mammalian target of rapamycin, experience disfigurement caused by skin lesions involving facial angiofibromas. Many have been left untreated because of a lack of therapeutic options that are less invasive than surgery or laser treatment.
Objective
To confirm the efficacy and safety of sirolimus gel, 0.2%, for treatment of patients with angiofibromas and/or skin lesions.
Design, Setting, and Patients
Multicenter, randomized clinical trial at 9 centers in Japan from December 2015 to October 2016 including 62 children and adults with TSC.
Interventions
Patients who developed angiofibromas were randomly assigned, in a 1:1 ratio, to receive sirolimus gel, 0.2%, or placebo, each applied topically twice daily for 12 weeks.
Main Outcomes and Measures
The primary end point was composite improvement in the size and color of angiofibromas in photographs at week 12 of treatment. It was assessed by an independent review committee comprising 3 blinded dermatologists who categorized patient results into the following 6 categories: “markedly improved,” “improved,” “slightly improved,” “unchanged,” “slightly aggravated,” and “aggravated.”
Results
Sixty-two patients (27 pediatric and 35 adult; 34 [55%] female; mean [SD] age, 22.5 [11.9] years) were enrolled and randomly assigned to receive sirolimus gel, 0.2% (30 patients), or placebo (32 patients). The response rates of angiofibromas at weeks 4, 8, and 12 of treatment were 0 each in the placebo group in contrast to 20% (95% CI, 8%-39%; P = .01), 43% (95% CI, 26%-63%; P < .001), and 60% (95% CI, 41%-77%; P < .001), respectively, in the sirolimus group. None of the 31 assessable patients in the placebo group were rated improved or better, and 26 of them (84%) were rated unchanged. In contrast, 5 (17%) and 13 (43%) patients in the sirolimus group were rated markedly improved and improved, respectively. Adverse events were mild to moderate and were observed in 27 (90%) and 22 (69%) patients in the sirolimus and placebo groups, respectively; however, none of the trial participants discontinued treatment. Acute pancreatitis developed as a serious adverse event in 1 patient in the sirolimus group, and the patient recovered soon after hospitalization without discontinuing treatment.
Conclusions and Relevance
Sirolimus gel, 0.2%, demonstrated a significant clinical benefit for patients with TSC involving angiofibromas, thus providing a promising therapeutic modality.
Trial Registration
ClinicalTrials.gov Identifier: NCT02635789
Introduction
Tuberous sclerosis complex (TSC), an autosomal-dominant disorder caused by the constitutive activation of mammalian target of rapamycin (mTOR), gives rise to hamartomas in multiple organs. Angiofibromas are the most predominant skin lesions observed in patients with TSC older than 5 years1,2,3 and characteristically consist of numerous pink to reddish papules or nodules that are typically located on the cheeks, nose, and chin. Most patients also develop plaques, hypomelanotic macules, ungual fibromas, and/or shagreen patches.3,4 These skin lesions are permanent, cause appearance concerns that considerably impair patient quality of life, and have been treated by invasive therapeutic modalities (eg, surgery and laser therapy) that are associated with a high risk for complications and sequelae.2 However, clinical outcomes of these treatments have been unsatisfactory due to the frequent recurrence of the lesions. Moreover, an invasive treatment may be difficult to administer in patients with extensive angiofibromas or severe intellectual impairment. In consequence, these patients frequently remain untreated even though they could benefit from a safe and effective therapy that is easy to administer.
Small clinical studies and single case reports of patients with TSC treated with topical sirolimus have been published.5,6,7,8,9,10,11,12,13,14,15,16 The majority were proof-of-concept studies that lacked either a positive control or placebo group. Moreover, their sample size was too small to provide adequate statistical power, and the medication was frequently prepared by the study site’s pharmacy.
We previously conducted a dose escalation, phase 2 randomized clinical trial of the sirolimus gel, 0.05%, 0.1%, and 0.2%, formulations in patients with TSC,17 and both pediatric and adult patients showed significant reductions in the size and color of angiofibromas. The objective of the present study was to confirm the efficacy and safety of sirolimus gel, 0.2%, for a larger number of patients with angiofibromas and/or skin lesions.
Methods
Study Design and Patients
The present study was designed as a phase 3, multicenter, randomized, double-blind, placebo-controlled trial consisting of 3 study phases: screening of patients, 12-week treatment, and 4-week follow-up. The study was conducted at 9 sites in Japan. Patients were eligible if they were aged 3 years or older, had a definitive diagnosis of TSC,18 displayed 3 or more reddish papules of facial angiofibromas (≥2 mm in diameter), and had difficulty or did not desire to undergo laser therapy and/or surgery. Written informed consent was obtained from competent adult patients and from legal representatives of patients younger than 20 years or patients with intellectual impairment. Exclusion criteria were (1) erosions, ulcers, or other skin lesions associated with angiofibromas; (2) inability to adequately photograph skin lesions; (3) significant comorbidities including poorly controlled dyslipidemia; and (4) local or systemic treatment with an mTOR inhibitor within 12 months prior to use of the investigational drug, or laser therapy or surgery within 6 months prior to use of the investigational drug. The trial protocol (Supplement 1) was reviewed by the Japanese Pharmaceutical and Medical Devices Agency and was approved by the institutional review board at each site. The trial was conducted under Good Clinical Practice and the Declaration of Helsinki. All patients were examined for screening before the initiation of treatment (baseline), at weeks 4, 8, and 12 of treatment, and at week 4 of follow-up. At each visit, patients were medically examined and their facial lesions were photographed with the same digital camera at all sites. A color chart with a scale was used to calibrate the color tones and clarity of all photographs taken and to measure the size of skin lesions.
The sirolimus gel, 0.2%, and the placebo of identical appearance were manufactured under Good Manufacturing Practice for Investigational New Drugs by Nobelpharma, Tokyo, Japan. The gel contained sirolimus, 0.2% (w/w), and additives including alcohol.
Randomization and Blinding
Eligible patients were randomly assigned (1:1) to receive sirolimus or placebo. During the study, patients remained masked to the sirolimus gel and placebo of identical appearance. The codes for permuted-block (size of 4) randomization were generated using SAS, version 9.4 (SAS Institute), by an independent statistician, stratified into 2 age subpopulations of pediatric (3-18 years) and adult (>18 years) patients, and incorporated into the web-based electronic data capture system. The codes were provided in electronic data capture registration order to each site, and the drug corresponding to the code was allocated by individual investigators to each patient. People involved with the study, including patients and investigators, were masked to treatment allocation until study completion.
Procedures
Each patient was instructed to evenly spread the investigational drug to skin lesions at the daily amounts of gel, that is, up to 400, 600, and 800 mg for patients younger than 6, 6 to 11, and older than 11 years, respectively, twice daily for 12 weeks. The concurrent use of the following medications was prohibited: any mTOR inhibitors, topical tacrolimus, topical steroids, topical antibiotics, topical vitamin D3, adapalene, benzoyl peroxide, ibuprofen piconol, resorcinol, and zinc-salicylic acid. Laser therapy and surgery of the site of topical application were also not permitted.
Clinical Outcomes
The primary end point was composite improvement in angiofibromas—combined improvements in size and color—at week 12 of treatment, which was categorized according to the criteria in eTable 1A and B in Supplement 2 into the following 6 categories: “markedly improved,” “improved,” “slightly improved,” “unchanged,” “slightly aggravated,” and “aggravated” by an independent review committee (IRC) comprising 3 blinded dermatologists. Namely, size and color constituted the primary end point in a composite fashion and were also variables for secondary end points. Improvement in the size of cephalic plaques was rated based on photographs according to the criteria in eTable 1A in Supplement 2 into the same 6 categories as secondary end points by the IRC as well. When the size and color of angiofibromas were assessed, changes in papule extension from baseline were examined. The same photographs were separately assessed by all 3 members of the IRC, and the final rating was determined either by majority vote when at least 2 assessments were identical or by consensual decision making when 3 different judgments were made. Regarding the secondary end points, the response rates were calculated as the proportions of patients who were rated improved or markedly improved. Furthermore, changes from baseline in the total score of Dermatology Life Quality Index (DLQI)19 and Children’s DLQI (CDLQI)20 were calculated to assess the quality of life of patients. The secondary end points were assessed at weeks 4, 8, and 12 of treatment and at the end of the 4-week follow-up. At the time of the hospital visit, the safety of investigational drugs was assessed based on adverse events (AEs), laboratory tests (hematology, blood chemistry, and urinalysis), and vital signs. Whole-blood sirolimus concentrations were measured at the central laboratory.
Sample Size
Prior to the present study, the photographs of 36 patients in the phase 2 clinical trial were reviewed according to the same 6-category criteria for efficacy assessment as described herein. Subsequently, improvements in angiofibromas in the study groups—the sirolimus group and the placebo group—were examined to respectively determine the required numbers of pediatric and adult patients. Consequently, required sample sizes per group were calculated to be 12 and 15 for pediatric and adult patients, respectively, under the following conditions: power for joint probability (1 − β) of at least 95%; α = .05 (2-sided); and the active-placebo ratio of 1:1. The target number of patients in total was set to 60 in consideration of potentially a few withdrawals, and the power for this target patient population was determined to be at least 99% to distinguish a between-group difference of composite improvement in angiofibromas.
Statistical Methods
Full analysis set principles were used to statistically assess the efficacy of treatment including all patients who were assigned to the present study. The safety of investigational drugs was assessed in all patients who received at least 1 application of the gel or placebo. The Wilcoxon rank sum test was performed to detect between-group differences in the composite improvement in angiofibromas, improvements in the size and color of angiofibromas and plaques (unassessable patients were excluded from statistical analyses), and changes in the total scores of DLQI/CDLQI. The Fisher exact test was conducted to detect between-group differences in response rates (unassessable patients were assessed as nonresponders in the denominator) and in the incidences of AEs. A 2-tailed P < .05 was considered statistically significant. All statistical analyses were made with the SAS software package.
Results
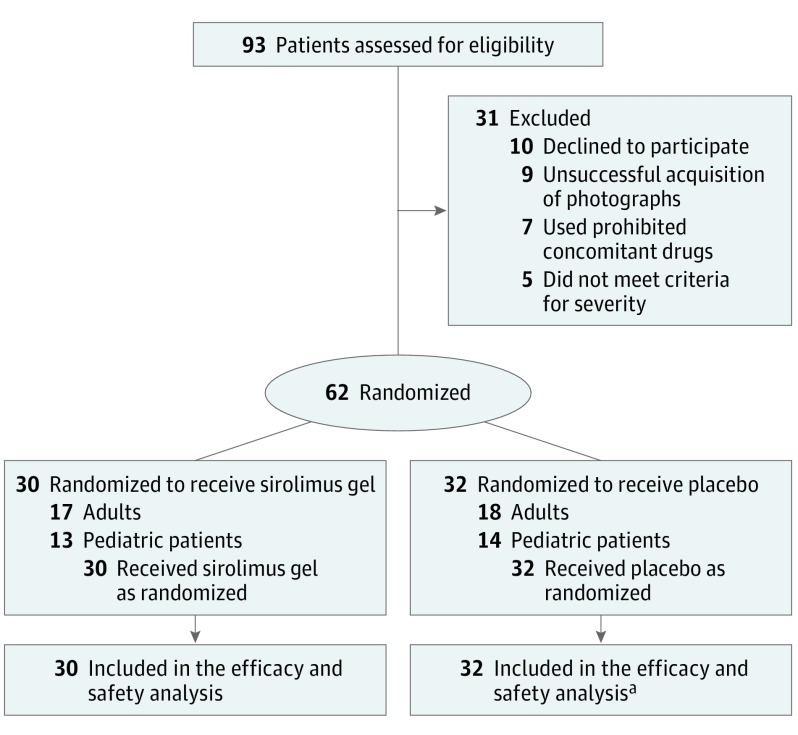
Between December 25, 2015, and October 21, 2016, 62 patients with TSC (mean [SD] age, 22.5 [11.9] years; range, 6-53 years) were screened and deemed eligible for inclusion, and 30 and 32 patients were randomly assigned to the sirolimus group and the placebo group, respectively (Table 1 and Figure 1). Discontinuation of treatment was not required for any of the participants. All patients received investigational drugs for 12 weeks and completed 4-week follow-up. Angiofibromas were found in all 62 patients, and cephalic plaques in 29 patients (13 in the sirolimus group and 16 in the placebo group). The IRC adjudicated 1 patient in the placebo group as unassessable due to the unfocused photographs of skin lesions at week 12 of treatment.
Table 1. Baseline Demographic and Clinical Characteristics of 62 Japanese Patients With Tuberous Sclerosis Complex.
| Characteristic | Sirolimus Group (n = 30) |
Placebo Group (n = 32) |
|---|---|---|
| Male sex, No. (%) | 17 (57) | 11 (34) |
| Age, y | ||
| No. (%) | ||
| 6-11 | 6 (20) | 6 (19) |
| 12-18 | 7 (23) | 8 (25) |
| 19-53 | 17 (57) | 18 (56) |
| Mean (SD) | 22 (11) | 23 (13) |
| Height, mean (SD), cm | 157 (15) | 156 (14) |
| Weight, mean (SD), kg | 49 (15) | 54 (17) |
| Assessed skin lesions, No. (%) | ||
| Facial angiofibromas | 30 (100) | 32 (100) |
| Cephalic plaques | 13 (43) | 16 (50) |
| Comorbidities, No. (%) | ||
| Intellectual impairmenta | 14 (47) | 12 (38) |
| Epilepsyb | 21 (70) | 16 (50) |
| Prior use of mTOR inhibitors, No. (%)c | 7 (23) | 11 (34) |
| Major features, No. (%) | ||
| ≥3 Hypomelanotic macules (≥5 mm in diameter) | 17 (57) | 22 (69) |
| ≥3 Angiofibromas or fibrous cephalic plaques | 30 (100) | 32 (100) |
| ≥2 Ungual fibromas | 14 (47) | 12 (38) |
| Shagreen patch | 19 (63) | 20 (63) |
| Multiple retinal hamartomas | 5 (17) | 9 (28) |
| Cortical dysplasias | 16 (53) | 21 (66) |
| Subependymal nodules | 19 (63) | 24 (75) |
| Subependymal giant cell astrocytoma | 4 (13) | 2 (6) |
| Cardiac rhabdomyoma | 3 (10) | 6 (19) |
| Lymphangioleiomyomatosis | 5 (17) | 7 (22) |
| ≥2 Angiomyolipomas | 18 (60) | 16 (50) |
| Minor features, No. (%) | ||
| “Confetti” skin lesions | 1 (3) | 3 (9) |
| ≥3 Dental enamel pits | 11 (37) | 5 (16) |
| ≥2 Intraoral fibromas | 5 (17) | 3 (9) |
| Retinal achromic patch | 2 (7) | 1 (3) |
| Multiple renal cysts | 6 (20) | 5 (16) |
| Nonrenal hamartomas | 5 (17) | 7 (22) |
| DLQI/CDLQI total score,d mean (SD) | 1.8 (3.7) | 1.9 (2.7) |
Abbreviations: CDLQI, Children’s Dermatology Life Quality Index; DLQI, Dermatology Life Quality Index; mTOR, mammalian target of rapamycin.
Severe intellectual impairment and autism were included.
Febrile convulsions, infantile spasms, seizures, and status epilepticus were included.
Everolimus or sirolimus.
DLQI for patients 16 years or older; CDLQI for patients younger than 16 years. Both surveys had 10 items rated on a 0-to-3 scale (for a maximum total score of 30), with higher scores implying poorer quality of life.
Figure 1. CONSORT Study Flow Diagram.
aIncludes 1 patient whose out-of-focus photograph obtained at week 12 of treatment was rated unassessable.
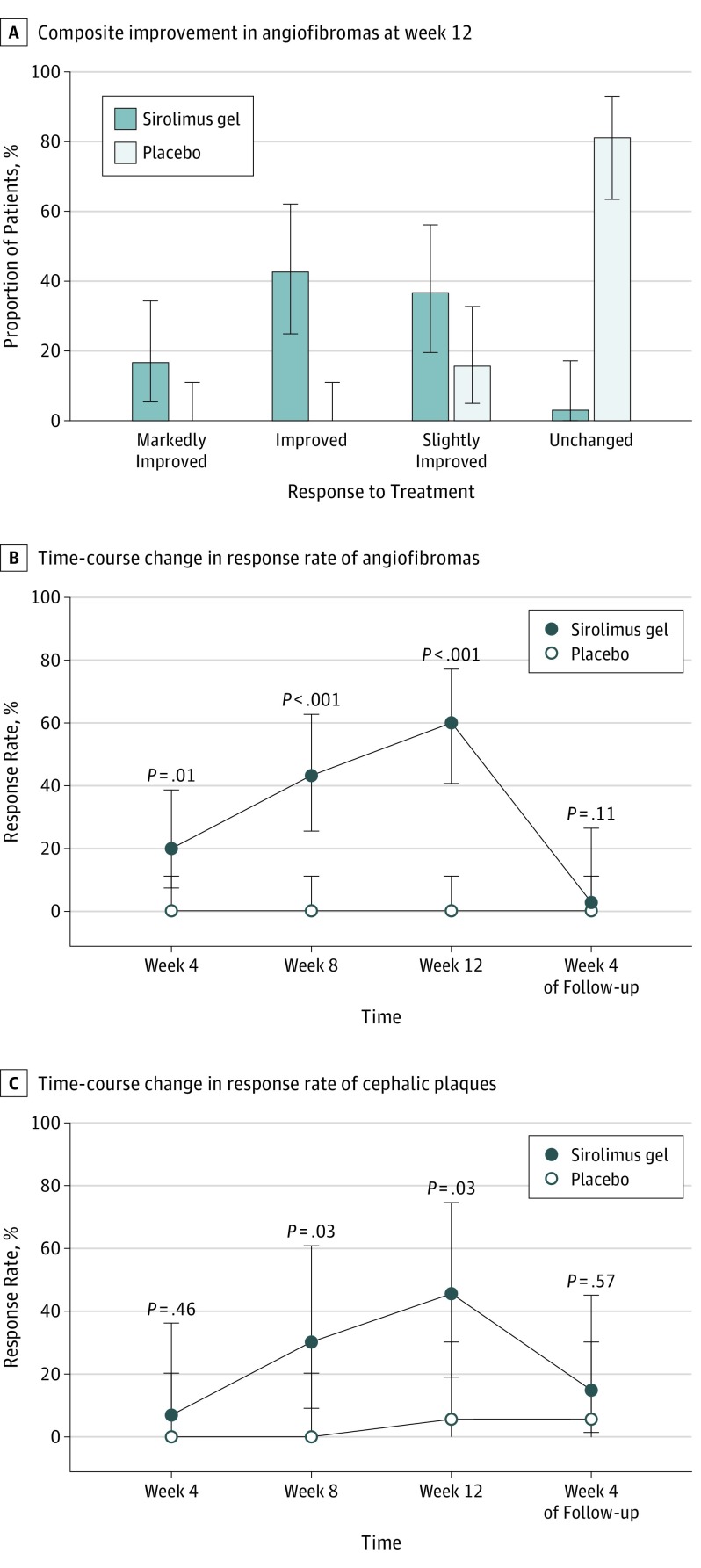
Regarding the primary end point of composite improvements in angiofibromas at week 12, none of the 31 assessable patients in the placebo group were rated improved or better, and 26 of them (84%) were rated unchanged. In contrast, 5 (17%) and 13 (43%) patients in the sirolimus group were rated markedly improved and improved, respectively (P < .001) (Figure 2A and eTable 2A in Supplement 2). The sirolimus group was also significantly superior (P < .001) to the placebo group in the subpopulations of pediatric and adult patients (pediatric, no patients in the placebo group improved or better vs 11 [85%] in the sirolimus group; adult, no patients in the placebo group improved or better vs 7 [41%] in the sirolimus group) (eFigure 1A in Supplement 2).
Figure 2. Efficacy of Sirolimus Gel, 0.2%, for Angiofibromas and Cephalic Plaques.
A, Composite improvement in angiofibromas at week 12 of treatment was significantly superior in the sirolimus group vs the placebo group for all patients (Wilcoxon rank sum test, P < .001). B and C, Time-course changes in the response rates of angiofibromas and cephalic plaques. The response rate was defined as the proportion of patients rated markedly improved or improved. Between-group differences were tested using the Fisher exact test. Error bars represent 95% confidence intervals.
Composite improvements in angiofibromas at weeks 4 and 8 and at week 4 of follow-up were significantly different between the 2 groups as with the primary end point, while almost all (12 of 13) of the patients rated improved at week 12 shifted to slightly improved at week 4 of follow-up in the sirolimus group. Improvements in size and color of angiofibromas were also superior in the sirolimus group compared with the placebo group at weeks 4, 8, and 12.
The response rates of angiofibromas at weeks 4, 8, and 12 of treatment were 0 each in the placebo group in contrast to 20% (95% CI, 8%-39%; P = .01), 43% (95% CI, 26%-63%; P < .001), and 60% (95% CI, 41%-77%; P < .001), respectively, in the sirolimus group (Figure 2B and eTable 2B in Supplement 2). In the sirolimus group, furthermore, the response rates were significantly higher in pediatric patients (85%; 95% CI, 55%-98%) than in adult patients (41%; 95% CI, 18%-67%) at week 12 of treatment (P = .03).
The response rates of the size of angiofibromas in the sirolimus group showed significant differences against those in the placebo group at weeks 4, 8, and 12 of treatment (eFigure 1B in Supplement 2) in the entire population, as well as in pediatric and adult subpopulations (eFigure 1C in Supplement 2). In the sirolimus group, moreover, the response rates were significantly higher in pediatric patients (85%; 95% CI, 55%-98%) than in adult patients (41%; 95% CI, 18%-67%) (P = .03) at week 12 of treatment. The response rates of the color of angiofibromas in the sirolimus group also indicated significant differences compared with those in the placebo group at weeks 8 and 12 of treatment in all populations (eFigure 1D in Supplement 2), as well as in pediatric and adult subpopulations (eFigure 1E in Supplement 2); these rates in the sirolimus group were nearly equal between pediatric (46%; 95% CI, 19%-75%) and adult (35%; 95% CI, 14%-62%) patients at week 12 of treatment.
Improvements in plaques in the sirolimus gel group at weeks 4, 8, and 12 were significantly superior to those in the placebo group. The response rates of plaques in the sirolimus group at weeks 8 (31%; 95% CI, 9%-61%) and 12 (46%; 95% CI, 19%-75%) were significantly higher (P = .03 for both) than in the placebo group (0; 95% CI, 0-21% and 6%; 95% CI, 0-30%, respectively) (Figure 2C and eTable 2C in Supplement 2) in all populations; however, the improvement rates were nearly comparable between pediatric and adult patients at week 12 of treatment (eFigure 1F in Supplement 2).
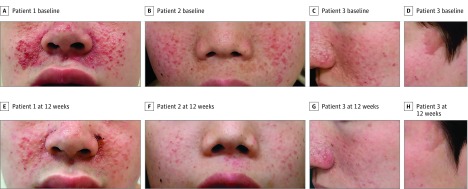
Photographs of representative patients in the sirolimus group are displayed in Figure 3. At week 12, 3 of the 18 patients who were rated improved or markedly improved showed reduced size and faded color of angiofibromas (patients 1-3) and a flattened plaque (patient 3) and had smoother skin than at baseline, and their skin color became close to normal.
Figure 3. Representative Photographs From Patients in the Sirolimus Group.
A-C, Angiofibromas on the cheek at baseline in patient 1, a teenage boy; patient 2, a young boy; and patient 3, a man in his 20s. D-F, After 12-week treatment with sirolimus gel, 0.2%, the angiofibromas were rated improved, improved, and markedly improved, respectively, showing reduced size and faded color. G and H, A cephalic plaque on the temple of patient 3 at baseline appeared flattened and was rated improved after 12-week treatment with sirolimus gel, 0.2%.
The total scores on the DLQI/CDLQI at week 12 in the sirolimus gel group and the placebo group showed mean (SD) changes of −0.2 (2.9) and −0.5 (2.3), respectively, with no statistical difference between the 2 groups.
Regarding whole-blood concentration of sirolimus, the highest level was 0.5 ng/mL in the sirolimus group (eFigure 2 in Supplement 2).
Adverse events were observed in 27 (90%) patients in the sirolimus group and 22 (69%) in the placebo group. Major adverse events (Table 2 for ≥2 cases; eTable 3 in Supplement 2 for any case) were dry skin, application site irritation, and pruritus. One man in his 20s in the sirolimus group developed acute pancreatitis and gastric hemorrhage that were reported as serious AEs; the patient recovered soon after hospitalization without discontinuing the gel application. None of the AEs found in the present study caused treatment discontinuation.
Table 2. Adverse Events With at Least 2 Cases.
| System Organ Class/Preferred Termsa | No. (%) | |||||
|---|---|---|---|---|---|---|
| Sirolimus | Placebo | |||||
| Total (n = 30) |
Pediatric (n = 13) |
Adult (n = 17) |
Total (n = 32) |
Pediatric (n = 14) |
Adult (n = 18) |
|
| Any adverse event | 27 (90) | 10 (77) | 17 (100) | 22 (69) | 10 (71) | 12 (67) |
| Influenza | 3 (10) | 3 (23) | 0 | 0 | 0 | 0 |
| Nasopharyngitis | 1 (3) | 1 (8) | 0 | 3 (9) | 3 (21) | 0 |
| Eye irritation | 1 (3) | 1 (8) | 0 | 2 (6) | 1 (7) | 1 (6) |
| Upper respiratory tract inflammation | 0 | 0 | 0 | 2 (6) | 1 (7) | 1 (6) |
| Stomatitis | 1 (3) | 0 | 1 (6) | 2 (6) | 0 | 2 (11) |
| Acne | 2 (7) | 0 | 2 (12) | 0 | 0 | 0 |
| Dry skin | 11 (37) | 4 (31) | 7 (41) | 4 (13) | 2 (14) | 2 (11) |
| Pruritus | 7 (23) | 1 (8) | 6 (35) | 4 (13) | 2 (14) | 2 (11) |
| Application site irritation | 11 (37) | 4 (31) | 7 (41) | 9 (28) | 3 (21) | 6 (33) |
| Skin abrasion | 0 | 0 | 0 | 2 (6) | 1 (7) | 1 (6) |
MedDRA (Medical Dictionary for Regulatory Activities), version 19.0.
Discussion
The results of this trial provide clinical evidence about the beneficial effects of sirolimus gel application on angiofibromas of patients with TSC as previously suggested by the phase 2 clinical trial.17 Twelve-week treatment with the sirolimus gel was significantly superior to placebo in improving angiofibromas. The response rate of angiofibromas was 60% in the sirolimus group in contrast to 0 in the placebo group.
Tuberous sclerosis complex is a rare multisystem disease that causes benign tumors including subependymal giant cell astrocytomas, lymphangioleiomyomatosis, angiomyolipomas, and angiofibromas. These benign tumors do not spontaneously resolve, although they are responsive to the inhibition of mTOR. Patients with subependymal giant cell astrocytomas21 or angiomyolipomas22 treated in placebo-controlled clinical studies with another mTOR inhibitor, everolimus, demonstrated good response rates of 35% and 42%, respectively, whereas the placebo groups did not exhibit any responses. Patients in our study also showed a good response to the gel and no response to the placebo, providing further proof of concept for the efficacy of mTOR inhibitors in treating hamartoma of patients with TSC.
The response rate was significantly higher in pediatric (85%) than in adult (41%) subpopulations concerning the size but not color (46% vs 35%) of angiofibromas in the sirolimus group—a finding that is in line with the results from our phase 2 clinical trial.17 Both studies complement a previous study by Foster et al,9 who noted an improvement in erythema of adult patients but only a minimal reduction in the size of nodular lesions following the topical application of sirolimus, 0.1%. Apparently age-related differences in response to sirolimus treatment reflect the increased size and fibrous content of angiofibromas in adult patients.
Plaques develop on the forehead of 40% of patients with TSC.4 Excepting single case reports,6,14,17 the efficacy of sirolimus has not been demonstrated. Therefore, the present study also demonstrated that topical sirolimus is effective for the treatment of facial fibrotic plaques.
All response rates in the present study decreased at week 4 of follow-up (Figure 2B and C and eFigure 1 in Supplement 2), reflecting the transient efficacy of mTOR inhibition by topical sirolimus. Angiomyolipomas23 in patients with TSC and lymphangioleiomyomatosis24 recurred after discontinuation of their oral sirolimus treatment. Taken together, these trials provide objective evidence that long-term treatment is required to maintain the therapeutic effect of sirolimus.
Concerning the DLQI/CDLQI scores, 42% of patients indicated a score of 0 (no problem) at baseline. The scores changed little from baseline to treatment completion; therefore, we consider assay sensitivity to the cutaneous lesions of TSC to be low.
The major AEs in this trial were dry skin, irritation, and pruritus; the incidence of dry skin was significantly higher in the sirolimus group (11 [37%]) than in the placebo group (4 [13%]; P = .04), indicating that this AE was probably caused by sirolimus. Although the mechanism by which topical sirolimus causes dry skin remains unknown, dry skin has been observed with patients who were treated with either oral sirolimus or everolimus.25 Skin irritation and pruritus were common in both the sirolimus gel and placebo groups; however, no statistically significant difference was found between the 2 groups. Alcohol, which is contained in the gel to improve the solubility and stability of sirolimus, may be responsible for these AEs. Although the ointment was considered to be less irritating than the gel,6,11 we adopted the latter because of its capability of facilitating greater transdermal absorption of sirolimus.15 Overall, these AEs were mild and did not lead to treatment discontinuation, thus indicating that sirolimus gel treatment was well tolerated. Nevertheless, moisturizers (eg, petrolatum and heparinoid) can be concurrently used to prevent or treat these AEs.
Whole-blood sirolimus concentrations were either low (≤0.5 ng/mL) or undetectable at week 4 of treatment and did not further increase during the entire study period. The concentrations after topical application were only less than one-tenth the value after oral administration (5-15 ng/mL),26 making negligible the risk that cutaneous exposure to sirolimus provokes systemic AEs. One patient in the sirolimus group developed 1 episode of gastric hemorrhage and acute pancreatitis that necessitated hospitalization. The patient recovered after the removal of pancreatic compression caused by aerophagia and severe constipation without requiring treatment discontinuation.
Limitations
Our study has several limitations. First, whether treatment with sirolimus gel for a period longer than 12 weeks improves skin lesions is unknown. Second, the long-term safety and tolerability of and responsiveness to the sirolimus gel are not sufficiently documented. Finally, the effects of combination treatment with topical and oral sirolimus remain to be elucidated because patients who were treated with oral sirolimus were excluded from the present study.
Conclusions
In this randomized clinical trial, sirolimus gel demonstrated a significant clinical benefit for patients with angiofibromas of TSC, compared with placebo, thus providing a promising therapeutic modality.
Trial Protocol
eTable 1. A. Rating criteria for improvements in cutaneous lesions from baseline
B. Rating criteria for composite improvement in angiofibromas based on efficacy variables
eTable 2. A. Composite improvement in angiofibromas at week 12 of treatment
B. Response rates of angiofibromas
C. Response rates of cephalic plaques
eTable 3. Adverse events
eFigure 1. A. Response rates of angiofibromas by age subpopulation
B. Response rates of the size of angiofibromas in all populations
C. Response rates of the size of angiofibromas by age subpopulation
D. Response rates of the color of angiofibromas in all populations
E. Response rates of the color of angiofibromas by age subpopulation
F. Response rates of plaques by age subpopulation
eFigure 2. Plots of whole blood sirolimus concentrations
References
- 1.National Organization for Rare Disorders (NORD) Tuberous sclerosis. https://rarediseases.org/rare-diseases/tuberous-sclerosis. Accessed December 7, 2016.
- 2.Schwartz RA, Fernández G, Kotulska K, Jóźwiak S. Tuberous sclerosis complex: advances in diagnosis, genetics, and management. J Am Acad Dermatol. 2007;57(2):189-202. [DOI] [PubMed] [Google Scholar]
- 3.Webb DW, Clarke A, Fryer A, Osborne JP. The cutaneous features of tuberous sclerosis: a population study. Br J Dermatol. 1996;135(1):1-5. [PubMed] [Google Scholar]
- 4.Wataya-Kaneda M, Tanaka M, Hamasaki T, Katayama I. Trends in the prevalence of tuberous sclerosis complex manifestations: an epidemiological study of 166 Japanese patients. PLoS One. 2013;8(5):e63910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haemel AK, O’Brian AL, Teng JM. Topical rapamycin: a novel approach to facial angiofibromas in tuberous sclerosis. Arch Dermatol. 2010;146(7):715-718. [DOI] [PubMed] [Google Scholar]
- 6.Wataya-Kaneda M, Tanaka M, Nakamura A, Matsumoto S, Katayama I. A topical combination of rapamycin and tacrolimus for the treatment of angiofibroma due to tuberous sclerosis complex (TSC): a pilot study of nine Japanese patients with TSC of different disease severity. Br J Dermatol. 2011;165(4):912-916. [DOI] [PubMed] [Google Scholar]
- 7.Mutizwa MM, Berk DR, Anadkat MJ. Treatment of facial angiofibromas with topical application of oral rapamycin solution (1 mg mL−1) in two patients with tuberous sclerosis. Br J Dermatol. 2011;165(4):922-923. [DOI] [PubMed] [Google Scholar]
- 8.DeKlotz CMC, Ogram AE, Singh S, Dronavalli S, MacGregor JL. Dramatic improvement of facial angiofibromas in tuberous sclerosis with topical rapamycin: optimizing a treatment protocol. Arch Dermatol. 2011;147(9):1116-1117. [DOI] [PubMed] [Google Scholar]
- 9.Foster RS, Bint LJ, Halbert AR. Topical 0.1% rapamycin for angiofibromas in paediatric patients with tuberous sclerosis: a pilot study of four patients. Australas J Dermatol. 2012;53(1):52-56. [DOI] [PubMed] [Google Scholar]
- 10.Kaufman McNamara E, Curtis AR, Fleischer AB Jr. Successful treatment of angiofibromata of tuberous sclerosis complex with rapamycin. J Dermatolog Treat. 2012;23(1):46-48. [DOI] [PubMed] [Google Scholar]
- 11.Salido R, Garnacho-Saucedo G, Cuevas-Asencio I, et al. . Sustained clinical effectiveness and favorable safety profile of topical sirolimus for tuberous sclerosis - associated facial angiofibroma. J Eur Acad Dermatol Venereol. 2012;26(10):1315-1318. [DOI] [PubMed] [Google Scholar]
- 12.Truchuelo T, Díaz-Ley B, Ríos L, Alcántara J, Jaén P. Facial angiofibromas treated with topical rapamycin: an excellent choice with fast response. Dermatol Online J. 2012;18(1):15. [PubMed] [Google Scholar]
- 13.Ebrahimi-Fakhari D, Müller CSL, Meyer S, Flotats-Bastardas M, Vogt T, Pföhler C. Topical rapamycin for facial angiofibromas in a child with tuberous sclerosis complex (TSC): a case report and long-term follow-up. Dermatol Ther (Heidelb). 2017;7(1):175-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tu J, Foster RS, Bint LJ, Halbert AR. Topical rapamycin for angiofibromas in paediatric patients with tuberous sclerosis: follow up of a pilot study and promising future directions. Australas J Dermatol. 2014;55(1):63-69. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka M, Wataya-Kaneda M, Nakamura A, Matsumoto S, Katayama I. First left-right comparative study of topical rapamycin vs vehicle for facial angiofibromas in patients with tuberous sclerosis complex. Br J Dermatol. 2013;169(6):1314-1318. [DOI] [PubMed] [Google Scholar]
- 16.Koenig MK, Hebert AA, Roberson J, et al. . Topical rapamycin therapy to alleviate the cutaneous manifestations of tuberous sclerosis complex: a double-blind, randomized, controlled trial to evaluate the safety and efficacy of topically applied rapamycin. Drugs R D. 2012;12(3):121-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wataya-Kaneda M, Nakamura A, Tanaka M, et al. . Efficacy and safety of topical sirolimus therapy for facial angiofibromas in the tuberous sclerosis complex: a randomized clinical trial. JAMA Dermatol. 2017;153(1):39-48. [DOI] [PubMed] [Google Scholar]
- 18.Northrup H, Krueger DA; International Tuberous Sclerosis Complex Consensus Group . Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013;49(4):243-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)--a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210-216. [DOI] [PubMed] [Google Scholar]
- 20.Lewis-Jones MS, Finlay AY. The Children’s Dermatology Life Quality Index (CDLQI): initial validation and practical use. Br J Dermatol. 1995;132(6):942-949. [DOI] [PubMed] [Google Scholar]
- 21.Franz DN, Belousova E, Sparagana S, et al. . Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2013;381(9861):125-132. [DOI] [PubMed] [Google Scholar]
- 22.Bissler JJ, Kingswood JC, Radzikowska E, et al. . Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2013;381(9869):817-824. [DOI] [PubMed] [Google Scholar]
- 23.Bissler JJ, McCormack FX, Young LR, et al. . Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med. 2008;358(2):140-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCormack FX, Inoue Y, Moss J, et al. ; National Institutes of Health Rare Lung Diseases Consortium; MILES Trial Group . Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med. 2011;364(17):1595-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Afinitor [package insert]. East Hanover, NJ: Novartis. http://www.pharma.us.novartis.com/product/pi/pdf/afinitor.pdf. Accessed January 24, 2017.
- 26.Rapamune [package insert]. Philadelphia, PA: Pfizer. http://labeling.pfizer.com/showlabeling.aspx?id=139. Accessed January 24, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. A. Rating criteria for improvements in cutaneous lesions from baseline
B. Rating criteria for composite improvement in angiofibromas based on efficacy variables
eTable 2. A. Composite improvement in angiofibromas at week 12 of treatment
B. Response rates of angiofibromas
C. Response rates of cephalic plaques
eTable 3. Adverse events
eFigure 1. A. Response rates of angiofibromas by age subpopulation
B. Response rates of the size of angiofibromas in all populations
C. Response rates of the size of angiofibromas by age subpopulation
D. Response rates of the color of angiofibromas in all populations
E. Response rates of the color of angiofibromas by age subpopulation
F. Response rates of plaques by age subpopulation
eFigure 2. Plots of whole blood sirolimus concentrations