Abstract
Transgenic Metarhizium pingshaense expressing the spider neurotoxin Hybrid (Met-Hybrid) kill mosquitoes faster and at lower spore doses than wild-type strains. In this study, we demonstrate that this approach dovetails with the cornerstone of current malaria control: pyrethroid-insecticides, which are the cornerstone of current malaria control. We used World Health Organization (WHO) tubes, to compare the impact on insecticide resistance of Met-Hybrid with red fluorescent M. pingshaense (Met-RFP), used as a proxy for the wild-type fungus. Insecticides killed less than 20% of Anopheles coluzzii and Anopheles gambiae s.s. mosquitoes collected in a malaria endemic region of Burkina Faso where pyrethroid use is common. Seven days post-infection, mortality for insecticide-sensitive and resistant mosquitoes averaged 94% with Met-Hybrid and 64% with Met-RFP, with LT80 values of 5.32±0.199 days and 7.76±0.183 days, respectively. Eighty nine percent of insecticide-resistant mosquitoes exposed to permethrin five days post-infection with Met-Hybrid died within 24 hours: only 22% died from Met-Hybrid alone over this 24-hour period. Compared to Met-RFP, Met-Hybrid also significantly reduced flight capacity of mosquitoes 3 to 5 days post-infection. Based on WHOPES phase I laboratory susceptibility bioassays, transgenic Met-Hybrid provides effective biological control for adult African malaria vectors that may be used to synergistically manage insecticide resistance with current methods.
Background
An estimated 2 billion people live in areas where mosquito borne diseases are endemic. In 2015, malaria infected an estimated 212 million people around the world, killing 429,000, primarily children and pregnant women in sub-Saharan Africa [1]. Pyrethroid insecticide treated bed nets (ITNs) and indoor residual spraying (IRS) are current mainstays of mosquito control programs and have facilitated impressive reductions in the global malaria burden [2]. However, these approaches are threatened by increasing resistance to insecticides [3]. The widespread emergence of ‘knockdown resistance’ (kdr) in African mosquitoes was associated with the early intensive use of DDT and pyrethroids in agriculture, as both insecticides share the same mode of action [3–6]. In May 2012, the World Health Organization (WHO) launched the Global Plan for Insecticide Resistance Management in Malaria Vectors (GPIRM) with five strategic pillars(7). The third pillar highlighted a medium-term (3 to 10 year) need for an improved understanding of insecticide resistance, development of tools to manage resistance, as well as strategies for deploying these tools to achieve sustainable vector control. To meet these objectives, we are developing multiple alternative tools and strategies for exploiting transgenic mosquitoes and transgenic Metarhizium [7–9].
Entomopathogenic fungi are being evaluated as an environmentally friendly alternative to chemical insecticides [10]. However, compared to chemical insecticides, entomopathogenic fungi are considered to have poor efficacy (slow kill, high dose requirement, poor persistence in the field due to abiotic stressors) [11]. The large spore dose required also increases the difficulty of achieving sufficient coverage of mosquito resting surfaces or sleeping spaces so as to target enough adult vectors for effective reductions in disease burden.
To remedy these constraints, Metarhizium spp. targeting different insects have been engineered with improved tolerance to environmental stress [12]. Moreover, a strain of Metarhizium has been engineered to block transmission of malaria parasites in insect by expressing a single-chain antibody fragment targeting the parasite [13]. However, most genetic engineering efforts have aimed to increase fungal virulence. A transgenic, broad host-range M. anisopliae strain expressing an insect neurotoxin (AaIT) from Androctonus australis reduced mobility and blood feeding interest of adult Aedes aegypti mosquitoes, with a 9-fold reduction in lethal spore dose [14]. Similarly, a strain of M. pingshaense expressing the spider toxin Hybrid was highly effective against wild-caught, insecticide-resistant Anopheles gambiae s.l., the main vector of malaria transmission in Africa [9]. We chose Hybrid, also known as VersitudeTM, for further development as it is effective and a well-characterized toxin: the US EPA has already approved it for use as a stand-alone insect control agent [15]. Furthermore, while pyrethroids and other chemical insecticides target neuronal voltage-gated sodium (NaV) channels. The CaV and KV channels targeted by Hybrid are previously unexploited insecticide targets, reducing the likelihood of pre-existing resistance.
We envision genetically modified (GM) Metarhizium being used in conjunction with other technologies, including ITNs that are still the gold standard of vector control tools, even though pyrethroid resistance is now at high enough levels to render ITNs largely ineffective. Ten hours of exposure to permethrin produced a mortality rate in Burkina Faso An. gambiae of 26% as compared to an LT50 <2 min for the susceptible laboratory strain [16]. In laboratory studies, Met-Hybrid is equally infectious to insecticide-resistant and susceptible mosquitoes(9). Current proposals for field release involve application of the fungus to black cotton sheets which can be hung in houses to provide a resting site for mosquitoes that have taken blood meals [17]. This is the same application site as chemical insecticides, which are applied to house walls or used to treat the ITNs, so mosquitoes will likely be simultaneously confronted by both the fungus and pyrethroids. To successfully combine transgenic biopesticides and chemical insecticides in an integrated pest management program, it is necessary to determine if concurrent exposure synergizes their activities and better manages insecticide resistance in malaria vectors.
In this study, we adapted standard WHO laboratory bioassays for insecticides to compare the efficacy of the parental wild type strain and transgenic Met-Hybrid against wild mosquitoes with and without commonly used insecticides [18]. Our results suggest that transgenic fungi and insecticides would complement each other in field application and significantly mitigate the buildup of resistance when co-applied.
Methods
Ethical considerations
Ethical permissions required for this study were obtained through the Institutional Review of Institut de Recherche en Science de la Santé (IRSS) and Centre Muraz ethics committee (A012-2014/CE-CM).
Mosquito colonies
For bioassays, we used F1 progeny of An. coluzzii and An. gambiae s.s. reared from larval collections at Kou Valley (11°23’ N, 4°24’ W) and Soumousso (11°04’ N, 4°03’ W), respectively. Mosquitoes from these areas are highly resistant to multiple insecticides [19]. We used the laboratory Kisumu colony of Anopheles gambiae s.s. as a pyrethroid-susceptible reference strain. Only non-blood-fed females, 2 to 5 days old, were used in bioassays. Approximately 100 mosquitoes (4 replicates of 25 mosquitoes each) were exposed to each fungal strain or a control without spores (Bioassays 1 and 2). All tests were carried out at 25°C±2°C and 80%±10% relative humidity.
PCR determination of kdr levels
The level of kdr resistance within a subsample of mosquitoes was performed using the PCR protocol and primer sequences previously described [20]. We only analyzed mutation L1014F because it is the commonest in West Africa, whereas the L1014S mutation is confined to East Africa [21]. The primers AgD1 (5′-ATA GAT TCC CCG ACC ATG-3′) and AgD3 (5′-AAT TTG CAT TAC TTA CGA CA-3′) amplified the resistant allele yielding 195 bp fragments. The susceptible allele was assayed using primers AgD2 (5′-AGA CAA GGA TGA TGA ACC-3′) and AgD4 (5′-CTG TAG TGA TAG GAA ATT TA-3′), which amplified a 137 bp fragment. The primer set AgD1 and AgD2 amplified a ubiquitous 293 bp fragment as a positive control. During amplification, denaturation was set at 94°C for 3 min followed by 35 cycles of denaturation, annealing and elongation (94°C for 30 s, 55°C for 30 s, 72°C for 10 s, respectively). The final elongation was set at 72°C for 5 min.
Fungal strains
An East African isolate of Metarhizium pingshaense was transformed to express either red fluorescent protein (RFP) or green fluorescent protein (GFP) under a constitutive promoter (GdpA). The GFP-expressing strains was also transformed to express Hybrid-toxin under the hemolymph-specific Mcl1 promoter [9]. The RFP-expressing strain demonstrates wild-type virulence and growth, and was used as a proxy for the wild type. Both strains were maintained on potato dextrose agar at 26°C with 70% relative humidity.
Fungal formulations for bioassays
Conidia were formulated in organic sesame oil for application onto black cotton cloth; both the oil and the cloth were obtained in the local market in Bobo-Dioulasso, Burkina Faso. Five mL of a 8.0x107 conidia/mL spore suspension containing 8% (v/v) sesame oil was spread onto 12 cm x 15 cm sections of black cloth to achieve ~2 × 106 conidia/cm2. Impregnated sheets were dried for 16 hours overnight at ambient temperature (26±1°C, 80±10% RH) before placement inside WHO bioassay tubes (15 cm long and 4 cm diameter).
First bioassay: Comparing the efficacy of fungi and pyrethroids to mosquitoes in WHO bioassay tubes
The insecticide-susceptible reference strain (An. gambiae s.s. Kisumu) and mosquitoes reared from larvae collected at field sites were bioassayed in WHO tubes following standard WHO procedures [18]. The tubes were loaded either with cotton cloths impregnated with Met-Hybrid or Met-RFP conidia (to infect mosquitoes with fungus), or test papers (supplied by the Universiti Sains Malaysia, Penang) impregnated with recommended dosages of permethrin (0.75%) or deltamethrin (0.05%). Control batches of mosquitoes were exposed to blank test papers (without insecticide) and/or to cotton sheets treated with an oil preparation without fungal spores. For each treatment and mosquito strain, 25 mosquitoes were used per replicate. This first bioassay was performed over 4 replicates, ~120 mosquitoes / treatment /mosquito strain.
After one hour of exposure to treated or untreated surfaces, mosquitoes were transferred to holding tubes and fed with 6% glucose. Fungal infection alone does not cause the instantaneous knockdown and mortality seen in insecticide-susceptible mosquitoes treated with chemical insecticides [20]. Beyond this, we recorded mortality daily over one week. For all bioassays, we observed fluorescent mycosis on cadavers to confirm death due to fungal treatments. Post-mortem, the legs of 3–5 days post-infection mosquitoes were removed and used for the Kdr frequency determination.
Second bioassay: The impact of fungal infection on the susceptibility of mosquitos to permethrin
Mosquitoes were exposed to fungal spores (Met-RFP and Met-Hybrid) at ~2x106 conidia/cm2 using the adapted WHO 2013 protocol described previously(9). Briefly, black cotton cloth impregnated with an oil formulation of conidia was stapled to cardstock for support and placed into a WHO tube with the cardstock facing outward. Mosquitoes were placed in the WHO tube for 1 hour to allow reliable fungal infection. One to five days after fungal infection, mosquitoes were placed in WHO tubes containing test paper impregnated with 0.75% permethrin. For each day post infection, an overall 120-mosquitoes/treatment/mosquito strain were used over 4 replicates for this second bioassay. Permethrin was chosen for this second bioassay because it is the main insecticide used on insecticide-treated bed nets (ITNs) currently mass distributed in Burkina Faso [2]. Any mosquito that died, became immobile and/or lost any part of any appendages before being transferred to the insecticide treatment was discarded according to WHOPES, 2013 requirements [18]. We followed knock-down during the course of a one-hour exposure time, after which mosquitoes were transferred to cages and mortality was followed for a further 24 hours. Mortalities were compared with both control batches of infected mosquitoes exposed to blank untreated paper and control batches of uninfected mosquitoes directly exposed to permethrin or to control paper. Wild-caught An. gambiae s.s., wild-caught An. coluzzii and lab-reared An gambiae Kisumu were used for this experiment.
Third bioassay: Irritability cone tests on the impact of fungal infection on the flight capacity of mosquitoes
Insecticides, such as pyrethroids and DDT, have irritant and excito-repellent properties beyond their knock-down effects. Knockdown and irritant effects on An coluzzii resulting from tarsal contact with netting were measured one through five days post-infection with Met-RFP or Met-Hybrid. The fibers of treated netting (Olyset Plus®, Sumitomo Chemicals Co. Ltd., Japan) incorporate 2% (w/w) permethrin combined with 1% piperonyl butoxide (PBO). PBO works synergistically with permethrin in order to counteract metabolic-based pyrethroid resistance of mosquitoes [22]. For each replicate, six WHO plastic cones were attached to each 25 cm × 25 cm piece of treated or untreated (PBO alone) netting and held together by two plastic boards, which were clamped together with two binder clips. The assembly was held flat on the table (S1 Fig). Using an aspirator, one non-blood-fed female aged two to five days was gently introduced into each cone and the entrances were plugged with pieces of cotton. Each mosquito was exposed for 5 min. During the exposure period, two observers used electronic timers (one for landing and one for flying) to record the amount of time each mosquito spent flying and resting on the netting. After the 5 min test period each mosquito was transferred into a labeled 150 ml plastic holding cup and provided with 6% sugar solution. Knockdown and mortality were recorded 60 min and 24 h after exposure.
Data management and analysis
All data were entered into Microsoft Windows Excel 2010, checked for accuracy, then imported to R studio version 2.11.1 for data manipulation, visualization and statistical analysis. (S1 File). Using Fisher’s exact test, P<0.05 was accepted as statistically significant. The susceptibility of the mosquitoes was evaluated on the basis of the 2013 WHOPES criteria of test mortality. Mosquitoes were considered to be alive if they could stand upright. A mosquito was classified as moribund if it cannot stand, cannot fly in a coordinated manner or takes off briefly, but falls immediately. A mosquito was classified as dead if were immobile, cannot stand or otherwise shows no signs of life [23]. The datasets generated during the current study, R codes (scripts) for all statistical analysis and visualizations (graphs) are available in this GitHub repository at https://github.com/EtienneBilgo/Transgenic_Metarhizium_Insecticides.
Results
Mosquito susceptibility to fungi and pyrethroids in WHO bioassay tubes
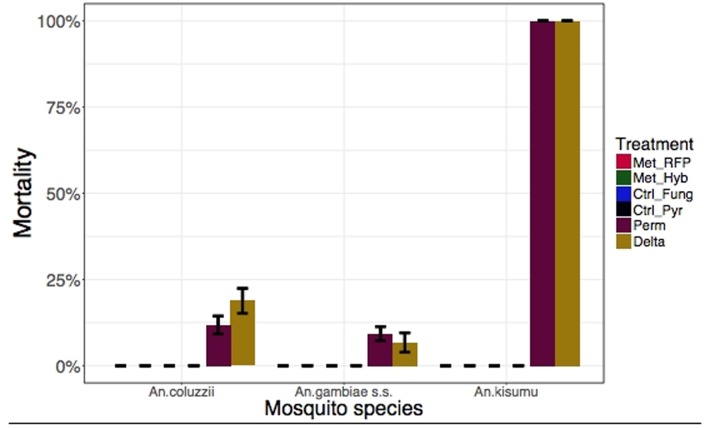
As expected, Permethrin and Deltamethrin killed 100% of the insecticide-susceptible strain (An. gambiae Kisumu), confirming its status as a reference. We found that 96.9% of the An. coluzzii and 81.5% of the An.gambiae s.s. used for bioassays carried the kdr resistance gene (Table 1). Consequentially, they were far less susceptible to permethrin with 24-hour mortality of An. coluzzii and An gambiae being 11.8±2.58% and 9.30±2.03%, respectively. Likewise, Deltamethrin caused low mortality in both An. coluzzii (18.8±3.61%) and An. gambiae s.s. (6.72±2.81%) (Fig 1 and S1 Table). The surviving mosquitoes were monitored for a week during which time there was no significant difference between their mortality and controls that were not exposed to insecticides (Fig 2 and S1 Table).
Table 1. Kdr 1014L mutation frequency in tested wild-caught mosquitoes.
| Genotypes | |||||
|---|---|---|---|---|---|
| Species | N | 1014L | 1014L | 1014F | f (1014F (%)) |
| 1014L | 1014F | 1014F | |||
| An. coluzzii | 291 | 4 | 10 | 277 | 0.969 |
| An.gambiae s.s. | 279 | 35 | 33 | 211 | 0.815 |
Fig 1. Results of World Health Organization (WHO) susceptibility tests for wild caught mosquitoes from Burkina Faso and laboratory Anopheles gambiae Kisumu strain.
Adult female mosquitos were exposed to the WHO diagnostic dose of insecticides and Metarhizium pingshaense for 1 h, and mortality rates were recorded 24 h later.
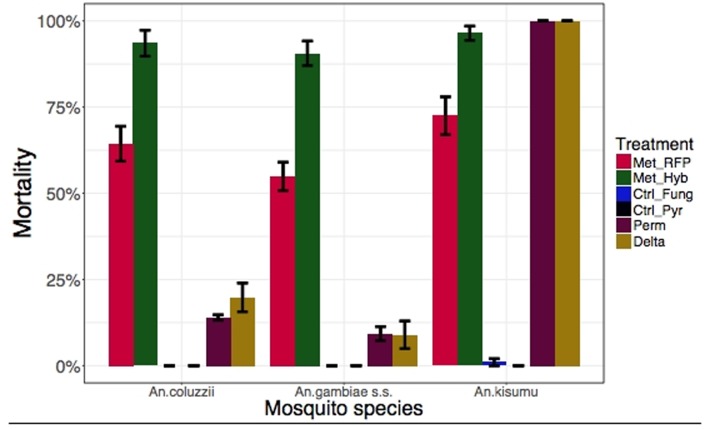
Fig 2. Results of World Health Organization (WHO) susceptibility tests for wild caught mosquitoes from Burkina Faso and laboratory Anopheles gambiae Kisumu strain.
Adult female mosquitos were exposed to the WHO diagnostic dose of insecticides and Metarhizium pingshaense for 1 h, and mortality rates were recorded 7 days later.
Since M. pingshaense spores take 2–3 days to penetrate host cuticle, Met-RFP and Met-Hybrid do not cause mortality 24 hours post-infection (Fig 1 and S1 Table). However, within one week post-infection Met-Hybrid (Met-RFP) killed 93.5±3.77% (64.4±5.06%) of the An. coluzzii, 90.6±3.55% (54.9±4.10%) of the An.gambiae s.s. and 96.4±2.06% (72.5±5.50%) of the An gambiae Kisumu. As previously shown9, insecticide-resistance did not alter susceptibility to Metarhizium for both An. coluzzii and An. gambiae s.s. There were no statistical differences between the very high mortalities produced by Met-Hybrid and Permethrin or Deltamethrin in the insecticide sensitive An gambiae Kisumu mosquitoes (P = 0.31). Met-RFP was significantly less virulent than Met-Hybrid but significantly more effective than insecticides against insecticide-resistant mosquitoes (Fig 2 and S1 Table).
Met-Hybrid is the only treatment that achieved >80% mortality within one week (Table 2) with LT80’s of 5.18±0.482 days, 5.54±0.326 days and 5.25±0.269 days for An. coluzzii, An. gambiae and An. Kisumu, respectively. A mortality of 80% is the WHO threshold for successful vector control agents [23].
Table 2. LT80 survival following WHO tube exposure for various treatments according to mosquito species.
SE: Standard error of the mean; Pairwise comparison of LT80 Treatments without letters in common are significant at P<0.05.
| Mosquito species | Treatment | LT80 Mean (Days) | SE | Grouping |
|---|---|---|---|---|
| Anopheles coluzzii | Met_RFP | 7.63 | 0.295 | a |
| Met_Hybrid | 5.18 | 0.482 | b | |
| Deltamethrin | - | - | - | |
| Permethrin | - | - | - | |
| Control1 | - | - | - | |
| Control2 | - | - | - | |
| Anopheles gambiae s.s. (Giles) | Met_RFP | 8.12 | 0.358 | a |
| Met_Hybrid | 5.54 | 0.326 | b | |
| Deltamethrin | - | - | - | |
| Permethrin | - | - | - | |
| Control1 | - | - | - | |
| Control2 | - | - | - | |
| Anophles gambiae Kisumu | Met_RFP | 7.52 | 0.288 | a |
| Met_Hybrid | 5.26 | 0.269 | b | |
| Deltamethrin | 0.769 | 0 | c | |
| Permethrin | 0.769 | 0 | c | |
| Control1 | - | - | - | |
| Control2 | - | - | - |
Impact of fungal infection on the susceptibility of mosquitos to permethrin
In a previous study, we have reported that within 2.5 days post-infection, mosquitoes exposed to Met-Hybrid died faster than those exposed to Met-RFP with LT80 values for Met-Hybrid and Met-RFP were 4.14 ± 5.47 ± 0.25 and 7.71 ± 0.16 days, respectively (mean ± standard error is reported) [9]. The purpose of the current bioassay was to check the mortality rate of surviving fungal infected mosquitoes in contact with insecticide (Permethrin treated net) versus control-net (without any insecticide) following each day post infections during this range of time (1 to 5 days post-infection).
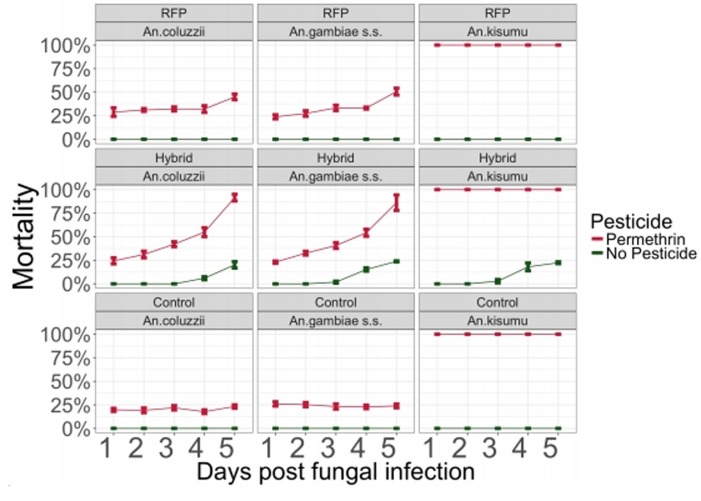
We used WHO tubes and standard WHO protocols to test the effects of 0.75% permethrin on batches of mosquitoes one to five days post-infection. One hundred percent of the insecticide sensitive An. gambiae kisumu died at day 1 through 5 (Fig 3). The susceptibility of both Anopheles coluzzii and Anopheles gambiae s.s. to permethrin one-day post-infection with Met-Hybrid or Met-RFP was low (mean 28.3%) (S2 Table). Uninfected control mosquitoes retained a similar level of susceptibility (~22%) throughout the experiment (Fig 3). However, 88.8±4.28% of mosquitoes exposed to permethrin 5 days post-infection with Met-Hybrid died within 24 hours as compared to 23.5±1.26% that died from pesticides alone one day following pesticide exposure (a 3.78-fold increase) (Fig 3 and S2 Table). A 5-day infection with Met-RFP increased mortality of insecticide-susceptible mosquito 2.03-fold to 47.8±2.45%.
Fig 3. Legend.
Curves of World Health Organization (WHO) susceptibility tests of the impact of fungal infections on the susceptibility of wild caught mosquitoes (An. coluzzii and An.gambiae s.s.) from Burkina Faso and laboratory Anopheles gambiae Kisumu strain. Following the first 5 days post-infection, living fungus-infected mosquitoes (Met_RFP and Met_Hybrid) versus uninfected mosquitoes (control) were exposed to the WHO diagnostic dose of permethrin treated net (pesticides) versus non-impregnated net (no pesticide) for 1 h, and mortality rates were recorded 24 h later.
Between 3 to 5 days post-infection, significantly more (P<0.05) Met-Hybrid infected mosquitoes died within 24 hours of exposure to insecticide-free paper than those infected with Met-RFP (no mortality was observed in uninfected controls). Further, 3.9-fold more insecticide-resistant mosquitoes died (88.8±4.28% in total) 5 days-post-infection with Met-Hybrid and one day-post-exposure to permethrin than were killed by either Met-Hybrid (22.0±1.75%) or the permethrin (23.5±1.26%) used individually (Fig 3 and S2 Table). While the impact of Met-RFP infection with permethrin exceeds the sum of their impacts individually by day 5, this effect is observed earlier (starting day 2 post-infection) and to a greater extent mosquitoes are exposed to Met-Hybrid with permethrin. Fluorescent Metarhizium mycosis was observed on fungus-exposed cadavers, confirming mortality due to treatment.
Effect of fungal infections on mosquitoes flying ability
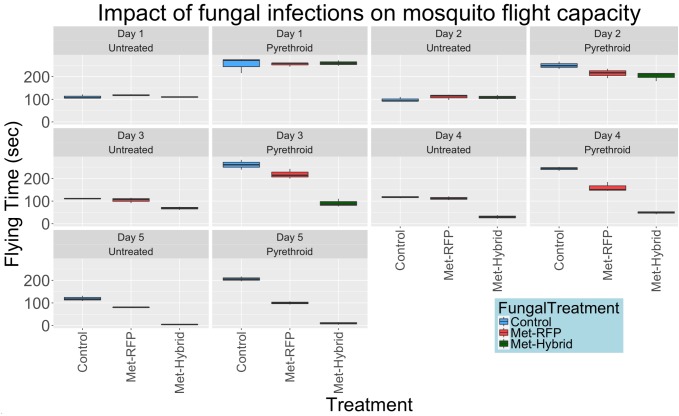
Fungal infection and Hybrid toxin expression may alter the ability of mosquitoes to sense and move away from chemical pesticides, thus altering exposure to pesticides on netting. Using WHO irritability cone tests, we individually assessed 15 mosquitoes per fungal treatment (Control, Met-RFP and Met-Hybrid) per day for 5 days against untreated and permethrin treated netting (a total of 450 mosquitoes). Uninfected mosquitoes took significantly (P<2.2x10-6) more flights when exposed to permethrin (S2 Table), confirming Permethrin irritates mosquitoes. Fig 4 represents how the first five days of fungal infection impacts flight capacity on both permethrin-treated and untreated netting. Overall, uninfected and infected mosquitoes spent less time flying with untreated netting as this lacked irritant and excito-repellent properties. Uninfected mosquitoes and mosquitoes infected for two days with Met-RFP or Met-Hybrid showed similar flying capacity (Table 3).
Fig 4. Results of World Health Organization (WHO) cone tests of the impact of fungal infections on the flight capacity of wild caught Anopheles coluzzii mosquitoes from Burkina Faso.
Following the first 5 days post-infection, adult female mosquitos were exposed to untreated versus permethrin treated netting in the WHO cone for 5 minutes, and flying /landing times were recorded.
Table 3. Flight time on WHO irritability cone test on Permethrin treated versus untreated net following the first 5 days post-fungal infection.
Following the first 5 days fungal post-infection, each female mosquito was exposed for 5 min on permethrin treated net versus untreated net. During the exposure period, the flying times were recorded.
| Net Treatment | Days Post-Infection | Fungal Treatment | Percent of time in flight (%) | S E (%) |
|---|---|---|---|---|
| Untreated | Day 1 | Met-Hybrid | 36.7 | 0.52 |
| Control | 37.0 | 1.71 | ||
| Met-RFP | 39.5 | 0.64 | ||
| Day 2 | Met-Hybrid | 36.2 | 1.92 | |
| Control | 32.7 | 2.01 | ||
| Met-RFP | 37.0 | 2.39 | ||
| Day 3 | Met-Hybrid | 22.8 | 1.36 | |
| Control | 37.0 | 0.15 | ||
| Met-RFP | 34.8 | 2.34 | ||
| Day 4 | Met-Hybrid | 9.98 | 1.54 | |
| Control | 39.1 | 0.86 | ||
| Met-RFP | 37.4 | 1.58 | ||
| Day 5 | Met-Hybrid | 1.49 | 0.41 | |
| Control | 39.7 | 2.24 | ||
| Met-RFP | 26.6 | 0.45 | ||
| Permethrin | Day 1 | Met-Hybrid | 86.5 | 2.33 |
| Control | 85.0 | 6.58 | ||
| Met-RFP | 84.8 | 1.72 | ||
| Day 2 | Met-Hybrid | 67.6 | 3.79 | |
| Control | 83.2 | 3.13 | ||
| Met-RFP | 71.7 | 4.02 | ||
| Day 3 | Met-Hybrid | 30.3 | 3.36 | |
| Control | 86.9 | 4.20 | ||
| Met-RFP | 72.8 | 4.23 | ||
| Day 4 | Met-Hybrid | 16.3 | 1.19 | |
| Control | 81.0 | 1.72 | ||
| Met-RFP | 53.4 | 4.11 | ||
| Day 5 | Met-Hybrid | 3.27 | 1.13 | |
| Control | 68.6 | 1.99 | ||
| Met-RFP | 33.3 | 1.35 |
Three to five days post-infection, when the fungus has entered the hemolymph and is expressing Met-Hybrid, mosquitoes infected with Met-Hybrid had significantly shorter flight times compared to uninfected controls and mosquitoes infected with Met-RFP (P<0.05; Table 3). Five days post-infection, mosquitoes infected with Met-RFP, but not exposed to pesticides, flew 13% less (~39 seconds) than the uninfected controls, and 25% (~75 seconds) more than mosquitoes infected with Met-Hybrid. These flight times differences were significantly different (P = 0.0045 for uninfected controls and P = 1.1x10-5 for Hybrid treated mosquitoes).
Fig 4 also illustrates the extent fungal infection impacts the flight capacity of mosquitoes exposed to permethrin treated netting. Met-Hybrid significantly reduced mosquito’s cumulative flight capacity 3 to 5 days post-infection compared to both uninfected controls and Met-RFP (Table 3). Five days-post-infection, mosquitoes infected with Met-Hybrid flew for 3.27±1.13% (9.80±3.40 seconds) of the bioassay, more than 10-fold less than mosquitoes infected by Met-RFP (33.3±1.35%, P = 8.7x10-8) or uninfected mosquitoes (68.6±1.99%, P = 8.9x10-6). The reduction in flight capacity due to Met-RFP (14.0% on Day 3 and 35.2% on Day 5) was significantly less (P < 0.05) than Met-Hybrid (56.6% on Day 3 and 65.3% on Day 5). By day 3 post-infection, there is no significant difference (P = 0.145) when comparing flight times between mosquitoes infected with Met-Hybrid on untreated (mean = 68.5) and permethrin treated net (mean = 90.8). However the flight times of mosquitoes infected with Met-RFP were significantly (P>0.05) higher on Permethrin treated net than untreated net each day following infection (Table 4). By day 3 post-infection, mosquitoes infected with Met-Hybrid no longer displayed irritability to Permethrin; however, these irritability effects were still observed in mosquitoes infected with Met-RFP and in control mosquitoes.
Table 4. Comparison of flight times of mosquitoes on untreated or permethrin treated net following each of the first 5 days post-fungal infection in a WHO cone bioassay.
| Day post- infection with fungi |
Mean mosquito flight times (sec) untreated Net |
Mean mosquito flight times (sec) Permethrin treated net |
P value (Welch two samples t. test) |
|
|---|---|---|---|---|
| 1 | 110.9 | 255.0 | 0.01374 | |
| 2 | 98.13 | 249.5 | 0.0004312 | |
| Control | 3 | 111.0 | 260.6 | 0.006944 |
| 4 | 117.3 | 243.1 | 0.0002404 | |
| 5 | 119.1 | 205.7 | 0.0006898 | |
| 1 | 118.5 | 254.5 | 0.0004294 | |
| 2 | 110.9 | 215.0 | 0.003794 | |
| Met-RFP | 3 | 104.3 | 218.5 | 0.003687 |
| 4 | 112.2 | 160.1 | 0.04591 | |
| 5 | 79.87 | 100.0 | 0.02823 | |
| 1 | 110.1 | 259.5 | 0.001436 | |
| 2 | 108.6 | 202.9 | 0.005336 | |
| Met-Hybrid | 3 | 68.53 | 90.80 | 0.1453 |
| 4 | 29.93 | 49.00 | 0.03386 | |
| 5 | 4.467 | 9.800 | 0.2534 |
Discussion
Current mosquito control technologies are increasingly inadequate, and novel technologies are needed to compliment them [10]. This will require a coordinated approach that integrates compatible technologies to build strengths synergistically and minimize risks. This is the first time that the efficacy of transgenic fungi against wild caught insecticide-resistant mosquitoes was compared with currently applied insecticides. The efficacy of the Hybrid-toxin against insecticide-resistant mosquitoes likely reflects Hybrid targeting two different neuron channels simultaneously in mosquitoes (Ca2+ and K+), compared to the single Na+ site targeted by permethrin and deltamethrin. S2 Fig. represents the mechanisms of action of Met-Hybrid and Pyrethroids. Mosquitoes exposed to WHO test papers impregnated with 0.4–1.6% insecticide pick up ~0.018–0.06μg insecticide/insect, respectively [24]. M. pingshaense spores are ~7μm by 3μm in size [25]. Estimating the volume of these spores using V = πr2h gives ~50 μm3. A yeast cell of this volume weighs ~53 pg [26,27]. The LD100 for Met-Hybrid is ~6 spores or ~318 pg of spores [9], which is ~58-fold less than the amount of insecticide picked up by a mosquito exposed to a 0.4–1.6% insecticide treated sheet.
Our results show that even with pyrethroid-resistant mosquitoes, transgenic fungi showed synergistic enhancement of insecticide-based control methods. It has already been shown that combining permethrin with wild-type fungi at very high concentrations increased mosquito mortality (M. anisopliae or Beauveria bassiana at 1×1011 spores/m2) [28]. Here we show that 5-days post-infection with a low spore dose (~130 transgenic M. pingshaense spores per mosquito) picked up from cloth impregnated with conidia increased the efficacy of permethrin or deltamethrin to insecticide resistant mosquitoes up to 91%. Previous studies on insect hosts other than mosquitoes have indicated fungi can act synergistically with insecticides [29]. Mixtures of M. anisopliae and deltamethrin displayed enhanced virulence when used together against ticks, indicating synergistic effects that would enhance effectiveness with lower concentrations of both deltamethrin and the fungus [29].
Metarhizium takes around 36 hours to breach the cuticle and enter the mosquito hemolymph [30]. The increased mortality of insecticide-resistant mosquitoes 3–5 days post inoculation with Met-Hybrid coincides with expression of the Hybrid-toxin in the mosquito hemolymph; the toxin is under the control of a hemolymph-specific promoter, so it is only expressed when the fungus is in contact with insect hemolymph [14]. Mosquitoes infected with Met-Hybrid showed accelerated loss of their flight capacity compared to those infected with Met-RFP. We previously used a blood feeding choice tunnel test to show that Hybrid-toxin significantly decreases the blood feeding propensity of mosquitoes within 3–5 days [9]. As the Hybrid-toxin acts on both sensory and motor neurons in the mosquito, reduced blood feeding is likely due to decreased sensation and mobility in Met-Hybrid infected mosquitoes.
Resistance to pyrethroids is typically mediated by the kdr genes. However, more potent biochemical, physiological and behavior resistance mechanisms have evolved and are now common in Burkina Faso [16,31]. In S1 Table, we report 11% and 18 of wild-caught An. coluzzii died 24 h post exposure to permethrin and Deltamethrin respectively. This suggests a small portion of An.coluzzii carrying the resistance gene (Kdr gene mutation, Table 1) demonstrated apparent susceptibility to insecticides in our bioassays: this may reflect limitations in the insecticide bioassays. Though following the WHO guidelines, the WHO cylinder Insecticidal bioassays are indeed sensitive to variations in temperature, humidity, time of day and physiological state, controlling for which necessitates standardized rearing and testing conditions [32]. Further, even though the Kdr mutation confers the resistance to pyrethroids, the correlation with WHO cone test mortality is not always identical and makes direct testing of wild-caught adult (female) mosquitoes problematic. Some studies suggested this gap could be readily filled by other molecular diagnostic markers, especially those targeting DNA [32] or new standardized bioassays that produce consistent dose-response measurements with a minimal number of mosquitoes [33]
In spite of this, Hybrid-expressing fungi significantly increased wild-caught mosquito susceptibly to insecticides: exceeding the 80% mortality threshold established by WHO [18]. The current study establishes that transgenic fungi meet the criteria established by GPIRM for malaria vector control, namely, they are suitable for combination in a mixture of IRS insecticides co-formulated into a single IRS product and can be combined with insecticide treated cloth or insecticide treated bed netting. Future modeling with transgenic fungi accounting for various mosquito ages and Plasmodium infection stages, as well as the coverage of ITN and IRS in communities, will facilitate the development of programs that combine transgenic fungi and chemical insecticides for malaria control in the field.
Supporting information
(TIFF)
(PDF)
(DOCX)
(DOCX)
(TIFF)
Acknowledgments
This work was supported by NIH-NIAID under award RO1-AI106998 to R.J.S. and A.D.
Data Availability
The datasets generated during the current study, R codes (scripts) for all statistical analysis and visualizations (graphs) are available in this GitHub repository at https://github.com/EtienneBilgo/Transgenic_Metarhizium_Insecticides.
Funding Statement
This work was supported by NIH-NIAID under award RO1-AI106998 to R.J.S. and A.D.
References
- 1.WHO (2015) World malaria report. http://www.who.int/malaria/publications/world-malaria-report-2016/report/en/
- 2.Lu G, Traoré C, Meissner P, Kouyaté B, Kynast-Wolf G, Beiersmann C, et al. Safety of insecticide-treated mosquito nets for infants and their mothers: randomized controlled community trial in Burkina Faso. Malar J. 10.1186/s12936-015-1068-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ranson H, Lissenden N. Insecticide Resistance in African Anopheles Mosquitoes: A Worsening Situation that Needs Urgent Action to Maintain Malaria Control. Trends Parasitol. 2016;32(3):187‑96. 10.1016/j.pt.2015.11.010 [DOI] [PubMed] [Google Scholar]
- 4.Bazie BVET. Contribution à l’évaluation de la resistance aux insecticides des vecteurs du paludisme et de la distribution du gène Kdr au Bukina. M.Sc. Thesis, The Université Polytechnique de Bobo Dioulasso, 2007. Available from: http://www.beep.ird.fr/collect/upb/index/assoc/IDR-2007-BAZ-CON/IDR-2007-BAZ-CON.pdf
- 5.Hien AS, Soma DD, Hema O, Bayili B, Namountougou M, Gnankiné O, et al. Evidence that agricultural use of pesticides selects pyrethroid resistance within Anopheles gambiae s.l. populations from cotton growing areas in Burkina Faso, West Africa. PLoS One. 2017;12(3):1‑15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hemingway J, Ranson H, Magill A, Kolaczinski J, Fornadel C, Gimnig J, et al. Averting a malaria disaster: Will insecticide resistance derail malaria control? Lancet. 2016;387(10029):1785‑8. 10.1016/S0140-6736(15)00417-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mnzava AP, Knox TB, Temu EA, Trett A, Fornadel C, Hemingway J, et al. (2015) Implementation of the global plan for insecticide resistance management in malaria vectors: progress, challenges and the way forward. Malaria journal 14: 173 10.1186/s12936-015-0693-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epopa PS, Millogo AA, Collins CM, North A, Tripet F, Benedict MQ, et al. The use of sequential mark-release-recapture experiments to estimate population size, survival and dispersal of male mosquitoes of the Anopheles gambiae complex in Bana, a west African humid savannah village. Parasit Vectors. 2017;10(1):376 10.1186/s13071-017-2310-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bilgo E, Lovett B, Fang W, Bende N, King GF, Diabate A, et al. Improved efficacy of an arthropod toxin expressing fungus against insecticide-resistant malaria-vector mosquitoes. Sci Rep. 2017;7(1):3433 10.1038/s41598-017-03399-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hancock PA. Combining fungal biopesticides and insecticide-treated bednets to enhance malaria control. PLoS Comput Biol. 2009;5(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farenhorst M, Knols BGJ. Fungal entomopathogens for the control of adult mosquitoes: a look at the issues. Proc Netherlands Entomol Soc Meet. 2007;18:51‑9. [Google Scholar]
- 12.Zhao H, Lovett B, Fang W. Genetically Engineering Entomopathogenic Fungi. Adv Genet. 2016;94:137‑63. 10.1016/bs.adgen.2015.11.001 [DOI] [PubMed] [Google Scholar]
- 13.Fang W., Vega-Rodríguez J., Ghosh A.K., acobs-Lorena M., et al. Development of transgenic fungi that kill human malaria parasites in mosquitoes. Science 331, 1074–7, (2011) 10.1126/science.1199115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang C, St Leger RJ. A scorpion neurotoxin increases the potency of a fungal insecticide. Nat Biotechnol. 2007;25(12):1455‑6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17994009%5Cnhttp://www.nature.com/nbt/journal/v25/n12/pdf/nbt1357.pdf 10.1038/nbt1357 [DOI] [PubMed] [Google Scholar]
- 15.National Biosafety Authority, Kenya. Guidelines and checklists for the Risk Assessment and Certification of facilities dealing with Genetically Modified Organisms Ref: NBA/TSD/ML/03 Revision No:00 Page 1 of 61.Available from: http://www.biosafetykenya.go.ke/index.php?option=com_content&view=article&id=18&Itemid=123
- 16.Toé KH, Jones CM, N’fale S, Ismai HM, Dabiré RK, Ranson H. Increased pyrethroid resistance in malaria vectors and decreased bed net effectiveness Burkina Faso. Emerg Infect Dis. 2014;20(10):1691‑6. 10.3201/eid2010.140619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scholte E-J, Knols BGJ, Takken W. Infection of the malaria mosquito Anopheles gambiae with the entomopathogenic fungus Metarhizium anisopliae reduces blood feeding and fecundity. J Invertebr Pathol. 2006;91(2006):43‑9. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization; Guidelines for laboratory and field-testing of long-lasting insecticidal nets. 2013;93 Available from: www.who.int/about/licensing/copyright_form/en/index.html [Google Scholar]
- 19.Namountougou M, Simard F, Baldet T, Diabaté A, Ouédraogo JB, Martin T, et al. Multiple Insecticide Resistance in Anopheles gambiae s.l. Populations from Burkina Faso, West Africa. PLoS One. 2012;7(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez-Torres D, Chandre F, Williamson MS, Darriet F, Bergé JB, Devonshire AL, et al. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Mol Biol. 1998;7(2):179‑84. [DOI] [PubMed] [Google Scholar]
- 21.Santolamazza F, Mancini E, Simard F, Qi Y, Tu Z, della Torre A. Insertion polymorphisms of SINE200 retrotransposons within speciation islands of Anopheles gambiae molecular forms. Malar J. 2008;7(1):163 10.1186/1475-2875-7-163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pennetier C, Bouraima A, Chandre F, Piameu M, Etang J, Rossignol M, et al. Efficacy of Olyset® Plus, a New Long-Lasting Insecticidal Net Incorporating Permethrin and Piperonil-Butoxide against Multi-Resistant Malaria Vectors. PLoS One. 2013;8(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHOPES. Guildelines for Laboratory and Field-Testing of Long-Lasting Insecticidal Nets. 2013;105. [Google Scholar]
- 24.Pennell JT, Miskus R, Craig R. the Use of Gas Chromatography for the Quantitative Determination of Micro-Amounts of Insecticide Picked Up By Mosquitos. Bull World Health Organ. 1964;30:91‑5. [PMC free article] [PubMed] [Google Scholar]
- 25.Bischoff JF, Rehner S a, Humber R a. A multilocus phylogeny of the Metarhizium anisopliae lineage. Mycologia. 2009;101(4):512‑30. [DOI] [PubMed] [Google Scholar]
- 26.Haddad AS and Lindegren CC. A method for determining the Weight of an individual yeast cell. Appl Microbiol. 1953. May; 1(3): 153–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phillips R, Kondev J, Theriot J.and Garcia H. Physical Biology of the Cell. 2nd Edition. 2012. Garland Science, Taylor and Francis Group LLC: New York: ISBN: (Paperback) 978–0815344506. 10.1111/boc.201100081 [DOI] [Google Scholar]
- 28.Farenhorst M, Knols BGJ, Thomas MB, Howard AF V, Takken W, Rowland M, et al. Synergy in efficacy of fungal entomopathogens and permethrin against west african insecticide-resistant anopheles gambiae mosquitoes. PLoS One. 2010;5(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ericsson JD, Kabaluk JT, Goettel MS, Myers JH. Spinosad interacts synergistically with the insect pathogen Metarhizium anisopliae against the exotic wireworms Agriotes lineatus and Agriotes obscurus (Coleoptera: Elateridae). J Econ Entomol. 2007;100(1):31‑8. [DOI] [PubMed] [Google Scholar]
- 30.Roberts DW, St. Leger RJ. Metarhizium spp., cosmopolitan insect-pathogenic fungi: Mycological aspects. Adv Appl Microbiol. 2004;54:1‑70. 10.1016/S0065-2164(04)54001-7 [DOI] [PubMed] [Google Scholar]
- 31.IRMAPPER. Insecticide susceptibility,2017.Available from: http://anopheles.irmapper.com/. Cited 17 August 2017.
- 32.Weetman D and Donnelly MJ, Evolution of insecticide resistance diagnostics in malaria vectors. Trans R Soc Trop Med Hyg 2015; 109: 291–293 10.1093/trstmh/trv017 [DOI] [PubMed] [Google Scholar]
- 33.Owusu HF, Jancčáryová D, Malone D and Müller P, Comparability between insecticide resistance bioassays for mosquito vectors: time to review current methodology? Parasites & Vectors (2015) 8:357 10.1186/s13071-015-0971-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIFF)
(PDF)
(DOCX)
(DOCX)
(TIFF)
Data Availability Statement
The datasets generated during the current study, R codes (scripts) for all statistical analysis and visualizations (graphs) are available in this GitHub repository at https://github.com/EtienneBilgo/Transgenic_Metarhizium_Insecticides.