Abstract
Background
Most electronic-cigarette liquids contain propylene glycol, glycerin, nicotine and a wide variety of flavors of which many are sweet. Sweet flavors are classified as saccharides, esters, acids or aldehydes. This study investigates changes in cariogenic potential when tooth surfaces are exposed to e-cigarette aerosols generated from well-characterized reference e-liquids with sweet flavors.
Methods
Reference e-liquids were prepared by combining 20/80 propylene glycol/glycerin (by volume fraction), 10 mg/mL nicotine, and flavors. Aerosols were generated by a Universal Electronic-Cigarette Testing Device (49.2 W, 0.2 Ω). Streptococcus mutans (UA159) were exposed to aerosols on tooth enamel and the biological and physiochemical parameters were measured.
Results
E-cigarette aerosols produced four-fold increase in microbial adhesion to enamel. Exposure to flavored aerosols led to two-fold increase in biofilm formation and up to a 27% decrease in enamel hardness compared to unflavored controls. Esters (ethyl butyrate, hexyl acetate, and triacetin) in e-liquids were associated with consistent bacteria-initiated enamel demineralization, whereas sugar alcohol (ethyl maltol) inhibited S. mutans growth and adhesion. The viscosity of the e-liquid allowed S. mutans to adhere to pits and fissures. Aerosols contained five metals (mean ± standard deviation): calcium (0.409 ± 0.002) mg/L, copper (0.011 ± 0.001) mg/L, iron (0.0051 ± 0.0003) mg/L, magnesium (0.017 ± 0.002) mg/L, and silicon (0.166 ± 0.005) mg/L.
Conclusions
This study systematically evaluated e-cigarette aerosols and found that the aerosols have similar physio-chemical properties as high-sucrose, gelatinous candies and acidic drinks. Our data suggest that the combination of the viscosity of e-liquids and some classes of chemicals in sweet flavors may increase the risk of cariogenic potential. Clinical investigation is warranted to confirm the data shown here.
Introduction
Electronic-cigarette (e-cigarette) use has steadily increased in prevalence over the past decade especially among millennials. E-cigarettes are now the most used tobacco product among U.S. middle- and high-school students, surpassing combustible cigarettes [1, 2]. The success of the e-cigarette industry, in part, can be attributed to its target market strategy to younger age group, the public’s perception that e-cigarettes are a safer alternative to traditional tobacco products, and readily available Do-It-Yourself (DIY) instructions and starter kits on social media platforms [3, 4]. E-liquids are available in a wide variety of candy-, beverage-, and fruit- like flavors, as well as traditional flavors such as tobacco and menthol [5]. E-liquids can be ordered without nicotine (AKA “pleasure without consequences”) which can be enticing to youth and young adults [6, 7]. E-cigarette use has been implicated in encouraging smoking initiation among tobacco-naïve individuals [8–10]. With passage of the 2009 Family Smoking Prevention and Tobacco Control Act, all flavors—except menthol—from conventional cigarettes have been banned in the U.S. [11]. Similarly, the European Union (E.U.) and all member states adopted E.U. Tobacco Products Directive (2014/40/EU) to prohibit characterizing flavors at the product level [12]. However, other flavored tobacco products—smokeless tobacco, little cigars and cigarillos, large cigars, hookah, dissolvables, and e-liquids—remain on the U.S., E.U., and many other global markets and continue to be readily available and prevalent [13–15].
Estimates indicate that there are over 10000 e-liquid formulations (in 2018) available from online and in-store vape shops [16]. Many research laboratories and online consumer forums have reported that the quality of currently available e-liquids varies significantly [17–23]. Inaccurate labels on products (e.g., incorrect nicotine concentration) or unintended contaminants are commonly found in commercially available e-liquids [19, 20, 23–25]. Recently, U.S. and E.U. regulations (2009 Tobacco Control Act and 2014 Tobacco Products Directive, respectively) have emphasized the need to raise e-liquid quality and manufacturing standards. The U.S. Food and Drug Administration (FDA)’s Center for Tobacco Products (CTP) issued an Advance Notice of Proposed Rulemaking (ANPRM) to obtain information related to the role that flavors play in tobacco products (Docket Number: FDA-2017-N-6565). However, internationally recognized standards (e.g., The International Organization for Standardization) on manufacturing and safety testing methods are still in an early developmental stage [26].
Although there are a wide variety of e-liquids, the basic core components of e-liquids are well-known: base, nicotine and flavors. The base is made from propylene glycol, glycerin or a mixture of the two in various ratios, diluted in purified water. The concentration of nicotine varies from 0 mg/mL to 18 mg/mL and the users typically choose their own nicotine strength. Flavors can be categorized by tastes/fragrances (e.g., bakery, beverages, fruits, menthol, and tobacco) or by their chemical compositions (e.g., saccharides, esters, acids, and aldehydes). Sucrose or sucralose is added for the sweet taste in e-liquids and sugar alcohol (e.g., ethyl maltol) is used for the sweet fragrance [27–29]. In a previous study, it was shown that the viscous base is a major cause of unintended compositional error during manufacturing and bottling processes [26]. E-liquids, especially those made from glycerin (1.412 Pa•s) have high viscosity properties. Aerosols generated from these e-liquids are likely to adhere to exposed surfaces. These surfaces include soft and hard tissues in oral cavity, nasal cavity, pharynx, epiglottis, larynx, trachea, lung (directly) and skin, hair, clothing, and indoor living spaces (indirectly). The interaction between the viscous aerosol and oral cavity is of particular interest for several reasons: (i) dental professionals have long been aware of the danger of tobacco products and nicotine on oral health, (ii) the oral cavity which includes lips, gingiva, teeth, palate and tongue is the first organ to directly interact with the e-cigarette aerosol, (iii) changes in tissue surface characteristics from eating glutinous food (e.g., caramels, licorices, or sour candies) and high sucrose intake (e.g., sodas) can lead to negative health consequences in oral cavity. Many e-liquids share similar physical and chemical properties to sugary and gelatinous foods that have been proven to be major risks for dental caries [30, 31], and recently (iv) a population-based cross-sectional study revealed that daily use of e-cigarettes is independently associated with poor oral health [32].
Although the etiological role and infectious transmission of Streptococcus mutans in the development of dental caries have been discovered more than 50 years ago [31], dental caries continues to be the most prevalent infectious disease in humans, affecting 97% of the world population during their lifetimes [33]. The persistence of the disease stems from the fact that dental caries cannot be attributed to a single cause. Dental caries progresses by pathogenic oral bacteria, such as S. mutans, metabolizing fermentable carbohydrate (e.g., glucose, fructose, sucrose, and maltose) to produce lactic acid [34]. At low or moderate concentration of the acid, saliva and components in saliva buffer and neutralize the low pH in oral environment [35]. However, excessive intake of sucrose disturbs the dynamic balance between pathological and protective oral factors and leads to an acidic environment where it is beyond the normal saliva buffering capacity [34]. The prolonged low pH condition promotes survival of aciduric and acidogenic bacteria such as S. mutans which have developed the ability to thrive in an acidic environment [36–38]. S. mutans also produce glucosyltransferases (GTFs) to catalyze synthesis of Intracellular Polysaccharides (IPS) and Extracellular Polymeric Substances (EPS) from sucrose [39]. These EPS significantly contribute to the formation and structural stability of oral biofilm [40]. The biofilm (AKA dental plaque) is shown to enhance attachment and protection of oral bacteria, and aid in retaining physiological nutrients including essential metal ions [41–44]. The accumulation of negative consequences and the formation of cariogenic biofilm eventually lead to break down of hard tissues (enamel and dentin) of teeth [34, 45].This study was designed to systematically evaluate whether aerosols generated from highly-characterized reference e-liquids with various sweet flavors can produce bacteria-initiated demineralization on healthy human enamel surfaces. The Universal Electronic-Cigarette Testing Machine (UECTM) was optimized to simulate human physiological parameters. A novel visualization method to quantify e-cigarette aerosol droplets and a sample preparation protocol to increase reproducibility in enamel surface adhesion measurement were developed.
Materials and methods
Universal electronic-cigarette testing machine (UECTM) and study design
Aerosols were generated by using a Universal Electronic-Cigarette Testing Machine (UECTM) developed by the American Dental Association (ADA) Foundation in collaboration with the University of Maryland, Department of Chemistry [26]. For all experiments, a commercial sub-ohm tank (Aspire Cleito: 0.2 Ω Kanthal coil with cotton wick) was used. Due to low resistance heating coils, sub-ohm tanks are designed to be run at higher wattages than previous generation devices. In this study, aerosols were generated at a power setting of 3.14 V (total of 49.2 W based on P = V2 / R) determined by the manufacturer’s manual (capable up to 55–70 W) and online “vaping power charts”. Each atomizer was used for ≤ 750 puffs (approximately 5 d usage) and replaced thereafter. If discoloration, excessive heat or abnormal sound was observed, the atomizer was immediately discarded and replaced. The entire e-cigarette system was disassembled, thoroughly cleaned with de-ionized H2O (diH2O), and dried after each experiment. Aerosols were generated using the published physiological human e-cigarette puffing topography: 50 mL puff volume in 4 s puff duration every 18 s [46]. For this study, we defined 10 puffs as one vaping session [47] and 150 puffs as one-day use (≈ 3mL / day) [48]. We acknowledge that no machine testing regime can represent all human vaping behavior and there is great variability across different users and devices.
E-liquid formulation
Flavor-free reference e-liquid was prepared following our previous work (20/80 propylene glycol/glycerin (by volume fraction) with 10 mg/mL nicotine) [26]. To increase reproducibility, gravimetric method was used (0.410 g propylene glycol, 2.000 g glycerin and 20.0 mg nicotine) [26]. The following flavors were added to the reference e-liquids separately: ethyl butyrate (11.1 mg/mL); ethyl maltol (27.2 mg/mL); hexyl acetate (2.5 mg/mL); sucralose (2.0 mg/mL); and triacetin (11.6 mg/mL) (Table 1). The flavored e-liquids were mixed for additional 24 h using a vertical rotator at 0.5 rad/s. Selecting 20/80 propylene glycol/glycerin ratio was based on a previous study [49] and understanding that newer, high-wattage sub-ohm tanks are designed to be compatible with high glycerin e-liquids [50].
Table 1. Flavored reference e-liquids.
| Name | Category | Taste/smell | Formula | Reported concentration (mg/mL) | Concentration used in this study (mg/mL) |
|---|---|---|---|---|---|
| Ethyl butyrate | Ester | Pineapple | C6H12O2 | 11.1 | 11.1 |
| Ethyl maltol | Sugar alcohol | Cotton candy | C7H8O3 | 27.1 | 27.1 |
| Hexyl acetate | Ester | Apple | C8H16O2 | 2.5 | 2.5 |
| Sucralose | Sugar substitute | Sweetener | C12H19Cl3O8 | 1–5 | 2.0 |
| Triacetin | Triester of glycerol and acetic acid | Velvety / smoky | C9H14O6 | N/K | 11.6 |
Gas chromatography–mass spectrometry (GC-MS) analysis
Chemical by-product identification was performed using PerkinElmer Clarus 680 Gas Chromatography–Mass Spectrometry (GC-MS) Detection (PerkinElmer, Waltham, MA) fitted with a Velocity DB 5 column (PerkinElmer N9306325). Testing parameters of the Gas Chromatography (GC) method were as follows: Sampling method = manual headspace, Inlet temperature = 210°C, Carrier gas = 1.43 L/min, Split = 1:5, Temperature ramp = initial: 40°C, hold 3 min, 6°C/min to 300°C, hold for 3 min, and Total analysis time = 49.33 min. Testing parameters for the MS method were as follows: MS detector = PerkinElmer Clarus, ionization source = El, Polarity = positive, Mass range = (44 to 600) m/z, Acquisition type = centroid, Solvent delay = (0.00 to 2.00) min, and Analysis time = (2.00 to 49.30) min.
Bacterial strain and culture conditions
Streptococcus mutans UA157 (ATCC) was used for all experiments. Frozen cells were plated on a 100 mm Brain Heart Infusion (BHI) agar plate. After overnight incubation (37 oC and 5% CO2), a single colony was inoculated in 3 mL BHI liquid media. BHI liquid media was used for all planktonic growth assays. 75 μL of the media with bacteria was transferred to a 96 well flat-bottomed plate.
Preparation of enamel disks
American Dental Association (ADA) Institutional Review Board has reviewed and approved the following study (MML-16-0052). Human teeth were collected during routine third molar extractions due to clinical indications, not for research purposes. Teeth were part of discarded surgical tissues and did not contain patient identifiers. Once extracted, teeth were pooled into a collection container and it was not possible for the investigators to identify the donors. Caries-free teeth were sectioned parallel to the long axis (average 5 mm thickness) and embedded in a 44 mm diameter x 5 mm thickness VariDur mounting acrylic resin (Buehler). The top of enamel disks was polished using grid 1000, 1200, 2400, and 4000 silicon carbide papers (Struers) under streaming water and which was followed by polishing with 3 μm and 1 μm sized polycrystalline diamond pastes (MetaDi, Buehler). The directionality of the disks was rotated 90o in between grid changes. The quality of polishing was checked using a light metallurgical microscope (Nikon) at 40X magnification. A proper polishing step is a major component of reproducibility. The samples should not be under- or over-polished. The polished disks should have uniform orientation and be free of cracks, deformations, scratches, steps and slopes. The direction of enamel rods should be carefully considered when selecting which part of the disks is to be measured. The final disks had tooth surfaces exposed from the top and bottom. This is important to improve reproductivity in subsequent hardness measurement.
Micro-indentation hardness tests
All measurements were performed using Knoop hardness number (KHN) following American Society for Testing and Materials (ASTM) testing methods E92-16 and E384-16. The mounted disks were placed under the Knoop indenter of a micro-indentation hardness tester (Buehler-Wilson Knoop/Vickers Hardness Tester, Tukon 1202) and subjected to a load of 50 g for 10 s. Hardness was determined at five sites between the surface of the tooth to the Dentino- Enamel Junction (DEJ). After e-cigarette exposure and subsequent bacterial attachment, another sets of KHN measurements were made on each disk on parallel tracks approximately 100 μm apart [51, 52]. The disks were thoroughly cleaned with diH2O prior to post-exposure measurement and blotted carefully with Kimwipes while avoiding desiccating the disks.
Biofilm formation assay
To form biofilm, we used the standard O’Toole-Kolter protocol [53]. Overnight culture was inoculated in Biofilm Formation (BF) media (25% TSB + 5 mg/mL yeast extract + 30 mol/L sucrose) [54]. S. mutans were allowed to attach to the surface and collected at 4 h. Unattached cells and media were removed, and the plates were washed with diH2O twice. 5 mL of 0.1% crystal violet solution was added and stained the attached cells for 15 min. Crystal violet solution was removed and the plates were washed with diH2O twice. The plates were dried in a biological safety cabinet overnight. 5 mL of 30% acetic acid (by volume) was added and incubated at room temperature for 15 min. 75 μL of the solubilized crystal violet solution was transferred to a 96 well flat-bottomed plate. Absorbance was measured at 550 nm using a plate reader (SpectraMax, Molecular Devices).
Scanning electron microscopy
Enamel disk samples were washed with PBS and stored at -80°C for 24 h. The samples were transferred and freeze dried in a pre-chilled lyophilizer (Freezemobile 25XL, VirTis) for 24 h. The dried samples were mounted on Scanning Electron Microscope (SEM) aluminum stubs and sputter coated in gold (Desk V HP, Denton Vacuum). The samples were imaged using SEM (JSM 5300, JEOL) with following parameters: Secondary Electron Imaging (SEI), 10.0 KV at 50X, 1,500X and 10,000X magnifications.
Aerosol droplet quantification
A UECTM was programed with the specified physiological parameters as described in the Study Design section. Aerosols were exposed on non-reflective vinyl surface (3M Temflex 1700). The exposed surface was captured with a stereoptical light microscope (Leica MZ16). Aerosol droplets were quantified using ImageJ (NIH) “Analyze Particles” feature from three randomly chosen locations. The averaged aerosol droplet counts were compared among (0, 10 and 150) puff samples.
Metal quantification
E-cigarette aerosol (150 puffs) was collected in 30 mL of 2% ultra-pure nitric acid using a gas condenser (Pyrex 1760–125). Inductively Coupled Plasma with Optical Emission Spectrometry (ICP-OES) analyses were performed as described previously [26]. The presence of 12 metals was evaluated: cadmium (Cd), calcium (Ca), chromium (Cr), cobalt (Co), copper (Cu), iron (Fe), lead (Pb), magnesium (Mg), manganese (Mn), nickel (Ni), palladium (Pd), and silicon (Si). The final results are shown in concentration (mg/L) after considering the dilution factor of the collecting liquid medium. Nitric acid was used during the metal quantification only.
Atomic force microscopy
The adhesive force between S. mutans and enamel surface was measured by a single-cell force spectroscopy through the atomic force microscope (AFM, Model: Bruker BioScope Resolve). Bruker NP-O10 cantilever probe with spring constants (k) of 0.06 N m-1 was used for the functionalization of cantilever tip. The cantilever was immersed in 10 mmol/L Tris-HCl buffer solution (pH 8.5) containing 4mg/mL dopamine hydrochloride for 1 h. In the force spectroscopy, the dopamine-coated cantilever tip was used to attach a single S. mutans cell, then the tip was pressed against the control or aerosol exposed enamel surfaces. The adhesive force was measured by separating the cell from the enamel surface at a pulling rate of 1 Hz.
Statistical analysis
Concentration, absorbance, adhesion force and count were quantified using mean±standard deviation (S.D.) from three independent measurements. Each experiment was performed in triplicate and was repeated at least three times. All statistical analyses were conducted using the MaxStat 3.6 statistical software (Jever-OT Cleverns, Germany). The significant level was indicated as p < 0.05 (*), p<0.005 (**), or p<0.0001 (***).
Results
E-cigarette deposits fine aerosol particles on surfaces
To characterize e-cigarette aerosol, we used a Universal Electronic-Cigarette Testing Machine (UECTM) and reference e-liquid to generate (10 and 150) puffs, set at the predetermined physiological parameters (50 mL puff volume in 4 s puff duration every 18 s) [46]. Several surfaces were evaluated, and it was found that non-reflective vinyl surface (3M Temflex 1700) gave the least background interference when a stereoptical light microscope (Leica MZ16) was used for imaging (Fig 1A). Aerosols from (0, 10 and 150) puffs deposited (5.7 ± 5.0, 175.5 ± 12.7, and 1051.25 ± 59.4) aerosol particles / mm2 respectively (Fig 1B). Diameters of the visible particles ranged from 1.3 μm to 30.5 μm.
Fig 1. Quantification of e-cigarette aerosol droplets.
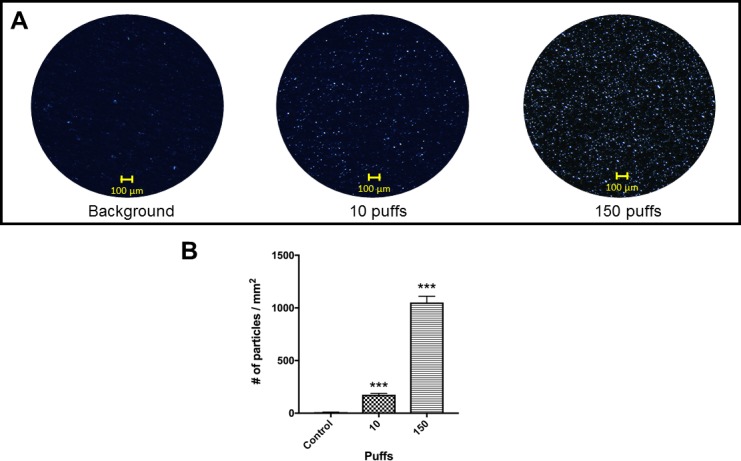
(A) Aerosols were delivered using a simulated human vaping topology (50 mL puff volume in 4 s puff duration every 18 s). Aerosol droplets were imaged by a stereoptical light microscope on a non-reflective vinyl surface (bar = 100 μm). (B) ImageJ was used to quantify the aerosol droplets. The number of particles for control, after 10 puffs, and 150 puffs were (5.7 ± 5.0, 175.5 ± 12.7 and 1051.2 ± 59.4) particles per mm2, respectively (mean ± S.D.). Student t-tests were performed control vs. individual puffing regime (*** = p<0.0001).
E-cigarette generates viscous aerosol and promotes Streptococcus mutans attachment
To test if e-cigarette aerosol leads to a biologically-relevant surface change, human tooth enamel disks were exposed following the standard protocol as described in the Study Design. Using the single-cell force spectroscopy, the adhesive force between S. mutans and enamel surface was measured under three different conditions: control (no exposure), 10 and 150 puffs. It was found that the adhesive force between the pathogenic bacteria and enamel surface increased significantly with 10 puffs (p < 0.0001) and 150 puffs (p < 0.0001) aerosol exposure compared to the unexposed control (Fig 2).
Fig 2. Adhesive force between S. mutans and enamel surface.
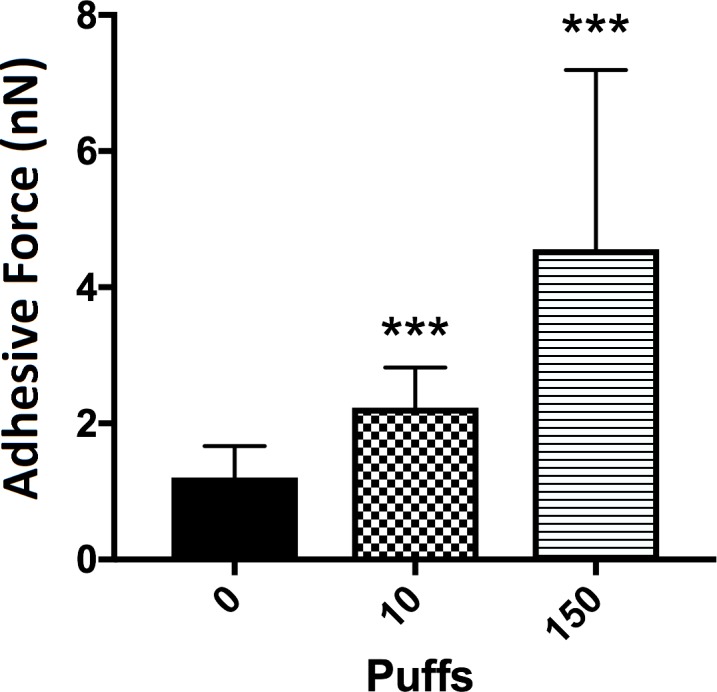
The adhesive forces were calculated by averaging 30 measurements on three individual surfaces. The forces for control, after 10 puffs and 150 puffs were (1.2 ± 0.4, 2.2 ± 0.5, and 4.5 ± 2.6) nN, respectively (mean ± S.D.). Student t-tests were performed control vs. individual puffing regime (*** = p<0.0001).
Certain flavors increase biofilm formation
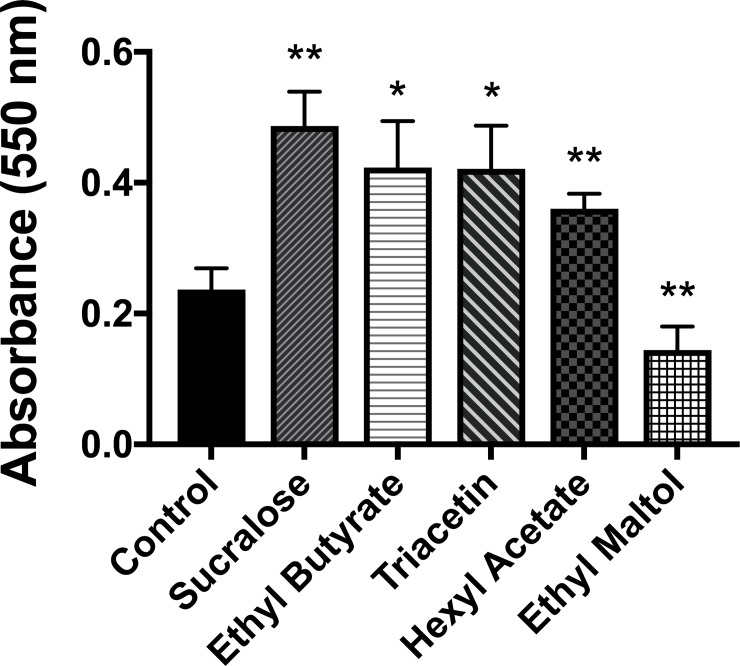
Dental plaque is a biofilm found on natural teeth. Dental plaque is implicated in dental caries which is associated with a shift in the balance of healthy oral microbiome, resulting in dysbiosis favoring disease-promoting bacteria including acid producing S. mutans [55]. The purpose of this study, therefore, was to test the effect of different e-cigarette flavor exposure to S. mutans biofilm formation. Five e-liquid flavors were pre-selected based on their high potential for cariogenicity (e.g., sweetness or low pH) and a previous study [28] which analyzed 30 commercial products: hexyl acetate (apple/plum), ethyl butyrate (pineapple), sucralose (sugar substitute), triacetin (“velvety” or “smoky” flavor) and ethyl maltol (cotton candy). Individually the five flavored e-liquids were aerosolized following the standard protocol as described in the Study Design. After forming biofilms, the amount of biofilm on each plate was quantified using the O’Toole-Kolter method. Four out of five flavors (sucralose, ethyl butyrate, triacetin, hexyl acetate) increased biofilm formation significantly compared to unflavored e-liquid control. Interestingly ethyl maltol, sugar alcohol, decreased biofilm development significantly compared to the control (Fig 3).
Fig 3. Biofilm quantification after flavored e-liquid aerosol exposures.
The absorbance for control, sucralose, ethyl butyrate, triacetin, hexyl acetate and ethyl maltol were (0.23 ± 0.03, 0.48 ± 0.05, and 0.42 ± 0.07, 0.42 ± 0.06, 0.36 ± 0.02, and 0.14 ± 0.03) AU, respectively (mean ± S.D.). Student t-tests were performed control vs. individual flavored e-liquid and statistical differences were indicated as: * = p<0.05 or ** = p<0.005.
E-cigarette aerosol occupies pits and fissures of human teeth and promotes bacterial attachment
To characterize how e-cigarette aerosol interacts with complex biological surfaces, caries-free extracted teeth (without polishing or cutting) were exposed using the standard protocol. After a 24 h incubation with S. mutans, a Scanning Electron Microscope (SEM) was used to visualize smooth surfaces, pits, and fissures–three areas on tooth enamel surface where bacteria can attach, form a biofilm, and lead to dental caries. Generally, bacteria were found more frequently in aerosol exposed pits and fissures compared to the unexposed controls (Fig 4). The aerosol exposed smooth surfaces also had more S. mutans compared to the unexposed control smooth surface but to a lesser degree than pits and fissures (Fig 4). Once S. mutans occupied pits and fissures, the oral bacteria thrived and formed very complex biofilm by secreting EPS (Fig 5).
Fig 4. Complex interaction among S. mutans, enamel surface and e-cigarette aerosol.
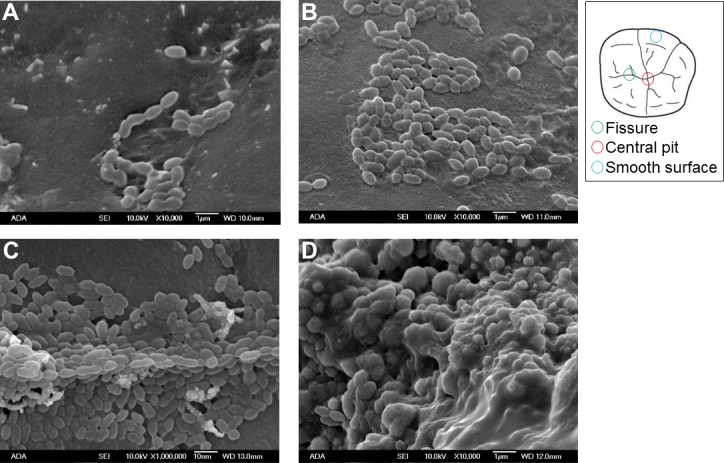
(A) Control: smooth enamel surface, unexposed. (B) Smooth enamel surface, exposed with 10 puff e-cigarette aerosol. (C) Fissure, exposed with 10 puff e-cigarette aerosol. (D) Central pit, exposed with 10 puff e-cigarette aerosol (SEM parameters: X10,000, 10.0 kV, and bar = 1 μm).
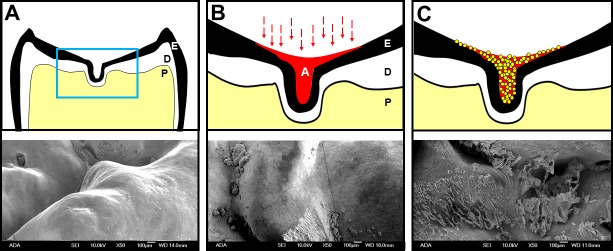
Fig 5. E-liquid aerosol pools into pits and fissures.
(A) Top: a cross section of a human tooth (E = enamel, outer layer, D = dentin, middle layer, and P = pulp, cellular component with nervous and vascular tissues), Bottom: control, unexposed enamel fissure. (B) Top: a tooth after e-cigarette aerosol exposure (A = aerosol, E = enamel, D = dentin, and P = pulp), Bottom: aerosol exposed enamel fissure. (C) Top: a tooth after e-cigarette aerosol exposure and subsequent S. mutans attachment (Spheres = S. mutans), Bottom: S. mutans colonizing fissure and secreting EPS (SEM parameters: X50, 10.0 kV, and bar = 100 μm).
Certain flavors demineralize enamel and decrease tooth hardness
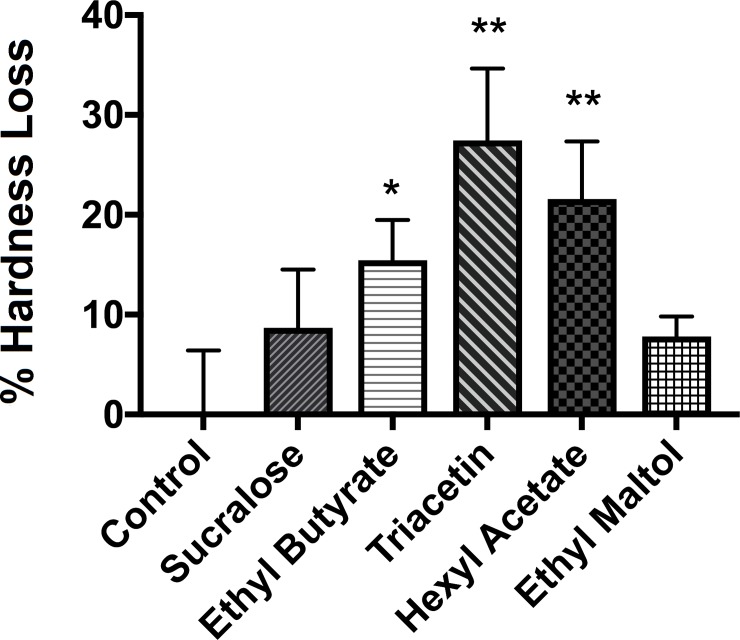
To determine if flavors in e-liquids will increase demineralization of healthy enamel surface, the hardness of the surface was compared among enamel disks exposed to five different e-liquid aerosols (Fig 6). The baseline values were recorded by measuring hardness (three enamel disks per condition, five random locations per disk) prior to the aerosol exposure. The enamel disks were exposed using the standard protocol. After a 6 h incubation with S. mutans, the hardness of the disks was re-assessed by performing new indentations within 100 μm from the initial indents (prior to aerosol exposure). The percentage hardness loss (%) for control, sucralose, ethyl butyrate, triacetin, hexyl acetate, and ethyl maltol were (0.0004 ± 6.4, 8.6 ± 5.8, 15.4 ± 4.0 (p<0.05), 27.4 ± 7.1 (p<0.005), 21.5 ± 5.7 (p<0.005) and 7.8 ± 2.0) %, respectively.
Fig 6. Enamel hardness loss after flavored e-liquid aerosol exposures.
The hardness loss for control, sucralose, ethyl butyrate, triacetin, hexyl acetate and ethyl maltol were (0.01 ± 6.41, 8.67 ± 5.84, and 15.45 ± 4.02, 27.45 ± 7.19, 21.57 ± 5.76, and 7.80 ± 2.00) %, respectively (mean ± S.D.). Student t-tests were performed control vs. individual flavored e-liquid and statistical differences were indicated as: * = p<0.05 or ** = p<0.005.
E-liquid base and flavors break down to smaller chemical by-products upon heating
Aerosols from control and five reference e-liquids were characterized using Gas Chromatography–Mass Spectrometry (GC-MS) (Table 2). Propylene glycol, nicotine, 2-propanol, diphenyl ether and nicotyrine were identified in aerosols generated from all six e-liquids. Generally, upon heating, e-liquids broke down to several alcohols, aromatic hydrocarbons, carboxylic acids, esters, aldehydes, ureas, carbonyl compounds, and ethers. Each flavor had at least one chemical by-product that was not present in the control aerosol (indicated by an asterisk). Ethyl maltol produced the most unique chemical by-products and sucralose aerosol had the least. Table 2 also contains Signal-to-Noise (S/N) ratios which are based on Total Ion Chromatogram (TIC) intensity peak values. S/N ratio is important parameter for sensitivity and chromatography quality evaluation. The chemical by-products shown in Table 2 are based on detectable peaks and suggested by the NIST Mass Spectral Library.
Table 2. GS-MS analyses of e-cigarette aerosols.
| Group | CAS number | Name | S/N ratio |
|---|---|---|---|
| Control | 57-55-6 | Propylene Glycol | 34.7 |
| 54-11-5 | Nicotine | 53.7 | |
| 20324-32-7 | 2-Propanol, 1-(2-methoxy-1-methylethoxy)- | 61.9 | |
| 101-84-8 | Diphenyl ether | 30.7 | |
| 487-19-4 | Nicotyrine | 17.2 | |
| Alcohol | |||
| 13588-28-8 | 1-Propanol, 2-(2-methoxypropoxy)- | 51.8 | |
| 22104-79-6 | 2-Nonen-1-ol | 7.62 | |
| 3944-36-3 | 2-Propanol, 1-(1-methylethoxy)- | 4.49 | |
| 116-09-6 | 2-Propanone, 1-hydroxy-(Hydroxyacetone) | 44.3 | |
| Aromatic hydrocarbon | |||
| 1014-60-4 | Benzene, 1,3-bis(1,1-dimethylethyl)- | 29.1 | |
| Sucralose (C12H19Cl3O8) | 57-55-6 | Propylene Glycol | 34.1 |
| 54-11-5 | Nicotine | 57.4 | |
| 20324-32-7 | 2-Propanol, 1-(2-methoxy-1-methylethoxy)- | 67 | |
| 101-84-8 | Diphenyl ether | 56.9 | |
| 487-19-4 | Nicotyrine | ||
| Alcohol | |||
| 22104-79-6 | *2-Nonen-1-ol | 7.18 | |
| Carboxylic acid | |||
| 55536-71-5 | *Di-tert-butyl 1,4-Dihydro-2,6-dimethyl-3,5-pyridinedicarboxylate | 15.1 | |
| Aromatic hydrocarbon | |||
| 1014-60-4 | *Benzene, 1,3-bis(1,1-dimethylethyl)- | 28.3 | |
| Ethyl Butyrate (C6H12O2) | 57-55-6 | Propylene glycol | 66.7 |
| 54-11-5 | Nicotine | 56.8 | |
| 20324-32-7 | 2-Propanol, 1-(2-methoxy-1-methylethoxy)- | 38.4 | |
| 101-84-8 | Diphenyl ether | 58.1 | |
| 487-19-4 | Nicotyrine | 27 | |
| Alcohol | |||
| 108-61-2 | *1-Propanol, 2,2'-oxybis- | 21.2 | |
| 104-76-7 | *1-Hexanol, 2-ethyl- | 9.04 | |
| Ester | |||
| 105-54-4 | *Ethyl butyrate | 96.1 | |
| Aromatic hydrocarbon | |||
| *Benzenemethanol, α,α-dimethyl- | 29.6 | ||
| Aldehyde | |||
| 124-19-6 | *Nonanal (C9H18O) | 38.1 | |
| Triacetin (C9H14O6) | 4254-14-2 | Propylene glycol | 68.2 |
| 54-11-5 | Nicotine | 67 | |
| 20324-32-7 | 2-Propanol, 1-(2-methoxy-1-methylethoxy)- | 43.9 | |
| 101-84-8 | Diphenyl ether | 55.8 | |
| 487-19-4 | Nicotyrine | 23.7 | |
| Alcohol | |||
| 116-09-6 | *2-Propanone, 1-hydroxy-(C3H6O2) | 38 | |
| 54305-61-2 | *2-Butanol, 3,3'-oxybis- | 35.1 | |
| Ester | |||
| 102-62-5 | *Glycerol 1,2-diacetate | 49.3 | |
| Aromatic hydrocarbon | |||
| 1014-60-4 | *Benzene, 1,3-bis(1,1-dimethylethyl)- | 15.5 | |
| Hexyl Acetate (C8H16O2) | 57-55-6 | Propylene glycol | 34.5 |
| 54-11-5 | Nicotine | 70.2 | |
| 20324-32-7 | 2-Propanol, 1-(2-methoxy-1-methylethoxy)- | 45.4 | |
| 101-84-8 | Diphenyl ether | 64.3 | |
| 478-19-4 | Nicotyrine | 23.9 | |
| Alcohol | |||
| 111-27-3 | *1-Hexanol | 28.3 | |
| Ester | |||
| 142-92-7 | *Hexyl acetate | 90.1 | |
| 95-92-1 | *Ethanedioic acid, diethyl ester | 62.8 | |
| Aromatic hydrocarbon | |||
| 1014-60-4 | *Benzene, 1,3-bis(1,1-dimethylethyl)- | 35.2 | |
| Aldehyde | |||
| 124-19-6 | *Nonanal (C9H18O) | 54.7 | |
| Carboxylic acid | |||
| 26164-26-1 | *Benzeneacetic acid, α-methoxy-, (S)- (C6H5CH(OCH3)CO2H) | 6.13 | |
| Urea | |||
| 598-50-5 | *N-Methylurea (CH3NHCONH2) | 33.3 | |
| Ethyl Maltol (C7H8O3) | 57-55-6 | Propylene Glycol | 32.2 |
| 54-11-5 | Nicotine | 57.9 | |
| 20324-32-7 | 2-Propanol, 1-(2-methoxy-1-methylethoxy)- | 60.2 | |
| 101-84-8 | Diphenyl ether | 32.8 | |
| 478-19-4 | Nicotyrine | ||
| Alcohol | |||
| 13588-28-8 | *1-Propanol, 2-(2-methoxypropoxy)- | 66.5 | |
| 54305-61-2 | *2-Butanol, 3,3'-oxybis- | 55 | |
| 4940-11-8 | *Ethyl maltol | 66.2 | |
| Aromatic hydrocarbon | |||
| 1014-60-4 | *Benzene, 1,3-bis(1,1-dimethylethyl)- | 49.1 | |
| 108-41-8 | *Benzene, 1-chloro-3-methyl- | 35.1 | |
| 95-49-8 | *Benzene, 1-chloro-2-methyl- | 43.8 | |
| Aldehyde | |||
| 124-19-6 | *Nonanal (C9H18O) | 24.3 | |
| Carbonyl compound | |||
| 1874-54-0 | *Psicofuranine (C11H15N5O5) | 29.3 | |
| Ether | |||
| 3386-87-6 | *3,3'-(Ethylenedioxy) dipropionitrile (C8H12N2O2) | 24.5 | |
* = a unique chemical by-product that is not present in the control aerosol. These chemical by-products are based on detectable peaks and suggested by the NIST Mass Spectral Library.
Sub-ohm e-cigarette aerosol contains metals
To identify types of metals in e-cigarette aerosol, Inductively Coupled Plasma with Optical Emission Spectrometry (ICP-OES) analyses were performed on 7.5 L (150 puffs) aerosol samples generated using the flavor-free reference e-liquid, a sub-ohm (0.2 Ω) heating element with a cotton-based wick. The presence of calcium (0.409 ± 0.002) mg/L, copper (0.011 ± 0.001) mg/L, iron (0.0051 ± 0.0003) mg/L, magnesium (0.017 ± 0.002) mg/L, and silicon (0.166 ± 0.005) mg/L was confirmed from the analysis of the aerosol. In contrast to previous findings, the new sub-ohm device did not emit lead or manganese [26]. The levels of cadmium (Cd), cobalt (Co), chromium (Cr), nickel (Ni), and palladium (Pd) were below the limit of ICP-OES detection. The results are summarized in Table 3 with the National Institute of Occupational Safety and Health (NIOSH) Daily Exposure Limit information.
Table 3. Metals in e-cigarette aerosol.
| Calcium | Copper | Iron | Magnesium | Silicon | |
|---|---|---|---|---|---|
|
Sub-ohm: 0.2 Ω 49.2 W 150 puffs (mg/L) a |
0.409 ± 0.002 | 0.011 ± 0.001 | 0.0051 ± 0.0003 | 0.017 ± 0.002 | 0.166 ± 0.005 |
|
NIOSH: Daily exposure limits (mg/L) |
5 | 1 | 5 | 15 | 5 |
a The concentrations of the metals (mg/L) are shown as (mean ± S.D.).
Discussion
Dental caries is a complex disease with many etiological factors including host genetics, oral microbiome, immune system, diet, oral hygiene, salivary function, community water fluoridation, and access to quality dental care [56]. The direct correlation between diet, especially the quantity of sucrose intake, and dental caries incidence has been intensely researched and supported by many studies [56, 57]. In addition to the quantity of sucrose intake, how it is delivered (e.g., sugar mixed in acidic beverages), how often it is delivered (e.g., sipping sugary beverage over an extended time), and how long the sucrose is in contact with the hard tissue surface (e.g., hard and sticky candies can lead to longer sucrose exposure in the oral cavity) can further increase the risk of initiation, progress, and severity of dental caries [58, 59]. Although there is strong scientific evidence showing that a diet high in sucrose is the most important factor in caries development, the similar sugary and acidic flavors (e.g., saccharides, esters, acids, and aldehydes) found in e-liquids have not been studied to the same extent. Since the main route of intake and sensory perception of foods (mastication via oral cavity to gastrointestinal tract) and e-liquids (inhalation via oral cavity to respiratory tract) are not exactly the same, caution should be taken when flavors of e-liquids are compared to actual foods and beverages. However, identification of specific flavors that increase cariogenic potential will facilitate the development of the oral health risk assessment of e-cigarette use and provide scientific evidence that there may be unintended consequences of using e-cigarettes.
In this study, Streptococcus mutans was exposed to flavored e-liquid aerosols to identify specific flavors and chemical by-products that may increase tooth surface damage. Through a combination of pure reference e-liquid and GC-MS based analysis, we discovered that ethyl butyrate, triacetin and hexyl acetate and their respective chemical by-products increase cariogenic potential. Ethyl butyrate, hexyl acetate, and triacetin are different types of esters. Ethyl butyrate possesses a strong pineapple scent and is naturally produced in many fruits [59]. Interestingly, several oral bacteria including Streptococcus salivarius and Lactococcus lactis actively produce ethyl butyrate [60]. This indicates S. mutans is frequently exposed to ethyl butyrate in oral biofilm and at minimum tolerates or as suggested by the results, can enhance biofilm development in response. Hexyl acetate has not been studied in the context of oral biofilm, dental caries or S. mutans. However, Streptococcus, Actinomyces, and Lactobacillus via classic glycolysis metabolize carbohydrates to acetate in oral biofilm [61]. With acetate being one of final principle products formed by biofilm bacteria [62], it is expected that S. mutans is able to thrive in an acetate-rich environment. Triacetin is found in fruits and cigarette filters and has a “velvety” or “smoky” flavor. Triacetin is mainly used as a food additive, humectant, plasticizer, and anti-knocking agent but not much is known in the context of oral biology [62]. It is, however, well known that S. mutans has esterase activities that degrade monomers in dental restorative materials such as resin composites and adhesives [63]. Based on these data, it is proposed that esters in e-liquid flavors provide an additional food source for S. mutans to flourish in oral biofilm environment. However, further metabolomic analysis should be performed to validate the findings described here.
Further examination of the biofilm assays, mechanical testing (adhesion force and hardness measurement), and SEM images show complex surface changes and biological responses upon e-cigarette aerosol exposure. The results demonstrate that an e-cigarette produces viscous aerosols which cover enamel surfaces. Surface characteristics such as surface roughness, tackiness, charge, and energy have a significant impact on how bacterial cells adhere to surfaces and subsequently form biofilm [64]. The data shown in this study are consistent with previous studies that adhesion of S. mutans to surfaces can be influenced by several factors (e.g., presence of acquired pellicles, salivary proteins, specific glucan synthesis, cell surface proteins, other oral bacteria and availability of sucrose) [65–67]. This study confirms that S. mutans-to-surface interactions can be altered by e-cigarette aerosols. Previous studies have shown that S. mutans are able to exploit the initial attachment to tooth surfaces by secreting Extracellular Polymeric Substances (EPS) [68–70]. EPS allow S. mutans to encapsulate itself on the surface, start multiplying in number and eventually forming biofilm [71, 72]. S. mutans in biofilm can rapidly metabolize carbohydrates into lactic acid, creating locally a low pH, leading to demineralization of enamel surface [56]. Our SEM and micro-indentation hardness analyses suggest that S. mutans attach to the e-cigarette exposed surface, metabolize e-liquid base and flavors to secrete EPS and rapidly form biofilm which demineralize the e-cigarette exposed enamel surfaces. Demineralization of enamel is of great importance to oral health because the demineralization is the first step of dental caries development.
S. mutans have evolved to survive in challenging environment by developing remarkable metabolic flexibility [73]. In this study, levels of 12 metal ions in e-cigarette aerosol have been measured. At a high concentration, metals can be toxic to bacteria and humans, though, at physiological levels, metal ions may serve as nutrients required for many important biological processes [73]. Oral bacteria including S. mutans require metal ions (e.g., copper, iron, and magnesium) as a co-factor to activate essential enzymes [73]. Pathogenic bacteria also have evolved numerous mechanisms for essential metal uptake to circumvent the host’s immune system [73]. By ICP-OES analyses, the following metals in e-cigarette aerosol were identified: calcium, copper, iron, magnesium, and silicon. Calcium, iron and copper ions are well-known modulators in biofilm formation and enamel remineralization / demineralization processes [74–76]. S. mutans is known to have a nutritional requirement for magnesium [77]. Our data suggest that the level of metals is well tolerated by S. mutans following the e-cigarette aerosol exposure as described in the Study Design.
The non-linear correlation between the absorbance data and the hardness loss data indicate that the mechanism of bacterial growth and enamel demineralization is complex. Therefore, in addition to the bacteria-initiated damage to the enamel surface, the chemical by-products may influence the hardness of the surface directly. Previous studies have demonstrated acidic drinks, citrus suckling behavior, and bulimia can lead to enamel surface damage directly [78].
Using AFM, it was found that the adhesive forces between S. mutans and enamel surface increases as a function of number of puffs. It was also found that with 10 puffs, aerosol droplets were evenly distributed which was verified by the force measurement and light microscope images. However, as the number of puffs increased, aerosol droplets started to aggregate, which may explain the larger variation recorded from 150 puff samples. Whether the local accumulation of droplets can be recapitulated in in-vivo system and whether it has important biological implications remain to be seen.
Ethyl maltol has a distinctive fragrance that resembles cotton candy. It is one of the strongest fragrances tested in this study and remains a popular additive among commercial e-liquids. It was unexpected to find that ethyl maltol in e-liquids acted as a potent antimicrobial agent against S. mutans. Although ethyl maltol was not investigated as a therapeutic antibiotic, Schved et al. have shown that ethyl maltol destabilizes the outer cell membrane of E. coli by chelating Mg2+ and/or Ca2+ in a pH dependent manner [79]. Thus, it is plausible that ethyl maltol may interfere with S. mutans cell membrane integrity in a similar fashion.
There are several limitations to this study. The primary limitation of this study is that the oral microbiome is a complex network of several hundred bacteria species. In this study, the biological responses were characterized only from one organism, Streptococcus mutans, a major cariogenic bacterium in oral cavity. Since other oral bacteria have different nutritional requirements and can tolerate different levels of environmental challenges such as pH changes or chemical exposures, they may respond differently to the flavored e-liquids tested here. Although the reference e-liquids intentionally used only one flavor per e-liquid, commercial e-liquids contain several additives, including sucrose, sugar substitutes and acids, some at much higher concentration [27–29, 80]. This suggests that the actual damage to the tooth enamel surface may vary with the constituents present in e-liquids, and could be higher or lower than measured in this study. Since e-liquid undergoes thermal degradation when aerosolized, the concentration of flavors in the resulting aerosol may be different from the concentration of flavors in the starting e-liquid. For example, Rosbrook et al. reported that the amount of sucralose in aerosols can be altered by e-cigarette delivery systems such as wick design and size of mouthpiece, and interestingly, not necessarily by voltage or resistance of the metal heating element [81]. This suggests that (1) the concentration of flavors in aerosols may be difficult to predict without actual experimental quantification, and (2) the concentration of flavors in aerosols depends on the constituents of the starting e-liquid as well as the design of the device. Recently, Krusemann et al. systematically classified commercial e-liquids into a comprehensive chart AKA “E-Liquid Flavor Wheel” [5]. The flavor wheel suggests there may be other flavors (e.g., alcohol, honey, or vanilla) which could damage tooth enamel as well. A limitation of the study is that the bacterial culturing protocol could not directly incorporate human saliva (for its buffering ability to counteract low pH challenges) into the in-vitro experimental design. To counteract this limitation, S. mutans were grown in buffered media. Although ICP-OES data suggested that the level of metals was below the National Institute of Occupational Safety and Health (NIOSH) Daily Exposure Limit (Table 3), the experimental conditions were ideal and conservative as possible. This was intentional to improve transparency and reproducibility of the research methods described in this study. In-vivo metal levels may vary depending on devices, e-liquids, flavors, puff and inter-puff durations, heating element resistance, wattage of the system and user’s behavior patterns (e.g., compliance to the manufacturer’s instruction). It is possible that the chronic metal exposure even at a low to moderate levels may lead to unintended microbial dysbiosis causing negative health consequences [82–85]. Finally, humans’ flavor perception is a complex neurophysiological phenomenon. Perceptions of the flavors of foods or beverages reflect information received from multiple sensory afferents, including gustatory (taste), olfactory (smell), and somatosensory fibers [86]. As such, some flavors in commercial e-liquids are added to enhance positive gustatory (e.g., sucralose) input or olfactory sensation (e.g. ethyl maltol) or a combination of both. Although descriptions of commercial e-liquids may resemble actual foods or beverages, the similarity between two products is mainly achieved by manipulating the olfaction of the users through inhalation. A fraction of aerosols will be dissolved by saliva and transported to the sweet taste receptors mainly located on the tongue and palate of oral cavity. In this study, we primarily focused on bacteria-induced cariogenicity from certain flavors, chemical by-products and viscosity of glycerin. However, the impact of flavors in e-cigarette products on human health may be more significant than previously described.
Despite these limitations, this study provides insight into potential unintended negative consequences of vaping on oral health, specifically teeth. Although tooth enamel is the hardest mineralized tissue in human body, once damaged beyond salivary buffering capacity, it has no way of regenerating itself. Based on the results and aforementioned limitations, there are at least two immediate needs to advance this work: (1) clinical investigations should be performed to confirm and translate the data shown here, and (2) case studies based on clinical observations from oral health providers will greatly enhance our understanding of the real cost of e-cigarette on oral health.
Conclusions
A novel finding of this study is that certain e-liquid ingredients interact with hard tissues of the oral cavity in such a way that resembles high-sucrose candies and acidic drinks that adversely affect teeth. This is an important finding that suggests the complexity of e-cigarettes on human health goes beyond respiratory and cardiac systems and may have significant implications on oral health. It is a common perception among e-cigarette users that vaping is less harmful or is without health risk. Though it is acknowledged here that e-cigarette aerosols contain less harmful and potentially harmful constituents compared to combustible tobacco products, the data suggests e-cigarettes produce viscous aerosols that change surface characteristics and have biological consequences. Viscous e-liquids made from propylene glycol and glycerin, along with sweet flavors facilitate attachment and provide additional food source which pathogenic oral bacteria such as S. mutans prefer. Youth and young adults are a uniquely vulnerable population to dental caries due to their high-sucrose diet and poor to minimal oral hygiene practice [87–90]. This study suggests that flavored e-cigarette products negatively affect teeth and pose potential oral health risk. These two facts advocate that there is an urgent need to further research e-cigarettes, e-liquids and flavors in the context of human health and disease.
Acknowledgments
Disclaimer: Certain commercial equipment, instruments, and materials are identified in this paper to describe the experiments performed. Such identification does not imply recommendation or endorsement by the ADA Foundation and/or the National Institute of Standards and Technology (NIST), nor does it imply that the materials or equipment identified are necessarily the best available for the purpose.
The authors thank Drago Skrtic and Thomas C. Hart for critically reviewing the manuscript, Christine Dillon for her administrative support, and Tiffany Cao for her effort in the revision process. The authors acknowledge the National Institute of Standards and Technology (NIST), U.S. Department of Commerce for sharing technical excellence, measurement expertise, core facilities and high-precision analytical instruments through CRADA (CN-1730). The authors thank the NIST Summer Undergraduate Research Fellowship (SURF) program, especially Rebecca Zangmeister (NIST).
Authors have provided detailed instructions on how to formulate and appropriately use the reference e-liquid in the main text. Should readers need further assistance, they can refer to www.adafoundation.org for more information.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by the ADA Foundation, CR97200019 (JJK).
References
- 1.Singh T, Arrazola RA, Corey CG, Husten CG, Neff LJ, Homa DM, et al. Tobacco Use Among Middle and High School Students—United States, 2011–2015. MMWR Morb Mortal Wkly Rep. 2016;65(14):361–7. doi: 10.15585/mmwr.mm6514a1 . [DOI] [PubMed] [Google Scholar]
- 2.Jamal A, Gentzke A, Hu SS, Cullen KA, Apelberg BJ, Homa DM, et al. Tobacco Use Among Middle and High School Students—United States, 2011–2016. MMWR Morb Mortal Wkly Rep. 2017;66(23):597–603. doi: 10.15585/mmwr.mm6623a1 ; PubMed Central PMCID: PMCPMC5657845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindblom EN. Effectively Regulating E-Cigarettes and Their Advertising—And the First Amendment. Food Drug Law J. 2015;70(1):55–92. . [PubMed] [Google Scholar]
- 4.Pearson JL, Richardson A, Niaura RS, Vallone DM, Abrams DB. e-Cigarette awareness, use, and harm perceptions in US adults. Am J Public Health. 2012;102(9):1758–66. 10.2105/AJPH.2011.300526 ; PubMed Central PMCID: PMCPMC3474361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krusemann EJZ, Boesveldt S, de Graaf K, Talhout R. An E-liquid Flavor Wheel: A Shared Vocabulary based on Systematically Reviewing E-liquid Flavor Classifications in Literature. Nicotine Tob Res. 2018. 10.1093/ntr/nty101 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ambrose BK, Day HR, Rostron B, Conway KP, Borek N, Hyland A, et al. Flavored Tobacco Product Use Among US Youth Aged 12–17 Years, 2013–2014. JAMA. 2015;314(17):1871–3. 10.1001/jama.2015.13802 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh T, Kennedy S, Marynak K, Persoskie A, Melstrom P, King BA. Characteristics of Electronic Cigarette Use Among Middle and High School Students—United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(5051):1425–9. doi: 10.15585/mmwr.mm655051a2 . [DOI] [PubMed] [Google Scholar]
- 8.Soneji SS, Sung HY, Primack BA, Pierce JP, Sargent JD. Quantifying population-level health benefits and harms of e-cigarette use in the United States. PLoS One. 2018;13(3):e0193328 10.1371/journal.pone.0193328 ; PubMed Central PMCID: PMCPMC5851558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schneider S, Diehl K. Vaping as a Catalyst for Smoking? An Initial Model on the Initiation of Electronic Cigarette Use and the Transition to Tobacco Smoking Among Adolescents. Nicotine Tob Res. 2016;18(5):647–53. 10.1093/ntr/ntv193 . [DOI] [PubMed] [Google Scholar]
- 10.Soneji S, Barrington-Trimis JL, Wills TA, Leventhal AM, Unger JB, Gibson LA, et al. Association Between Initial Use of e-Cigarettes and Subsequent Cigarette Smoking Among Adolescents and Young Adults: A Systematic Review and Meta-analysis. JAMA Pediatr. 2017;171(8):788–97. 10.1001/jamapediatrics.2017.1488 ; PubMed Central PMCID: PMCPMC5656237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.H.R. 1256-111th Congress: Family Smoking Prevention and Tobacco Control Act In: HR 1256: GovTrack.us (database of federal legislation); 2009.
- 12.European Comission. The Tobacco Products Directive (2014/40/EU). 2014 http://ec.europa.eu/health/tobacco/docs/dir_201440_en.pdf. (accessed Aug. 2, 2018)
- 13.Villanti AC, Johnson AL, Ambrose BK, Cummings KM, Stanton CA, Rose SW, et al. Flavored Tobacco Product Use in Youth and Adults: Findings From the First Wave of the PATH Study (2013–2014). Am J Prev Med. 2017;53(2):139–51. 10.1016/j.amepre.2017.01.026 ; PubMed Central PMCID: PMCPMC5522636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Food, Drug Administration HHS. Deeming Tobacco Products To Be Subject to the Federal Food, Drug, and Cosmetic Act, as Amended by the Family Smoking Prevention and Tobacco Control Act; Restrictions on the Sale and Distribution of Tobacco Products and Required Warning Statements for Tobacco Products. Final rule. Fed Regist. 2016;81(90):28973–9106. . [PubMed] [Google Scholar]
- 15.Kowitt SD, Meernik C, Baker HM, Osman A, Huang LL, Goldstein AO. Perceptions and Experiences with Flavored Non-Menthol Tobacco Products: A Systematic Review of Qualitative Studies. Int J Environ Res Public Health. 2017;14(4). 10.3390/ijerph14040338 ; PubMed Central PMCID: PMCPMC5409539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu SH, Sun JY, Bonnevie E, Cummins SE, Gamst A, Yin L, et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control. 2014;23 Suppl 3:iii3–9. 10.1136/tobaccocontrol-2014-051670 ; PubMed Central PMCID: PMCPMC4078673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goniewicz ML, Kuma T, Gawron M, Knysak J, Kosmider L. Nicotine levels in electronic cigarettes. Nicotine Tob Res. 2013;15(1):158–66. 10.1093/ntr/nts103 . [DOI] [PubMed] [Google Scholar]
- 18.Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23(2):133–9. 10.1136/tobaccocontrol-2012-050859 ; PubMed Central PMCID: PMCPMC4154473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hutzler C, Paschke M, Kruschinski S, Henkler F, Hahn J, Luch A. Chemical hazards present in liquids and vapors of electronic cigarettes. Arch Toxicol. 2014;88(7):1295–308. 10.1007/s00204-014-1294-7 . [DOI] [PubMed] [Google Scholar]
- 20.Trehy ML, Ye W, Hadwiger ME, Moore TW, Allgire JF, Woodruff JT, et al. Analysis of Electronic Cigarette Cartridges, Refill Solutions, and Smoke for Nicotine and Nicotine Related Impurities. Journal of Liquid Chromatography & Related Technologies. 2011;34(14):1442–58. 10.1080/10826076.2011.572213 PubMed PMID: WOS:000296230900012. [DOI] [Google Scholar]
- 21.Cobb NK, Byron MJ, Abrams DB, Shields PG. Novel nicotine delivery systems and public health: the rise of the "e-cigarette". Am J Public Health. 2010;100(12):2340–2. Epub 2010/11/12. 10.2105/AJPH.2010.199281 ; PubMed Central PMCID: PMC2978165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cameron JM, Howell DN, White JR, Andrenyak DM, Layton ME, Roll JM. Variable and potentially fatal amounts of nicotine in e-cigarette nicotine solutions. Tob Control. 2014;23(1):77–8. Epub 2013/02/15. 10.1136/tobaccocontrol-2012-050604 . [DOI] [PubMed] [Google Scholar]
- 23.Cheah NP, Chong NW, Tan J, Morsed FA, Yee SK. Electronic nicotine delivery systems: regulatory and safety challenges: Singapore perspective. Tob Control. 2014;23(2):119–25. Epub 2012/12/04. 10.1136/tobaccocontrol-2012-050483 . [DOI] [PubMed] [Google Scholar]
- 24.Etter JF, Zather E, Svensson S. Analysis of refill liquids for electronic cigarettes. Addiction. 2013;108(9):1671–9. Epub 2013/05/25. 10.1111/add.12235 . [DOI] [PubMed] [Google Scholar]
- 25.Hadwiger ME, Trehy ML, Ye W, Moore T, Allgire J, Westenberger B. Identification of amino-tadalafil and rimonabant in electronic cigarette products using high pressure liquid chromatography with diode array and tandem mass spectrometric detection. J Chromatogr A. 2010;1217(48):7547–55. Epub 2010/10/29. 10.1016/j.chroma.2010.10.018 . [DOI] [PubMed] [Google Scholar]
- 26.Kim JJ, Sabatelli N, Tutak W, Giuseppetti A, Frukhtbeyn S, Shaffer I, et al. Universal electronic-cigarette test: physiochemical characterization of reference e-liquid. Tob Induc Dis. 2017;15:14 10.1186/s12971-017-0119-x ; PubMed Central PMCID: PMCPMC5314484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soussy S, El-Hellani A, Baalbaki R, Salman R, Shihadeh A, Saliba NA. Detection of 5-hydroxymethylfurfural and furfural in the aerosol of electronic cigarettes. Tob Control. 2016;25(Suppl 2):ii88–ii93. 10.1136/tobaccocontrol-2016-053220 . [DOI] [PubMed] [Google Scholar]
- 28.Tierney PA, Karpinski CD, Brown JE, Luo W, Pankow JF. Flavour chemicals in electronic cigarette fluids. Tob Control. 2016;25(e1):e10–5. 10.1136/tobaccocontrol-2014-052175 ; PubMed Central PMCID: PMCPMC4853541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.https://www.nudenicotine.com/product/sucralose-solutions-5-15/ (accessed Aug. 2, 2018).
- 30.Frostell G, Keyes PH, Larson RH. Effect of various sugars and sugar substitutes on dental caries in hamsters and rats. J Nutr. 1967;93(1):65–76. 10.1093/jn/93.1.65 . [DOI] [PubMed] [Google Scholar]
- 31.Keyes PH. Dental caries in the Syrian hamster. VI. Minimal dental caries activity in animals fed presumably cariogenic rations. J Dent Res. 1954;33(6):830–41. 10.1177/00220345540330061101 . [DOI] [PubMed] [Google Scholar]
- 32.Huilgol P, Bhatt SP, Biligowda N, Wright NC, Wells JM. Association of e-cigarette use with oral health: a population-based cross-sectional questionnaire study. J Public Health (Oxf). 2018. 10.1093/pubmed/fdy082 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berg JH. The marketplace for new caries management products: dental caries detection and caries management by risk assessment. BMC Oral Health. 2006;6 Suppl 1:S6 10.1186/1472-6831-6-S1-S6 ; PubMed Central PMCID: PMCPMC2147594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Featherstone JD. The science and practice of caries prevention. J Am Dent Assoc. 2000;131(7):887–99. . [DOI] [PubMed] [Google Scholar]
- 35.Zero DT. Dental caries process. Dent Clin North Am. 1999;43(4):635–64. . [PubMed] [Google Scholar]
- 36.Leung V, Dufour D, Levesque CM. Death and survival in Streptococcus mutans: differing outcomes of a quorum-sensing signaling peptide. Front Microbiol. 2015;6:1176 10.3389/fmicb.2015.01176 ; PubMed Central PMCID: PMCPMC4615949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lemos JA, Burne RA. A model of efficiency: stress tolerance by Streptococcus mutans. Microbiology. 2008;154(Pt 11):3247–55. 10.1099/mic.0.2008/023770-0 ; PubMed Central PMCID: PMCPMC2627771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith EG, Spatafora GA. Gene regulation in S. mutans: complex control in a complex environment. J Dent Res. 2012;91(2):133–41. 10.1177/0022034511415415 . [DOI] [PubMed] [Google Scholar]
- 39.Schilling KM, Bowen WH. Glucans synthesized in situ in experimental salivary pellicle function as specific binding sites for Streptococcus mutans. Infect Immun. 1992;60(1):284–95. ; PubMed Central PMCID: PMCPMC257534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bowen WH, Koo H. Biology of Streptococcus mutans-derived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms. Caries Res. 2011;45(1):69–86. 10.1159/000324598 ; PubMed Central PMCID: PMCPMC3068567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tatevossian A. Facts and artefacts in research on human dental plaque fluid. J Dent Res. 1990;69(6):1309–15. 10.1177/00220345900690061801 . [DOI] [PubMed] [Google Scholar]
- 42.Hayacibara MF, Koo H, Vacca-Smith AM, Kopec LK, Scott-Anne K, Cury JA, et al. The influence of mutanase and dextranase on the production and structure of glucans synthesized by streptococcal glucosyltransferases. Carbohydr Res. 2004;339(12):2127–37. 10.1016/j.carres.2004.05.031 . [DOI] [PubMed] [Google Scholar]
- 43.Flemming HC, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010;8(9):623–33. 10.1038/nrmicro2415 . [DOI] [PubMed] [Google Scholar]
- 44.Wilson RF, Ashley FP. Relationships between the biochemical composition of both free smooth surface and approximal plaque and salivary composition and a 24-hour retrospective dietary history of sugar intake in adolescents. Caries Res. 1990;24(3):203–10. 10.1159/000261266 . [DOI] [PubMed] [Google Scholar]
- 45.Featherstone JD. The continuum of dental caries—evidence for a dynamic disease process. J Dent Res. 2004;83 Spec No C:C39–42. . [DOI] [PubMed] [Google Scholar]
- 46.Behar RZ, Hua M, Talbot P. Puffing topography and nicotine intake of electronic cigarette users. PLoS One. 2015;10(2):e0117222 10.1371/journal.pone.0117222 ; PubMed Central PMCID: PMCPMC4321841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spindle TR, Breland AB, Karaoghlanian NV, Shihadeh AL, Eissenberg T. Preliminary results of an examination of electronic cigarette user puff topography: the effect of a mouthpiece-based topography measurement device on plasma nicotine and subjective effects. Nicotine Tob Res. 2015;17(2):142–9. 10.1093/ntr/ntu186 ; PubMed Central PMCID: PMCPMC4838000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Etter JF. A longitudinal study of cotinine in long-term daily users of e-cigarettes. Drug Alcohol Depend. 2016;160:218–21. 10.1016/j.drugalcdep.2016.01.003 . [DOI] [PubMed] [Google Scholar]
- 49.Li Q, Zhan Y, Wang L, Leischow SJ, Zeng DD. Analysis of symptoms and their potential associations with e-liquids' components: a social media study. BMC Public Health. 2016;16:674 10.1186/s12889-016-3326-0 ; PubMed Central PMCID: PMCPMC4967297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.http://vaping360.com/pg-vs-vg-what-is-the-difference-and-what-should-i-use/ (accessed Aug. 2, 2018).
- 51.Lippert F, Lynch RJ. Comparison of Knoop and Vickers surface microhardness and transverse microradiography for the study of early caries lesion formation in human and bovine enamel. Arch Oral Biol. 2014;59(7):704–10. 10.1016/j.archoralbio.2014.04.005 . [DOI] [PubMed] [Google Scholar]
- 52.Craig RG, Gehring PE, Peyton FA. Relation of structure to the microhardness of human dentin. J Dent Res. 1959;38(3):624–30. 10.1177/00220345590380032701 . [DOI] [PubMed] [Google Scholar]
- 53.O'Toole GA. Microtiter dish biofilm formation assay. J Vis Exp. 2011;(47). 10.3791/2437 ; PubMed Central PMCID: PMCPMC3182663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lopez Perez D, Baker PJ, Pintar AL, Sun J, Lin NJ, Lin-Gibson S. Experimental and statistical methods to evaluate antibacterial activity of a quaternary pyridinium salt on planktonic, biofilm-forming, and biofilm states. Biofouling. 2017;33(3):222–34. 10.1080/08927014.2017.1286476 . [DOI] [PubMed] [Google Scholar]
- 55.Kilian M, Chapple IL, Hannig M, Marsh PD, Meuric V, Pedersen AM, et al. The oral microbiome—an update for oral healthcare professionals. Br Dent J. 2016;221(10):657–66. 10.1038/sj.bdj.2016.865 . [DOI] [PubMed] [Google Scholar]
- 56.Pitts NB, Zero DT, Marsh PD, Ekstrand K, Weintraub JA, Ramos-Gomez F, et al. Dental caries. Nat Rev Dis Primers. 2017;3:17030 10.1038/nrdp.2017.30 . [DOI] [PubMed] [Google Scholar]
- 57.Sheiham A, James WP. Diet and Dental Caries: The Pivotal Role of Free Sugars Reemphasized. J Dent Res. 2015;94(10):1341–7. 10.1177/0022034515590377 . [DOI] [PubMed] [Google Scholar]
- 58.Anderson CA, Curzon ME, Van Loveren C, Tatsi C, Duggal MS. Sucrose and dental caries: a review of the evidence. Obes Rev. 2009;10 Suppl 1:41–54. 10.1111/j.1467-789X.2008.00564.x . [DOI] [PubMed] [Google Scholar]
- 59.Moynihan P, Petersen PE. Diet, nutrition and the prevention of dental diseases. Public Health Nutr. 2004;7(1A):201–26. . [DOI] [PubMed] [Google Scholar]
- 60.Liu SQ, Holland R, Crow VL. Ethyl butanoate formation by dairy lactic acid bacteria. International Dairy Journal. 1998;8(7):651–7. 10.1016/S0958-6946(98)00100-9 PubMed PMID: WOS:000077404700008. [DOI] [Google Scholar]
- 61.Sasaki K, Suzuki O, Takahashi N, Stashenko P. Interface oral health science 2011: proceedings of the 4th International Symposium for Interface Oral Health Science, Held in Sendai, Japan, Between March 7 and 8, 2011 and the Harvard-Forsyth-Tohoku Research Workshop, Held in Cambridge, USA, Between January 6 and 7, 2011. Tokyo; New York: Springer; 2012. xx, 422 pages p.
- 62.Kim JN, Ahn SJ, Burne RA. Genetics and Physiology of Acetate Metabolism by the Pta-Ack Pathway of Streptococcus mutans. Appl Environ Microbiol. 2015;81(15):5015–25. 10.1128/AEM.01160-15 ; PubMed Central PMCID: PMCPMC4495203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bourbia M, Ma D, Cvitkovitch DG, Santerre JP, Finer Y. Cariogenic bacteria degrade dental resin composites and adhesives. J Dent Res. 2013;92(11):989–94. 10.1177/0022034513504436 ; PubMed Central PMCID: PMCPMC3797536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tuson HH, Weibel DB. Bacteria-surface interactions. Soft Matter. 2013;9(18):4368–80. 10.1039/C3SM27705D ; PubMed Central PMCID: PMCPMC3733390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Orstavik D, Kraus FW, Henshaw LC. In vitro attachment of streptococci to the tooth surface. Infect Immun. 1974;9(5):794–800. ; PubMed Central PMCID: PMCPMC414887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Diaz-Garrido N, Lozano C, Giacaman RA. Frequency of sucrose exposure on the cariogenicity of a biofilm-caries model. Eur J Dent. 2016;10(3):345–50. 10.4103/1305-7456.184163 ; PubMed Central PMCID: PMCPMC4926586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matsumoto-Nakano M. Role of Streptococcus mutans surface proteins for biofilm formation. Jpn Dent Sci Rev. 2018;54(1):22–9. 10.1016/j.jdsr.2017.08.002 ; PubMed Central PMCID: PMCPMC5884221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guggenheim B. Extracellular polysaccharides and microbial plaque. Int Dent J. 1970;20(4):657–78. . [PubMed] [Google Scholar]
- 69.Hamada S, Slade HD. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980;44(2):331–84. ; PubMed Central PMCID: PMCPMC373181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Loesche WJ. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50(4):353–80. ; PubMed Central PMCID: PMCPMC373078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Banas JA, Vickerman MM. Glucan-binding proteins of the oral streptococci. Crit Rev Oral Biol Med. 2003;14(2):89–99. . [DOI] [PubMed] [Google Scholar]
- 72.Sutherland IW. Biotechnology of microbial exopolysaccharides Cambridge; New York: Cambridge University Press; 1990. viii, 163 p. p. [Google Scholar]
- 73.Passalacqua KD, Charbonneau ME, O'Riordan MX. Bacterial Metabolism Shapes the Host-Pathogen Interface. Microbiol Spectr. 2016;4(3). 10.1128/microbiolspec.VMBF-0027-2015 ; PubMed Central PMCID: PMCPMC4922512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leitao TJ, Cury JA, Tenuta LMA. Kinetics of calcium binding to dental biofilm bacteria. PLoS One. 2018;13(1):e0191284 10.1371/journal.pone.0191284 ; PubMed Central PMCID: PMCPMC5791987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garcia SS, Du Q, Wu H. Streptococcus mutans copper chaperone, CopZ, is critical for biofilm formation and competitiveness. Mol Oral Microbiol. 2016;31(6):515–25. 10.1111/omi.12150 ; PubMed Central PMCID: PMCPMC5123798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Berlutti F, Ajello M, Bosso P, Morea C, Petrucca A, Antonini G, et al. Both lactoferrin and iron influence aggregation and biofilm formation in Streptococcus mutans. Biometals. 2004;17(3):271–8. . [DOI] [PubMed] [Google Scholar]
- 77.Aranha H, Strachan RC, Arceneaux JE, Byers BR. Effect of trace metals on growth of Streptococcus mutans in a teflon chemostat. Infect Immun. 1982;35(2):456–60. ; PubMed Central PMCID: PMCPMC351061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Spear F. A patient with severe wear on the anterior teeth and minimal wear on the posterior teeth. J Am Dent Assoc. 2008;139(10):1399–403. . [DOI] [PubMed] [Google Scholar]
- 79.Schved F, Pierson MD, Juven BJ. Sensitization of Escherichia coli to nisin by maltol and ethyl maltol. Lett Appl Microbiol. 1996;22(3):189–91. . [DOI] [PubMed] [Google Scholar]
- 80.http://e-liquid-recipes.com/ (accessed Aug. 2, 2018).
- 81.Rosbrook K, Erythropel HC, DeWinter TM, Falinski M, O'Malley S, Krishnan-Sarin S, et al. The effect of sucralose on flavor sweetness in electronic cigarettes varies between delivery devices. PLoS One. 2017;12(10):e0185334 10.1371/journal.pone.0185334 ; PubMed Central PMCID: PMCPMC5624589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gaetke LM, Chow CK. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology. 2003;189(1–2):147–63. . [DOI] [PubMed] [Google Scholar]
- 83.Rosenfeld CS. Gut Dysbiosis in Animals Due to Environmental Chemical Exposures. Front Cell Infect Microbiol. 2017;7:396 10.3389/fcimb.2017.00396 ; PubMed Central PMCID: PMCPMC5596107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou J, Jiang N, Wang Z, Li L, Zhang J, Ma R, et al. Influences of pH and Iron Concentration on the Salivary Microbiome in Individual Humans with and without Caries. Appl Environ Microbiol. 2017;83(4). 10.1128/AEM.02412-16 ; PubMed Central PMCID: PMCPMC5288818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pietroiusti A, Magrini A, Campagnolo L. New frontiers in nanotoxicology: Gut microbiota/microbiome-mediated effects of engineered nanomaterials. Toxicol Appl Pharmacol. 2016;299:90–5. 10.1016/j.taap.2015.12.017 . [DOI] [PubMed] [Google Scholar]
- 86.Small DM, Prescott J. Odor/taste integration and the perception of flavor. Exp Brain Res. 2005;166(3–4):345–57. 10.1007/s00221-005-2376-9 . [DOI] [PubMed] [Google Scholar]
- 87.Peres MA, Sheiham A, Liu P, Demarco FF, Silva AE, Assuncao MC, et al. Sugar Consumption and Changes in Dental Caries from Childhood to Adolescence. J Dent Res. 2016;95(4):388–94. 10.1177/0022034515625907 . [DOI] [PubMed] [Google Scholar]
- 88.Rugg-Gunn AJ, Hackett AF, Appleton DR, Jenkins GN, Eastoe JE. Relationship between dietary habits and caries increment assessed over two years in 405 English adolescent school children. Arch Oral Biol. 1984;29(12):983–92. . [DOI] [PubMed] [Google Scholar]
- 89.Rosinger A, Herrick K, Gahche J, Park S. Sugar-sweetened Beverage Consumption Among U.S. Youth, 2011–2014. NCHS Data Brief. 2017;(271):1–8. . [PubMed] [Google Scholar]
- 90.Ervin RB, Kit BK, Carroll MD, Ogden CL. Consumption of added sugar among U.S. children and adolescents, 2005–2008. NCHS Data Brief. 2012;(87):1–8. . [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.