Abstract
Various upcoming techniques can be used in replacement of experiments requiring animal sacrifice or products of animal sacrifice. In many instances these techniques provide more reproducibility and control of parameter, compared to experiments involving animal or animal products. Use of these techniques can avoid the question of the animal sacrifice during experiment and subsequently permission of ethical approval. In silico simulation, informatics, 3D cell culture models, organ-on-chips are some innovative technology which can reduce the number of animals sacrifice. Scientist evolved some innovative culture procedures and production of animal friendly affinity reagents which are free from the product of animal sacrifice. Direct investigation on human body for treatment as well as further research, electronic health record is also helpful in the reduction of animals sacrifice in biomedical investigations. These techniques and strategies of research can be more cost effective as well as more relevant to various issues related to the human health. Some medical blunder has also been reported after the successful testing of drugs on animal’s model. Hence, the reliability of animal experiment in context with human health is questionable.
Alternative to animal experiments help to reduce the number of animals required for research up to certain extent but is not able to eliminate the need for animals in research completely. Wisely use of animals in teaching & research is expected and the importance of animal experimentation in futuristic development in life science cannot be ignored.
Keywords: Alternative, Animal, Experimentation, Sacrifice, Test
1. Introduction
In the present scenario of rules followed in scientific institutions or industry, it is not considered unethical to sacrifice an animal if the experiment is conducted for human welfare. (Shanks and Green, 2004). Although philosophers can debate on the importance of humans over animals, it is arguable that the humans are more important than animals (Hadley, 2005) Humans who are convicted of grave crimes and also the volunteers can be used for experimentation purposes. On the other hand some researchers feel frustrated that they have to take permissions for experimentations even for animal species which are otherwise slaughtered for their meat as food. The institutions are like a separate individual under law, responsible for acts formally done on its behalf, therefore institutions have devised rules for sacrifice of animal for experiments, based on law & commonly acceptable code of ethics (see Fig 1.).
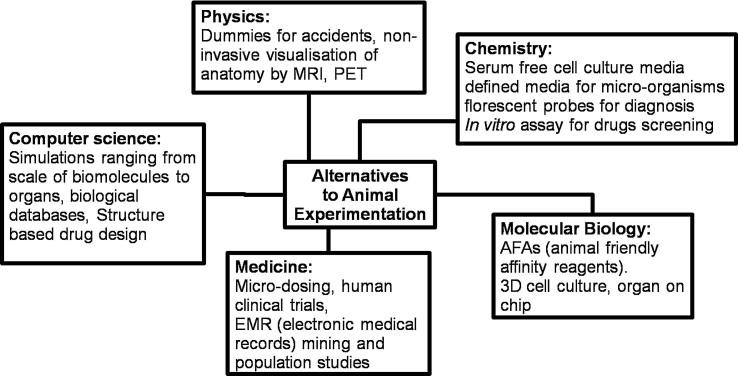
Fig. 1.
Upcoming technique contributed by various scientific disciplines for reducing animal sacrifice in science.
Charles Hume founded the Universities Federation for Animal Welfare (UFAW) and made a proposal in 1954 for three R’s of alternatives for animal testing i.e., refine reduce and replace (Fenwick and Fraser, 2005). Many international organizations are established for developing alternative techniques to avoid animal experimentation, for example National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs) (www.caat.jhsph.edu), European Centre for the Validation of Alternative Methods (ECVAM) (Marafante et al., 1994), Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM) (Stokes et al., 2002), Universities Federation for Animal Welfare (UFAW) (www.ufaw.org.uk), Center for Alternatives to Animal Testing (CAAT) (www.caat.jhsph.edu) etc.
Since beginning, animals have been used in experiments for teaching and research purposes and this has benefitted research in different areas (Danielski et al., 2011). It is estimated that, annually around 115 million animals are used in various biomedical industries worldwide (Taylor et al., 2008). It is reported that various scientist were engaged in animal dissection right from ancient times (www.icmr.nic.in). Two-thirds of Nobel laureates in physiology or medicine since 1901 have relied on animal data for their research (Burggren and Warburton, 2007). Rats, mice and other rodents make up 95% of all animals used, and primate’s make up one-third of one percent of all animals used. Beside the genetic constitution, there are lots of anatomical similarity between animals and human being as they have a same set of organs like heart, liver, kidney, lungs and other tissues. They possess the similar internal body mechanism and physiology like, blood circulation, respiration, nervous, endocrine system etc. So these similarities logically make some animals prone to dissection in the laboratory and provide basic training to the young scholars in relevant field of life sciences. According to Aysha Akhtar, the experiments performed on animals are not predictive of human results and they cannot be relied on for predicting the course of various diseases. Hence, the animal experimentation is inadequate for predicting the treatment related to human diseases (Akhtar, 2015).
Here, the authors provides an overview of upcoming techniques and products which can be used for avoiding animal sacrifice. This will not only reduce ethical issues as far as permissions related to procedures are concerned but also address shortcomings in alternative to experiment on animals, leading to an overall positive impact on the progression of biomedical research.
2. Development of new products and techniques to avoid animal sacrifice in research
2.1. In silico simulations and informatics
The term ‘in silico’ is a modern word usually used for experimentation performed by computer and is related to the more commonly known biological terms in vivo and in vitro (Ekins et al., 2007). Simulations can reduce the number of experiments required, although simulations cannot completely replace the experiments because all the principles governing a system are generally not known and simulations use approximations at some level of detail owing to limitation of computer and human life. Despite being not deterministic, simulation and informatics are becoming essential in all scientific research fields for making efficient use of existing knowledge in experiment design. Computer models have been constructed to model human metabolism, to study plaque build-up and cardiovascular risk, and to evaluate toxicity of drugs, tasks for which animals are also used (Washio et al., 2013). Toxicity and absorption, distribution, metabolism & excretion (ADME) of environment pollutants and cosmetics can be predicted using computational tools for physiologically based bio-kinetic (PBBK) modeling (Lipinski et al., 2001, Raunio, 2011). Similarly at various scale of details, the proteins, receptors, lipid bilayer cell, brain etc are often simulated to predict their behavior or response to physical conditions and stimulation or chemicals.
Computational simulation and informatics methods have minimized the number of animals sacrificed in drug discovery by narrowing down the potential drug candidate molecules (Ekins et al., 2007, Adler et al., 2011). Similarly it has also reduced the number of animal experiments required in basic biological sciences by efficient use of existing knowledge (Kwong et al., 2017). High definition 3D computer models of anatomy have been developed to level of details that they can replace animal dissection for teaching anatomy (Azer and Azer, 2016).
2.2. 3D cell-culture models and organs-on-chips
In the present scenario of advancement in life science some tissue models have been built by using 3D cell culture as well as so some chips containing models of organ (Huh et al., 2011). These models are generally built with the help of human cells which makes them more applicable for applications in humans (Huh et al., 2012). These models also provide better control on conditions as well as faster and convenient experimentation. It has been observed that beside the alternatives to animal experimentation, this technology in combination with the emerging induced pluripotent stem cell (IPSC) is moving towards making implantable organs and tissues (de-Stolpe and den Toonder, 2013). Models of multi-organ systems of heart, muscle, skin, brain, testis, marrow, gut, kidney, lungs, liver as well as individual organ have been made in microfluidic channels along with re-creating relevant physical and chemical micro environments (Huh et al., 2012). These techniques also called biomedical or biological microelectromechanical systems (Bio-MEMS) or lab-on-a-chip (LOC) and micro total analysis systems (μTAS) will replace animal testing in commercial laboratories in the pharmaceutical, biotechnology, chemistry and environmental safety industries.
3D cell cultures grow cells into 3D aggregates or spheroids using a scaffold, matrix or in a scaffold free manner (Edmondson et al., 2014). The 3D culture condition can be modified to include factors or proteins found in particular tissue or tumor microenvironment. The matrices contain ECM components that lead to increased cell–cell contact, communication, and signaling pathway activation such that functional and morphological differentiation of cell can be largely restored to what is seen in-vivo (Edmondson et al., 2014). The gene and protein expression levels of cells and thus the cellular behaviors are similar to in-vivo levels. Therefore, bridges the gap between in-vitro and in-vivo drug screening, possibly decreasing the use of animal models.
2.3. Microbial culture media without products of animal sacrifice
The culture of different micro-organisms is required in research as well as clinical diagnosis. The media required for these microbial cultures contain peptone as nutrient source of protein/peptide/amino acid. The peptone is often made by proteolysis of meat from farmed animals. But now different suppliers are coming up with peptone from plant and yeast source. These plant peptones costs same as animal peptone but plant peptone is more eco-friendly as it require less water and less plant material compared to food grain required to feed cattle which are slaughtered for producing peptone. In 2003, Himedia has introduced a range HiVeg products for replacing animal peptone and other nutrients required for microbial cultures in research and clinical diagnosis (www.hiveg.com.). Himedia is selling around 1500 formulations of microbial culture media for different microorganisms and assays, which are free from animal products. Many of these media give better microbial growth yield and has no risk of bovine spongiform encephalopathy infection. Animal free culture media prepared from plant or microbial source of nutrients for microbes is also offered by Thermo Fisher, BD Biosciences, Sigma Aldrich etc (Olivieri et al., 2007, Wright et al., 2012). In 1959, Pardee et al. (1959) developed minimal media (M9 salts) of E. coli, which is generally used with supplements too like thiamine, casamino acid (milk protein hydrolysate), glucose, calcium and magnesium. Such chemically defined formulations are also free from any meat product and are used for more controlled experiments and better reproducibility of results.
2.4. Serum free animal cell cultures
The cell culture from humans is done in a media that often contains fetal calf serum (FCS). The process of producing fetal calf serum involves extreme suffering to animal (cow) as a pregnant live cow is operated to take out the fetus and then harvest the serum from blood of this fetus. This fetal calf serum is not exposed to antigens or pathogens so it is used for cell culture to avoid contamination and immunological reactions as well as get all the mix of nutrients required for cell growth. Since fetal calf serum is a natural medium rich in all known and unknown nutrients required, it makes cell growth more likely to be successful. But scientists are identifying the nutrients and factors required for growth of different types of cells. It’s becoming feasible to culture almost any cell line completely in chemically defined medium without any animal product. The results of experiments using chemically defined media are more reproducible than animal serum media because the composition of serum will change from batch to batch due to animal’s health, age gender, genetic makeup and weather. Humane Research Australia Inc. is a not for profit organization which provides an information on alternatives to animal experiments and cell culture methods without FCS (www.humaneresearch.org.au). Almost all cell lines used in research can be cultured in synthetic media, the searchable database of such cell lines and culture media is available at http://www.sefrec.com/.
2.5. Alternatives to animal derived antibodies
Animal-friendly affinity (AFA) reagents are alternatives to antibodies, produced without immunization of animal. These antibodies are typically selected in vitro by phage, ribosome, or yeast display, but they also include non-antibody reagents such as DARPins, affibodies, monobodies, anticalins etc (Taussig et al., 2007, Dübel et al., 2010). In a recent review by Gray et al., (2016); a comparative analysis is done between animal derived antibodies and AFAs. AFAs are less time consuming, superior in quality, more reliable, reproducible and cost effective except that initial investment and expertise is required to move from animal antibodies to AFAs. The private entities like YUMAB has come up to provide custom made recombinant antibodies (www.yumab.com). Universities consortium recombinant antibody network (RAN) has mission to develop recombinant antibodies for all human proteins (Hornsby et al., 2015, www.recombinant-antibodies.org, 2017). The DNA for these antibodies can be procured from RAN for bacterial expression antibody. Missions like these will make animal-free antibodies commercially available to everybody. At present US$ 80-billion industry exists that creates millions of animal-derived monoclonal and polyclonal antibodies for use in diagnostics and detection. Furthermore, undetermined numbers of antibodies are generated through custom-made production by companies and research institutes. Despite the alternatives available such as naive B lymphocyte or recombinant antibodies expressed by phage display, animal immunization is still authorized for production of these antibodies.
3. Direct investigation on human body for treatment & further research
It has already proved by various meta-analysis that animals are not good models for human physiology. Now a day, various non-invasive and less hazardous indirect methods for experimentation on humans can be used beside it, there is a wealth of information available from clinical data which shall be mined with better informatics and these databases need to be further enriched.
3.1. Non invasive & indirect testing
Non-invasive methods like magnetic resonance Imaging (MRI) and positron emission tomography (PET) can look inside the human body and brain without causing any significant harm. Microdosing is a technique for studying the behavior of drugs in humans through the administration of doses so low that they are unlikely to produce whole-body effects, but it is enough to allow the cellular response to be studied by high sensitivity techniques (Wilding and Bell, 2005). Human tissues and organs obtained after post mortems has been main resource for discoveries on brain regeneration and the effects of multiple sclerosis and Parkinson’s disease. The tissues donated and obtained in surgery (e.g. biopsies, cosmetic surgery and transplants) are also used for research of direct human relevance. For example, skin and eye models made from reconstituted human skin and other tissues have been developed and are used to replace the cruel rabbit irritation tests. Companies such as Cell Systems, Mattek, GmbH and Episkin now produce these tests in easy to use kits for testing cosmetics and other substances. The late Dr. Bjӧrn Ekwall (Cytotoxicology Laboratory in Sweden) developed a replacement for the LD50 test using donated human tissue that measured toxicity at a 77-84% accuracy whereas in mice test 52-60% accuracy obtained (Rangantha and Kuppast, 2015).
Electronic health record (EHR) along with development of cost effective genotyping techniques has presented a wealth of information for research. Although there are some challenges in using medical transcription generated lot of EHR like data availability, missing data, incorrect data, and vast quantities of unstructured narrative text data etc. With ever increasing computational power and improving informatics techniques, using EHR is becoming a valuable resource for medical research (Denny, 2012). The association between SNPs in the 9p21 region and cardiovascular phenotypes in morbid obesity is discovered only by using electronic health record linked to genetic information (Wood et al., 2008). More of such success stories can be realized by advancements in natural language processing, accurate collections of cases and controls for a given disease.
3.2. Toxicity & authenticity animal test
Health information exchanges can be used to provide the necessary information and more institutional investment is required in DNA bio-banks. Developments in this direction of using data from humans is also important as 95% of drugs that enter clinical trials do not make it to the market, despite of all promise of the (animal) models used to develop them (Hartung, 2013). Even the results of experiments on rats and mice cannot be predicted with more than 60% accuracy (Olson et al., 2000). Rather new approaches that rely on molecular pathways of human toxicity currently are emerging as toxicology for the 21st Century. Consequently, in Europe, despite increasing R&D expenditure, animal use by pharmaceutical companies dropped by more than 25% from 2005 to 2008. Funding agencies in USA are also focusing on human-on-a-chip approaches (Hartung and Zurlo, 2012). Cell culture studies are also irrelevant to human diseases due to questionable cell authenticity, over-passaging, mycoplasma infections, and lack of differentiation as well as non- homeostatic and non-physiologic culture conditions. Cell culture based genotoxicity assays were demonstrated to have negligible specificity; it showed 90% false positive for non-carcinogens and 90% sensitivity for rat carcinogens (Pottenger et al., 2007). Toxicity testing traditionally involves animal experiments as well as cell culture experiments using fetal calf serum as media component. Different meta studies has shown that these methods involving animals are not relevant for human physiology and thereby the drugs and cosmetics fail at last stages of FDA approval (Astashkina et al., 2012).
Products like soft drinks, baby foods, paints, gardening products, cosmetics and shampoos, contain numerous synthetic chemicals as preservatives, dyes, active ingredients, or as contaminants. Their toxicity is largely tested on animal like rabbits, mice, rats and dogs. A comparative study found that the genomic response to inflammatory stresses from different etiologies in humans and mouse models correlate poorly. The mice orthologs of genes that changed significantly in humans, were responding close to random in matching their human counterparts (Seok et al., 2013). There is low productivity of animal responses in neuro degeneration, stroke, sepsis and inflammation therefore modern toxicology has embraced in vitro methods, omics technologies and systems biology approaches (Leist and Hartung, 2013).Through human randomized trials on even 76 highly cited animal studies, it was found that 14 (18%) contradicted, 34 (45%) remain untested, and only 28 (37%) could be replicated (Hackam and Redelmeier, 2006). Studies on rats, hamsters, guinea pigs, mice, monkeys, and baboons found no link between glass fibres and cancer whereas human studies related the two which resulted in the labeling of glass fibres as carcinogenic by Occupational Safety and Health Administration (OSHA). The 7th amendment to the EU cosmetics directive prohibited to put animal-tested cosmetics on the market in Europe after 2013 in anticipation that non-animal methods will be developed for toxicokinetics, repeated dose toxicity, carcinogenicity, skin sensitization, and reproductive toxicity (Adler et al., 2011).
4. Shortcoming of animals experiments & adverse impact on human health
Although certain physiological, cytological, biochemical and or biological factor make animal experiment more reliable for human health & diseases. But now day’s reliability of animal experiment in context with human health is questioned. Humans are harmed because of misleading animal testing results. During late fifties, drug thalidomide a sedative was prescribed to pregnant women, some of these women delivered babies without limbs, a condition known as phocomelia. The same drug was tested on approximately all experimental animal models like mice, rats, rabbits, hamsters, cats, dogs, armadillos, guinea pig, swine, ferrets etc., and it was observed that the teratogenic effects had been induced only occasionally (Schardein, 1976). In 2006, a compound TGN 1412 was designed to dampen the immune system, when injected to six human volunteer, a severe adverse reaction resulting from a life-threatening cytokine storm that led to catastrophic systemic organ failure, but it was successfully tested in mice, rabbits, rats, and Non human primates (Akhtar, 2015). Bailey (2008) observed that there are about 90 HIV vaccines that succeeded in animals failed in humans. In Parkinson’s disease, several therapies that appeared promising in both NHPs and rat models observed inappropriate outcome in humans (Lane and Dunnett, 2008). The role of animal experiment in relevance to human health, animal experimentation’s efficacy has been subjected to little systematic scrutiny (Akhtar, 2015, Akhtar et al., 2009). Standardization of laboratory settings and procedures (Macleod et al., 2004, O’ Neil et al., 1999) highlight the systematic differences in the results of experiments in these labs (Crabbe et al., 1999). Factors like, environmental condition and stress related physiological parameter, age of experimental models etc, can switch on and off specific gene which are not only specific to animals models but also varies in stains (Akhtar, 2015). These findings question the authenticity of animals experiment and its objectives in context with human health. Neglecting these parameters during animals experiment, investigators may come out with significant result but imprecise outcome.
Humans are a highly evolved and unique animal and its uniqueness becomes predominant in the entire animal kingdom. Albeit, human have almost similar morphological, anatomical, physiological or biochemical properties but not same with various laboratory animals. Animal models are widely used to predict the metabolic behavior of new compounds before pre-clinical study. In 1995, Kararli (Kararli, 1995) studied the various parameter of gastrointestinal (G.I.) tract of the human and common laboratory animals which cause significant variation in absorption of drug absorption via oral route. He found the comparable differences not only in the anatomy but also in physiological, and biochemical differences which have significantly impact on drug metabolism and absorption. The enzyme P450 which often play a critical role in the metabolism and pharmacokinetics of xenobiotics, but the activities of same enzyme in human and rat are very different (Yamazaki et al., 2011). However, animal testing still considered as gold slandered and is not completely replaced.
5. Shortcoming in alternatives to animals experiment
The upcoming challenges of alternative to animal experiment open a new era of biological development over worldwide. Multidisciplinary approach of research along with certain bioethical issues emerged from traditional method of research in medical and biological research. Computer simulations, modulation, in vitro testing technique are widely applicable. However these techniques are not glittering gold (Ashton et al., 2014). Alternative to animals experiment have some shortcoming i.e., there is no any suitable system to study the metabolic response which can replace animals models. To study the bodily response and metabolism of drug, and inability to study transplant models and idiosyncratic responses are still a big challenges to persist (Arora et al., 2011). If we review recent developments, it is observed that these alternative experiments up to certain extent help to reduce the number of animals required for research but are not able to eliminate the need for animals in research completely (Arora et al., 2011).
Inhuman behavior towards laboratory animals drew the attention of the public, worldwide, including many non-government organizations and animal lovers. They usually oppose dissection in the classroom, and even convinced some countries to make strict guidelines for use of animals for experimental purpose. In the world wide various countries issued the directive or even made legislation to ban the animal dissection. In India, University Grant Commission (UGC), New Delhi, issued new guidelines for the discontinuation of dissection and animal experimentation in the zoology/life sciences curricula in a phased manner. Dissection and vivisection are two important parameters of biological classes to understand the vital processes of life. It not only provides the basic training to the young learners, but also lays the foundation for all other research. The importance of animal dissections in teaching & research and for the parallel development of medical and biological science cannot be ignored.
Now days not only ethical issues are too important in animals experimentation, there are certain other factor like lack of skilled manpower, time consuming protocol and cost of experimentation are equally important (Doke and Dhawale, 2015). In context to Indian higher education system availability of experienced and expertise in the field of these alternative to animals experiment is still a big challenge. UGC has banned the animal dissection, so it is its moral responsibility to provide alternatives training in appropriate manner through academic staff college, university departments. As per the earlier guideline, UGC levied the responsibility for capacity building through training program in the form of 3-5 days workshops through Academic Staff College, University departments or colleges, but was not successfully executed. Wisely use of animals in teaching & research is expected. The importance of animal experimentation in futuristic development in life science must not be ignored. In the present paradigm, we cannot say that, if use of animals in research is stopped completely, it will create knowledge gaps that cannot be filled soon enough by developments in other fields of science.
6. Conclusion
The animals have been used in teaching & research since back from ancient times. Earlier animals were scarified for getting knowledge related to animal’s anatomy, physiology and certain other biological facts. Now days the role of animals’ dissection is moreover shifted from basic knowledge to advanced research. In the modern biological research and drug discovery based on the result obtained from animals based experiments. Permission for the clinical trial of any medicine is granted on the basis of studies on animals models. It seems that the study of effects as well as metabolism any chemical compound in animals is prerequisite.
In the era of technology, sometimes it seems that we have over and unethical used animals during some experiment procedures. During the development process several alternatives were devised to reduce or refine the experiments. Advanced technologies have been developed to fill the gap between in-vitro and in vivo technology and even soon or later replace animal testing. Worldwide concern of ethical use of animals in science & technology, traditional methodology has been challenged and refined; use of animals’ products is replaced by certain other alternatives in microbial and medical diagnosis industries. Recombinant technology plays a vital role in the reduction of animal scarification. Drug designing & development nowadays based on electronic health records, which are used with the help of some bioinformatics software. Some drugs have failed in clinical trial after the performance of success story in animals testing.
The scientific concern towards the inhumane use of laboratory animals came out in the form of certain directive or even strict legislation worldwide. Even some countries banned the use of animals in basic classroom teaching which may be prove a barrier in the progressive journey of biological science.
Conflict of interest
The authors declare that the research paper was written in the absence of any commercial or financial relationships that could be construed as real or potential conflict of interest.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article.
Footnotes
Peer review under responsibility of King Saud University.
References
- Adler S., Basketter D., Creton S., Pelkonen O., van Benthem J., Zuang V., Andersen K.E., Angers-Loustau A., Aptula A., Bal-Price A., Benfenati E., Bernauer U., Bessems J., Bois F.Y., Boobis A., Brandon E., Bremer S., Broschard T., Casati S., Coecke S., Corvi R., Cronin M., Daston G., Dekant W., Felter S., Grignard E., Gundert-Remy U., Heinonen T., Kimber I., Kleinjans J., Komulainen H., Kreiling R., Kreysa J., Leite S.B., Loizou G., Maxwell G., Mazzatorta P., Munn S., Pfuhler S., Phrakonkham P., Piersma A., Poth A., Prieto P., Repetto G., Rogiers V., Schoeters G., Schwarz M., Serafimova R., Tähti H., Testai E., van Delft J., van Loveren H., Vinken M., Worth A., Zaldivar J.M. Alternative (non-animal) methods for cosmetics testing: current status and future prospects-2010. Arch. Toxicol. 2011;85:367–48510. doi: 10.1007/s00204-011-0693-2. [DOI] [PubMed] [Google Scholar]
- Akhtar A. The flaws and human harms of animal experimentations. Camb. Q. Healthc. Ethics. 2015;24:407–419. doi: 10.1017/S0963180115000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar A.Z., Pippin J.J., Sandusky C.B. Animal studies in spinal cord injury: A systematic review of methylprednisolone. Altern. Lab. Anim. 2009;37:43–62. doi: 10.1177/026119290903700108. [DOI] [PubMed] [Google Scholar]
- Arora T., Mehta A.K., Joshi V., Mehta K.D., Rathor N., Mediratta P.K., Sharma K. Substitute of animals in drug research: an approach towards fulfillment of 4R's. Indian J. Pharm. Sci. 2011;73:1–6. doi: 10.4103/0250-474X.89750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton R., De Wever B., Fuchs H.W., Gaca M., Hill E., Krul C., Poth A., Roggen E.L. State of the art on alternative methods to animal testing from an industrial point of view: ready for regulation? ALTEX. 2014;31:357–363. doi: 10.14573/altex1403121. [DOI] [PubMed] [Google Scholar]
- Astashkina A., Mann B., Grainger D.W. A critical evaluation of in vitro cell culture models for high-throughput drug screeningand toxicity. Pharmacol. Ther. 2012;134:82–106. doi: 10.1016/j.pharmthera.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Azer S.A., Azer S. 3D anatomy models and impact on learning: A review of the Quality of the Literature. Health Professions Education. 2016;2:80–98. [Google Scholar]
- Bailey J. An assessment of the role of chimpanzees in AIDS vaccine research. Altern. Lab. Anim. 2008;36:381–428. doi: 10.1177/026119290803600403. [DOI] [PubMed] [Google Scholar]
- Burggren W.W., Warburton S. Amphibians as animal models for laboratory research in physiology. ILAR J. 2007;48:260–269. doi: 10.1093/ilar.48.3.260. [DOI] [PubMed] [Google Scholar]
- Crabbe J.C., Wahlsten D., Dudek B.C. Genetics of mouse behavior: Interactions with laboratory environment. Science. 1999;284:1670–1672. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- Danielski, J.C.R., Barros, D.M., Carvalho, H.A.H.O., 2011. Animal use for teaching and research purposes: pros and cons. R. Eletr. De. Com. Inf. Inov. Saúde. Rio de Janeiro. 5, 72.
- Denny, J.C., 2012. Chapter 13: mining electronic health records in the genomics era. PLoS Computational Biology, 8(12), e1002823. http://doi.org/10.1371/journal.pcbi.1002823 [DOI] [PMC free article] [PubMed]
- de-Stolpe A.V., den Toonder J. Workshop meeting report organs-on-chips: human disease models. Lab Chip. 2013;13:3449–3470. doi: 10.1039/c3lc50248a. [DOI] [PubMed] [Google Scholar]
- Doke S.K., Dhawale S.C. Alternatives to animal testing: A review. Saudi Pharm. J. 2015;23:223–229. doi: 10.1016/j.jsps.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dübel S., Stoevesandt O., Taussig M.J., Hust M. Generating recombinant antibodies to the complete human proteome. Trends Biotechnol. 2010;28:333–339. doi: 10.1016/j.tibtech.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Edmondson R., Broglie J.J., Adcock A.F., Yang L. Three- dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev. Technol. 2014;12:207–218. doi: 10.1089/adt.2014.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekins S., Mestres J., Testa B. In silico pharmacology for drug discovery: methods for virtual ligand screening and profiling. Br. J. Pharmacol. 2007;152:9–20. doi: 10.1038/sj.bjp.0707305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick N.P., Fraser D. The Three Rs in the pharmaceutical industry: perspectives of scientists and regulators. Anim. Welf. 2005;14:367–377. [Google Scholar]
- Gray A.C., Sidhu S.S., Chandrasekera P.C., Hendriksen C.F., Borrebaeck C.A. Animal-friendly affinity reagents: replacing the needless in the haystack. Trends Biotechnol. 2016;34:960–969. doi: 10.1016/j.tibtech.2016.05.017. [DOI] [PubMed] [Google Scholar]
- Hackam D.G., Redelmeier D.A. Translation of research evidence from animals to humans. JAMA. 2006;296:1731–1732. doi: 10.1001/jama.296.14.1731. [DOI] [PubMed] [Google Scholar]
- Hadley J. Why (some philosophers think) using animals in scientific research is seriously wrong. Anzccart News. 2005;18:1–5. https://www.adelaide.edu.au/ANZCCART/docs/news/archive/AN18_1.pdf [Google Scholar]
- Hartung T. Look back in anger - what clinical studies tell us about preclinical work. ALTEX. 2013;30:275–291. doi: 10.14573/altex.2013.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung T., Zurlo J. Alternative approaches for medical countermeasures to biological and chemical terrorism and warfare. ALTEX. 2012;29:251–260. doi: 10.14573/altex.2012.3.251. [DOI] [PubMed] [Google Scholar]
- Hornsby M., Paduch M., Miersch S., Sääf A., Matsuguchi T., Lee B., Wells J. A high through put platform for recombinant antibodies to folded proteins. Mol. Cell. Proteomics. 2015;14:2833–2847. doi: 10.1074/mcp.O115.052209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh D., Hamilton G.A., Ingber D.E. From three-dimensional cell culture to organs-on-chips. Trends Cell Biol. 2011;21:745–754. doi: 10.1016/j.tcb.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh D., Torisawa Y., Hamilton G.A., Kim H.J., Ingber D.E. Micro engineered physiological biomimicry: Organs-on-chips. Lab Chip. 2012;12:2156–2164. doi: 10.1039/c2lc40089h. [DOI] [PubMed] [Google Scholar]
- Kararli T.T. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm. Drug. Dispos. 1995;16:351–380. doi: 10.1002/bdd.2510160502. [DOI] [PubMed] [Google Scholar]
- Kwong P.D., Chuang G.Y., DeKosky B.J., Gindin T., Georgiev I.S., Lemmin T., Schramm C.A., Sheng Z., Soto C., Yang A.S., Mascola J.R., Shapiro L. Antibodyomics: bioinformatics technologies for understanding B-cell immunity to HIV- 1. Immunol. Rev. 2017;275:108–128. doi: 10.1111/imr.12480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane E., Dunnett S. Animal models of Parkinson’s disease and L-dopa induced dyskinesia: How close are we to the clinic? Psychopharmacology. 2008;199:303–312. doi: 10.1007/s00213-007-0931-8. [DOI] [PubMed] [Google Scholar]
- Leist, M., Hartung, T., 2013. Reprint: Inflammatory findings on species extrapolations: humans are definitely no 70kg mice. ALTEX.30, 227–230. [DOI] [PubMed]
- Lipinski C.A., Lombardo F., Dominy B.W., Feeney P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug. Deliv. Rev. 2001;46:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- Macleod M.R., O’Collins T., Howells D.W., Donnan G.A. Pooling of animal experimental data reveals influence of study design and publication bias. Stroke. 2004;35:1203–1208. doi: 10.1161/01.STR.0000125719.25853.20. [DOI] [PubMed] [Google Scholar]
- Marafante E., Smyrniotis T., Balls M. ECVAM: the European centre for the validation of alternative methods. Toxicol. In Vitro. 1994;8:803–805. doi: 10.1016/0887-2333(94)90072-8. [DOI] [PubMed] [Google Scholar]
- O’ Neil B.J., Kline J.A., Burkhart K., Younger J. Research fundamentals: V. The use of laboratory animal models in research. Acad. Emerg. Med. 1999;6:75–82. doi: 10.1111/j.1553-2712.1999.tb00099.x. [DOI] [PubMed] [Google Scholar]
- Olivieri, R., Sabbatini, F., Kontakou, M., Tagliaferri, L., A Giglioli, A, Rappuoli, R., 2007. Culture medium with soy bean extract as amino acid source and no protein complexes of animal origin. Patent EP0983342B1.
- Olson H.G., Betton D., Robinson C., HartmanYeager S., Blosky M.A., Krum W., Carey D.J., Skelding K.A., Benotti P., Stewart W.F., Gerhard G.S. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul. Toxicol. Pharmacol. 2000;32:56–67. doi: 10.1006/rtph.2000.1399. [DOI] [PubMed] [Google Scholar]
- Pardee A.B., Jacob F., Monod J. The genetic control and cytoplasmic expression of “inducibility” in the synthesis of ß-galactosidase in E. coli. J. Mol. Biol. 1959;1:165–178. [Google Scholar]
- Pottenger L.H., Bus J.S., Gollapudi B.B. Genetic toxicity assessment: employing the best science for human safety evaluation Part VI: When salt and sugar and vegetables are positive, how can genotoxicity data serve to inform risk assessment? Toxicol. Sci. 2007;98:327–331. doi: 10.1093/toxsci/kfm068. [DOI] [PubMed] [Google Scholar]
- Rangantha N., Kuppast I.J. A review on alternatives to animal testing methods in drug development. Int. J. Pharm. Pharm. Sci. 2015;4:28–32. [Google Scholar]
- Raunio H. In silico toxicology – non-testing methods. Front. Pharmacol. 2011;2:33. doi: 10.3389/fphar.2011.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schardein, J., 1976. Drugs as Teratogens CRC Press, Cleveland.
- Seok, J., Warren, H.S., Cuenca, A.G., Mindrinos, M.N., Baker, H.V., Xu, W., Richards, D.R., McDonald-Smith, G.P., Gao, H., Hennessy, L., Finnerty, C.C., López, C.M., Honari, S., Moore, E.E., Minei, J.P., Cuschieri, J., Bankey, P.E., Johnson, J.L., Sperry, J., Nathens, A.B., Billiar, T.R., West, M.A., Jeschke, M.G., Klein, M.B., Gamelli, R.L., Gibran, N.S., Brownstein, B.H., Miller-Graziano, C., Calvano, S.E., Mason, P.H., Cobb, J.P., Rahme, L.G., Lowry, S.F., Maier, R.V., Moldawer, L.L., Herndon, D.N., Davis, R.W., Xiao,W., Tompkins, R.G., 2013. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. USA.110, 3507–3512. [DOI] [PMC free article] [PubMed]
- Shanks, N., Green, K., 2004. Evolution and the ethics of animal research. Essays Philos. 5 (2), Article 30. http://commons.pacificu.edu/cgi/viewcontent.cgi?article=1168&context=eip
- Stokes W.S., Schechtman L.M., Hill R.N. The interagency coordinating committee on the validation of alternative methods (ICCVAM): a review of the iccvam test method evaluation process and current international collaborations with the European centre for the validation of alternative methods (ECVAM) Altern. Lab. Anim. 2002;30:23–32. doi: 10.1177/026119290203002S04. [DOI] [PubMed] [Google Scholar]
- Taussig M.J., Stoevesandt O., Borrebaeck C.A., Bradbury A.R., Cahill D., Cambillau C., de Daruvar A., Dübel S., Eichler J., Frank R., Gibson T.J., Gloriam D., Gold L., Herberg F.W., Hermjakob H., Hoheisel J.D., Joos T.O., Kallioniemi O., Koegl M., Konthur Z., Korn B., Kremmer E., Krobitsch S., Landegren U., van der Maarel S., McCafferty J., Muyldermans S., Nygren P.A., Palcy S., Plückthun A., Polic B., Przybylski M., Saviranta P., Sawyer A., Sherman D.J., Skerra A., Templin M., Ueffing M., Uhlén M. Proteome Binders: planning a European resource of affinity reagents for analysis of the human proteome. Nat. Methods. 2007;4:13–17. doi: 10.1038/nmeth0107-13. [DOI] [PubMed] [Google Scholar]
- Taylor K., Gordon N., Langley G., Higgins W. Estimates for worldwide laboratory animal use in 2005. Altern. Lab. Anim. 2008;36:327–342. doi: 10.1177/026119290803600310. [DOI] [PubMed] [Google Scholar]
- Washio T., Okada J., Takahashi A., Yoneda K., Kadooka Y., Sugiura S., Toshiaki H. Multiscale heart simulation with cooperative stochastic cross-bridge dynamics and cellular structures. Multiscale. Model. Simul. 2013;11:965–999. [Google Scholar]
- Wilding I., Bell J. Improved early clinical development through human microdosing studies. Drug Discov. Today. 2005;10:890–894. doi: 10.1016/S1359-6446(05)03509-9. [DOI] [PubMed] [Google Scholar]
- Wood G.C., Still C.D., Chu X., Susek M., Erdman R., Hartman C., Yeager S., Blosky M.A., Krum W., Carey D.J., Skelding K.A., Benotti P., Stewart W.F., Gerhard G.S. Association of chromosome 9p21 SNPs with cardiovascular phenotypes in morbid obesity using electronic health record data. Genomic. Med. 2008;2:33–43. doi: 10.1007/s11568-008-9023-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright A.K.A., Ferreira D.M., Gritzfeld J.F., Wright A.D., Armitage K., Jambo K.C., Gordon S.B. Human nasal challenge with streptococcus pneumoniae is immunizing in the absence of carriage. PLoS Pathogens. 2012;8:e1002622. doi: 10.1371/journal.ppat.1002622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- www.caat.jhsph.edu. (Accessed on 15 May, 2017).
- www.hiveg.com. (Accessed on 15 May, 2017).
- www.humaneresearch.org.au. (Accessed on May, 2017).
- www.icmr.nic.in/bioethics/cc_biothics/presentations/sym_pune/For%20PGs/Animal%20ethics.pdf. (Accessed on 15 September 2016).
- www.recombinant-antibodies.org. (Accessed on 15 May, 2017).
- www.ufaw.org.uk. (Accessed on 15 May, 2017).
- www.yumab.com. Accessed on 15 May, 2017.
- Yamazaki K., Suzuki M., Itoh T., Yamamoto K., Kanemitsu M., Matsumura C., Nakano T., Sakaki T., Fukami Y., Imaishi H., Inui H. Structural basis of species differences between human and experimental animal CYP1A1s in metabolism of 3,3',4,4',5-pentachlorobiphenyl. J. Biochem. 2011;149:487–494. doi: 10.1093/jb/mvr009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article.