Abstract
Renal tubular acidosis (RTA) represents a group of diseases characterized by: 1) a normal anion gap metabolic acidosis; 2) abnormalities in renal HCO3− absorption or new renal HCO3− generation; 3) changes in renal NH4+, Ca2+, K+ and H2O homeostasis; and 4) extrarenal manifestations that provide etiologic diagnostic clues. The focus of this review is to give a general overview of the pathogenesis of the various clinical syndromes causing RTA with a particular emphasis on Type I (hypoklemic distal RTA) and Type II (proximal) RTA while reviewing their pathogenesis from a physiological “bottom” up approach. In addition, the factors involved in the generation of metabolic acidosis in both Types I and II RTA are reviewed highlighting the importance of altered renal ammonia production/partitioning and new HCO3− generation. Our understanding of the underlying tubular transport and extrarenal abnormalities has significantly improved since the first recognition of RTA as a clinical entity because of significant advances in clinical acid-base chemistry, whole tubule and single cell H+/base transport, and the molecular characterization of the various transporters and channels that are functionally affected in patients with RTA. Despite these advances, additional studies are needed to address the underlying mechanisms involved in hypokalemia, altered ammonia production/partitioning, hypercalciuria, nephrocalcinosis, cystic abnormalities, and CKD progression in these patients.
Keywords: renal tubular acidosis, transport, acid-base, bicarbonate, hypercalciuria, hypokalemia, nephrocalcinosis
Introduction
In 1935 in an autopsy series of 850 patients, Lightwood et al first characterized pathological findings in 6 infants he termed “calcium infarction” of the kidneys that is considered the first report of renal tubular acidosis with nephrocalcinosis (1). Butler described additional patients with acidosis and nephrocalcinosis (2). In 1946 Albright considered these patients to have “renal acidosis resulting from tubular insufficiency” and suggested citrate be used as a therapy (3). Studies by Elkinton and colleagues emphasized the presence of systemic acidosis associated with a defect in urine acidification (4–6). Pines et al were first to coin the term “renal tubular acidosis (RTA)”. Stapleton reported a patient who rather than having an inability to generate a steep urine pH gradient, the underlying abnormality appeared to be a defect in proximal tubule bicarbonate absorption (7). Rodriguez-Soriano and Edelmann expanded on these findings and classified patients into having a defect either in proximal tubular bicarbonate absorption or in distal tubule urinary acidification (8, 9). In a review article Morris introduced the term Type I RTA for patients with distal or classic RTA, Type II RTA proximal RTA, and Type III RTA designating patients with both proximal and distal abnormalities (10). Mcsherry et al showed the transient nature of the proximal tubule defect in certain patients with Type III RTA that was attributed to developmental immaturity (11). When it became recognized subsequently that there are patients with distal RTA who are hyperkalemic due to aldosterone deficiency in contradistinction to patients with Type I RTA who are hypokalemic, a fourth type group called Type IV RTA or hyperkalemic distal RTA was added to the classification scheme1 (12,13, 14, 15,16). It is now known that various transport abnormalities in the distal nephron independent of aldosterone cause hyperkalemic distal RTA (17,18).
Proximal Tubule H+/Base and Bicarbonate Transport
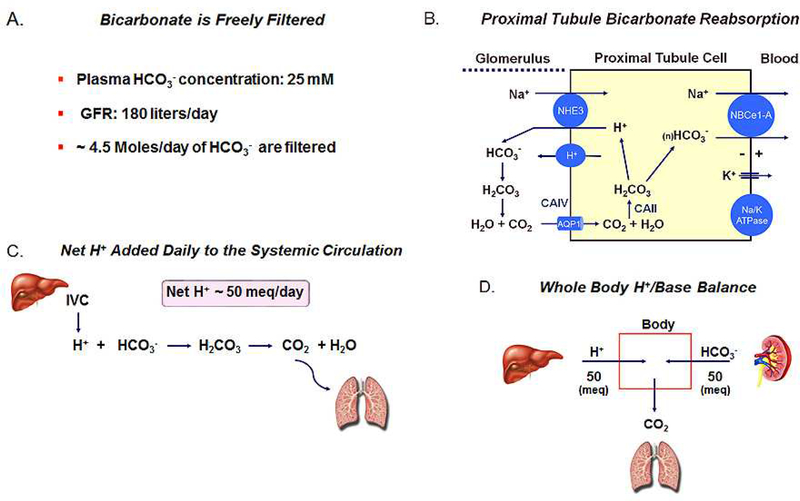
The various tubular transport abnormalities causing RTA syndromes impair the ability of the kidney to maintain the blood bicarbonate concentration within the normal range. Bicarbonate is essentially freely filtered by the glomerulus (Fig. 1), and at a plasma concentration of 25 meq/L, assuming a glomerular filtration rate of ~180 L/day, ~4.5 Moles of bicarbonate are filtered through the ~ 2 million glomeruli in both the kidneys in a 24-hr period (19,20). This represents the approximate amount of bicarbonate contained in a box of Arm & Hammer baking soda. If the filtered bicarbonate were not reabsorbed by the nephron, in approximately 4 hrs the plasma bicarbonate would decrease to zero. It is the function of nephron and particularly the proximal tubule to reclaim virtually all the filtered bicarbonate load such that only a very small fraction of the filtered bicarbonate load is excreted. The proximal tubule reclaims approximately 80% of the filtered bicarbonate load whereas the thick ascending limb and collecting duct absorbing 15% and 5% respectively. Fig. 1 shows the proximal tubular ‘transport machinery’ in mammals that efficiently transfers HCO3− from the lumen to the basolateral side. It is currently thought that the majority of luminal HCO3− is absorbed indirectly across the apical cell membrane via the passive CO2 flux that is formed following the reaction of luminal HCO3− with H+ secreted mainly by apical NHE3 (SLC9A3) (21) and to a lesser extent by an apical vacuolar H+-ATPase (22). Recent evidence suggests that a small amount of HCO3− may be directly absorbed via the NBCn2 (SLC4A10) transporter (23). The luminal production of CO2 is accelerated by the GPI anchored CAIV enzyme (24). CO2 flux from lumen to cell drives the reverse reaction that is catalyzed by soluble CAII thereby regenerating H+ and bicarbonate ions (25). Protons are then recycled across the apical membrane whereas HCO3− is absorbed across the basolateral cell membrane via the electrogenic sodium bicarbonate cotransporter NBCe1-A (SLC4A4) (26). The basolateral membrane potential (VBL) is the driving force for HCO3− efflux given that the chemical gradients for both Na+ and HCO3− are directed intracellularly and oppose the cellular efflux of HCO3−. VBL is generated by the electrogenic basolateral Na+-K+-ATPase coupled to a K+ diffusion potential that appears to be mediated in part by basolateral TASK2 K+ channels (27).
Figure 1.
(A) HCO3− is freely filterable (~4.5 moles/day); (B) Proximal tubule cell bicarbonate transport processes. In patients, CAII and NBCe1 mutations can inhibit transepithelial HCO3− absorption causing Type II (proximal) RTA. (C) Dietary net metabolic production of H+ depletes whole body HCO3− generating CO2 which is excreted by the lungs; (D) Schematic depiction of the equality of dietary net metabolic production of H+ and new HCO3− produced by the kidney that had not been consumed in the urea cycle. Approximately 60% of total new HCO3− generation is utilized to match metabolic production of H+ production and ~40% is consumed in the urea cycle with NH4+ generated by the kidney.
Renal Generation of New Bicarbonate from α-ketoglutarate
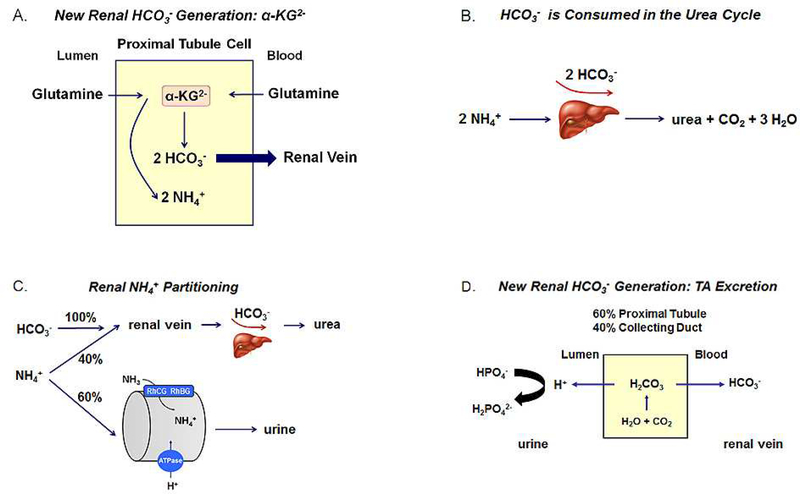
In addition reclaiming the filtered bicarbonate thereby preventing its urinary excretion, the kidney also plays a key role in counteracting the acidifying effect of metabolic H+ production from dietary metabolism (28,29). These processes generate ~ 50 meq of H+ per day (Fig. 1) that would otherwise gradually result in the titration of extracellular and intracellular bicarbonate and non-bicarbonate buffers were it not for the renal generation of new HCO3− (30). In the proximal tubule cell, α-ketoglutarate derived predominantly from glutamine is metabolized during gluconeogenesis or the Kreb’s cycle to two HCO3− ions (Fig. 2) (30). These new HCO3− ions are transported by basolateral NBCe1-A to the peritubular blood compartment. New HCO3− is also generated during the conversion of HPO4− to HPO4, so-called titratable acid formation that takes place in the proximal tubule (~60%) and the collecting duct (~40%). It is important to consider that in the formation of α-ketoglutarate from glutamine via glutamine and glutamate dehydrogenase, 2 NH4+ ions are generated (30,31)2. Were all the NH4+ an equimolar quantity of newly generated bicarbonate derived from α-ketoglutarate metabolism would be consumed in the liver in the urea cycle where 2 NH4+ + 2 HCO3− → urea + CO2 + 3 H2O (Fig. 2) (33,34). This would result in a futile cycle from an acid-base perspective with glutamine being converted into urea without new HCO3− being generated to help counter the H+ produced during dietary metabolism. In 1921 Nash and Owen showed that the kidney releases ammonia into the systemic circulation (35). In normal humans not all NH4+ generated is transferred to the renal vein such that the concept of NH4+ partitioning arose wherein ~ 60% of the NH4+ produced exits the kidney in urine and the remainder via the renal vein (Fig. 2) (36–38). Therefore, only approximately 60% of the new bicarbonate generated from α-ketoglutarate is available to counter daily dietary H+ production; the remainder is consumed in the urea cycle. A series of complex inter-nephron transport processes determine what percent of the NH4+ produced predominantly in the proximal tubule will be transferred to the urine for excretion from the body (31). As stated above new bicarbonate is also generated during the conversion of HPO42− into H2PO4−, so-called titratable acid (TA) formation (Fig. 2) that takes place in the proximal tubule (~60%) and the collecting duct (~40%)3, 4. Given the pH of normal urine, free H+ excretion cannot contribute in any significant fashion to matching the daily metabolic H+ load.
Figure 2.
(A) α-ketoglutarate production from glutamine in mitochondria. Each α-ketoglutarate molecule generates 2 HCO3− in gluconeogenesis (or the Kreb’s cycle) along with NH4+; (B) Schematic depiction of the consumption of 2 HCO3− and 2 NH4+ in ureagenesis; (C) Normal partitioning of ammonia produced in the kidney between the urine (~ 60%) and the renal vein (~40%). Of the total new HCO3− generated by the kidney, the portion remaining that is not consumed with NH4+ during ureagenesis is available for preventing the plasma HCO3− from decreasing as a resulting of dietary H+ production; (D) Titratable acid (TA) formation and excretion. Approximately 60% of TA is generated in the proximal tubule and ~ 40% is generated in the collecting duct.
Autosomal Recessive Isolated Proximal RTA: NBCe1 Mutations
Proximal RTA can occur in an isolated manner on a genetic or acquired basis, and in the context of additional proximal tubular transport, defects termed Fanconi’s syndrome. Isolated autosomal recessive proximal RTA is caused by mutations in the SLC4A4 gene encoding the proximal tubule basolateral HCO3− transporter NBCe1-A (39). Additional mutations have since been described in the N-terminal and transmembrane domains that alter function, result in misfolded proteins that are retained intracellularly, and that affect plasma membrane targeting (40). One of the interesting missense mutations is the NBCe1-A T485S mutation in the ion coordination region of the transporter which demonstrates why wildtype NBCe1-A cannot be electroneutral (41). Wild-type NBCe1-A is electrogenic carrying a negative transport current that senses the basolateral proximal membrane potential (VBL). The negative basolateral VBL drives Na+ and HCO3− across the basolateral membrane against their respective chemical gradients. The electroneutral T485S mutant fails to sense VBL resulting in defective bicarbonate absorption. Patients have an extra-renal phenotype in addition to proximal RTA because the majority of mutations affect other SLC4A4 gene transcripts (40). These extra-renal manifestations include short stature, band keratopathy, cataracts, glaucoma, basal ganglia calcifications, migraine headaches, mental retardation, and tooth enamel defects. This constellation of findings is diagnostic of patients with NBCe1 mutations.
In all RTA syndromes whether proximal or distal, when extra-renal manifestations are present, they provide important diagnostic clues as to the underlying etiology. In some instances, the phenotype is not diagnostic. For example, growth retardation is not unique to these patients and occurs in numerous diseases associated with a congenital metabolic acidosis. Similarly, IQ abnormalities and abnormal brain development although characteristically seen in these patients is again not diagnostic. Calcified basal ganglia is also detected in patients with carbonic anhydrase (CA) II mutations (42) and is thought to result from an increased local pH resulting in local calcium phosphate precipitation. The eye phenotype in patients with NBCe1 mutations consists of band keratopathy, glaucoma and cataracts. The mechanism for these abnormalities remain poorly understood. With regards to band keratopathy, normal eyelid opening results in a loss of CO2 that increases the pH of the corneal tear film. We have hypothesized that NBCe1 (the -B variant) in corneal endothelial cells ameliorates the increase in pH by transporting bicarbonate into the aqueous humor. In patients with NBCe1 mutations, defective NBCe1-B transport impairs normal corneal pH regulation resulting in calcium phosphate precipitation (band keratopathy) in the central cornea (the exposed region of the cornea during eyelid opening). NBCe1-B is expressed in the lens epithelium and ciliary body; the mechanism for lens cataract formation and glaucoma in these patients, however, is not known (43). In concert with the eye findings, the tooth enamel abnormalities are diagnostic and studies in mice have shown that NBCe1 is required for normal enamel formation (44). As in other mouse models that aren’t exact phenocopies of human disease, mice with global loss of NBCe1, unlike humans, have a severe intestinal GI phenotype and short survival (45) suggesting that either compensatory mechanisms mask the phenotype, and/or NBCe1 plays a less important transport role in the human GI tract.
Brenes et al reported patients with autosomal dominant proximal RTA (46,47). These patients differ phenotypically from patients with NBCe1 mutations (48). The cause of this syndrome is currently unknown with mutations in many genes including PAT1(CFEX), NHERF1 and NHERF2, CA II, CA IV, CA XIV, NHE3, NHE8, and NBCe1, having been ruled out (49).
Patients with mutations in CAII (Guibaud-Vainsel syndrome or marble brain disease) have a combined proximal and distal RTA (Type III RTA) although the relative magnitude of proximal and distal RTA is variable (42,50,51). Extrarenal manifestations include osteopetrosis, increased fractures, growth abnormalities, intellectual impairment, intracerebral calcification, and excessive bone growth resulting in conduction deafness and optic nerve compression. Patients with CAIV mutations have a rod/cone dystrophy whereas proximal RTA has not been described in these patients (52). Mice lacking NHE3 have a moderate impairment in proximal tubule bicarbonate reclamation, however, mutations in humans have not yet been described (53). Loss of the basolateral TASK2 K+ channel in mice impairs proximal tubule bicarbonate absorption likely due to decreased NBCe1-A function (27). Similar to NHE3, mutations in TASK2 have not been described in humans. Heterotetrameric Kir4.1/Kir5.1 K+ channels play an important role as a K+ sensor in the DCT (54). Mice with targeted loss of Kir5.1 have hypokalemic RTA with hypercalciuria, decreased urine NH4+ excretion, and an acidic urine pH (55). Although the role of Kir5.1 in the proximal tubule is unknown, the electrolyte abnormalities in these mice suggest an additional impairment in proximal tubule HCO3− and NH4+ production.
Diagnostically, patients with isolated proximal RTA have a normal anion gap metabolic acidosis and are typically normokalemic prior to receiving therapy. To diagnose a proximal tubule bicarbonate reclamation defect, sodium bicarbonate is administered (2.75% NaHCO3 IV (4 ml/kg/hr)) with a goal of normalizing the plasma bicarbonate concentration and the fractional excretion of bicarbonate (FEHCO3) is then measured according to: [urine HCO3 X plasma Cr / plasma HCO3 X urine Cr]. Given that proximal tubules absorb ~ 80% of the filtered bicarbonate load (~3.6 Moles/day), a FEHCO3 greater than 20% would imply that there must be a proximal tubule effect6. Depending on the magnitude of the proximal defect and whether children (during growth) versus adults are being treated, up to 10–15 meq/kg/day (children) of alkali therapy in the form of sodium citrate or a combination of sodium and potassium citrate may be required. In general sodium citrate is administered initially and potassium citrate can be given concomitantly if a patient becomes hypokalemic during therapy.
Mechanism(s) of Generation of Metabolic Acidosis in Proximal RTA
The initial loss of bicarbonate in urine following which a new steady state is reached represents the classic and major mechanism underlying the normal anion gap metabolic acidosis in patients with proximal RTA (10). It has become increasingly apparent that a second mechanism unrelated to urine HCO3− loss is involved in the induction of metabolic acidosis in proximal RTA. Although information regarding urine NH4+ excretion in proximal RTA is limited, previous studies in patients with autosomal dominant pRTA documented normal urine NH4+ excretion in the steady state but a lack of an appropriate increase in urine NH4+ excretion following NH4Cl loading (46,47). Similarly, NBCe1−/− mice have decreased urine NH4+ excretion in the presence of an acidic urine pH associated with decreased phosphate-dependent glutaminase and phosphoenolpyruvate carboxykinase expression, and increased expression of glutamine synthetase (56,57). These findings are compatible with a defect in proximal tubule from NH4+ production from glutamine and an associated decrease in new HCO3− generation from α-ketoglutarate. It has been previously hypothesized that patients with proximal RTA resulting from defective basolateral HCO3− efflux (such as with NBCe1-A mutations) would be predicted to have an increased proximal tubule cell pH (58). Were the proximal tubule cell mitochondrial pH also increased in this scenario, NH4+ produced from glutamine, given the pH sensitivity of glutaminase and glutamate dehydrogenase, would be predicted to be decreased as would the generation of new HCO3− from α-ketoglutarate (59). Furthermore, in addition to decreased α-ketoglutarate generation from glutamine, enhanced urinary loss of α-ketoglutarate in mice with targeted disruption of NBCe1 represents a third potential mechanism for the generation of metabolic acidosis i.e. metabolizable organic anion loss in the urine (60,61).
Thick Ascending Limb H+/base, Bicarbonate and Ammonia Transport
The loop of Henle absorbs approximately 10–20% of the filtered HCO3− load. The major site of active HCO3− absorption is in the medullary and cortical thick ascending limb (mTAL and cTAL respectively) (62). The TAL shares some of its transport properties with the proximal tubule. Specifically, apical NHE3 plays the same role in luminal HCO3− absorption as in the proximal tubule, with both NHE2 and a vacuolar H+-ATPase also localized to the apical membrane (63–65). An apical K+-dependent HCO3− transport process opposing transepithelial HCO3− absorption has also been described (63) in addition to K+-dependent ATP-ase activity (67). TAL cells express various carbonic anhydrase isoforms including cytoplasmic CAII and XV, apical and basolateral CAIV, basolateral CAXII, and apical CAXIV (68). AE2 mediated anion exchange contributes to cell HCO3− efflux and transepithelial TAL HCO3− absorption (69). Basolateral membrane cell HCO3− uptake in the mTAL is mediated in part by NBCn1, that plays a role in cellular NH3 + H+ efflux and ammonia absorption (70). A basolateral K+-dependent HCO3− transport process has also been reported in the rat mTAL (71). Basolateral ClC-K1 and ClC-K2 chloride channels (or their human orthologs CLC-Ka and CLC-Kb, respectively), contribute to transcellular Cl− absorption (72). NH4+ produced in the proximal tubule is transported from the lumen of the TAL to the peritubular interstitium for subsequent secretion via RhCG and RhBG into the collecting duct or delivery to the renal vein (31). NKCC2 and potentially ROMK transport NH4+ across the apical membrane that possesses a very low apical membrane NH3 permeability preventing NH3 backflux from cell to lumen (73). NHE1 and NHE4 are expressed basolaterally, and NHE4 is also thought to contribute to the peritubular efflux of NH4+ (74).
Renal Tubular Acidosis Secondary to Abnormal Thick Ascending Limb Transport and Decreased Medullary NH4+ Concentration
In humans, renal tubular acidosis attributed to defective TAL transport per se has not been described. Mice with loss of NHE4 have normal acid-base status at baseline and lower than control urine pH but when challenged with NH4Cl, develop a normal anion gap metabolic acidosis associated with an inability to increase NH4+ and net acid excretion appropriately (74). Loss of NHE4 function leads to decreased TAL ammonia transport, outer/inner medulla tissue ammonia content, and predicted impaired collecting duct ammonia secretion (74). RTA due to decreased collecting duct ammonia secretion also underlies the abnormality in mice with medullary interstitial sulfatide (highly charged anionic glycosphingolipids) deficiency with disruption of cerebroside sulfotransferases (Cst) UDP-galactose:ceramide galactosyltransferase and UDP-glucose:ceramide glucosyltransferase (Ugcg) genes (75). In Ugcg/Cst-deficient mice at baseline blood pH and HCO3− levels were normal, however HCl loading induced a greater decrease in blood pH, HCO3− concentration, and urine pH than controls with a decreased capacity to increase urine NH4+ and net acid excretion (75). It is hypothesized that renal sulfatide-deficient mice have an impaired ability to electrostatically maintain a high medullary concentration ammonia content. As discussed below in more detail, the inability to generate a high medullary ammonia concentration gradient has implications with regards to the kidney’s ability to modulate the partitioning of ammonia between the collecting duct (urine) and renal vein (see below) at baseline and in response to extrarenal H+ loads (see below). An impaired ability to generate a high medullary-collecting duct gradient may also play a role in a subset of patients diagnosed with incomplete RTA (see below).
Cellular H+/base and Ammonia Transport Processes in the Distal Nephron Collecting Duct System: Distal Convoluted Tubule (DCT); Connecting Tubule (CNT); Collecting Duct (Cortical (CCD), Outer Medullary (OMCD) and Inner Medullary Collecting Ducts (IMCD))
Among mammalian species, heterogeneity often exists in both the expression of H+/base transporters and the functional properties of the various nephron segments in the distal nephron collecting duct system. The distal convoluted tubule (DCT) is the next nephron segment after the TAL that can transport bicarbonate (76). The luminal H+/base transport processes differ depending on the segment.
Apical H+ secretion in the early DCT (DCT1) is mediated by the NHE2 Na+/H+ exchanger and H+ATPase activity (77). In the latter portion of the DCT (DCT2), both an H+-ATPase and H+-K+-ATPase mediate H+ secretion (77,78). The H+-K+- ATPase colonic variant has also been localized to the CNT and early CCD (79,80). A third type of intercalated cell in the CNT called non-A non-B ICs has express apical H+-ATPase and pendrin, but not basolateral AE1 (81–84).
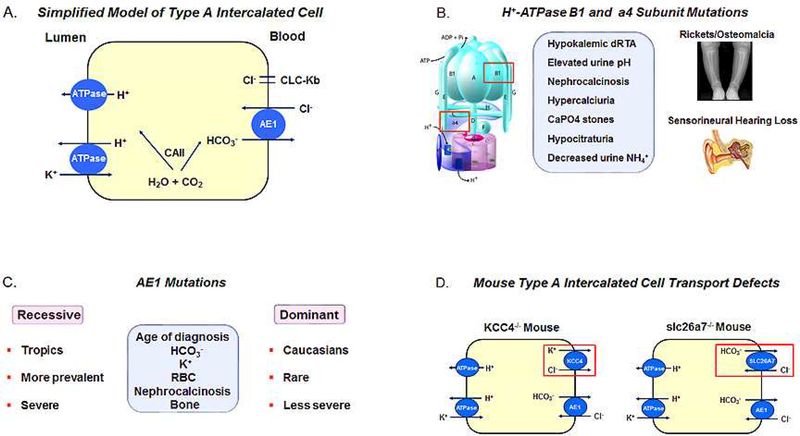
In the cortical collecting duct (CCD) ~ 60% of the cells are principal cells (PCs) with approximately 40% of the cells intercalated cells (ICs) that are further subdivided into Type A and Type B subtypes. Type A ICs absorb bicarbonate whereas Type B ICs mediate bicarbonate secretion (85). Fig 3. shows a simplified cell model of the Type A cells which secrete H+ via apical H+- ATPase and to a lesser extent H+-K+-ATPase. Basolateral bicarbonate efflux is mediated predominantly by the anion exchanger AE1. In Type B ICs apical Cl−/HCO3− exchange is mediated by pendrin (SLC26A4) and is coupled to Na+-driven Cl−/HCO3 mediated by NDCBE when transporting NaCl electroneutrally (86). Unlike the Type A cell, the H+-ATPase is expressed diffusely in the cytoplasm and basolateral membrane (87). Cells that don’t fit the cell models of Type A or Type B ICs have also been described. ICs cells in the CCD (γ or G cells) have been identified functionally with basolateral Na+-independent Cl−/HCO3− exchange (88,89). The apical transporter is thought to be pendrin, whereas the transporter responsible for basolateral Cl−/HCO3− exchange is not known. In Type A ICs, apical KBAT may function as an electrogenic Cl− extruder thereby enhancing H+-ATPase secretion and basolateral CLC-Kb Cl− channels coupled with AE1 may mediate Cl− recycling (90). In Type B ICs, CLC-Kb Cl− channels are expressed on the basolateral membrane and contribute to cAMP stimulatable transcellular Cl− transport (91).
Figure 3.
(A) Type A intercalated cell H+/base transport processes; (B) Patients with H+-ATPase B1 or a4 mutations have Type I RTA with sensorineural deficits, hypokalemia, hypercalciuria, nephrocalcinosis and renal calculi. Bone abnormalities i.e. rickets versus osteomalacia are age dependent; (C) AE1 mutations are more commonly inherited in an autosomal recessive pattern with a greater frequency in the tropics. Autosomal dominant disease is less common and less severe than patients with recessive mutations, and typically is diagnosed in Caucasians. Patients lack sensorineural hearing abnormalities and the phenotype in general is otherwise similar to patients with H+-ATPase subunit mutations; (D) Loss of KCC4 and slc26a7 in the mouse Type A IC cause distal acidification defects. Mutations in these transporters in humans have not been documented.
Unlike the CCD, the OMCD absorbs HCO3− only (92). In the outer stripe portion (OMCDos) ~ 2/3 of the cells are PCs and 1/3 are Type A ICs (93). In the OMCD, Type A ICs are responsible for generating the lumen positive transepithelial voltage due to electrogenic apical H+ secretion. Like Type A ICs in the CCD, these cells express basolateral AE1 Cl−/HCO3− exchanger coupled to Cl− recycling through basolateral Cl− channels (94). Type A ICs in the OMCD also express the basolateral SLC26A7 anion exchanger (95). These cells also have H+-K+-ATPase transport activity (96,97). Differences in the structural and functional properties of the OMCDos and inner stripe portion (OMCDis) have been documented (98–100).
The IMCD is divided anatomically into an initial (IMCDi) and terminal segment (IMCDt), or categorized into thirds (IMCD1, IMCD2, and IMCD3) (101,102). Species and functional differences exist where IMCD1 cells in the rabbit are of a single type and resemble OMCDis cells with respect to their staining positive for carbonic anhydrase and Na+-K+-ATPase (100), whereas in rat ~ 10% of the cells are Type A ICs (101,102). Unlike Type A ICs, IMCD cells lack staining for the H+-ATPase, AE1, and H+-K+ATPase. Basolateral base transport is mediated by a Cl−/HCO3− exchanger (possibly AE2) (103). There is functional evidence for a Na+/H+ exchange process (104) and a HCO3− conductive pathway of unknown molecular identity (105).
Ammonia Production and Urine/Renal Vein Partitioning in Response to Extrarenal H+ Loads
In response to an increased systemic H+ load (or HCO3−) loss that would potentially cause a metabolic acidosis, the normal kidney responds several ways in the chronic setting to ameliorate the changes in acid-base chemistry7. These adaptive responses include: 1) Increased α-ketoglutarate production from glutamine associated with an up to ~10-fold increase in NH4+ production (106,107) and new HCO3− generation in gluconeogenesis; 2) Increased urine/renal vein NH4+ partitioning such that ~80% of renal NH4+ production is excreted in the urine (Table I) (37); 3) Increased TA excretion and concomitant new HCO3− generation up to ~ 3-fold (107); and 4) Decreased ammonia consumption in periportal hepatocytes (urea synthesis) with an increase in perivenous hepatocytes (glutamine synthesis) such that HCO3− consumption in ureagenesis is decreased (108).
Table I.
Renal Ammonia Production and Partitioning
| Cause | Urine | Renal Vein | Urine/Renal Vein | Production | Reference |
|---|---|---|---|---|---|
| Type I RTA | ↓ | ↑a | ↓a | ↑a,b | --- |
| Acetazolamide | ↓ | ↑ | NC | ↓ | 172 |
| Metabolic Acidosis | ↑ | NC | ↑ | ↑ | 36,37 |
| Hypokalemia | ↑ | ↑ | NC | ↑ | 173 |
| Metabolic Alkalosis | ↓ | ↑ | ↓ | NC | 171 |
Predicted
Total renal ammonia production would be predicted to be increased as a result of both a chronic metabolic acidosis and hypokalemia. Increased blood ammonia levels have been documented in patients with Type I RTA attributed to increased renal vein ammonia delivery (Refs 185–189).
NC No change
By modulating the partitioning of ammonia between the urine and renal vein the kidney can regulate the magnitude of HCO3− consumption in the urea cycle. In chronic extrarenal metabolic acidosis the majority of ammonia produced in the proximal tubule and absorbed by the TAL is transferred to the urine (Table I) (37). Collecting duct NH3 secretion is enhanced because of an increase in TAL NH4+ transport (109) thereby increasing the interstitial to collecting duct NH3 concentration gradient, coupled with enhanced H+ secretion by Type A intercalated cells that shifts the luminal NH4+-NH3 equilibrium towards NH4+) (31). NH3 is secreted across the collecting duct plasma membrane via specific rhesus (Rh) membrane proteins. In the CNT, Type A ICs, and non-A, non-B cells (110,111) express RhBG basolaterally, whereas RhCG is expressed on both apically and basolaterally in DCT, CNT, Type A ICs, and non-A, non-B cells (112,113). In extrarenal metabolic acidosis increased RhCG expression in both the OMCD and IMCD ICs (114) and in the OMCDis increased apical RhCG expression in ICs and PCs contributes to enhanced urine NH4+excretion and therefore altered urine/renal vein ammonia partitioning (115). Although humans with renal tubular acidosis due to mutations in RhCG and RhBG have not been reported, in mice various studies have established the role of RhBg and RhCg in the renal response to extrarenal metabolic acidosis. Specifically, mice with combined collecting duct loss RhBG/RhCG proteins have normal acid-base base parameters but following acid-loading have a more severe metabolic acidosis than controls (116).
Type I Distal Renal Tubular Acidosis: Type A Intercalated Cell Transport Defects
The apical vacuolar H+-ATPase in Type A ICs is the predominant luminal acidification process in the collecting duct (Fig. 3). The pump is assembled into two domains: A Vo transmembrane domain (subunits A-H) and a V1 cytoplasmic domain (ATP6V1) composed of subunits a, c, c”, d, and (117,118). At the B/A subunit interface of the V1 domain ATP hydrolysis occurs. In the V0 domain H+ are translocated between the a- and c-ring. In 1999 Karet et al reported patients with autosomal recessive hypokalemic distal RTA and mutations in the B1 (subunit (V1 domain)(119). Patients with B1 subunit mutations typically present in infancy with polyuria, nephrocalcinosis, hypercalciuria, hypocitraturia, decreased urine NH4+ excretion, an elevated urine pH, bilateral sensorineural hearing loss, and rickets or osteomalacia (depending on the age of presentation) (Fig. 3). Subsequently additional patients were described with autosomal recessive mutations in the a4 (ATP6V0A4) V-ATPase subunit (V0 domain) who were initially thought to be characterized by the absence of sensorineural hearing loss, however subsequent studies indicated that these patients can also develop hearing abnormalities (Fig. 3) (120,121). Alkali therapy does not alter the course of hearing loss (121,122). It has been suggested that patients with a4 mutations may have a more severe phenotype. Interestingly, mice with loss of the a4 subunit (123,124) have a severe metabolic acidosis unlike mice with loss of the B1 subunit due to the adaptive increased expression of B2 subunit containing H+-ATPase transporters (125,126)8. In addition, the a4 subunit is expressed in the proximal tubule and a4-/- mice have proximal tubule transport defects including phosphaturia and albuminuria, which thus far have not been reported in humans (123). The latter may be due to compensatory changes in the a1 and a2 subunits (and to a lesser extent the a3 subunit) in proximal tubules of patients (128). Proximal tubule transport defects resulting in molecular weight proteinuria, generalized aminoaciduria, phosphaturia, and uricosuria have been documented in Type I RTA patients including siblings with H+-ATPase B1 subunit mutations and are typically reversed following alkali therapy (129–131).
Bicarbonate efflux across the basolateral membrane of type A ICs is mediated predominantly by AE1 (SLC4A1) although there is evidence in mice for an additional role for slc26a7 mediated anion exchange (Fig. 3). Bruce et al characterized the first patients with autosomal dominant Type I RTA due to mutations in AE1 (132). Unlike mutations in the H+-ATPase B1 subunit, Type I RTA caused by AE1 mutations is inherited in both an autosomal dominant and recessive fashion In Caucasians AE1 mutations are rare and typically inherited in an autosomal dominant fashion, whereas in Asians an autosomal dominant inheritance is uncommon (Fig. 3) (133–135). Mutations in AE1 either cause Type I RTA or red cell abnormalities but rarely both (136,137). The severity of the phenotype in these patients that including age of diagnosis and severity of the metabolic acidosis, hypokalemia, nehrocalcinosis, rickets/osteomalacia, and red cell abnormalities is greater in patients with autosomal recessive inheritance or compound heterozygotes who are typically diagnosed in the tropics (138). It has been hypothesized that mutations in the transporter in the regions of the world confer a selective advantage for prevention of malaria.
HCO3− transport in the collecting duct is sensitive to carbonic anhydrase inhibition (92,139,140). ICs in the collecting duct in all species stain for cytoplasmic CAII (141) with species differences in the expression of membrane anchored CAIV (142,143). In addition to its enzymatic role, studies in mice suggest that CAII is important for normal IC cell development in that in CAII-/- mice there is a loss of Type A and Type B ICs (144). As discussed, patients with CAII mutations have a combined proximal and distal RTA (Type III RTA) with a variable component of each disorder(42,50,51). Borthwick et al reported a phenocopy of CAII deficiency with two separate and penetrant recessive disorders where the osteopetrosis was caused by a homozygous deletion in TCIRG1 (encoding an osteoclast specific H+ATPase a subunit), whereas the distal RTA was caused by a homozygous ATP6V1B1 mutation (145).
The Forkhead transcription factor Foxi1 is necessary for expression of the H+-ATPase subunits A1, E2 and a4 in the collecting duct (146). Mice with loss of Foxi1 develop overt type 1 RTA following a chronic acid load and their kidneys lack ATP6B1, AE4, as well as Pds and AE1 (147). Enerbäck recently reported patients with homozygous mutations in Foxi1 who have Type I RTA, normokalemia, nephrocalcinosis, medullary cystic abnormalities and one patient had hypercalciuria (148).
Additional Mouse Models of Impaired Type A Intercalated Cell H+/Base Transport
Several additional mouse models of impaired type A IC H+ secretion have been reported which are not yet known to be involved in human disease. These include mice with loss of KCC4 (Slc12a7) (149), slc26a7(90), Hensin (DMBT1)-CXCL12 complex (150), GPR4 (151,152), NBCe2 (153,154) and ATP6ap2 (155). Species differences also exist in that mice are not necessarily exact phenocopy models of human disease. For example AE1−/− mice have a normal anion gap metabolic acidosis with nephrocalcinosis and hypercalciuria as do patients with AE1 mutations,; yet unlike patients, there blood K+ concentration is greater than normal and the urine NH4+/Cr ratio is not decreased (156). AE1 R607H knockin mice (a model the human R589H mutation) have a distal acidification defect (detected following an acid load) with reduced basolateral membrane expression of AE1 and reduced B1 apical subunit H+ATPase expression in Type A ICs (157). Mice with loss of the basolateral KCl cotransporter KCC4 develop a distal acidification defect and deafness (Fig. 3) (149). These findings suggest that KCC4 in addition to CLC-Kb is coupled to AE1 in Type A ICs membrane in contributing to basolateral membrane Cl− recycling. In mice with loss of KCC4, it is thought that AE1 mediated HCO3− absorption is decreased as a result of a decreased peritubular-to-cell Cl− gradient. It is also possible that loss of KCC4 impairs basolateral NH4+ uptake from the interstitium (158). Mice with loss slc26a7 also develop a distal acidification defect (Fig. 3) (90). These findings suggest that in the mouse slc26a7 plays an important role in Type IC HCO3− absorption and that loss of slc26a7 function cannot be compensated by AE1. The role of SLC26A7 in humans has not been determined and mutations in the transporter have not been detected. Clearly loss of AE1 transport in patients with type I RTA cannot be compensated by SLC26A7.
Categorization of Patients with Incomplete Distal Renal Tubular Acidosis
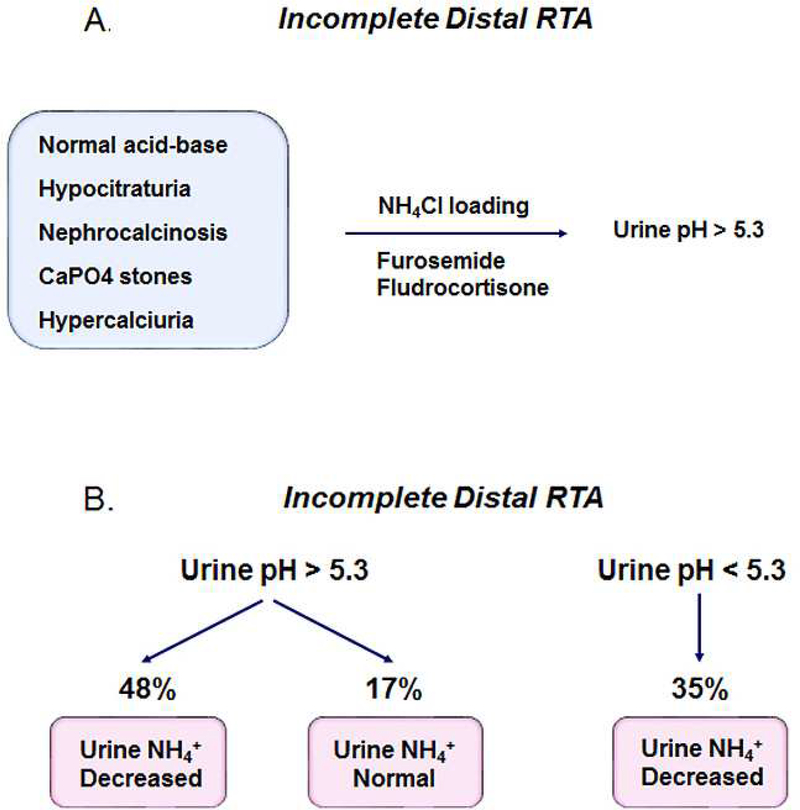
In 1959 patients were described who have normal acid-base parameters at baseline but following an extrarenal NH4Cl acid load, are unable to decrease their urine pH to < 5.3 (159)9. Since the original description of incomplete distal RTA it has become increasingly apparent that these patients have findings in common that can include: 1) hypocitraturia; 2) nephrocalcinosis; 3) renal calculi (calcium phosphate) and 4) hypercalciuria (Fig. 4) (160–163). Skeletal abnormalities including osteopenia, osteoporosis, and growth retardation have also been reported (162,163,165). More recently Forni Ogna et al reported that patients can be divided into three separate groups based on the urinary pH and ammonia excretion in response to a urine acidification challenge (Fig. 4) (166). 65% of the total patients were unable to lower their urine pH < 5.3. 74% of these patients had a decreased urine NH4+ (< 33 umole/min) whereas 17% had a normal excretion rate (> 33 umole/min). 26% of the patients were capable of acidifying their urine normally and all had decreased NH4+ excretion. Whether the latter patients are miscategorized and have a proximal tubule NH4+ and concomitant new HCO3− production impairment is unknown. The latter patients could also have an abnormality in thick ascending limb to collecting duct ammonia secretion with as yet to be documented mutations in NHE4, RhBG/RhCG, and cerebroside sulfotransferases.
Figure 4.
Incomplete distal RTA. Patients have characteristics in common including normal acid base status, hypocitraturia, hypercalciuria, nephrocalcinosis and renal calculi. Following ammonium chloride loading or furosemide/fludrocortisone administration, patients with incomplete distal RTA are unable to lower the urine pH to < 5.3 (159,196). More recently it has been shown the patients fall into 3 categories based on their urine pH and ammonia excretion during a furosemide/fludrocortisone test (166). The group with normal urine acidification and defective urine ammonia excretion may involve abnormalities in NHE4, RhBG/RhCG, and cerebroside sulfotransferases that are known to play an important role in ammonia transfer from the thick ascending limb to the collecting duct in the mouse.
Although in the majority of patients, the molecular basis of incomplete distal RTA is unknown, carriers with a p.Phe468fsX487 mutation in ATP6V1B1 encoding the H+-ATPase B1 subunit at baseline have normal systemic acid-base parameters with an inappropriately high urine pH, hypocitraturia, and hypercalciuria individually or in combination (167). Some heterozygotes also were unable to acidify their urine normally following acute ammonium chloride loading and had an abnormal U-B pCO2 gradient. In addition, recurrent calcium phosphate kidney stones formers who carry a p.E161K SNP encoding the B1 H+-ATPase subunit have incomplete distal RTA (168). Imai et al recently described a patient with a heterozygous ATP6V0A4 gene H+-ATPase a4 subunit p.S544L mutation who had hypokalemia, normal acid-base values, CKD, an alkaline urine, and nephrocalcinosis with an abnormal NH4Cl acid load test, furosemide/fludrocortisone acidification test and U-B pCO2 test (169).
Mechanism of the Generation of Metabolic Acidosis in Type I Renal Tubular Acidosis
The generation of metabolic acidosis in distal RTA can be viewed as an experiment of nature demonstrating the importance of the kidney in producing the appropriate amount of new HCO3− to match the daily hepatic dietary H+ load and in partitioning ammonia between the urine and renal vein appropriately to prevent excessive hepatic HCO3− consumption in the urea cycle. In Type I RTA two phenomena are thought to contribute: 1) decreased new HCO3− generation from H2PO4− (TA) excretion and 2) decreased urine ammonia excretion. We have postulated that this is associated with enhanced renal vein ammonia delivery (with concomitant HCO3− consumption in the urea cycle) as a result of an inappropriately high pH in the collecting duct lumen (170) (Table I). The latter effect, in our opinion, is quantitatively the most important factor in the generation of the systemic acidemia. Similar phenomena i.e. decreased TA excretion and shunting of urine ammonia to the renal vein resulting from an acute increase in collecting duct luminal pH are associated with the administration of a systemic HCO3− load (171) or the administration of acetazolamide where urine ammonia excretion can decrease significantly to ~ 10% of total renal production (Table I) (172). As a compensatory response, the proximal tubule should respond (as it does following an extrarenal H+ load) by increasing glutamine utilization, NH4+ and α-ketoglutarate production and new HCO3− generation. In addition, these patients are often chronically hypokalemic, which is an additional stimulus for glutamine utilization, NH4+ and α-ketoglutarate production, and new HCO3− generation (173); responses that would tend to decrease the severity of the systemic acidemia in any patient with metabolic acidosis. Renal vein cannulation would be required to assess abnormal urine/renal vein ammonia partitioning and compensatory increased total ammonia production (urine + renal vein). Several studies have emphasized the diagnostic utility of measuring the urine anion or osmolar gaps as an indirect assessment of the urine ammonia concentration/excretion (174–181). It is now recognized that the direct measurement of urine ammonia is preferable (182,183), however it should be recognized that even if one were clinically routinely able to measure the urine ammonia concentration and excretion directly (which is still problematic in many hospitals), one can still not determine whether a decrease in urine ammonia excretion resulted from impaired proximal tubule ammonia production and/or abnormal urine/renal vein ammonia partitioning. Vasuvattakul reported a method in dogs to indirectly assess total ammonia production (without measure renal vein ammonia delivery directly). Its accuracy and general utility in patients is unknown (184).
Given the typical patient with hypokalemic distal RTA with abnormal urine/renal vein ammonia partitioning and compensatory enhanced renal ammonia production (due to metabolic acidosis and hypokalemia (Table I) it would be expected that renal vein ammonia delivery to the systemic circulation would be increased. Of interest, an increased blood ammonia level has been occasionally documented in these patients compatible with increased NH4+ delivery systemically (renal vein) that was not matched by enhanced hepatic uptake (185–189). Whether the absolute blood ammonia level or the ratio of blood to urine ammonia concentrations is helpful diagnostically in patients with normal anion gap metabolic acidosis remains to be studied.
What is the diagnostic utility of assessing the urine ammonia concentration in comparison to U-B pCO2 and/or minimal urine pH following an acidification stimulus? In reality, each of these measurements assesses a different aspect of renal acid-base phenomenology (190). The urine ammonia excretion reflects renal ammonia production and urine/renal vein ammonia partitioning whereas the U-B pCO2 measurement following administration of NaHCO3 IV (2.75% 4 ml/Kg/hr) reflects the maximal quantity of H+ secreted into the collecting duct lumen at an alkaline lumen pH (191–194)10. The minimal urine pH reflects the maximal collecting duct cell to lumen H+ gradient that can be generated and maintained. The classic NH4Cl acid load test (0.1 gm/kg po) because of its gastrointestinal side effects has been replaced by a furosemide (40 mg) + fludrocortisone urine acidification test (1 mg) (urine pH < 5.3 and urine ammonia excretion > 33 umole/min) (196). In mice furosemide induced urinary acidification has been attributed to increased H+ secretion by Type A ICs in the CNT (197) and enhanced TAL NHE3 mediated apical transport (198). In humans the urinary acidification response to furosemide can be completely abrogated by amiloride (199, 200), which blocks Type A IC H+ secretion by hyperpolarizing its apical membrane through circular intraepithelial current flows via a direct inhibition of PC ENaC channels (201). These findings indicate that normal ENaC activity is required for H+ secretion to be stimulated by this test.
Mechanism of Development of Hypokalemia in Distal Renal Tubular Acidosis
Gill et al first described patients with hypokalemic distal RTA whose hypokalemia was ameliorated with sodium bicarbonate or phosphate but not sodium chloride (202). Sebastian et al hypothesized that these patients have an associated Na+ transport defect with volume depleted leading to mineralocorticoid stimulation of collecting duct K+ secretion and showed that the associated hypokalemia is not corrected by correcting the metabolic acidosis (203). Dafnis found that vanadate, a non-specific H+-K+-ATPAse inhibitor could induce hypokalemia and metabolic acidosis similar to that seen in patients with classic RTA in rats suggesting impaired H+-K+-ATPase activity in patients (204). Since that time several factors that can potentially perturb renal K+ transport in patients with metabolic acidosis that might be playing a role in patients with hypokalemic distal RTA have been considered including: 1) Decreased proximal tubule paraceellular/transcellular NaCl transport and passive K+ absorption; 2) Impaired thick ascending limb K+ absorption associated with decreased ROMK transport; and 3) Increased Na+ delivery, luminal flow and aldosterone level enhancing collecting duct K+ secretion. More recently Oguejiofor et al reported a patient with hypokalemic distal RTA with metabolic acidosis and hypokalemia where treatment with amiloride helped correct the electrolyte abnormalities suggesting an important role for enhanced CNT and/or CCD K+ secretion (205). Inhibition of basolateral Kir4.1/5.1 K+ channel heterotetramers by a decrease in intracellular pH would be predicted to impair NCC activity in DCT1 and DCT2 with a concomitant increase in ENaC mediated Na+ transport in the DCT2-CNT-CCD segments (206,207). Of note, protons per se can stimulate ENaC activity by decreasing Na+ self-inhibition (208). The stimulation of ENaC activity would lead to enhanced K+ secretion. In mice with loss of the B1 subunit, it has been proposed that dysfunction of the Type B IC rather than the Type A IC plays a key role via a paracrine ATP/PGE2 signaling mechanism in enhancing K+ secretion and excretion through flow-dependent BKCa channels and ENaC-driven ROMK channels (209). These findings would not explain the hypokalemia found in patients with Type A IC AE1 mutations (138).
Hypercalciuria, Calcium Phosphate Stones and Nephrocalcinosis
Lemann et al (210) and Sutton et al (211) first demonstrated that the hypercalciuria observed in acute metabolic acidosis is mediated predominantly by a change in renal tubular transport of calcium independent of parathyroid hormone. Rodriguez-Soriano showed that the urine calcium excretion rate varies inversely with the plasma HCO3− concentration in patients with Type I RTA (212). Studies by Hoenderop et al have documented the importance of the TRPV5 calcium channel in the DCT2-CNT portion of the distal tubule in mediating the calciuric response to metabolic acidosis (213). Intracellular and extracellular acidification decrease the single channel conductance and open probability of TRPV5 channels (214,215). Moreover, the plasma membrane expression of the channel is decreased following a decrease in extracellular pH (216,217). In mice with loss of TRPV5, baseline calcium excretion is significantly increased and the calciuric response to metabolic acidosis induced by acetazolamide or NH4Cl loading is entirely blunted (213). The presence and magnitude of hypercalciuria in patients with nephrocalcinosis with H+-ATPase B1 and a4 subunit mutations may be dependent on the amount of dietary sodium intake (120,218,219). The low sodium content of formula could potentially account for the absence of hypercalciuria in some infants.
In murine animal models Ca2+ has been shown to impair collecting duct water absorption by decreasing membrane expression of AQP2 through CaSR signaling (220,221). Ca2+ has been reported to also stimulate Type A IC H+ secretion in the mouse through CaSR signaling (222). CaSR signaling appears to modulate Type B IC HCO3− secretion (223). Whether these factors reduce the risk of calcium stone formation in humans with hypercalciuria is controversial (224). TRPV5−/− mice have hypercalciuria and hyperphosphaturia yet don’t develop kidney stones whereas TRPV5−/− with the additional ablation of the collecting duct specific H+-ATPase B1 subunit develop severe medullary calcium phosphate precipitation despite polyuria (222). These finding suggest that patients with Type I RTA lacking a normal H+ secretory response are by inference more at risk for renal calculi formation and nephrocalcinosis. Type I RTA, unlike Type II RTA patients, have hypocitraturia and relatively alkaline urine in the steady state which favors calcium phosphate stone formation whereas Type IV RTA patients are thought to be protected against stone formation because of decreased calcium and uric acid excretion (225,226). In treating hypercalciuria in patients with Type I RTA we currently lack compounds that can modulate the function and membrane expression of TRPV5 channels directly. In order to normalize both urine citrate and calcium excretion patients are typically treated with K+ citrate (1–4 meq/kg/day) until the plasma bicarbonate increases to ~ 22 meq/l. A dose of 4 meq/kg/day in children was shown to be sufficient for normalizing the urine calcium excretion, citrate excretion, and elevated urinary calcium oxalate saturation but not the urinary calcium phosphate saturation (227). Despite concurrent reductions in urinary calcium and phosphate excretion and increased urinary citrate excretion, these changes could not counterbalance the effect of elevated urinary pH resulting from citrate administration. If during the course of therapy the urine pH increases > 6.5, thiazides can be started. If treated at a young age, nephrocalcinosis can respond to therapy (218) and is histologically due to plugging of inner medullary collecting ducts IMCD and Bellini ducts with deposits of calcium phosphate (apatite) coupled with epithelial cell injury/loss (228).
Urinary Citrate Excretion in Type I and Type II RTA
In patients with Type I RTA, decreased citrate excretion is thought to play a role in nephrocalcinosis and Ca2+ stone formation (227). In proximal RTA, the rate of citrate excretion is less well documented and might be expected to differ depending on the effect on intracellular pH of apical versus basolateral transport defects11 (58). The proximal tubule plays a key role in absorbing the filtered citrate load reabsorption and in determining urinary citrate excretion (229–231). Apical citrate2− uptake coupled to 3 Na+ is mediated by NaDC-1 and metabolic acidosis increases apical NaDC-1 expression and stimulates luminal citrate uptake (60,232–234). Basolateral citrate3− uptake coupled to 3 Na+ is mediated by NaDC-3.
This process is also stimulated by metabolic acidosis. Patients with autosomal dominant proximal RTA had normal urine citrate excretion despite systemic acidemia (48). It was hypothesized that these patients had a higher proximal tubule intracellular pH than might otherwise be expected possibly because of a basolateral HCO3− transport defect (58). Interestingly mice with loss of NBCe1 have decreased NaDC-1 and NaDC-3 expression and low urinary citrate excretion (60) suggesting the presence of additional compensatory citrate transport process(es). Hering‐Smith et al. has characterized a Ca2+‐regulated apical transport process that is stimulated during metabolic acidosis and may play a role (235). In the steady state prior to treatment, the low urine pH in patients with proximal RTA likely plays an important role in preventing intratubular calcium phosphate precipitation, nephrocalcinosis, and Ca2+ stone formation.
CKD in Type I Renal Tubular Acidosis
Although Type I RTA is generally considered a benign disease with respect to a decline in GFR, there is increasing evidence that there is an increased frequency of CKD in Type I RTA patients (236). The specific mechanism(s) are currently unkown. Duration of disease, nephrocalcinosis, hypokalemia, and repeated episodes of volume depletion have been considered as causal factors. A previous study of renal cortical and medullary biopsies that included patients with idiopathic Type I RTA (transport defect undefined) demonstrated glomerular, tubular, and interstitial abnormalities, and intratubular calcium phosphate deposits suggesting that the development of CKD in these patients is multifactorial (237). Radiologically, medullary cysts have been reported in children with Type I RTA (131) and patients with B1 and a4 mutations can also have radiologic findings of medullary sponge kidney (238). Whether these abnormalities and/or the severity of nephrocalcinosis alter the progression of CKD in these patients is currently unknown.
Acknowledgement
Supported in part by funds from the NIH (R01-DK077162), the Allan Smidt Charitable Fund, and the Factor Family Foundation Chair in Nephrology
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
It should be noted that some authors only use the term Type IV RTA to refer to those diseases causing hyperkalemic distal RTA caused by abnormal collecting duct aldosterone signaling.
Another more less well known pathway whose relative importance is species dependent is the glutaminase II consisting of glutamine transaminase coupled to a ω-amidase which generates αketoglutarate and one NH4+ such that ratio of “new” bicarbonate (from α-ketoglutarate metabolism) to NH4+ is expected to be 2:1 (32).
The urinary excretion of a greater ratio of H2PO4− to HPO4 than is present in blood would tend to remove H+ from the systemic circulation if there was no input of H+. However, given that ~ 50% of total urine phosphate excretion is due to the addition of H2PO4− systemically (30), at a whole body level, this component of titratable acid excretion does not contribute in a net sense to new whole body bicarbonate addition.
In addition to HPO4 −, a component of TA (and therefore new HCO3 ) is also generated from protonation of creatinine (pKa ~5.0) in the collecting duct.
NBCe1-A likely transports CO32- rather than HCO3; the two ions are used interchangeably in the text.
An FEHCO3 greater than 20% cannot per se rule out a proximal tubule abnormality in combination with impaired thick ascending limb and/or collecting duct defects.
In acute (24-h) metabolic acidosis following NH4Cl loading in humans, glutamine extraction by the kidney is not increased and the increment in ammonia production is thought to reflect increased glycine and ornithine uptake (36).
Studies of urinary exosomes in controls and patients with acquired Type I RTA and ATP6V1B1 subunit mutations showed no change in B2 subunit abundance following acute NH4Cl acid loading (127)
These findings are analogous to patients with defective free water excretion detected by a water load test, who are capable of handling their daily water intake and are therefore not dysnatremic under basal conditions.
Some patients with autosomal dominant mutations in AE1 causing mistargeting of mutated AE1 to the apical membrane have a greater than normal U-B pCO2 that has been proposed to be due to increased HCO3− secretion (195).
It would be predicted that in the generation phase of proximal RTA prior to reaching a steady state, impaired proximal tubule apical H+ secretion would lead to a decrease in intracellular pH whereas impaired basolateral HCO3− transport would increase intracellular pH. Given the effect of intracellular pH on citrate transport, citrate uptake would be theoretically enhanced in the former and decreased in the latter. However, luminal pH also modulates proximal tubule citrate uptake. Moreover, the development of systemic acidemia will tend to decrease intracellular pH. These factors make it difficult to assign predictable differences in urine citrate excretion to specific proximal tubule transport defects.
References
- 1.Lightwood R Calcium infarction of the kidneys in infants. Arch Did Child. 1935; 10:205. [Google Scholar]
- 2.Butler AM, Wilson JL, Farber S. Dehydration and acidosis with calcification at renal tubules. J Ped. 1936; 8(4):489–499. [Google Scholar]
- 3.Albright F, Burnett CH, et al. Osteomalacia and late rickets; the various etiologies met in the United States with emphasis on that resulting from a specific form of renal acidosis, the therapeutic indications for each etiological sub-group, and the relationship between osteomalacia and Milkman's syndrome. Medicine (Baltimore). 1946;25:399–479. [DOI] [PubMed] [Google Scholar]
- 4.Elkinton JR. Renal acidosis. Am J Med. 1960;28:165–168. [DOI] [PubMed] [Google Scholar]
- 5.Elkinton JR, Huth EJ, Webster GD Jr., Mc CR. The renal excretion of hydrogen ion in renal tubular acidosis. I. quantitative assessment of the response to ammonium chloride as an acid load. Am J Med. 1960;29:554–575. [DOI] [PubMed] [Google Scholar]
- 6.Elkinton JR. The kidney and hydrogen ion metabolism. Bibl Paediatr. 1960;74:99–123. [PubMed] [Google Scholar]
- 7.Stapleton T Idiopathic renal acidosis in an infant with excessive loss of bicarbonate in the urine. Lancet. 1949;1:683–685. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez Soriano J, Boichis H, Stark H, Edelmann CM Jr., Proximal renal tubular acidosis. A defect in bicarbonate reabsorption with normal urinary acidification. Pediatr Res. 1967;1:81–98. [DOI] [PubMed] [Google Scholar]
- 9.Soriano JR, Boichis H, Edelmann CM Jr., Bicarbonate reabsorption and hydrogen ion excretion in children with renal tubular acidosis. J of Peds. 1967;71:802–813. [DOI] [PubMed] [Google Scholar]
- 10.Morris RC Jr., Renal tubular acidosis. Mechanisms, classification and implications. N Engl J Med. 1969;281:1405–1413. [DOI] [PubMed] [Google Scholar]
- 11.McSherry E, Sebastian A, Morris RC Jr., Renal tubular acidosis in infants: the several kinds, including bicarbonate-wasting, classic renal tubular acidosis. J Clin Invest. 1972;51:499–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perez GO, Oster JR, Vaamonde CA. Renal acidosis and renal potassium handling in selective hypoaldosteronism. Am J Med. 1974;57:809–816. [DOI] [PubMed] [Google Scholar]
- 13.Sebastian A, Schambelan M, Lindenfeld S, Morris RC Jr. Amelioration of metabolic acidosis with fludrocortisone therapy in hyporeninemic hypoaldosteronism. N Engl J Med. 1977;297:576–583. [DOI] [PubMed] [Google Scholar]
- 14.Batlle DC, Arruda JAL, Kurtzman NA. Hyperkalemic distal renal tubular acidosis associated with obstructive uropathy. N Engl J Med. 1981; 304:373–380. [DOI] [PubMed] [Google Scholar]
- 15.Arruda JA, Batlle DC, Sehy JT, Roseman MK, Baronowski RL, Kurtzman NA. Hyperkalemia and renal insufficiency: role of selective aldosterone deficiency and tubular unresponsiveness to aldosterone. Am J Nephrol. 1981;1(3–4):160–167. [DOI] [PubMed] [Google Scholar]
- 16.Batlle DC. Hyperkalemic hyperchloremic metabolic acidosis associated with selective aldosterone deficiency and distal renal tubular acidosis. Seminars in Nephrology, 1981; 1(3), 260–274. [Google Scholar]
- 17.Karet FE. Mechanisms in hyperkalemic renal tubular acidosis. JASN. 2009;20:251–254. [DOI] [PubMed] [Google Scholar]
- 18.Harris AN, Grimm PR, Lee HW, et al. Mechanism of hyperkalemia-induced metabolic acidosis. JASN. 2018. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertram JF, Douglas-Denton RN, Diouf B, Hughson MD, Hoy WE. Human nephron number: implications for health and disease. Pediatr Nephrol. 2011;26:1529–1533. [DOI] [PubMed] [Google Scholar]
- 20.Kurtz I Molecular mechanisms and regulation of urinary acidification. Compr Physiol. 2014;4:17371774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarker R, Cha B, Kovbasnjuk O, et al. Phosphorylation of NHE3-S(719) regulates NHE3 activity through the formation of multiple signaling complexes. Mol Biol Cell. 2017;28:1754–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cotter K, Stransky L, McGuire C, Forgac M. Recent Insights into the Structure, Regulation, and Function of the V-ATPases. Trends Biochem Sci. 2015;40:611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo YM, Liu Y, Liu M, et al. Na+/HCO3- cotransporter NBCn2 mediates HCO3- reclamation in the apical membrane of renal proximal tubules. JASN. 2017;28:2409–2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu XL, Sly WS. Carbonic anhydrase IV from human lung. Purification, characterization, and comparison with membrane carbonic anhydrase from human kidney. J Biol Chem. 1990;265:8795–8801. [PubMed] [Google Scholar]
- 25.Hurst TK, Wang D, Thompson RB, Fierke CA. Carbonic anhydrase II-based metal ion sensing:Advances and new perspectives. BBA. 2010;1804:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huynh KW, Jiang J, Abuladze N, et al. CryoEM structure of the human SLC4A4 sodium-coupled acid-base transporter NBCe1. Nat Commun. 2018;9:900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warth R, Barriere H, Meneton P, et al. Proximal renal tubular acidosis in TASK2 K+ channel-deficient mice reveals a mechanism for stabilizing bicarbonate transport. PNAS. 2004;101:8215–8220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sebastian A, Frassetto LA, Sellmeyer DE, Merriam RL, Morris RC Jr., Estimation of the net acid load of the diet of ancestral preagricultural Homo sapiens and their hominid ancestors. Am J Clin Nutr. 2002;76:1308–1316. [DOI] [PubMed] [Google Scholar]
- 29.Remer T, Manz F. Potential renal acid load of foods and its influence on urine pH. J Am Diet Assoc. 1995;95:791–797. [DOI] [PubMed] [Google Scholar]
- 30.Halperin ML, Jungas RL. Metabolic production and renal disposal of hydrogen ions. Kid Int. 1983;24:709–713. [DOI] [PubMed] [Google Scholar]
- 31.Weiner ID, Verlander JW. Ammonia transporters and their role in acid-base balance. Physiol Rev. 2017;97:465–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cooper AJ, Kuhara T. Alpha-ketoglutaramate: an overlooked metabolite of glutamine and a biomarker for hepatic encephalopathy and inborn errors of the urea cycle. Metab Brain Dis. 2014;29:9911006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Atkinson DE, Bourke E. The role of ureagenesis in pH homeostasis. Trends Biochem Sci. 1984;9:297–300. [Google Scholar]
- 34.Halperin ML, Cheemadhadli S, Chen CB, West ML, Jungas RL. Is urea formation regulated primarily by acid-base-balance. Kid Int. 1986;29:367–367. [DOI] [PubMed] [Google Scholar]
- 35.Nash TP, Benedict SR. The ammonia content of the blood, and its bearing on the mechanism of acid neutralization in the animal organism. JBC. 1921;48:463–488. [Google Scholar]
- 36.Tizianello A, Deferrari G, Garibotto G, Robaudo C, Acquarone N, Ghiggeri GM. Renal ammoniagenesis in an early stage of metabolic acidosis in man. J Clin Invest. 1982;69:240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garibotto G, Sofia A, Robaudo C, et al. Kidney protein dynamics and ammoniagenesis in humans with chronic metabolic acidosis. JASN. 2004;15:1606–1615. [DOI] [PubMed] [Google Scholar]
- 38.Owen EE, Robinson RR. Amino acid extraction and ammonia metabolism by the human kidney during the prolonged administration of ammonium chloride. J Clin Invest.1963;42:263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Igarashi T, Inatomi J, Sekine T, et al. Mutations in SLC4A4 cause permanent isolated proximal renal tubular acidosis with ocular abnormalities. Nat Gen. 1999;23:264–266. [DOI] [PubMed] [Google Scholar]
- 40.Kurtz I NBCe1 as a model carrier for understanding the structure-function properties of Na+ coupled SLC4 transporters in health and disease. Pflugers Archiv 2014;466:1501–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu Q, Shao XM, Kao L, et al. Missense mutation T485S alters NBCe1-A electrogenicity causing proximal renal tubular acidosis. Amer J Physiol Cell Physiol. 2013;305:C392–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sly WS, Hewett-Emmett D, Whyte MP, Yu YS, Tashian RE. Carbonic anhydrase II deficiency identified as the primary defect in the autosomal recessive syndrome of osteopetrosis with renal tubular acidosis and cerebral calcification. PNAS. 1983;80:2752–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bok D, Schibler MJ, Pushkin A, et al. Immunolocalization of electrogenic sodium-bicarbonate cotransporters pNBC1 and kNBC1 in the rat eye. Amer J Physiol Ren Physiol. 2001;281:F920–935. [DOI] [PubMed] [Google Scholar]
- 44.Lacruz RS, Nanci A, White SN, et al. The sodium bicarbonate cotransporter (NBCe1) is essential for normal development of mouse dentition. J Biol Chem. 2010;285:24432–24438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gawenis LR, Bradford EM, Prasad V, et al. Colonic anion secretory defects and metabolic acidosis in mice lacking the NBC1 Na+/HCO3- cotransporter. J Biol Chem. 2007;282:9042–9052. [DOI] [PubMed] [Google Scholar]
- 46.Brenes LG, Brenes JN, Hernandez MM. Familial proximal renal tubular acidosis. A distinct clinical entity. Am J Med. 1977;63:244–252. [DOI] [PubMed] [Google Scholar]
- 47.Brenes LG, Sanchez MI. Impaired urinary ammonium excretion in patients with isolated proximal renal tubular acidosis. JASN. 1993;4:1073–1078. [DOI] [PubMed] [Google Scholar]
- 48.Lemann J Jr., Adams ND, Wilz DR, Brenes LG. Acid and mineral balances and bone in familial proximal renal tubular acidosis.Kid Int. 2000;58:1267–1277. [DOI] [PubMed] [Google Scholar]
- 49.Katzir Z, Dinour D, Reznik-Wolf H, Nissenkorn A, Holtzman E. Familial pure proximal renal tubular acidosis--a clinical and genetic study. Nephrol Dial Transplant. 2008;23:1211–1215. [DOI] [PubMed] [Google Scholar]
- 50.Guibaud P, Larbre F, Freycon MT, Genoud J. [Osteopetrosis and renal tubular acidosis. 2 cases of this association in a sibship]. Arch Fr Pediatr. 1972;29:269–286. [PubMed] [Google Scholar]
- 51.Vainsel M, Fondu P, Cadranel S, Rocmans C, Gepts W. Osteopetrosis associated with proximal and distal tubular acidosis. Acta Paediatr Scand. 1972;61:429–434. [DOI] [PubMed] [Google Scholar]
- 52.Rebello G, Ramesar R, Vorster A, et al. Apoptosis-inducing signal sequence mutation in carbonic anhydrase IV identified in patients with the RP17 form of retinitis pigmentosa. PNAS. 2004;101:66176622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li HC, Du Z, Barone S, et al. Proximal tubule specific knockout of the Na+/H+ exchanger NHE3: effects on bicarbonate absorption and ammonium excretion. J Mol Med (Berl). 2013;91:951–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cuevas CA, Su XT, Wang MX, et al. Potassium sensing by renal distal tubules requires Kir4.1. JASN. 2017;28:1814–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paulais M, Bloch-Faure M, Picard N, et al. Renal phenotype in mice lacking the Kir5.1 (Kcnj16) K+ channel subunit contrasts with that observed in SeSAME/EAST syndrome. PNAS. 2011;108:1036110366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Handlogten ME, Osis G, Lee HW, Romero MF, Verlander JW, Weiner ID. NBCe1 expression is required for normal renal ammonia metabolism. Amer J Physiol Ren Physiol. 2015;309:F658–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee HW, Osis G, Harris AN, et al. NBCe1-A regulates proximal tubule ammonia metabolism under basal conditions and in response to metabolic acidosis. JASN. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Halperin ML, Kamel KS, Ethier JH, Magner PO. What is the underlying defect in patients with isolated, proximal renal tubular acidosis? Amer J Nephrol. 1989;9:265–268. [DOI] [PubMed] [Google Scholar]
- 59.Nissim I Newer aspects of glutamine/glutamate metabolism: the role of acute pH changes. The American journal of physiology. 1999;277:F493–497. [DOI] [PubMed] [Google Scholar]
- 60.Osis G, Handlogten ME, Lee HW, et al. Effect of NBCe1 deletion on renal citrate and 2-oxoglutarate handling. Physiol Rep. 2016;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brown JC, Packer RK, Knepper MA. Role of organic anions in renal response to dietary acid and base loads.Amer J Physiol. 1989;257:F170–176. [DOI] [PubMed] [Google Scholar]
- 62.Capasso G, Unwin R, Rizzo M, Pica A, Giebisch G. Bicarbonate transport along the loop of Henle: molecular mechanisms and regulation. J Nephrol. 2002;15 Suppl 5:S88–96 [PubMed] [Google Scholar]
- 63.Amemiya M, Loffing J, Lotscher M, Kaissling B, Alpern RJ, Moe OW. Expression of NHE-3 in the apical membrane of rat renal proximal tubule and thick ascending limb. Kid Intl. 1995;48:1206–1215 [DOI] [PubMed] [Google Scholar]
- 64.Brown D, Hirsch S, Gluck S. Localization of a proton-pumping ATPase in rat kidney. J Clin Invest. 1988;82:2114–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang T, Hropot M, Aronson PS, Giebisch G. Role of NHE isoforms in mediating bicarbonate reabsorption along the nephron. Amer J Physiol Ren Physiol. 2001;281:F1117–1122. [DOI] [PubMed] [Google Scholar]
- 66.Watts BA, 3rd, Good DW. An apical K+-dependent HCO3- transport pathway opposes transepithelial HCO3- absorption in rat medullary thick ascending limb. Amer J Physiol Ren Physiol.. 2004;287:F57–63. [DOI] [PubMed] [Google Scholar]
- 67.Younes-Ibrahim M, Barlet-Bas C, Buffin-Meyer B, Cheval L, Rajerison R, Doucet A. Ouabain-sensitive and -insensitive K-ATPases in rat nephron: effect of K depletion.Amer J Physiol. 1995;268:F1141–1147. [DOI] [PubMed] [Google Scholar]
- 68.Purkerson JM, Schwartz GJ. The role of carbonic anhydrases in renal physiology. Kid. Int 2007;71:103–115. [DOI] [PubMed] [Google Scholar]
- 69.Frische S, Zolotarev AS, Kim YH, et al. AE2 isoforms in rat kidney: immunohistochemical localization and regulation in response to chronic NH4Cl loading. Amer J Physiol Ren Physiol. 2004;286:F1163–1170. [DOI] [PubMed] [Google Scholar]
- 70.Vorum H, Kwon TH, Fulton C, et al. Immunolocalization of electroneutral Na-HCO3- cotransporter in rat kidney. Amer J Physiol Ren Physiol. 2000;279:F901–909. [DOI] [PubMed] [Google Scholar]
- 71.Blanchard A, leviel F, Bichara M, Podevin RA, Paillard M. Interactions of external and internal K+ with K+-HCO3- cotransporter of rat medullary thick ascending limb. Amer J Physiol.. 1996;271:C218–225. [DOI] [PubMed] [Google Scholar]
- 72.Fahlke C, Fischer M. Physiology and pathophysiology of ClC-K/barttin channels. Front Physiol. 2010;1:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dimke H, Schnermann J. Axial and cellular heterogeneity in electrolyte transport pathways along the thick ascending limb. Acta Physiol (Oxf). 2018. [DOI] [PubMed] [Google Scholar]
- 74.Bourgeois S, Meer LV, Wootla B, et al. NHE4 is critical for the renal handling of ammonia in rodents. J Clin Invest. 2010;120:1895–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stettner P, Bourgeois S, Marsching C, et al. Sulfatides are required for renal adaptation to chronic metabolic acidosis. PNAS. 2013;110:9998–10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kunau RT Jr., Walker KA. Total CO2 absorption in the distal tubule of the rat. Amer J Physiol. 1987;252:F468–473. [DOI] [PubMed] [Google Scholar]
- 77.Wang T, Malnic G, Giebisch G, Chan YL. Renal bicarbonate reabsorption in the rat. IV. Bicarbonate transport mechanisms in the early and late distal tubule. J Clin Invest. 1993;91:2776–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wesson DE. Na/H exchange and H-K ATPase increase distal tubule acidification in chronic alkalosis. Kid Int. 1998;53:945–951. [DOI] [PubMed] [Google Scholar]
- 79.Fejes-Toth G, Naray-Fejes-Toth A. Immunohistochemical localization of colonic H-K-ATPase to the apical membrane of connecting tubule cells. Amer J Physiol Ren Physiol. 2001;281:F318–325. [DOI] [PubMed] [Google Scholar]
- 80.Verlander JW, Moudy RM, Campbell WG, Cain BD, Wingo CS. Immunohistochemical localization of H-K-ATPase α2c-subunit in rabbit kidney. Amer J Physiol Ren Physiol. 2001;281:F357–365. [DOI] [PubMed] [Google Scholar]
- 81.Kim J, Kim YH, Cha JH, Tisher CC, Madsen KM. Intercalated cell subtypes in connecting tubule and cortical collecting duct of rat and mouse. JASN. 1999;10:1–12. [DOI] [PubMed] [Google Scholar]
- 82.Madsen KM, Verlander JW, Kim J, Tisher CC. Morphological adaptation of the collecting duct to acid-base disturbances. Kidney Int Suppl. 1991;33:S57–63. [PubMed] [Google Scholar]
- 83.Kim YH, Kwon TH, Frische S, et al. Immunocytochemical localization of pendrin in intercalated cell subtypes in rat and mouse kidney. Amer J Physiol Ren Physiol. 2002;283:F744–754. [DOI] [PubMed] [Google Scholar]
- 84.Teng-umnuay P, Verlander JW, Yuan W, Tisher CC, Madsen KM. Identification of distinct subpopulations of intercalated cells in the mouse collecting duct. JASN. 1996;7:260–274. [DOI] [PubMed] [Google Scholar]
- 85.Edwards A, Crambert G. Versatility of NaCl transport mechanisms in the cortical collecting duct. Amer J Physiol Ren Physiol. 2017;313:F1254–F1263. [DOI] [PubMed] [Google Scholar]
- 86.Leviel F, Hübner CA, Houillier P, Morla L, El Moghrabi S, Brideau G, Hassan H, Parker MD, Kurth I,Kougioumtzes A, Sinning A, Pech V, Riemondy KA, Miller RL, Hummler E, Shull GE, Aronson PS, Doucet A, Wall SM, Chambrey R, Eladari D. The Na+-dependent chloride-bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. J Clin Invest. 2010; 120(5):1627–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Alper SL, Natale J, Gluck S, Lodish HF, Brown D. Subtypes of intercalated cells in rat kidney collecting duct defined by antibodies against erythroid band 3 and renal vacuolar H+-ATPase. PNAS. 1989;86:5429–5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Emmons C, Kurtz I. Functional characterization of three intercalated cell subtypes in the rabbit outer cortical collecting duct. J Clin Invest. 1994;93:417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Weiner ID, Weill AE, New AR. Distribution of Cl−/HCO3- exchange and intercalated cells in rabbit cortical collecting duct. Amer J Physiol. 1994;267:F952–964. [DOI] [PubMed] [Google Scholar]
- 90.Xu J, Song P, Nakamura S, et al. Deletion of the chloride transporter slc26a7 causes distal renal tubular acidosis and impairs gastric acid secretion. J Biol Chem. 2009;284:29470–29479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zaika O, Tomilin V, Mamenko M, Bhalla V, Pochynyuk O. New perspective of ClC-Kb/2 Cl− channel physiology in the distal renal tubule. Amer J Physiol Ren Physiol. 2016;310:F923–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lombard WE, Kokko JP, Jacobson HR. Bicarbonate transport in cortical and outer medullary collecting tubules. Amer J Physiol. 1983;244:F289–296. [DOI] [PubMed] [Google Scholar]
- 93.Koeppen BM. Conductive properties of the rabbit outer medullary collecting duct: outer stripe. Amer J Physiol. 1986;250:F70–76. [DOI] [PubMed] [Google Scholar]
- 94.Schuster VL, Fejes-Toth G, Naray-Fejes-Toth A, Gluck S. Colocalization of H+-ATPase and band 3 anion exchanger in rabbit collecting duct intercalated cells. Amer J Physiol. 1991;260:F506–517. [DOI] [PubMed] [Google Scholar]
- 95.Petrovic S, Barone S, Xu J, et al. SLC26A7: a basolateral Cl−/HCO3- exchanger specific to intercalated cells of the outer medullary collecting duct. Amer J Physiol Ren Physiol. 2004;286:F161169. [DOI] [PubMed] [Google Scholar]
- 96.Wingo CS, Madsen KM, Smolka A, Tisher CC. H-K-ATPase immunoreactivity in cortical and outer medullary collecting duct. Kid. Int. 1990;38:985–990. [DOI] [PubMed] [Google Scholar]
- 97.Armitage FE, Wingo CS. Luminal acidification in K-replete OMCDi: contributions of H-K-ATPase and bafilomycin-A1-sensitive H-ATPase. Amer J Physiol. 1994;267:F450–458. [DOI] [PubMed] [Google Scholar]
- 98.Koeppen BM. Conductive properties of the rabbit outer medullary collecting duct: inner stripe. Amer J Physiol. 1985;248:F500–506. [DOI] [PubMed] [Google Scholar]
- 99.Verlander JW, Madsen KM, Tisher CC. Structural and functional features of proton and bicarbonate transport in the rat collecting duct. Sem Nephrol. 1991;11:465–477. [PubMed] [Google Scholar]
- 100.Ridderstrale Y, Kashgarian M, Koeppen B, et al. Morphological heterogeneity of the rabbit collecting duct. Kid Int.. 1988;34:655–670. [DOI] [PubMed] [Google Scholar]
- 101.Clapp WL, Madsen KM, Verlander JW, Tisher CC. Morphologic heterogeneity along the rat inner medullary collecting duct. Lab Invest. 1989;60:219–230. [PubMed] [Google Scholar]
- 102.Madsen KM, Clapp WL, Verlander JW. Structure and function of the inner medullary collecting duct. Kid Int. 1988;34:441–454. [DOI] [PubMed] [Google Scholar]
- 103.Stuart-Tilley AK, Shmukler BE, Brown D, Alper SL. Immunolocalization and tissue-specific splicing of AE2 anion exchanger in mouse kidney. JASN. 1998;9:946–959. [DOI] [PubMed] [Google Scholar]
- 104.Hering-Smith KS, Cragoe EJ Jr., Weiner D, Hamm LL. Inner medullary collecting duct Na+-H+ exchanger. Amer J Physiol. 1991;260:C1300–1307. [DOI] [PubMed] [Google Scholar]
- 105.Stanton BA. Characterization of apical and basolateral membrane conductances of rat inner medullary collecting duct. Amer J Physiol Ren Physiol. 1989;256:F862–868. [DOI] [PubMed] [Google Scholar]
- 106.Madison LL, Seldin DW. Ammonia excretion and renal enzymatic adaptation in human subjects, as disclosed by administration of precursor amino acids. J Clin Invest. 1958;37:1615–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sartorius OW, Roemmelt JC, Pitts RF. The renal regulation of acid-base balance in man; the nature of the renal compensations in ammonium chloride acidosis. J Clin Invest. 1949;28:423–439. [PubMed] [Google Scholar]
- 108.Nissim I, Cattano C, Lin Z, Nissim Ic. Acid-base regulation of hepatic glutamine metabolism and ureagenesis: study with 15N. JASN. 1993;3:1416–1427. [DOI] [PubMed] [Google Scholar]
- 109.Good DW. Adaptation of HCO-3 and NH+4 transport in rat MTAL: effects of chronic metabolic acidosis and Na+ intake. Amer J Physiol. 1990;258:F1345–1353. [DOI] [PubMed] [Google Scholar]
- 110.Han KH, Lee HW, Handlogten ME, et al. Expression of the ammonia transporter family member, Rh B Glycoprotein, in the human kidney. Amer J Physiol Ren Physiol. 2013;304:F972–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Verlander JW, Miller RT, Frank AE, Royaux IE, Kim YH, Weiner ID. Localization of the ammonium transporter proteins RhBG and RhCG in mouse kidney. Amer J Physiol Ren Physiol. 2003;284:F323337. [DOI] [PubMed] [Google Scholar]
- 112.Han KH, Croker BP, Clapp WL, et al. Expression of the ammonia transporter, rh C glycoprotein, in normal and neoplastic human kidney. JASN. 2006;17:2670–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kim HY, Verlander JW, Bishop JM, et al. Basolateral expression of the ammonia transporter family member Rh C glycoprotein in the mouse kidney. Amer J Physiol Ren Physiol. 2009;296:F543–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Seshadri RM, Klein JD, Kozlowski S, et al. Renal expression of the ammonia transporters, Rhbg and Rhcg, in response to chronic metabolic acidosis. Amer J Physiol Ren Physiol. 2006;290:F397–408. [DOI] [PubMed] [Google Scholar]
- 115.Seshadri RM, Klein JD, Smith T, et al. Changes in subcellular distribution of the ammonia transporter, Rhcg, in response to chronic metabolic acidosis. Amer J Physiol Ren Physiol. 2006;290:F1443–1452. [DOI] [PubMed] [Google Scholar]
- 116.Lee HW, Verlander JW, Handlogten ME, Han KH, Weiner ID. Effect of collecting duct-specific deletion of both Rh B Glycoprotein (Rhbg) and Rh C Glycoprotein (Rhcg) on renal response to metabolic acidosis. Amer J Physiol Ren Physiol. 2014;306:F389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Breton S, Brown D. Regulation of luminal acidification by the V-ATPase. Physiol. 2013;28:318–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Forgac M Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol. 2007;8:917–929. [DOI] [PubMed] [Google Scholar]
- 119.Karet FE, Finberg KE, Nelson RD, et al. Mutations in the gene encoding B1 subunit of cause renal tubular acidosis with sensorineural deafness. Nat Gen.. 1999;21:84–90. [DOI] [PubMed] [Google Scholar]
- 120.Smith AN, Skaug J, Choate KA, et al. Mutations in ATP6N1B, encoding a new kidney vacuolar proton pump 116-kD subunit, cause recessive distal renal tubular acidosis with preserved hearing. Nat Gen. 2000;26:71–75. [DOI] [PubMed] [Google Scholar]
- 121.Stover EH, Borthwick KJ, Bavalia C, et al. Novel ATP6V1B1 and ATP6V0A4 mutations in autosomal recessive distal renal tubular acidosis with new evidence for hearing loss. J Med Genet. 2002;39:796803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mohebbi N, Vargas-Poussou R, Hegemann SC, et al. Homozygous and compound heterozygous mutations in the ATP6V1B1 gene in patients with renal tubular acidosis and sensorineural hearing loss. Clin Genet. 2013;83:274–278. [DOI] [PubMed] [Google Scholar]
- 123.Hennings JC, Picard N, Huebner AK, et al. A mouse model for distal renal tubular acidosis reveals a previously unrecognized role of the V-ATPase a4 subunit in the proximal tubule. EMBO Mol Med. 2012;4:1057–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Norgett EE, Golder ZJ, Lorente-Canovas B, Ingham N, Steel KP, Karet Frankl FE. Atp6v0a4 knockout mouse is a model of distal renal tubular acidosis with hearing loss, with additional extrarenal phenotype. PNAS. 2012;109:13775–13780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Finberg KE, Wagner CA, Bailey MA, et al. The B1-subunit of the H+ ATPase is required for maximal urinary acidification. PNAS. 2005;102:13616–13621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Paunescu TG, Russo LM, Da Silva N, et al. Compensatory membrane expression of the V-ATPase B2 subunit isoform in renal medullary intercalated cells of B1-deficient mice. Amer J Physiol Ren Physiol. 2007;293:F1915–1926. [DOI] [PubMed] [Google Scholar]
- 127.Pathare G, Dhayat NA, Mohebbi N, Wagner CA, Bobulescu IA, Moe OW, Fuster DG. Changes in V-ATPase subunits of human urinary exosomes reflect the renal response to acute acid/alkali loading and the defects in distal renal tubular acidosis. 2018; 93(4):871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schulz N, Dave MH, Stehberger PA, Chau T, Wagner CA. Differential localization of vacuolar H+ATPases containing a1, a2, a3, or a4 (ATP6V0A1–4) subunit isoforms along the nephron. Cell Physiol Biochem. 2007;20:109–120. [DOI] [PubMed] [Google Scholar]
- 129.Watanabe T Proximal renal tubular dysfunction in primary distal renal tubular acidosis. Pediatr Nephrol. 2005;20:86–88. [DOI] [PubMed] [Google Scholar]
- 130.Tasic V, Korneti P, Gucev Z, Hoppe B, Blau N, Cheong HI. Atypical presentation of distal renal tubular acidosis in two siblings. Pediatr Nephrol. 2008;23:1177–1181. [DOI] [PubMed] [Google Scholar]
- 131.Besouw MTP, Bienias M, Walsh P, et al. Clinical and molecular aspects of distal renal tubular acidosis in children. Pediatr Nephrol. 2017;32:987–996. [DOI] [PubMed] [Google Scholar]
- 132.Bruce LJ, Cope DL, Jones GK, et al. Familial distal renal tubular acidosis is associated with mutations in the red cell anion exchanger (Band 3, AE1) gene. J Clin Invest. 1997;100:1693–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bruce LJ, Wrong O, Toye AM, et al. Band 3 mutations, renal tubular acidosis and South-East Asian ovalocytosis in Malaysia and Papua New Guinea: loss of up to 95% band 3 transport in red cells. Biochem J. 2000;350 Pt 1:41–51. [PMC free article] [PubMed] [Google Scholar]
- 134.Shao L, Xu Y, Dong Q, Lang Y, Yue S, Miao Z. A novel SLC4A1 variant in an autosomal dominant distal renal tubular acidosis family with a severe phenotype. Endocrine. 2010;37:473–478. [DOI] [PubMed] [Google Scholar]
- 135.Ito N, Ihara K, Kamoda T, et al. Autosomal dominant distal renal tubular acidosis caused by a mutation in the anion exchanger 1 gene in a Japanese family. CEN Case Rep. 2015;4:218–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Karet FE, Gainza FJ, Gyory AZ, et al. Mutations in the chloride-bicarbonate exchanger gene AE1 cause autosomal dominant but not autosomal recessive distal renal tubular acidosis. PNAS.1998;95:6337–6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tanphaichitr VS, Sumboonnanonda A, Ideguchi H, et al. Novel AE1 mutations in recessive distal renal tubular acidosis. Loss-of-function is rescued by glycophorin A. J Clin Invest. 1998;102:2173–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Khositseth S, Bruce LJ, Walsh SB, et al. Tropical distal renal tubular acidosis: clinical and epidemiological studies in 78 patients. QJM. 2012;105:861–877. [DOI] [PubMed] [Google Scholar]
- 139.McKinney TD, Davidson KK. Bicarbonate transport in collecting tubules from outer stripe of outer medulla of rabbit kidneys. Amer J Physiol. 1987;253:F816–822. [DOI] [PubMed] [Google Scholar]
- 140.Richardson RM, Kunau RT Jr., Bicarbonate reabsorption in the papillary collecting duct: effect of acetazolamide. Amer J Physiol. 1982;243:F74–80. [DOI] [PubMed] [Google Scholar]
- 141.Lonnerholm G, Wistrand PJ. Carbonic anhydrase in the human kidney: a histochemical and immunocytochemical study. Kid Int. 1984;25:886–898. [DOI] [PubMed] [Google Scholar]
- 142.Brown D, Zhu XL, Sly WS. Localization of membrane-associated carbonic anhydrase type IV in kidney epithelial cells. PNAS. 1990;87:7457–7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lonnerholm G, Wistrand PJ. Membrane-bound carbonic anhydrase CA IV in the human kidney. Acta Physiol Scand. 1991;141:231–234 [DOI] [PubMed] [Google Scholar]
- 144.Breton S, Alper SL, Gluck SL, Sly WS, Barker JE, Brown D. Depletion of intercalated cells from collecting ducts of carbonic anhydrase II-deficient (CAR2 null) mice. Amer J Physiol. 1995;269:F761–774. [DOI] [PubMed] [Google Scholar]
- 145.Borthwick KJ, Kandemir N, Topaloglu R, et al. A phenocopy of CAII deficiency: a novel genetic explanation for inherited infantile osteopetrosis with distal renal tubular acidosis. J Med Genet. 2003;40:115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vidarsson H, Westergren R, Heglind M, Blomqvist SR, Breton S, Enerback S. The forkhead transcription factor Foxi1 is a master regulator of vacuolar H+-ATPase proton pump subunits in the inner ear, kidney and epididymis. PloS One. 2009;4:e4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Blomqvist SR, Vidarsson H, Fitzgerald S, et al. Distal renal tubular acidosis in mice that lack the forkhead transcription factor Foxi1. J Clin Invest. 2004;113:1560–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Enerback S, Nilsson D, Edwards N, et al. Acidosis and deafness in patients with recessive mutations in FOXI1. JASN. 2018;29:1041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Boettger T, Hubner CA, Maier H, Rust MB, Beck FX, Jentsch TJ. Deafness and renal tubular acidosis in mice lacking the K-Cl co-transporter Kcc4. Nature. 2002;416:874–878. [DOI] [PubMed] [Google Scholar]
- 150.Gao X, Eladari D, Leviel F, et al. Deletion of hensin/DMBT1 blocks conversion of β- to α-intercalated cells and induces distal renal tubular acidosis.PNAS. 2010;107:21872–21877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sun X, Yang LV, Tiegs BC, et al. Deletion of the pH sensor GPR4 decreases renal acid excretion. JASN. 2010;21:1745–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sun X, Stephens L, DuBose TD Jr., Petrovic S. Adaptation by the collecting duct to an exogenous acid load is blunted by deletion of the proton-sensing receptor GPR4. Amer J Physiol Ren Physiol. 2015;309:F120–136. [DOI] [PubMed] [Google Scholar]
- 153.Groger N, Vitzthum H, Frohlich H, et al. Targeted mutation of SLC4A5 induces arterial hypertension and renal metabolic acidosis. Hum Mol Genet. 2012;21:1025–1036. [DOI] [PubMed] [Google Scholar]
- 154.Wen D, Yuan Y, Cornelius RJ, et al. Deficient acid handling with distal RTA in the NBCe2 knockout mouse. Amer J Physiol Ren Physiol. 2015;309:F523–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Trepiccione F, Gerber SD, Grahammer F, et al. Renal Atp6ap2/(Pro)renin Receptor Is Required for Normal Vacuolar H+-ATPase Function but Not for the Renin-Angiotensin System. JASN. 2016;27:33203330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Stehberger PA, Shmukler BE, Stuart-Tilley AK, Peters LL, Alper SL, Wagner CA. Distal renal tubular acidosis in mice lacking the AE1 (band3) Cl−/HCO3- exchanger (slc4a1). JASN. 2007;18:1408–1418. [DOI] [PubMed] [Google Scholar]
- 157.Mumtaz R, Trepiccione F, Hennings JC, et al. Intercalated cell depletion and vacuolar H+-ATPase mistargeting in an Ae1 R607H Knockin Model. JASN. 2017;28:1507–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bergeron MJ, Gagnon E, Wallendorff B, Lapointe JY, Isenring P. Ammonium transport and pH regulation by K+-Cl− cotransporters. Amer J Physiol Ren Physiol. 2003;285:F68–78. [DOI] [PubMed] [Google Scholar]
- 159.Wrong O, Davies HE. The excretion of acid in renal disease. Q J Med. 1959;28:259–313. [PubMed] [Google Scholar]
- 160.Buckalew VM, Jr., McCurdy DK, Ludwig GD, Chaykin LB, Elkinton JR. Incomplete renal tubular acidosis. Physiologic studies in three patients with a defect in lowering urine pH. Amer J Med. 1968;45:32–42. [DOI] [PubMed] [Google Scholar]
- 161.Tannen RL, Falls WF Jr., Brackett NC Jr., Incomplete renal tubular acidosis: some clinical and physiological features. Nephron. 1975;15:111–123. [DOI] [PubMed] [Google Scholar]
- 162.Weger M, Deutschmann H, Weger W, Kotanko P, Skrabal F. Incomplete renal tubular acidosis in 'primary' osteoporosis. Osteopor Int. 1999;10:325–329. [DOI] [PubMed] [Google Scholar]
- 163.Choi JS, Kim CS, Park JW, Bae EH, Ma SK, Kim SW. Incomplete distal renal tubular acidosis with nephrocalcinosis. Chonnam Med J. 2011;47:170–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Weger W, Kotanko P, Weger M, Deutschmann H, Skrabal F. Prevalence and characterization of renal tubular acidosis in patients with osteopenia and osteoporosis and in non-porotic controls. NDT. 2000;15:975–980. [DOI] [PubMed] [Google Scholar]
- 165.Sharma AP, Sharma RK, Kapoor R, Kornecki A, Sural S, Filler G. Incomplete distal renal tubular acidosis affects growth in children. NDT. 2007;22:2879–2885. [DOI] [PubMed] [Google Scholar]
- 166.Forni Ogna V, Blanchard A, Vargas-Poussou R, et al. Signification of distal urinary acidification defects in hypocitraturic patients. PloS One. 2017;12:e0177329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhang J, Fuster DG, Cameron MA, et al. Incomplete distal renal tubular acidosis from a heterozygous mutation of the V-ATPase B1 subunit.Amer J Physiol Ren Physiol. 2014;307:F1063–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dhayat NA, Schaller A, Albano G, et al. The Vacuolar H+-ATPase B1 subunit polymorphism p.E161K associates with impaired urinary acidification in recurrent stone formers. JASN. 2016;27:1544–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Imai E, Kaneko S, Mori T, Okado T, Uchida S, Tsukamoto Y. A novel heterozygous mutation in the ATP6V0A4 gene encoding the V-ATPase a4 subunit in an adult patient with incomplete distal renal tubular acidosis. Clin Kid J. 2016;9:424–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kurtz I, Dass PD, Cramer S. The importance of renal ammonia metabolism to whole body acid-base balance: a reanalysis of the pathophysiology of renal tubular acidosis. Miner Electrolyte Metab. 1990;16(5):331–340. [PubMed] [Google Scholar]
- 171.Tizianello A, Deferrari G, Garibotto G, et al. Renal ammoniagenesis in man with acute metabolic alkalosis. Contr Neph. 1988;63:105–113. [DOI] [PubMed] [Google Scholar]
- 172.Owen EE, Tyor MP, Flanagan JF, Berry JN. The kidney as a source of blood ammonia in patients with liver disease: the effect of acetazolamide. J Clin Invest. 1960;39:288–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tizianello A, Garibotto G, Robaudo C, et al. Renal ammoniagenesis in humans with chronic potassium depletion. Kid Int. 1991;40:772–778. [DOI] [PubMed] [Google Scholar]
- 174.Goldstein MB, Bear R, Richardson RM, Marsden PA, Halperin ML. The urine anion gap: a clinically useful index of ammonium excretion. Am J Med Sci. 1986; 292(4):198–202. [DOI] [PubMed] [Google Scholar]
- 175.Halperin ML, Margolis BL, Robinson LA, Halperin RM, West ML, Bear RA. The urine osmolal gap: a clue to estimate urine ammonium in "hybrid" types of metabolic acidosis. Clin Invest Med. 1988; 11(3):198–202. [PubMed] [Google Scholar]
- 176.Dyck RF, Asthana S, Kalra J, West ML, Massey KL. A modification of the urine osmolal gap: an improved method for estimating urine ammonium. Am J Neph. 1990;10:359–362. [DOI] [PubMed] [Google Scholar]
- 177.Inase N, Ozawa K, Sasaki S, Marumo F. Is the urine anion gap a reliable index of urine ammonium excretion in most situations? Nephron. 1990;54:180–181. [DOI] [PubMed] [Google Scholar]
- 178.Kim GH, Han JS, Kim YS, Joo KW, Kim S, Lee JS. Evaluation of urine acidification by urine anion gap and urine osmolal gap in chronic metabolic acidosis.Amer J Kid Dis. 1996;27:42–47. [DOI] [PubMed] [Google Scholar]
- 179.Kirschbaum B, Sica D, Anderson FP. Urine electrolytes and the urine anion and osmolar gaps.J Lab Clin Med. 1999;133:597–604. [DOI] [PubMed] [Google Scholar]
- 180.Kim S, Lee JW, Park J, et al. The urine-blood PCO2 gradient as a diagnostic index of H+-ATPase defect distal renal tubular acidosis. Kid Int. 2004;66:761–767. [DOI] [PubMed] [Google Scholar]
- 181.Batlle D, Ba Aqeel SH, Marquez A. The urine anion gap in context. Clin J Am Soc Nephrol. 2018; 13(2):195–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ha LY, Chiu WW, Davidson JS. Direct urine ammonium measurement: time to discard urine anion and osmolar gaps. Ann Clin Biochem. 2012;49:606–608. [DOI] [PubMed] [Google Scholar]
- 183.Raphael KL, Gilligan S, Ix JH. Urine anion gap to predict urine ammonium and related outcomes in kidney disease. Clin J Am Soc Nephrol. 2018; 13(2):205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Vasuvattakul S, Gougoux A, Halperin ML. A method to evaluate renal ammoniagenesis in vivo.Clin Invest Med. 1993;16:265–273. [PubMed] [Google Scholar]
- 185.Miller SG, Schwartz GJ. Hyperammonaemia with distal renal tubular acidosis. Arch Did Child. 1997;77:441–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Seracini D, Poggi GM, Pela I. Hyperammonaemia in a child with distal renal tubular acidosis. Ped Neph. 2005;20:1645–1647. [DOI] [PubMed] [Google Scholar]
- 187.Pela I, Seracini D. Hyperammonemia in distal renal tubular acidosis: is it more common than we think? Clin Nephrol. 2007;68:109–114. [DOI] [PubMed] [Google Scholar]
- 188.Saito T, Hayashi D, Shibata S, Jogamoto M, Kamoda T. Novel compound heterozygous ATP6V0A4 mutations in an infant with distal renal tubular acidosis. Eur J Ped. 2010;169:1271–1273. [DOI] [PubMed] [Google Scholar]
- 189.Ripoli C, Pinna A, Marras S, Fenu ML, Nurchi AM. A distal renal tubular acidosis showing hyperammonemia and hyperlactacidemia. La Pediatr Med e Chirur: Med and Surg Ped. 2012;34:198201. [DOI] [PubMed] [Google Scholar]
- 190.Vasuvattakul S, Nimmannit S, Shayakul C, Vareesangthip K, Halperin ML. Should the urine PCO2 or the rate of excretion of ammonium be the gold standard to diagnose distal renal tubular acidosis? Amer J Kid Dis. 1992;19:72–75. [DOI] [PubMed] [Google Scholar]
- 191.Halperin ML, Goldstein MB, Haig A, Johnson MD, Stinebaugh BJ. Studies on the pathogenesis of type I (distal) renal tubular acidosis as revealed by the urinary PCO2 tensions. 1974; J Clin Invest. 53:669–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Batlle D, Grupp M, Gaviria M, Kurtzman NA. Distal renal tubular acidosis with intact capacity to lower urinary pH. 1982; Am J Med. 72:751–758. [DOI] [PubMed] [Google Scholar]
- 193.Du Bose TD Jr, Pucacco LR, Green JM. Hydrogen ion secretion by the collecting duct as a determinant of the urine to blood PCO2 gradient in alkaline urine. 1982; J Clin Invest. 69: 145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Du Bose TD Jr, Caflisch CR. Validation of the differences in urine and blood carbon dioxide tension during bicarbonate loading as an index of distal nephron acidification in experimental models of distal renal tubular acidosis. 1985; J Clin Invest. 75:1116–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Vasuvattakul S Molecular approach for distal renal tubular acidosis associated AE1 mutations. E & BP. 2010;8:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Walsh SB, Shirley DG, Wrong OM, Unwin RJ. Urinary acidification assessed by simultaneous furosemide and fludrocortisone treatment: an alternative to ammonium chloride. Kid Int. 2007;71:13101316. [DOI] [PubMed] [Google Scholar]
- 197.Kovacikova J, Winter C, Loffing-Cueni D, et al. The connecting tubule is the main site of the furosemide-induced urinary acidification by the vacuolar H+-ATPase. Kid Int. 2006;70:1706–1716. [DOI] [PubMed] [Google Scholar]
- 198.de Bruijn PI, Larsen CK, Frische S, et al. Furosemide-induced urinary acidification is caused by pronounced H+ secretion in the thick ascending limb. Amer J Physiol Ren Physiol. 2015;309:F146–153. [DOI] [PubMed] [Google Scholar]
- 199.Batlle DC. Segmental characterization of defects in collecting tubule acidification. Kid Int. 1986; 30(4):546–554. [DOI] [PubMed] [Google Scholar]
- 200.Bech AP, Wetzels JFM, Nijenhuis T. Use of the furosemide fludrocortisone test to clinically assess distal tubular acidification. Amer J Kid Dis. 2017;70:589–591. [DOI] [PubMed] [Google Scholar]
- 201.Koeppen BM. Electrophysiological identification of principal and intercalated cells in the rabbit outer medullary collecting duct. Pflugers Archiv. 1987;409:138–141. [DOI] [PubMed] [Google Scholar]
- 202.Gill JR Jr., Bell NH, Bartter FC. Impaired conservation of sodium and potassium in renal tubular acidosis and its correction by buffer anions. Clin Sci. 1967;33:577–592. [PubMed] [Google Scholar]
- 203.Sebastian A, McSherry E, Morris RC Jr., Renal potassium wasting in renal tubular acidosis (RTA): its occurrence in types 1 and 2 RTA despite sustained correction of systemic acidosis. J Clin Invest. 1971;50:667–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Dafnis E, Spohn M, Lonis B, Kurtzman NA, Sabatini S. Vanadate causes hypokalemic distal renal tubular acidosis. Amer J Med. 1992;262:F449–453. [DOI] [PubMed] [Google Scholar]
- 205.Oguejiofor P, Chow R, Yim K, Jaar BG. Successful management of refractory type 1 renal tubular acidosis with amiloride. Case Rep Nephrol. 2017;2017:8596169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Tanemoto M, Kittaka N, Inanobe A, Kurachi Y. In vivo formation of a proton-sensitive K+ channel by heteromeric subunit assembly of Kir5.1 with Kir4.1. J Physiol. 2000;525 Pt 3:587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Pessia M, Imbrici P, D'Adamo MC, Salvatore L, Tucker SJ. Differential pH sensitivity of Kir4.1 and Kir4.2 potassium channels and their modulation by heteropolymerisation with Kir5.1. J Physiol. 2001;532:359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Collier DM, Snyder PM. Extracellular protons regulate human ENaC by modulating Na+ selfinhibition. J Biol Chem. 2009;284:792–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Gueutin V, Vallet M, Jayat M, Peti-Peterdi J, Cornière N, Leviel F, Sohet F, Wagner CA, Eladari D, Chambrey R. Renal β-intercalated cells maintain body fluid and electrolyte balance. 2013: J Clin Invest. 123(10):4219–4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lemann J, Litzow JR, Lennon EJ. Studies of the mechanism by which chronic metabolic acidosis augments urinary calcium excretion in man. J Clin Invest. 1967;46:1318–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Sutton RA, Wong NL, Dirks JH. Effects of metabolic acidosis and alkalosis on sodium and calcium transport in the dog kidney. Kid Int. 1979;15:520–533. [DOI] [PubMed] [Google Scholar]
- 212.Rodriguez-Soriano J, Vallo A, Castillo G, Oliveros R. Natural history of primary distal renal tubular acidosis treated since infancy. J Ped. 1982;101:669–676. [DOI] [PubMed] [Google Scholar]
- 213.Hoenderop JG, van Leeuwen JP, van der Eerden BC, et al. Renal Ca2+ wasting, hyperabsorption, and reduced bone thickness in mice lacking TRPV5. J Clin Invest. 2003;112:1906–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Yeh BI, Sun TJ, Lee JZ, Chen HH, Huang CL. Mechanism and molecular determinant for regulation of rabbit transient receptor potential type 5 (TRPV5) channel by extracellular pH. J Biol Chem. 2003;278:51044–51052. [DOI] [PubMed] [Google Scholar]
- 215.Cha SK, Jabbar W, Xie J, Huang CL. Regulation of TRPV5 single-channel activity by intracellular pH. J Mem Biol. 2007;220:79–85. [DOI] [PubMed] [Google Scholar]
- 216.Nijenhuis T, Renkema KY, Hoenderop JG, Bindels RJ. Acid-base status determines the renal expression of Ca2+ and Mg2+ transport proteins. JASN. 2006;17:617–626. [DOI] [PubMed] [Google Scholar]
- 217.Lambers TT, Oancea E, de Groot T, Topala CN, Hoenderop JG, Bindels RJ. Extracellular pH dynamically controls cell surface delivery of functional TRPV5 channels. Mol Cell Biol. 2007;27:14861494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Tsai HY, Lin SH, Lin CC, Huang FY, Lee MD, Tsai JD. Why is hypercalciuria absent at diagnosis in some children with ATP6V1B1 mutation? Pediatr Nephrol. 2011;26:1903–1907. [DOI] [PubMed] [Google Scholar]
- 219.Gil H, Santos F, Garcia E, et al. Distal RTA with nerve deafness: clinical spectrum and mutational analysis in five children. Pediatr Nephrol. 2007;22:825–828. [DOI] [PubMed] [Google Scholar]
- 220.Procino G, Mastrofrancesco L, Tamma G, et al. Calcium-sensing receptor and aquaporin 2 interplay in hypercalciuria-associated renal concentrating defect in humans. An in vivo and in vitro study. PloS One. 2012;7:e33145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Sands JM, Flores FX, Kato A, et al. Vasopressin-elicited water and urea permeabilities are altered in IMCD in hypercalcemic rats. Amer J Physiol. 1998;274:F978–985. [DOI] [PubMed] [Google Scholar]
- 222.Renkema KY, Velic A, Dijkman HB, et al. The calcium-sensing receptor promotes urinary acidification to prevent nephrolithiasis. JASN. 2009;20:1705–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Yasuoka Y, Sato Y, Healy JM, Nonoguchi H, Kawahara K. pH-sensitive expression of calcium-sensing receptor (CaSR) in type-B intercalated cells of the cortical collecting ducts (CCD) in mouse kidney.Clin Exp Neph. 2015;19:771–782. [DOI] [PubMed] [Google Scholar]
- 224.Bergsland KJ, Coe FL, Gillen DL, Worcester EM. A test of the hypothesis that the collecting duct calcium-sensing receptor limits rise of urine calcium molarity in hypercalciuric calcium kidney stone formers. Amer J Physiol Ren Physiol. 2009;297:F1017–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Hamm LL, Hering-Smith KS. Pathophysiology of hypocitraturic nephrolithiasis. Endocrinol Metab Clin North Am. 2002;31:885–893, viii. [DOI] [PubMed] [Google Scholar]
- 226.Uribarri J, Oh MS, Pak CY. Renal stone risk factors in patients with type IV renal tubular acidosis. Am J Kidney Dis. 1994;23:784–787. [DOI] [PubMed] [Google Scholar]
- 227.Domrongkitchaiporn S, Khositseth S, Stitchantrakul W, Tapaneya-olarn W, Radinahamed P. Dosage of potassium citrate in the correction of urinary abnormalities in pediatric distal renal tubular acidosis patients. Am J Kidney Dis. 2002;39:383–391. [DOI] [PubMed] [Google Scholar]
- 228.Evan AP, Lingeman J, Coe F, et al. Renal histopathology of stone-forming patients with distal renal tubular acidosis. Kid Int. 2007;71:795–801. [DOI] [PubMed] [Google Scholar]
- 229.Pajor AM. Citrate transport by the kidney and intestine. Sem Nephrol. 1999;19:195–200. [PubMed] [Google Scholar]
- 230.He Y, Chen X, Yu Z, et al. Sodium dicarboxylate cotransporter-1 expression in renal tissues and its role in rat experimental nephrolithiasis. J Neph. 2004;17:34–42. [PubMed] [Google Scholar]
- 231.Unwin RJ, Capasso G, Shirley DG. An overview of divalent cation and citrate handling by the kidney. Neph Physiol. 2004;98:p15–20. [DOI] [PubMed] [Google Scholar]
- 232.Simpson DP. Citrate excretion: a window on renal metabolism. Amer J Physiol. 1983;244:F223–234. [DOI] [PubMed] [Google Scholar]
- 233.Hamm LL. Renal handling of citrate. Kid Int. 1990;38:728–735. [DOI] [PubMed] [Google Scholar]
- 234.Hamm LL, Simon EE. Roles and mechanisms of urinary buffer excretion. Amer J Physiol. 1987;253:F595–605. [DOI] [PubMed] [Google Scholar]
- 235.Hering-Smith KS, Mao W, Schiro FR, Coleman-Barnett J, Pajor AM, Hamm LL. Localization of the calcium-regulated citrate transport process in proximal tubule cells. Urolith. 2014;42:209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Palazzo V, Provenzano A, Becherucci F, et al. The genetic and clinical spectrum of a large cohort of patients with distal renal tubular acidosis. Kid Int. 2017;91:1243–1255. [DOI] [PubMed] [Google Scholar]
- 237.Evan AP, Lingeman JE, Coe FL, et al. Crystal-associated nephropathy in patients with brushite nephrolithiasis. Kid Int. 2005;67:576–591. [DOI] [PubMed] [Google Scholar]
- 238.Carboni I, Andreucci E, Caruso MR, et al. Medullary sponge kidney associated with primary distal renal tubular acidosis and mutations of the H+-ATPase genes. NDT. 2009;24:2734–2738. [DOI] [PubMed] [Google Scholar]