Abstract
The urinary bladder stores urine until the time of urination. Systemic administration of drugs to treat bladder diseases faces several limitations. Therefore, intravesical drug delivery is a promising alternative route of administration. An in-situ gel is used to form a gel inside the bladder cavity and ensure continuous release of the drug even after urination. The objective of the present study was to optimize an in-situ gel formulation of poloxamer and chitosan for intravesical delivery of ketorolac tromethamine. The gelling temperature of the prepared combinations ranged from 20.67 to 25.8 °C. In-vitro release of KT was sustained for up to 7 h using a poloxamer concentration ranging from 17% to 19% and a chitosan concentration ranging from 1% to 2%. Design-Expert® 10 was used to select the optimized formulation (poloxamer/chitosan 17/1.589% w/w) which significantly (p < 0.05) extended the drug release more than each polymer alone. An ex-vivo study showed the ability of the optimized formulation to sustain drug release after emptying two times to mimic urination. Furthermore, the formed gel adhered to the bladder tissue throughout the time period of the experiment. Intravesical administration of the optimized formulation to rabbits via catheter showed no obstruction of urine flow and continuous release of the drug for 12 h.
Keywords: Intravesical, In-situ gel, Ketorolac, Poloxamer 407, Chitosan
1. Introduction
The urinary bladder has an important role storing urine formed by the kidney and preventing systemic reabsorption of urine components from the bladder cavity until urination (Lewis, 2000). Different diseases such as interstitial cystitis (Davis et al., 2014), overactive bladder syndrome (Geoffrion, 2012), urinary tract infection (Zacche et al., 2015), and bladder cancer (Kamat et al., 2017) affect the bladder’s normal function. Patients with these diseases have symptoms that affect the bladder and cause discomfort such as urinary storage problems and pain. Treatment of urinary bladder diseases with systemic drug administration suffers from several limitations such as poor bioavailability and first pass metabolism leading to a low drug concentration in bladder tissue and the subsequent need for high drug doses which may increase side effects (Tyagi et al., 2006).
Intravesical drug delivery systems (IDDS) can be delivered via urethral catheter as an alternative route of drug administration. Intravesical administration of drugs leads to high drug concentrations in bladder tissue which increases the efficacy of treatment (GuhaSarkar and Banerjee, 2010). However, the drug will be washed out within 2 h after intravesical administration by urination (Lin et al., 2016). Repeated administration of the drug via frequent catheterization increases risk of infection and causes patient discomfort. Therefore, developing an IDDS that can be retained in the bladder cavity even after urination is important to ensure continuous release of the drug (Tyagi et al., 2016).
One of the recent IDDS approaches is using an in-situ gel to increase drug residence time after intravesical administration (Tyagi et al., 2016). A vital advantage of the in-situ gel is that it possesses low viscosity during storage and forms a gel only after intravesical administration. These systems are designed to form the gel in response to different stimuli such as a change in temperature, pH, and ion concentration (Nagarwal and Pandit, 2008).
Among various in-situ gel systems, thermosensitive polymers have been widely used. Thermosensitive polymers, such as poloxamers, are available as a solution before and during administration and form a gel only in response to body temperature. Poloxamers are composed of poly(ethylene oxide)-block-poly(propylene oxide)-block-poly(ethylene oxide) copolymers (Klouda and Mikos, 2008). Using poloxamer 407 in the formulation of in-situ gels extended the release of different intravesical drugs compared with drugs administered without poloxamer 407 (Tyagi et al., 2004). In a previous study, the poloxamer-based formulation for intravesical administration had an in vitro release time of up to 3.5 h for adriamycin (Lin et al., 2016). In another study, it was found that poloxamer alone suffered from rapid erosion. Upon addition of HPMC, the release time of adriamycin was extended up to 10 h (Lin et al., 2014). Previous studies support the need of adjacent polymers to be added with poloxamer to form a firmer gel after intravesical administration.
Another IDDS approach is the use of mucoadhesive polymers. In a previous study, an in-situ gel formulation containing gellan as a mucoadhesive polymer for intravesical administration was developed. Gellan was able to adhere to the bladder wall after administration and was superior to drug solutions in increasing the concentration of the drug in bladder tissues (GuhaSarkar et al., 2017).
Therefore, using a combination of poloxamer and mucoadhesive polymers would has the potential to solve the aforementioned limitation of poloxamer. In the current study, chitosan was utilized as a mucoadhesive polymer because it is biocompatible and biodegradable. Chitosan has been widely used in ophthalmic (Gratieri et al., 2010) and nasal (Ravi et al., 2015) in-situ gel formulations. The proposed combination of poloxamer and chitosan should avoid rapid erosion of poloxamer with minimal risk of urinary obstruction.
Ketorolac tromethamine (KT), a nonsteroidal anti-inflammatory drug, has been used to study intravesical therapy (Williams et al., 2014). For patients undergoing urethral stenting, a study showed that pain reduction due to ketorolac was superior to lidocaine and oxybutynin (Beiko et al., 2004).
In the present study, poloxamer 407 and chitosan were used in an in-situ gel formulation containing KT for intravesical administration. This combination has never been used in in-situ gel formulations for bladder diseases. Gelling temperature and in vitro drug release were studied to select the optimized formulation using Design-Expert® 10. In addition, ex-vivo and in vivo release studies were performed to evaluate the optimized formulation.
2. Materials and methods
2.1. Materials
KT was kindly supplied by Amriya Pharmaceutical Industries (Alexandria, Egypt). Poloxamer 407 was obtained from Spectrum (Germany) and chitosan high viscosity grade (M.W. 600,000) was obtained from Winlab (England). All other chemicals were pharmaceutical grade. Freshly isolated bladder tissue of slaughtered sheep was used in the ex-vivo study.
2.2. Methods
2.2.1. Experimental design and selection of optimized formulation
Randomized full 32 factorial experimental design (Design-Expert® 10, Stat-Ease Inc., Minneapolis, MN, USA) was used to characterize the relationship between poloxamer and chitosan concentration and measured in-situ gel properties by polynomial fitting and analysis of variance (ANOVA). Poloxamer concentration (X1) and chitosan concentration (X2) were the two independent variables selected to study their impact on the attributes of the prepared in-situ gels containing KT. Appropriate models were selected by comparing p values and coefficient of determination (R2) values. The matrix of 32 full factorial design for preliminary study of KT in-situ gel formulations is shown in Table 1. Response surface methodology (RSM) was used to investigate the effect of the independent variables (X1 and X2) on a range of dependent variables, including gelling temperature Y1 and percent of drug release at 2 h (D2 %), 4 h (D4 %), and 7 h (D7 %) designated as Y2, Y3, and Y4, respectively. Response surfaces were constructed using the obtained equations and used as an aid in the selection of the optimized formulation (Fopt) based on gelling temperature and percent of drug release.
Table 1.
32 full factorial design for poloxamer/chitosan combinations.
| Chitosan (X2) |
||||
|---|---|---|---|---|
| 1% | 1.5% | 2% | ||
| Poloxamer (X1) | 17% | F 1 | F 2 | F3 |
| 18% | F 4 | F 5 | F 6 | |
| 19% | F 7 | F 8 | F 9 | |
2.2.2. Preparation of in-situ gels
All formulations were prepared based on w/w % calculation. Poloxamer solutions were prepared by dissolving the weighed amount of poloxamer in water and kept in a refrigerator overnight. In-situ gel formulations were prepared by dissolving chitosan in 0.5% v/v acetic acid, then adding the calculated amount of KT (2.5 mg/g of the prepared formulation). Finally, poloxamer was added, and the preparation was kept in a refrigerator until poloxamer completely dissolved.
2.2.3. Determination of effect of different polymer concentration on viscosity
The viscosity of different concentration of poloxamer (17, 18, and 19% w/w) and chitosan (1.0, 1.5, and 2.0% w/w) was measured using a viscometer (SV-10 Vibro Viscometer, Company Limited, Japan) at room temperature of 22 ± 0.5 °C. Before using the viscometer, calibration was done with purified water according to the Japan Calibration Service System (JCSS). All measurements were repeated three times.
2.2.4. Determination of gelling temperature
The gelling temperature of the prepared in-situ gel formulations was determined using a Brookfield viscometer (RVDV-II+; Brookfield, Engineering Laboratories Inc., Stoughton, MA, USA) with small sample adaptor equipped with a water jacket through which water from a Brookfield temperature controller water bath (TC-202) could be circulated. Fifteen mL from each formulation was gradually heated. The gelling temperature was determined from the sharp inflection point in the viscosity versus temperature curve which results from the sudden increase of viscosity accompanying gel formation (Lin et al., 2014).
2.2.5. Assay of drug content
Directly after gel preparation, half gram of formulation was dissolved in 50 mL distilled water in a volumetric flask. Then, 1 mL of dissolved formulation was transferred to 25 mL volumetric flask and further diluted with distilled water. The drug concentration was measured using the developed ultra high-performance liquid chromatography (UHPLC®) method.
2.2.6. In-vitro drug release
Two grams of each formulation was placed on grooved circular disc (5 cm diameter). Each disc was placed in the bottom of dissolution apparatus vessels (USP-II; Caleva, England). In each vessel, 400 mL of the simulated urine fluid (SUF, composed of NaCl 13.75, MgSo4 1.69, MgCl2 0.83, CaCl2 0.67, KCl 0.38, and urea 17.40 gm/L, pH 7.50) (Stolarz et al., 2005) was used as the dissolution medium and maintained at 37 °C. A two mL sample was withdrawn at predetermined time intervals (0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 4.0, 5.0, 6.0, and 7.0 h) and was filtered using 10 µm filter tips (Logan Instruments Corp., USA) (Lin et al., 2014). The drug concentration was measured using the developed UHPLC® method.
2.2.7. Ex-vivo study
A modified method described by Lin et al. (2014) was used to study adhesion of the optimized formulation and simulating the physiological condition of urination. A grooved circular disc was covered with bladder tissue, then two grams of the formulation was placed on the disc. Each disc was placed in bottom of dissolution apparatus vessels (USP-II; Caleva, England). The dissolution experiment was started with 200 mL of SUF placed in the dissolution vessel (zero time), then SUF was added using a peristaltic pump (Watson-Marlow, USA) at a rate of 2 mL/min. Once the volume reached 400 mL (at 100 min), the vessels were completely emptied. Then, fresh SUF was added at the same rate till the volume reached 400 mL (at 300 min), then the vessels were completely emptied again. This procedure was repeated for a third time (at 500 min). During the experiment, a two mL sample was withdrawn at predetermined time intervals (25, 50, 75, 100, 125, 150, 175, 200, 225, 250, 275, 300, 350, 375, 400, 425, 450, 475, and 500 min) and was filtered using 10 µm filter tips (Logan Instruments Corp., USA). After dilution, the drug concentration was measured using the developed UHPLC® method.
2.2.8. In-vivo study
Nine male rabbits (2.5–3 kg) were selected. Six rabbits were maintained in a metabolic cage 22 ± 2 °C temperature and 50–70% RH with free access to fresh water and commercially available pellet feed during all experiments. The optimized formulation was stored in the refrigerator before injection into the bladder. The rabbits were restrained on a desk, and 1 g of optimized formulation was administered intravesically through an urethral catheter (CH 10, 40 cm). Urine samples were collected via the metabolic cage for 3 days before and after intravesical administration. The volume, pH, and amount of drug released in the rabbits’ urine were measured. The other three rabbits were sacrificed 15 min after intravesical administration of colored optimized formulation in order to visualize and photograph the formed gel inside the bladder.
2.2.9. Developed UHPLC method for determination of KT
Dionex™ ultra high-performance liquid chromatography (UHPLC) equipped with a Dionex™ automatic sample manager and diode array ultraviolet detector (Thermo Scientific, Bedford, MA, USA) was used for determination of KT during the in vitro, ex-vivo, and in vivo studies. KT was separated using an Acquity UHPLC® BEH C18 column (2.1 ∗ 50 mm, 1.7 μm; Waters Corp., Milford, MA, USA). The column temperature was maintained at 45 ± 0.5 °C. The method used an isocratic mobile phase comprised of a mixture of 0.05 M ammonium formate buffer (containing 0.1% trifluoroacetic acid): acetonitrile (68:32). The flow rate was maintained at 0.4 mL min−1, and the detection wavelength was 320 nm.
-
a)
For analysis of in vitro samples:
An autosampler was used to inject 1.0 μL sample, and the concentration of drug calculated based on the calibration curve between the concentration of KT and the measured UV absorbance. The obtained calibration curve was linear in the concentration range of 0.05–5.0 μg mL−1 with a R2 value of 0.9997.
-
b)
For analysis of ex-vivo and in vivo samples:
A 500 μL sample was spiked with 50 μL of apigenin (internal standard; 10.0 μg/mL dissolved in methanol), then 450 μL of acetonitrile was added. The sample was centrifuged for 5 min at 13,000 rpm, and 5 μL of supernatant was injected. The drug concentration was calculated based on the calibration curve plotted between the concentration of KT and the peak area ratio of KT to apigenin. An obtained calibration curve was linear in the concentration range of 0.1–5.0 μg mL−1 with a R2 value of 0.9994. The proposed UHPLC® method was validated in terms of “linearity, accuracy, precision, robustness, sensitivity, reproducibility and specificity.”
3. Results and discussion
3.1. In-situ gel content
In-situ gels containing 2.5 mg KT/gram were successfully prepared as described previously. The drug content of the prepared in-situ gels for each formula ranged from 94.45 to 102.7% of the claimed content.
3.2. Effect of polymers on the viscosity
The viscosity of 17, 18, and 19% w/w poloxamers was 57.8 ± 0.2, 107 ± 1.0, and 167 ± 0.0 cp, respectively. The viscosity of 1.0, 1.5, and 2.0% w/w chitosan was 254.7 ± 1.6, 642 ± 1.0, and 1150 ± 0.0 cp, respectively. It was found that increasing the concentration of the polymer resulted in a significant increase in viscosity. Chitosan had a more pronounced effect on the viscosity than poloxamer at the studied ranges.
3.3. Gelling temperature
Poloxamer in aqueous solution has a low viscosity at low temperature and converted to gel after an increase in temperature. An increase in poloxamer concentration (17, 18, 19, and 20% w/w) was found to lead to a decrease in the gelling temperature (25.33, 24, 22.33, and 20.33 °C, respectively). Poloxamer formulations with a concentration of less than or equal to 16% w/w displayed a gelling temperature of more than 40 °C. Table 2 shows the gelling temperature of different poloxamer/chitosan combinations (F1-F9). A linear model was suggested by Design-Expert® 10 for the gelling temperature of the combinations with a significant p value (Table 3). Eq. (1) represents the actual effect of each factor on the gelling temperature.
| (1) |
Table 2.
Gelling temperature of poloxamer/chitosan combinations.
| Chitosan concentration |
||||
|---|---|---|---|---|
| 1% | 1.5% | 2% | ||
| Poloxamer concentration | 17% | 25.8 ± 0.76 | 25.5 ± 0.66 | 24.9 ± 0.76 |
| 18% | 23.8 ± 0.76 | 23.3 ± 0.76 | 23.1 ± 0.63 | |
| 19% | 21.3 ± 0.76 | 21.2 ± 0.76 | 20.7 ± 0.3 | |
Table 3.
ANOVA analysis for the selected models for different responses of KT in-situ gel.
| Response | Source | Sum of squares | DF | Mean square | F-value | p-value |
|---|---|---|---|---|---|---|
| Y1 (gelling temperature) | Linear model | 28.97 | 2 | 14.49 | 641.21 | <0.0001 |
| Y2 (drug release at 2 h (D2 %)) | Quadratic model | 289.24 | 5 | 77.85 | 641.21 | <0.0001 |
| Y3 (drug release at 4 h (D4 %)) | Quadratic model | 846.41 | 5 | 169.28 | 498.17 | 0.0001 |
| Y4 (drug release at 7 h (D7 %)) | Quadratic model | 764.18 | 5 | 152.84 | 375.23 | 0.0002 |
Fig. 1(A) shows that increasing the concentration of either polymer resulted in a significant decrease in gelling temperature. In addition, Eq. (1) shows that the effect of poloxamer concentration on gelling temperature is more pronounced than the effect of chitosan concentration. This could be observed from coefficient factor 2.17 and 0.37 for poloxamer and chitosan, respectively. Eq. (2) can be used to predict the gelling temperature at any level of the two factors within the selected range.
| (2) |
Fig. 1.
Three dimensional surface plot represent (A) gelling temperature (B, C and D) percent of drug released at 2, 4 and 7 h respectively of poloxamer/chitosan combinations.
These findings are similar to what has been reported by Gratieri et al. (2010) who developed and optimized an in-situ gel formulation for ocular application. Gratieri et al. (2010) studied the gelling temperature of poloxamer formulation, and it was found that an increase in poloxamer concentration from 16 to 20% resulted in a decrease in gelling temperature. Moreover, the gelation of poloxamer after increasing the temperature is due to a decrease in the solubility of the polypropylene oxide block of poloxamer (Klouda and Mikos, 2008). Poloxamer alone suffers from rapid erosion and lacks bioadhesive properties so addition of other polymers will help in the formation of a firm gel. In the present study, a polymer with mucoadhesive property (chitosan) was used in combination with poloxamer to avoid urinary tract obstruction while enhancing the gel’s bioadhesive properties. In another study, Malli et al. (2017) developed an in-situ gel formulation for delivery of metronidazole inside the vagina. Increasing the concentration of chitosan from 0 to 1.0% resulted in a decrease in the gelling temperature from 21.9 to 21.1 °C. Hence, chitosan slightly affected the gelling temperature of poloxamer which is in agreement with our finding.
3.4. In-vitro drug release
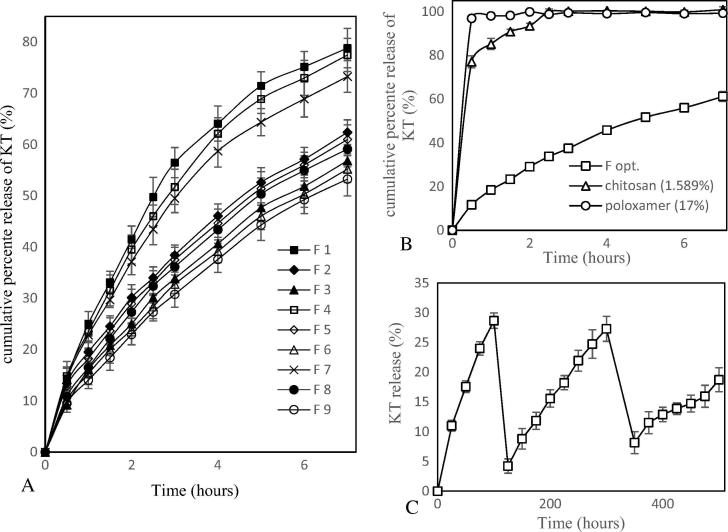
Design-Expert® 10 was used to determine the degree of effect of each polymer on the percent of drug release at different time intervals (2, 4, and 7 h). Table 3 shows the model suggested for each time interval and the degree of significance. Fig. 2(A) shows the in vitro release of KT from poloxamer/chitosan combinations (F1- F9). Formulations containing 1% chitosan suffered from more rapid erosion than formulations with 1.5 and 2% chitosan. Eqs. (3), (4), (5) represent the actual effect of each factor on the percent of drug release at different time intervals (2, 4, and 7 h, respectively).
| (3) |
| (4) |
| (5) |
Fig. 2.
(A) In-vitro release of KT from F1 - F9 in SUF at 37 °C, (B) In-vitro release of KT from Fopt, 17% poloxamer and 1.589% chitosan in SUF at 37 °C and (C) Ex-vivo release of KT from Fopt.
It is clear from Fig. 1 (B, C, and D) that both polymers decrease the percent of drug release. In addition, Eqs. (3), (4), (5) show that chitosan has a more pronounced effect on the percent of drug released than poloxamer concentration at selected time intervals. This could be observed from coefficient factor of poloxamer and chitosan. There is an interaction between the two factors on the percent of drug release. Eqs. (6), (7), (8) can be used to predict percent of drug release after 2, 4, and 7 h, respectively, at any level of the two factors within the selected range.
| (6) |
| (7) |
| (8) |
In agreement with our findings, Qian et al. (2014) developed an in-situ gel formulation for intranasal administration of tacrine which contained poloxamer and chitosan as the mucoadhesive polymer. The percent of drug released after 600 min from the formulation containing 20% poloxamer was 90%, while after addition of 0.5% chitosan, the percent of drug released was 80%. In another study, Zaki et al. (2007) developed an in-situ gel formulation for intranasal administration of metoclopramide using poloxamer and a mucoadhesive polymer. It was found that 18% poloxamer solution released 100% of the drug at 150 min while the formulation after the addition of 0.5% chitosan released 75% of the drug after 450 min.
3.5. Selection of optimized formulation
In order to optimize a formulation with better properties, Design-Expert® 10 was used to target a formulation with maximum gelling temperature and minimum drug release. The maximum gelling temperature ensures enough time to administer the formulation while minimum drug release ensures the release of drug for a longer period of time. The suggested formulation (Fopt; poloxamer/chitosan 17/1.589%) was prepared and evaluated for gelling temperature and percent of drug release. Table 4 shows the results of the observed values were close to the predicted values of responses and fall within 95% prediction intervals. Fig. 2(B) shows the in vitro release of Fopt and each polymer alone. Using combinations of both polymers significantly decreased drug release than use of each polymer alone.
Table 4.
Validation of the experimental design model using the formulation [poloxamer/chitosan (17/1.589%w/w)].
| Response | S.D. | n | Predicted mean | 95% PI low |
Actual data mean | 95% PI high |
|---|---|---|---|---|---|---|
| Gelling temp | 0.150308 | 3 | 25.3903 | 25.10 | 25.67 | 25.68 |
| D% 2hr | 0.154365 | 3 | 28.9227 | 28.46 | 29.04 | 29.38 |
| D% 4hr | 0.582929 | 3 | 44.5566 | 42.81 | 45.73 | 46.30 |
| D% 7hr | 0.638209 | 3 | 60.8655 | 58.96 | 61.15 | 62.77 |
3.6. Ex-vivo study from optimized formulation
This method was used to simulate physiological conditions to get a release pattern similar to what happens in vivo. This simulation was attained by using a disc covered with bladder tissue to observe adhesion. In addition, the vessel was periodically emptied then refilled with fresh SUF to simulate urination. Gel from optimized formulation still adhered to tissue until the end of the experiments. Fig. 3(C) shows the ability to sustain release of the drug from prepared formulations even after successive pouring. The amount of KT released from the optimized formulation reached 28.67% (at 100 min) before the first pouring. After the first pouring, the gel continued to release drug, and 27.3% of KT was released before the second pouring (at 300 min). Another 18.74% was released between the second pouring and the end of experiment (at 500 min).
Fig. 3.
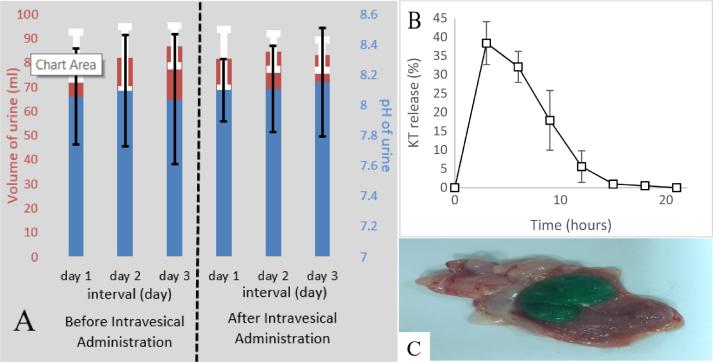
(A) Mean pH and volume of urine for rabbits before and after intravesical administration of Fopt, (B) KT released from in-situ gel after intravesical administration of Fopt (C) Photographs of formed gel inside bladder cavity after intravesical administration of Fopt.
3.7. In-vivo study
To evaluate obstruction capability, the volume of urine was measured for 3 days before and after intravesical administration of the optimized formulation. In addition, pH was measured to ensure gelation of chitosan after intravesical administration. The average volume of urine for the six rabbits was 83.83 ± 2.65 and 83.05 ± 1.4 mL before and after intravesical administration, respectively. The average pH was 8.06 ± 0.03 and 8.12 ± 0.028 before and after intravesical administration, respectively. All six rabbits had normal urine volume and pH before and after intravesical administration of the optimized formulation as shown in Fig. 3(A).
The rabbits’ urine was collected at different intervals, and the amount of drug released was measured using developed UHPLC® method. Fig. 3(B) shows the ability of the optimized formulation to extend the drug release for up to 12 h in the bladder cavity. The amount of drug released was found to be 38.37, 32.07, 17.87, and 5.06% after 3, 6, 9, and 12 h, respectively.
Furthermore, an additional three rabbits were sacrificed 15 min after intravesical administration of the optimized formulation. The gel was photographed to confirm in-situ formation. Fig. 3(C) shows the gel in the bladder cavity was firm and adhered to the bladder wall.
The normal flow of urine for 3 days after intravesical administration was not disturbed, indicating the success of this formulation in avoiding urinary bladder obstruction. The urine pH was not affected by intravesical administration of the optimized formulation. In addition, the pH was higher than 7.5 which ensured gelation of chitosan after intravesical administration of the optimized formulation. The optimized formulation was able to extend drug release for 12 h with frequent urination which ensured continuous release of the drug and avoided rapid elimination of the drug after urination. In addition, the photograph of the bladder cavity showed that the formed gel was firm enough to resist drastic conditions during urination and urine collection.
4. Conclusion
In this study, an optimized novel in-situ gel formulation for intravesical administration of KT was successfully developed. The in-situ gel consists of a thermosensitive polymer (poloxamer 407) which ensures the formation of gel in urinary bladder cavity. A mucoadhesive polymer (chitosan) was used in addition to poloxamer to avoid rapid erosion and ensure adhesion to the bladder wall to prevent urinary bladder obstruction. An ex-vivo study showed that the optimized formulation is able to adhere to the mucous membrane of the bladder tissue and can sustain drug release for up to 8 h. An in vivo study showed the formulation allowed a continuous flow of urine with no obstruction and sustained the release of drug for up to 12 h.
Acknowledgements
This project was financially supported by King Saud University, Vice Deanship of Research, Kayyali Chair for Pharmaceutical Industry through the grant number ABD-2017. The authors thank RSSU at King Saud University for their technical support.
Footnotes
Peer review under responsibility of King Saud University.
References
- Beiko D.T., Watterson J.D., Knudsen B.E., Nott L., Pautler S.E., Brock G.B., Razvi H., Denstedt J.D. Double-blind randomized controlled trial assessing the safety and efficacy of intravesical agents for ureteral stent symptoms after extracorporeal shockwave lithotripsy. J. Endourol. 2004;18:723–730. doi: 10.1089/end.2004.18.723. [DOI] [PubMed] [Google Scholar]
- Davis N.F., Brady C.M., Creagh T. Interstitial cystitis/painful bladder syndrome: epidemiology, pathophysiology and evidence-based treatment options. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014;175:30–37. doi: 10.1016/j.ejogrb.2013.12.041. [DOI] [PubMed] [Google Scholar]
- Geoffrion R. Treatments for overactive bladder: focus on pharmacotherapy. J. Obstet. Gynaecol. Can. 2012;34:1092–1101. doi: 10.1016/S1701-2163(16)35440-8. [DOI] [PubMed] [Google Scholar]
- Gratieri T., Gelfuso G.M., Rocha E.M., Sarmento V.H., De Freitas O., Lopez R.F. A poloxamer/chitosan in situ forming gel with prolonged retention time for ocular delivery. Eur. J. Pharm. Biopharm. 2010;75:186–193. doi: 10.1016/j.ejpb.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Guhasarkar S., Banerjee R. Intravesical drug delivery: challenges, current status, opportunities and novel strategies. J. Control Release. 2010;148:147–159. doi: 10.1016/j.jconrel.2010.08.031. [DOI] [PubMed] [Google Scholar]
- Guhasarkar S., More P., Banerjee R. Urothelium-adherent, ion-triggered liposome-in-gel system as a platform for intravesical drug delivery. J. Control Release. 2017;245:147–156. doi: 10.1016/j.jconrel.2016.11.031. [DOI] [PubMed] [Google Scholar]
- Kamat A.M., Bagcioglu M., Huri E. What is new in non-muscle-invasive bladder cancer in 2016? Turk. J. Urol. 2017;43:9–13. doi: 10.5152/tud.2017.60376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klouda L., Mikos A.G. Thermoresponsive hydrogels in biomedical applications. Eur. J. Pharm. Biopharm. 2008;68:34–45. doi: 10.1016/j.ejpb.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S.A. Everything you wanted to know about the bladder epithelium but were afraid to ask. Am. J. Physiol. Renal. Physiol. 2000;278:F867–F874. doi: 10.1152/ajprenal.2000.278.6.F867. [DOI] [PubMed] [Google Scholar]
- Lin T., Wu J., Zhao X., Lian H., Yuan A., Tang X., Zhao S., Guo H., Hu Y. In situ floating hydrogel for intravesical delivery of adriamycin without blocking urinary tract. J. Pharm. Sci. 2014;103:927–936. doi: 10.1002/jps.23854. [DOI] [PubMed] [Google Scholar]
- Lin T., Zhang Y., Wu J., Zhao X., Lian H., Wang W., Guo H., Hu Y. Re: A floating hydrogel system capable of generating CO2 bubbles to diminish urinary obstruction after intravesical instillation. J. Urol. 2016;195:525–526. doi: 10.1016/j.juro.2015.10.158. [DOI] [PubMed] [Google Scholar]
- Malli S., Bories C., Pradines B., Loiseau P.M., Ponchel G., Bouchemal K. In situ forming pluronic® F127/chitosan hydrogel limits metronidazole transmucosal absorption. Eur. J. Pharm. Biopharm. 2017;112:143–147. doi: 10.1016/j.ejpb.2016.11.024. [DOI] [PubMed] [Google Scholar]
- Nagarwal R.C., Pandit J.K. Phase transition system: novel oral in-situ gel. Curr. Drug Deliv. 2008;5:282–289. doi: 10.2174/156720108785914952. [DOI] [PubMed] [Google Scholar]
- Qian S., Wong Y.C., Zuo Z. Development, characterization and application of in situ gel systems for intranasal delivery of tacrine. Int. J. Pharm. 2014;468:272–282. doi: 10.1016/j.ijpharm.2014.04.015. [DOI] [PubMed] [Google Scholar]
- Ravi P.R., Aditya N., Patil S., Cherian L. Nasal in-situ gels for delivery of rasagiline mesylate: improvement in bioavailability and brain localization. Drug Deliv. 2015;22:903–910. doi: 10.3109/10717544.2013.860501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolarz A., Alonso A., De Bolle W., Kuhn H., Richter S., Quetel C., Ponzevera E., Verbruggen A., Wellum R. Joint Research Centre; Belgium: 2005. Preparation of Simulated Urine Samples Containing Certified Uranium for the NUSIMEP 4 Campaign; p. 6. [Google Scholar]
- Tyagi P., Kashyap M., Hensley H., Yoshimura N. Advances in intravesical therapy for urinary tract disorders. Expert Opin. Drug Deliv. 2016;13:71–84. doi: 10.1517/17425247.2016.1100166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi P., Li Z., Chancellor M., De Groat W.C., Yoshimura N., Huang L. Sustained intravesical drug delivery using thermosensitive hydrogel. Pharm. Res. 2004;21:832–837. doi: 10.1023/b:pham.0000026436.62869.9c. [DOI] [PubMed] [Google Scholar]
- Tyagi P., Tyagi S., Kaufman J., Huang L., De Miguel F. Local drug delivery to bladder using technology innovations. Urol. Clin. North Am. 2006;33:519–530. doi: 10.1016/j.ucl.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Williams N.A., Bowen J.L., Al-Jayyoussi G., Gumbleton M., Allender C.J., Li J., Harrah T., Raja A., Joshi H.B. An ex vivo investigation into the transurothelial permeability and bladder wall distribution of the nonsteroidal anti-inflammatory ketorolac. Mol. Pharm. 2014;11:673–682. doi: 10.1021/mp400274z. [DOI] [PubMed] [Google Scholar]
- Zacche M.M., Srikrishna S., Cardozo L. Novel targeted bladder drug-delivery systems: a review. Res. Rep. Urol. 2015;7:169–178. doi: 10.2147/RRU.S56168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki N.M., Awad G.A., Mortada N.D., Elhady S.S.A. Enhanced bioavailability of metoclopramide HCl by intranasal administration of a mucoadhesive in situ gel with modulated rheological and mucociliary transport properties. Eur. J. Pharm. Sci. 2007;32:296–307. doi: 10.1016/j.ejps.2007.08.006. [DOI] [PubMed] [Google Scholar]