Abstract
Background
Hyperkalemia is a commonly encountered medical problem. The treatment of hyperkalemia involves the use of pharmacological agents with different mechanism of actions. Sodium Polystyrene sulfonate (SPS) is a cation-exchange resin that exchanges sodium for potassium. In 2009, the United States Food and Drug Administration issued warning against the use of SPS with sorbitol due to risk of colonic necrosis. We present a case of SPS induced colonic necrosis in the absence of sorbitol and risk factors deemed to increase risk of colonic necrosis.
Case report
Here we report a 64-year old male with past medical history of kidney stones who was admitted for treatment of colitis which was complicated by septic shock requiring vasopressors. His course was further complicated by hyperkalemia attributed to acute kidney injury. One dose 30 gm of SPS was administered which normalized his serum potassium. The patient’s course was complicated by duodenal ulcer, and colonic perforation. The initial pathology findings of the resected specimen were suggestive of inflammatory bowel disease which resulted in starting patient on mesalamine. The patient then developed fistula which was resected and sent for pathology. SPS induced colonic necrosis was made based on the pathology findings.
Conclusion
SPS is commonly used to decrease potassium levels. SPS has been reported to be associated with several gastrointestinal complications. FDA issued warning against the use of SPS in patients at risk for complications. Here we report a case with SPS induced colonic necrosis in the absence of risk factors reported in the literature.
Keywords: Sodium polystyrene sulfonate, Intestinal necrosis, Hyperkalemia
1. Introduction
Sodium polystyrene sulfonate (SPS) was approved in 1958 and is considered “grandfathered drug”, which is a drug product that was on the market prior to the passage of the 1938 Food, Drug, and Cosmetic Act. This Act mandated a pre- market approval of all new drugs requiring manufacturers to prove to the FDA that their drug is safe (Administration, 2009). Prior to the passage of this act, drugs were not tested for safety and efficacy before they become available for consumption. SPS, a cation-exchange resin administered orally or via the rectum as an enema, exchanges sodium for potassium; however, it is not selective for sodium only and can also bind to calcium and magnesium (Drug, 2010). In 2009, the United States Food and Drug Administration (FDA) issued a warning against the concomitant use of sodium polystyrene sulfonate with sorbitol due to cases of colonic necrosis and other gastrointestinal adverse events (Administration, 2009). Patients at risk for such adverse events were identified as those with inadequate gastrointestinal function, a history of constipation, and a history of impaction. SPS with sorbitol appears to carry a higher risk of gastrointestinal complications compared to SPS without sorbitol as reported by Harel et al. (Harel et al., 2013). Nevertheless, several case reports reported the risk of intestinal obstruction and necrosis following the administration of SPS without sorbitol (Tongyoo et al., 2013, Kato et al., 2013, Goutorbe et al., 2011). Aside from intestinal necrosis, SPS can cause bezoar formation, however, there are few reports of intestinal obstruction due to SPS bezoar formation reported in the literature (Lai et al., 2013). Here we report a case of SPS without sorbitol induced intestinal necrosis secondary to SPS without sorbitol.
2. Case report
A 64 year old male with past medical history of genetic neutropenia, hypertension, and tongue cancer in remission presented to our Emergency Department (ED) with a history of lower abdominal pain of unknown duration. In the week prior to this admission, he experienced hematuria prompting an appointment with his urologist. At this outpatient appointment, a CT scan showed right renal stone and the urologist scheduled an elective lithotripsy. However, before the scheduled lithotripsy session he presented to our ED with chills, fever, and colic pain. Upon his presentation, he complained of nausea and vomiting. His vitals and labs on admission were as follows: Maximum temperature (Tmax): 102 F, respiratory rate (RR): 20, blood pressure (BP): 180/98, oxygen saturation (SpO2): 98% potassium (K): 3.7 mEq/L, creatinine (Cr):1.50 mg/dL, blood urea nitrogen (BUN): 23 mg/dL (Table 1). Home medications prior to admission are shown in (Table 2). An abdominal CT scan showed colitis for which he was started on intravenous (IV) levofloxacin 500 mg every 24 h, IV metronidazole 500 mg every 6 h and fluid resuscitation was initiated for acute kidney injury secondary to his kidney stones. The following day (day 2 of admission), he became hypotensive despite the fluid resuscitation and continued to complain of abdominal pain, which was more centered in the suprapubic area. His labs on the second day were as follow: K: 5.6 mEq/L, Cr: 3.40 mg/dL, BUN: 40 mg/dL (Table 1). The decline in kidney function was believed to be multifactorial due to sepsis and administration of non-steroidal anti-inflammatory medications. The patient was subsequently transferred to the ICU and vasopressors were initiated as well as hydromorphone 0.5 mg IV as needed every 4 h for increasing pain. A repeat CT scan showed ascending colitis with no signs of perforation. Due to the patient’s worsening condition, general surgery was consulted and because no perforation was seen on the CT scan, favored conservative management with intravenous fluids and antibiotics. Labs were repeated upon ICU admission (day 2) and the patient was found to have hyperkalemia (6.2 mEq/L) (Table 1). No ECG was done at that time, however, due to the elevated blood potassium level, a single 30 g dose of SPS without sorbitol was administered orally. Labs were repeated the next day (day 3) and the serum potassium level was still elevated (5.9 mEq/L) for which the patient received a single dose of 10 units IV regular insulin with 25 g (50 ml of Dextrose 50% in water) (Table 1). On the fifth day of admission, vasopressors were discontinued and he began to clinically improve. The patient was transferred to the floor and his kidney function further improved and was discharged home on day 9. His labs upon discharge were as follow: K: 3.4 mEq/L, Cr: 1.10 mg/dL, BUN: 37 mg/dL. Three days after discharge, however, he presented with weakness and fever and was found to be anemic with hemoglobin of 7.9 g/dL. Upon direct admission to the ICU, two units of packed red blood cells were transfused and the Gastrointestinal (GI) services were consulted, as there was a concern of GI bleeding. An abdominal CT scan was repeated and showed marked wall thickening in the right hemicolon and proximal transverse colon compatible with colitis, either infectious, ischemic or due to an inflammatory process. The patient was started on broad-spectrum antibiotics, which included vancomycin IV 1 g every 12 h and piperacillin-tazobactam IV 3.375 mg every 6 h.
Table 1.
Laboratory values for first admission.
| Laboratory parameter | Day 1 | Day 2 | Day 3 | Day 3 |
|---|---|---|---|---|
| Potassium (K+) | 3.7 mEq/l | 6.2 mEq/l | 5.9 mEq/l | 3.8 mEq/l |
| Serum creatinine | 1.50 mg/dL | 3.40 mg/dL | 3.60 mg/dL | 3 mg/dL |
| Blood urea nitrogen | 23 mg/dL | 40 mg/dL | 65 mg/dL | 105 mg/dL |
Table 2.
Home medications.
| Medication | Frequency | Dose |
|---|---|---|
| Citalopram | Daily | 20 mg |
| Lorazepam | Three times daily | 0.5 mg |
| Omeprazole | Daily | 20 mg |
The next day of admission (day 2 of admission), the patient experienced three bloody stools and a colonoscopy was performed on the ninth day of admission as patient had low ANC (less than 1000). Findings were suggestive of right colon colitis with possible etiology of ischemia and necrotic appearing mucosa. His hemoglobin was 9.6 g/dL, however, overnight the patient experienced melena which resulted in a sudden drop in his hemoglobin (6.7 g/dL). GI services were then re-consulted to do esophagogastroduodenoscopy (EGD), which showed 15 mm duodenal ulcer with edematous and erythematous borders. The ulcer was injected with epinephrine (2 ml of 1:10,000) and the patient was started on pantoprazole IV drip at 8 mg/hr for72 h after which pantoprazole IV 40 mg twice a day was initiated as the size of ulcer precluded clip placement. General surgery was consulted but preferred to observe the patient and to manage his condition conservatively. Thirteen days after admission, the patient reported abdominal discomfort on the right side and spiked one fever (101.8). Another CT scan was obtained and findings were compatible with a perforation of the colon with an air-fluid collection in the right hemiabdomen containing enteric contrast (Image 1). General surgery was notified and patient was taken to the operation room (OR). Exploratory laparotomy was done and patient was found to have abundant adhesions involving the small bowel. There was frank perforation of the ascending colon and stool spoilage along large portion of the right paracolic gutter. Colon specimens were collected and were sent for pathology. On post-operation day one patient developed abdominal pain and hypotension and sepsis protocol was started. Fluconazole IV 400 mg daily was started in addition to piperacillin-tazobactam. On post-operation day five, abdominal pain was better; however, the drainage from the hemicolectomy was found to be turbid and increasing in volume. Abdominal CT scan was ordered and results were suggestive of intra-abdominal abscesses and collection within the wound which subsequently opened and purulent material was removed from wound. The pathological findings from the resected colon were suggestive of inflammatory bowel disease and due to these findings, GI services started patient on mesalamine for presumptive ulcerative colitis. Thirty- four days after admission patient was discharged home. Nineteen days after discharge, he presented again to the ED with fever, chills, drainage and, redness around surgical site. An abdominal CT scan was done with findings suggestive of phlegmonous changes, seroma and cellulitis for which he was started on piperacillin-tazobactam 3.375 g IV extended infusion every 8 h and was discharged on oral amoxicillin-clavulanic acid 875 mg–125 mg every 12 h. During outpatient follow up, the patient consulted different rheumatologists and was taken off mesalamine as it was determined that he did not have ulcerative colitis. Four months later, the patient underwent fistulogram due to the non-healing nature of seroma, which showed transverse colon to skin fistula. This fistula was attributed to inflammatory bowel disease vs. vasculitis and patient was started on prednisone with outpatient management by a rheumatologist.Five months later, the patient presented again to the ED complaining of fever and was found to be hypotensive. He was started on ceftriaxone IV 1 g daily and metronidazole IV 500 mg every 6 h which was subsequently changed to cefepime IV 2 g every 8 h. An abdominal CT scan was done and findings were consistent with enterocutaneous fistula (Image 2). On day 9 of this admission, the patient underwent exploratory laparotomy where the enterocutaneous fistula tract was removed with a subsequent small bowel resection with anastomosis. The fistula tract was sent for pathology where the specimen was found to be granulated and contain SPS crystals. The patient’s clinical status improved significantly after the resection and anastomosis and he was discharged 10 days after surgery.
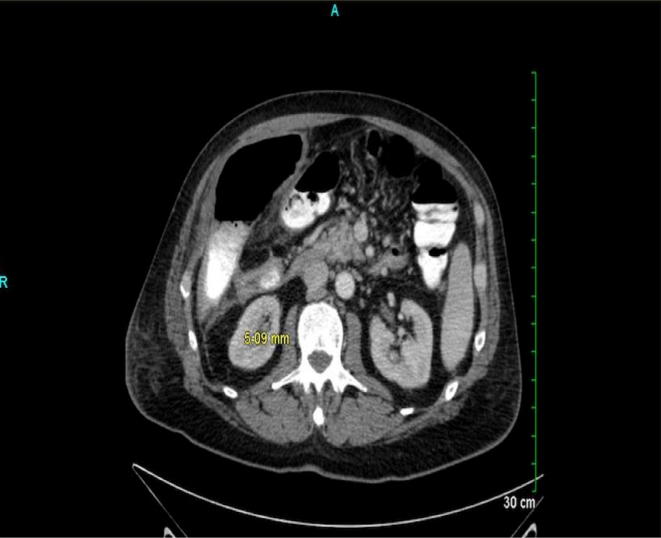
Image 1.
Abdominal CT scan showing perforated colon with air-fluid collection in the hemiabdomen.
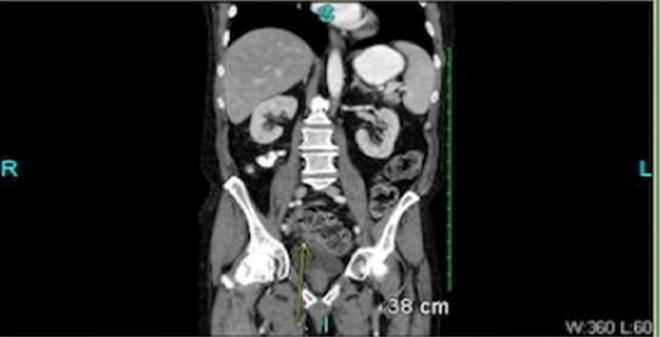
Image 2.
Abdominal CT scan showing enterocutanous fistula.
3. Discussion
SPS is a cation-exchange resin administered orally or via the rectum as an enema that has been available in the market since 1958 for treatment of hyperkalemia. SPS is available with and without sorbitol (70% and 33%) with the rationale behind the use of sorbitol is to induce an osmotic cathartic effect to prevent the SPS impaction or obstruction of gastrointestinal tract. SPS induced colonic necrosis was first reported in 1987 by Lillemoe et al. in five uremic patients (Lillemoe et al., 1987). In September 2009, the FDA recommended against the concomitant use of SPS with sorbitol due to the increase risk of colonic necrosis and other gastrointestinal complications (Administration, 2009). However, this warning for the SPS/sorbitol 70% didn’t include SPS/sorbitol 33% products. Initial cases reported colonic necrosis associated SPS in patients with uremia, kidney transplantation, gastrointestinal surgery, or a history of constipation [(Lillemoe et al., 1987); (Gerstman et al., 1992); (Rashid and Hamilton, 1997); (Wootton et al., 1989); (McGowan et al., 2009); (Bomback et al., 2009)]. However, McGown et al. retrospectively reported all gastrointestinal specimens containing SPS crystals while SPS/sorbitol induced colonic necrosis seems to be higher in selected population as described above, colonic necrosis may still occur even in the absence of uremia, gastrointestinal surgery, or other significant morbidities (McGowan et al., 2009). Here we report a case of colonic necrosis following the administration of single dose of SPS orally without sorbitol in the absence of risk factors. One explanation for increased risk of colonic necrosis following SPS administration could be due to the decrease of colonic motility following the administration of opiates, which resulted in increase the contact time between SPS and colonic mucosa. Although patient received multiple antibiotics and antifungals during his different admissions, no significant interaction with SPS actually exists. SPS induced colonic necrosis symptoms usually start hours to several days after administration (McGowan et al., 2009). In our case symptoms started three days after SPS administration and the diagnosis of SPS induced colonic necrosis was delayed. SPS should not be used in patients with hyperkalemia with risk factors that increase the risk of SPS induced-gastrointestinal complications. Additionally, patients with the absence of such risk factors should be assessed carefully and other measures to reverse/manage hyperkalemia may be considered before prescribing SPS. Alternative medications that were found to be effective in treating hyperkalemia in addition to SPS include patiromer (Veltassa®) and Sodium Zirconium Cyclosilicate. Patiromer a nonabsorbable polymer that binds potassium in exchange for calcium in the distal colon was approved in October 2015 for treatment of hyperkalemia (Drug, 2015). Weir MR et al. conducted multinational, two-phases, single blinded study to evaluate the safety and efficacy of patiromer in chronic kidney disease patients who had hyperkalemia (Weir et al., 2015). Patients with potassium levels of 5.1 to less than 5.5 mmol per liter received 4.2 g twice daily, while patients with potassium levels of 5.5 to less than 6.5 mmol per liter received 8.4 g twice daily. Among patients with potassium levels of 5.5 to less than 6.5 mmol per liter, the mean potassium was less than 5.5 by day two after the start of patiromer. No fatal adverse events were reported and the most common adverse event was constipation. Some patient population reported in the literature to be at risk for GI complication following SPS were excluded from this clinical trial (patients with gastrointestinal disorders and kidney transplant patients) and the long-term safety of patiromer is unknown. Additionally, potassium levels were measured on day 3 of starting therapy with the goal of the study to evaluate the mean change in the serum potassium levels from baseline to week 4. Altogether, these limitations from the study design may preclude, at this point, the use of patiromer in acute hyperkalemia setting where the need for urgent reduction of serum potassium is needed. Clinical trials are needed to evaluate it’s efficacy in acute settings. Sodium Zirconium Cyclosilicate (ZS-9), another potassium lowering agent, works through entrapping potassium in exchange for hydrogen and sodium. In studies, this potassium lowering efficacy of this agent was tested after 48 h of administration. As with patiromer, the long-term safety in high risk patients for GI complications is unknown and more studies may be needed (Packham et al., 2015).
We think this case is interesting and worth reporting as the initial diagnosis of SPS necrosis was delayed as the patient didn’t have risk factors for colonic necrosis except for concomitant opiates administration for the severe pain when he was first admitted for colitis.
Acknowledgments
Acknowledgments
Abdulaziz Almulhim is grateful to King Faisal University in Saudi Arabia for providing financial support for his scholarship.
Financial disclosure
This work was not financially supported by any means.
Footnotes
Peer review under responsibility of King Saud University.
References
- Administration, F., D., 2009. Kayexalate ® Cation-Exchange Resin.
- Bomback A.S., Woosley J.T., Kshirsagar A.V. Colonic necrosis due to sodium polystyrene sulfate (Kayexalate) Am. J. Emerg. Med. 2009;27 doi: 10.1016/j.ajem.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drug, F., A. (FDA), 2015. VELTASSA (patiromer) [WWW Document]. URL https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/205739s000lbl.pdf (accessed 8.25.17).
- Drug, F., A. (FDA), 2010. Kayexalate® Sodium Polystyrene Sulfonate, USP Cation-Exchange Resin [WWW Document]. URL https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/011287s023lbl.pdf (accessed 8.24.17).
- Gerstman B.B., Kirkman R., Platt R. Intestinal necrosis associated with postoperative orally administered sodium polystyrene sulfonate in sorbitol. Am. J. Kidney Dis. 1992;20:159–161. doi: 10.1016/s0272-6386(12)80544-0. [DOI] [PubMed] [Google Scholar]
- Goutorbe P., Montcriol A., Lacroix G., Bordes J., Meaudre E., Souraud J.B. Intestinal necrosis associated with orally administered calcium polystyrene sulfonate without sorbitol. Ann. Pharmacother. 2011;45 doi: 10.1345/aph.1M547. [DOI] [PubMed] [Google Scholar]
- Harel, Z., Harel, S., Shah, P.S., Wald, R., 2013. Gastrointestinal adverse events with sodium polystyrene sulfonate (Kayexalate) use : a systematic review. AJM 126, 264.e9-264.e24. 10.1016/j.amjmed.2012.08.016 [DOI] [PubMed]
- Kato S., Ono Y., Takagi T., Yoshida J., Hirakawa M., Ito T., Nakanishi K., Ogino J., Hasegawa T. Case report; a case of ileus due to ileal stenosis caused by oral intake of calcium polystyrene sulfonate. Nihon Naika Gakkai Zasshi. 2013;102:150–152. doi: 10.2169/naika.102.150. [DOI] [PubMed] [Google Scholar]
- Lai T.P., Yang C.W., Siaop F.Y., Yen H.H. Calcium polystyrene sulfonate bezoar in the ileum: diagnosis and treatment with double-balloon endoscopy. Endoscopy. 2013 doi: 10.1055/s-0033-1344835. [DOI] [PubMed] [Google Scholar]
- Lillemoe K.D., Romolo J.L., Hamilton S.R., Pennington L.R., Burdick J.F., Williams G.M. Intestinal necrosis due to sodium polystyrene (Kayexalate) in sorbitol enemas: clinical and experimental support for the hypothesis. Surgery. 1987;101:267–272. [PubMed] [Google Scholar]
- McGowan C.E., Saha S., Chu G., Resnick M.B., Moss S.F. Intestinal necrosis due to sodium polystyrene sulfonate (Kayexalate) in sorbitol. South. Med. J. 2009;102:493–497. doi: 10.1097/SMJ.0b013e31819e8978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packham D.K., Rasmussen H.S., Lavin P.T., El-Shahawy M.A., Roger S.D., Block G., Qunibi W., Pergola P., Singh B. Sodium zirconium cyclosilicate in hyperkalemia. New Engl. J. Med. 2015;372:222–231. doi: 10.1056/NEJMoa1411487. [DOI] [PubMed] [Google Scholar]
- Rashid A., Hamilton S.R. Necrosis of the gastrointestinal tract in uremic patients as a result of sodium polystyrene sulfonate (Kayexalate) in sorbitol: an underrecognized condition. Am. J. Surg. Pathol. 1997;21:60–69. doi: 10.1097/00000478-199701000-00007. [DOI] [PubMed] [Google Scholar]
- Tongyoo Assanee, Ekkapak Sriussadaporn, Palin Limpavitayaporn, Chatchai M. Case report acute intestinal obstruction due to kalimate, a potassium-lowering agent : a case report and literature review. J. Med. Assoc. Thai. 2013;96:1617–1620. [PubMed] [Google Scholar]
- Weir M.R., Bakris G.L., Bushinsky D.A., Mayo M.R., Garza D., Stasiv Y., Wittes J., Christ-Schmidt H., Berman L., Pitt B. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. New Engl. J. Med. 2015;372:211–221. doi: 10.1056/NEJMoa1410853. [DOI] [PubMed] [Google Scholar]
- Wootton F.T., Rhodes D.F., Lee W.M., Fitts C.T. Colonic necrosis with Kayexalate-sorbitol enemas after renal transplantation. Ann. Intern. Med. 1989;111:947–949. doi: 10.7326/0003-4819-111-11-947. [DOI] [PubMed] [Google Scholar]