Abstract
Purpose
Development of a new dosage-form of antiepileptic-drugs appropriated for children.
Methods
Clonazepam (Cl) was formulated as cubosomal-gel (cub-gel) to be used as a patch reservoir through transdermal-route. Cubosomes prepared using glycerol-mono-oleate(GMO)/Pluronic-F127(PF127) mixture. An actual-statistical design was used to investigate the effect of different stabilizing agents (Ethanol and PVA) and surfactant concentration on cubosomes’ particle size and entrapping-efficiency. The selected formulae were evaluated by testing particle-morphology, in vitro drug release and stability. Cub-gel was prepared using selected cubosome formulae. The optimal cub-gel subjected to in vitro dissolution, ex-vivo permeation and skin deposition studies followed by studying its pharmacological effect.
Results
Using PVA or Et as stabilizers with PF127 significantly decreases the average cubosomes’PS (352 ± 2.8 and 264 ± 2.16 nm) and increases EE (58.97 ± 4.57% and 54.21 ± 3.89%). Cubosomes increase the initial release rate of Cl to ensure rapid therapeutic effect (37.39% and 46.04% in the first hour) followed by a prolonged release till 4 h. Cub-gel containing PVA showed significantly higher Cl-transdermal permeation when compared to Cl-suspension. Moreover, increases the retention-time (89.57% at 48 h) and skin-deposition up to 6-times. It also reduces the epileptic seizures and alters the behavioral parameters induced by pilocarpine.
Conclusions
Cubosomal-gel could be considered an innovative dosage-form for Cl through the transdermal route.
Keywords: Epilepsy, Antiepileptic, Clonazepam, Cubosomes, Cubogels
Abbreviations: Cl, Clonazepam; GMO, glycerol-mono-oleate; PF127, Poloxamer 407; Cubs, cubosomes; Et, ethanol; PVA, polyvinyl alcohol; EE, entrapping efficiency; PS, particle size; CNS, Central Nervous System; TDDS, Transdermal Drug Delivery System; PBS, phosphate buffer saline; PDI, polydispersity index; I.P, Intraperitoneal injections; SMS, stereotyped movements signs; PCS, peripheral cholinergic signs
1. Introduction
Epilepsy is a disturbance of electrical activity in CNS described usually by different symptom; seizures, consciousness lost and convulsion (Letcher, 2005). The epilepsy prevalence is very high in children. It was reported that from 187 epileptic patients 41 individual are children (Camfield and Camfield, 2015). In addition, they are approximately doubling the adults or middle age patients (Benamer and Grosset, 2009). The incidence in children is consistently reported to be highest in the first year of life by the end of the first decade.
The development of new dosage forms which are appropriate for children can present significant challenges. Oral solid dosage forms such as tablets or capsules will be undesirable for them. Many children also prefer monotherapy because they only have to remember to take the medication only one time. Previous studies evidenced the potential advantages of the transdermal administration over conventional dosage forms, especially with different benzodiazepines kinds and using patches could lead to reducing stress for the child (Kreider et al., 2001, Mura et al., 2014, Wu et al., 2012).
TDDS is a preferred dosage form due to their ability to avoid intravenous therapy or incompatibility of other routes of administration like emptying time, gastric pH and first pass metabolism. For children, transdermal patches are usually very accepted due to its ease applying and represent a valuable alternative to an oral administration. All children, including neonates, were born with full-term skin which has a thin epidermal layer control the water loss but perform the same adult skin function (Gratieri et al., 2013, Delgado and Guy, 2001).
Clonazepam will be chosen as a model. It is long-acting benzodiazepine derivative. It is structurally related to chlordiazepoxide hydrochloride, diazepam, and nitrazepam (Lasoń et al., 2013). Recently, it has been used in the treatment of a variety of psychiatric disorders (Dell’osso and Lader, 2013, Wang et al., 2016).
Clonazepam available in various dosage forms. The most common dosage form in epilepsy attack is injection form. Although intravenous administration provides rapid seizure suppression, however intravenous and oral route is considered impractical and/or inconvenient drug delivering routes. Therefore alternative drug delivery route is needed (Hart and Sibai).
Clonazepam (Cl) transdermal administration is recommended, due to its irregular absorption and bioavailability. It has low solubility in water which leads to low dissolution rate high. Excessive first-pass metabolism considers another reason as it was administrated in low size dose for long-term. The latter produce wide blood level alternations (Miyamoto et al., 2017, Wu et al., 2012).
A transdermal multilayered patch consists mainly of basic components; polymers and drugs formulated in drug reservoir layer in the patch system. The drug reservoir layer is the most important one as it is the carrier of drug molecules and the controller of the drug release pattern (Ali, 2017, Nguyen et al., 2017).
Cubosomes will be used as a drug reservoir system as they are successfully used for drugs transdermal delivery system (Thakkar et al., 2016). Cubosomes are cubic shape liquid crystals dispersion. They have unique nanostructure of a highly twisted, uninterrupted lipid bilayer and two congruent non-transecting water channels (Kim et al., 1997, Pan et al., 2013). The cubosomes particles’ penetration enhancing effect and the subcutaneous fluidizing effect were mainly due to the cubosomes’ lipid phase. The lipid phase with its cubic phase structure mix with the subcutaneous lipids due to their similar structure (He et al., 2017, Peng et al., 2015). In addition, it was previously reported that the delivery of CNS drugs was significantly increased in lipid nanoparticle structure as the human plasma interacts with the monoolein-based cubosomes to enhance the drug absorption (Natarajan et al., 2017, Peng et al., 2010, Peng et al., 2015).
One of the main cubosome limitations, it was in a liquid form product. It is particle is susceptible to aggregation. Furthermore, GMO intrinsic properties increase the cubosome stickiness and stiffness (Gupta et al., 2017). Increasing the system viscosity via preparing cubosomes as hydro-gels can solve the liquid cubosomes’ limitations. Hydrogels have high water content and soft consistency, which is similar to natural tissue (Dal Magro et al.). The later improve the drugs biocompatibility (Knipe et al., 2015). The elastic nature of the hydrogels after application minimizes irritation to the surrounding tissues (Blount Iv and Bhattarai, 2016). It was found that the low interfacial tension between the hydrogel surface and the site of administration (skin) fluid reduces protein adsorption and cell adhesion (Bhattarai et al., 2010). Hydrogels provide a suitable drug protection especially in the release site due to the large applied surface area and lower viscosity (Mallick, 2011).
In view of the above-mentioned, the existing work was aimed to develop a new effective and innovative Cl-cubosomes' transdermal delivery system. Different cubosomal formulae were developed and incorporation the optimal cubosomal formulae in hydrogels (cubogels). The optimized obtained gel formula could be used as a reservoir in the transdermal patch system.
2. Methodology
2.1. Materials
Clonazepam, Cl (FIS- Fabbrica Italiana Sintetici S.P.A, Italy) was gently supplied by Amoun pharmaceutical company (Cairo, Egypt). Glyceryl monooleate (GMO), polyvinyl alcohol and carbopol 934 were obtained from Sigma Chemical Co. (St. Louis, USA). Poloxamer 407 (PF127) was purchased from BASF chemical company (Germany). Methanol, ethanol, sodium hydroxide, disodium hydrogen phosphate, and triethanolamine were purchased from El Nasr pharmaceutical company (Cairo, Egypt). Spectra/Pore dialysis membrane (12,000–14,000 molecular weight cut-off) was purchased from Spectrum Laboratories Inc. (USA).
2.2. Preparation of Cl loaded cubosomes (Cl-Cubs)
Cubosome was prepared by emulsification of monoglyceride/Poloxamer 407 (PF127) as lipid phase surfactant mixture in water (Esposito et al., 2003). The lipid phase (GMO/PF127 mixture) was 5%w/w of the total dispersion weight. The surfactant was used in a concentration range from 0% to 10% w/w from the total disperse weight. Polyvinyl alcohol (PVA) and ethanol (Et) were used as stabilizing agents in different concentrations (0%, 2.5%, or 5%w/w with respect to the disperse phase). The used Cl concentration was 0.25%w/w (about 20 times the pediatric effective dose (Caselli et al., 2014). The prepared Cl-cubosomes composition is presented in Table 1.
Table 1.
The composition of different Cl cubosome formulae.
| F | 5%(w/w) GMO/PF127* |
%Stabilizer** |
||
|---|---|---|---|---|
| GMO* | PF127* | PVA | Ethanol | |
| CA | 97.5 | 2.5 | 0 | – |
| C2 | 95 | 2.5 | 2.5 | – |
| C3 | 92.5 | 2.5 | 5 | – |
| C4 | 95 | 5 | 0 | – |
| C5 | 92.5 | 5 | 2.5 | – |
| C6 | 90 | 5 | 5 | – |
| C7 | 90 | 10 | 0 | – |
| C8 | 87.5 | 10 | 2.5 | – |
| C9 | 85 | 10 | 5 | – |
| C10 | 95 | 2.5 | – | 2.5 |
| C11 | 92.5 | 2.5 | – | 5 |
| C12 | 92.5 | 5 | – | 2.5 |
| C13 | 90 | 5 | – | 5 |
| C14 | 87.5 | 10 | – | 2.5 |
| C15 | 85 | 10 | – | 5 |
Notes: The drug is solubilized in the organic phase.
All formulae contain; 0.25% (w/w) clonazepam 95% (w/w) water.
The GMO/PF127 concentration is constant 5%w/w of the total Cubosome weight.
The stabilizer concentration is (w/w) percentage form the disperse phase.
On the basis of the preliminary trials a 2-factor, 3-level actual statistical design was conducted to study the effect of each independent variable (PF127 and stabilizer concentration) on the dependent variables (entrapment efficiency, and particle size) using design expert 10.0.3. The design repeated twice for each stabilizer (PVA and ethanol). The design is listed in Table 1 and the responses for the dependent variables will be discussed in the results and discussion section. One-way ANOVA followed by LSD test was used to investigate the surfactant concentration and stabilizer concentration combined effect on the dependence factor. The difference significant at P < 0.05 will be considered.
Briefly, cubosomes are prepared using emulsification method (Luo et al., 2015). GMO/PF127 and Cl (0.25% w/w) were melted on a hot plate (oil phase). PVA or Et was added at 60 °C in the aqueous phase in different concentrations. The oil phase was then added drop-wise to the aqueous phase under a mechanical stirring at 1500 rpm for 2 h at 70 °C (a water bath) until homogeneous emulsion obtained, then the obtained hot emulsion was dispersed rapidly into cold distilled water (0–2 °C) about 20 ml. the system stirred in an with magnetic at 1000 rpm for 1 h in an ice bath.
2.3. Cl-HPLC analysis method
The Cl samples were assayed using a modified Sadanshio et al., 2015 HPLC analysis method (Sadanshio et al., 2015). HPLC Agilent 1100 system with an ultraviolet detector was used. The analytical column was a reverse phase C18 column (Thermo ® BDS, 250X4.6 mm, 5 µ) was used at 20 °C. The mobile phase was a mixture of acetonitrile and 20 mM phosphate buffer solution pH 7 (55:45 v/v) at 2 ml/min flow rate. The injection volume was 20 ml and Cl detection was carried out at 313 nm.
2.4. In vitro Cl loaded cubosomes characterization
2.4.1. Particle size distribution
The mean particle size and polydispersity index (PDI) characteristics of the prepared cubosomes in the current study were determined by light scattering based on laser diffraction using the Malvern Mastersizer 2000 Ver. 2.00 (Malvern Instruments, Malvern, UK). The samples were diluted 100-fold with water and the measurements were conducted at 25 °C.
2.4.2. Determination of Cl entrapment efficiency
Free drug concentration was measured in the aqueous phase after separation from cubosomes systems by dialysis using a previously described (HPLC) method. In brief, 2 g Cl-cubosome were deposited into a dialysis tubing cellulose membrane (14 kDa MW cutoff). The whole set then dialyzed into 1000 ml of 10% ethanolic water for 2 h at 25◦C. The media replaced after 1 h. Samples were conducted three times and the values reported as the mean ± S.D. The Cl entrapped in the Cl-Cubs (WE) was calculated by subtraction the amount of free drug from the calculated total incorporated drug weight. The encapsulation efficiency (EE%) can be calculated as:
| (1) |
where WE is the mass of Cl entrapped in the Cl-Cubs, and WA is the weight of Cl in the system (Dian et al., 2013).
2.4.3. In vitro release Cl from the prepared cubosomes and the kinetic data analysis
The in vitro Cl release from the prepared cubosomes was determined in order to evaluate the effect of various compositions on the Cl release rate. In vitro release of Cl from cubosomes was conducted by adding Cl-Cubs equivalent to 2 mg of Cl into a cellophane membrane. The whole set, then immersed in 200 ml phosphate buffer saline (PBS) pH 7.4 containing 10% ethanol to ensure sink conditions and stirred at a speed 50 rpm for 4hrs. Samples were collected at appropriate time intervals. The Cl release amount was measured by the previously described HPLC method. The results are the mean values of three experiments. A blank experiment for the drug assay was carried out concurrently using free drug cubosomes. The Cl release from the cubosome formula was compared with the pure drug (Cl) release. The release of free Cl was done in the same condition as a control.
For interpretation the release data and ideal kinetic model development, various kinetic models were applied to obtain the best fit mathematical model. To explain the mechanism of Cl release from the cubosomes; Higuchi, first-order, zero-order, Weibull, Hixson–Crowell, and Ritger–Peppas equations were used (D’Souza et al., 2014). To differentiate statistically between the studied release profiles of the selected formulae, paired t-test was performed (p < 0.05).
2.4.4. Imaging of the optimized cubosomal dispersion formula by TEM
The morphological aspects of Cl loaded cubosomes were visualized by using transmission electron microscopy TEM (JEOL JEM-HR-2100, Japan). The droplets were negatively stained with 1% (w/v) phosphotungstic acid and air-dried before imaging. All experiments were conducted three times (Enin et al., 2016).
2.4.5. Stability study
The cubosomes physical stability was conducted according to Chinese Pharmacopoeia 2010 (part 2, Appendix XIX C). The cubosomal samples were subjected to a strong light beam (4500 ± 500 lx) and high temperature (60 °C) in a drug stability chamber for 10 days. In 5th and 10th days, samples were examined for homogeneity, particle size and PDI.
2.5. Preparation of Cl-cubogels
Cubogels were prepared using carbopol 934. Carbopol 934 (10% w/v from the total cubosome volume used) was added to a specified amount of liquid Cl-cubosome and stirred with a magnetic stirrer. A sufficient quantity of triethanolamine was added for neutralization.
2.6. In vitro characterization of prepared cubogels
2.6.1. Physical evaluation of the prepared cubogels
The freshly prepared cubo-gels were examined by visual inspection, where the prepared formulae were examined for their physical characteristics (e.g., color and homogeneity). In addition, the pH of a 10% aqueous solution of the Cl-cubogels was measured.
2.6.2. Rheological properties evaluation of the prepared Cl-cubogels
The prepared Cl-cubogels were evaluated a rotational Brookfield viscometer of cone and plate structure, using spindle CPE-41 at 25 ± 2 °C. About 0.5 g of the tested formula was applied to the plate and settings the speed range from 0.3 to 60 rpm or 0.5 to 100 rpm with 10 s between each two successive speeds. when the torque was within 10–100% (the acceptable range) rheological data (the viscometer were shear stress and viscosity at different shear rate values) were recorded (Azhari et al., 2016).
Studying the rheological behavior using the rheological data was by fitting the power law model:
| (2) |
where is the shear stress, is the rate of shear, K is the consistency index (sec), and n is the flow index. When n in the range (zero to one) this means a shear thinning fluid, while if approaches one it is in the case of Newtonian system and exceeds one in a dilatant system (El-Dahmy et al., 2014).
The non-Newtonian system was described using Bingham, Casson, and Carreau equations using regression coefficient as a point of comparison.
Bingham equation was used to describe linear plastic system:
| (3) |
where is the yield value.
While the non-linear plastic system was described by Casson Equation:
| (4) |
While Carreau’s model was used for pseudo-plastic shear thinning systems description:
| (5) |
where and and K were determined from the shear rate–viscosity curve. and , refer to viscosity values at the lowest and highest rate of shear values, respectively. K is a constant parameter where 1/K is the rate of shear when the viscosity begins to decrease. While m is a dimensionless constant indicating the degree of pseudoplasticity (Brandl and Massing, 2016).
2.6.3. In vitro Cl release from the prepared Cl-cubogels and kinetic analysis of the release results
This study was carried out using a modified USP dissolution apparatus II. A 5 g of Cl-cubogels (equivalent to 2 mg Cl) was evenly distributed on the surface of glass dish attached to a glass cube and tightly covered with stainless wire cloth 120 mesh (0.12 mm aperture). This set was dipped in 250 ml PBS, pH 7.4 containing 10% ethanol. The release study was carried out with rotation speed at 50 rpm at 37 °C. The set was repeated but with replacing the Cl-cubogels with a pure drug suspension and the selected Cl-cubs formulae all equivalent to 2 mg Cl respectively. 2 ml sample was withdrawn at a different time interval from the vessel with replacing by fresh media. Previously described HPLC method used to analyze the samples. The release data were examined with zero-order, first-order, and diffusion kinetic models to determine the order of the release pattern (D’Souza et al., 2014). A blank experiment for the drug assay was carried out concurrently using free drug cubosomes, free-drug cubo-gel and drug-free plain gel to determine the main factor that effect on the drug release.
2.6.4. Ex-vivo permeation study
Ex-vivo permeation procedures of the selected Cl-cubs, Cl-cubogels, and pure drug suspension were determined using Franz diffusion cells (Hanson Microette System). The rabbits’ abdominal skin was used as the membrane model after shaving the hair and maximally removing the subcutaneous fats. Each sample was applied to the diffusion surface of the skin (2.5 cm2 available diffusion area) in the donor compartment equivalence to 2 mg Cl. The receptor compartment filled with 7.5 ml 10% ethanol buffer system, (PBS, pH 7.4), stirred at 50 rpm, and maintained at 32 °C ± 0.5 °C. Ethanol was added to maintain the sink condition and increase Cl solubility. The test was performed for 48 h, and the samples were withdrawn and the drug amount was determined by previously described HPLC.
At the steady state condition the apparent permeability coefficient (cm/h) was calculated according to the following equation:
| (6) |
where J (mg/cm2 h) is the steady-state transdermal flux, Kp (cm/h) is the permeability coefficient, and Co is the initial drug concentration in mg (the donor phase).
2.6.5. Skin deposition study
After Ex-vivo permeation study finishes carefully removed used skin was cleaned with fresh receptor media. The Clonazepam retained in the skin was extracted with 5 ml of ethanol and sonicated for 15 min. The samples were filtered using 0.22 mm syringe filter. The yielding clonazepam amount deposited in the rabbit skin was determined by HPLC (Clares et al., 2014).
2.7. Pharmacological evaluation
The SE can be chemically induced by pilocarpine (chemo-convulsants agent). Pilocarpine induces seizures by cholinergic hyper-activation (Turski et al., 1984). Male mice weighing 18–24 g were used. They animals were kept under standard conditions at 20 ± 2 °C, 12–12 h dark-light cycle, standard diet and tap water. All experiences were conducted according to the 1996 NIH Guide for the Care and Use of Laboratory and performed between 8 a.m. and 3p.m.
The animals were divided into four groups each of 12 individual: the negative control group, shaved back animals only (−ve); test group (T-Cl) an amount of Cl-cubogel corresponding to 25 mg/kg Cl was applied to the shaved backs of the tested animals; control group in which a free drug cubogel applied topically to the shaved backs (C-Cl); Cl suspension (25 mg/kg) oral, used as a reference group (R-Cl).
The test was done as reported by Turski et al. (1984). Seizures were induced in mice via IP 400 mg/kg pilocarpine injection. Continuous noticing was performed for 60 min to monitor the latency time to the beginning of the first seizures, and the latency of development of status epilepticus (SE). The subsequent development of behavioral symptoms was studied such as piloerection, tremors, diarrhea, salivation, and falling: as a peripheral cholinergic signs (PCS); scratching, rearing (lift), chewing, head bobbing as a stereotyped movements signs (SMS) and myoclonic movements of the forelimbs, convulsions which progressed to motor seizures with SE (Luszczki et al., 2007, Turski, 1999). In addition, the deaths individuals number was recorded for 24 h after administration (Freitas et al., 2006).
All the data are presented as the percentage and the mean ± SE. Results were statistically evaluated using two-way analysis of variance (ANOVA) followed by Fisher's exact comparison test. P < 0.05 was considered statistically significant.
3. Results and discussion
3.1. Preparation and evaluation of Cl cubosomes (Cl-Cubs)
The liquid crystalline bi-continuous cubic phase nanoparticles were mainly affected by the preparation component. Different surfactant and stabilizer ratios were used for preparing the Cl-Cubs. For formation the cubic phase nanoparticles, a preliminary study was conducted; considering the cubosomes have a homogenous milky like consistency only is the accepted formulae. Therefore, formula CA was excluded (two-phase separation). It contains 2.5% surfactant only. It was evident that the bi-continuous cubic phases form unstable aqueous dispersions. This may be due to the exposure the hydrophobic domains’ to the aqueous medium surrounding the particles disturb the inner crystalline structure. This can be prevented by adding amphiphilic dispersion agents to maintain the inner cubic structure. In our case, this was done by increasing the PF127 concentration and adding a stabilizer in different concentration. The latter explains the homogeneity of the other formula.
3.2. In vitro characterization of prepared Cl loaded cubosomes
The particle size (PS) and entrapment efficiency (EE) values were evaluated by polynomial analysis using quadratic model. Signal to noise ratio and precision was determined and calculated by Design-Expert software. A ratio greater than 4 indicates the validity of the used model to identify the design space (Ahmad et al., 2015, Aslam et al., 2016).
3.2.1. Particle size distribution
To confirm that the prepared cubosome particles are all of the nanometer range, particle size was measured and summarizes the result of the statistical design in Table 2. All formulae were in the nanometer range. The average particle size values range was 215.3 nm to 686.4 nm, with a polydispersity index <1 except formula CA. The PdI value indicates the particle size uniform distribution and the particles dispersion homogeneity of the Cl-cubs (Jain et al., 2012). Table 2 showed that the PF127 and stabilizer concentration had a significant effect on the average cubosomes’ particle size (p < 0.05).
Table 2.
The Composition of the prepared clonazepam Cubosomes Cl-Cu formulae and the measured parameter (n = 3).
| F | Response |
PDI | |
|---|---|---|---|
| PS (nm) | EE% | ||
| CA | 1089 ± 1.01 | 9.54 ± 1.95 | 1.67 ± 0.02 |
| C2 | 485 ± 2.12 | 25.87 ± 1.95 | 0.32 ± 0.01 |
| C3 | 358 ± 2.84 | 45.98 ± 2.63 | 0.45 ± 0.05 |
| C4 | 378-±3.21 | 11.85 ± 1.57 | 0.45 ± 0.01 |
| C5 | 352 ± 1.91 | 51.08 ± 3.82 | 0.39 ± 00.05 |
| C6 | 215.3 ± 2.85 | 68.97 ± 4.57 | 0.58 ± 0.02 |
| C7 | 686 ± 4.12 | 21.58 ± 2.99 | 0.63 ± 0.03 |
| C8 | 251 ± 2.09 | 65.87 ± 4.58 | 0.66 ± 0.04 |
| C9 | 361 ± 3.11 | 74.25 ± 5.21 | 0.44 ± 0.01 |
| C10 | 512 ± 4.12 | 24.56 ± 2.01 | 0.85 ± 0.04 |
| C11 | 364 ± 3.78 | 34.51 ± 2.91 | 0.79 ± 0.05 |
| C12 | 297 ± 2.17 | 49.54 ± 2.99 | 0.68 ± 0.02 |
| C13 | 264 ± 2.16 | 69.21 ± 3.98 | 0.43 ± 0.01 |
| C14 | 384 ± 3.06 | 64.45 ± 5.29 | 0.58 ± 0.02 |
| C15 | 375 ± 3.45 | 73.98 ± 5.35 | 0.38 ± 0.01 |
Predicted R2 was calculated to determine the model accuracy and to predict a response value through comparing the calculated value with the adjusted R2 value (Shamma and Elsayed, 2013). For PS in case of using stabilizer, the precision was 28.47 and 29.434 with the reasonable difference between the predicted R2 (0.8972 and 0.8260) and the adjusted R2 (0.9749 and 0.9707) for PVA and ethanol (Et) respectively.
Eqs. (7), (8) for PVA and Et respectively are the regression equations identify the PF127 (A), and stabilizer (B) concentrations effect on the cubosomes particle size (Y) in terms of coded values.
| (7) |
And
| (8) |
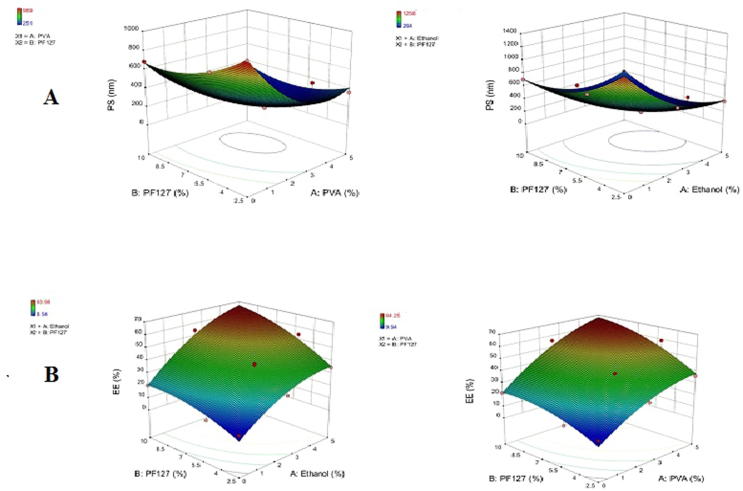
From the previous equation, the results proved that increasing the PF127 and PVA or Et concentrations from 2.5% to 5% significantly decrease the average PS (p-value < 0.0001). Further increasing in their concentration up to 10% resulted in a significant increase in the cubosomes particle size. However, increasing stabilizer concentration from 0% to 2.5% resulted in a significant decrease in average particle size. Further increase in stabilizer concentration from 2.5% to 5% resulted in a significant increase in particle size as shown in Fig. 1A.
Fig. 1.
Response surface plot for the effect of stabilizer and Pluronic F127 (B) concentration on (A) the particle size (PS) and (B) the entrapping efficiency (EE).
This result is in agreement with the results previously reported by Esposito et al., 2003 (Esposito et al., 2003). The combined effects of the PF127 in the presence of different concentrations of PVA or Et could have an additional effect on particle size; this effect was evaluated by performing a one-way ANOVA.
Increasing the total surfactant percent from 2.5% to 10% resulted in a significant decrease in the cubosomes average particle size (p < 0.05). These results suggested that the combined high concentrations of poloxamer and PVA could influence the steric stability of the system and lead to disruption of the crystalline structure of the dispersion (Esposito et al., 2003). The lowest PS was noticed in the formula (C6, C8, and C13). Paired t-test, applying on the formulae C6 and C13 using SPSS 19.0® as both containing 5%w/w PF127 and 5%w/w or 5%w/w Et respectively was found that a significant difference in PS value on using Et or PVA as a stabilizer was observed.
3.2.2. Cl entrapment efficiency determination
EE percentages of the prepared Cl cubosomes were between 25.87% and 74.25% and 24.56% to 69.21% for PVA and Et respectively as shown in Table 2 and Fig. 1B. The calculated EE values were analyzed using a polynomial quadratic model with an adequate precision 23.122, 27.64 and the reasonable difference between the predicted R2 (0.6604 and 0.7715) and the adjusted R2 (0.9435 and 0.9603). The calculated EE analysis equation was:
| (9) |
And
| (10) |
It could be recognized from the statistical analysis that increasing the EE was significantly recognized when PF127 and stabilizer concentration increases (p-value < 0.0001). This might be because PVA or Et in the aqueous dispersion medium assumed to stabilize the formed cubosomal nano-vesicles by forming a coat over them. The formed coat could retain an excess Cl amount so increasing its entrapment. In addition, the strong attraction between the poorly soluble drug and the hydrophobic domain in the cubic phase bilayer facilitate a high drug entrapping in the cubic system.
Statistical comparison between the PVA effect on EE and the ethanol effect on the EE using paired t-test showed no significant difference between them at p < 0.001.
3.2.3. Optimized cubosomal formula selection
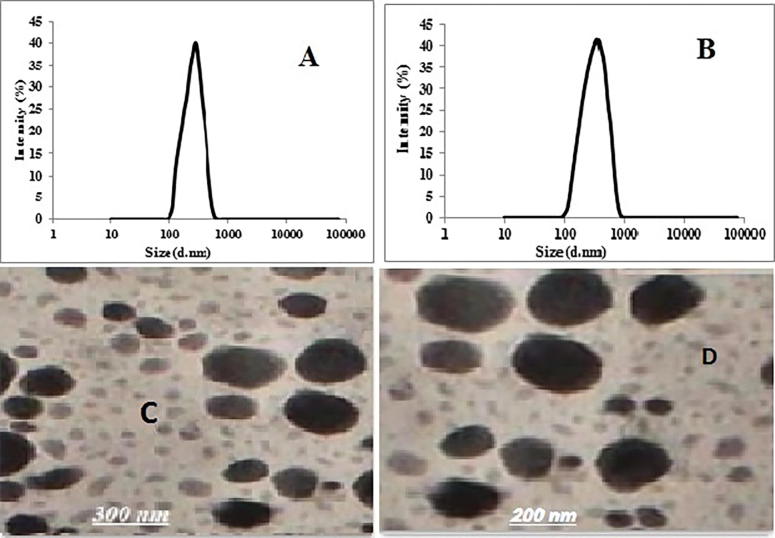
For formulae optimization higher priority was given to PS, PDI and EE as they are the studied responses due to the significant effects of the latter factors. Desirability was estimated to predict the optimum composition with the minimum PS, PDI, and maximum EE (Singh et al., 2012). The greatest desirability value will be found in formulae C6 and C13. Fig. 2A and B showed the Size distribution of Cl-Cub for formulae C6 and C13. They have the uniform PS distribution with high intensity 40.09% and 42.87% for C6 and C13 respectively. They were selected for further study as they showed the lowest particle size and the highest EE.
Fig. 2.
Size distribution of Cl-Cubosome (A) C6 and (B) C13. Transmission electron micrographs of the selected Cubosome at magnification of 100,000. (C) C6 and (D) C13.
3.2.4. Imaging of the optimized cubosomal formula by TEM
To confirm the cubic structures formation of the selected formulae C6 and C13, the practical morphology was examined using TEM. The obtained photomicrographs of Cl-loaded cubosomes presented in Fig. 2C and D demonstrated that the cubosomes are well dispersed individual particles, having irregular cubic shape, and in the nano-size range. The result ratifies the particle size measurement results.
3.2.5. In vitro Cl release from the prepared Cl-cubogels and kinetic analysis of the release results
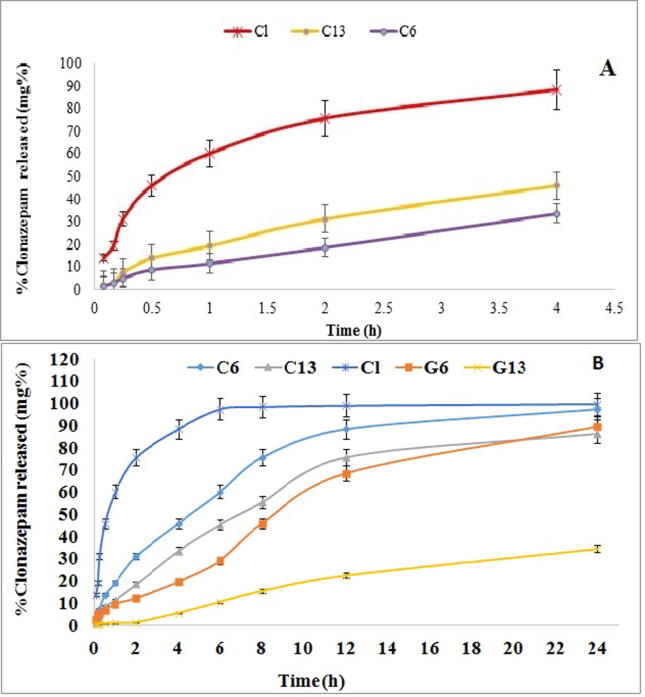
The Cl release profiles from the selected cubosome formulae in comparison with the pure Cl in 10% alcoholic PBS (pH 7.4) were illustrated in Fig. 3A. The drug release from the selected formula was found to be significantly slower than that of the pure Cl with a p-value < 0.001. The release of pure drug completed within 4 h (the test time), while the selected formulae released only 37.39% and 46.04% for formulae C6 and C13 during the same release period respectively. This could be due to the existence of ethanol in the dissolution media, which facilitate the pure Cl release in the dissolution medium than the incorporated Cl in the cubosomal formulae (Jantratid and Vertzoni, 2016).
Fig. 3.
(A) Cumulative percentage of Cl release profiles from the selected cubosomal formula in comparison with the drug (Cl) in phosphate buffer (pH 7) for 4 h. (B) Cumulative percentage of Cl release profiles from cubogels in phosphate buffer pH 7.4 (n = 3).
The Cl release profiles from the cubosomal formulae were illustrated in Fig. 3A. The drug release from formula C13 (46.04%) in rate higher than from formula C6 (33.568%). The Cl release profile from the selected formula C6 and C13 was significantly different (p < 0.05). The Cl release profile is following Higuchi’s equation (Akhlaghi et al., 2016) indicated a diffusion rate (R2=0.99).
The release of the selected formulae begins relatively fast followed by a slower release rate. Apparently, cubosomes could conserve a sustained release profile for Cl. The initial fast release rate attributed to free Cl on the cubosome particle surface, while drug incorporated into the particle core has been released in a prolonged mode (Zhang et al., 2008, Zhuang et al., 2010). In addition, GMO as a basic cubosomes component might lead to decreasing the drug partitioning rate from the oily medium to the aqueous one when compared to the pure drug which could diffuse easily to the dissolution media (Nguyen et al., 2017, Patil et al., 2015, Sadanshio et al., 2015). This result was in agreement with the entrapping efficiency result.
Formula C6 has lower release efficiencies, although its smaller PS than formula C13 because PVA is a polyol which considered a recommended stabilizer and size controlling agent (Rarokar et al., 2016, Singh et al., 2012).
3.2.6. Stability study
The stability test was done by stress test according to Chinese Pharmacopoeia 2010 (part 2, Appendix XIX C). There was no change in the homogeneity of the prepared formulae. There were no phase separation or oil drops on the surface of the cubosome formulae. The cubosome particles were maintained as a milky white emulsion without observable accumulation. Table 3 explained that the stabilization in the cubosomes’ particle sizes during the stability period (10 days). Therefore, the stability test could confirm that the cubosome is a thermodynamically stable system for Cl.
Table 3.
Cubosomes particle sizes during the 10-days stress test stability study.
| Formulation | Time (days) | High light beam (4500 ± 500 L×) |
Temperature (up to 60 °C) |
||
|---|---|---|---|---|---|
| Diameter (nm) | PDI | Diameter (nm) | PDI | ||
| C6 | 0 | 252 | 0.58 | 252 | 0.58 |
| 5 | 252.6 | 0.55 | 255 | 0.59 | |
| 10 | 253.8 | 0.57 | 254 | 0.56 | |
| C13 | 0 | 264 | 0.43 | 264 | 0.43 |
| 5 | 263.4 | 0.44 | 267 | 0.42 | |
| 10 | 265.6 | 0.45 | 269 | 0.42 | |
3.3. Preparation and characterization of the prepared cubo-gels
3.3.1. Characterization of the prepared cubo-gels
Carbopol 934 as gelling agent were used to decrease the cubosome nanoparticles aggregation and to increase their physical stability via enhancing the systems viscosity moreover, increase the retention time of cubosome to be used as a reservoir in the transdermal patches (Sinko and Martin, 2009). The prepared cubogels (G6 and G13) were white viscous creamy preparations with a smooth and homogenous appearance. Carbopol cubogels had a pH value of 8 due to the presence of triethanolamine.
3.3.2. Evaluation of rheological properties of the prepared cubogels
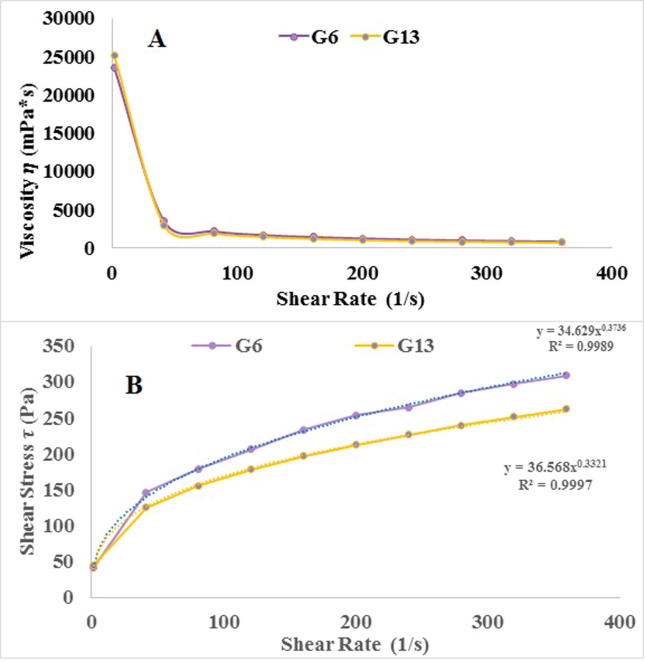
The rheological properties study of the prepared cubo-gels revealed that the selected formula had shear thinning flow. The later observed from decreasing gel viscosity by shear rate increasing as shown in Fig. 4A and B. As demonstrated in Table 4; formulae G6 and G13 follow non-newtonian and shear thinning behavior (n-values less than 1). The highest regression coefficient was fitting to Carreau equation yielded pseudoplastic flow (according to the power law equation). This flow could achieve system physical stability during shelf-life, ease application and increase the system retention after application (Nutan and Reddy, 2010, Piriyaprasarth and Sriamornsak, 2011).
Fig. 4.
Rheological characteristics of the prepared cubosomal gel formulae.
Table 4.
Rheological properties of prepared cubogels.
| Formula | Flow index (n) | Regression coefficient (r2) Type of flow |
Flow type | ||
|---|---|---|---|---|---|
| Bingham (linear plastic) |
Casson (non-linear plastic) |
Carreau (pseudoplastic) |
|||
| G6 | 0.3736 | 0.767 | 0.6158 | 0.9987 | Shear thinning pseudoplastic |
| G13 | 0.3321 | 0.8669 | 0.8307 | 0.9911 | Shear thinning pseudoplastic |
3.3.3. In vitro Cl release from the prepared cubo-gels
The in vitro release data of Cl from the prepared cubo-gel formulae are illustrated in Fig. 3B. It is obvious that the cubogels had a slower release rate than pure Cl suspension and Cl-cubs selected formulae. This result was expected as increasing gel viscosity could retard the drug diffusion from the lipid phase (GMO) to the diffusion media (Chanp et al., 1997, Song et al., 2008). In addition, GMO increased significantly the in vitro permeation/retention of the used drug, therefore, the cubo-gel formulations achieved sustained action of Cl. This was agreed with previous studies which achieved the cubosomal formulation could produce a sustained effect (Steluti et al., 2005).
Formula G13 has a higher release rate than formula G6 as shown in Fig. 3B. This may be due to formula G13 containing ethanol. Clonazepam has a higher molar solubility in ethanol than in polyols (Song et al., 2008). In addition, the solubility of PVA in alcohol (dissolution media contains 10% alcohol) is approximately low. It fell in the interval insoluble or insoluble (Elsayed et al., 2006).
3.3.4. Ex-vivo permeation study
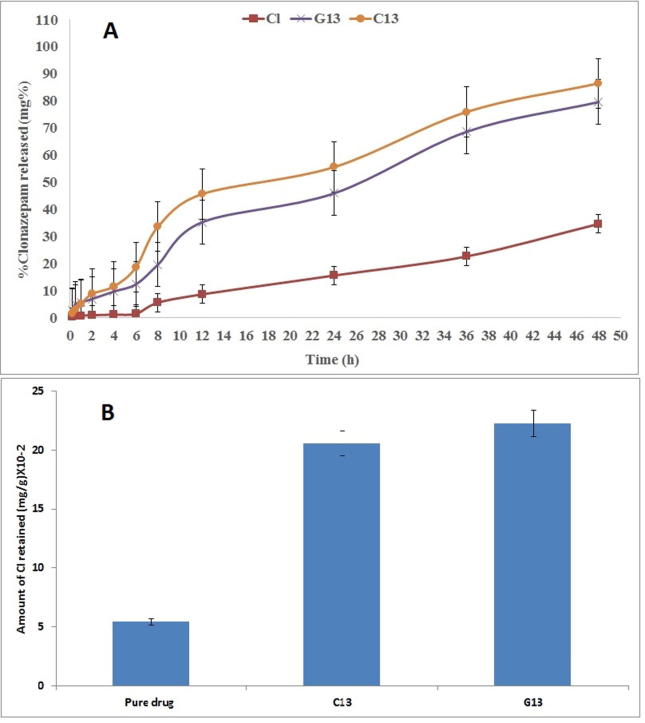
Ex-vivo permeation study helps in indicating the drug amount diffused through the skin. Ex-vivo permeation studies were monitored for predicting the formulae in vivo behavior. The rabbit skin is considered a successful ex-vivo model for the permeation effect studying of the different drug carrier systems (El Maghraby, 2008, Elsayed et al., 2006). It has been used as commonly substitutes for human skin (Godin and Touitou, 2007). Formulae C13 and, G13 were selected for ex-vivo permeation study through the skin due to their acceptable physical properties. They compared to the pure drug suspension. The diffusion experiment was done by franz diffusion cells as previously reported.
The drug permeation from the cubosomes formulae (C13 and G13) was significantly higher in both rate and extent than the pure drug suspension, as shown in Fig. 5A. This may be due to the previously reported that cubosomes could maintain higher drug permeation and retention time (Lopes et al., 2006). There nano-size particles and existence of PF127, and Et are acting as permeation enhancer factors (Shamma and Elsayed, 2013). Also, GMO may compose the cubosomal wall more elastic to allow the nano-vesicles to pass through the small pores existing at the skin membrane.
Fig. 5.
(A) Cumulative percentage of Cl permeated from the selected formulae through rabbit skin over 48 h at 32 °C ± 0.5 compared to its aqueous suspension. (B) Amount of Cl deposited from the selected formulae in the rabbit skin.
Statistical analyses of the given results show a significant difference between formula C13 and G13 in the diffusion percent. The release rate of formula G13 is less than formula C13. It was expected as the increase in the system viscosity upon, hindering the Cl permeability. Therefore, the cubosomal gel formulae achieved higher and sustained skin retention comparing to the pure drug.
The cubosomal formulae had a significant improvement effect on Cl skin permeation. As presented in Table 5, all the studied formulae revealed that a higher transdermal flux value (J) than the pure drug (3.357 and 2.347 mg/cm2 h for C13 and G13 respectively). Therefore, these results revealed that the predominance of the prepared formulae in sustaining the Cl release and enhancing the skin permeation 6 folds more than the drug suspension (Brinon et al., 1999, Cappel and Kreuter, 1991).
Table 5.
Transdermal permeation parameters of Cl-cubosomal formulae through a rabbit skin compared to the pure drug aqueous suspension.
| Experiment | J* (mg/cm2 h) | Kpa** (cm/h) × 10−2 | Enhancement factor |
|---|---|---|---|
| Pure drug | 0.554777 | 27.73885 | – |
| C13 | 3.3568 | 167.84 | 6.050719 |
| G13 | 2.347165 | 117.3582 | 4.230825 |
J* = transdermal flux value; Kpa** = the permeability coefficient.
3.3.5. Skin deposition
All the selected formulae C13 and G13 showed an increase in the skin deposition (Fig. 5B). The highest skin deposition was in the C13 formulation up to 7 times compared to the pure drug. Remarkably, the hydrophobic and hydrophilic domain mixed effect has a great influence. Non-ionic surfactants can enhance Cl partition and deposition in the skin layers as it can combine with the stratum corneum lipid bilayer along with denaturation of skin protein (Cappel and Kreuter, 1991). GMO had also an optimistic effect on the drug retention as well as the permeation enhancer activity of monoolein. The GMO dual effect was markedly facilitating the skin retention and transdermal delivery system (Lopes et al., 2005, Lopes et al., 2007).
3.4. Pharmacological evaluation
After pilocarpine administration, the animals in the negative control group showed increased PCS and SMS which continued from 10 to 15 min. These behavioral changes advanced to motor seizures for 20 min and then advanced to SE (Table 6). All animals in the −ve control group (pilocarpine only) have no significant difference statistically when compared to the C-CL group according to the Fisher test. No reduction in PCS and SMS was noticed or change in any tested parameters.
Table 6.
Behavioural changes in the animals in pilocarpine model of seizures.
| Group | % PCS | % SMS | Seizures latency (min) |
% Seizures | SE latency (min) |
Death latency (min) |
% Death |
|---|---|---|---|---|---|---|---|
| −ve group | 100 | 96.4 | 6.56 ± 0.66 | 96.4 | 13.18 ± 1.48 | 17.0 ± 1.71 | 82 |
| C-CL | 100 | 95.9 | 6.16 ± 0.63 | 95.9 | 13.33 ± 1.23 | 17.8 ± 1.68 | 81 |
| R-CL | 99.9 | 77.1 | 21.71 ± 2.34 | 40 | 24.71 ± 2.15 | 24.17 ± 2.23 | 68 |
| T-CL | 99.7 | 46.7 | 33.17 ± 3.33 | 17.5 | 47.17 ± 3.21 | 51.25 ± 4.13 | 25 |
SE = status epileptics; −ve group = pilocarpine 400 mg/kg, IP, n = 15; C-CL = GMOPF127 mixture, t.d., n = 15; R-CL = Clonazepam of 100 mg/g, 40 mg/kg, orally, n = 15; T-CL = CL-cubogel of 50 mg/g, 40 mg/kg, t.d., n = 15. The results were expressed as the mean ± standard error and percentage (%).
The development of seizures was prevented in 82.5% and 60% of animals in R-CL and T-CL groups receiving oral and transdermal CL, respectively. There were 32% survivors in R-CL group, with a deaths inhibition difference approximately 13% in relation to −ve control group or the C-Cl (Table 6). CL-cubogel had a surviving rate of 75% (43% lower compared to groups receiving oral CL). Cl-cubogel could increase the onset of the first seizure latency, latency for the SE and the death of the animals in relation to the other groups (p = 0.05).
The previous results indicated the high efficiency of transdermal Cl-cubogel to reduce epileptic seizures and alter the behavioral parameters induced by pilocarpine, than oral Cl. The high percentage of Cl absorbed through the transdermal route could be due to the cubosome particles nano size, as well as their elevated surfactant content. The later contributes to enhancing Cl permeation through the skin layers and interacts with the stratum cornea, which facilitated the disruption of the lipids’ skin (Damasceno et al., 2011, Silva et al., 2010).
4. Conclusion
From the previous study, we concluded that using 2.5% PF127 produces a non-homogenous cubosome formula. Adding PVA or Et with increasing PF127 concentration produce a homogenous cubosomes with irregular cubic shape, well dispersed as individual particles and with nanoparticle range. 5% PF127 and 5% PVA or 5% Et led to significant PS decreasing (p-value < 0.0001) and a significant increase in the EE capacity of the cubosomes. The prepared cubosomes formula comprised a thermodynamically stable system, there was no change in the homogeneity of the prepared formulae, no phase separation or oil drops on the surface for 10 days under stress condition. Converting cubosomes into cubogels improving the cubosomes’ retention time and flow. The cubosomes formulae produce a sustained release system. PVA has a negative impact on the release rate as it slows the release rate than ethanol. The drug permeation from the Cubo-gel and cubosome formulae was significantly higher in the rate and extent than the pure drug, due to the nanoparticle size and the existence of PF127, and Et as they act as a permeation enhancer. Cubosomal clonazepam formulae possibly bent a superior opportunity for Cl transdermal delivery with an entrapping ratio up to 6 times, in comparison to the pure drug suspension. Cl-cubogel reduces the epileptic seizures and alters the behavioral parameters induced by pilocarpine than oral Cl.
Finally, the present study provides a novel insight into clonazepam skin penetration via the cubic phase of cubosomal gel or cubosome for effective management of seizers with the benefit of reducing the risk of oral route. The cubosomal formulae could be considered as an innovative dosage form for Cl easily used by children through the transdermal route as a reservoir in patches as the transdermal route has probable application for chronic drug therapy as epilepsy. Based on these results, further clinical pharmacodynamics /pharmacokinetic and toxicological studies are required to investigate the clinical possibility of the prepared system.
Acknowledgement
The authors thank, Jamjoom Pharma for its support and help to complete this project.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahmad A., Alkharfy K.M., Wani T.A., Raish M. Application of Box-Behnken design for ultrasonic-assisted extraction of polysaccharides from Paeonia emodi. Int. J. Biol. Macromol. 2015;72:990–997. doi: 10.1016/j.ijbiomac.2014.10.011. [DOI] [PubMed] [Google Scholar]
- Akhlaghi S.P., Ribeiro I.R., Boyd B.J., Loh W. Impact of preparation method and variables on the internal structure, morphology, and presence of liposomes in phytantriol-Pluronic® F127 cubosomes. Colloids Surf., B. 2016;145:845–853. doi: 10.1016/j.colsurfb.2016.05.091. [DOI] [PubMed] [Google Scholar]
- Ali, B.A., 2017. Formulation and In-vitro evaluation of baclofen transdermal patches ahmed. Asian Journal of Pharmaceutics (AJP): Free full text articles from Asian J. Pharm. 11.
- Aslam M., Aqil M., Ahad A., Najmi A.K., Sultana Y., Ali A. Application of Box-Behnken design for preparation of glibenclamide loaded lipid based nanoparticles: optimization, in vitro skin permeation, drug release and in vivo pharmacokinetic study. J. Mol. Liq. 2016;219:897–908. [Google Scholar]
- Azhari H., Strauss M., Hook S., Boyd B.J., Rizwan S.B. Stabilising cubosomes with Tween 80 as a step towards targeting lipid nanocarriers to the blood–brain barrier. Eur. J. Pharm. Biopharm. 2016;104:148–155. doi: 10.1016/j.ejpb.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Benamer H.T.S., Grosset D.G. A systematic review of the epidemiology of epilepsy in Arab countries. Epilepsia. 2009;50:2301–2304. doi: 10.1111/j.1528-1167.2009.02058.x. [DOI] [PubMed] [Google Scholar]
- Bhattarai N., Gunn J., Zhang M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Del. Rev. 2010;62:83–99. doi: 10.1016/j.addr.2009.07.019. [DOI] [PubMed] [Google Scholar]
- Blount Iv R.P., Bhattarai N. Natural polysaccharide-based hydrogels for controlled localized drug delivery, GELS HANDBOOK: fundamentals, properties and applications volume 3: application of hydrogels in drug delivery and biosensing. World Sci. 2016:35–59. [Google Scholar]
- Brandl M., Massing U. Vesicular phospholipid gels. Lipos. Technol. 2016;1:241–260. [Google Scholar]
- Brinon L., Geiger S., Alard V., Doucet J., Tranchant J.-F., Couarraze G. Percutaneous absorption of sunscreens from liquid crystalline phases. J. Control. Release. 1999;60:67–76. doi: 10.1016/s0168-3659(99)00054-1. [DOI] [PubMed] [Google Scholar]
- Camfield P., Camfield C. Incidence, prevalence and aetiology of seizures and epilepsy in children. Epileptic Disord. 2015;17:117–123. doi: 10.1684/epd.2015.0736. [DOI] [PubMed] [Google Scholar]
- Cappel M.J., Kreuter J. Effect of nonionic surfactants on transdermal drug delivery: II. Poloxamer and poloxamine surfactants. Int. J. Pharm. 1991;69:155–167. [Google Scholar]
- Caselli D., Rosati A., Faraci M., Podda M., Ripaldi M., Longoni D., Cesaro S., Nigro L.L., Paolicchi O., Maximova N. Risk of seizures in children receiving busulphan-containing regimens for stem cell transplantation. Biol. Blood Marrow Transpl. 2014;20:282–285. doi: 10.1016/j.bbmt.2013.10.028. [DOI] [PubMed] [Google Scholar]
- Chanp L.W., Heng W.S., Wan L.S.C. Effect of cellulose derivatives on alginate micro spheresprepared by emulsification. J. Microencapsul. 1997;14:545–555. doi: 10.3109/02652049709006808. [DOI] [PubMed] [Google Scholar]
- Clares B., Calpena A.C., Parra A., Abrego G., Alvarado H., Fangueiro J.F., Souto E.B. Nanoemulsions (NEs), liposomes (LPs) and solid lipid nanoparticles (SLNs) for retinyl palmitate: effect on skin permeation. Int. J. Pharm. 2014;473:591–598. doi: 10.1016/j.ijpharm.2014.08.001. [DOI] [PubMed] [Google Scholar]
- D’Souza S., Faraj J.A., Giovagnoli S., DeLuca P.P. In vitro–in vivo correlation from lactide-co-glycolide polymeric dosage forms. Prog. Biomater. 2014;3:131–142. doi: 10.1007/s40204-014-0029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Magro, R., Ornaghi, F., Cambianica, I., Beretta, S., Re, F., Brambilla, A., Barbero, F., Musicanti, C., Cagnotto, A., Donzelli, E., Enhanced brain targeting of engineered solid lipid nanoparticles.
- Damasceno, B., Silva, J.A., Oliveira, E.E., Silveira, W., Araújo, I.B., Oliveira, A.G.d., Egito, E., 2011. Microemulsão: um promissor carreador para moléculas insolúveis. Revista de Ciências Farmacêuticas Básica e Aplicada, pp. 9–18.
- Dell’osso B., Lader M. Do benzodiazepines still deserve a major role in the treatment of psychiatric disorders? A critical reappraisal. Eur. Psychiatry. 2013;28:7–20. doi: 10.1016/j.eurpsy.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Dian L., Yang Z., Li F., Wang Z., Pan X., Peng X., Huang X., Guo Z., Quan G., Shi X. Cubic phase nanoparticles for sustained release of ibuprofen: formulation, characterization, and enhanced bioavailability study. Int. J. Nanomed. 2013;8:845. doi: 10.2147/IJN.S40547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Dahmy R.M., Elsayed I., Elshafeey A.H., El Gawad N.A.A., El-Gazayerly O.N. Optimization of long circulating mixed polymeric micelles containing vinpocetine using simple lattice mixture design, in vitro and in vivo characterization. Int. J. Pharm. 2014;477:39–46. doi: 10.1016/j.ijpharm.2014.10.003. [DOI] [PubMed] [Google Scholar]
- El Maghraby G.M. Transdermal delivery of hydrocortisone from eucalyptus oil microemulsion: effects of cosurfactants. Int. J. Pharm. 2008;355:285–292. doi: 10.1016/j.ijpharm.2007.12.022. [DOI] [PubMed] [Google Scholar]
- Elsayed M.M.A., Abdallah O.Y., Naggar V.F., Khalafallah N.M. Deformable liposomes and ethosomes: mechanism of enhanced skin delivery. Int. J. Pharm. 2006;322:60–66. doi: 10.1016/j.ijpharm.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Enin H.A.A., El Nabarawy N.A., Elmonem R.A.A. Treatment of radiation-induced oral mucositis using a novel accepted taste of prolonged release mucoadhesive Bi-medicated double-layer buccal films. AAPS PharmSciTech. 2016:1–13. doi: 10.1208/s12249-016-0533-z. [DOI] [PubMed] [Google Scholar]
- Esposito E., Eblovi N., Rasi S., Drechsler M., Di Gregorio G.M., Menegatti E., Cortesi R. Lipid-based supramolecular systems for topical application: a preformulatory study. AAPS PharmSci. 2003;5:62–76. doi: 10.1208/ps050430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas R.M., Sousa F.C.F., Viana G.S.B., Fonteles M.M.F. Effect of gabaergic, glutamatergic, antipsychotic and antidepressant drugs on pilocarpine-induced seizures and status epilepticus. Neurosci. Lett. 2006;408:79–83. doi: 10.1016/j.neulet.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Godin B., Touitou E. Transdermal skin delivery: predictions for humans from in vivo, ex vivo and animal models. Adv. Drug Del. Rev. 2007;59:1152–1161. doi: 10.1016/j.addr.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Gratieri T., Alberti I., Lapteva M., Kalia Y.N. Next generation intra-and transdermal therapeutic systems: using non-and minimally-invasive technologies to increase drug delivery into and across the skin. Eur. J. Pharm. Sci. 2013;50:609–622. doi: 10.1016/j.ejps.2013.03.019. [DOI] [PubMed] [Google Scholar]
- Gupta U., Sharma S., Khan I., Gothwal A., Sharma A.K., Singh Y., Chourasia M.K., Kumar V. Enhanced apoptotic and anticancer potential of paclitaxel loaded biodegradable nanoparticles based on chitosan. Int. J. Biol. Macromol. 2017;98:810–819. doi: 10.1016/j.ijbiomac.2017.02.030. [DOI] [PubMed] [Google Scholar]
- Hart, L.A., Sibai, B.M., Seizures in pregnancy: epilepsy, eclampsia, and stroke, fourth ed., Elsevier, pp. 207–224. [DOI] [PubMed]
- He, H., Rahimi, K., Zhong, M., Mourran, A., Luebke, D.R., Nulwala, H.B., Möller, M., Matyjaszewski, K., 2017. Cubosomes from hierarchical self-assembly of poly (ionic liquid) block copolymers. Nat. Commun. pp. 8. [DOI] [PMC free article] [PubMed]
- Jain V., Swarnakar N.K., Mishra P.R., Verma A., Kaul A., Mishra A.K., Jain N.K. Paclitaxel loaded PEGylated gleceryl monooleate based nanoparticulate carriers in chemotherapy. Biomaterials. 2012;33:7206–7220. doi: 10.1016/j.biomaterials.2012.06.056. [DOI] [PubMed] [Google Scholar]
- Jantratid, E., Vertzoni, M., 2016. 12 dissolution testing to forecast in vivo performance of immediate-release formulations. Oral Drug Absorpt., pp. 224.
- Kim M.K., Chung S.J., Lee M.H., Cho A.R., Shim C.K. Targeted and sustained delivery of hydrocortisone to normal and stratum corneum-removed skin without enhanced skin absorption using a liposome gel. J. Control. Release. 1997;46:243–251. [Google Scholar]
- Knipe J.M., Chen F., Peppas N.A. Enzymatic biodegradation of hydrogels for protein delivery targeted to the small intestine. Biomacromolecules. 2015;16:962–972. doi: 10.1021/bm501871a. [DOI] [PubMed] [Google Scholar]
- Kreider K.A., Stratmann R.G., Milano M., Agostini F.G., Munsell M. Reducing children's injection pain: lidocaine patches versus topical benzocaine gel. Pediatric Dent. 2001;23:19–23. [PubMed] [Google Scholar]
- Lasoń W., Chlebicka M., Rejdak K. Research advances in basic mechanisms of seizures and antiepileptic drug action. Pharmacol. Rep. 2013;65:787–801. doi: 10.1016/s1734-1140(13)71060-0. [DOI] [PubMed] [Google Scholar]
- Letcher M.G. second ed. Gale Virtual Reference Libraray, Brigham Narins; 2005. Epilepsy, the Gale Encyclopedia of Generic Disorders; pp. 422–425. [Google Scholar]
- Lopes L.B., Collett J.H., Bentley M.V.L.B. Topical delivery of cyclosporin A: an in vitro study using monoolein as a penetration enhancer. Eur. J. Pharm. Biopharm. 2005;60:25–30. doi: 10.1016/j.ejpb.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Lopes L.B., Lopes J.L.C., Oliveira D.C.R., Thomazini J.A., Garcia M.T.J., Fantini M.C.A., Collett J.H., Bentley M.V.L.B. Liquid crystalline phases of monoolein and water for topical delivery of cyclosporin A: characterization and study of in vitro and in vivo delivery. Eur. J. Pharm. Biopharm. 2006;63:146–155. doi: 10.1016/j.ejpb.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Lopes L.B., Speretta F.F.F., Bentley M.V.L.B. Enhancement of skin penetration of vitamin K using monoolein-based liquid crystalline systems. Eur. J. Pharm. Sci. 2007;32:209–215. doi: 10.1016/j.ejps.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Luo Q., Lin T., Zhang C.Y., Zhu T., Wang L., Ji Z., Jia B., Ge T., Peng D., Chen W. A novel glyceryl monoolein-bearing cubosomes for gambogenic acid: preparation, cytotoxicity and intracellular uptake. Int. J. Pharm. 2015;493:30–39. doi: 10.1016/j.ijpharm.2015.07.036. [DOI] [PubMed] [Google Scholar]
- Luszczki J.J., Jankiewicz K., Jankiewicz M., Czuczwar S.J. Influence of aminophylline on the anticonvulsive action of gabapentin in the mouse maximal electroshock seizure threshold model. J. Neural Transm. 2007;114:1539–1545. doi: 10.1007/s00702-007-0795-4. [DOI] [PubMed] [Google Scholar]
- Delgado-Charro M.B., Guy R.H. Taylor & Francis; London and New York: 2001. Transdermal Drug Delivery. [Google Scholar]
- Mallick, S.P., 2011. Gelatin based emulsion hydrogels as a matrix for controlled delivery.
- Miyamoto M., Iwasaki S., Chisaki I., Nakagawa S., Amano N., Hirabayashi H. Comparison of predictability for human pharmacokinetics parameters among monkeys, rats, and chimeric mice with humanised liver. Xenobiotica. 2017:1–12. doi: 10.1080/00498254.2016.1265160. [DOI] [PubMed] [Google Scholar]
- Mura P., Bragagni M., Mennini N., Cirri M., Maestrelli F. Development of liposomal and microemulsion formulations for transdermal delivery of clonazepam: effect of randomly methylated β-cyclodextrin. Int. J. Pharm. 2014;475:306–314. doi: 10.1016/j.ijpharm.2014.08.066. [DOI] [PubMed] [Google Scholar]
- Natarajan J., Baskaran M., Humtsoe L.C., Vadivelan R., Justin A. Enhanced brain targeting efficacy of Olanzapine through solid lipid nanoparticles. Artif. Cells Nanomed. Biotechnol. 2017;45:364–371. doi: 10.3109/21691401.2016.1160402. [DOI] [PubMed] [Google Scholar]
- Nguyen H.T.P., Soucé M., Perse X., Vial F., Perrier T., Yvergnaux F., Chourpa I., Munnier E. Lipid-based submicron capsules as a strategy to include high concentrations of a hydrophobic lightening agent in a hydrogel. Int. J. Cosmet. Sci. 2017 doi: 10.1111/ics.12397. [DOI] [PubMed] [Google Scholar]
- Nutan M.T.H., Reddy I.K. Pharmaceutical Suspensions. Springer; 2010. General principles of suspensions; pp. 39–65. [Google Scholar]
- Pan X., Han K., Peng X., Yang Z., Qin L., Zhu C., Huang X., Shi X., Dian L., Lu M. Nanostructed cubosomes as advanced drug delivery system. Curr. Pharm. Des. 2013;19:6290–6297. doi: 10.2174/1381612811319350006. [DOI] [PubMed] [Google Scholar]
- Patil, P.M., Wankhede, S.B., Chaudhari, P.D., 2015. A validated stability indicating HPLC method estimation of clonazepam in the bulk drug and pharmaceutical dosage form, Pharm. Anal. Acta.
- Peng X., Wen X., Pan X., Wang R., Chen B., Wu C. Design and in vitro evaluation of capsaicin transdermal controlled release cubic phase gels. AAPS PharmSciTech. 2010;11:1405–1410. doi: 10.1208/s12249-010-9481-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X., Zhou Y., Han K., Qin L., Dian L., Li G., Pan X., Wu C. Characterization of cubosomes as a targeted and sustained transdermal delivery system for capsaicin. Drug Des. Dev. Therapy. 2015;9:4209. doi: 10.2147/DDDT.S86370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piriyaprasarth S., Sriamornsak P. Flocculating and suspending properties of commercial citrus pectin and pectin extracted from pomelo (Citrus maxima) peel. Carbohyd. Polym. 2011;83:561–568. [Google Scholar]
- Rarokar N.R., Saoji S.D., Raut N.A., Taksande J.B., Khedekar P.B., Dave V.S. Nanostructured cubosomes in a thermoresponsive depot system: an alternative approach for the controlled delivery of docetaxel. AAPS PharmSciTech. 2016;17:436–445. doi: 10.1208/s12249-015-0369-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadanshio P.P., Wankhede S.B., Chaudhari P.D. A validated stability–indicating HPLC method estimation of ketoprofen in the presence of preservative in the bulk drug and formulated gel. World J. Pharm. Res. 2015;4:947–966. [Google Scholar]
- Shamma R.N., Elsayed I. Transfersomal lyophilized gel of buspirone HCl: formulation, evaluation and statistical optimization. J. Lipos. Res. 2013;23:244–254. doi: 10.3109/08982104.2013.801489. [DOI] [PubMed] [Google Scholar]
- Silva J.A., ApolináRio A.C., Souza M.S.R., Damasceno B.P.G.L., Medeiros A.C.D. Administração cutânea de fármacos: desafios e estratégias para o desenvolvimento de formulações transdérmicas. Revista de Ciências Farmacêuticas básica e aplicada. 2010;31:125–131. [Google Scholar]
- Singh G., Pai R.S., Devi V.K. Optimization of pellets containing solid dispersion prepared by extrusion/spheronization using central composite design and desirability function. J. Young Pharm. 2012;4:146–156. doi: 10.4103/0975-1483.100020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinko P.J., Martin A.N. Lippincott Williams & Wilkins; 2009. Martin’s Physical Pharmacy and Pharmaceutical Sciences: Physical Chemical and Biopharmaceutical Principles in the Pharmaceutical Sciences. [Google Scholar]
- Song X., Zhao Y., Wu W., Bi Y., Cai Z., Chen Q., Li Y., Hou S. PLGA nanoparticles simultaneously loaded with vincristine sulfate and verapamil hydrochloride: systematic study of particle size and drug entrapment efficiency. Int. J. Pharm. 2008;350:320–329. doi: 10.1016/j.ijpharm.2007.08.034. [DOI] [PubMed] [Google Scholar]
- Steluti R., De Rosa F.S., Collett J., Tedesco A.C., Bentley M.V.L.B. Topical glycerol monooleate/propylene glycol formulations enhance 5-aminolevulinic acid in vitro skin delivery and in vivo protophorphyrin IX accumulation in hairless mouse skin. Eur. J. Pharm. Biopharm. 2005;60:439–444. doi: 10.1016/j.ejpb.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Thakkar V., Korat V., Baldaniya L., Gohel M., Gandhi T., Patel N. Development and characterization of novel hydrogel containing antimicrobial drug for treatment of burns. Int. J. Pharm. Invest. 2016;6:158. doi: 10.4103/2230-973X.187343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turski W.A. Pilocarpine-induced seizures in rodents–17 years on. Pol. J. Pharm. 1999;52:63–65. [PubMed] [Google Scholar]
- Turski W.A., Cavalheiro E.A., Bortolotto Z.A., Mello L.M., Schwarz M., Turski L. Seizures produced by pilocarpine in mice: a behavioral, electroencephalographic and morphological analysis. Brain Res. 1984;321:237–253. doi: 10.1016/0006-8993(84)90177-x. [DOI] [PubMed] [Google Scholar]
- Wang S.-M., Kim J.-B., Sakong J.K., Suh H.-S., Oh K.S., Woo J.-M., Yoo S.-W., Lee S.M., Lee S.-Y., Lim S.-W. The efficacy and safety of clonazepam in patients with anxiety disorder taking newer antidepressants: a multicenter naturalistic study. Clin. Psychopharmacol. Neurosci. 2016;14:177. doi: 10.9758/cpn.2016.14.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Li J., Zhang Q., Yan X., Guo L., Gao X., Qiu M., Jiang X., Lai R., Chen H. A novel small Odorranalectin-bearing cubosomes: preparation, brain delivery and pharmacodynamic study on amyloid-β 25–35-treated rats following intranasal administration. Eur. J. Pharm. Biopharm. 2012;80:368–378. doi: 10.1016/j.ejpb.2011.10.012. [DOI] [PubMed] [Google Scholar]
- Zhang P., Gao W., Zhang L., Chen L., Shen Q., Wang X., Cui Y. In vitro evaluation of topical microemulsion of capsaicin free of surfactant. Biol. Pharm. Bull. 2008;31:2316–2320. doi: 10.1248/bpb.31.2316. [DOI] [PubMed] [Google Scholar]
- Zhuang C.-Y., Li N., Wang M., Zhang X.-N., Pan W.-S., Peng J.-J., Pan Y.-S., Tang X. Preparation and characterization of vinpocetine loaded nanostructured lipid carriers (NLC) for improved oral bioavailability. Int. J. Pharm. 2010;394:179–185. doi: 10.1016/j.ijpharm.2010.05.005. [DOI] [PubMed] [Google Scholar]