Abstract
Antibiotic resistance in bacterial species is opening new avenues to search for alternative modes of antimicrobial treatment, medicinal plant extracts being one among them. The aim of this study was to access the possibility of medicinal plant extract from Shih in the manufacture of pharmaceutical preparations for oral hygiene specifically for the prevention and treatment of dental caries due to Streptococcus mutans. Antimicrobial effects of crude organic extract of Shih on S. mutans isolated from the saliva were examined by taking S. mutans with culture media only (−ve control); S. mutans treated with the antibiotic gentamicin (+ve control) and S. mutans treated with Shih. Minimal Inhibitory Concentration (MIC) and Minimal Bactericidal Concentration (MBC) were Determination by Iodonitrotetrazolium chloride (INT) colorimetric assay Time-kill dynamic assay was performed using broth microdilution method. The metabolic reason behind the bacteriostatic and bactericidal effect were studied by measuring the glucose utilization by the microbes, pH as a measure of acid production, nucleic acids quantitation to check the DNA status and inhibition of water-insoluble glucan synthesis were undertaken. Shih MIC for S. mutans was at 2.5 mg/ml and MBC was 4 mg/ml. S. mutans bacterial population started reclining within 60 min of incubation with Shih at MBC. Utilization of added glucose was very high at MIC due to bacteria overcoming the stress, whereas at MBC its utilization was less. Accordingly pH also became acidic to 2.9 with MIC and 4.03 with MBC. There was a great degree of inhibition in the formation of nucleic acids indicating this crude extract interferes with DNA replication. Inhibition of glucan synthesis was to the tune of 45% as compared to control. Thus we conclude that Shih has potentially effective antibacterial activity hence it can be proposed as a potentially effective antiplaque and anticariogenic agent in the form of mouth wash or gum paint. However, the cytotoxicity of the extract needs to be evaluated in in-vitro and in-vivo conditions before it is considered as a safe antiplaque and anticariogenic agent.
Keywords: Artemisia herba-alba (Shih), Streptococcus mutans, Dental caries, Antimicrobial effect, Metabolic effects
1. Introduction
Streptococcus mutans, the human pathogen that resides in the oral cavity is a gram-positive bacterium that has low content of G + C. It is believed to be the principal etiological cause in the development of dental caries (Loesche, 1986). For the survival in the dental plaque S. mutans has developed a biofilm existence that forms on the tooth surface (Hamada and Slade, 1980). This pathogen is a potential inducer of infective endocarditis, wherein to about 14% of viridians streptococcus induced endocarditis are caused by S. mutans (Loesche, 1986, Hamada and Slade, 1980, Ullman et al., 1988). The dietary carbohydrate sucrose is converted to a sticky polysaccharides known as glucans by S. mutans (Banas and Vickerman, 2003, Kuramitsu, 1993). S. mutans colonizes the oral cavity with the help of surface linked proteins which bind to glucan, resulting in the formation of biofilms on the tooth surface, commonly known as dental plaque. Several mechanisms have been evolved by this organism to sustain its existence in the oral cavity (Carlsson and Hamilton, 1994) and to resist extreme environmental anomalies (Ahn et al., 2006, Lemos et al., 2005).
There has been a worldwide increase in the number of streptococci resistant to various antibiotics (Emslie, 1974) and the incidence of resistance among S. mutans is also increasing (Ferretti and Ward, 1976). Increase in the frequency of therapeutic failure is due to continuous emergence of gram-positive multi drug resistant bacteria which drastically reduce the efficacy of our antibiotic armory (Rice, 2006). Hence there has been a global urge to scrutinize alternatives to antibiotics in the treatment and prevention of dental caries caused by S. mutans.
Though many anticariogenic agents have been used to control caries activity, none possess the ideal cariogenic properties. Saudi Arabian population uses numerous medicinal herbs to treat oral and periodontal diseases. Their effectiveness is well known; however, not all have been validated scientifically. This paper aims to validate the effectiveness of a commonly used Arabian medicinal herb, Artemisia herba-alba (Shih – An Arabic term used locally) on the inhibition of salivary Streptococcus mutans. A secondary objective is to investigate the mechanism of action of Shih. Shih is an aromatic herb known for bitter tasting leaves commonly known as Wormwood. Many species of wormwood are used medicinally in various parts of the world including the Arabian Peninsula. One Armenian worm wood is perennial and one of the most common plants of semi-desert areas. Used as a fuel and a pasture plant, Armenian wormwood is also well-known to the Bedouins as a healing plant. Santolina alcohol is the active component in Artemisia herba-alba (Shih) that exhibits the antimicrobial action. Armenian wormwood is used by Bedouins as a medicine often by inhaling the smoke. It has also been used in “toothpaste” which not only polished the teeth but also counteracted decay, treated bad breath and protected the mouth. One of the aims of this study was to investigate the mechanism of action of this antimicrobial herb whether they are inhibiting DNA synthesis or metabolic pathway or destroying cell membrane.
2. Materials and methods
2.1. Study design
A Cross sectional study utilizing non-probable convenient sampling.
2.2. Study population
Fifty (50) systemically healthy patients of various nationalities and ethnicities attending Dental Clinics were recruited for this study.
2.2.1. Inclusion criteria
-
1.
Equal number of male and female patients between the age group of 18–50 years.
-
2.
Patients with a DMFT of 1 or more.
-
3.
Patients with at least 25 permanent teeth.
-
4.
Patients willing to participate in the study.
2.2.2. Exclusion criteria
-
1.
Patients with systemic diseases (including diabetes mellitus) and syndromic disorders.
-
2.
Patients with periodontal disease.
-
3.
Immuno-compromised patients.
-
4.
Patients on corticosteroids and/or hormones as medication.
-
5.
Patients who had suffered from upper respiratory tract infection one week prior to saliva collection or have used antibiotic for any other reason.
-
6.
Patients with salivary flow rate less than 0.1 ml/min.
2.3. Ethics approval
Ethical approval for the study has been granted by the Research Ethics Committee of the institute vide order number SRC/REG/2016–2017/45.
2.4. Personal and demographic data
Personal data of the study population was recorded including the date of birth, gender, nationality and past medical history. The study population was informed regarding the ‘Objectives’, and ‘Expected Outcome’ of the study through an Informed consent form. The patients were asked to read and sign the printed informed consent in Arabic and English prior to sample collection.
2.5. Collection of saliva samples
Unstimulated whole saliva samples were collected by passive drooling method. Separate dental cubicles at Dental Diagnostic Clinics were assigned for the sample collection. All samples were collected between 2 and 4 pm according the method previously described (Silwood et al., 2002). The study subjects were instructed not to eat or drink 1–2 h before saliva collection. The study subjects were made to sit upright with head tilted forward for optimum collection of saliva. They were asked to rinse the mouth thoroughly with water and spit out completely. 5 min later the study participants were asked to collect saliva in the floor of the mouth and spit in a sterile labeled graduated container. The collected samples was frozen immediately and transported to the laboratory. At the laboratory the samples were stored at −80 °C. After all the samples were collected, saliva samples were thawed and used for the determination of antimicrobial effect of shih on salivary Streptococcus mutans and the biochemical mechanisms involved in it.
2.6. Distribution of study groups
The antimicrobial effects of the crude organic extract of the medicinal plant Shih on Streptococcus mutans isolated from the saliva was studied and examined under three groups
Group 1 (G1): S. mutans with culture media only – Negative control.
Group 2 (G2): S. mutans treated with the antibiotic Gentamicin – Positive control.
Group 3 (G3): S. mutans treated with Artemisia herba-alba (Shih) – Experimental sample.
2.7. Laboratory procedures
2.7.1. Plant material and preparation of crude extract
The aerial parts of Artemisia herba-alba (Shih) were collected from Farasan Island of Red Sea during the peak harvest season viz. in the month of March 2017, and were presented for identification by the Botanical experts. The herb was air dried and 100 g of this medicinal plant was pulverized in an electric mixer to obtain a fine powder of the material. This powder was macerated and the plant oils extracted in 1 L of Dioxane (ether) at 50 °C using a continuous stirrer for 24 h. The solvent was then removed at 70 °C under reduced pressure in a rotary evaporator to yield a light green colour residue weighing 9.4 g.
2.7.2. Growth media
Brain Heart Infusion (BHI) broth was used in the preparation of plaque bacteria suspension and Columbia Nutrient agar (CNA) was used to selectively grow Streptococcus species in plaque bacteria. Colonies of streptococci were identified by gram staining from which the Optochin resistant colonies of Streptococcus mutans were isolated and stored for further use.
2.7.3. Determination of Minimal Inhibitory Concentration (MIC) and Minimal Bactericidal Concentration (MBC) by iodonitrotetrazolium chloride (INT) colorimetric assay
Minimal Inhibitory Concentration (MIC) of the plant extract on Streptococcus mutans was determined using broth microdilution method described by Kuete et al. (2008) with some modifications (Mativandlela et al., 2006). Briefly, plant extract, and reference antibiotic gentamicin were dissolved in dimethyl sufoxide (DMSO)-Mueller Hinton Broth (MHB) (10:90). The microbial growth is not affected if DMSO is lower than 2.5% as the final concentration in the inoculated media (Kuete et al., 2008). 100 μL of serially diluted plant extract (80–1.25 mg/mL) were added to each of the wells of a sterile Nunc 96-well polystyrene ELISA plate. One hundred microlitre (100 μL) of inoculum at 1.5 × 106 CFU/mL prepared in appropriate MHB was then added to each of the well (Tereschuk et al., 1997). Wells containing DMSO-MHB with bacterial inoculum only served as the bacterial growth control (−ve control) and MHB with different concentrations of gentamicin (80–1.25 µg/ml) served as reference antibiotic control (+ve control). Additional controls included MHB alone (medium sterility control) and bacterial inoculum. A sterile plate sealer was used to cover the plates, and the contents of the wells were mixed by agitating over a plate shaker. After 18 h incubation at 37 °C the MIC of samples was detected. This was done by addition of 40 μL of 0.2 mg/mL p-iodonitrotetrazolium chloride (INT) and incubation at 37 °C for 30 min. Conversion of the dye to a pink color from yellow indicated viable bacteria. Attempts to determine the turbidity of the microcultures with a microplate reader failed because the cells of the test organisms clumped at the bottom of the well, precipitation of compounds present in extracts of plant and the green colour of the extract also caused problems. Hence the use of the tetrazolium salt p-iodonitrotetrazolium chloride (INT) which changes its colour was detected visibly and was used to determine the MIC and MBC (Eloff, 1998). MIC was confirmed in that well (sample concentration) which did not show the color change of the medium. The MBC was determined by adding 50 μL aliquots of the preparations, from the concentration which did not show any growth after incubation during MIC assays upwards (2.5–10 mg/ml of plant extract and 2.5–25 µg/ml of gentamicin), to 150 μL of the broth. These preparations were incubated at 37 °C for 48 h. The lowest concentration of extract that did not show any color change upon adding INT was taken as the Minimum Bactericidal Concentration (MBC) (Zgoda and Porter, 2001).
2.7.4. Time-kill dynamic curves against Streptococcus mutans
Time-kill dynamic assay was performed using broth microdilution method as previously described (Avila et al., 1999) with minor modifications. The final concentration of suspension of S. mutans was adjusted to 1 × 106 CFU/mL. The crude medicinal plant extract, and gentamicin were used in the time-kill dynamic experiment. Cells treated with concentrations corresponding to ½ MIC; MIC and MBC of each sample were incubated at 37 °C for 0, 30, 60, 120, 240, 480, and 960 min. The final concentration of DMSO was 2.5%. A control sample was made using DMSO 2.5% and the inoculum. 50 μL of liquid was removed at each incubation time point for ten-fold serial dilution. The number of CFU/mL was counted by spreading 25 μL liquid from each dilution on the surface of the MHA plates and incubated at 37 °C for 24 h. Time-kill curves were built by plotting the number of CFU/mL against time (min).
2.7.5. Glucose metabolism and measurement of pH
Glucose metabolism was studied in order to evaluate the inhibitory effects of Artemisia herba-alba on S. mutans. Samples measuring 10 ml from the culture media were collected at regular intervals of 6, 8, 12, 24, and 48 h in a pre-autoclaved candle jar in which an anaerobic atmosphere was created by lighting a candle inside the jar to consume part of the oxygen. Initial pH of the samples was noted and neutralized to pH 7.0 using 0.1 M KOH. 300 mg/dl of glucose solution was prepared in sterile distilled water and 10 ml of this solution was added to the jar containing the S. mutans culture broth. The amount of glucose used by S. mutans was measured using a commercially available kit, which uses the enzymes glucose oxidase and peroxidase in the presence of 4-aminoantipyrine and p-hydroxybenzoate. This reaction is based on the oxidation of the glucose molecule with the consequent production of a compound (quinoneimine) that was spectrophotometrically quantified using Lambert-Beer's law at 510 nm. A decrease in glucose concentration with time was an indication of bacteria actively fermenting the carbohydrate and vice versa. Acid production as a result of glucose metabolism by the bacteria in the samples was measured at 37 °C in a pH stat, the decrease in pH from 7 was recorded over 15 min time.
2.7.6. Quantification of nucleic acids
Samples from the culture dishes were taken at regular intervals of 6, 8, 12, and 24 h and nucleic acids were quantitated by absorption spectrophotometry at 260 nm. Using the Beer-Lambert Law it is possible to relate the amount of light absorbed to the concentration of the nucleic acids in the culture media. At a wavelength of 260 nm, the average extinction coefficient for double-stranded DNA is 0.020 (µg/ml)/−1 cm−1, for single-stranded DNA it is 0.027 (µg/ml)/−1 cm−1, for single-stranded RNA it is 0.025 (µg/ml)/−1 cm−1 and for short single-stranded oligonucleotides it is dependent on the length and base composition. Thus, an Absorbance (A) of 1 corresponds to a concentration of 50 µg/ml for double-stranded DNA, for ssDNA it is 37 µg/ml and for ssRNA it is 40 µg/ml. Sample Purity was assessed by using the OD ratios 260:280/260:230. Quantity of nucleic acids in each of the samples tested was inferred for the inhibition of DNA synthesis by Shih or vice versa.
2.7.7. Inhibition of water-insoluble glucan synthesis
The assay and preparation of crude glucosyltransferase (GTase) was based on the method previously described by Koo et al. (2000). The cell- free enzymes were precipitated from culture supernatant of S. mutans. After filtering the culture supernatant of S. mutans using a 0.2 µm membrane filter, the cell-free enzymes were extracted and precipitated by Amicon ultra centrifugal filter (MWCO 30 kDa, Millipore, USA). The crude enzymes were restored at −80 °C and used for synthesis of water-insoluble glucan. A reaction mixture consisting of 20 µL of crude enzyme, 180 µL of the diluted herbal medicine extract in 800 µL of 62.5 mM potassium phosphate buffer (pH 6.5) containing 12.5 g of sucrose and 0.25 g of sodium azide were incubated at 37 °C for 30 h. After incubation, the fluid was removed, and the contents that stick to tube wall were washed with sterile water and dispersed by a sonicator. The total amount of water-insoluble glucan was measured by the absorbance at 890 nm in spectrophotometer. Percent inhibition of the synthesis of glucan by the plant extract was compared with that of gentamicin and control having only S. mutans with the culture media.
2.8. Statistical analysis
The data was analyzed using Statistical Package for Social Sciences (SPSS), Windows version 21.0, 2015. Independent t-test was used to compare group means. Statistical significance was set at P ≤ 0.05.
3. Results
The Minimum Inhibitory Concentration (MIC) of Shih organic extract on S. mutans was 2.5 mg/ml where as for the positive control gentamicin was 7.5 µg/ml and the Minimum Bactericidal Concentration (MBC) for the organic extracts of Shih was 4.0 mg/ml and for the reference antibiotic gentamicin on salivary S. mutans was 15 µg/ml. Further experiments were carried out taking these values into consideration.
3.1. Time-kill dynamic curves against Streptococcus mutans
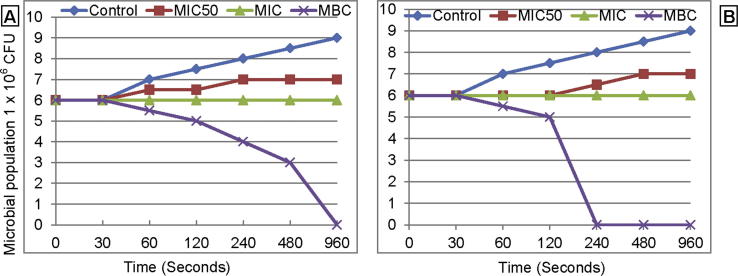
The time-kill dynamic curves for shih and gentamicin against S. mutans are presented in Fig. 1. The bacterial concentration increased steadily with time in control upto 1 × 109 CFU/ml in 960 min. At MIC50 there was non-statistical increase in the bacterial population to 1 × 107 from the initial inoculum of 1 × 106 CFU/ml. At MBC there was a slower decrease in the bacterial population with Shih as compared to gentamicin which was much faster and reached null within 240 min.
Fig. 1.
The time-kill dynamic curves against Streptococcus mutans by Shih oil extract (A) and gentamicin (B).
3.2. Measurement of glucose metabolism
Table 1 shows the values of glucose and pH respectively. The mean glucose concentration reduced drastically with time in the control where there was neither any antibiotic gentamicin nor any shih plant extract. The reduction in glucose level in the culture media of control sample from 18 h after incubation was statistically significant (P < 0.05). However it was not statistically significant between 48 and 72 h indicating that there was not much growth of bacteria either due to lack of dilution of the media or constriction in the space. When S. mutants was inoculated with the antibiotic gentamicin at MIC the glucose levels in the culture media reduced much more as compared to the control indicating that the microbes were striving to overcome the effect of the drug using more energy for the enhancement of the various metabolic activities. The decrease in the level of glucose was statistically significant from 18 h of incubation but it did not show any difference statistically with that of control at 72 h of incubation. However gentamicin containing culture at MBC did not use up any glucose as most of the bacterial population ceased to survive from 12 h of incubation. Crude Shih extract at MIC showed increased usage of glucose on par with gentamicin again owing to the fact that the cells tried to survive the effect of the plant extract. Likewise crude Shih organic extract inoculated with MBC did not show significant use of glucose throughout the culture period due to death of the cells.
Table 1.
Glucose concentration (mg/dl) in culture media by use of shih oil extract on S. mutans.
| Treatment | Time (h) |
||||||
|---|---|---|---|---|---|---|---|
| 0 | 6 | 12 | 18 | 24 | 48 | 72 | |
| −ve control | 300a ± 0 | 279a ± 2.33 | 255a ± 4.41 | 180b ± 3.08 | 105c ± 2.9 | 57d ± 3.67 | 29.5d ± 0.66 |
| +ve Control at MIC | 300a ± 0 | 249a ± 8.68 | 209a ± 5.99 | 118c ± 7.33 | 84e ± 10.05 | 26f ± 0.85 | 18fd ± 2.78 |
| +ve control at MBC | 300a ± 0 | 276a ± 3.68 | 260a ± 1.87 | 254a ± 1.85 | 246a ± 1.93 | 237a ± 3.4 | 228a ± 6.44 |
| Plant extract at MIC | 300a ± 0 | 243a ± 7.69 | 210a ± 8.99 | 126c ± 6.03 | 83d ± 7.15 | 29f ± 0.85 | 19fd ± 1.25 |
| Plant extract at MBC | 300a ± 0 | 275a ± 3.56 | 256a ± 1.65 | 199b ± 7.57 | 194b ± 4.52 | 193b ± 4.75 | 191b ± 0.48 |
(1) −ve control = S. mutans in MHB; +ve control = Gentamicin (7.5 µg/ml MIC and 15 µg/ml MBC); Plant extract Shih (2.5 mg/ml MIC and 4 mg/ml MBC).
(2) Values are mean ± Standard error.
(3) Values in the same row and same time period bearing the same superscript do not differ significantly (P < 0.05).
3.3. Measurement of pH
pH was measured to access the acid production as a result of the metabolic activity by the cells which is presented in Table 2. pH values increased statistically (P < 0.05) to about 10.54 in −ve control and 10.65 in the plant extract treatment after 6 h of incubation. However in +ve control samples with gentamicin, the pH dropped down to 6.63 at MIC and 7.8 with MBC which was statistically insignificant. The pH became acidic in the negative control samples with increase in incubation time due to increase in acid production as a result of continuous metabolism by the cells. Plant extract treatment with MIC significantly reduced the pH to 2.9 after 72 h of incubation, indicating higher metabolic activity of the cells in a bid to survive the stress. Whereas at MBC of the plant extract the pH dropped only to 4.03 as most of the cells were dead.
Table 2.
pH values in culture media by use of shih oil extract on S. mutans.
| Treatment | Time |
||||||
|---|---|---|---|---|---|---|---|
| 0 | 6 | 12 | 18 | 24 | 48 | 72 | |
| −ve control | 7a ± 0 | 10.54c ± 0.55 | 7.63a ± 0.26 | 6.6a ± 0.04 | 5.48d ± 0.05 | 4.65bd ± 0.07 | 3.55e ± 0.03 |
| +ve Control at MIC | 7a ± 0 | 6.53a ± 0.25 | 5.38b ± 0.21 | 4.08b ± 0.09 | 3.3b ± 0.09 | 3.03b ± 0.06 | 2.93d ± 0.05 |
| +ve control at MBC | 7a ± 0 | 7.8a ± 0.15 | 6.53a ± 0.25 | 6.45a ± 0.24 | 6.38a ± 0.21 | 6.55a ± 0.16 | 6.15a ± 0.07 |
| Plant extract at MIC | 7a ± 0 | 10.65c ± 0.07 | 5.1b ± 0.17 | 4.63b ± 0.13 | 3.2b ± 0.09 | 3.18b ± 0.16 | 2.9d ± 0.04 |
| Plant extract at MBC | 7a ± 0 | 10.65c ± 0.07 | 5.1b ± 0.17 | 4.63b ± 0.13 | 4.25b ± 0.12 | 4.1b ± 0.19 | 4.03b ± 0.28 |
(1) −ve control = S. mutans in MHB; +ve control = Gentamicin (7.5 µg/ml MIC and 15 µg/ml MBC); Plant extract Shih (2.5 mg/ml MIC and 4 mg/ml MBC).
(2) Values are mean ± Standard error.
(3) Values in the same row and same time period bearing the same superscript do not differ significantly (P < 0.05).
3.4. Quantification of nucleic acids
Nucleic acid levels in the culture media at different time intervals with various groups are shown in Table 3. The nucleic acid levels significantly (P < 0.05) increased only in negative control samples with advancement in time of incubation and reached 20.5 µg/ml. Whereas in the treatment groups both the plant extract and gentamicin treated samples did not show any significant change in the nucleic acid levels due to inhibition in DNA replication.
Table 3.
Nucleic acid levels (µg/ml) in culture media by use of shih oil extract on S. mutans.
| Treatment | Time |
||||||
|---|---|---|---|---|---|---|---|
| 0 | 6 | 12 | 18 | 24 | 48 | 72 | |
| −ve control | 9a ± 0 | 11.75a ± 1.25 | 13.50a ± 0.29 | 15.75b ± 1.44 | 16.25b ± 0.85 | 17.25b ± 1.11 | 20.50c ± 0.65 |
| +ve Control at MIC | 9a ± 0 | 10a ± 0.41 | 10.5a ± 0.29 | 10.75a ± 0.63 | 9.25a ± 0.48 | 9a ± 0.41 | 8.5a ± 0.29 |
| +ve control at MBC | 9a ± 0 | 8.75a ± 0.48 | 8.5a ± 0.29 | 7.75a ± 0.49 | 7.75a ± 0.49 | 7.25a ± 0.25 | 7a ± 0.4 |
| Plant extract at MIC | 9a ± 0 | 9.75a ± 0.25 | 10a ± 0.41 | 10.5a ± 0.66 | 10a ± 0.41 | 9.5a ± 0.65 | 9a ± 0.4 |
| Plant extract at MBC | 9a ± 0 | 9a ± 0.41 | 8.75a ± 0.75 | 8.5a ± 0.65 | 8.5a ± 0.66 | 8a ± 0.41 | 7.25a ± 0.25 |
(1) −ve control = S. mutans in MHB; +ve control = Gentamicin (7.5 µg/ml MIC and 15 µg/ml MBC); Plant extract Shih (2.5 mg/ml MIC and 4 mg/ml MBC).
(2) Values are mean ± Standard error.
(3) Values in the same row and same time period bearing the same superscript do not differ significantly (P < 0.05).
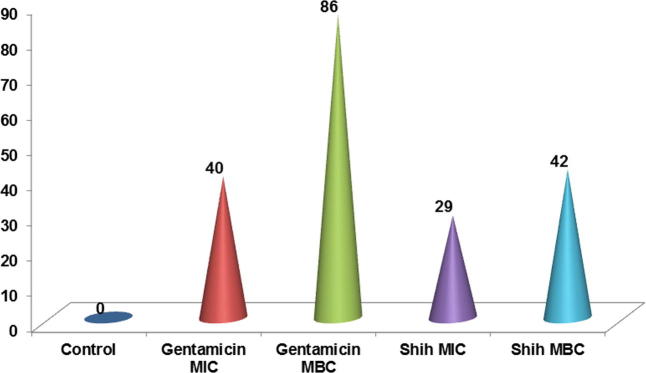
3.5. Inhibition of water-insoluble glucan synthesis
The percent inhibition of water-insoluble glucan synthesis is shown in Fig. 2. The inhibition of glucan synthesis was more with gentamicin at MBC (86%) followed by Shih with MBC (42%) which was tailed with gentamicin at MIC (40%) and the least inhibitory effect was with Shih at MIC (29%).
Fig. 2.
The percent inhibition of cell wall insoluble glucan due to the effect of Shih oil extract on S. mutans.
4. Discussion
Many researchers have made efforts to develop new treatment modalities using natural sources for the management of oral diseases, thus preventing subsequent systemic illnesses. Various materials and drugs have been used for the prevention and treatment oral conditions, exclusively by elimination of bacteria either by way of inhibition or by preventing the formation of biofilm, which is vital for bacterial survival (Hanus et al., 2005, Al-Rowais, 2002). Further, the severe rise in the resistance of microbes to conventional therapeutic agents opens new avenues to look into various approaches for exploring new drugs from the existing native natural resources. The use of Shih is common among Saudis, but no scientific data has been documented on its clinical use and the biochemical mechanism of antibacterial action. According to results of this study crude Shih (Artemisia herba-alba) plant organic extract, was able to inhibit the growth of S. mutans at 2.5 mg/ml concentration and cease bacterial growth at 4 mg/ml concentration. As mentioned in Fig. 1, S. mutans failed to grow in the presence of diffused components of Artemisia herba-alba crude organic extract. All plants used in conventional medicine that show the minimum inhibitory concentration (MIC) values of less than 8 mg/mL are designated as active (Fabry et al., 1998). Based on the above criteria, it can be concluded that the plant extract from Shih has a good antibacterial activity as its MIC value tested in this trial is 2.5 mg/ml (below 8 mg/ml). Out work is in agreement with other workers who reported similar results with other plant extracts (Chatterjee et al., 2017, Kanth et al., 2016, Hasan et al., 2015).
The other objective of the study was to assess the mechanism of action of this medicinal plant extract’s bacteriostatic and bactericidal effect. Hence we studied the metabolic activity of S. mutans including glucose utilization, pH change, nucleic acid levels and inhibition of water in-soluble glucans synthesis. S. mutans used higher amounts of glucose at MIC both with the plant extract and the antibiotic gentamicin indicating that the microbial cells were thriving hard to overcome the stress due to these drugs. At MBC the utilization of added glucose did not disappear from the media in gentamicin treated group due to early death of the cells whereas in Shih treated group at MBC there was little utilization of glucose because the death of S. mutants was little delayed.
pH became very alkaline both in the positive control (10.54) and plant extract treated cells (10.65) initial after 6 h of incubation and this can be attributed to the cessation of metabolic activities of the cells during the lag phase. The pH became acidic with time and reached a value of about 2.9 after 72 h of incubation with MIC of the plant extract due to higher rate of anaerobic fermentation of both the added sugar and glucose released from starch contained in the media by the bacterial membrane enzymes. However the pH of 4.03 seen with MBC at 72 h is due to the death of the cells, but the glycolytic enzymes continued the reactions producing the acid but at a slower rate.
There was significant difference in the nucleic acid levels when compared to negative control samples with the treatment groups indicating that these drugs interfere with DNA replication. In S. mutans, there is a very high degree of coordination between DNA replication and cell division (Huisman and D’Ari, 1981, Liu et al., 2001). Chromosomal aberration or disruption of DNA replication hampers with proper cell division which eventually results in blockage of DNA synthesis (Higgins et al., 1974, Patel and Weaver, 2006) and an expansion in cell surface area. The cause for enlargement of cell shape, as per our speculation is that treatment of S. mutans cells with Shih extract blocks DNA replication leading to improper cell division. Enlargement of cell shape perturbs normal cell envelope integrity. The bacterial cell envelope is the most vital cellular structures whose integrity needs to be preserved at all times under any situations in order to survive. Several environmental stresses such as high osmotic pressure, toxic chemicals like antibiotics, and acidic pH impair the integrity of the cell envelope. To cope with the cell envelope damage, bacteria have evolved various mechanisms to detect perturbations to the envelope.
It is known that many antibiotics acts as a cationic detergent, binding to lipopolysaccharides and other negatively charged molecules in the bacterial cell wall (Tsang et al., 1976). This is consistent with observations by other investigators who have shown representative strains of serotype b to be the most electronegative of the S. mutans strains (Olsson et al., 1976). If serotype b strains have a greater negative charge, their increased susceptibility to Shih oil extract could be a result of greater affinity for the molecule. Bacitracin is reported to inhibit bacterial cell wall synthesis by binding to a membrane phospholipid and thus blocking peptidoglycan synthesis Goodman and Gilman, 1975). Recently, Higerd et al. found that serotype a strains contain a fatty acid unique to this serotype (Higerd et al., 1977). Therefore, this fatty acid may be involved in the binding of bacitracin to membrane phospholipid, and thus contribute to the greater susceptibility of serotype-a strains to bacitracin. Our findings indicate that certain antibiotics, and perhaps other agents which act on the bacterial cell surface, may not be equally effective against representative strains of S. mutans. This range in activity could be due to differences in cell wall components among the serotypes. Further, our finding that the Shih extract inhibits the synthesis of water insoluble glucan to about 46% is a sufficient proof that binding of the S. mutans to the tooth enamel is very unlikely resulting in non-formation of biofilms thus preventing dental caries.
5. Conclusion
We conclude that crude Shih (Artemisia herba-alba) organic extract which is supposed to contain the plant oils, has potentially effective antibacterial activity and this action is mediated by inhibition in the DNA replication, disruption of the cell wall integrity owing to its detergent like property and also due to obstruction in the synthesis of water insoluble glucan. Also, according to the results shih extract can be proposed as a potentially effective antiplaque and anticariogenic agent in the form of toothpaste or mouth wash or gum paint. However, the cytotoxicity of the extract needs to be evaluated in in-vitro and in-vivo conditions before it is considered as a safe antiplaque and anticariogenic agent.
Source(s) of support
This work was financed by the “Deanship of Scientific Research, King Khalid University, Abha, Saudi Arabia”. (G.R.P. 215 - 38).
Conflict of interest
None.
Authors contribution
Dr Mohammed Amanullah: Head of the project and main operating person. Undertaken all the laboratory procedures including extraction, microbiological and biochemical works. Compilation of data, drafting and revision the article and submission to the journal. Dr Syed Sadatullah: Development of the research idea, drafting the research project, planning of the experimental design, Selection of patients and collection of salivary samples. Compilation of data and editing the manuscript. Dr Syed M. Yasin: Selection of patients, taking the history of the patients, collection of saliva samples. Compilation of data and editing the manuscript. Dr Ali Azhar Dawasaz: Collection of the plant material and confirmation from the botanist. Compilation of data, statistics and editing the manuscript.
Acknowledgment
The authors are grateful to Dr. Syed Mairajuddin Ahmed, Assistant Professor, Department of Chemistry, College of Basic Sciences, King Khalid University, Abha, Saudi Arabia, for providing the necessary facilities and extending personal help, support and expertise in the preparation of Shih organic extract.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahn S.J., Wen Z.T., Burne R.A. Multilevel control of competence development and stress tolerance in Streptococcus mutans UA159. Infect. Immun. 2006;74:1631–1642. doi: 10.1128/IAI.74.3.1631-1642.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Rowais N.A. Herbal medicine in the treatment of diabetes mellitus. Saudi Med. J. 2002;23:1327–1331. [PubMed] [Google Scholar]
- Avila J.G., de Liverant J.G., Martínez A., Martínez G. Mode of action of Buddleja cordata verbascoside against Staphylococcus aureus. J. Ethnopharmacol. 1999;66:75–78. doi: 10.1016/s0378-8741(98)00203-7. [DOI] [PubMed] [Google Scholar]
- Banas J.A., Vickerman M.M. Glucan binding proteins of the oral streptococci. Crit. Rev. Oral Biol. Med. 2003;14:89–99. doi: 10.1177/154411130301400203. [DOI] [PubMed] [Google Scholar]
- Carlsson, J., Hamilton, I.R., 1994. Metabolic Activity of Oral Bacteria. 2. Munksgaard, Copenhagen, Denmar.
- Chatterjee Tanushree, Mohanty Suprava, Das Premananda. Antimicrobial efficacy of some medicinal plant extract against Streptococcus mutans causing dental caries. J. Med. Plants Stud. 2017;5(2):315–317. [Google Scholar]
- Eloff J.N. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998;64:711–713. doi: 10.1055/s-2006-957563. [DOI] [PubMed] [Google Scholar]
- Emslie J.A.N. Tetracycline-resistant group A streptococci. Br. Med. J. 1974;3:467. doi: 10.1136/bmj.3.5928.467-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabry W., Okemo P.O., Ansorg R. Antibacterial activity of East African medicinal plants. J. Ethnopharmacol. 1998;60:79–84. doi: 10.1016/s0378-8741(97)00128-1. [DOI] [PubMed] [Google Scholar]
- Ferretti J.J., Ward M. Susceptibility of Streptococcus mutans to antimicrobial agents. Antimicrob. Agents Chemother. 1976;10:274–276. doi: 10.1128/aac.10.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman L.S., Gilman A. fifth ed. The Macmillan Co.; New York: 1975. The Pharmacological Basis of Therapeutics. [Google Scholar]
- Hamada S., Slade H.D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol. Rev. 1980;44:331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanus L.O., Rezanka T., Dembitsky V.M., Moussaieff A. Myrrh — Commiphora chemistry. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2005;149:3–27. doi: 10.5507/bp.2005.001. [DOI] [PubMed] [Google Scholar]
- Hasan S., Danishuddin M., Khan A.U. Inhibitory effect of zingiber officinale towards Streptococcus mutans virulence and caries development: in vitro and in vivo studies. BMC Microbiol. 2015;151:1–5. doi: 10.1186/s12866-014-0320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higerd T.B., Priest D.G., Murray J., Stillway L.W. Heterogeneity of Streptococcus mutans. J. Dent. Res. 1977;56(special issue B):118. [Google Scholar]
- Higgins M.L., DaneoMoore L., Boothby D., Shockman G.D. Effect of inhibition of deoxyribonucleic acid and protein synthesis on the direction of cell wall growth in Streptococcus faecalis. J. Bacteriol. 1974;118:681–692. doi: 10.1128/jb.118.2.681-692.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman O., D’Ari R. An inducible DNA replication cell division coupling mechanism in E. coli. Nature. 1981;290:797–799. doi: 10.1038/290797a0. [DOI] [PubMed] [Google Scholar]
- Kanth M. Rajini, Prakash A. Ravi, Sreenath G. Efficacy of specific plant products on microorganisms causing dental caries. J. Clin. Diagnostic Res. 2016;12:ZM01–ZM3. doi: 10.7860/JCDR/2016/19772.9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo H., Vacca Smith A.M., Bowen W.H., Rosalen P.L., Cury J.A., Park Y.K. Effects of Apis mellifera propolis on the activities of streptococcal glucosyltransferases in solution and adsorbed onto saliva-coated hydroxyapatite. Caries Res. 2000;34:418–426. doi: 10.1159/000016617. [DOI] [PubMed] [Google Scholar]
- Kuete V., Ngameni B., Fotso Simo C.C. Antimicrobial activity of the crude extracts and compounds from Ficus chlamydocarpa and Ficus cordata Moraceae. J. Ethnopharmacol. 2008;120:17–24. doi: 10.1016/j.jep.2008.07.026. [DOI] [PubMed] [Google Scholar]
- Kuramitsu H. Virulence factors of mutans streptococci: role of molecular genetics. Crit. Rev. Oral Biol. Med. 1993;4:159–176. doi: 10.1177/10454411930040020201. [DOI] [PubMed] [Google Scholar]
- Lemos J.A., Abranches J., Burne R.A. Responses of cariogenic streptococci to environmental stresses. Curr. Issues Mol. Biol. 2005;7:95–107. [PubMed] [Google Scholar]
- Liu G., Begg K., Geddes A., Donachie W.D. Transcription of essential cell division genes is linked to chromosome replication in Escherichia coli. Mol. Microbiol. 2001;40:909–916. doi: 10.1046/j.1365-2958.2001.02434.x. [DOI] [PubMed] [Google Scholar]
- Loesche W.J. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 1986;50:353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mativandlela S.P.N., Lall N., Meyer J.J.M. Antibacterial, antifungal and antitubercular activity of the roots of) Pelargonium reniforme (CURT) and Pelargonium sidoides (DC) (Geraniaceae) root. S. Afr. J. Bot. 2006;72:232–237. [Google Scholar]
- Olsson J., Glantz P.O., Krasse B. Electrophoretic mobility of oral streptococci. Arch. Oral Biol. 1976;21:605–609. doi: 10.1016/0003-9969(76)90030-3. [DOI] [PubMed] [Google Scholar]
- Patel S., Weaver K.E. Addiction toxin Fst has unique effects on chromosome segregation and cell division in Enterococcus faecalis and Bacillus subtilis. J. Bacteriol. 2006;188:5374–5384. doi: 10.1128/JB.00513-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice L.B. Unmet medical needs in antibacterial therapy. Biochem. Pharmacol. 2006;71:991–995. doi: 10.1016/j.bcp.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Silwood C.J., Lynch E., Claxson A.W., Grootveld M.C. 1H and (13C) NMR spectroscopic analysis of human saliva. J. Dent. Res. 2002;81(6):422–427. doi: 10.1177/154405910208100613. [DOI] [PubMed] [Google Scholar]
- Tereschuk M.L., Riera M.V.Q., Castro G.R., Abdala L.R. Antimicrobial activity of flavonoid from leaves of Tagetes minuta. J. Ethnopharmacol. 1997;56:227–232. doi: 10.1016/s0378-8741(97)00038-x. [DOI] [PubMed] [Google Scholar]
- Tsang J.C., Weber D.A., Brown D.A. Evidences for complex formation between polymyxin B and lipopolysaccharides from Serratia marcescens. J. Antibiot. 1976;29:735–742. doi: 10.7164/antibiotics.29.735. [DOI] [PubMed] [Google Scholar]
- Ullman R.F., Miller S.J., Strampfer M.J., Cunha B.A. Streptococcus mutans endocarditis: report of three cases and review of the literature. Heart Lung. 1988;17:209–212. [PubMed] [Google Scholar]
- Zgoda J.R., Porter J.R. A convenient microdilution method screening natural products against bacteria and fungi. Pharmaceut. Biol. 2001;39:221–225. [Google Scholar]