Abstract
Kramecyne (KACY), a polymer isolated from Krameria cytisoides Cav, has anti-inflammatory, anti-nociceptive, anti-arthritic and anti-ulcerogenic properties. As a part of standard preclinical safety tests, the present study sought to determine potential developmental toxicity (in female rats) and genotoxicity (in male mice) of KACY. Pregnant female rats were divided into six groups: the negative control (vehicle), the positive control (250 mg/kg of acetylsalicylic acid (ASA)), and four experimental groups (50, 250, 500 and 1000 mg/kg of KACY). To evaluate genotoxicity by in vivo micronuclei (MN) and sister chromatid exchange (SCE) tests, male mice were divided into five groups: the negative control (vehicle), the positive control (1.5 and 2.5 mg/kg of doxorubicin for MN and SCE, respectively), and three experimental groups (50, 500 and 1000 mg/kg of KACY). All treatments were administered by oral gavage. A slight maternal toxicity was evidenced by lower weight gain for rats receiving 500 and 1000 mg/kg of KACY, but no fetal malformations were found. However, there were less live fetuses/litter and greater post-implantation loss/litter at these two doses. Manifestations of developmental toxicity were limited to a higher rate of skeletal alterations. The MN tests did not evidence genotoxicity or cytotoxicity. KACY caused a slightly but significantly increased frequency of SCE. Although KACY-treated rats had skeletal alterations, these apparently were not caused by a mechanism of genotoxicity. Furthermore, the same administration in adult male mice did not produce genotoxicity. Hence, KACY herein proved to be safe for rats during the period of organogenesis.
Keywords: Kramecyne, Teratogenic study, Micronuclei, Sister chromatid exchange
1. Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are among the medications often taken by pregnant women (Kassaw and Wabe, 2012), especially during the first trimester of pregnancy (Olesen et al., 1999). Those most commonly used are non-selective COX-2 inhibitors, including aspirin, ibuprofen, diclofenac, indomethacin and naproxen (Kassaw and Wabe, 2012).
Animal studies suggest that the inhibition of COX-1 by these NSAIDs may lead to several disorders during embryonic fetal development (Gupta et al., 2003, Burdan and Bełzek, 2001). Clinical trials have established an association between exposure to NSAIDs and developmental anomalies. The children of women who took NSAIDs during the first trimester of pregnancy have presented a greater risk of suffering congenital anomalies such as musculoskeletal deformities (Ofori et al., 2006), cardiac defects and orofacial clefts (Ericson and Källén, 2001). Similarly, some in vivo and in vitro assays have shown that genotoxicity can be generated by various NSAIDs, including paracetamol (Hongslo et al., 1988), sulindac (Franco et al., 2007), ketorolac (Galar et al., 2016) and ibuprofen (Tripathi et al., 2012).
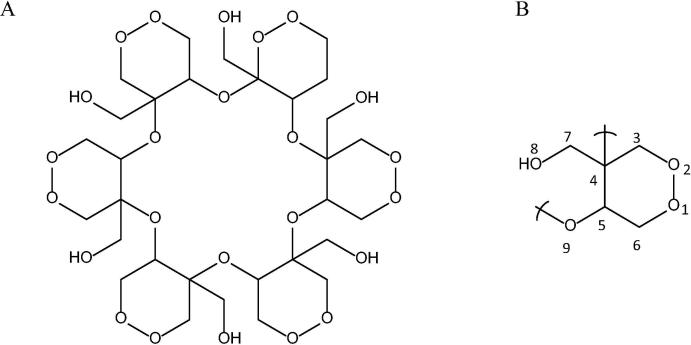
Krameria cytisoides Cav (Krameriaceae) is used in the traditional medicine of the Otomíes (in Mexico) to treat inflammatory processes, hemorrhoids, diarrhea, sore nipples, a sore throat, sore gums, diseases of the digestive tract (e.g., bleeding gums, stomach pain and stomach cancer), and distinct types of pains and wounds (Pérez et al., 2012, Sánchez et al., 2008). Kramecyne (KACY), having the formula C30H48O24 (Fig. 1), is a new hexamer polymer isolated and purified from a methanol extract of this plant. It is composed of six monomers of epoxide with the formula C30H8O4 (Pérez et al., 2012). This compound has not only demonstrated activity similar to Krameria cytisoides Cav, but also produced other beneficial effects according to pharmacological studies on animals (Sánchez et al., 2013, Alonso et al., 2015a, Alonso et al., 2015b). An in vivo experiment reported that at a dose of 250 μg/mL in a culture of macrophages, this polymer induced a low level of toxicity, evidenced by an LD50 greater than 5000 mg/kg (Pérez et al., 2012), and a low level of cytotoxicity (Sánchez et al., 2013).
Fig. 1.
Structural formula of kramecyne; a cyclic hexamer polymer (A) and a monomer (B).
Considering the broad pharmacological activities of KACY, it is important to determine whether or not it compromises embryonic development and DNA stability, as do many other molecules with anti-inflammatory activity. The present study was designed to explore the possibility that KACY elicits embryonic dysmorphogenesis in Wistar rats and/or genotoxic effects in male CD1 mice.
2. Material and methods
2.1. Teratogenicity study
2.1.1. Chemicals
Acetylsalicylic acid (ASA), alizarin red and alcian blue were purchased from Sigma-Aldrich Chemicals Co. (St. Louis, MO, USA). Kramecyne (KACY) was obtained from the Department of Biological Systems, Universidad Autónoma Metropolitana (Mexico City, Mexico). Ethanol, urea, and potassium hydroxide were acquired from J.T. Baker Chemicals (Mexico City, Mexico). Acetic acid, picric acid and formaldehyde were purchased from Merck, S.A. (Mexico City, Mexico).
2.1.2. Animals and housing
Ninety female and 10 male Wistar rats (ten weeks old) were obtained from the UPEAL bioterium of the Universidad Autónoma Metropolitana, Unidad Xochimilco (Mexico City, Mexico). Three females were placed in each cage and the males were housed individually. The animals were kept at 23 ± 1 °C and 55–60% relative humidity, on a 12/12 h light-dark cycle (lights on at 10:00 AM). They had access to food (Rodent Lab Chow 5001, Purina, St. Louis, MO, U.S.A.) and purified water ad libitum.
After 2 weeks of acclimatization, 3 females were placed with a male during 3 h, just before initiating the illumination period. At the end of the 3 h, the estrous cycle of each female was examined by vaginal smear. The presence of a vaginal plug or spermatozoa in the estrus phase indicated that mating had occurred, and this was designated as gestational day (GD) 0. Each mated animal was assigned an identification number by coat staining and randomly distributed to one of six groups of 15 pregnant females, all individually housed.
KACY was dissolved in water and administered by gastric intubation at doses of 0 (control), 50, 250, 500 and 1000 mg/kg at a constant volume of 10 mL/kg. The two highest doses were selected because a previous study of acute toxicity found an LD50 value greater than 5000 mg/kg (Pérez et al., 2012). The lowest dose was chosen based on a report specifying 50 mg/kg as the dose at which a pharmacological anti-inflammatory effect begins in animals (Alonso et al., 2015a). Solutions of KACY were prepared each day immediately before drug administration. The negative control group was given the vehicle only (purified water), and the positive control group received 250 mg/kg of ASA in the same volume. All rats were treated from GD6 to GD15 and observed for abnormalities in their condition, behavior, mortality and morbidity. Body weight was recorded on GD0, GD6, GD11, GD15 and GD19.
On GD19, dams were euthanized in a CO2 chamber after sedation with sodium pentobarbital (40 mg/kg), and their uterine horns were removed. Embryos were carefully explanted from uterine tissue and inspected for external malformations. The diameter and weight of the placenta, the length of the umbilical cord, and the length and sex of each fetus were recorded, as was the number of pregnant females, females with live fetuses, live fetuses, resorptions and dead fetuses. The implantation site was noted and post-implantation loss was calculated.
After hypothermic sacrifice, approximately two thirds of the fetuses in each litter were fixed in Bouin solution for visceral examination by using the Wilson free-hand slicing method (Wilson, 1965). The other fetuses, designated for the determination of skeleton and cartilage abnormalities (Staples and Schnell, 1964), were skinned and eviscerated prior to being fixed in 95% alcohol, cleared with potassium hydroxide, and stained by Peters’ double staining method with alizarin red and alcian blue (Peters, 1977). The skeletons were scrutinized under a stereomicroscope to assess the degree of ossification, the presence/absence of bones, bone deformation and the presence of additional elements such as rudimentary ribs (Menegola et al., 2001).
2.1.3. Statistical analysis
The maternal body weight and the fetal variables (umbilical cord length, placental weight, placental diameter, fetal weight and fetal length) were analyzed with one-way ANOVA followed by a post hoc Student-Newman-Keuls test. The parameters corresponding to the number and type of implants (early resorptions, late resorptions, total resorptions, live fetuses, dead fetuses and total fetuses) were scrutinized with ANOVA on Ranks (Kruskal-Wallis test), and the data corresponding to visceral and skeletal alterations were examined with the X2 test followed by Fisher’s exact test. Data were processed with SigmaPlot® statistical software, version 12.0, and significance was considered at p ≤ 0.05.
2.3. Genotoxicity study
2.3.1. Chemicals
Doxorubicin (DXO), Höechst 33258, 5-bromo-2-deoxyuridine (BrdU) and colchicine were purchased from Sigma-Aldrich Chemicals Co. (St. Louis, MO, USA), while acetic acid, methanol and Giemsa stain were acquired from Merck, S.A. (Mexico City, Mexico). Sodium citrate, potassium chloride, sodium chloride, potassium phosphate and sodium phosphate were obtained from J.T. Baker, S.A. (Mexico City, Mexico).
2.4. Animals and housing
2.4.1. Determination of micronuclei in mice
Thirty CD1 male mice (mean weight, 24 g) were obtained from the bioterium of the Universidad Autónoma del Estado de Hidalgo (Pachuca de Soto, Hidalgo, Mexico). Groups of six animals each were placed in polypropylene cages at 23 ± 1 °C and 50–60% relative humidity, on a 12/12 h dark-light cycle (lights on at 7 AM). Animals were allowed free access to food (Rodent Lab Chow 5001, Purina, St. Louis, MO, USA) and purified water. Mice were acclimatized in the Animal House of the Genetics Laboratory for two weeks before starting the experiment.
For the micronuclei examination, the animals were designated to five groups (n = 6): the negative control group received 10 mL/kg of purified water (the vehicle of the drugs tested), the positive control group was given 1.5 mg/kg of DXO (a tetracycline antibiotic), and three experimental groups were treated with KACY at doses of 50, 500 and 1000 mg/kg. All treatments were intragastrically (ig) administered, except DXO. The latter compound was injected intraperitoneally (ip). The doses were selected under the same guidelines used in the developmental toxicity assay.
Before drug administration (0 h) and at 24, 48, 72 and 96 h post-administration, blood was taken from the tail of each mouse. One drop was placed on each of two clean slides to make an extension. Cells were fixed in 96% ethanol for 3 min and then washed under running water (briefly), dried, and processed for 12 min with 5% Giemsa stain in PBS. Subsequently, slides were washed under running water, dried, and carefully kept for microscopic analysis. To evaluate the genotoxic potential of KACY, a determination was made of the number of micronucleated polychromatic erythrocytes (MNPE) per 1000 polychromatic erythrocytes (PE) in each mouse. To take advantage of the assay, the bone marrow cytotoxic effect of KACY was also assessed by calculating the proportion (PE/NE) of young PE cells and mature normochromic erythrocytes (NE) per 1000 cells in each mouse.
2.5. Statistical analysis
The data was examined with two-way ANOVA for repeated measures followed by a post hoc Student-Newman-Keuls test. All statistical analyses were performed with SigmaPlot® statistical software, version 12.0, and significance was considered at p ≤ 0.05.
2.5.1. Determination of sister chromatid exchange (SCE) in mice
Male CD1 mice (mean weight, 24 g) were used under conditions similar to those described for the micronuclei assay. KACY (50, 500 and 1000 mg/kg) was ig administered to the experimental groups of mice, while DXO (2.5 mg/kg) was injected ip into positive control animals. The control group received 10 mL/kg of purified water.
Thirty tablets were prepared that contained 45 mg of 5-bromo-2′-deoxyuridine (BrdU) each, and then 60% of the tablet surface was coated with paraffin. Mice were slightly anesthetized with ether and the tablets subcutaneously implanted in the abdominal zone. The wound was subsequently closed with surgical clips. At 30 min post-surgery, the test substances were administered to mice, and at 22 h post-surgery colchicine (7.5 µg/g) was ip injected. After another two hours and 45 min, the animals were sacrificed by cervical dislocation and the bone marrow of each femur was obtained and placed in a solution of KCl (0.075 M) at 37 °C for 60 min. Samples were centrifuged at 1500 rpm for 10 min, the supernatant was discarded, and Carnoy’s fixative solution was added. The mixture was left to stand for 10 min. Centrifugation was again carried out at 1500 rpm for 10 min and the supernatant discarded, followed by two additional centrifugation/fixation processes (1500 rpm, 10 min). Three drops of the cell suspension were then placed on each slide to continue the staining process.
Chromatid differential staining was performed according to previously reported procedures (Stults et al., 2014). Initially, Höechst 33,258 solution (0.5 mL) and a coverslip were placed on a slide, which was kept in the dark for 30 min. Subsequently, the solution was discarded and the differentiation buffer was placed over the coverslip to shield the cells before exposing the preparation to black light for 90 min. At the end of that period, the coverslip was detached by immersing the preparation several times in distilled water at 24 °C. Slides were dried at room temperature and introduced into a Coplin jar with a saline solution of citrates and left to stand for 15 min at 60 °C. Afterwards, they were briefly immersed in a water bath at 60 °C and another one at 24 °C, then dried and processed with 5% Giemsa stain in PBS.
To examine the genotoxic potential of KACY, the frequency of SCE was determined in 30 s-division metaphases per mouse. To assess cytotoxicity, the mitotic index was calculated by registering the number of dividing cells per 1000 cells in each mouse. Finally, to evaluate cell proliferation kinetics, the average generation time (AGT) was ascertained in 100 mitoses per mouse. Cells were classified into first cellular division stage (M1), second division stage (M2) and third division stage (M3). The AGT was calculated by using the following formula: AGT = [22/(M1) + (2)(M2) + (3)(M3)] × 100.
2.6. Statistical analysis
Data was scrutinized with one-way ANOVA followed by a post hoc Student-Newman-Keuls test. All statistical analyses were carried out with SigmaPlot® statistical software, version 12.0, and significance was considered at p ≤ 0.05.
The current teratogenicity and mutagenicity protocols were approved by the Bioethics Committee of the Escuela Nacional de Ciencias Biológicas (Mexico City, Mexico).
3. Results
3.1. Teratogenicity study
There were no signs of toxicity or miscarriage related to KACY treatment of female rats. Physical appearance was normal and all animals survived to the end of the study. The only observable adverse effect was the occurrence of episodes of diarrhea (which were not permanent) with the doses of 500 and 1000 mg/kg. At sacrifice, no apparent abnormalities were found in maternal organs such as liver, lung, brain, heart, kidneys and stomach.
Before the administration of KACY or the vehicles, the number of mated and pregnant females and their corresponding indexes were normal for all groups (Table 1). Oral treatment with KACY at a dose of 50 mg/kg during the period of organogenesis apparently had no adverse effect, as evidenced by the gestational data. Females receiving the 1000 mg/kg dose of KACY displayed a small but significant difference (versus the control) in live fetuses/litter, resorptions and dead fetuses/litter, and post-implantation loss/litter (%). ASA at 250 mg/kg did indeed present significant differences in the number of live fetuses, resorptions and dead fetuses, resorptions and dead fetuses/litter, post-implantation loss/litter (%) and the number of dams with resorptions. Regarding maternal weight gain from GD0 to GD19, the control group showed a mean increase of 132.86 ± 5.68 g, while the groups receiving KACY at doses of 500 and 1000 mg/kg showed a weight gain of 97.61 ± 8.06 g and 94.80 ± 10.64 g, respectively (representing a dose-dependent effect as well as a statistically significant difference compared to the control). In contrast, the weight gain of the ASA 250 group was drastically reduced, being slightly over a third of that of control animals (56.08 ± 6.06 g).
Table 1.
Parameters of gravid female rats administered KACY.
| Parameter | Reproductive performance from GD6 to GD15 (dose in mg/kg) |
|||||
|---|---|---|---|---|---|---|
| Control | ASA 250 | KACY 50 | KACY 250 | KACY 500 | KACY 1000 | |
| No. of mated females | 15 | 15 | 15 | 15 | 15 | 15 |
| No. of pregnant females | 15 | 15 | 15 | 15 | 15 | 15 |
| Pregnancy index (%) | 100 | 100 | 100 | 100 | 100 | 100 |
| Fertility index (%) | 100 | 100 | 100 | 100 | 100 | 100 |
| Maternal weight gain (g) (GD0-19) | 132.86 ± 5.68b | 56.08 ± 6.06a | 127.38 ± 4.78b | 111.51 ± 4.77b | 97.61 ± 9.06a,b | 94.80 ± 10.64a,b |
| No. of females with live fetuses | 15 | 10a | 15b | 15b | 15b | 12 |
| Implantation sites | 199 | 223 | 198 | 206 | 206 | 217 |
| Implantations/per litter | 13.26 ± 0.82 | 14.86 ± 0.69 | 13.2 ± 0.83 | 13.73 ± 0.71 | 13.73 ± 0.69 | 14.46 ± 0.60 |
| Live fetuses | 178 | 87a | 180b | 186b | 179b | 176b |
| Live fetuses/litter | 11.86 ± 0.78 | 5.8 ± 0.57 | 12.00 ± 0.77 | 12.4 ± 0.55 | 11.93 ± 0.77 | 11.33 ± 1.45a |
| Resorptions and dead fetuses | 21 | 136a | 18b | 20b | 27b | 47b |
| Resorptions and dead fetuses/litter | 1.40 ± 0.42 | 9.06 ± 0.78a | 1.20 ± 0.31 | 1.33 ± 0.37 | 1.8 ± 0.26b | 3.13 ± 1.15a,b |
| Post-implantation loss/per litter (%) | 10.55 | 60.98a | 9.09b | 9.70b | 13.10b | 18.89a,b |
| Dams with resorptions | 9 | 15a | 10 | 9 | 13 | 10 |
The values are given as the mean ± SEM. ASA = acetylsalicylic acid; KACY = kramecyne. A significant difference (p < 0.05) in relation to the acontrol and/or bASA at 250 mg/kg.
Pregnancy index = (No. of females with live fetuses/No. of mated females) x 100.
Fertility index = (No. of pregnant females/No. of mated females) × 100.
Post-implantation loss = (No. of implantations - No. of live fetuses/n of implantations) × 100.
Regarding fetal parameters, a significant difference (p < 0.05) existed in the fetal crown-rump length and umbilical cord length between the highest doses of KACY (500 and 1000 mg/kg) and the control group (Table 2). Moreover, the 1000 mg/kg dose of KACY as well as the positive control dose of DXO produced significantly lower values for fetal and placental weights.
Table 2.
Fetal parameters of rats administered KACY from GD6 to GD15.
| Groups (dose in mg/kg) |
||||||
|---|---|---|---|---|---|---|
| Control | ASA 250 | KACY 50 | KACY 250 | KACY 500 | KACY 1000 | |
| Fetuses examined | 178 | 87 | 180 | 186 | 179 | 176 |
| Fetal weight (g) | 2.32 ± 0.02 | 1.29 ± 0.02a | 2.32 ± 0.01b,c | 2.24 ± 0.01b,c | 2.24 ± 0.02b,c | 2.10 ± 0.01a,b |
| Fetal crown-rump length (cm) | 2.94 ± 0.01 | 2.26 ± 0.02a,c | 2.92 ± 0.01b,c | 2.68 ± 0.01a,b,c | 2.85 ± 0.01a,b,c | 2.79 ± 0.01a,b |
| Placental weight (g) | 0.54 ± 0.01 | 0.43 ± 0.01a | 0.54 ± 0.01b,c | 0.51 ± 0.01b,c | 0.54 ± 0.01b,c | 0.49 ± 0.01a,b |
| Placental diameter (cm) | 1.33 ± 0.01 | 1.27 ± 0.02 | 1.31 ± 0.01 | 1.29 ± 0.01 | 1.34 ± 0.01 | 1.29 ± 0.03 |
| Umbilical cord length (cm) | 2.29 ± 0.02 | 1.30 ± 0.03a | 2.32 ± 0.02b | 1.85 ± 0.07a,b | 2.24 ± 0.02a,b | 2.08 ± 0.02a,b |
| Male/female fetuses | 84/94 | 41/46 | 86/94 | 92/94 | 90/89 | 86/90 |
| Sex ratio (male/female) | 0.89 | 0.89 | 0.91 | 0.97 | 1.01 | 0.95 |
Evaluations of the fetuses from fifteen pregnant rats per group. ASA = acetylsalicylic acid; KACY = kramecyne. Significant difference (p < 0.05) in relation to the acontrol, bASA at 250 mg/kg and/or cKACY at 1000 mg/kg.
Concerning the external and visceral malformations found in the fetuses, only in the group that received ASA 250 were there statistical differences versus the control. Malformations included exencephaly, craniorachischisis, gastroschisis (Fig. 2, Table 3), visceral dilation of the cerebral ventricles, and renal pelvis (Fig. 3). In the KACY-treated groups, no morphological alteration was observed.
Fig. 2.
External anomalies: (A) a normal fetus of the negative control; (B) a fetus from a female rat administered KACY at 1000 mg/kg shows small size; (C–E) fetuses treated with ASA at 250 mg/kg show exencephaly (C), craniorachischisis (D), and gastroschisis and exencephaly (E).
Table 3.
Frequency of external and visceral malformations in fetuses from female rats administered KACY during days 6 through 15 of gestation.
| Groups (mg/kg) |
||||||
|---|---|---|---|---|---|---|
| Control | ASA 250 | KACY 50 | KACY 250 | KACY 500 | KACY 1000 | |
| External | ||||||
| Fetuses analyzed | 178 | 87 | 180 | 186 | 179 | 176 |
| Exencephaly | 0 (0%) | 26 (30%)a | 0 (0%)b | 0 (0%)b | 0 (0%)b | 0 (0%)b |
| Craniorachischisis | 0 (0%) | 13 (15%)a | 0 (0%)b | 0 (0%)b | 0 (0%)b | 0 (0%)b |
| Gastroschisis | 0 (0%) | 17 (20%)a | 0 (0%)b | 0 (0%)b | 0 (0%)b | 0 (0%)b |
| Kinked tail | 0 (0%) | 1 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Visceral | ||||||
| Fetuses analyzed | 123 | 61 | 123 | 128 | 124 | 125 |
| Hydrocephalus | 0 (0%) | 17 (28%)a | 0 (0%)b | 0 (0%)b | 0 (0%)b | 0 (0%)b |
| Hydronephrosis | 0 (0%) | 7 (11%)a | 0 (0%)b | 0 (0%)b | 0 (0%)b | 0 (0%)b |
| Ectopic kidney | 0 (0%) | 2 (3%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
Values in parenthesis denote percentages. ASA = acetylsalicylic acid; KACY = kramecyne. Significant difference (p < 0.05) in relation to the acontrol, bASA at 250 mg/kg and/or cKACY at 1000 mg/kg.
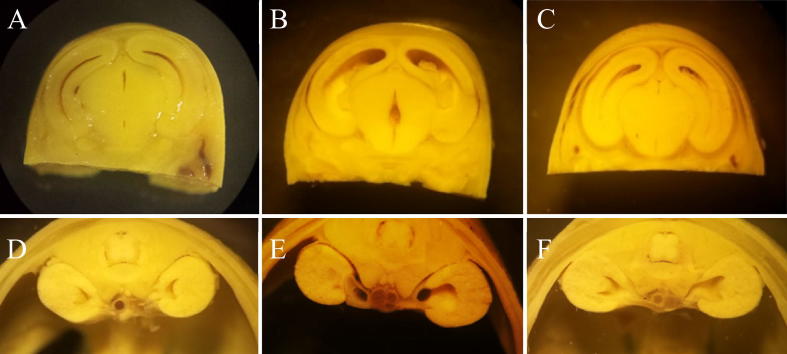
Fig. 3.
Transverse sections of brain and kidneys of rat fetuses. The brain (A) and kidney (D) sections of control rat fetuses. The brain (B) and kidney (E) sections of fetuses from female rats treated with ASA (250 mg/kg). Dilation of the lateral and central brain ventricles can be appreciated, as well as dilation of the renal pelvis. The brain (C) and kidney (F) sections of fetuses from females treated with KACY (1000 mg/kg). A slight dilation of the renal pelvis is noticeable.
Significant skeletal variations were identified in the groups administered the different doses of KACY (Table 4). For the dose of 1000 mg/kg, these consisted of incomplete ossification of some skull bones (Fig. 4), pelvis bones, sternum bones and vertebrae, as well variations in the ribs (Fig. 5). Some of these variations were also found at doses of 250 and 500 mg/kg, representing a significant difference compared to the negative control group. On the other hand, a statistically significant difference was detected for all measured skeletal parameters between the female rats receiving ASA (250 mg/kg) and those in the negative control group.
Table 4.
Skeletal alterations in the fetuses from female rats administered KACY during days 6 through 15 of gestation.
| Treatment (mg/kg) |
||||||
|---|---|---|---|---|---|---|
| Control | ASA 250 | KACY 50 | KACY 250 | KACY 500 | KACY 1000 | |
| Fetuses analyzed | 55 | 26 | 57 | 58 | 55 | 51 |
| Skull bones | ||||||
| Incomplete ossification in the following bones: | ||||||
| Frontal | 0 (0%) | 14 (54%)a | 5 (9%)b,c | 8 (14%)a,b,c | 18 (33%)a | 23 (45%)a |
| Parietal | 0 (0%) | 14 (54%)a | 5 (9%)b,c | 16 (28%)a,b,c | 37 (67%)a | 31 (61%)a |
| Interparietal | 0 (0%) | 14 (54%)a | 9 (16%)b,c | 11 (19%)a,b,c | 32 (56%)a | 38 (75%)a |
| Exoccipital | 0 (0%) | 18 (69%)a | 5 (9%)b | 2 (3%)b | 4 (7%)b | 2 (4%)b |
| Supraoccipital | 2 (4%) | 21 (81%)a | 4 (7%)b | 4 (7%)b | 3 (5%)b | 5 (10%)b |
| Acrania | 0 (0%) | 9 (35%)a | 0 (0%)b | 0 (0%)b | 0 (0%)b | 0 (0%)b |
| Pelvis bones | ||||||
| Incomplete ossification in the following bones: | ||||||
| Pelvis | 18 (33%) | 13 (50%) | 22 (39%) | 21 (36%) | 15 (27%) | 23 (45%) |
| Ilium | 2 (4%) | 7 (27%)a | 0 (%)b | 0 (0%)b | 3 (5%)b | 2 (4%)b |
| Ischium | 3 (5%) | 11 (42%)a | 1 (2%)b | 1 (2%)b | 3 (5%)b | 8 (16%)b |
| Sternum bones | ||||||
| Incomplete ossification in the following bones: | ||||||
| Sternebrae I | 15 (27%) | 1 (4%)a | 17 (30%)b | 21 (36%)b | 25 (45%)b | 25 (49%)a |
| Sternebrae II | 20 (36%) | 2 (8%)a | 35 (61%)b | 38 (66%)b | 29 (53%)b | 36 (71%)a |
| Sternebrae III | 12 (22%) | 3 (12%) | 21 (37%)b | 20 (34%) | 28 (51%)a,b | 26 (51%)a,b |
| Asymmetrical bones: | ||||||
| Sternebrae I | 2 (4%) | 12 (46%)a | 1 (2%)b,c | 6 (10%)b | 5 (9%)b | 13 (25%)a,b |
| Sternebrae II | 1 (2%) | 12 (46%)a | 2 (4%)b,c | 5 (9%)b | 5 (9%)b | 8 (16%)a,b |
| Sternebrae III | 3 (5%) | 12 (46%)a | 2 (4%)b,c | 7 (12%)b | 6 (11%)b | 13 (25%)a,b |
| Fused bones: | ||||||
| Sternebrae I | 0 (0%) | 8 (31%)a | 0 (0%)b | 0 (0%)b | 0 (0%)b | 0 (0%)b |
| Sternebrae II | 0 (0%) | 8 (31%)a | 0 (0%)b | 0 (0%)b | 0 (0%)b | 0 (0%)b |
| Sternebrae III | 0 (0%) | 8 (31%)a | 0 (0%)b | 0 (0%)b | 0 (0%)b | 0 (0%)b |
| Hyperplastic and cleaved bones: | ||||||
| Sternebrae I | 0 (0%) | 5 (19%)a | 0 (0%)b | 0 (0%)b | 0 (0%)b | 0 (0%)b |
| Sternebrae II | 0 (0%) | 5 (19%)a | 0 (0%)b | 0 (0%)b | 0 (0%)b | 0 (0%)b |
| Sternebrae III | 0 (0%) | 5 (19%)a | 0 (0%)b | 0 (0%)b | 0 (0%)b | 0 (0%)b |
| Vertebrae | ||||||
| Cleaved bones: | ||||||
| Cervical | 0 (0%) | 5 (19%)a | 0 (0%)b | 0 (0%)b | 0 (0%)b | 0 (0%)b |
| Thoracic | 0 (0%) | 12 (46%)a | 0 (0%)b | 0 (0%)b | 0 (0%)b | 0 (0%)b |
| Lumbar | 0 (0%) | 13 (50%)a | 0 (0%)b | 0 (0%)b | 0 (0%)b | 0 (0%)b |
| Sacred | 0 (0%) | 1 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Fused bones: | ||||||
| Cervical | 0 (0%) | 3 (12%)a | 0 (0%)b | 0 (0%)b | 0 (0%)b | 0 (0%)b |
| Thoracic | 0 (0%) | 4 (15%)a | 0 (0%)b | 0 (0%)b | 0 (0%)b | 0 (0%)b |
| Ribs | ||||||
| Cleaved right ribs | 0 (0%) | 10 (38%)a | 0 (0%)b | 0 (0%)b | 0 (0%)b | 0 (0%)b |
| Cleaved left ribs | 0 (0%) | 16 (62%)a | 0 (0%)b | 0 (0%)b | 0 (0%)b | 0 (0%)b |
| Extra ribs | 0 (0%) | 1 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Rudimentary right ribs | 1 (2%) | 14 (54%)a | 3 (5%)b | 3 (5%)b | 4 (7%)b | 3 (6%)b |
| Rudimentary left ribs | 1 (2%) | 10 (38%)a | 2 (4%)b | 2 (3%)b | 3 (5%)b | 3 (6%)b |
| Undulate right ribs | 0 (0%) | 4 (15%)a | 0 (0%)b | 0 (0%)b | 1 (2%)b | 3 (6%) |
| Undulate left ribs | 0 (0%) | 4 (15%)a | 0 (0%)b | 1 (2%)b | 2 (4%)b | 2 (4%)b |
Values in parenthesis denote percentages. ASA = acetylsalicylic acid; KACY = kramecyne. Significant difference (p < 0.05) in relation to the acontrol, bASA at 250 mg/kg and/or cKACY at 1000 mg/kg.
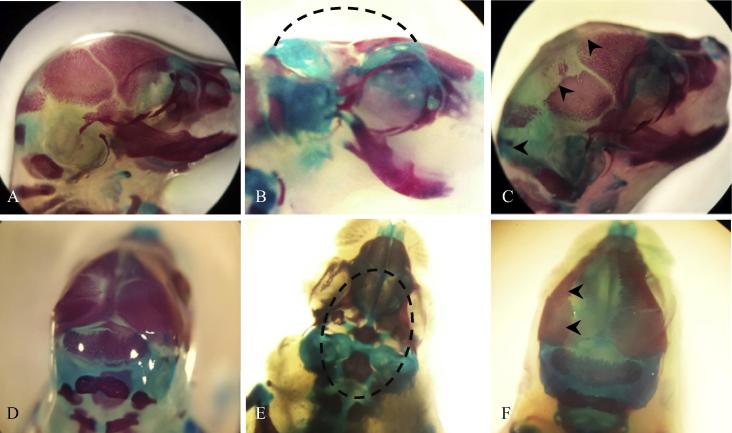
Fig. 4.
Fetal skeletal anomalies: A and D, a fetus of the negative control group. B and E, a fetus showing acrania (dashed line), extracted from a female rat treated with 250 mg/kg of ASA. C and F, a fetus showing poor ossification of frontal, parietal and supraoccipital bones (arrows heads), extracted from a female rat treated with 1000 mg/kg of KACY.
Fig. 5.
Fetal skeletal anomalies in sternebrae (A–C) and ribs (D–F). Fetus of the negative control (A, D); fetus from a female rat treated with 250 mg/kg of ASA, showing poor ossification in sternebrae (B, arrows heads) as well as wavy ribs, lobed ribs and poorly ossified vertebrae (E, arrows heads); fetus from a female rat treated with 1000 mg/kg of KACY, showing poor ossification in sternebrae (C, arrows heads) as well as rudimentary ribs (F, arrows heads).
3.2. Micronucleus test: Geno/cytotoxicity
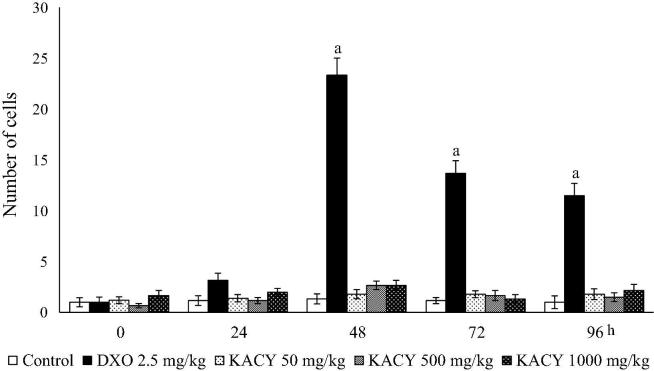
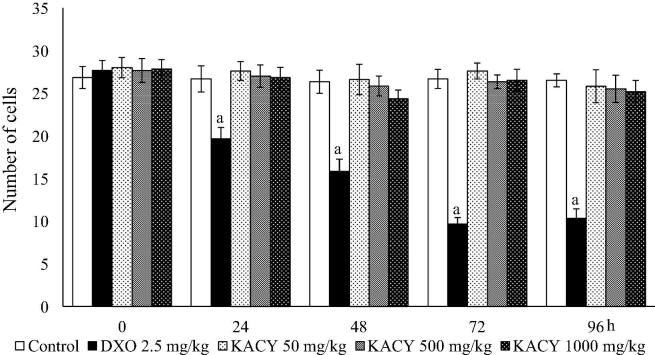
Concerning the generation of MNPE, a small number of micronuclei (no more than a mean of 1.33 MNPE/1000 PE) was detected in the animals of the negative control (administered purified water only) throughout the experiment (Fig. 6, Fig. 7). The mice given DXO exhibited a slightly higher level of MNPE at 24 h, a large and significant boost at 48 h (23.33 MNPEs/1000), and a subsequent decrease at 72 and 96 h. No significant difference existed in this parameter between the control and KACY-treated animals during the entire 96 h of exposure. Interestingly, the greatest damage produced by KACY was shown at 48 h, similar to the pattern observed with DXO.
Fig. 6.
Number of micronucleated polychromatic erythrocytes (MNPE) found in the peripheral blood of control, DXO-treated and KACY-treated mice. Each bar represents the mean ± SEM of MNPE in 1000 polychromatic erythrocytes per mouse (n = 6). DXO = doxorubicin; KACY = kramecyne. aA significant difference (p < 0.001) in relation to the control.
Fig. 7.
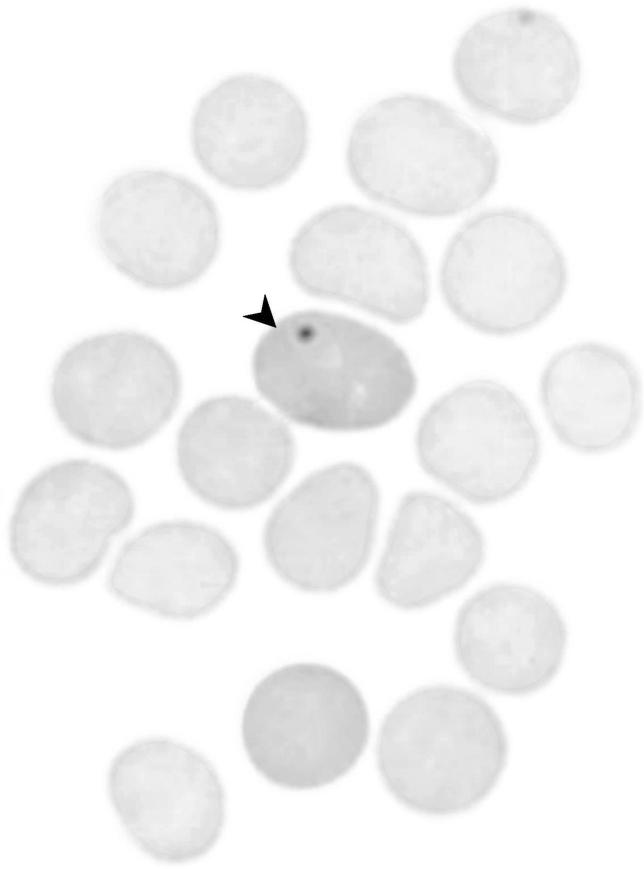
A mouse peripheral blood smear showing a polychromatic erythrocyte with a micronucleus.
Regarding cytotoxic effects, similar values were found for the PE/NE index in the control and KACY-treated groups throughout the experiment (a mean of 26.58). The highest dose of the test compound caused a slight, non-significant decline in this index (Fig. 8). Contrarily, the antineoplastic compound provoked cytotoxicity at all measurement times, with a much lower value of the PN/NE index at 72 and 96 h (9.66 and 10.33, respectively).
Fig. 8.
Number of polychromatic erythrocytes in mouse peripheral blood from the control, DXO-treated and KACY-treated groups. Each bar depicts the mean ± SEM found in 1000 polychromatic erythrocytes per mouse (n = 6). DXO = doxorubicin; KACY = kramecyne. aA significant difference (p < 0.001) in relation to the control.
3.3. SCE, mitotic index and AGT
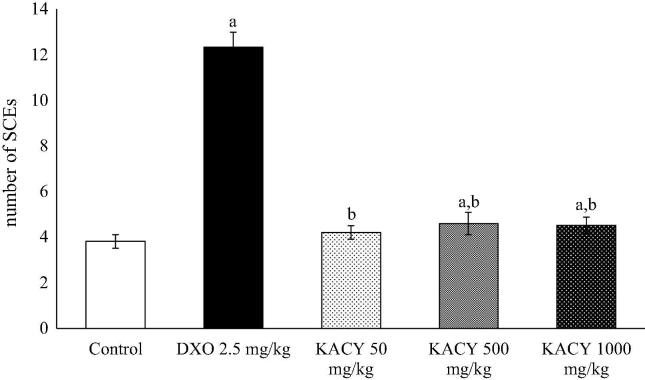
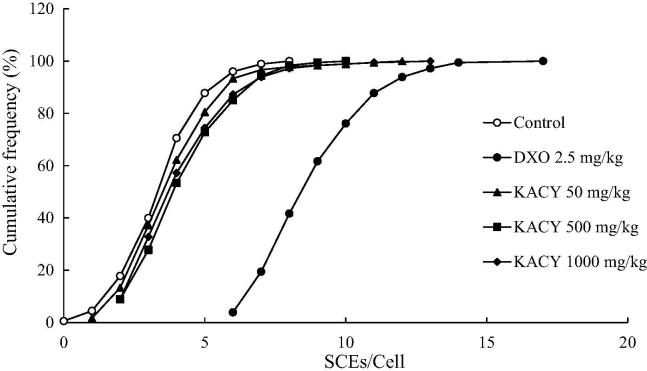
There was a moderate increase in SCEs/cell when using the anti-inflammatory test compound (Fig. 9). In contrast, the value of 9.1 SCEs/cell displayed by DXO-treated animals represented over twice the value found in the control group, which exhibited a mean frequency of 3.81 SCEs/cell. At 50, 500 and 1000 mg/kg of KACY, mean values of 4.2, 4.6 and 4.5 SCE/metaphase were detected, respectively. These values were moderately higher than the control and statistically significant. At 500 mg/kg, the cumulative count of SCEs/cells revealed a displacement of the DXO curve in relation to the control group. For example, about 8 SCEs/cell were detected when observing 40% of DXO-treated cells, while about 2 and 3 SCEs/cell were encountered with this same percentage of control and KACY-treated (at 1000 mg/kg) cells, respectively (Fig. 10, Fig. 11).
Fig. 9.
Sister chromatid exchange (SCE) found in the bone marrow cells of control, DXO-treated and KACY-treated mice. Each bar represents the mean ± SEM detected in 30 s-division metaphases (n = 6). DXO = doxorubicin; KACY = kramecyne. A significant difference (p < 0.05) in relation to the acontrol and/or bDXO at 2.5 mg/kg.
Fig. 10.
Cumulative frequency of sister chromatid exchanges (SCEs) per bone marrow cell of control, DXO-treated and KACY-treated mice. Each line portrays the cumulative frequency of SCE displayed in 30 s-division metaphases per group (n = 6). DXO = doxorubicin; KACY = kramecyne.
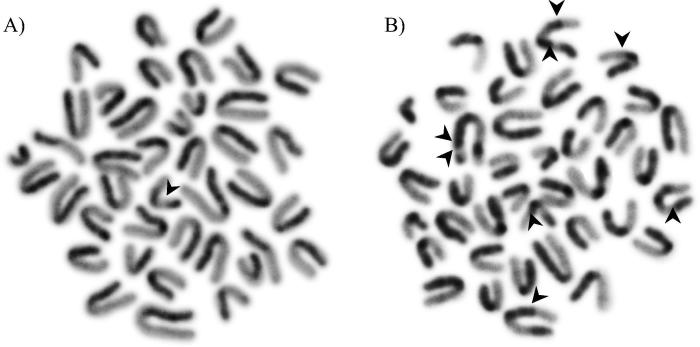
Fig. 11.
A second-division bone marrow mitosis showing sister chromatid differentiation: a single sister-chromatid exchange (A), and eight sister-chromatid exchanges (B).
KACY treatments did not induce any bone marrow lethal potency (Table 5), as can be appreciated by the similar MI values for control animals (4.6% of mitosis per 1000 cells) and the three test doses of the anti-inflammatory agent (50, 500 and 1000 mg/kg, resulting in 4.8, 4.3 and 4.4%, respectively). In contrast, DXO displayed a 3.8% decrease in the mean MI value in relation to the control group.
Table 5.
The mitotic index and the average generation time induced by kramecyne (KACY) in mouse bone marrow cells.
| Mitotic index | Metaphase classification |
AGT | |||
|---|---|---|---|---|---|
| (%) | M1 | M2 | M3 | ||
| Control | 4.6 | 36.3 ± 1.2 | 47.8 ± 1.2 | 15.8 ± 0.7 | 12.26 ± 0.10 |
| DXO 2.5 mg/kg | 3.8 | 25.8 ± 1.2a | 65.0 ± 2.2a | 9.2 ± 1.4a | 12.00 ± 0.01 |
| KACY 50 mg/kg | 4.8 | 36.8 ± 1.1 | 50.0 ± 1.8 | 13.1 ± 1.8 | 12.48 ± 0.17 |
| KACY 500 mg/kg | 4.3 | 36.8 ± 1.1 | 49.6 ± 1.9 | 13.5 ± 1.1 | 12.45 ± 0.09 |
| KACY 1000 mg/kg | 4.4 | 37.0 ± 1.7 | 51.0 ± 1.7 | 12.0 ± 1.1 | 12.58b ± 0.17 |
Mitotic index values represent the mean ± SEM evaluated in 1000 cells per mouse (n = 6).
To obtain the average generation time (AGT), 100 metaphases were initially classified into first, second and third cellular division (M1, M2 and M3, respectively), and then the following formula was applied: AGT = [22/(M1) + (2)(M2) + (3)(M3)] × 100. DXO = Doxorubicin; KACY = Kramecyne. One-way ANOVA followed by the post hoc Student-Newman-Keuls test revealed a significant difference (p < 0.05) in relation to the acontrol and/or bDXO 2.5 mg/kg.
The values for AGT were also similar between the control and KACY-treated animals. A variation of 12.26–12.58 h was usually found in untreated mice, indicating no influence of the test drug on cell progression. Contrarily, DXO showed a lower AGT value (12.00 h), representing a statistical difference from the rest of the groups.
4. Discussion
The purpose of the current study was to evaluate KACY as a candidate drug for treating inflammatory, nociceptive, arthritic and ulcerogenic disorders. This compound is isolated from Krameria cytisoides Cav, a plant widely used in the ethnomedicinal practices of the Otomíes in Mexico. Hence, the potential teratogenicity of KACY was tested in female Wistar rats, as was its genotoxicity in male adult mice.
The oral administration of KACY did not lead to mortality in the dams, even at a dose of 1000 mg/kg. However, maternal toxicity is suggested by the negative effects on body weight gain at the doses of 500 and 1000 mg/kg. Since there was no apparent effect on the macroscopic structures of the organs of female rats herein examined, the weight loss was likely due to diarrhea, which was caused by the two highest doses.
KACY administration during the organogenesis period did not cause any deleterious effects for gravid females, as demonstrated by the pregnancy index, fertility index, and the number of implantations and resorptions. Between the control rats and animals that received 1000 mg/kg of KACY, statistical differences were indeed detected in regard to live fetuses/litter, resorptions, dead fetuses/litter and post-implantation loss/litter, which concurs with the decline in the number of females that had live fetuses at the same dose.
Concerning the possible teratogenic effects of KACY, an assessment was made of external and skeletal variations as well as visceral malformations. Growth retardation was observed in the fetuses of KACY-treated groups compared to the negative control. Additionally, the values for the crown-rump length, placental weight and umbilical cord length were different from those of the negative control. Although KACY did not cause sex-selective effects (evidenced by the similar gender-based ratios in the four groups), a high incidence of skeletal variations was induced by the 500 and 1000 mg/kg dose (especially the highest dose). In some cases, there was incomplete ossification of the skull, sternum and squamosal bones of embryos. Thus, it is reasonable to suggest that KACY induced a decrease in calcium utilization by the dams during pregnancy or maternal stress, and not a dysfunction in bone growth.
The evaluation of possible teratogenic effects of compounds with anti-inflammatory activity, such as KACY, is important due to the contradictory reports on the toxic effect of NSAIDs during fetal development. The latter have been investigated in humans and various animal models, with some studies finding developmental alterations (Ofori et al., 2006, Ericson and Källén, 2001) and others detecting no deleterious effects (Cleves et al., 2004, Nielsen et al., 2001).
To explore the genotoxic potential of KACY, two in vivo assays were carried out for the determination of MN and SCE. MN furnishes information on chromosome breakage and whole chromosomes abnormally segregated during cell division. Because of the reliability of the information, it is one of the most commonly used genotoxicity assays (Hayashi, 2016). The same procedure also yields insights into bone marrow cytotoxicity. The SCE assay, on the other hand, is related to DNA replication when two sister chromatids break and rejoin, at which time they physically exchange regions of the parental strands in the duplicated chromosomes (Perry and Wolff, 1974). SCE generally results from S-dependent lesions, which during the S-phase are eventually modified to double-strand breaks that may be repaired by a homologous recombination mechanism (Wilson and Thomson, 2007, Mourelatos, 2016). For decades, this assay has provided information about the genotoxic potential of numerous agents.
In KACY groups, no increase was found in micronuclei nor was there any bone marrow proliferative damage. While no adverse effect was evidenced by the mitotic index or the AGT value, a slightly higher level of SCE was detected. Despite the statistical difference in the SCE data between the control and KACY-treated groups, the mean difference was 1.1+ SCEs/metaphase, indicating little real biological significance.
The outcome of the genotoxic assay lends itself to two interpretations. Regarding its preclinical safety, no significant deleterious damage was apparent. Thus, the compound can be recommended for further research to eventually reach a conclusion about its possible administration to patients. Secondly, genotoxicity does not seem to be the underlying mechanism to explain the developmental damage observed in the size and weight or the skeleton of the fetuses of KACY-treated rats.
The negligible genotoxic potential determined for KACY in the present study is interesting in the context of the genetic or chromosomal damage reported for various commercial drugs with similar pharmacological qualities. For example, an antinociceptive agent, paracetamol, was found to augment the level of SCE in V79 Chinese hamster cells after 2 h of exposure. Tramadol, on the other hand, has proven to significantly increase the rate of chromosomal aberrations and micronuclei along with decreasing the mitotic index in mouse bone marrow (Hongslo et al., 1988, Maleek et al., 2015). An antiulcerogenic agent, ranitidine, leads to hepatic DNA fragmentation in rodents, probably related to an intragastric nitrosation effect (Brambilla et al., 1983). A non-steroidal anti-inflammatory drug, sulindac, causes DNA breaks and cell cycle anomalies in Aspergillus nidulans (Franco et al., 2007), while three AINE drugs (etodolac, nimesulide and naproxen) elevate the level of SCE in dental patients (Köseoǧlu et al., 2008).
5. Conclusion
With the present oral administration, KACY did not induce either maternal or developmental toxicity in pregnant rats (during the period of organogenesis) or genotoxicity in adult male mice. The only significant alterations found were slight fetal skeletal variations and differences in the height and weight of adult females. These alterations do not appear to derive from a genotoxic mechanism, since KACY did not give rise to any substantial alterations in DNA or any significant cytotoxicity to bone marrow. Contrarily, other drugs currently on the market that exhibit activity similar to KACY have shown relevant genotoxic damage, including the inhibition of certain enzymes or the triggering of oxidative stress, which may lead to deviations in embryonic development.
Further research is needed on other animal species to explore the safety of prescribing KACY to patients. The fact that the administration of this compound to animals produces low toxicity in relation to embryonic development and does not disrupt the stability of genetic material provides a solid basis for intensifying investigation.
Acknowledgment
We thank Bruce Allan Larsen, a native English speaker, for proofreading the manuscript. We also thank Mr. Armando Casas for animal care.
Acknowledgments
Author disclosure statemen
The authors declare that there are no conflicts of interest.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alonso A.J., Zavala M.A., Pérez J., Sánchez S., Pérez S. Antinociceptive and anti-arthritic effects of kramecyne. Life Sci. 2015;121:70–77. doi: 10.1016/j.lfs.2014.11.015. [DOI] [PubMed] [Google Scholar]
- Alonso A.J., Pérez J., Sánchez E., Pérez C., Pérez S. Effects of kramecyne on LPS induced chronic inflammation and gastric ulcers. Drug Dev. Res. 2015;76:185–193. doi: 10.1002/ddr.21254. [DOI] [PubMed] [Google Scholar]
- Brambilla G., Cavanna M., Faggin P., Maura A., Pino A., Ricci R., Robbiano L. Genotoxic effects in rodents given high oral doses of ranitidine and sodium nitrite. Carcinogenesis. 1983;4(10):1281–1285. doi: 10.1093/carcin/4.10.1281. [DOI] [PubMed] [Google Scholar]
- Burdan F., Bełzek A. Current opinions on embryotoxic and teratogenic effects of ibuprofen. Pol. Merkur. Lekarski. 2001;11(63):266–270. [PubMed] [Google Scholar]
- Cleves M.A., Savell V.H., Raj S., Zhao W., Correa A., Werler M.M., Hobbs C.A. Maternal use of acetaminophen and non-steroidal anti-inflammatory drugs (NSAIDs), and muscular ventricular septal defects. Birth Defects Res. A. Clin. Mol. Teratol. 2004;70:107–113. doi: 10.1002/bdra.20005. [DOI] [PubMed] [Google Scholar]
- Ericson A., Källén B.A.J. Nonsteroidal anti-inflammatory drugs in early pregnancy. Reprod. Toxicol. 2001;15:371–375. doi: 10.1016/s0890-6238(01)00137-x. [DOI] [PubMed] [Google Scholar]
- Franco C.C.D.S., Miyamoto C.T., De Sant'Anna J.R., De Castro-Prado M.A.A. Genotoxicity of the cyclo-oxygenase-inhibitor sulindac sulfide in the filamentous fungus aspergillus nidulans. Braz. J. Microbiol. 2007;38(3):430–434. [Google Scholar]
- Galar M., García S., Gómez L.M., Pérez I., Mendoza D.J., Arrazola R.E. Oxidative stress and genotoxicity induced by ketorolac on the common carp Cyprinus carpio. Environ. Toxicol. 2016;31(9):1035–1043. doi: 10.1002/tox.22113. [DOI] [PubMed] [Google Scholar]
- Gupta U., Cook J.C., Tassinari M.S., Hurtt M.E. Comparison of developmental toxicology of aspirin (acetylsalicylic acid) in rats using selected dosing paradigms. Birth Defects Res. B. Dev Reprod. Toxicol. 2003;68(1):27–37. doi: 10.1002/bdrb.10007. [DOI] [PubMed] [Google Scholar]
- Hayashi M. The micronucleus test-most widely used in vivo genotoxicity test. Genes Environ. 2016;38(18) doi: 10.1186/s41021-016-0044-x. eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongslo J.K., Christensen T., Brunborg G., Bjørnstad C., Holme J.A. Genotoxic effects of paracetamol in V79 Chinese hamster cells. Mutat. Res. 1988;204(2):333–341. doi: 10.1016/0165-1218(88)90108-5. [DOI] [PubMed] [Google Scholar]
- Kassaw C., Wabe N.T. Pregnant women and non-steroidal antiInflammatory drugs: knowledge, perception and drug consumption pattern during pregnancy in Ethiopia. N. Am. J. Med. Sci. 2012;4(2):72–76. doi: 10.4103/1947-2714.93377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köseoǧlu B.G., Öztürk S., Koçak H., Palanduz S., Çefle K. The effects of etodolac, nimesulid and naproxen sodium on the frequency of sister chromatid exchange after enclused third molars surgery. Yonsei Med. J. 2008;49(5):742–747. doi: 10.3349/ymj.2008.49.5.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleek M.I., Safaa A.F., Marwa M.K. Genotoxicity of dactinomycin and tramadol on mice bone marrow. IJSER. 2015;4(10):9–14. [Google Scholar]
- Menegola E., Broccia M.I., Giavini E. Atlas of rat fetal skeleton double stained for bone and cartilage. Teratology. 2001;64:125–133. doi: 10.1002/tera.1055. [DOI] [PubMed] [Google Scholar]
- Mourelatos D. Sister chromatid exchange assay as predictor of tumor chemoresponse. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2016;803–804:1–12. doi: 10.1016/j.mrgentox.2016.03.011. [DOI] [PubMed] [Google Scholar]
- Nielsen G.L., Sørensen H.T., Larsen H., Pedersen L. Risk of adverse birth outcome and miscarriage in pregnant users of non-steroidal anti-inflammatory drugs: population based observational study and case-control study. BMJ. 2001;322:266–270. doi: 10.1136/bmj.322.7281.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofori B., Oraichi D., Blais L., Rey E., Bérard A. Risk of congenital anomalies in pregnant users of non-steroidal anti-inflammatory drugs: a nested case-control study. Birth Defects Res B. Dev. Reprod. Toxicol. 2006;77:268–279. doi: 10.1002/bdrb.20085. [DOI] [PubMed] [Google Scholar]
- Olesen C., Steffensen F.H., Nielsen G.L., de Jong-van den Berg L., Olsen J., Sørensen H.T. Drug use in first trimester and lactation: a population-based survey among Danish women. Eur. J. Clin, Pharmacol. 1999;55(2):139–144. doi: 10.1007/s002280050608. [DOI] [PubMed] [Google Scholar]
- Pérez S., Sánchez E., Martínez D., Zavala M.A., Pérez C. Kramecyne a new anti-inflammatory compound isolated from Krameria cytisoides. Molecules. 2012;17:2049–2057. doi: 10.3390/molecules17022049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry P., Wolff S. New Giemsa method for the differential staining of sister chromatids. Nature. 1974;251:156–158. doi: 10.1038/251156a0. [DOI] [PubMed] [Google Scholar]
- Peters P.W.J. Double staining of fetal skeletons for cartilage and bone. In: Neubert D., Merker H.J., Kwasigroch T.E., editors. Methods in prenatal toxicology. Georg Thieme; Stuttgart: 1977. pp. 153–154. [Google Scholar]
- Sánchez A., Granados D., Simón N. Uso medicinal de las plantas por los Otomíes del Municipio de Nicolás Flores, Hidalgo. México. Rev. Chap. S. Horti. 2008;14(3):271–279. [Google Scholar]
- Sánchez E., Lemus J., Pérez S., Pérez J. Effect of kramecyne on the inflammatory response in lipopolysaccharide-stimulated peritoneal macrophages. Based. Complement. Alternat. Med Evid. 2013 doi: 10.1155/2013/762020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples R.E., Schnell V. Refinements in rapid clearing technic in the KOH-alizarin red S method for fetal bone. Stain Technol. 1964;39:61–63. [PubMed] [Google Scholar]
- Stults D.M., Killen M.W., Pierce A.J. The sister chromatid exchange (SCE) assay. Methods Mol. Biol. 2014;1105:439–455. doi: 10.1007/978-1-62703-739-6_32. [DOI] [PubMed] [Google Scholar]
- Tripathi R., Pancholi S.S., Tripathi P. Genotoxicity of ibuprofen in mouse bone marrow cells in vivo. Drug Chem. Toxicol. 2012;35(4):389–392. doi: 10.3109/01480545.2011.630670. [DOI] [PubMed] [Google Scholar]
- Wilson D.M.I.I.I., Thomson L.H. Molecular mechanisms of sister-chromatid exchange. Mutat. Res. 2007;616:11–23. doi: 10.1016/j.mrfmmm.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Wilson J.G. Teratology principles and techniques. University Pres; Chicago: 1965. pp. 262–277. [Google Scholar]