Summary
In injured tissues, regeneration is often associated with cell fate plasticity, such that cells deviate from their normal lineage paths. It is becoming increasingly clear that this plasticity often creates alternative strategies to restore damaged or lost cells. Alternatively, cell fate plasticity is also part and parcel of pathologic tissue transformations that accompany disease. In this Perspective, we summarize a few illustrative examples of physiologic and aberrant cellular plasticity. Then we speculate on how one could enhance endogenous plasticity to promote regeneration and reverse pathologic plasticity, perhaps inspiring interest in a new class of therapies targeting cell fate modulation.
Introduction
In recent years, it has become evident that while cell fate conversions during homeostasis follow certain prescribed paths of differentiation, injury renders cell fate significantly more plastic. Early pioneering work in the late 1800s identified that newt retina, lens, and even limbs could regenerate, implying that some vertebrates retain extraordinary cell fate plasticity (reviewed in Del Rio-Tsonis and Tsonis, 2003). More modern work, which includes extensive genetic lineage tracing, has helped reveal that seemingly similar organisms can invoke different forms of plasticity while reaching phenomenologically similar forms of regeneration. There is no more spectacular example of regeneration than the restoration of a limb following its amputation. In both red spotted newt and axolotl larvae, regeneration following limb amputation involves the formation of an undifferentiated set of cells termed a blastema. In the adult newt, differentiated cells dedifferentiate and become proliferative while maintaining their original lineage commitment and form cells faithful to the fate of the original progenitor. That is to say, for example, myofibers become proliferative, migratory mononuclear cells that then generate more muscle. The red spotted newt’s close cousin, the axolotl, has comparable regenerative capabilities, and also does so through the formation of a seemingly undifferentiated set of blastema cells. However, in this case, the origin of muscle cells has been shown to be resident PAX7+ muscle stem cells, as opposed to the dedifferentiating myocytes of the red spotted newt (Sandoval-Guzman et al., 2013; Tanaka et al., 2016). Interestingly, large numbers of transcripts unique to axolotl blastema have been detected, suggesting that a mechanism distinctly evolved in these organisms (Haas and Whited, 2017). We emphasize this example to draw attention to the notion that even in the context of urodele amphibian regeneration, proliferation of lineage-restricted cells, stem cells, and dedifferentiating cells can be variously deployed to effect the same fundamental regenerative end. It seems as though evolution is capable of harnessing a deep homology concerning cell fate interconvertibility. It has become increasingly clear that plasticity is a cardinal mode of injury-response in myriad organs in mammals. Particularly prominent examples are found in epithelia where a focused effort has been made to identify plasticity phenomenon.
In addition to being a regenerative phenomenon, cell fate plasticity is also evident in disease states. Metaplastic transformation of tissues accompanies both malignant and non-malignant pathologies. Most notably cancer and its antecedents have been associated with arrested or aberrant differentiation. This conceptual piece points to the possibilities that (1) regeneration could be stimulated by enhancing effective plasticity and that (2) pathological plasticity could be reversed by modulating cell fate. We speculate how insights into cell plasticity may inspire next-generation therapeutics that move into the clinic.
Physiologic Cell Fate Plasticity
Dedifferentiation in Epithelia
While amphibians and teleost fish are known for their prodigious ability to regenerate, we will focus on a few illustrative examples of mammalian cell fate plasticity. Most of the well-documented examples of mammalian plasticity, proven using indelible lineage tracing in the mouse, occur in epithelia. These tissues have historically been described as having parental stem cells that appear “undifferentiated” and functional differentiated cells such as secretory, absorptive, ciliated, and sensory cells. Recent studies show that genetic ablation of specific cell types or physiologic damage to tissues can cause differentiated cells to revert into stem cells or assume a facultative stem cell function to repair tissues. In other cases, differentiated cells can assume alternative fates directly, despite the fact that these lineage paths do not exist normally in the steady state tissue.
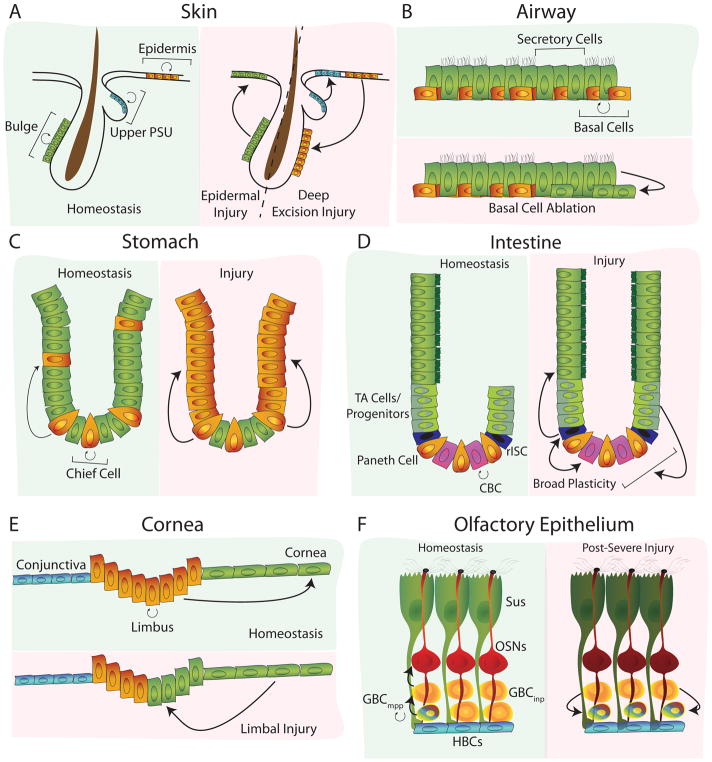
In the epidermis, it has been reported that lineage restricted progenitors can acquire the potential for forming cells of other lineages following epidermal injury. For example, bulge stem cells are normally restricted to hair follicles, but extensive injury induces them to transiently contribute to the epidermal compartment during the acute phase of wound repair (Ito et al., 2005) (Figure 1A). On the other hand, the reverse has also been shown, where extensive injury induces fate plasticity in which epidermal cells of the interfollicular epidermis surrounding a wound generate hair follicles through a WNT dependent mechanism reminiscent of development (Ito et al., 2007)(Figure 1A). Similarly, the stem cells of the upper pilosebaceous unit are also lineage restricted, until punch biopsy injury, after which they contribute long term to the interfollicular epidermis (Page et al., 2013)(Figure 1A).
Figure 1.
Dedifferentiation in Epithelia. (A) In the epidermis, bulge, interfollicular epidermal, and upper pilosebaceous stem cells (PSU) normally produce differentiated cells restricted to their respective compartments. After injury, this restriction is lifted, allowing for bulge stem cells to contribute transiently to the epidermal compartment, epidermal stem cells to contribute to the generation of new hair follicles, and upper PSU stem cells to contribute to the interfollicular epidermis. (B) In the airway, differentiated secretory cells can dedifferentiate after ablation of the basal stem cell population. (C) In the stomach, Chief cells, which produce pepsinogen and chymosin, can generate all of the cell types of the tissue. This ability is enhanced significantly after injury. (D) In the intestine, differentiated cells are capable of broad levels of plasticity. After injury, reserve intestinal stem cells (rISC), along with early transit amplifying (TA) cells and secretory progenitors, are capable of gaining stem cell function. (E) In the cornea, ablation of the stem cells in the limbus induces dedifferentiation and migration of corneal cells to restore the limbus. (F) In the olfactory epithelium, severe injury involving loss of the Sus and olfactory sensory neurons (OSNs) induces dedifferentiation in neuronally specified progenitors (GBCinp) to become multipotent progenitors (GBCmpp).
In the airway, we have demonstrated that differentiated secretory cells can dedifferentiate after ablation of the pre-existing basal stem cell population, restoring the lost basal stem cells. Indeed, dedifferentiated cells that have adopted a basal fate are functionally identical to pre-existing stem cells, giving rise to long lived progeny that persisted for at least 2 months (Tata et al., 2013)(Figure 1B). Interestingly, there was an inverse relationship between the likelihood of dedifferentiation and the maturity of the differentiated cell, suggesting that cell fate becomes progressively “locked” with time. In the lung, it has also been shown using Hopx-CreER lineage tracing, that some type 1 lineage-traced cells can generate type 2 alveolar stem cells after pneumonectomy. Whether this is an example of dedifferentiation or labeling of a sub-population of type 1 cells remains to be elucidated (Jain et al., 2015).
In the gastrointestinal tract, several different populations of cells have demonstrated lineage plasticity. Most strikingly, differentiated TROY+ chief cells of the stomach that produce pepsinogen and chymosin, have also been shown to be capable of generating all of the cell types of the tissue, and this function is enhanced following injury (Stange et al., 2013)(Figure 1C). In the intestine itself, differentiated cells appear to have broadly “open” chromatin that allows their interconversion based on Notch target gene expression (Kim et al., 2014). Perhaps such permissive genomic architecture underlies the ability of Alpi+ enterocytes, Bmi1+ enteroendocrine, early transit-amplifying, and secretory progenitor cells to dedifferentiate into LGR5+ stem cells of the crypts (Buczacki et al., 2013; van Es et al., 2012; Jadhav et al., 2017; Tetteh et al., 2016; Tian et al., 2011; Yan et al., 2017)(Figure 1D).
More recently, it has been shown that depletion of ectodermally-derived corneal stem cells induces the dedifferentiation of committed corneal cells into stem cells to restore the lost stem cell compartment, remarkably reminiscent of the stem cell ablation in the airway. Interestingly, this study also provided a unique insight into mechanism by demonstrating that destruction of the limbal stroma prevented dedifferentiation, suggesting that some aspects of cell fate plasticity can be negotiated by adjacent mesenchyme (Nasser et al., 2018)(Figure 1E).
Finally, the murine olfactory epithelium, a pseudostratified neuroepithelial sensory tissue that is responsible for the sense of smell, utilizes dedifferentiation to accelerate tissue repair. The epithelium is comprised of sensory neurons that are unique in that they directly contact the external environment to bind odorants, which exposes them to damage (Shipley, 1985). To compensate for the continual turnover of damaged olfactory neurons, the epithelium is armed with several different pools of stem cells that act together throughout adult life to generate neurons and maintain olfaction (Fletcher et al., 2011; Huard et al., 1998; Schnittke et al., 2015). While the tissue contains a full complement of previously described conventional active and reserve stem cell pools, in times of significant injury, the tissue can also call upon neuronally specified progenitors to dedifferentiate into multipotent stem cells that then contribute to multi-lineage tissue repair using developmentally relevant pathways. These cells downregulate the epigenetic effector Ezh2, up-regulate Sox2, Klf4, and Pax6, and traverse the normal neurogenic path in reverse, until ultimately converting into a multipotent stem cell. This immediate injury response effectively increases the proliferative pool of multipotent stem cells available to the tissue during the initial week post-injury, accelerating overall tissue regeneration (Lin et al., 2017) (Figure 1F). Once again, the importance of epigenetic factors in cell plasticity phenomenon is evident.
These examples of injury responses suggest that dedifferentiation is ubiquitously deployed in varying degrees amongst many epithelia. Notably, all of the aforementioned tissues are exposed to constant environmental damage, and effective repair is crucial for organ homeostasis and survival. It remains to be seen whether these tissue-specific modes of regeneration share an underlying core mechanism, although in aggregate, it is likely that a common set of epigenetic principles determine which types of cell fate transitions are permitted (or favored) in a given injury in a given tissue. In the absence of direct genetic lineage tracing in humans, newer technologies will have to be employed to definitively establish plasticity phenomenon. These include clone determination using the tracking of somatic or mitochondrial mutations, or in the absence of an indelible genetic stamp, computational reconstruction (Bendall et al., 2014; Greaves et al., 2006; Horns et al., 2016; Treutlein et al., 2014; Woodworth et al., 2017).
Not all tissues employ dedifferentiation as the mechanism of choice for tissue regeneration, although it appears widespread in epithelia containing resident stem cells. In some tissues that experience low levels of turnover, the primary mode of regeneration may be transdifferentiation. Upon ablation of insulin-producing β cells of the pancreas, glucagon-producing α cells can transdifferentiate into the lost β cells to supplement those cells produced by the replication of existing β cells. Oddly, juvenile mice do not employ this α cell transdifferentiation. Instead, somatostatin-positive δ cells engage a developmentally relevant pathway to dedifferentiate and regenerate lost β cells (Chera et al., 2014; Thorel et al., 2010). This suggests that lineage plasticity, even within one mammalian organ, can follow different courses with age and maturity. As in the trachea, developmental maturity, either with reference to the age of an organism or the age of a particular cell, seems to have a profound influence of cell plasticity (Tata et al., 2013).
Metaplasia and Pathologic Cell Fate Plasticity
Epithelial metaplasia
Metaplasia has been classically defined as a tissue type transformation, and this is how the term is used by pathologists to grossly describe the phenotypes of tissues. In this context, the term does not directly imply a particular form of cell plasticity and merely refers to the presence of novel cells or disproportionate numbers of normal cells within a tissue compartment. The best examples arise from human tissues. The pathologist’s definition of metaplasia also does not explicitly denote whether the tissue type transformation is beneficial or harmful. It has been speculated that metaplasias are associated with a beneficial temporary benefit, but can become the harbingers of pathologic metaplasia and cancer. The exact mechanisms governing the loss of metaplasia reversibility remain to be elucidated. Similarly, there is little understanding of why metaplasia leads to transformation and frank cancer. As an example, airway exposure to noxious stimuli like cigarette smoke leads to an increase in the numbers of squamous cells. These squamous cells are presumed to subtend a protective epithelial barrier function. Since squamous cells are not present in the unperturbed airway, a tissue transformation is grossly evident, but the source of new cells is still mysterious. It has been shown that in patients that have stopped smoking, squamous differentiation is less evident, suggesting that this form of metaplasia can be physiologically reversible (Lapperre et al., 2007; Rigden et al., 2016; Schamberger et al., 2015). At the same time, squamous metaplasia is thought to presage lesions with cytologic atypia that are on their way to becoming frankly dysplastic and eventually giving rise to squamous cell cancer. Little is known about the possible conversion of physiologic protective squamous metaplastic into pre-cancerous metaplasia. Indeed, it remains a formal possibility that these are distinct states that resemble one another histologically.
Although the appearance of a novel cell type may represent the emergence of a new cell fate from a stem cell or a transdifferentiation of a pre-existing mature cell type into a novel cell type, it is also possible that unidentified rare progenitor cell populations expand and provide a source of novel cell types. Thus, tissue level metaplasia may arise from cellular plasticity or involve the expansion of cell types that are not evident in the steady state. Indeed, the latter appears to be the case in Barrett’s esophagus, perhaps the most classic example of human metaplasia, in which continuous acid and bile reflux cause an adaptive change in tissue composition. In this case, the distal esophageal epithelium adopts an intestinal phenotype presumed to protect the mucosa from injury. Many hypotheses have been raised for the origin of this metaplasia; however, recently a novel transitional basal cell population has been identified. These transitional basal cells, which are TRP63+, KRT5+, and KRT7+, are distinct from normal squamous basal cells of the esophagus or stem cells of the adjacent stomach. This transitional epithelium expands following injury and is particularly receptive to CDX2-mediated intestinal metaplasia. Interestingly, similar cells are found at other squamocolumnar junctions, such as those of the anus and cervix (Jiang et al., 2017)(Figure 2A), implying that many epithelial metaplasias may be a result of the expansion of a rare cell population (with or without subsequent cell plasticity events).
Figure 2.
Pathologic Cell Fate Plasticity. (A) In Barrett’s esophagus, rare transitional basal cells in the squamocolumnar junction (SCJ) expand to generate intestinal metaplasia. (B) In mucous metaplasia of the airway, chronic inflammation induces secretory cells to differentiate into goblet cells that produce excess mucous. (C) In progressive osseous heteroplasia, activating mutations in the BMP pathway induce adiopocyte progenitors to induce bone forming transcriptional programs and deposit calcium. (D) in calciphylaxis, vascular smooth muscle cells (VSMCs) in the tunica media induce ectopic Runx1 and begin to deposit calcium. (E) In normal hematopoiesis, hematopoietic stem cells (HSCs) generate the common myeloid progenitor (CMP) that then give rise to myeloblasts, which can then generate monocytes or granulocytes. In acute myeloid leukemia, this differentiation step in myeloblasts is blocked, and large amounts of myeloblasts are generated.
Other forms of epithelial metaplasia are also considered beneficial injury responses. In addition to the squamous metaplasias alluded to above, mucous metaplasia arises in tissues such as the airway or nasal epithelium. In this setting, the epithelia are characterized by increased numbers of goblet cells and basal stem cells. Thus, the tissue is characterized as metaplastic based on these gross changes in cell proportionality of normally resident cell populations. The excess of goblet cells, stimulated by inflammatory cells following infection or allergy, are thought to be needed to produce mucous to increase barrier function alongside the squamous metaplasia that often accompanies the same injury (Curran and Cohn, 2010)(Figure 2B). The source of new goblet cells is debated, but likely includes the differentiation of pre-existing secretory cells. Goblet cell metaplasia is reversible at the histologic tissue level, but once again it is unclear whether the goblet cells die, are extruded, undergo apoptosis, or whether they revert into secretory cells. Thus, even this simple form of beneficial metaplasia is poorly understood at a cellular level. Additionally, why squamous and mucous metaplasia arise in the same setting in the airway, and what dictates which metaplasia arises in a given locale within the airway remains mysterious. In aggregate, these examples illustrate that the basis of tissue level metaplasias as defined by histologic tissue transformation are not understood at the cellular level. At the end of the day, all we can say is that metaplasias may arise from plasticity phenomena, but they may also arise from the expansion of unknown cell populations.
Mesenchymal metaplasia: Transdifferentiation Yielding Ectopic Calcification and Bone Tissue
Some striking examples of tissue metaplasia can occur in mesenchymal tissues where fibrosis or, in extreme situations, ectopic mineralization and bone formation can occur. Fibrosis, at times may be associated with epithelial-to-mesenchymal transition (EMT), but often also occurs because of an expansion of non-epithelial cell populations, including fibroblasts of various sources, smooth muscle cells, and pericytes (Nieto et al., 2016). Recent studies suggest some forms of fibrosis result from the expansion and over-activity of a cell type present in or near the tissue, rather than a cell fate change (Dulauroy et al., 2012; Zepp et al., 2017). In addition to fibrosis, forms of metaplasia such as calcification and bone formation can occur outside the skeletal system as hereditary or non-hereditary. Calcification can arise from systemic mineral imbalance, where elevated levels of phosphorous and calcium can form mineralized deposits without known transdifferentiation events (Block et al., 1998; Giachelli, 1999). However, in hereditary and non-hereditary heterotopic ossification and in vascular calcification, it is becoming clear that specific populations of tissue resident cells are adopting a bone regulatory program, which includes activation of BMP or Hedgehog signaling or of bone associated transcription factors such as Osterix or Runx2 (Lin et al., 2015; Regard et al., 2013; Zhang et al., 2017). The hereditary forms of heterotopic ossification include fibrodysplasia ossificans progressiva (FOP) and progressive osseous heteroplasia (POH), and they are caused by gain-of-function mutations in ACVR1 resulting in activated BMP signaling, and inactivating mutations in GNAS which result in activated Hedgehog signaling (Regard et al., 2013; reviewed in Shore and Kaplan, 2010). Although both diseases are characterized by the progressive formation of bone in extraskeletal connective tissues, they have distinguishing features. POH can occur more often in the dermis near adipose tissue and moves to deeper regions of connective tissue and muscle, whereas ossification in FOP is found in tendons, ligaments, muscle, and fascia and can be compounded by injury involving the immune system. Lineage tracing experiments suggest non-endothelial interstitial Tie2+/Pdgfrα+ fibro-adipogenic progenitors, rather than smooth muscle cells or muscle cells, contribute to heterotopic bone (Dey et al., 2016; Lees-Shepard et al., 2018; Lounev et al., 2009; Wosczyna et al., 2012). In addition, expression of activating ACVR1 mutations in Scleraxis-derived tendon cells rather than in other endothelial or myofibroblast cell types causes ectopic expression of Sox9 and Osterix and subsequent bone formation (Agarwal et al., 2017) (Figure 2C).
The ability of tendon and ligament cells to form bone is not limited to these hereditary ectopic bone disorders. Cartilage and bone can form in tendons, ligaments, and their attachment sites because of overuse conditions, genetic defects in matrix components, or X-linked hypophosphatemia (AMEYE et al., 2002; Archambault et al., 2007; Benjamin and Ralphs, 1998; Liang et al., 2009). Interestingly, spinal cord, traumatic brain, and burn injuries as well as major surgical events such as hip arthroplasty can initiate heterotopic ossification in soft connective tissue in joints unrelated to the injury site (Ranganathan et al., 2015). The mechanisms underlying these lesions are not well understood, but are thought to involve the inflammatory response and changes in the local microenvironment. For example, ossification of the ligamentum flavum, which can lead to spinal stenosis, was linked to increased expression of the pro-inflammatory cytokine, TNFα. Culture with TNFα upregulated Osx and promoted osteoblast differentiation in primary cells cultured from the ligamentum flavum (Zhang et al., 2017). Changes in mechanical signals also have effects on gene expression in connective tissues (Archambault et al., 2007), consistent with the idea that the mechanical environment can shift cell identity (Engler et al., 2006). Tendon, ligament, and joint tissues appear particularly susceptible to shifting fates towards cartilage and bone programs. Whether this stems from their close developmental relationship (Blitz et al., 2013; Soeda et al., 2010; Sugimoto et al., 2013) or an inherent connection between skeletal and connective tissue regulatory programs is currently unknown, but it is interesting to note that there is specificity in the cell types that can be acted upon to form bone. This observation could provide important clues as to the underlying biology of the cell types capable of transdifferentiation, as well as to the local environmental signals eliciting such dramatic cell fate changes.
In addition to ectopic bone, two primary types of vascular calcification have been described in adult disease: (1) intimal calcification associated with atherosclerosis, myocardial infarction, and stroke; and (2) medial calcification associated with chronic kidney disease, and diabetes (Amann, 2008; Otsuka et al., 2014). Calciphylaxis is a rare, but well documented syndrome, characterized by the calcification of microvessels (40–600 micrometers) in dermal and subcutaneous adipose tissue and distinctive painful skin lesions (Chen et al., 2014; Nigwekar et al., 2015). Analyses of biopsies reveal medial wall calcification leading to ischemia with further occlusion by thrombosis resulting in necrosis and the hallmark skin lesions (Magro et al., 2010; Nigwekar et al., 2008, 2015; Weenig, 2008).
The predominant cell type within the tunica media, the site of medial vascular calcification and calciphylaxis, is the vascular smooth muscle cell (VSMC). Under normal homeostasis, the function of this contractile cell is to maintain vessel wall integrity and regulate arterial tone. However, VSMCs exhibit remarkable plasticity even in adulthood. Evidence suggests contractile VSMCs can adopt an osteogenic phenotype causing vascular calcification (Goiko et al., 2013; Gomez and Owens, 2012; Malhotra et al., 2015; Shanahan et al., 1994; Shankman et al., 2015; Speer et al., 2010). This phenotypic switch is characterized by loss of smooth muscle cell markers (e.g. SM22, α-smooth muscle actin, calponin), and induction of osteogenic cell markers (e.g. RUNX2 and osteopontin)(Speer et al., 2002, 2010; Steitz et al., 2001). The triggers for this phenotypic switch are myriad, including aging, oxidative stress, inflammatory cytokines, high phosphate concentrations, and paracrine signaling (Chang et al., 2008; Chen et al., 2014; Kapustin and Shanahan, 2016). Unfortunately, the specific trigger for calciphylaxis remains unknown, though it has been hypothesized that it may be acute tissue trauma in the context of risk factors like inflammation and high phosphate. It is known, however, that expression of the transcription factor RUNX2 is associated with the development of calciphylaxis (Kramann et al., 2013; Nigwekar et al., 2017; Speer et al., 2010). Subsequent to osteogenic phenotype switching, VSMCs secrete matrix vesicles containing calcium phosphate that serve as a nidus for medial vascular calcification (Kapustin et al., 2015). Altogether, VSMC transdifferentiation – likely mediated by Runx2 – is implicated in the pathophysiology of calciphylaxis (Figure 2D).
Interestingly, pathological cues can induce heart valves, which contain both endothelial and interstitial cells, to undergo valvular calcification. Interstitial cells can undergo a phenotypic transition to an osteoblast-like cell that produces bone matrix and signaling molecules, ultimately promoting valvular calcification. This process mirrors vascular calcification involving BMP-induced Runx2 (Leopold, 2012; Rajamannan et al., 2003). On the other hand, endothelial cells lining the valve surface can also undergo a phenotypic transition to an osteoblast-like cell. To do this, they first pass through an endothelial-to-mesenchymal transformation, mimicking a developmentally critical process for normal valvulogenesis (Hjortnaes et al., 2015; Wylie-Sears et al., 2011). The mechanism underlying this phenomenon has been well characterized—it is known that calcification is normally inhibited in endothelial cells by shear stress, which activates Notch signaling and induces expression of calcification-inhibiting Matrix Gla protein (White et al., 2015). Accordingly, loss-of-function mutations in Notch1 have been shown to result in severe aortic valve calcification in adults (Theodoris et al., 2015). Altogether, these studies and others demonstrate the importance of cell fate plasticity in a wide range of calcification pathologies.
Differentiation Arrest and Cancer
The concept of differentiation arrest pre-dates our molecular and genetic understanding of cancer. Since the advent of microscopy, it has been recognized that malignant cells appear morphologically less-differentiated, or aberrantly-differentiated, and have been termed dysplastic. Furthermore, in many circumstances the degree of visible differentiation correlates with the clinical aggressiveness of disease, forming the basis of current tumor grading schemas. Indeed, the most concerning tumors are referred to as anaplastic, denoting cells that have lost many or all the characteristics of their tissue-type of origin. In the most undifferentiated cases, these anaplastic malignant cells lose all identifiable features, and appear only as “small-blue-round-cell-tumors”, a histopathological category unto themselves.
Differentiation arrest has been best exemplified in disorders of blood cells, mostly owing to years of research contributing to a deep understanding of hematopoietic stem cell biology and the normal, step-wise developmental process of hematopoiesis. Even prior to the identification of the first translocations and mutations contributing to acute myeloid leukemia (AML), there was the recognition that the leukemic “blast” cells from different patients appeared variably mature under the microscope (Bennett et al., 1976) (Figure 2E). Originally, this sub-division of AML was merely academic, but is now recognized as therapeutically relevant.
Therapeutic Modulation: Forms of Therapeutic Cellular Conversions
Differentiation Therapy for Cancer
Differentiation therapy promises to target and to overcome the differentiation roadblock present in disorders such as AML, prompting the corrupted cancer cell to resume its normal process of maturation, development, and death. Furthermore, because the tumor-initiating cells are believed to be particularly stem-like, differentiation therapy might preferentially target this critical reservoir, with curative potential. Originally, all patients with AML received the same chemotherapy regardless of differentiation state. However, it was recognized that patients with acute promyelocytic leukemia (M3) presented with a clinically distinct disease.
Upon microscopic examination, the AML M3 leukemic blasts were typically packed with darkly staining primary granules, apparently frozen at a developmental state half-way between a non-descript myeloid blast and a terminally-differentiated neutrophil. Ultimately, advances in karyotyping would demonstrate that every M3 leukemia was marked by a recurrent translocation involving the retinoic acid receptor (RAR), typically t(15;17), which results in production of the PML/RAR-alpha chimeric oncoprotein.
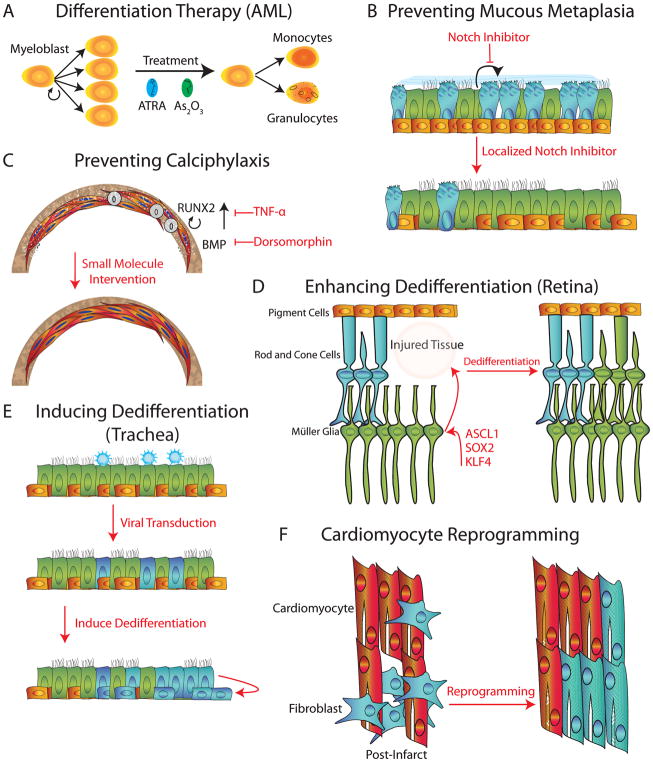
This recurrent translocation also predicted sensitivity to treatment with high doses of all-trans retinoic acid (ATRA). Remarkably, ATRA relieved the transcriptionally repressive program of PML/RAR-alpha, and the leukemic cells were freed from differentiation arrest, and rapidly progressed down the normal developmental course to mature neutrophils, despite the fact that they still carried the abnormal translocation (Wang and Chen, 2008). Therapy with ATRA has since been combined with arsenic trioxide, which seems to target the PML/RAR-alpha fusion for degradation. This combination of molecularly-targeted pro-differentiation agents has resulted in curative therapy for the majority of patients, obviating the need for conventional chemotherapy. Where acute promyelocytic leukemia once carried the worst prognosis of all the forms of AML, it now carries the best, and this complete inversion of the survival curve underscores the most successful prototype for differentiation therapy as a whole (de The, 2017) (Figure 3A).
Figure 3.
Therapeutic Modulation of Cell Fate. (A) An example of differentiation therapy in which all-trans retinoic acid (ATRA) and arsenic trioxide (As2O3) are used in acute myeloid leukemia (AML), to force the differentiation of aberrant myeloblasts into monocytes and granulocytes. (B) Localized administration of Notch inhibitors could prevent the differentiation of secretory cells into goblet cells, removing the source of excess mucous in airways diseases. (C) Localized TNF-α or dorsomorphin could block Runx2 or BMP, respectively, and reverse calcification. (D) Restoring the ability of Muller glia to express Ascl1, Sox2, and KLF4 could yield cells capable of dedifferentiation and restoration of lost rod and cone cells in the retina. (E) Viral infection of the airway selectively targets short-lived luminal cells, preventing long lasting treatment. Subsequent basal cell ablation or outright induction of dedifferentiation of virally transduced secretory cells could generate long-lasting basal stem cells carrying the proper gene therapy. (F) Cardiac infarct generates fibroblast scarring in heart tissue alongside the death of cardiomyocytes. Directed reprogramming of scar fibroblasts into cardiomyocytes could be used to treat both the scar and restore the myocardium.
Given the successes in acute promyelocytic leukemia, investigators have turned to other subtypes of AML. AML is a genetically diverse malignancy, and categorizing recurrent mutations has confirmed that many of these disrupt the function of proteins known to be involved in normal maturation, suggesting that directed small-molecule therapy may be able to overcome differentiation arrest. This has prompted the development of recently-approved therapies targeting mutant IDH (Stein et al., 2017), as well as new investigational targets including LSD1 (Schenk et al., 2012), DOT1l, and EZH2 in clinical development.
Other investigators, have taken an unbiased approach to identifying new differentiation therapies in AML, relying on phenotypic screens rather than target-directed screens (Banerji et al., 2012; Pikman et al., 2016; Radomska et al., 2015; Stegmaier et al., 2004; Sykes et al., 2016). This approach presupposes that different leukemias may share a common node of differentiation arrest despite their genetic heterogeneity. New targets have emerged from these screens, and the clinical utility of small molecules targeting SYK, GSK3-alpha, MTHFD2, and DHODH are all under investigation.
Taking the paradigm one step further, one might hypothesize that many solid cancers arise in the setting of arrested differentiation. If so, what then are the barriers to developing differentiation therapy in non-hematologic malignancies? The clinical success in AML builds on a foundation of deep understanding of normal hematopoiesis including the predictable expression of transcription factors and cell-surface proteins effectively marking each stage of differentiation. This same roadmap is not available in all tissue or tumor-types, making it difficult to devise a similar differentiation screening strategy. Despite the technical challenges inherent to phenotypic screens, we feel strongly that these have the potential to not only identify new biology, but also to identify therapies with a clinically-relevant therapeutic window. Our current reliance on screens with a simple viability read-out must make way to more sophisticated screens that report on cell-fate decisions. This can facilitate the path towards identifying small molecules that can bias a malignant progenitor towards “proper” differentiation.
Indeed, large-scale genomic analysis of colorectal cancer has yielded a potential path to treat PTPRK-RSPO3-fusion positive tumors that is strikingly reminiscent of the path taken for AML. In this subset of tumors, it has been shown in xenograft models that anti-Rspondin treatment results in tumor growth inhibition. Furthermore, treatment resulted in a downregulation of stem-cell markers and an increase in mature crypt differentiation markers as seen through RNAseq, and also exhibited a differentiated appearance. This study and others have provided a proof-of-principle for differentiation therapy in non-hematologic malignancies (Chartier et al., 2016; Storm et al., 2016).
We can also imagine that an effective way for cancer to evade chemotherapy might involve a cell fate switch. Indeed, cellular plasticity in the form of EMT has long been recognized as a phenotype encountered in resistant cancers. As the mesenchymal state is being characterized, one could imagine blocking the transdifferentiation of epithelia into more mesenchymal cells. More shockingly, epithelial cancers have been demonstrated to undergo wholesale conversion from one tissue type to another. Indeed EGFR+ lung adenocarcinomas have converted into small cell cancers (Niederst et al., 2015; Oser et al., 2015) and prostate cancers can gain resistance to anti-androgen treatment by shifting from an androgen receptor (AR) dependent luminal state to AR-independent basal cell state mediated through loss of Trp53 and Rb1 whose loss is promoted by Sox2 (Ku et al., 2017; Mu et al., 2017). If these forms of cellular plasticity represent a common escape mechanism from targeted therapies, it follows that blocking transdifferentiation might prevent escape.
Reversing Transdifferentiation
Based on the initial success of differentiation therapies in the setting of leukemia, one can imagine similar strategies for reversing pathologic metaplasias. In mucous metaplasia, such as encountered in asthma, COPD, or cystic fibrosis, repetitive insults or infection invoke an inflammatory response, triggering goblet cell hyperplasia. Most strategies that have been designed for treating diseases associated with pathologic mucous metaplasia have aimed to halt the inciting immunoinflammatory stimuli. In the case of asthma, corticosteroids and antibiotics, along with more selective immunomodulatory agents such as leukotriene antagonists and anti-IL-13 monoclonal antibodies are often effective but carry significant side effect profiles (Curran and Cohn, 2010; Nguyen et al., 2017; Walker, 2003). We propose that targeting the actual goblet cell differentiation event, and thus directly blocking the cell fate transitions that result in excess mucous-producing goblet cells, might be of therapeutic value regardless of whether the underlying pathology is stimulated by an allergen in asthma or smoke in COPD-related coughing. Indeed, the Notch pathway is critical for cell fate decisions in the airway epithelium and in promoting airway mucous metaplasia (Chiba et al., 2009; Miklossy et al., 2013). Furthermore, inhibition using Notch antagonists has been shown to block IL13-induced mucous metaplasia. Together, this suggests that a local Notch inhibitor, potentially delivered as an aerosol, could specifically reverse mucous metaplasia by rebalancing the ratio of goblet cells to normal (Danahay et al., 2015; Guseh et al., 2009)(Figure 3B). Of note, since this form of differentiation blockade involves a balance of multiple cell types, one must be careful not to alter the normal differentiation of a necessary cell type that is not involved in the pathology. This stands in sharp contrast to the situation with cancer in which it is preferable that all pathologic cells are removed. Additionally, one must note that signaling pathway modifiers may have untoward effects in various tissues throughout the body. Some form of localized therapy is likely to be needed, including topical or aerosolized, or non-absorbable formulations for the skin, lungs, and GI tract respectively.
The lack of effective therapies for a disease such as calciphylaxis or the hereditary heterotopic bone disorders suggests the possibility that reversing the osteogenic phenotype might be used therapeutically. In this case, there is the potential to utilize the existing knowledge of the mechanisms underlying transdifferentiation and vascular calcification to develop therapies that could inhibit or even reverse pathologic vascular calcification. Existing research described above suggests that downregulating RUNX2 (such as through TNF-α) (Gilbert et al., 2002) or inhibiting BMP signaling in vessel walls would be effective at preventing VSMC transdifferentiation (Figure 3C). With FOP in particular, there is a focus on therapeutic agents that can interfere with BMP receptor activation (Luo et al., 2016; Yu et al., 2008). Finally, inhibition of paracrine signaling or cytokine-mediated fate changes or restoring normal mechanical signals in different forms of heterotopic bone formation could prevent or reverse the underlying osteogenic pathology. Ultimately, however, a greater understanding of the molecular mechanisms that induce altered pathologic cell fates are required to identify more specific targets.
Promoting Dedifferentiation
While we have speculated about reversing pathologic cell plasticity, one might consider harnessing cell plasticity as a direct form of therapy. There are several potential avenues for the future. It’s long been noted that “lower” organisms often possess greater regenerative potential than their mammalian counterparts. Leveraging what we know about cell plasticity in lower organisms might allow us to coax mammalian tissues in a similar vein. For example, in lower animals, the sensory epithelia of the olfactory system, retina, and inner ear are all known to possess regenerative capacity after injury. Interestingly, the olfactory epithelium of the mouse is the only sensory epithelium that has retained its regenerative potential, and does so through the induction of a subset of reprogramming factors (Sox2 and Klf4 along with c-Myc target genes). In contrast, although the zebrafish inner ear and retina are capable of regenerating after injury (Millimaki et al., 2010; Ramachandran et al., 2010), the mammalian cochlear epithelium and retina are incapable of significant regeneration of hair and photoreceptor cells, respectively. Zebrafish and embryonic chick Muller glia of the retina express sox2, along with the other reprogramming factors nanog, oct4, and c-mycA (Fischer et al., 2010; Gallina et al., 2014; Ramachandran et al., 2010). Perhaps the expression of these factors could facilitate adult chick retinal regeneration. Similarly, mammalian retina, expresses only Sox2 (Fischer et al., 2010) and perhaps re-expression of the missing reprogramming factors would permit retinal repair (Figure 3D). Indeed, there are suggestions that a brief overexpression of Oct4, Sox2, and Klf4 in fibroblasts, along with modulation of signaling cascades associated with the adult myocardial niche, results in effective reprogramming towards a cardiomyocyte lineage. However that has yet to be done successfully in vivo (Efe et al., 2011). An alternative strategy is also yielding fruit as recent work in the cochlea has demonstrated the feasibility of small molecule mediated expansion of LGR5+ supporting cells followed by their directed differentiation into hair cells (McLean et al., 2017).
We also speculate that dedifferentiation could be an attractive method of affecting long-lasting gene therapy in tissues with high turnover. In both the skin and the airway, it is difficult to virally transduce the underlying stem cell population, resulting in short-lived effects due to constant turnover of the targeted cells. However, efficient dedifferentiation of infected mature epithelial cells into basal stem cells would allow a durable repair of an epithelium (Figure 3E).
Directed Transdifferentiation
We would be remiss in discussing therapeutic approaches aimed at manipulating cell fate plasticity if we did not touch upon outright reprogramming or transdifferentiation in vivo (Srivastava and DeWitt, 2016). Of particular interest are efforts aimed at reducing the fibrosis that occurs after an infarct in the heart. If it were possible to convert cardiac fibroblasts directly into functional, beating cardiomyocytes, this would solve two problems at once. Indeed, direct reprogramming of cardiac fibroblasts using GATA4, MEF2C, and TBX5 after an infarct attenuated injury and reduced scar area (Qian et al., 2012). However, it appears that human cells require further factors for efficient repair (Patel et al., 2016). Similarly, research in reprogramming cardiomyocytes into functional pacemaker cells has yielded promising results in pig, where adenoviral delivery of TBX18 generated sufficient numbers of pacemaker cells to overcome complete heart block. Further research is required to refine this process, as the pacemaker function was transient, lasting only 2 weeks (Hu et al., 2014; Meyers et al., 2016). In the nervous system, the direct conversion of reactive glia into functional neurons would hold similar promise (reviewed in Li and Chen, 2016), and it is possible to imagine myriad opportunities in disease states where functional cells are lost, such as in type 1 Diabetes.
Conclusion
Differentiation therapy has already been effective in treating leukemias, but such approaches have only recently received attention in the treatment of solid tumors. Perhaps modulating plasticity may emerge as an important method to prevent EMT or recently identified gross cell fate conversions of one tumor type into another, which is increasingly recognized as a method of escape. We have laid out a map for imagining how encouraging or restraining cell fate plasticity could be deployed in pathologic settings, including those characterized by non-cancerous metaplasia or fibrosis. This points to the more general need to enumerate and understand what cell types exist in any given pathology, whether they are disproportionately represented by normal cell types or novel pathologic cell types that do not normally exist. Emerging technologies led by single cell transcriptomics should provide ample data in this regard. As with all therapies, encouraging beneficial plasticity may be associated with the risk of engendering pathologic cell plasticity, including cancer. Furthermore, any cell modulation therapy must be carefully deployed to act solely on a given tissue without altering the proportions of other necessary cell types of that tissue or other off -target tissues.
In this Perspective, Lin et al. highlight examples of plasticity during normal regeneration and in aberrant situations in a variety of tissues. The authors also discuss the merits of enhancing endogenous plasticity to promote regeneration as well as reversing pathologic plasticity as potential therapeutic strategies.
Acknowledgments
J.R. is a Howard Hughes Medical Institute Faculty Scholar, a New York Stem Cell Foundation Robertson Investigator, a Maroni Research Scholar at Massachusetts General Hospital, and a member of the Ludwig Institute for Cancer Research of Harvard Medical School. This work was supported by grants from the NIH R01HL116756, R01HL118185. We would also like to recognize Katrina Armstrong and Mark Fishman who encouraged the collaboration of clinicians and scientists to think about the application of developmental thinking to disease in the MGH Pathways program. Finally, we dedicate this review to two MGH medicine residents who inspired this form of intellectual collaboration, Lauren Zeitels and Victor Federov. We apologize to the myriad authors whose work we could not include in this brief review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agarwal S, Loder SJ, Cholok D, Peterson J, Li J, Breuler C, Cameron Brownley R, Hsin Sung H, Chung MT, Kamiya N, et al. Scleraxis-Lineage Cells Contribute to Ectopic Bone Formation in Muscle and Tendon. Stem Cells. 2017;35:705–710. doi: 10.1002/stem.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann K. Media calcification and intima calcification are distinct entities in chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1599–1605. doi: 10.2215/CJN.02120508. [DOI] [PubMed] [Google Scholar]
- AMEYE L, ARIA D, JEPSEN K, OLDBERG A, XU T, YOUNG MF. Abnormal collagen fibrils in tendons of biglycan/fibromodulin-deficient mice lead to gait impairment, ectopic ossification, and osteoarthritis. FASEB J. 2002;16:673–680. doi: 10.1096/fj.01-0848com. [DOI] [PubMed] [Google Scholar]
- Archambault JM, Jelinsky SA, Lake SP, Hill AA, Glaser DL, Soslowsky LJ. Rat supraspinatus tendon expresses cartilage markers with overuse. J Orthop Res. 2007;25:617–624. doi: 10.1002/jor.20347. [DOI] [PubMed] [Google Scholar]
- Banerji V, Frumm SM, Ross KN, Li LS, Schinzel AC, Hahn CK, Kakoza RM, Chow KT, Ross L, Alexe G, et al. The intersection of genetic and chemical genomic screens identifies GSK-3α as a target in human acute myeloid leukemia. J Clin Invest. 2012;122:935–947. doi: 10.1172/JCI46465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendall SC, Davis KL, Amir EAD, Tadmor MD, Simonds EF, Chen TJ, Shenfeld DK, Nolan GP, Pe’Er D. Single-cell trajectory detection uncovers progression and regulatory coordination in human b cell development. Cell. 2014;157:714–725. doi: 10.1016/j.cell.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin M, Ralphs JR. Fibrocartilage in tendons and ligaments - An adaptation to compressive load. J Anat. 1998;193:481–494. doi: 10.1046/j.1469-7580.1998.19340481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett JM, Catovsky D, Daniel M-T, Flandrin G, Galton DAG, Gralnick HR, Sultan C. Proposals for the Classification of the Acute Leukaemias French-American-British (FAB) Co-operative Group. Br J Haematol. 1976;33:451–458. doi: 10.1111/j.1365-2141.1976.tb03563.x. [DOI] [PubMed] [Google Scholar]
- Blitz E, Sharir A, Akiyama H, Zelzer E. Tendon-bone attachment unit is formed modularly by a distinct pool of Scx - and Sox9 -positive progenitors. Development. 2013;140:2680–2690. doi: 10.1242/dev.093906. [DOI] [PubMed] [Google Scholar]
- Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: A national study. Am J Kidney Dis. 1998;31:607–617. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- Buczacki SJA, Zecchini HI, Nicholson AM, Russell R, Vermeulen L, Kemp R, Winton DJ. Intestinal label-retaining cells are secretory precursors expressing lgr5. Nature. 2013;495:65–69. doi: 10.1038/nature11965. [DOI] [PubMed] [Google Scholar]
- Chang HB, Javed A, Dai Q, Kappes JC, Clemens TL, Darley-Usmar VM, McDonald JM, Chen Y. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J Biol Chem. 2008;283:15319–15327. doi: 10.1074/jbc.M800021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartier C, Raval J, Axelrod F, Bond C, Cain J, Dee-Hoskins C, Ma S, Fischer MM, Shah J, Wei J, et al. Therapeutic targeting of tumor-derived r-spondin attenuates b-catenin signaling and tumorigenesis in multiple cancer types. Cancer Res. 2016;76:713–723. doi: 10.1158/0008-5472.CAN-15-0561. [DOI] [PubMed] [Google Scholar]
- Chen NX, O’Neill K, Akl NK, Moe SM. Adipocyte induced arterial calcification is prevented with sodium thiosulfate. Biochem Biophys Res Commun. 2014;449:151–156. doi: 10.1016/j.bbrc.2014.05.005. [DOI] [PubMed] [Google Scholar]
- Chera S, Baronnier D, Ghila L, Cigliola V, Jensen JN, Gu G, Furuyama K, Thorel F, Gribble FM, Reimann F, et al. Diabetes recovery by age-dependent conversion of pancreatic δ-cells into insulin producers. Nature. 2014;514:503–507. doi: 10.1038/nature13633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba Y, Todoroki M, Nishida Y, Tanabe M, Misawa M. A novel STAT6 inhibitor AS1517499 ameliorates antigen-induced bronchial hypercontractility in mice. Am J Respir Cell Mol Biol. 2009;41:516–524. doi: 10.1165/rcmb.2008-0163OC. [DOI] [PubMed] [Google Scholar]
- Curran DR, Cohn L. Advances in mucous cell metaplasia: A plug for mucus as a therapeutic focus in chronic airway disease. Am J Respir Cell Mol Biol. 2010;42:268–275. doi: 10.1165/rcmb.2009-0151TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danahay H, Pessotti AD, Coote J, Montgomery BE, Xia D, Wilson A, Yang H, Wang Z, Bevan L, Thomas C, et al. Notch2 is required for inflammatory cytokine-driven goblet cell metaplasia in the lung. Cell Rep. 2015;10:239–252. doi: 10.1016/j.celrep.2014.12.017. [DOI] [PubMed] [Google Scholar]
- Dey D, Bagarova J, Hatsell SJ, Armstrong KA, Huang L, Ermann J, Vonner AJ, Shen Y, Mohedas AH, Lee A, et al. Two tissue-resident progenitor lineages drive distinct phenotypes of heterotopic ossification. Sci Transl Med. 2016;8:1–14. doi: 10.1126/scitranslmed.aaf1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulauroy S, Di Carlo SE, Langa F, Eberl G, Peduto L. Lineage tracing and genetic ablation of ADAM12 + perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nat Med. 2012;18:1262–1270. doi: 10.1038/nm.2848. [DOI] [PubMed] [Google Scholar]
- Efe JA, Hilcove S, Kim J, Zhou H, Ouyang K, Wang G, Chen J, Ding S. Conversion of mouse fibroblasts into cardiomyocytes using a direct reprogramming strategy. Nat Cell Biol. 2011;13:215–222. doi: 10.1038/ncb2164. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- van Es JH, Sato T, van de Wetering M, Lyubimova A, Yee Nee AN, Gregorieff A, Sasaki N, Zeinstra L, van den Born M, Korving J, et al. Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nat Cell Biol. 2012;14:1099–1104. doi: 10.1038/ncb2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Zelinka C, Scott MA. Heterogeneity of glia in the retina and optic nerve of birds and mammals. PLoS One. 2010;5 doi: 10.1371/journal.pone.0010774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher RB, Prasol MS, Estrada J, Baudhuin A, Vranizan K, Choi YG, Ngai J. P63 Regulates Olfactory Stem Cell Self-Renewal and Differentiation. Neuron. 2011;72:748–759. doi: 10.1016/j.neuron.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallina D, Todd L, Fischer AJ. A comparative analysis of Muller glia-mediated regeneration in the vertebrate retina. Exp Eye Res. 2014;123:121–130. doi: 10.1016/j.exer.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giachelli CM. Ectopic Calcification. Am J Pathol. 1999;154:671–675. doi: 10.1016/S0002-9440(10)65313-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert L, He X, Farmer P, Rubin J, Drissi H, Van Wijnen AJ, Lian JB, Stein GS, Nanes MS. Expression of the osteoblast differentiation factor RUNX2 (Cbfa1/AML3/Pebp2αA) is inhibited by tumor necrosis factor-α. J Biol Chem. 2002;277:2695–2701. doi: 10.1074/jbc.M106339200. [DOI] [PubMed] [Google Scholar]
- Goiko M, Dierolf J, Gleberzon JS, Liao Y, Grohe B, Goldberg HA, De Bruyn JR, Hunter GK. Peptides of matrix gla protein inhibit nucleation and growth of hydroxyapatite and calcium oxalate monohydrate crystals. PLoS One. 2013;8:1–11. doi: 10.1371/journal.pone.0080344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez D, Owens GK. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc Res. 2012;95:156–164. doi: 10.1093/cvr/cvs115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves LC, Preston SL, Tadrous PJ, Taylor RW, Barron MJ, Oukrif D, Leedham SJ, Deheragoda M, Sasieni P, Novelli MR, et al. Mitochondrial DNA mutations are established in human colonic stem cells, and mutated clones expand by crypt fission. Proc Natl Acad Sci U S A. 2006;103:714–719. doi: 10.1073/pnas.0505903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guseh JS, Bores SA, Stanger BZ, Zhou Q, Anderson WJ, Melton DA, Rajagopal J. Notch signaling promotes airway mucous metaplasia and inhibits alveolar development. Development. 2009;136:1751–1759. doi: 10.1242/dev.029249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas BJ, Whited JL. Advances in Decoding Axolotl Limb Regeneration. Trends Genet. 2017;xx:1–13. doi: 10.1016/j.tig.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjortnaes J, Shapero K, Goettsch C, Hutcheson JD, Keegan J, Kluin J, Mayer JE, Bischoff J, Aikawa E. Valvular interstitial cells suppress calcification of valvular endothelial cells. Atherosclerosis. 2015;242:251–260. doi: 10.1016/j.atherosclerosis.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horns F, Vollmers C, Croote D, Mackey SF, Swan GE, Dekker CL, Davis MM, Quake SR. Lineage tracing of human B cells reveals the in vivo landscape of human antibody class switching. Elife. 2016;5:1–20. doi: 10.7554/eLife.16578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu YF, Dawkins JF, Cho HC, Marban E, Cingolani E. Biological pacemaker created by minimally invasive somatic reprogramming in pigs with complete heart block. Sci Transl Med. 2014;6:245ra94–245ra94. doi: 10.1126/scitranslmed.3008681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huard JM, Youngentob SL, Goldstein BJ, Luskin MB, Schwob JE. Adult olfactory epithelium contains multipotent progenitors that give rise to neurons and non-neural cells. J Comp Neurol. 1998;400:469–486. [PubMed] [Google Scholar]
- Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, Cotsarelis G. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE, Cotsarelis G. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–320. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- Jadhav U, Saxena M, Neill NKO, Herbert Z, Murata K, Jadhav U, Saxena M, Neill NKO, Saadatpour A, Yuan G. Dynamic Reorganization of Chromatin Accessibility Signatures during Dedifferentiation of Secretory Precursors into Lgr5 + Intestinal Stem Cells Article Dynamic Reorganization of Chromatin Accessibility Signatures during Dedifferentiation of Secretory. Prec Stem Cell. 2017:1–13. doi: 10.1016/j.stem.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R, Barkauskas CE, Takeda N, Bowie EJ, Aghajanian H, Wang Q, Padmanabhan A, Manderfield LJ, Gupta M, Li D, et al. Plasticity of Hopx+ type I alveolar cells to regenerate type II cells in the lung. Nat Commun. 2015;6:6727. doi: 10.1038/ncomms7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Li H, Zhang Y, Yang Y, Lu R, Liu K, Lin S, Lan X, Wang H, Wu H, et al. Transitional basal cells at the squamous–columnar junction generate Barrett’s oesophagus. Nature. 2017 doi: 10.1038/nature24269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapustin AN, Shanahan CM. Emerging roles for vascular smooth muscle cell exosomes in calcification and coagulation. J Physiol. 2016;594:2905–2914. doi: 10.1113/JP271340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapustin AN, Chatrou MLL, Drozdov I, Zheng Y, Davidson SM, Soong D, Furmanik M, Sanchis P, De Rosales RTM, Alvarez-Hernandez D, et al. Vascular smooth muscle cell calcification is mediated by regulated exosome secretion. Circ Res. 2015;116:1312–1323. doi: 10.1161/CIRCRESAHA.116.305012. [DOI] [PubMed] [Google Scholar]
- Kim TH, Li F, Ferreiro-Neira I, Ho LL, Luyten A, Nalapareddy K, Long H, Verzi M, Shivdasani RA. Broadly permissive intestinal chromatin underlies lateral inhibition and cell plasticity. Nature. 2014;506:511–515. doi: 10.1038/nature12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramann R, Brandenburg VM, Schurgers LJ, Ketteler M, Westphal S, Leisten I, Bovi M, Jahnen-Dechent W, Knuchel R, Floege J, et al. Novel insights into osteogenesis and matrix remodelling associated with calcific uraemic arteriolopathy. Nephrol Dial Transplant. 2013;28:856–868. doi: 10.1093/ndt/gfs466. [DOI] [PubMed] [Google Scholar]
- Ku SY, Rosario S, Wang Y, Mu P, Seshadri M, Goodrich ZW, Goodrich MM, Labbe DP, Gomez EC, Wang J, et al. Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science (80- ) 2017;355:78–83. doi: 10.1126/science.aah4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapperre TS, Sont JK, van Schadewijk A, Gosman MME, Postma DS, Bajema IM, Timens W, Mauad T, Hiemstra PS. Smoking cessation and bronchial epithelial remodelling in COPD: a cross-sectional study. Respir Res. 2007;8:1–9. doi: 10.1186/1465-9921-8-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees-Shepard JB, Yamamoto M, Biswas AA, Stoessel SJ, Nicholas SAE, Cogswell CA, Devarakonda PM, Schneider MJ, Cummins SM, Legendre NP, et al. Activin-dependent signaling in fibro/adipogenic progenitors causes fibrodysplasia ossificans progressiva. Nat Commun. 2018;9:471. doi: 10.1038/s41467-018-02872-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold JA. Cellular mechanisms of aortic valve calcification. Circ Cardiovasc Interv. 2012;5:605–614. doi: 10.1161/CIRCINTERVENTIONS.112.971028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Chen G. Perspective In Vivo Reprogramming for CNS Repair: Regenerating Neurons from Endogenous Glial Cells. Neuron. 2016;91:728–738. doi: 10.1016/j.neuron.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang G, Katz LD, Insogna KL, Carpenter TO, MacIca CM. Survey of the enthesopathy of X-linked hypophosphatemia and its characterization in Hyp mice. Calcif Tissue Int. 2009;85:235–246. doi: 10.1007/s00223-009-9270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B, Coleman JH, Peterson JN, Zunitch MJ, Jang W, Herrick DB, Schwob JE. Injury Induces Endogenous Reprogramming and Dedifferentiation of Neuronal Progenitors to Multipotency. Cell Stem Cell. 2017:1–14. doi: 10.1016/j.stem.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin ME, Chen T, Leaf EM, Speer MY, Giachelli CM. Runx2 Expression in Smooth Muscle Cells Is Required for Arterial Medial Calcification in Mice. Am J Pathol. 2015;185:1958–1969. doi: 10.1016/j.ajpath.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounev VY, Ramachandran R, Wosczyna MN, Yamamoto M, Maidment ADA, Shore EM, Glaser DL, Goldhamer DJ, Kaplan FS. Identification of rogenitor cells that contribute to eterotopic skeletogenesis. J Bone Jt Surg - Ser A. 2009;91:652–663. doi: 10.2106/JBJS.H.01177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Alsamarah A, Zhang K, Hao J. Development of New Therapeutic Agents for Fibrodysplasia Ossificans Progressiva. Curr Mol Med. 2016;16:4–11. doi: 10.2174/1566524016666151222142446. [DOI] [PubMed] [Google Scholar]
- Magro CM, Simman R, Jackson S. Calciphylaxis: A review. J Am Col Certif Wound Spec. 2010;2:66–72. doi: 10.1016/j.jcws.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra R, Burke MF, Martyn T, Shakartzi HR, Thayer TE, O’Rourke C, Li P, Derwall M, Spagnolli E, Kolodziej SA, et al. Inhibition of bone morphogenetic protein signal transduction prevents the medial vascular calcification associated with matrix gla protein deficiency. PLoS One. 2015;10:1–21. doi: 10.1371/journal.pone.0117098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean WJ, Yin X, Lu L, Lenz DR, McLean D, Langer R, Karp JM, Edge ASB. Clonal Expansion of Lgr5-Positive Cells from Mammalian Cochlea and High-Purity Generation of Sensory Hair Cells. Cell Rep. 2017;18:1917–1929. doi: 10.1016/j.celrep.2017.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers JD, Jay PY, Rentschler S. Reprogramming the conduction system: Onward toward a biological pacemaker. Trends Cardiovasc Med. 2016;26:14–20. doi: 10.1016/j.tcm.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklossy G, Hilliard TS, Turkson J. Therapeutic modulators of STAT signalling for human diseases. Nat Rev Drug Discov. 2013;12:611–629. doi: 10.1038/nrd4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millimaki BB, Sweet EM, Riley BB. Sox2 is required for maintenance and regeneration, but not initial development, of hair cells in the zebrafish inner ear. Dev Biol. 2010;338:262–269. doi: 10.1016/j.ydbio.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu P, Zhang Z, Benelli M, Karthaus WR, Hoover E, Chen CC, Wongvipat J, Ku SY, Gao D, Cao Z, et al. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science (80- ) 2017;355:1–6. doi: 10.1126/science.aah4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasser W, Amitai-Lange A, Soteriou D, Hanna R, Tiosano B, Fuchs Y, Shalom-Feuerstein R. Corneal-Committed Cells Restore the Stem Cell Pool and Tissue Boundary following Injury. Cell Rep. 2018;22:323–331. doi: 10.1016/j.celrep.2017.12.040. [DOI] [PubMed] [Google Scholar]
- Nguyen LP, Al-Sawalha NA, Parra S, Pokkunuri I, Omoluabi O, Okulate AA, Windham Li E, Hazen M, Gonzalez-Granado JM, Daly CJ, et al. β2-Adrenoceptor signaling in airway epithelial cells promotes eosinophilic inflammation, mucous metaplasia, and airway contractility. Proc Natl Acad Sci. 2017;114:E9163–E9171. doi: 10.1073/pnas.1710196114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederst MJ, Sequist LV, Poirier JT, Mermel CH, Lockerman EL, Garcia AR, Katayama R, Costa C, Ross KN, Moran T, et al. RB loss in resistant EGFR mutant lung adenocarcinomas that transform to small-cell lung cancer. Nat Commun. 2015;6:199–203. doi: 10.1038/ncomms7377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto MA, Huang RYYJ, Jackson RAA, Thiery JPP. Emt: 2016. Cell. 2016;166:21–45. doi: 10.1016/j.cell.2016.06.028. [DOI] [PubMed] [Google Scholar]
- Nigwekar SU, Wolf M, Sterns RH, Hix JK. Calciphylaxis from nonuremic causes: A systematic review. Clin J Am Soc Nephrol. 2008;3:1139–1143. doi: 10.2215/CJN.00530108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigwekar SU, Kroshinsky D, Nazarian RM, Goverman J, Malhotra R, Jackson VA, Kamdar MM, Steele DJR, Thadhani RI. Calciphylaxis: Risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133–146. doi: 10.1053/j.ajkd.2015.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigwekar SU, Jiramongkolchai P, Wunderer F, Bloch E, Ichinose R, Nazarian RM, Thadhani RI, Malhotra R, Bloch DB. Increased Bone Morphogenetic Protein Signaling in the Cutaneous Vasculature of Patients with Calciphylaxis. Am J Nephrol. 2017;46:429–438. doi: 10.1159/000484418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oser MG, Niederst MJ, Sequist LV, Engelman JA. Transformation from non-small-cell lung cancer to small-cell lung cancer: molecular drivers and cells of origin. Lancet Oncol. 2015;16:e165–e172. doi: 10.1016/S1470-2045(14)71180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka F, Sakakura K, Yahagi K, Joner M, Virmani R. Has our understanding of calcification in human coronary atherosclerosis progressed? Arterioscler Thromb Vasc Biol. 2014;34:724–736. doi: 10.1161/ATVBAHA.113.302642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page ME, Lombard P, Ng F, Gottgens B, Jensen KB. The epidermis comprises autonomous compartments maintained by distinct stem cell populations. Cell Stem Cell. 2013;13:471–482. doi: 10.1016/j.stem.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V, Mathison M, Singh VP, Yang J, Rosengart TK. Direct Cardiac Cellular Reprogramming for Cardiac Regeneration. Curr Treat Options Cardiovasc Med. 2016;18 doi: 10.1007/s11936-016-0480-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikman Y, Puissant A, Alexe G, Furman A, Chen LM, Frumm SM, Ross L, Fenouille N, Bassil CF, Lewis CA, et al. Targeting MTHFD2 in acute myeloid leukemia. J Exp Med. 2016;213:1285–1306. doi: 10.1084/jem.20151574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, Conway SJ, Fu JD, Srivastava D. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485:593–598. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomska HS, Jernigan F, Nakayama S, Jorge SE, Sun L, Tenen DG, Kobayashi SS. A cell-based high-throughput screening for inducers of myeloid differentiation. J Biomol Screen. 2015;20:1150–1159. doi: 10.1177/1087057115592220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajamannan NM, Subramaniam M, Rickard D, Stock SR, Donovan J, Springett M, Orszulak T, Fullerton DA, Tajik AJ, Bonow RO, et al. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation. 2003;107:2181–2184. doi: 10.1161/01.CIR.0000070591.21548.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Fausett BV, Goldman D. Ascl1a regulates Muller glia dedifferentiation and retinal regeneration through a Lin-28-dependent, let-7 microRNA signalling pathway. Nat Cell Biol. 2010;12:1101–1107. doi: 10.1038/ncb2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan K, Peterson J, Agarwal S, Oluwatobi E, Loder S, Forsberg JA, Davis TA, Buchman SR, Wang SC, Levi B. Role of Gender in Burn-Induced Heterotopic Ossification and Mesenchymal Cell Osteogenic Differentiation. Plast Reconstr Surg. 2015;135:1631–1641. doi: 10.1097/PRS.0000000000001266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regard JB, Malhotra D, Gvozdenovic-Jeremic J, Josey M, Chen M, Weinstein LS, Lu J, Shore EM, Kaplan FS, Yang Y. Activation of hedgehog signaling by loss of GNAS causes heterotopic ossification. Nat Med. 2013;19:1505–1512. doi: 10.1038/nm.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigden HM, Alias A, Havelock T, O’Donnell R, Djukanovic R, Davies DE, Wilson SJ. Squamous metaplasia is increased in the bronchial epithelium of smokers with chronic obstructive pulmonary disease. PLoS One. 2016;11:1–11. doi: 10.1371/journal.pone.0156009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio-Tsonis K, Tsonis PA. Eye regeneration at the molecular age. Dev Dyn. 2003;226:211–224. doi: 10.1002/dvdy.10224. [DOI] [PubMed] [Google Scholar]
- Sandoval-Guzman T, Wang H, Khattak S, Schuez M, Roensch K, Nacu E, Tazaki A, Joven A, Tanaka EM, Simon A. Fundamental Differences in Dedifferentiation and Stem Cell Recruitment during Skeletal Muscle Regeneration in Two Salamander Species. Cell Stem Cell. 2013:1–14. doi: 10.1016/j.stem.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Schamberger AC, Staab-Weijnitz CA, Mise-Racek N, Eickelberg O. Cigarette smoke alters primary human bronchial epithelial cell differentiation at the air-liquid interface. Sci Rep. 2015;5:1–9. doi: 10.1038/srep08163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk T, Chen WC, Gollner S, Howell L, Jin L, Hebestreit K, Klein HU, Popescu AC, Burnett A, Mills K, et al. Inhibition of the LSD1 (KDM1A) demethylase reactivates the all-trans-retinoic acid differentiation pathway in acute myeloid leukemia. Nat Med. 2012;18:605–611. doi: 10.1038/nm.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnittke N, Herrick DB, Lin B, Peterson J, Coleman JH, Packard AI, Jang W, Schwob JE. Transcription factor p63 controls the reserve status but not the stemness of horizontal basal cells in the olfactory epithelium. Proc Natl Acad Sci USA. 2015 doi: 10.1073/pnas.1512272112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan CM, Cary NR, Metcalfe JC, Weissberg PL. High expression of genes for calcification-regulating proteins in human atherosclerotic plaques. J Clin Invest. 1994;93:2393–2402. doi: 10.1172/JCI117246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM, Swiatlowska P, Newman AAC, Greene ES, Straub AC, et al. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med. 2015;21:628–637. doi: 10.1038/nm.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley MT. Transport of molecules from nose to brain: Transneuronal anterograde and retrograde labeling in the rat olfactory system by wheat germ agglutinin-horseradish peroxidase applied to the nasal epithelium. Brain Res Bull. 1985;15:129–142. doi: 10.1016/0361-9230(85)90129-7. [DOI] [PubMed] [Google Scholar]
- Shore EM, Kaplan FS. Inherited human diseases of heterotopic bone formation. Nat Rev Rheumatol. 2010;6:518. doi: 10.1038/nrrheum.2010.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeda T, Deng JM, De Crombrugghe B, Behringer RR, Nakamura T, Akiyama H. Sox9-expressing precursors are the cellular origin of the cruciate ligament of the knee joint and the limb tendons. Genesis. 2010;48:635–644. doi: 10.1002/dvg.20667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer MY, McKee MD, Guldberg RE, Liaw L, Yang HY, Tung E, Karsenty G, Giachelli CM. Inactivation of the Osteopontin Gene Enhances Vascular Calcification of Matrix Gla Protein– deficient Mice. J Exp Med. 2002;196:1047–1055. doi: 10.1084/jem.20020911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer MY, Li X, Hiremath PG, Giachelli CM. Runx2/Cbfa1, but not loss of myocardin, is required for smooth muscle cell lineage reprogramming toward osteochondrogenesis. J Cell Biochem. 2010;110:935–947. doi: 10.1002/jcb.22607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava D, DeWitt N. In Vivo Cellular Reprogramming: The Next Generation. Cell. 2016;166:1386–1396. doi: 10.1016/j.cell.2016.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange DE, Koo BK, Huch M, Sibbel G, Basak O, Lyubimova A, Kujala P, Bartfeld S, Koster J, Geahlen JH, et al. Differentiated troy(+) chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell. 2013;155:357–368. doi: 10.1016/j.cell.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmaier K, Ross KN, Colavito SA, O’Malley S, Stockwell BR, Golub TR. Gene expression-based high-throughput screening (GE-HTS) and application to leukemia differentiation. Nat Genet. 2004;36:257–263. doi: 10.1038/ng1305. [DOI] [PubMed] [Google Scholar]
- Stein EM, DiNardo CD, Pollyea DA, Fathi AT, Roboz GJ, Altman JK, Stone RM, Deangelo DJ, Levine RL, Flinn IW, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130:722–731. doi: 10.1182/blood-2017-04-779405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz SA, Speer MY, Curinga G, Yang HY, Haynes P, Aebersold R, Schinke T, Karsenty G, Giachelli CM. Smooth Muscle Cell Phenotypic Transition Associated With Calcification: Upregulation of Cbfa1 and Downregulation of Smooth Muscle Lineage Markers. Circ Res. 2001;89:1147–1154. doi: 10.1161/hh2401.101070. [DOI] [PubMed] [Google Scholar]
- Storm EE, Durinck S, De Sousa E, Melo F, Tremayne J, Kljavin N, Tan C, Ye X, Chiu C, Pham T, Hongo JA, et al. Targeting PTPRK-RSPO3 colon tumours promotes differentiation and loss of stem-cell function. Nature. 2016;529:97–100. doi: 10.1038/nature16466. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Takimoto A, Akiyama H, Kist R, Scherer G, Nakamura T, Hiraki Y, Shukunami C. Scx+/Sox9+ progenitors contribute to the establishment of the junction between cartilage and tendon/ligament. Development. 2013;140:2280–2288. doi: 10.1242/dev.096354. [DOI] [PubMed] [Google Scholar]
- Sykes DB, Kfoury YS, Mercier FE, Wawer MJ, Law JM, Haynes MK, Lewis TA, Schajnovitz A, Jain E, Lee D, et al. Inhibition of Dihydroorotate Dehydrogenase Overcomes Differentiation Blockade in Acute Myeloid Leukemia. Cell. 2016:171–186. doi: 10.1016/j.cell.2016.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka HV, Ng NCY, Yang Yu Z, Casco-Robles MM, Maruo F, Tsonis PA, Chiba C. A developmentally regulated switch from stem cells to dedifferentiation for limb muscle regeneration in newts. Nat Commun. 2016;7:11069. doi: 10.1038/ncomms11069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata PR, Mou H, Pardo-Saganta A, Zhao R, Prabhu M, Law BM, Vinarsky V, Cho JL, Breton S, Sahay A, et al. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature. 2013;503:218–223. doi: 10.1038/nature12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetteh PW, Basak O, Farin HF, Wiebrands K, Kretzschmar K, Begthel H, van den Born M, Korving J, de Sauvage F, van Es JH, et al. Replacement of Lost Lgr5-Positive Stem Cells through Plasticity of Their Enterocyte-Lineage Daughters. Cell Stem Cell. 2016;18:203–213. doi: 10.1016/j.stem.2016.01.001. [DOI] [PubMed] [Google Scholar]
- de The H. Differentiation therapy revisited. Nat Rev Cancer. 2017;18:117–127. doi: 10.1038/nrc.2017.103. [DOI] [PubMed] [Google Scholar]
- Theodoris CV, Li M, White MP, Liu L, He D, Pollard KS, Bruneau BG, Srivastava D. Human disease modeling reveals integrated transcriptional and epigenetic mechanisms of NOTCH1 haploinsufficiency. Cell. 2015;160:1072–1086. doi: 10.1016/j.cell.2015.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorel F, Nepote V, Avril I, Kohno K, Desgraz R, Chera S, Herrera PL. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature. 2010;464:1149–1154. doi: 10.1038/nature08894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, De Sauvage FJ. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255–259. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treutlein B, Brownfield DG, Wu AR, Neff NF, Mantalas GL, Espinoza FH, Desai TJ, Krasnow Ma, Quake SR. Reconstructing lineage hierarchies of the distal lung epithelium using single-cell RNA-seq. Nature. 2014 doi: 10.1038/nature13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MM. Is intestinal metaplasia of the stomach reversible? Gut. 2003;52:1–4. doi: 10.1136/gut.52.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Chen Z. Acute promyelocytic leukemia : from highly fatal to highly curable ASH 50th anniversary review Acute promyelocytic leukemia : from highly fatal to highly curable. Hematology. 2008;111:2505–2515. doi: 10.1182/blood-2007-07-102798. [DOI] [PubMed] [Google Scholar]
- Weenig RH. Pathogenesis of calciphylaxis: Hans Selye to nuclear factor ? B J Am Acad Dermatol. 2008;58:458–471. doi: 10.1016/j.jaad.2007.12.006. [DOI] [PubMed] [Google Scholar]
- White MP, Theodoris CV, Liu L, Collins WJ, Blue KW, Lee JH, Meng X, Robbins RC, Ivey KN, Srivastava D. NOTCH1 regulates matrix gla protein and calcification gene networks in human valve endothelium. J Mol Cell Cardiol. 2015;84:13–23. doi: 10.1016/j.yjmcc.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth MB, Girskis KM, Walsh CA. Building a lineage from single cells: genetic techniques for cell lineage tracking. Nat Rev Genet. 2017;18:230–244. doi: 10.1038/nrg.2016.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wosczyna MN, Biswas AA, Cogswell CA, Goldhamer DJ. Multipotent progenitors resident in the skeletal muscle interstitium exhibit robust BMP-dependent osteogenic activity and mediate heterotopic ossification. J Bone Miner Res. 2012;27:1004–1017. doi: 10.1002/jbmr.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie-Sears J, Aikawa E, Levine RA, Yang JH, Bischoff J. Mitral Valve Endothelial Cells With Osteogenic Differentiation Potential. Arterioscler Thromb Vasc Biol. 2011;31:598–607. doi: 10.1161/ATVBAHA.110.216184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan KS, Gevaert O, Zheng GXY, Anchang B, Probert CS, Larkin KA, Davies PS, Cheng Z, Kaddis JS, Han A, et al. Intestinal Enteroendocrine Lineage Cells Possess Homeostatic and Injury-Inducible Stem Cell Activity. Cell Stem Cell. 2017;21:78–90. e6. doi: 10.1016/j.stem.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu PB, Deng DY, Lai CS, Hong CC, Cuny GD, Bouxsein ML, Hong DW, McManus PM, Katagiri T, Sachidanandan C, et al. BMP type I receptor inhibition reduces heterotopic ossification. Nat Med. 2008;14:1363–1369. doi: 10.1038/nm.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zepp JA, Zacharias WJ, Frank DB, Cavanaugh CA, Zhou S, Morley MP, Morrisey EE. Distinct Mesenchymal Lineages and Niches Promote Epithelial Self-Renewal and Myofibrogenesis in the Lung. Cell. 2017;170:1134–1148. e10. doi: 10.1016/j.cell.2017.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Chen Z, Meng X, Li M, Zhang L, Huang A. The involvement and possible mechanism of pro-inflammatory tumor necrosis factor alpha (TNF-α) in thoracic ossification of the ligamentum flavum. PLoS One. 2017;12:1–13. doi: 10.1371/journal.pone.0178986. [DOI] [PMC free article] [PubMed] [Google Scholar]