Abstract
In neurons, long-distance communication between axon terminals and cell bodies is a critical determinant in establishing and maintaining neural circuits. Neurotrophins are soluble factors secreted by post-synaptic target tissues that retrogradely control axon and dendrite growth, survival, and synaptogenesis of innervating neurons. Neurotrophins bind Trk receptor tyrosine kinases in axon terminals to promote endocytosis of ligand-bound phosphorylated receptors into signaling endosomes. Trk-harboring endosomes function locally in axons to acutely promote growth events, and can also be retrogradely transported long-distances to remote cell bodies and dendrites to stimulate cytoplasmic and transcriptional signaling necessary for neuron survival, morphogenesis, and maturation. Neuronal responsiveness to target-derived neurotrophins also requires the precise axonal targeting of newly synthesized Trk receptors. Recent studies suggest that anterograde delivery of Trk receptors is regulated by retrograde neurotrophin signaling. In this review, we summarize current knowledge on the functions and mechanisms of retrograde trafficking of Trk signaling endosomes, and highlight recent discoveries on the forward trafficking of nascent receptors.
Introduction
The development and maturation of neural circuits relies on communication between neurons and their post-synaptic targets, often located millimeters or even meters away from neuronal cell bodies. During embryonic and postnatal development, axons navigate toward final targets and upon reaching their destinations, establish connections with target tissues. An excess of neurons produced during development is scaled back by interactions with targets to ensure a matching of neuronal numbers with the size and demands of the target. Additionally, innervated target tissues also relay retrograde signals to influence dendrite morphogenesis and synaptogenesis. Diffusible factors secreted by targets shape multiple aspects of this neuronal developmental program. The family of neurotrophins provide a well-characterized example of these target-derived instructive cues. Neurotrophins include nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT3), and neurotrophin-4 (NT4). Each neurotrophin binds with high-affinity to a specific member of the Trk Receptor Tyrosine Kinase (RTK) family with NGF binding to TrkA, NT4 and BDNF binding to TrkB, and NT3 binding to TrkC (Huang and Reichardt, 2003). NT3 also binds with lower affinity to the other Trk receptors, and all neurotrophins bind and activate a common p75 receptor, which can either enhance or diminish Trk signaling depending on the cellular context. In addition to mature neurotrophins that bind selectively to Trk receptors to promote trophic signaling, there are un-processed forms of neurotrophins called pro-neurotrophins that act via p75 to largely promote apoptotic events. There are several excellent reviews on p75 and pro-neurotrophins (Hempstead, 2014; Meeker and Williams, 2015), that discuss their functions and trafficking, which will not be covered here.
A remarkable aspect of neurotrophin signaling is that although neurotrophins bind their cognate Trk receptors on axons, they also stimulate transcriptional responses and other cellular events that take place at considerable distances away in cell bodies and dendrites. How neurotrophins, impinging on nerve terminals, are capable of relaying signals to remote somatodendritic compartments is a question that has long fascinated cell biologists and neurobiologists. Currently, several lines of evidence suggest that the retrograde neurotrophin signal is communicated via internalized neurotrophin-Trk receptor complexes that are housed in endocytic vesicles (Barford et al., 2017; Yamashita and Kuruvilla, 2016). These specialized vesicles called signaling endosomes act acutely in axons to regulate growth and branching events. Furthermore, long-distance transport of these vesicles carries the neurotrophin signal from the axons to cell bodies to control transcriptional events necessary for survival, growth, and maturation. A subset of axon-derived Trk endosomes are also transported to dendritic compartments to regulate synaptic events (Lehigh et al., 2017; Sharma et al., 2010). In addition to retrograde transport of autophosphorylated Trk receptors, nascent receptors, synthesized in cell bodies, must undergo anterograde trafficking to axons to ensure continued responses to target-derived ligands. Compared to the intense focus on retrograde trafficking, relatively little is known about anterograde delivery of Trk receptors. This review summarizes the current knowledge on the functions and mechanisms of retrograde Trk trafficking from axons to somatodendritic compartments. In addition, we discuss recent evidence that provides insights into the mechanisms and regulation of the anterograde receptor delivery of naïve Trk receptors.
Functions of retrograde neurotrophin signalling
Neuronal survival
The ability of neurotrophins to transmit retrograde signals from distal axons to neuronal soma has been classically studied in the context of neuronal survival. Removal of target tissues or treatment with neutralizing antibodies against NGF, the founding member of the neurotrophin family, leads to the death of neuronal populations in the peripheral nervous system (Cowan, 2001; Hamburger, 1992; Levi-Montalcini and Booker, 1960). Genetic ablation of NGF or its TrkA receptors elicits profound loss of sympathetic and sensory neurons in embryonic and neonatal mice (Crowley et al., 1994; Smeyne et al., 1994). Further genetic studies in mice lacking Bdnf, Nt-3, or Nt-4 also pointed to other peripheral neuronal populations that relied on neurotrophins for survival (Snider, 1994). These, and other experiments, provided support for the neurotrophic factor hypothesis, which states that during development, neurons are overproduced and compete for limiting amounts of neurotrophins secreted by their targets for survival (Oppenheim, 1989).
How then could neurotrophins, secreted from peripheral tissues located millimeters to meters away from the cell body, promote survival? Early in vivo studies showed that injection of radiolabeled neurotrophins into target fields resulted in accumulation of the label in neuronal cell bodies, suggesting that part of the retrograde signal involved the ligand itself (Hendry et al., 1974). Additionally, phosphorylated Trk receptors could be retrogradely transported in vivo by a process dependent on endogenous ligand found in target tissues (Bhattacharyya et al., 1997; Ehlers et al., 1995). Ultrastructural analyses of peripheral nerves revealed localization of neurotrophins and phosphorylated Trk receptors in vesicular organelles (Bhattacharyya et al., 2002; Sandow et al., 2000), although there has been considerable debate about the identity of these organelles, that is discussed in detail in the Mechanisms of retrograde neurotrophin signaling section below. The use of compartmentalized neuron culture systems, where cell bodies and axons are separated in distinct fluidic chambers, demonstrated that neurotrophins added exclusively to distal axon compartments were both necessary and sufficient to promote neuronal survival (Campenot, 1982; Mok and Campenot, 2007; Ye et al., 2003). This survival signal required activation of Trk receptors in axons and initiation of RTK signaling cascades including the phosphatidylinositol 3-kinase (PI-3 kinase)- and MAPK-mediated pathways (Kuruvilla et al., 2000; Watson et al., 2001; Ye et al., 2003). Application of neurotrophins to distal axons of compartmentalized neuronal cultures elicited the retrograde accumulation of autophosphorylated Trk receptors in cell bodies (Riccio et al., 1997; Watson et al., 1999). Co-precipitation studies showed that Trk receptors were retrogradely co-transported in a complex with the ligand (Tsui-Pierchala and Ginty, 1999; Watson et al., 1999). Notably, interfering with Trk kinase activity and downstream trophic signaling in cell bodies, following neurotrophin application to axons, resulted in apoptosis (Heerssen et al., 2004; Kuruvilla et al., 2000; Ye et al., 2003). Although evidence from several laboratories support the notion of axonal transport of ligand-receptor complexes as the primary mode of retrograde NGF survival signaling, other models have proposed a non-endosomal mechanism of retrograde NGF signal propagation. These models were based on observations that NGF immobilized on beads, and thus incapable of internalization, is capable of retrogradely promoting neuronal survival (MacInnis and Campenot, 2002). These alternative models include the retrograde transport of Trk signaling effectors, retrogradely propagating waves of Trk phosphorylation along the plasma membrane, or retrograde propagation of regenerating calcium waves (Howe and Mobley, 2004). Whether these mechanisms exist in parallel with the transport of signaling endosomes and the extent to which they contribute to target-derived trophic support remains unclear.
Retrograde NGF signaling promotes survival in cell bodies by regulating expression of anti-apoptotic factors and metabolism-related proteins (Deppmann et al., 2008; Mandai et al., 2009; Pazyra-Murphy et al., 2009), via activation of downstream transcription factors including CREB and MEF2D (Pazyra-Murphy et al., 2009; Riccio et al., 1997) (Figure 1). A remarkable aspect of retrograde neurotrophin signaling is the activation of positive feedback loops that help to distinguish the neurons that survive from those that undergo apoptosis during development (Ascano et al., 2012). In sympathetic neurons, retrograde NGF signaling enhances the transcription of its own TrkA receptor and endosomal effectors involved in retrograde signaling (Deppmann et al., 2008) (Figure 1). Thus, small differences in the initial strengths of NGF signaling are amplified in competing neurons to establish differential responsiveness to ligand. Retrograde NGF signaling also augments the expression of pro-death factors that act in a paracrine manner to kill neighboring neurons with low levels of NGF signaling (Deppmann et al., 2008). Thus, these feedback mechanisms enhance the competitive ability of the “winning” neurons, and weaken neighboring neurons that do not gain sufficient access to NGF.
Figure 1. Retrograde Trk signaling endosomes regulate diverse developmental events in multiple neuronal compartments.
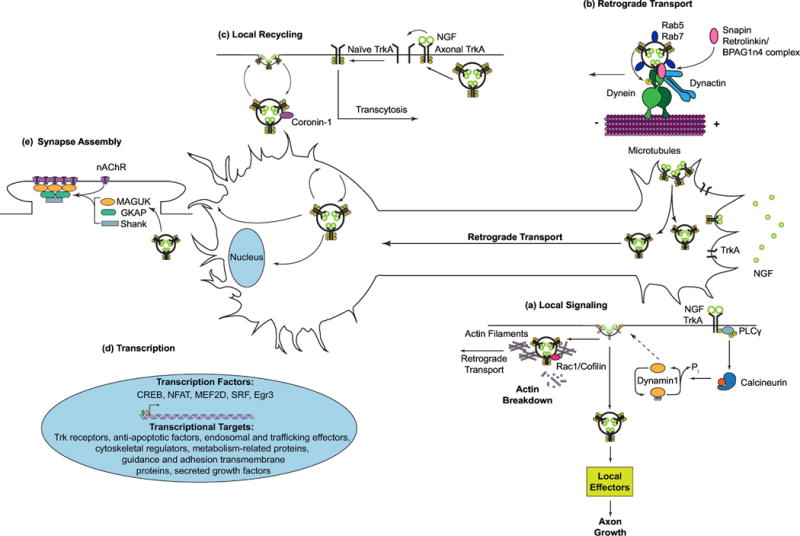
(a) In axon terminals, NGF promotes endocytosis of its TrkA receptors by activating a signaling pathway that involves PLCγ1, calcineurin, and dynamin1. NGF-TrkA-containing signaling endosomes promote local growth events and are also retrogradely transported back to neuronal soma. TrkA endosomal signaling controls its own vesicular trafficking by stimulating breakdown of a dense actin meshwork in axon terminals. (b) Activated Trk receptors are transported along axonal microtubules in endosomes, positive for Rab5 or Rab7, by dynein motors. Trk receptors interact with dynein motors either directly or indirectly via adaptors. Trk signaling actively recruits dynein motors via phosphorylation of dynein intermediate chains. (c) In cell bodies, axon-derived Trk endosomes are exocytosed to soma surfaces where they interact with nascent receptors to influence their forward trafficking to axons (transcytosis). Persistent Trk signaling may be facilitated by Coronin-1-mediated local recycling, which allows receptors to evade lysosomal fusion and degradation. (d) Somatic signaling by Trk endosomes activates transcriptional programs necessary for survival and growth. (e) A sub-set of axon-derived Trk endosomes signal locally in dendrites to modulate the assembly of post-synaptic components.
Axon growth
In addition to controlling neuronal survival, target-derived neurotrophins also regulate axon growth. Developing axons rely on neurotrophic cues secreted by intermediate and final targets to navigate toward and innervate final target fields. During sympathetic neuron development, axon extension along the vasculature is guided by vascular cues including the neurotrophin NT-3, artemin, a member of the glial-derived neurotrophic factor (GDNF) family, and endothelin (Glebova and Ginty, 2005; Makita et al., 2008). Upon reaching final targets, sympathetic axons extend and arborize extensively to completely innervate target fields, and become completely reliant on limiting amounts of target-derived NGF. Sympathetic and sensory innervation of final target fields is either absent or incomplete in mice lacking NGF (Glebova and Ginty, 2004; Kuruvilla et al., 2004; Patel et al., 2000). Conversely, over-expression of NGF in target tissues enhances peripheral nerve growth into the targets (Davis et al., 1994; Edwards et al., 1989; Hassankhani et al., 1995). Interestingly, although the two neurotrophins, NT-3 and NGF, mediate distinct stages of sympathetic axon growth, they do so by acting through a common TrkA receptor (Kuruvilla et al., 2004). This difference in functional outcomes is related to the differential abilities of the two ligands to initiate retrograde signaling. In contrast to NGF, NT-3 is incapable of activating retrograde transport of TrkA receptors, and promotes axon outgrowth predominantly via local signaling mechanisms (Harrington et al., 2011; Kuruvilla et al., 2004).
The importance of axon-intrinsic mechanisms in mediating neurotrophin-dependent growth has been appreciated through the use of compartmentalized cultures. In Campenot chamber cultures, the barrier separating the cell body and distal axon compartments is >1 mm, thus long neurites that traverse this length are most likely axons, while the cell body compartments consist of cell bodies, dendrites, and proximal axons. Application of NGF exclusively to sympathetic axons promoted axon growth, while application to cell bodies did not (Campenot, 1977). Importantly, if NGF is removed from the distal axons, but left in the medium bathing the cell bodies, the axons retract although the neurons survive. Therefore, neurotrophin signaling initiated in axons is both necessary and sufficient to support axon growth. Trk signaling instructs axonal growth by impinging on the axonal cytoskeleton through activation of the MAPK and PI-3K/Akt signaling pathways (Atwal et al., 2000), and their downstream effectors including Rac1, the Arp2/3 complex, glycogen synthase kinase 3β (GSK3β), and adenomatous polyposis coli (APC) (Spillane et al., 2012; Zhou et al., 2004). In distal axons, the growth-promoting effects of NGF may be mediated by signaling from surface TrkA receptors or by endosomal signaling from internalized receptors. In sympathetic axons, NGF promotes growth by activating an endocytic pathway that involves regulation of dynamin1. NGF-TrkA signaling stimulates calcineurin, a calcium-dependent phosphatase, to dephosphorylate dynamin1, driving receptor internalization (Bodmer et al., 2011). Calcineurin-dynamin1-mediated endocytosis of TrkA receptors is required for axon growth and final target innervation in the sympathetic nervous system (Bodmer et al., 2011).
In addition to regulation of cytoskeletal and endocytic trafficking pathways, a key mechanism underlying the growth-promoting effects of neurotrophins involves intra-axonal protein synthesis (Spillane et al., 2013; Terenzio et al., 2017). In compartmentalized cultures, focal neurotrophin stimulation of distal axons results in axonal localization of diverse classes of mRNAs involved in cytoskeletal regulation, mitochondrial functions, signaling, and components of the translational machinery (Andreassi et al., 2010; Willis et al., 2007). RNA-binding proteins such as SFPQ mediate the transport of RNA granules to axons in response to neurotrophin stimulation (Cosker et al., 2016). Notably, neurotrophin signaling in distal axons stimulates the local synthesis of proteins that are critical for axon viability. For example, Impa1, a gene critical for lipid metabolism, is one of the most abundant transcripts in sympathetic axons (Andreassi et al., 2010). Impa1 mRNA is targeted to axons and locally translated in response to NGF stimulation of axons (Andreassi et al., 2010). Silencing of axonal Impa1 synthesis elicits axonal degeneration even in the presence of NGF (Andreassi et al., 2010).
Retrograde neurotrophin signaling also promotes axon growth and branching via activation of transcriptional programs. Knockout mice lacking the transcription factors CREB, Serum Response Factor (SRF), NFAT, or Early Growth Response 3 (Egr3), exhibit severe abnormalities in axon outgrowth and target innervation in neurotrophin-responsive neuronal populations (Eldredge et al., 2008; Graef et al., 2003; Lonze and Ginty, 2002; Wickramasinghe et al., 2008). Growth-promoting genes regulated by retrograde neurotrophin signaling include the neurotrophin receptors themselves, membrane proteins of the leucine-rich repeat and immunoglobulin (LIG) family, cytoskeletal regulators, and secreted Wnt proteins that act in an autocrine fashion to instruct axon arborizaton of final target fields (Bodmer et al., 2009; Deppmann et al., 2008; Estrach et al., 2002; Mandai et al., 2009) (Figure 1).
Dendrite growth and synapse assembly
Early studies demonstrated that dendrite arbors of post-ganglionic sympathetic neurons increase in complexity upon axon innervation of peripheral target tissues, suggesting a role for retrograde signaling in the process (Rubin, 1985; Voyvodic, 1987). In vivo administration of NGF enhanced dendrite complexity during development, while neutralizing NGF had opposite effects (Ruit and Snider, 1991; Snider, 1988). Similar results were seen in adult mice where systemic NGF administration, or treatment with NGF blocking antibodies, altered dendrite elaboration (Ruit et al., 1990). Taken together, these results suggest that retrograde NGF signaling promotes the elaboration and maintenance of dendrites. However, the molecular underpinnings and transcriptional targets necessary for this process remain undefined.
An additional level of control of sympathetic nervous system circuitry by retrograde NGF signaling comes from the regulation of synaptic connectivity between pre- and post-ganglionic neurons. Dendrites of post-ganglionic sympathetic neurons receive synaptic input from pre-ganglionic sympathetic axons. Recent studies indicate that retrograde NGF signaling promotes the development of post-synaptic specializations, and the assembly of synaptic machinery components in sympathetic neuron dendrites (Lehigh et al., 2017; Sharma et al., 2010). Axon-derived TrkA signaling endosomes are required locally in dendritic compartments of postganglionic sympathetic neurons to maintain their synaptic inputs with preganglionic neurons in vivo (Lehigh et al., 2017).
Mechanisms of retrograde neurotrophin signaling
It is now appreciated that several functions of target-derived neurotrophins are mediated by the retrograde transport of neurotrophin-bound Trk receptors in signaling endosomes from distal axons to neuronal soma and dendrites (Ascano et al., 2012; Harrington and Ginty, 2013). The formation of Trk endosomes begins with internalization of ligand-bound active receptors in axon terminals, which can occur either by clathrin-mediated endocytosis, (Howe et al., 2001) or Pincher-mediated micropinocytosis (Shao et al., 2002; Valdez et al., 2007). Both modes of internalization require the endocytic GTPase, dynamin. In sympathetic axons, NGF-TrkA signaling regulates dynamin-mediated endocytosis via a signaling pathway that involves recruitment of the downstream effector, Phospholipase Cγ1, and subsequent activation of the calcineurin phosphatase (Bodmer et al., 2011) (Figure 1a). Calcineurin dephosphorylates neuron-specific splicing isoforms of dynamin-1 to trigger detachment of TrkA endosomes from the plasma membrane (Bodmer et al., 2011). To traverse a dense actin meshwork in axon terminals, TrkA endosomes recruit the actin-modulatory proteins, Rac1 and cofilin, to promote actin severing to facilitate retrograde transport (Harrington et al., 2011) (Figure 1a). These findings suggest that Trk receptor-mediated signaling events actively regulate the endocytic machinery and axonal cytoskeleton to drive its own trafficking.
The question of the identity of the endocytic vesicles responsible for retrograde transport of neurotrophin signals has generated significant debate in the field. Early ultrastructural analyses revealed active TrkA receptors being retrogradely transported in heterogeneous vesicles of different sizes, both coated and uncoated vesicles, and multi-vesicular bodies (Bhattacharyya et al., 2002). Biochemical isolation of NGF-TrkA containing vesicles from sciatic nerve preparations showed the presence of Rab5, an early endosome marker, in these vesicles (Delcroix et al., 2003). Other studies have argued that retrograde Trk transport is mediated by Rab7-positive late endosomes (Deinhardt et al., 2006). Together, these findings might reflect a dynamic maturation of Trk-positive vesicles from early to late endosomes during the transport process or the coexistence of distinct organelles that carry the retrograde signal. Several lines of evidence have also supported the role of multi-vesicular bodies (MVBs) in retrograde transport of Trk receptors (Weible and Hendry, 2004). Early ultra-structural studies showed that 125I-NGF, added to distal axons of compartmentalized sympathetic neurons, was predominantly found in MVBs in cell bodies (Claude et al., 1982). Similarly, gold-labeled NGF injected into peripheral targets or phosphorylated TrkA receptors were found to associate with MVBs in axons in vivo (Bhattacharyya et al., 2002; Sandow et al., 2000). These early studies have been strengthened by more recent evidence using sympathetic neurons isolated from a FLAG-TrkA knock-in mouse line, where the majority (>70%) of retrogradely transported TrkA organelles in axons were found to be MVBs that are positive for Rab7 (Ye et al., 2018). Immuno-electron microcopy analyses suggest that the TrkA receptors, found both at the MVB limiting membrane and in the intraluminal vesicles, are axonally transported in a phosphorylated state and associated with signaling effectors such as phosphorylated PLCγ. Since the topology of TrkA receptors in MVB intraluminal vesicles would be restrictive for relaying signals to cytoplasmic effectors and to ultimately influence transcriptional events, the authors propose a model where MVBs mature into single-membrane vesicles that are signaling-competent upon reaching cell bodies (Ye et al., 2018). Intriguingly, in cortical and hippocampal neurons, Rab7-positive autophagosomes have been postulated to be the retrograde carriers for active TrkB receptors (Kononenko et al., 2017). Retrograde transport of TrkB-containing autophagosomes is mediated by a protein complex consisting of LC3, an autophagosome-associated protein, the p150-Glued subunit of the dynactin complex, and AP2, an endocytic adaptor protein. Autophagosomes may be well-positioned to carry the retrograde neurotrophin signal since they are preferentially generated in distal axons, undergo vectorial transport exclusively in the retrograde direction, and organelle maturation during transport to the soma (Maday et al., 2012), similar to observations made for retrogradely transported Trk vesicles. Conversely, one argument against autophagosomes as being the retrograde carriers of the Trk signal is that they are derived from axonal Endoplasmic Reticulum (ER) domains, and not from the plasma membrane at least under basal conditions (Maday et al., 2012). The observations of heterogenous Trk-containing vesicular structures raise the possibility of functionally distinct classes of Trk-signaling endosomes that may be specialized to mediate different biological outcomes.
The translocation of active Trk receptors from distal processes to cell bodies occurs by dynein motor-driven transport along axonal microtubules (Heerssen et al., 2004) (Figure 1b). In axons, microtubules are arranged as parallel arrays, with their minus ends oriented toward the soma and plus ends toward axon terminals. Live imaging analyses revealed distal axon-derived TrkA endosomes moving exclusively in the retrograde direction in a saltatory manner with intermittent pauses and an average speed of 1.2 μm/sec (Lehigh et al., 2017), consistent with the speed of dynein-based transport. Recruitment of dynein motors to Trk signaling endosomes is mediated either by direct interactions of Trk receptors with the dynein light chains, (Yano et al., 2001) or indirect interactions with adaptor proteins such as snapin and retrolinkin (Liu et al., 2007; Zhou et al., 2012). Additionally, the recruitment of dynein motors to Trk endosomes involves Trk receptor kinase-mediated phosphorylation of dynein intermediate chains on a conserved serine (Mitchell et al., 2012), suggesting that Trk signaling regulates its own retrograde transport (Figure 1b). Intriguingly, dynein cofactors, Lis1 and p150 Glued, are locally translated in axons in a manner dependent on neurotrophin signaling, and their intra-axonal synthesis was necessary for retrograde transport of multiple cargo, including neurotrophin endosomes (Villarin et al., 2016). This presents a novel mechanism by which neurotrophin signaling regulates the availability of dynein motor components in axons to control retrograde transport.
The trafficking fate of Trk-harboring endosomes upon reaching soma has only begun to be elucidated (Figure 1c). Live imaging analyses reveal that on reaching cell bodies, axon-derived Trk endosomes slow down, stall, and accumulate in the soma (Lehigh et al., 2017). Interestingly, one of the fates of retrogradely transported active Trk receptors is to undergo exocytosis to the soma surface (Suo et al., 2014; Yamashita et al., 2017), where these receptors interact with naïve Trk receptors to influence their anterograde transcytosis to axons (Yamashita et al., 2017 (Figure 1c), (discussed in detail in Anterograde trafficking of Trk receptors section below). Trk signaling endosomes are also capable of persistent signaling in cell bodies to activate transcriptional changes necessary for survival and growth (Suo, 2014 #3339) (Figure 1d). The sustained signaling may be facilitated, in part, by local recycling of axon-derived Trk receptors in the soma. Retrogradely transported TrkA receptors shuttle between the soma surface and interior in a manner dependent on the endosomal effector, Coronin-1 (Suo et al., 2014) (Figure 1c). Coronin-1-mediated somatic recycling has been postulated to extend the lifetime of Trk signaling endosomes by allowing them to evade lysosomal fusion and degradation (Barford et al., 2017; Suo et al., 2014). It remains unclear if the axon-derived TrkA receptors that interact with naïve receptors on the soma surface to promote transcytosis are the same population that recycle in cell bodies for persistent trophic signaling, or if these effects reflect distinct sub-sets of axon-derived receptors.
In addition to somatic functions, a small subset (4.2%) of axon-derived Trk endosomes further translocate to dendrites of sympathetic neurons (Lehigh et al., 2017). The dynamics of TrkA endosome transport in dendrites is distinct from that in axons, with frequent bi-directional and oscillatory movements, and an overall reduced speed (Lehigh et al., 2017). Axon-derived TrkA receptors were phosphorylated in dendrites, suggesting that they were signaling-competent and they were juxtaposed to clusters of post-synaptic density proteins, MAGUK, Shank, GKAP, and nAChRs (Lehigh et al., 2017; Sharma et al., 2010). Notably, axon-derived TrkA receptors signal locally in dendritic compartments in a manner independent of transcription to regulate the maintenance of synaptic clusters (Figure 1e). About 20% of the dendritic endosomes were found to be stationary, suggesting these endosomes may demarcate the sites of synaptic assembly.
Together, these studies support the notion that axon-derived Trk endosomes have far-reaching effects in morphologically complex neurons and they exert control of the neuronal development program at multiple levels.
Anterograde trafficking of Trk receptors
An essential feature of neurotrophin signaling is having Trk receptors in the right place at the right time. Internalization of TrkA receptors in axon terminals and retrograde transport to cell bodies for trophic communication must be balanced by the anterograde delivery of newly synthesized or somatic recycling receptors to axons to ensure continued responses to target-derived ligand. Compared to the intense focus on retrograde trafficking of TrkA receptors, relatively little is known about their anterograde trafficking.
Secretory pathway trafficking
It has been assumed that the majority of membrane proteins, including Trk receptors, are constitutively delivered to axons via the secretory pathway where proteins are shipped directly to axons in Golgi-derived vesicles after biosynthesis in the ER-Golgi network in cell bodies. Limited studies conducted so far on Trk receptors have favored this model of constitutive receptor transport through the secretory pathway. Trk receptors physically associate with sortilin, a Vps10 domain family member that regulates receptor sorting at the Golgi apparatus (Vaegter et al., 2011). Sortilin knockout mice exhibit impaired anterograde Trk transport, deficits in neurotrophin-induced signaling, and neurite outgrowth in sensory neurons (Vaegter et al., 2011). How sortilin affects Trk transport is unclear, but several roles have been proposed including facilitating receptor exit from the Trans-Golgi-Network (TGN) (Figure 2a), or acting as a scaffold protein to link Trks to anterograde kinesin motors (Vaegter et al., 2011). Sortilin likely functions in concert with binding partners such as ARHGAP33, a brain-enriched sorting nexin, in promoting Trk receptor biosynthetic trafficking (Figure 2a). Loss of ARHGAP33 results in retention of the TrkB receptor in the Golgi apparatus, and ARHGAP33-deficient mice show aberrant spine morphogenesis in hippocampal granule cells in the dentate gyrus, and behavioral deficits (Nakazawa et al., 2016), emphasizing the critical function of anterograde TrkB trafficking in development and functions of brain circuits.
Figure 2. Mechanisms for anterograde delivery of Trk receptors.
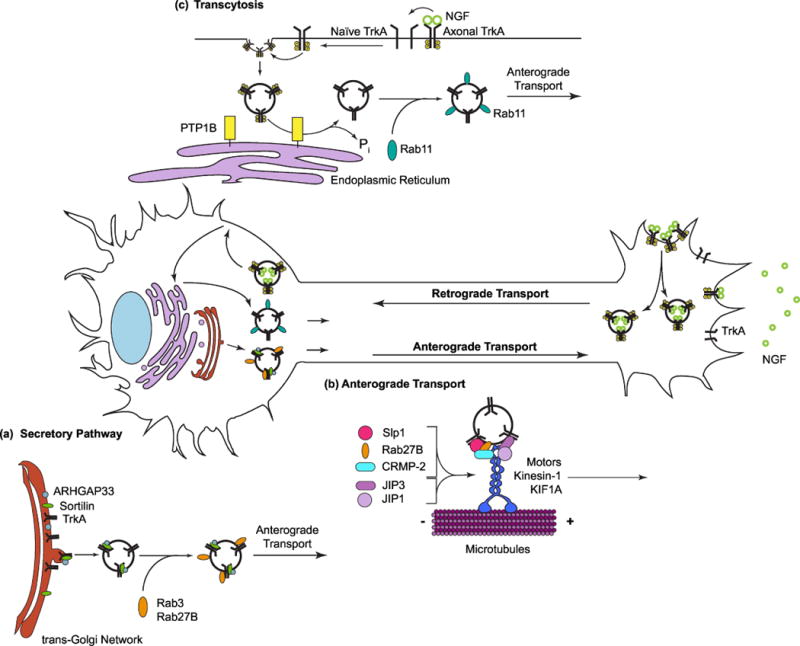
(a) Constitutive delivery of Trk receptors to axons via the secretory pathway. Exit of Trk receptors from the trans-Golgi-network may be mediated by interactions with sortilin, and its binding partner, ARHGAP33. (b) Anterograde transport of Trk receptors in secretory vesicles that are positive for Rab3 or Rab27B, mediated by kinesin motors, KIF1A or Kinesin-1. Trk receptors are coupled to kinesin motors with the help of adaptors such as Slp1, CRMP-2, JIP1 or JIP3. (c) In addition to the canonical secretory pathway, Trk receptors undergo axonal targeting via transcytosis, where soma surface-resident receptors are internalized and transported to axons in Rab11-positive recycling endosomes. Retrograde NGF signaling is necessary for anterograde transcytosis of TrkA receptors. Active axon-derived TrkA receptors are inserted on soma surfaces where they elicit phosphorylation and subsequent endocytosis of naive soma surface receptors. Endocytosed receptors are dephosphorylated by PTP1B, an Endoplasmic Reticulum-resident protein tyrosine phosphatase, to ensure axon targeting of inactive receptors to engage with ligand at terminals.
Of the 45 microtubule plus-end directed kinesin motors, KIF1A and kinesin-1 have been identified as the motors that transport Trk receptors as cargo (Figure 2b). In sensory neurons, TrkA receptors are transported in Rab3-positive secretory vesicles by KIF1A (Tanaka et al., 2016). Adult mice that are haplo-insufficient for KIF1A exhibit a progressive and specific loss of TrkA-positive sensory neurons, resulting in diminished nociceptive behaviors (Tanaka et al., 2016). In hippocampal neurons, an adaptor complex consisting of the Rab27B GTPase, it’s effector, Slp1, and a microtubule-binding protein, CRMP-2, links TrkB vesicles to Kinesin-1 for anterograde transport (Arimura et al., 2009). These studies suggest that specific secretory Rab GTPases might mediate the recognition and sorting of individual Trk family members. In addition to the Rab27B-Slp1-CRMP-2 complex, other adaptors such as JIP3 and JIP1 may function to transport Trk receptors in axons. JIP3 binds directly to TrkB and serves to couple the receptor to the kinesin light chain (Huang et al., 2011). JIP3 may function cooperatively with JIP1 to relieve auto-inhibition of kinesin (Sun et al., 2017). Knockdown of either JIP3 or JIP1 diminishes TrkB anterograde transport in axons, but not in dendrites of hippocampal neurons (Huang et al., 2011; Sun et al., 2017). Together, these findings support the notion that physical association of Trk receptors with the anterograde transport machinery regulates their forward trafficking.
Transcytosis
In addition to the classical Golgi-derived secretory pathway, axon targeting of membrane proteins is accomplished by an endocytosis-dependent mechanism called transcytosis, where proteins are initially delivered to somatodendritic compartments, then internalized and anterogradely transported for insertion into axon terminals (Winckler and Mellman, 2010). Transcytosis has been best characterized in polarized epithelial cells where endocytic trafficking from basolateral to apical surfaces is the primary mode for delivery of newly synthesized apical membrane proteins (Tuma and Hubbard, 2003). In neurons, transcytotic delivery has been demonstrated for a limited number of membrane proteins including NgCAM, a cell adhesion molecule important for axon guidance (Wisco et al., 2003), β1-integrin receptors, known to mediate growth cone motility and neurite outgrowth (Eva et al., 2010), the type 1 cannabinoid receptor (CB1R), an abundant G-protein coupled receptor implicated in synaptic plasticity (Leterrier et al., 2006), and the TrkA neurotrophin receptor (Ascano et al., 2009). Membrane flux through the endocytic pathway has been estimated to be ten times greater than via the secretory pathway (Horton and Ehlers, 2003). Thus, transcytosis might be more efficient for rapid responses to extrinsic cues than the classical pathway. The considerable heterogeneity of endocytic organelles compared to secretory vesicles might also allow for more plasticity in regulation of axonal trafficking.
In sympathetic neurons, naïve TrkA receptors resident on plasma membrane of cell bodies are internalized and trafficked to axons via Rab11-positive recycling vesicles (Ascano et al., 2009). Remarkably, anterograde TrkA transcytosis is triggered by NGF itself acting on axons (Ascano et al., 2009; Yamashita et al., 2017), suggesting a positive feedback mechanism that might serve to dynamically scale up axonal receptor levels to enhance neuronal sensitivity to limiting amounts of target-derived ligand. Mechanistically, anterograde TrkA transcytosis is controlled by the activity of retrogradely transported TrkA signaling endosomes (Figure 2c). Upon reaching cell bodies, axon-derived active receptors are exocytosed to soma cell surface membranes where they promote the phosphorylation of resident naive receptors resulting in their internalization (Yamashita et al., 2017). TrkA transcytosis is also primed by the activity of an ER-resident protein tyrosine phosphatase, PTP1B (Yamashita et al., 2017) (Figure 2c). PTP1B has its catalytic domain on the cytosolic face and dephosphorylates active RTKs harbored in endosomes as they transit past the ER (Stuible and Tremblay, 2010). Internalized somatic TrkA receptors are dephosphorylated by PTP1B prior to axonal transport. PTP1B inactivation prevents TrkA exit from soma and causes receptor degradation (Yamashita et al., 2017), suggesting a “gate-keeper” mechanism that ensures targeting of inactive receptors to axons to engage with ligand. Together, these findings suggest a positive feedback loop by which target-derived NGF recruits its own receptors to nerve terminals to amplify neuronal responsiveness during a developmental competition for survival and innervation of end-organs.
These findings raise several key questions regarding how TrkA receptor transcytosis is initiated in neuronal cell bodies, the endocytic itinerary of transcytosing receptors following internalization, their trafficking dynamics in axons, and whether TrkA receptors are co-transported with other membrane proteins known to undergo transcytosis. It also remains unclear if different modes of axonal targeting of Trk receptors co-exist or operate at different stages in the lifetime of the neuron. An attractive hypothesis is that, for sympathetic axons, constitutive delivery via the secretory pathway might serve to transport TrkA receptors prior to target innervation. However, when sympathetic axons reach final NGF-expressing targets, retrograde NGF signaling endosomes actively trigger transcytosis to augment delivery of nascent TrkA receptors.
Discussion
Retrograde Trk receptor signaling exemplifies the significance of endosomal signaling by receptor tyrosine kinases in the formation, maintenance and functions of the nervous system. From the canonical view of retrograde Trk trafficking mediating neuron survival via transcriptional signaling, the repertoire of functions executed by Trk signaling endosomes has expanded to include new roles in diverse sub-cellular locales throughout the neuron, from promoting growth at axon terminals, to regulating synaptogenesis in dendritic compartments, to communicating with naïve receptors on the soma membrane. Despite progress, several questions and challenges remain in gaining a thorough understanding of the scope of functions mediated by Trk signaling endosomes and the mechanistic underpinnings. Are there distinct signaling endosomes specialized for each function? Which signaling effectors act downstream of Trk-harboring endosomes in axons, soma, and dendrites to facilitate compartment-specific functions? Are there compartment-specific differences in the transport machinery, such as different adaptors, RAB GTPases, tethering factors, SNAREs, and motor proteins, that contribute to the distinct behaviors and motilities of Trk endosomes in different neuronal sub-domains? How does Trk signaling regulate its own trafficking and sorting from other axonal cargo? How are Trk endosomes capable of sustained intracellular signaling, and how is the signal ultimately terminated? Interestingly, a recent study proposed a coupling between the retrograde flux of Trk signaling endosomes and synaptic activity (Wang et al., 2016). Trk receptor endocytosis is known to be influenced by neuronal activity and Ca2+ influx (Du et al., 2003). Together, these results imply that more active neurons have a competitive advantage in relaying the retrograde survival signal to neuronal soma. Elucidating the precise molecular mechanisms by which synaptic activity regulates Trk receptor endocytosis, sorting, and axonal transport is an area of future interest.
The forward trafficking of Trk receptors remains an even more poorly researched area. Elucidating the regulatory mechanisms controlling the delivery of Trk receptors to their functional destinations in axons is essential for a fundamental understanding of the biology of neurotrophins, and will also provide a foundation for the general understanding of axonal targeting of membrane proteins. In particular, recent findings that Trk receptor trafficking is regulated by PTP1B, an ER-resident protein tyrosine phosphatase (Yamashita et al., 2017), support the idea that communication between Trk-harboring endosomes and other cellular organelles such as the ER influences receptor sorting decisions and signaling.
While endocytic trafficking has been established to be essential for neurotrophin-mediated nervous system development under normal circumstances, disturbances in trafficking have been postulated to contribute to neuronal dysfunction in neurodevelopmental disorders and neurodegenerative diseases (Chen et al., 2018; Cosker and Segal, 2014). Increased gene dosage for amyloid precursor protein (APP) and RCAN1, an endogenous inhibitor of calcineurin, have been linked to aberrant retrograde neurotrophin trafficking and neuronal atrophy or loss in basal forebrain cholinergic neurons and sympathetic neurons, respectively, in mouse models of Down syndrome (Patel et al., 2015; Salehi et al., 2006). Missense Rab7 mutations are linked to Charcot-Marie-Tooth type 2B, a peripheral neuropathy, and disease-associated Rab7 variants perturb TrkA trafficking, signaling and neurite outgrowth (BasuRay et al., 2010; Zhang et al., 2013). In mice, deletion of an endosomal sodium proton exchanger, NHE6, linked to an autism-related neurodevelopmental disorder called Christianson’s Syndrome, results in defects in neuronal morphogenesis in the developing hippocampus and cortex, likely due to over-acidification of endosomes and enhanced degradation of TrkB (Ouyang et al., 2013). Furthermore, defective retrograde transport of BDNF and TrkB receptors has been documented in striatal dendrites and in cortical axons in mouse models of Huntington Disease (Liot et al., 2013; Zhao et al., 2016). Together, these findings underscore the importance of fully elucidating the molecular underpinnings of polarized trafficking of neurotrophin receptors in neurons.
Highlights.
Retrograde neurotrophin signaling instructs diverse developmental events
Axon-derived Trk endosomes exert local and long-range effects in neurons
Trk signaling directs its own trafficking
Axonal targeting of Trk receptors is regulated
Acknowledgments
We thank Erica Boehm for critical reading of this manuscript. We apologize to authors whose work could not be cited due to space limitations. The authors’ work is supported by NIH grants R01 DK108267 and R01 NS073751 to R. Kuruvilla.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreassi C, Zimmermann C, Mitter R, Fusco S, De Vita S, Saiardi A, Riccio A. An NGF-responsive element targets myo-inositol monophosphatase-1 mRNA to sympathetic neuron axons. Nature neuroscience. 2010;13:291–301. doi: 10.1038/nn.2486. [DOI] [PubMed] [Google Scholar]
- Arimura N, Kimura T, Nakamuta S, Taya S, Funahashi Y, Hattori A, Shimada A, Menager C, Kawabata S, Fujii K, et al. Anterograde transport of TrkB in axons is mediated by direct interaction with Slp1 and Rab27. Developmental cell. 2009;16:675–686. doi: 10.1016/j.devcel.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Ascano M, Bodmer D, Kuruvilla R. Endocytic trafficking of neurotrophins in neural development. Trends in cell biology. 2012;22:266–273. doi: 10.1016/j.tcb.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascano M, Richmond A, Borden P, Kuruvilla R. Axonal targeting of Trk receptors via transcytosis regulates sensitivity to neurotrophin responses. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:11674–11685. doi: 10.1523/JNEUROSCI.1542-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwal JK, Massie B, Miller FD, Kaplan DR. The TrkB-Shc site signals neuronal survival and local axon growth via MEK and P13-kinase. Neuron. 2000;27:265–277. doi: 10.1016/s0896-6273(00)00035-0. [DOI] [PubMed] [Google Scholar]
- Barford K, Deppmann C, Winckler B. The neurotrophin receptor signaling endosome: Where trafficking meets signaling. Developmental neurobiology. 2017;77:405–418. doi: 10.1002/dneu.22427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BasuRay S, Mukherjee S, Romero E, Wilson MC, Wandinger-Ness A. Rab7 mutants associated with Charcot-Marie-Tooth disease exhibit enhanced NGF-stimulated signaling. PloS one. 2010;5:e15351. doi: 10.1371/journal.pone.0015351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya A, Watson F, Bradlee T, Pomeroy S, Stiles C, Segal R. Trk receptors function as rapid retrograde signal carriers in the adult nervous system. J Neuroscience. 1997;17:7007–7016. doi: 10.1523/JNEUROSCI.17-18-07007.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya A, Watson FL, Pomeroy SL, Zhang YZ, Stiles CD, Segal RA. High-resolution imaging demonstrates dynein-based vesicular transport of activated Trk receptors. Journal of neurobiology. 2002;51:302–312. doi: 10.1002/neu.10062. [DOI] [PubMed] [Google Scholar]
- Bodmer D, Ascano M, Kuruvilla R. Isoform-specific dephosphorylation of dynamin1 by calcineurin couples neurotrophin receptor endocytosis to axonal growth. Neuron. 2011;70:1085–1099. doi: 10.1016/j.neuron.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodmer D, Levine-Wilkinson S, Richmond A, Hirsh S, Kuruvilla R. Wnt5a mediates nerve growth factor-dependent axonal branching and growth in developing sympathetic neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:7569–7581. doi: 10.1523/JNEUROSCI.1445-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campenot RB. Local control of neurite development by nerve growth factor. Proc Natl Acad Sci. 1977;74:4516–4519. doi: 10.1073/pnas.74.10.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campenot RB. Development of sympathetic neurons in compartmentalized cultures. II. Local control of neurite survival by nerve growth factor. Developmental biology. 1982;93:13–21. doi: 10.1016/0012-1606(82)90233-0. [DOI] [PubMed] [Google Scholar]
- Chen XQ, Sawa M, Mobley WC. Dysregulation of neurotrophin signaling in the pathogenesis of Alzheimer disease and of Alzheimer disease in Down syndrome. Free Radic Biol Med. 2018;114:52–61. doi: 10.1016/j.freeradbiomed.2017.10.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claude P, Hawrot E, Dunis DA, Campenot RB. Binding, internalization, and retrograde transport of 125I-nerve growth factor in cultured rat sympathetic neurons. J Neurosci. 1982;2:431–442. doi: 10.1523/JNEUROSCI.02-04-00431.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosker KE, Fenstermacher SJ, Pazyra-Murphy MF, Elliott HL, Segal RA. The RNA-binding protein SFPQ orchestrates an RNA regulon to promote axon viability. Nature neuroscience. 2016;19:690–696. doi: 10.1038/nn.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosker KE, Segal RA. Neuronal signaling through endocytosis. Cold Spring Harbor perspectives in biology. 2014;6 doi: 10.1101/cshperspect.a020669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan WM. Viktor Hamburger and Rita Levi-Montalcini: the path to the discovery of nerve growth factor. Annual review of neuroscience. 2001;24:551–600. doi: 10.1146/annurev.neuro.24.1.551. [DOI] [PubMed] [Google Scholar]
- Crowley C, Spencer SD, Nishimura MC, Chen KS, Pitts MS, Armanini MP, Ling LH, MacMahon SB, Shelton DL, Levinson AD. Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell. 1994;76:1001–1011. doi: 10.1016/0092-8674(94)90378-6. [DOI] [PubMed] [Google Scholar]
- Davis BM, Albers KM, Seroogy KB, Katz DM. Overexpression of nerve growth factor in transgenic mice induces novel sympathetic projections to primary sensory neurons. The Journal of comparative neurology. 1994;349:464–474. doi: 10.1002/cne.903490310. [DOI] [PubMed] [Google Scholar]
- Deinhardt K, Salinas S, Verastegui C, Watson R, Worth D, Hanrahan S, Bucci C, Schiavo G. Rab5 and Rab7 control endocytic sorting along the axonal retrograde transport pathway. Neuron. 2006;52:293–305. doi: 10.1016/j.neuron.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Delcroix JD, Valletta JS, Wu C, Hunt SJ, Kowal AS, Mobley WC. NGF signaling in sensory neurons: evidence that early endosomes carry NGF retrograde signals. Neuron. 2003;39:69–84. doi: 10.1016/s0896-6273(03)00397-0. [DOI] [PubMed] [Google Scholar]
- Deppmann CD, Mihalas S, Sharma N, Lonze BE, Niebur E, Ginty DD. A model for neuronal competition during development. Science. 2008;320:369–373. doi: 10.1126/science.1152677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Feng L, Zaitsev E, Je HS, Liu XW, Lu B. Regulation of TrkB receptor tyrosine kinase and its internalization by neuronal activity and Ca2+ influx. The Journal of cell biology. 2003;163:385–395. doi: 10.1083/jcb.200305134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RH, Rutter WJ, Hanahan D. Directed expression of NGF to pancreatic beta cells in transgenic mice leads to selective hyperinnervation of the islets. Cell. 1989;58:161–170. doi: 10.1016/0092-8674(89)90412-1. [DOI] [PubMed] [Google Scholar]
- Ehlers MD, Kaplan DR, Price DL, Koliatsos VE. NGF-stimulated retrograde transport of TrkA in the Mammalian Nervous System. J Cell Bio. 1995;130:149–156. doi: 10.1083/jcb.130.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldredge LC, Gao XM, Quach DH, Li L, Han X, Lomasney J, Tourtellotte WG. Abnormal sympathetic nervous system development and physiological dysautonomia in Egr3-deficient mice. Development. 2008;135:2949–2957. doi: 10.1242/dev.023960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrach S, Schmidt S, Diriong S, Penna A, Blangy A, Fort P, Debant A. The Human Rho-GEF trio and its target GTPase RhoG are involved in the NGF pathway, leading to neurite outgrowth. Current biology : CB. 2002;12:307–312. doi: 10.1016/s0960-9822(02)00658-9. [DOI] [PubMed] [Google Scholar]
- Eva R, Dassie E, Caswell PT, Dick G, ffrench-Constant C, Norman JC, Fawcett JW. Rab11 and its effector Rab coupling protein contribute to the trafficking of beta 1 integrins during axon growth in adult dorsal root ganglion neurons and PC12 cells. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:11654–11669. doi: 10.1523/JNEUROSCI.2425-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glebova NO, Ginty DD. Heterogeneous requirement of NGF for sympathetic target innervation in vivo. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:743–751. doi: 10.1523/JNEUROSCI.4523-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glebova NO, Ginty DD. Growth and survival signals controlling sympathetic nervous system development. Annual review of neuroscience. 2005;28:191–222. doi: 10.1146/annurev.neuro.28.061604.135659. [DOI] [PubMed] [Google Scholar]
- Graef IA, Wang F, Charron F, Chen L, Neilson J, Tessier-Lavigne M, Crabtree GR. Neurotrophins and netrins require calcineurin/NFAT signaling to stimulate outgrowth of embryonic axons. Cell. 2003;113:657–670. doi: 10.1016/s0092-8674(03)00390-8. [DOI] [PubMed] [Google Scholar]
- Hamburger V. History of the discovery of neuronal death in embryos. Journal of neurobiology. 1992;23:1116–1123. doi: 10.1002/neu.480230904. [DOI] [PubMed] [Google Scholar]
- Harrington AW, Ginty DD. Long-distance retrograde neurotrophic factor signalling in neurons. Nature reviews Neuroscience. 2013;14:177–187. doi: 10.1038/nrn3253. [DOI] [PubMed] [Google Scholar]
- Harrington AW, St Hillaire C, Zweifel LS, Glebova NO, Philippidou P, Halegoua S, Ginty DD. Recruitment of actin modifiers to TrkA endosomes governs retrograde NGF signaling and survival. Cell. 2011;146:421–434. doi: 10.1016/j.cell.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassankhani A, Steinhelper ME, Soonpaa MH, Katz EB, Taylor DA, Andrade-Rozental A, Factor SM, Steinberg JJ, Field LJ, Federoff HJ. Overexpression of NGF within the heart of transgenic mice causes hyperinnervation, cardiac enlargement, and hyperplasia of ectopic cells. Developmental biology. 1995;169:309–321. doi: 10.1006/dbio.1995.1146. [DOI] [PubMed] [Google Scholar]
- Heerssen HM, Pazyra MF, Segal RA. Dynein motors transport activated Trks to promote survival of target-dependent neurons. Nature neuroscience. 2004;7:596–604. doi: 10.1038/nn1242. [DOI] [PubMed] [Google Scholar]
- Hempstead BL. Deciphering proneurotrophin actions. Handb Exp Pharmacol. 2014;220:17–32. doi: 10.1007/978-3-642-45106-5_2. [DOI] [PubMed] [Google Scholar]
- Hendry IA, Stockel K, Thoenen H, Iversen LL. The retrograde axonal transport of nerve growth factor. Brain Res. 1974;68:103–121. doi: 10.1016/0006-8993(74)90536-8. [DOI] [PubMed] [Google Scholar]
- Horton AC, Ehlers MD. Neuronal polarity and trafficking. Neuron. 2003;40:277–295. doi: 10.1016/s0896-6273(03)00629-9. [DOI] [PubMed] [Google Scholar]
- Howe CL, Mobley WC. Signaling endosome hypothesis: A cellular mechanism for long distance communication. Journal of neurobiology. 2004;58:207–216. doi: 10.1002/neu.10323. [DOI] [PubMed] [Google Scholar]
- Howe CL, Valletta JS, Rusnak AS, Mobley WC. NGF signaling from clathrin-coated vesicles: evidence that signaling endosomes serve as a platform for the Ras-MAPK pathway. Neuron. 2001;32:801–814. doi: 10.1016/s0896-6273(01)00526-8. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annual review of biochemistry. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Huang SH, Duan S, Sun T, Wang J, Zhao L, Geng Z, Yan J, Sun HJ, Chen ZY. JIP3 mediates TrkB axonal anterograde transport and enhances BDNF signaling by directly bridging TrkB with kinesin-1. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:10602–10614. doi: 10.1523/JNEUROSCI.0436-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kononenko NL, Classen GA, Kuijpers M, Puchkov D, Maritzen T, Tempes A, Malik AR, Skalecka A, Bera S, Jaworski J, et al. Retrograde transport of TrkB-containing autophagosomes via the adaptor AP-2 mediates neuronal complexity and prevents neurodegeneration. Nature communications. 2017;8:14819. doi: 10.1038/ncomms14819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuruvilla R, Ye H, Ginty DD. Spatially and functionally distinct roles of the PI3-K effector pathway during NGF signaling in sympathetic neurons. Neuron. 2000;27:499–512. doi: 10.1016/s0896-6273(00)00061-1. [DOI] [PubMed] [Google Scholar]
- Kuruvilla R, Zweifel LS, Glebova NO, Lonze BE, Valdez G, Ye H, Ginty DD. A neurotrophin signaling cascade coordinates sympathetic neuron development through differential control of TrkA trafficking and retrograde signaling. Cell. 2004;118:243–255. doi: 10.1016/j.cell.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Lehigh KM, West KM, Ginty DD. Retrogradely Transported TrkA Endosomes Signal Locally within Dendrites to Maintain Sympathetic Neuron Synapses. Cell reports. 2017;19:86–100. doi: 10.1016/j.celrep.2017.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leterrier C, Laine J, Darmon M, Boudin H, Rossier J, Lenkei Z. Constitutive activation drives compartment-selective endocytosis and axonal targeting of type 1 cannabinoid receptors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:3141–3153. doi: 10.1523/JNEUROSCI.5437-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi-Montalcini R, Booker B. Destruction of the Sympathetic Ganglia in Mammals by an Antiserum to a Nerve-Growth Protein. Proceedings of the National Academy of Sciences of the United States of America. 1960;46:384–391. doi: 10.1073/pnas.46.3.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liot G, Zala D, Pla P, Mottet G, Piel M, Saudou F. Mutant Huntingtin alters retrograde transport of TrkB receptors in striatal dendrites. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:6298–6309. doi: 10.1523/JNEUROSCI.2033-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JJ, Ding J, Wu C, Bhagavatula P, Cui B, Chu S, Mobley WC, Yang Y. Retrolinkin, a membrane protein, plays an important role in retrograde axonal transport. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:2223–2228. doi: 10.1073/pnas.0602222104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- MacInnis BL, Campenot RB. Retrograde support of neuronal survival without retrograde transport of nerve growth factor. Science. 2002;295:1536–1539. doi: 10.1126/science.1064913. [DOI] [PubMed] [Google Scholar]
- Maday S, Wallace KE, Holzbaur EL. Autophagosomes initiate distally and mature during transport toward the cell soma in primary neurons. The Journal of cell biology. 2012;196:407–417. doi: 10.1083/jcb.201106120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makita T, Sucov HM, Gariepy CE, Yanagisawa M, Ginty DD. Endothelins are vascular-derived axonal guidance cues for developing sympathetic neurons. Nature. 2008;452:759–763. doi: 10.1038/nature06859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandai K, Guo T, St Hillaire C, Meabon JS, Kanning KC, Bothwell M, Ginty DD. LIG family receptor tyrosine kinase-associated proteins modulate growth factor signals during neural development. Neuron. 2009;63:614–627. doi: 10.1016/j.neuron.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker RB, Williams KS. The p75 neurotrophin receptor: at the crossroad of neural repair and death. Neural Regen Res. 2015;10:721–725. doi: 10.4103/1673-5374.156967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DJ, Blasier KR, Jeffery ED, Ross MW, Pullikuth AK, Suo D, Park J, Smiley WR, Lo KW, Shabanowitz J, et al. Trk activation of the ERK1/2 kinase pathway stimulates intermediate chain phosphorylation and recruits cytoplasmic dynein to signaling endosomes for retrograde axonal transport. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:15495–15510. doi: 10.1523/JNEUROSCI.5599-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok SA, Campenot RB. A nerve growth factor-induced retrograde survival signal mediated by mechanisms downstream of TrkA. Neuropharmacology. 2007;52:270–278. doi: 10.1016/j.neuropharm.2006.07.032. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Hashimoto R, Sakoori K, Sugaya Y, Tanimura A, Hashimotodani Y, Ohi K, Yamamori H, Yasuda Y, Umeda-Yano S, et al. Emerging roles of ARHGAP33 in intracellular trafficking of TrkB and pathophysiology of neuropsychiatric disorders. Nature communications. 2016;7:10594. doi: 10.1038/ncomms10594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim RW. The neurotrophic theory and naturally occurring motoneuron death. Trends in neurosciences. 1989;12:252–255. doi: 10.1016/0166-2236(89)90021-0. [DOI] [PubMed] [Google Scholar]
- Ouyang Q, Lizarraga SB, Schmidt M, Yang U, Gong J, Ellisor D, Kauer JA, Morrow EM. Christianson syndrome protein NHE6 modulates TrkB endosomal signaling required for neuronal circuit development. Neuron. 2013;80:97–112. doi: 10.1016/j.neuron.2013.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A, Yamashita N, Ascano M, Bodmer D, Boehm E, Bodkin-Clarke C, Ryu YK, Kuruvilla R. RCAN1 links impaired neurotrophin trafficking to aberrant development of the sympathetic nervous system in Down syndrome. Nature communications. 2015;6:10119. doi: 10.1038/ncomms10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel TD, Jackman A, Rice FL, Kucera J, Snider WD. Development of sensory neurons in the absence of NGF/TrkA signaling in vivo [see comments] Neuron. 2000;25:345–357. doi: 10.1016/s0896-6273(00)80899-5. [DOI] [PubMed] [Google Scholar]
- Pazyra-Murphy MF, Hans A, Courchesne SL, Karch C, Cosker KE, Heerssen HM, Watson FL, Kim T, Greenberg ME, Segal RA. A retrograde neuronal survival response: target-derived neurotrophins regulate MEF2D and bcl-w. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:6700–6709. doi: 10.1523/JNEUROSCI.0233-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio A, Pierchala B, Ciarallo C, Ginty DD. An NGF-TrkA-Mediated retrograde signal to transcription factor CREB in sympathetic neurons. Science. 1997;227:1097–1100. doi: 10.1126/science.277.5329.1097. [DOI] [PubMed] [Google Scholar]
- Rubin E. Development of the rat superior cervical ganglion: ganglion cell maturation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1985;5:673–684. doi: 10.1523/JNEUROSCI.05-03-00673.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruit KG, Osborne PA, Schmidt RE, Johnson EM, Jr, Snider WD. Nerve growth factor regulates sympathetic ganglion cell morphology and survival in the adult mouse. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1990;10:2412–2419. doi: 10.1523/JNEUROSCI.10-07-02412.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruit KG, Snider WD. Administration or deprivation of nerve growth factor during development permanently alters neuronal geometry. The Journal of comparative neurology. 1991;314:106–113. doi: 10.1002/cne.903140110. [DOI] [PubMed] [Google Scholar]
- Salehi A, Delcroix JD, Belichenko PV, Zhan K, Wu C, Valletta JS, Takimoto-Kimura R, Kleschevnikov AM, Sambamurti K, Chung PP, et al. Increased App expression in a mouse model of Down’s syndrome disrupts NGF transport and causes cholinergic neuron degeneration. Neuron. 2006;51:29–42. doi: 10.1016/j.neuron.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Sandow SL, Heydon K, Weible MW, 2nd, Reynolds AJ, Bartlett SE, Hendry IA. Signalling organelle for retrograde axonal transport of internalized neurotrophins from the nerve terminal. Immunol Cell Biol. 2000;78:430–435. doi: 10.1046/j.1440-1711.2000.00924.x. [DOI] [PubMed] [Google Scholar]
- Shao Y, Akmentin W, Toledo-Aral JJ, Rosenbaum J, Valdez G, Cabot JB, Hilbush BS, Halegoua S. Pincher, a pinocytic chaperone for nerve growth factor/TrkA signaling endosomes. The Journal of cell biology. 2002;157:679–691. doi: 10.1083/jcb.200201063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Deppmann CD, Harrington AW, St Hillaire C, Chen ZY, Lee FS, Ginty DD. Long-distance control of synapse assembly by target-derived NGF. Neuron. 2010;67:422–434. doi: 10.1016/j.neuron.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeyne RJ, Klein R, Schnapp A, Long LK, Bryant S, Lewin A, Lira SA, Barbacid M. Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. Nature. 1994;368:246–248. doi: 10.1038/368246a0. [DOI] [PubMed] [Google Scholar]
- Snider WD. Nerve growth factor enhances dendritic arborization of sympathetic ganglion cells in developing mammals. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1988;8:2628–2634. doi: 10.1523/JNEUROSCI.08-07-02628.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider WD. Functions of the neurotrophins during nervous system development. Cell. 1994;77:627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Spillane M, Ketschek A, Donnelly CJ, Pacheco A, Twiss JL, Gallo G. Nerve growth factor-induced formation of axonal filopodia and collateral branches involves the intra-axonal synthesis of regulators of the actin-nucleating Arp2/3 complex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:17671–17689. doi: 10.1523/JNEUROSCI.1079-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillane M, Ketschek A, Merianda TT, Twiss JL, Gallo G. Mitochondria coordinate sites of axon branching through localized intra-axonal protein synthesis. Cell reports. 2013;5:1564–1575. doi: 10.1016/j.celrep.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuible M, Tremblay ML. In control at the ER: PTP1B and the down-regulation of RTKs by dephosphorylation and endocytosis. Trends in cell biology. 2010;20:672–679. doi: 10.1016/j.tcb.2010.08.013. [DOI] [PubMed] [Google Scholar]
- Sun T, Li Y, Li T, Ma H, Guo Y, Jiang X, Hou M, Huang S, Chen Z. JIP1 and JIP3 cooperate to mediate TrkB anterograde axonal transport by activating kinesin-1. Cellular and molecular life sciences : CMLS. 2017;74:4027–4044. doi: 10.1007/s00018-017-2568-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suo D, Park J, Harrington AW, Zweifel LS, Mihalas S, Deppmann CD. Coronin-1 is a neurotrophin endosomal effector that is required for developmental competition for survival. Nature neuroscience. 2014;17:36–45. doi: 10.1038/nn.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Niwa S, Dong M, Farkhondeh A, Wang L, Zhou R, Hirokawa N. The Molecular Motor KIF1A Transports the TrkA Neurotrophin Receptor and Is Essential for Sensory Neuron Survival and Function. Neuron. 2016;90:1215–1229. doi: 10.1016/j.neuron.2016.05.002. [DOI] [PubMed] [Google Scholar]
- Terenzio M, Schiavo G, Fainzilber M. Compartmentalized Signaling in Neurons: From Cell Biology to Neuroscience. Neuron. 2017;96:667–679. doi: 10.1016/j.neuron.2017.10.015. [DOI] [PubMed] [Google Scholar]
- Tsui-Pierchala BA, Ginty DD. Characterization of an NGF-P-TrkA retrograde signaling complex and age-dependent regulation of TrkA phosphorylation in sympathetic neurons. J Neurosci. 1999;19:8207–8218. doi: 10.1523/JNEUROSCI.19-19-08207.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuma P, Hubbard AL. Transcytosis: crossing cellular barriers. Physiological reviews. 2003;83:871–932. doi: 10.1152/physrev.00001.2003. [DOI] [PubMed] [Google Scholar]
- Vaegter CB, Jansen P, Fjorback AW, Glerup S, Skeldal S, Kjolby M, Richner M, Erdmann B, Nyengaard JR, Tessarollo L, et al. Sortilin associates with Trk receptors to enhance anterograde transport and neurotrophin signaling. Nature neuroscience. 2011;14:54–61. doi: 10.1038/nn.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez G, Philippidou P, Rosenbaum J, Akmentin W, Shao Y, Halegoua S. Trk-signaling endosomes are generated by Rac-dependent macroendocytosis. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:12270–12275. doi: 10.1073/pnas.0702819104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarin JM, McCurdy EP, Martinez JC, Hengst U. Local synthesis of dynein cofactors matches retrograde transport to acutely changing demands. Nature communications. 2016;7:13865. doi: 10.1038/ncomms13865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voyvodic JT. Development and regulation of dendrites in the rat superior cervical ganglion. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1987;7:904–912. doi: 10.1523/JNEUROSCI.07-03-00904.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Martin S, Nguyen TH, Harper CB, Gormal RS, Martinez-Marmol R, Karunanithi S, Coulson EJ, Glass NR, Cooper-White JJ, et al. Flux of signalling endosomes undergoing axonal retrograde transport is encoded by presynaptic activity and TrkB. Nature communications. 2016;7:12976. doi: 10.1038/ncomms12976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson FL, Heerssen HM, Bhattacharyya A, Klesse L, Lin MZ, Segal RA. Neurotrophins use the Erk5 pathway to mediate a retrograde survival response. Nature neuroscience. 2001;4:981–988. doi: 10.1038/nn720. [DOI] [PubMed] [Google Scholar]
- Watson FL, Heerssen HM, Moheban DB, Lin MZ, Sauvageot CM, Bhattacharyya A, Pomeroy SL, Segal RA. Rapid nuclear responses to target-derived neurotrophins require retrograde transport of ligand-receptor complex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:7889–7900. doi: 10.1523/JNEUROSCI.19-18-07889.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weible MW, 2nd, Hendry IA. What is the importance of multivesicular bodies in retrograde axonal transport in vivo? Journal of neurobiology. 2004;58:230–243. doi: 10.1002/neu.10318. [DOI] [PubMed] [Google Scholar]
- Wickramasinghe SR, Alvania RS, Ramanan N, Wood JN, Mandai K, Ginty DD. Serum response factor mediates NGF-dependent target innervation by embryonic DRG sensory neurons. Neuron. 2008;58:532–545. doi: 10.1016/j.neuron.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis DE, van Niekerk EA, Sasaki Y, Mesngon M, Merianda TT, Williams GG, Kendall M, Smith DS, Bassell GJ, Twiss JL. Extracellular stimuli specifically regulate localized levels of individual neuronal mRNAs. The Journal of cell biology. 2007;178:965–980. doi: 10.1083/jcb.200703209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winckler B, Mellman I. Trafficking guidance receptors. Cold Spring Harbor perspectives in biology. 2010;2:a001826. doi: 10.1101/cshperspect.a001826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisco D, Anderson ED, Chang MC, Norden C, Boiko T, Folsch H, Winckler B. Uncovering multiple axonal targeting pathways in hippocampal neurons. The Journal of cell biology. 2003;162:1317–1328. doi: 10.1083/jcb.200307069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita N, Joshi R, Zhang S, Zhang ZY, Kuruvilla R. Phospho-Regulation of Soma-to-Axon Transcytosis of Neurotrophin Receptors. Developmental cell. 2017;42:626–639. doi: 10.1016/j.devcel.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita N, Kuruvilla R. Neurotrophin signaling endosomes: biogenesis, regulation, and functions. Current opinion in neurobiology. 2016;39:139–145. doi: 10.1016/j.conb.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano H, Lee FS, Kong H, Chuang J, Arevalo J, Perez P, Sung C, Chao MV. Association of Trk neurotrophin receptors with components of the cytoplasmic dynein motor. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:RC125. doi: 10.1523/JNEUROSCI.21-03-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye H, Kuruvilla R, Zweifel LS, Ginty DD. Evidence in support of signaling endosome-based retrograde survival of sympathetic neurons. Neuron. 2003;39:57–68. doi: 10.1016/s0896-6273(03)00266-6. [DOI] [PubMed] [Google Scholar]
- Ye M, Lehigh KM, Ginty DD. Multivesicular bodies mediate long-range retrograde NGF-TrkA signaling. eLife. 2018;7 doi: 10.7554/eLife.33012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Fishel Ben Kenan R, Osakada Y, Xu W, Sinit RS, Chen L, Zhao X, Chen JY, Cui B, Wu C. Defective axonal transport of Rab7 GTPase results in dysregulated trophic signaling. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:7451–7462. doi: 10.1523/JNEUROSCI.4322-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Chen XQ, Han E, Hu Y, Paik P, Ding Z, Overman J, Lau AL, Shahmoradian SH, Chiu W, et al. TRiC subunits enhance BDNF axonal transport and rescue striatal atrophy in Huntington’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:E5655–5664. doi: 10.1073/pnas.1603020113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Cai Q, Xie Y, Sheng ZH. Snapin recruits dynein to BDNF-TrkB signaling endosomes for retrograde axonal transport and is essential for dendrite growth of cortical neurons. Cell reports. 2012;2:42–51. doi: 10.1016/j.celrep.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FQ, Zhou J, Dedhar S, Wu YH, Snider WD. NGF-induced axon growth is mediated by localized inactivation of GSK-3beta and functions of the microtubule plus end binding protein APC. Neuron. 2004;42:897–912. doi: 10.1016/j.neuron.2004.05.011. [DOI] [PubMed] [Google Scholar]


