Hematopoietic stem cell transplantation (HSCT) has become a standard of care for the treatment of many hematological disorders [1, 2]. However, current myeloablative conditioning regimens are associated with significant morbidity and mortality [1]. In addition, outcomes of transplantation can be suboptimal owing to limiting stem cell numbers, especially in umbilical cord blood (UCB) transplantation [1] or autologous transplantation [2]. Thus, novel means that optimize the engraftment of hematopoietic stem cells (HSCs) in the bone marrow (BM) of transplant recipients with attenuated toxicities are needed to maximize the benefits of HSCT.
Engraftment is a process of competition between endogenous and infused donor HSCs in the BM niche, the primary residence of HSCs [3]. In HSCT, engraftment of transplanted donor stem cells is often limited by the availability of BM niches normally occupied by host HSCs [3, 4]. Traditional myeloablative conditioning makes niche available by non-specifically destroying endogenous HSCs and disrupting niche structures [5]. Several studies have shown that depletion of host HSCs in a directed fashion could vacate BM niches and facilitate engraftment of donor HSCs [4–8]. However, the clinical utility of HSC mobilization agents, such as the cytokine granulocyte colony-stimulating factor (G-CSF) or the CXCR4 antagonist AMD3100, does not improve niche availability, probably owing to their concomitant pro-proliferative activity, resulting in rapid reoccupation of the BM niches by donor HSCs [9, 10].
We previously showed that conditional knockout of the Rho GTPase Cdc42 in murine HSCs led to defective F-actin polymerization, reduced adhesion to fibronectin matrix or stroma cells, and a massive egress of hematopoietic stem/progenitor cells (HSPCs) from BM [11]. More recently, we rationally identified a Cdc42 activity specific inhibitor, CASIN, that directly acts on the guanine nucleotide exchange site of Cdc42, specifically inhibits intracellular Cdc42 activity, and transiently induces murine HSC mobilization by suppressing actin polymerization, cell polarity, adhesion, and directional migration of HSCs, conferring Cdc42 knockout phenotypes [12].
To test whether Cdc42 targeting could allow access to functional niches in the BM, we first performed BM transplantation with WT BM cells and assessed donor engraftment after conditional deletion of Cdc42 in recipient HSCs (Fig. 1a). Four months post transplantation, donor engraftment in Cdc42-deleted hosts were significantly higher in both BM and peripheral blood (PB) than Cdc42+/+ host mice (Fig. 1b, left). The chimera was found in all blood lineages including Lin−Sca1+c-Kit+ (LSK) compartment (Fig. 1b, right), indicating successful multi-lineage reconstitution. Secondary transplants demonstrated long-term (LT) repopulating capacity and multi-lineage differentiation of donor HSCs (Figure S1). Thus, CDC42 gene deletion in recipient mice allows access of donor HSCs to functional niche in the BM.
Fig. 1.
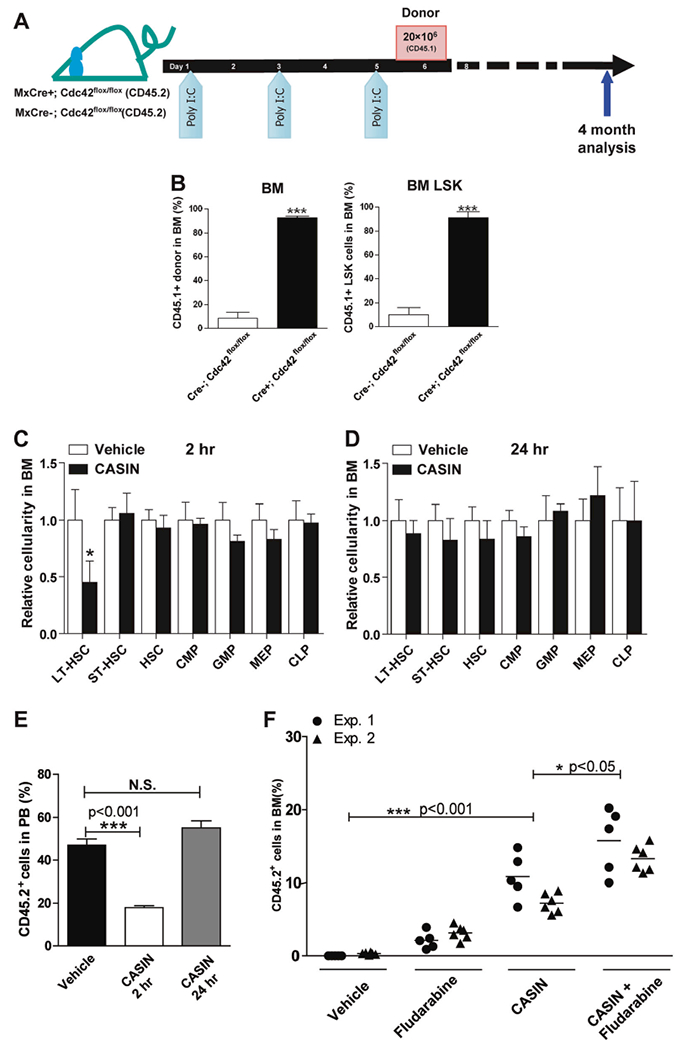
Cdc42 targeting opens BM niche. a, b A single dose of donor BM cell infusion effectively engrafts recipient mice upon deletion of Cdc42 in host BM. Recipient mice (Mx:Cre−/−; Cdc42flox/flox v.s. Mx: Cre+/+; Cdc42flox/flox) were injected with Poly I:C three times 1 day apart and transplanted with 2 × 107 congenic BM cells (CD45.1+) 1 day after the last injection a. Percentage of donor-derived chimerism (CD45.2+) in total BM cells (left panel) and LSK BM cells (right panel) of CASIN-treated BoyJ recipients (CD45.1+) v.s. vehicle 4 months after transplantation b. Results are means plus or minus SD from three independent experiments (n = 6 for each experimental group). *** p < 0.001. c–e CASIN administration phenocopies the effect of Cdc42 gene targeting in host mice to facilitate donor HSC engraftment. Relative changes in the number of phenotypic long term (LT)-HSC (Lin−IL-7R−Sca1+c-kit+CD34−) and various progenitor subpopulations were measured in CASIN- or vehicle-treated mouse BM (1.2 mg/kg, IV) at 2 h c or 24 h d. Results are representative of three independent experiments (n = 4). * p < 0.05. The BM cells harvested from these donor mice (3 × 106) were competitively transplanted into BoyJ recipients (CD45.1+) at a 1:1 ratio with CD45.1+ BM cells, and the chimera were analyzed 10 months after the competitive transplantation e. Results are representative of three independent experiments (n = 8 for each recipient group). *** p < 0.001. f CASIN promotes LT-HSC engraftment and synergizes with Fludarabine in a non-myeloablative conditioning regimen. Two separate cohorts of syngeneic BoyJ recipient mice were conditioned with vehicle, CASIN (twice at 1.2 mg/Kg), Fludarabine (three times at 75 mg/kg, IP), or CASIN together with Fludarabine prior to transplantation with congenic CD45.2+ BM cells (5 × 106). Percentages of donor-derived mononuclear cells in the PB of the recipients were measured 4 months after transplantation. * p < 0.05; *** p < 0.001
We reasoned that HSC mobilization by Cdc42 targeting may transiently vacate BM niche and facilitate the establishment of LT-donor chimerism upon HSCT. In support of this notion, we found that the Cdc42 inhibitor, CASIN [12], induced a transient decrease of phenotypic LT-HSCs (Lin−c-kit+ Sca1+IL7Rα−CD34−) in BM 2 h after CASIN administration (Fig. 1c), which returned to normal after 24 h (Fig. 1d). A transient reduction of HSCs in BM of CASIN-treated animals was further supported by a functional reduction of competitive repopulating HSC activity within BM isolated at the 2-h, but not the 24-h interval, after CASIN administration to the donors (Fig. 1e). We subsequently tested whether CASIN synergizes with commonly used Fludarabine (Flu)-based preparative regimen [13]. CASIN in combination with Flu allowed significantly higher engraftment of donor cells than CASIN or Flu alone (Fig. 1f), suggesting an additive effect of a combination of immunosuppressant with CASIN in establishing engraftment under reduced-intensity conditioning [13]. These data demonstrate that transient pharmacological inhibition of Cdc42 by CASIN can vacate functional BM niche and allows for LT-engraftment of donor HSCs, in part mimicking the effect of CDC42 gene deletion in HSCs.
CASIN significantly inhibited the downstream signaling of Cdc42 including PAK1, WASP, and a-PKC, mimicking that of Cdc42 knockdown in human primitive hematopoietic cells (Figure S2A). We then examined the effects of CASIN on mobilization of human hematopoietic cells by intrafemoral injection of CD34+ UCB cells into NSG mice [14]. We found that although G-CSF and CASIN had similar effect in mobilizing human CFU activity (Figure S2B), CASIN conditioning led to ~ 10-fold increase in human CFU progenitor mobilization relative to PBS control, whereas G-CSF alone did not show a conditioning advantage over the control (Figure S2C). These results indicate that CASIN is effective in mobilizing human blood stem/progenitor cells in xenograft mice without obvious cytotoxicity in the course of HSC mobilization (Figure S2D, S2E).
Similar to murine hematopoietic progenitor cells, CASIN inhibited Cdc42 activity in CD34+ UCB progenitor cells without affecting Rac1 activity (Figure S3A) and blocked SDF-1α-induced F-actin polymerization in a dose-dependent fashion without an additive effect in Cdc42 knockdown cells (Figure S3B). CASIN significantly reduced adhesion (Figure S3C, Left) and migratory activity (Figure S3D, left) of UCBs, similar to that seen with Cdc42 knockdown (Figures S3C, S3D, right). These data suggest that CASIN is useful in selectively inhibiting Cdc42 activity in human blood progenitor cells. CASIN administration significantly mobilized human CD34+ hematopoietic progenitor cells to the PB 1 h after CASIN injection (Fig. 2a, left) and resulted in a ~ 10-fold increase in colony-forming progenitors compared with controls (Fig. 2a, right). Conditioning with CASIN facilitated subsequent engraftment of human blood progenitors in a transplant model that does not include myeloablation, as the overall engraftment of CD45+ UCB cells (Fig. 2b, left) and human CD34+ hematopoietic progenitors (Fig. 2b, right) at 4 months were significantly higher in the CASIN-conditioned recipients than those seen in the vehicle-treated group (Fig. 2b). These results show that pharmacological inhibition of Cdc42 promotes the engraftment of CD34+ UCB cells into recipients without myeloablation.
Fig. 2.
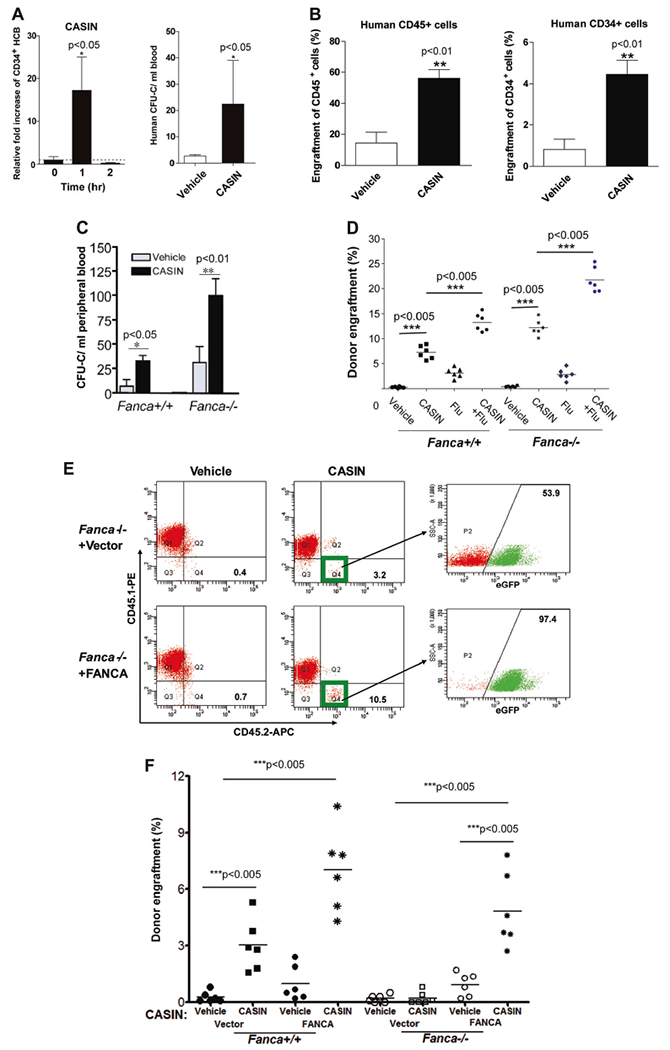
CASIN application to human cord blood and FA transplantation. a CASIN mobilizes CD34+ HCB cells in xenografted NSG mice. CD34+ HCB cells were transplanted into sublethally irradiated NSG recipient mice. Four month later, xenografted mice were treated with CASIN (1.2 mg/kg, iv). Peripheral blood (PB) were then obtained at the indicated time points and subjected to Flow Cytometry analysis for hCD34. Relative fold increase of CD34+ HCB chimerism (left) plus or minus SD (n = 13 in CASIN groups and n = 10 in control group) and CFU-C numbers in PB of mice (right) are shown. n = 5 in CASIN group and four in vehicle group. * p < 0.05. b CASIN enhances engraftment of CD34+ HCB cells in immunodeficient NSG mice without myeloablation. Recipient NSG mice were conditioned with vehicle or CASIN (1.2 mg/kg) 24 and 2 h prior to BM transplantation and then transplanted with 2 × 105 CD34+ HCB cells by intrafemoral injection. Percentages of donor-derived human CD45+ in PB (left) or CD34+ in BM (right) were assessed by Flow Cytometry 4 months after transplantation. n = 5 per group. ** p < 0.01. c CASIN further increased spontaneous mobilization of Fanca−/− BM progenitors. WT or Fanca−/− mice were injected with CASIN (1.2 mg/kg, IP) or Vehicle, and 48 h later, PB from the treated mice were subjected to progenitor assay for CFU-C activity. Results are means plus or minus SD from three independent experiments (n = 9 per group). * p < 0.05; ** p < 0.01. d CASIN synergizes with Fludarabine (Flu) on HSC engraftment. Fanca+/+ and Fanca−/− recipients were conditioned with CASIN (1.2 mg/kg, IP, 24 and 2 h prior to BMT), Fludarabine (75 mg/kg, i.p. administration, 72, 48, and 24 h prior to BMT) or CASIN plus Flu in parallel with vehicle controls, and then transplanted with congenic BM cells (5 × 106 cells/mouse). Percentages of donor-derived cells in BM of recipients were determined by Flow Cytometry analysis. Results are representative of three independent experiments (n = 6 per group). *** p < 0.005. e CASIN promotes engraftment of gene-corrected Fanca−/− HSCs in wild-type recipients. BoyJ recipients were pre-conditioned with vehicle or CASIN (twice at 1.2 mg/kg), and transplanted with 2 × 105 Fanca−/− Lin− BM cells transduced with retrovirus expressing eGFP only (Vector) or FANCA. Four months later, PB cells from the transplanted mice were stained with antibodies against CD45.1 and CD45.2, and donor-derived CD45.2+ cells were gated and analyzed by flow cytometry for GFP-positive and GFP-negative cell populations. Note that > 90% of donor-derived cells in the CASIN group are gene-corrected (eGFP-positive) cells. f CASIN promotes engraftment of gene-corrected Fanca−/− HSCs in Fanca−/− recipients. Similar donor cells and conditioning regimens as in e were used except that the transplant recipients were either Fanca+/+ or Fanca−/− mice. Donor engraftments were assayed by FACS analysis of eGFP+ cells in the recipient BM 4 months posttransplant. Results are representative of three independent experiments (n = 6 per group). *** p < 0.005
Fanconi anemia (FA) is a devastating BM failure syndrome characterized by decreased engraftment ability of HSCs and increased susceptibility to a variety of cellular stresses including DNA damage [15]. We found that CASIN could further inhibit Cdc42 activity, but not the closely related Rac1 activity, in Fanca−/− BM cells (Figure S4A). Correspondingly, CASIN mobilized significantly more colony-forming progenitors in Fanca−/− mice than in WT mice (Fig. 2c) and decreased LSK cells in the BM of Fanca−/− mice (Figure S4B). These data indicate that CASIN further decreases Cdc42 activity and enhances spontaneous BM HSPC mobilization of Fanca−/− mice. Furthermore, CASIN-conditioned mice showed significantly increased chimera relative to vehicle-control treated animals, with Fanca−/− mice, demonstrating an even greater engraftment than WT recipients, suggesting that CASIN may prove particularly useful in conditioning HSCT recipients with FA (Figure S4C).
Flu in combination with low-dose total body irradiation have shown a benefit in allogeneic HSCT for FA patients [13]. We found that CASIN in combination with Flu allowed significantly higher engraftment of donor cells in Fanca+/+ recipients than CASIN or Flu alone (Fig. 2d). The effect on engraftment was even greater in Fanca−/− recipients (Fig. 2d), suggesting that a CASIN-based conditioning regimen may be of value in facilitating engraftment of transplanted stem cells, and have particular efficacy in transplantation of FA patients.
To address the potential value of CASIN in gene therapy using autologous HSCs in FA [15], we tested the engraftment of gene-corrected Fanca−/− Lin− BM cells in CASIN-conditioned congenic mice. First, we found that CASIN enhanced the engraftment of FANCA-corrected Fanca−/− Lin− cells (Figures S5A, B) in BM of the recipients, to an extent comparable to that of WT donor cells (Fig. 2e). Second, 92.20 ± 4.5% of donor-derived cells in gene therapy group were FANCA-corrected eGFP+ cells, significantly higher than 52.97 ± 3.0% in the empty vector control group (Fig. 2e), indicating an engraftment advantage of gene-corrected Fanca−/−/FANCA cells over non-corrected ones. Importantly, we found that the gene-corrected Fanca−/−/FANCA stem cells were as effective as WT HSCs in engrafting in CASIN-conditioned Fanca−/− recipients (Fig. 2f). Furthermore, these gene-corrected Fanca−/−/FANCA HSCs exhibited much higher donor engraftment in CASIN-conditioned Fanca−/− recipients than in vehicle-treated group (Fig. 2f). These results indicate that CASIN treatment is useful in facilitating the engraftment of gene-corrected autologous HSCs of FA, similar to that of WT HSCs in allogeneic setting of HSCT.
Efficient delivery of HSCs to BM niche with minimum toxicity has been the goal of clinical HSCT as its first use in treating patients [1, 2, 5]. Appropriate preparative regimen is critical for a successful HSCT, and in the setting of non-malignant disease where eradication of malignant cells is not needed, sustained engraftment of donor cells with minimum toxicity is a goal. Conventional myeloablative regimens achieve optimal engraftment by non-specifically destroying recipient HSCs as well as other BM components, and cause significant acute and chronic toxicity, and associated morbidity and mortality [3]. In addition, the related tissue damage caused by myeloablative regimens can contribute to the pro-inflammatory environment that may facilitate graft versus host disease and hinder engraftment in allo-HSCT [3, 5]. In the present study, we found that pharmacological inhibition of Cdc42 by CASIN could transiently phenocopy Cdc42 gene deletion in host mice to facilitate donor HSC engraftment without myeloablative conditioning. Our studies offer a novel conditioning regimen for HSCT by pharmacological targeting Cdc42 to transiently open up the recipient HSC BM niche, thus allowing potential applications for human stem cell and FA patient HSCT [1, 15] with reduced exposure to cytotoxic chemotherapy.
Supplementary Material
Acknowledgements
We thank Dr. Madeleine Carreau (Laval University) for the Fanca+/− mice, the Vector Core of the Cincinnati Children’s Research Foundation (Cincinnati Children’s Hospital Medical Center) for the preparation of retroviruses, and the Comprehensive Mouse and Cancer Core of the Cincinnati Children’s Research Foundation (Cincinnati Children’s Hospital Medical Center) for bone marrow transplantation service. This work was supported by NIH grants R01 CA193350, R01 DK104814, R01 CA150547, R01 HL076712, and R01 HD089932. Q.P. and J.M. were Leukemia and Lymphoma Scholar. W.D. was supported by the NIH T32 HL091805 training grant. The studies were further supported by a NIH center grant P30 DK090971.
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1038/s41375-018-0200-3) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Stanevsky A, Goldstein G, Nagler A. Umbilical cord blood transplantation: pros, cons and beyond. Blood. 2009;23:199–204. [DOI] [PubMed] [Google Scholar]
- 2.Pusic I, Jiang SY, Landua S, Uy GL, Rettig MP, Cashen AF, et al. Impact of mobilization and remobilization strategies on achieving sufficient stem cell yields for autologous transplantation. Biol Blood Marrow Transplant. 2008;14:1045–56. [DOI] [PubMed] [Google Scholar]
- 3.Stewart FM, Zhong S, Wuu J, Hsieh C, Nilsson SK, Quesenberry PJ. Lymphohematopoietic engraftment in minimally myeloablated hosts. Blood. 1998;91:3681–7. [PubMed] [Google Scholar]
- 4.Czechowicz A, Kraft D, Weissman IL, Bhattacharya D. Efficient transplantation via antibody-based clearance of hematopoietic stem cell niches. Science. 2007;318:1296–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quesenberry PJ, Stewart FM, Becker P, D’Hondt L, Frimberger A, Lambert JF, et al. Stem cell engraftment strategies. Ann N Y Acad Sci. 2001;938:54–61. [DOI] [PubMed] [Google Scholar]
- 6.Xue X, Pech NK, Shelley WC, Srour EF, Yoder MC, Dinauer MC. Antibody targeting KIT as pretransplantation conditioning in immunocompetent mice. Blood. 2010;116:5419–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chandrakasan S, Jayavaradhan R, Ernst J, Shrestha A, Loberg A, Dexheimer P, et al. KIT blockade is sufficient for donor hematopoietic stem cell engraftment in Fanconi anemia mice. Blood. 2017;129:1048–52. [DOI] [PubMed] [Google Scholar]
- 8.Wang Z, Li G, Tse W, Bunting KD. Conditional deletion of STAT5 in adult mouse hematopoietic stem cells causes loss of quiescence and permits efficient nonablative stem cell replacement. Blood. 2009;113:4856–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao LQ, Liu L, Xu LP, Zhang XH, Wang Y, Fan QZ, et al. Correlation between pediatric donor characteristics and cell compositions in mixture allografts of combined G-CSF-mobilized PBSCs and bone marrow allografts. Bone Marrow Transplant. 2018;53:108–10. [DOI] [PubMed] [Google Scholar]
- 10.Dar A, Schajnovitz A, Lapid K, Kalinkovich A, Itkin T, Ludin A, et al. Rapid mobilization of hematopoietic progenitors by AMD3100 and catecholamines is mediated by CXCR4-dependent SDF-1 release from bone marrow stromal cells. Leukemia. 2011;25:1286–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang L, Wang L, Geiger H, Cancelas JA, Mo J, Zheng Y. Rho GTPase Cdc42 coordinates hematopoietic stem cell quiescence and niche interaction in the bone marrow. Proc Natl Acad Sci USA. 2007;104:5091–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu W, Du W, Shang X, Wang L, Evelyn C, Florian MC et al. Rational identification of a Cdc42 inhibitor presents a new regimen for long-term hematopoietic stem cell mobilization. Leukemia. Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thakar MS, Kurre P, Storb R, Kletzel M, Frangoul H, Pulsipher MA, et al. Treatment of Fanconi anemia patients using fludarabine and low-dose TBI, followed by unrelated donor hematopoietic cell transplantation. Bone Marrow Transplant. 2011;46:539–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Notta F, Doulatov S, Laurenti E, Poeppl A, Jurisica I, Dick JE. Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science. 2011;333:218–21. [DOI] [PubMed] [Google Scholar]
- 15.Adair JE, Becker PS, Chandrasekaran D, Choi G, Woolfrey AE, Burroughs L, et al. Gene therapy for fanconi anemia in Seattle: clinical experience and next steps. Blood. 2016;128:3510. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


