Abstract
Purpose
To identify baseline characteristics and long-term prognostic factors in non-transplant patients with unresectable hepatocellular carcinoma (HCC) who had prolonged survival after treatment with yttrium-90 radioembolization (Y90).
Materials and Methods
67 "Super Survivors" (defined as ≥3 years survival after Y90) were identified within our 1,000-patient Y90 database (2003–2017). Baseline imaging and follow-up occurred at 1 months and every 3 months thereafter. Overall survival (OS) was calculated with Kaplan-Meier estimates with log-rank test in sub-groups: Child-Pugh (CP) score, distribution of disease, portal vein thrombus (PVT), and technique (segmental vs lobar Y90).
Results
Median age 69.5 years (range: 45–94 years); 69% male; 60% solitary HCC; 79% unilobar disease; 12% PVT; 10% ascites; Barcelona Clinic Liver Cancer Stage A-54%/B-28%/C-16%/D-2%; CP A-70%/B-28%/C-2%. Longest baseline tumor diameter was 5.4 ± 4.0cm (mean ± SD). All patients had an imaging response (either partial or complete response). Median OS was 67.5 months (95% confidence interval; 55.2–82.5). CP score and main PVT stratified median OS (p=0.0007 and p=0.0187, respectively). Beyond 3 years, segmental vs lobar Y90 was associated with improved OS with a median OS of 80.2 vs 46.7 months, respectively (p=0.0024). Dosing >200Gy was not a significant predictor of improved OS.
Conclusions
Super survivors spanning the BCLC Staging System maintained durable OS after radioembolization that was stratified by the extent of underlying liver disease. The common variable among all patients was an imaging response. Segmental vs lobar Y90 may have a long-term associated OS benefit.
Keywords: radioembolization, hepatocellular carcinoma, yttrium-90, survival
INTRODUCTION
Yttrium-90 radioembolization (Y90) is a minimally invasive outpatient procedure offered for unresectable hepatocellular carcinoma (HCC). While once reserved only for the salvage setting in patients with multifocal disease and/or portal vein tumor thrombus, radioembolization is now applied across the spectrum of early to advanced HCC Barcelona Clinic Liver Cancer Stages [1–4]. Most patients with HCC are never eligible for curative surgery like hepatic resection or transplantation owing to the extent of disease at presentation, presence of portal hypertension, and the limited availability of transplant organs. In BCLC Stage A patients, chemoembolization and radioembolization have demonstrated similar median survivals of 54.2 months and 53.4 months, respectively [5,6]. In this retrospective study, we aimed to identify factors associated with prolonged survival after Y90 in the absence of surgical resection or transplantation. Among 1,000 patients treated with Y90, a subgroup of non-surgical HCC patients who had atypically long survival of three or more years after intra-arterial therapy with Y90 were defined as “Super Survivors” [4]. Our hypothesis based on the existing Y90 evidence in Barcelona Clinic Liver Cancer (BCLC) Stage A patients was that Super Survivors would be young, with excellent Eastern Cooperative Oncology Group (ECOG) performance status (0–1) and have primarily preserved liver function at baseline (Child-Pugh Class A), solitary, non-invasive disease, and the majority would often go on to eventually receive ablation per the American Association for the Study of Liver Diseases (AASLD) guidelines for BCLC Stage A disease [7].
METHODS
The study was a retrospective observational cohort study under our open-label protocol. We queried the Interventional Oncology Registry for all patients who underwent radioembolization (NCT00532740) and complied with the Health Insurance Portability and Accountability Act. Of 1000 HCC patients treated with Y90 between December 1, 2003 and March 31, 2017, 67 patients met the inclusion criteria as Super Survivors. Each patient was reviewed and discussed by a multidisciplinary tumor board including hepatology, oncology, interventional radiology, and transplant surgery.
Study eligibility
Inclusion criteria included (i) image- or biopsy-proven HCC according to AASLD guidelines [7]; (ii) treatment with Y90, as allocated by a multidisciplinary team; (iii) survival of 3 or more years. Exclusion criterion was (i) surgery including resection or transplantation at any time during 3-year follow up.
Evaluation and Staging
Patient demographics, risk factors, etiology of liver disease, ECOG performance status, BCLC staging, albumin-bilirubin score and Child-Pugh class were recorded.
Y90 Treatments
Visceral angiography and technetium-99m scintigraphy were used to estimate lung shunting, identify extrahepatic perfusion and perform coil embolization if necessary. Included data and the definition for technical success follow previously published reporting standards for radioembolization [8]. Glass microspheres (TheraSphere®, BTG International) were used in each case with treatment on an outpatient basis. Infusions were completed at the segmental or lobar level with adjustments as previously described for radiation segmentectomy and extended-shelf-life (ESL) protocols [9–12].
Outcome Variables
Baseline Characteristics. Baseline characteristics included patient demographics, underlying etiology and extent of liver disease, presence of vascular invasion, liver function, performance status, tumor size and distribution, and cancer staging per BCLC.
Treatment. The treatment characteristics included type of treatment (segmental or lobar), dose, vial size (GBq), embolic load defined as vial size on calibration (40k–70k microspheres per GBq) per treatment mass (GBq/kg), lung shunt fraction, and lung dose.
Laboratory toxicity. Patients received 1 month follow-up in clinic with measurements of serum albumin, bilirubin, alkaline phosphatase, alanine and aspartate transaminase to assess for biochemical toxicities. Lymphopenia and elevated creatinine after treatment were also recorded.
Response rate. Contrast-enhanced MRI or triphasic CT was used to determine World Health Organization (WHO) and European Association for the Study of the Liver (EASL) assessments [13–15]. Reported outcomes include time to response (either partial response or complete response), response at 6–9 months and response at 12 months applying the primary index lesion methodology.
Time-to-Progression. Time-to-progression (TTP) from the date of treatment was calculated using the Kaplan-Meier (KM) method with progression defined as increased size by WHO, increased enhancement by EASL, new lesions meeting HCC criteria adjudicated to the date of first detection, or extrahepatic metastases.
Overall Survival. Survival was identified by the Social Security Death Index with analyses were calculated from the day of first radioembolization to death (in months) by the KM method.
Interval Treatment. The median time to subsequent treatment, the number of treatments, and the conversion to other liver-directed therapies (denoted by “Y90->X” for Y90 followed by X) were recorded for each patient. The maximum and minimum treatment-free-interval was also calculated for each patient and defined as the longest and shortest period between any two treatments, respectively.
Statistics
Medians are reported with 95% confidence intervals (CI) and means ± standard deviation. Overall survival and TTP outcomes were estimated using the KM method and sub-groups were compared with the log-rank test. The hazard ratio and 95% CI was estimated using Cox proportional hazards regression. All analyses were performed using STATA v14.0 (StataCorp, College Station, Tx); p<0.05 was considered significant.
RESULTS
Baseline Characteristics
The baseline characteristics for the cohort are shown in Table 1. Solitary lesions were present in 59.7% of patients. Previous liver-directed therapy was received in 13.4% (n=9) of patients. Thirteen patients (19.4%) were referred for treatment from outside hospitals.
Table 1.
Baseline Characteristics
| Characteristic | No. Patients | % |
|---|---|---|
| Demographics | ||
| Age (years)* | 48 | (65, 71) |
| < 65 y | 19 | 28.4 |
| ≥ 65 y | 48 | 71.6 |
| Gender | ||
| Male | 46 | 68.7 |
| Female | 21 | 31.3 |
| Ethnicity | ||
| Caucasian | 48 | 71.6 |
| African American | 6 | 9.0 |
| Hispanic | 5 | 7.5 |
| Asian | 7 | 10.4 |
| Declined | 1 | 1.5 |
| Risk Factors | ||
| Etiology | ||
| Alcohol | 14 | 20.9 |
| HCV | 21 | 31.3 |
| HCV + Alcohol | 3 | 4.5 |
| Hemachromatosis | 1 | 1.5 |
| Hemachromatosis + Alcohol | 1 | 1.5 |
| HBV | 5 | 7.5 |
| NASH | 7 | 10.4 |
| NASH + Alcohol | 1 | 1.5 |
| Autoimmune Hepatitis | 1 | 1.5 |
| Acute Intermittent Porphyria | 1 | 1.5 |
| Acetaminophen | 1 | 1.5 |
| Cryptogenic | 5 | 7.5 |
| Unknown | 6 | 9.0 |
| Cirrhosis | ||
| Present | 52 | 77.6 |
| Absent | 15 | 22.4 |
| Distribution | ||
| Unilobar | 53 | 79.1 |
| Bilobar | 14 | 20.9 |
| No. of lesions | ||
| Solitary | 40 | 59.7 |
| Multifocal | 27 | 40.3 |
| Largest Tumor Size (cm) | ||
| Median (IQR) | 4.0 | 4.9 |
| Mean (95% CI) | 5.4 | (4.4, 6.4) |
| AFP (ng/mL) | ||
| <200 | 55 | 82.1 |
| ≥ 200 | 12 | 17.9 |
| Previous Therapies | ||
| None | 58 | 86.6 |
| Resection | 5 | 7.5 |
| RF ablation | 1 | 1.5 |
| Percutaneous Ethanol Injection and TACE | 1 | 1.5 |
| TACE | 1 | 1.5 |
| Drug-eluting Beads | 1 | 1.5 |
| Previous Cancer History | ||
| None | 45 | 67.2 |
| One | 20 | 29.9 |
| Two or more | 2 | 3.0 |
| ECOG Performance Status | ||
| 0 | 46 | 68.7 |
| 1 | 20 | 29.9 |
| 2 | 1 | 1.5 |
| Liver Function | ||
| Bilirubin, total serum (mg/dL)* | 0.9 | (1.0, 1.4) |
| Albumin (g/dL)* | 3.3 | (3.1, 3.4) |
| Ascites | ||
| Present | 7 | 10.4 |
| Absent | 60 | 89.6 |
| Portal Vein Thrombosis | ||
| Main | 3 | 4.5 |
| Branch | 5 | 7.5 |
| Absent | 59 | 88.1 |
| Staging | ||
| BCLC | ||
| A | 36 | 53.7 |
| B | 19 | 28.4 |
| C | 11 | 16.4 |
| D | 1 | 1.5 |
| Child-Pugh | ||
| A | 47 | 70.1 |
| B7 | 11 | 16.4 |
| B8 | 5 | 7.5 |
| B9 | 3 | 4.5 |
| C | 1 | 1.5 |
| Method of Diagnosis | ||
| Biopsy | 37 | 55.2 |
| Imaging | 30 | 44.8 |
Note. –
Values expressed as median and 95% confidence intervals.
BCLC, Barcelona Clinic Liver Cancer; ECOG, Eastern Cooperative Oncology Group; HBV, hepatitis B virus; HCV, hepatitis C virus; NASH, non-alcoholic steatohepatitis; RFA, radiofrequency ablation; TACE, transarterial chemoembolization.
Treatment and Dosimetry
All 67 patients were treated successfully. Segmental Y90 was performed in 45/67 (67%) patients; 22 (33%) were lobar treatments. The average number of Y90 treatments was 2.52±1.69 over the course of follow up. The mean dose was 116Gy for lobar and 206Gy for segmental radioembolization (Supplemental Table 1 online only). The extended-shelf life protocol was applied in 28/50 (56%) patients. The median embolic load (GBq/kg liver) was significantly lower at 10.2 (95% CI, 7.9–12.4) in non-extended shelf-life infusions versus 32.3 (95% CI, 25.3–39.2) for extended-shelf-life infusions.
Laboratory Toxicities
Supplemental Table 2 (online only) summarizes laboratory toxicities. Grade 3+ toxicities were elevated AST 2/67 (3%), hypoalbuminemia 1/67 (1%), and elevated bilirubin 4/67 (6%).
Imaging Outcomes
Supplemental Table 3 (online only) presents imaging response. Follow-up imaging was available in 100% of patients (67/67) with 61 of 67 patients (91%) at 6–9 months and 59 of 67 patients (88%) at 12 months. The number of patients achieving WHO response over follow up was 65/67 (97%) and median time to response was 6.1 months (95% CI, 4.7–8.7 months). EASL response was observed in 67/67 patients (100%). Compared to WHO criteria, the median time to PR/CR was significantly earlier for EASL criteria at 2.6 months (95% CI, 1.2–3.4). Dosing of 200Gy or more did not significantly shorten EASL response (1.2 vs 3.0 months, p=0.1903) or WHO response (5.9 months vs 6.2 months, p=0.9081). Representative cases are showing in Supplemental Figures S1 and S2 (online only).
Time-to-progression
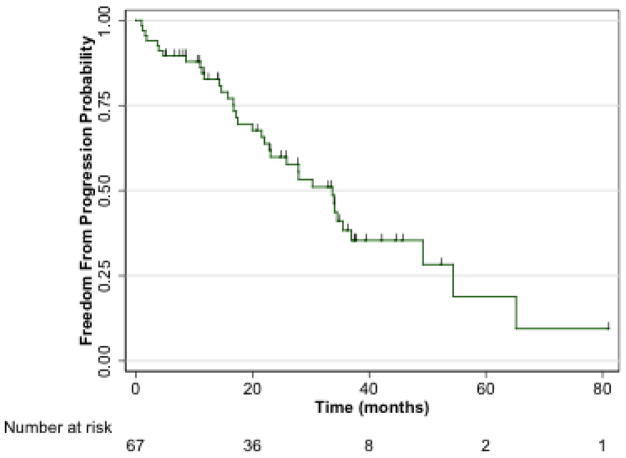
Figure 1 shows TTP. The rate of progression was 36/67 patients (53.7%) over the course of follow up and the median TTP was 33.7 months (95% CI, 22.0–36.9). Primary index lesion progression occurred in 14/67 (20.9%) and 13/67 (19.4%) by WHO and EASL criteria, respectively. Nine patients (13.4%) developed new lesions.
Fig 1.
The median TTP was 33.7 months (95% CI, 22.0–36.9).
Overall Survival
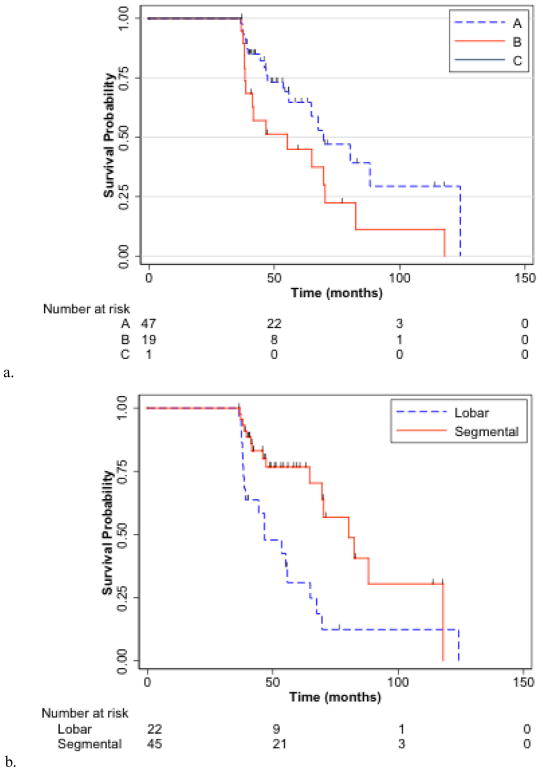
Figures 2a and b present KM OS curves. Median OS was 67.5 months (95% CI, 55.2–82.5). CP score stratified median OS at 88.1, 69.6, 69.6, 38.1, and 38.6 months for CP scores of 5, 6, 7, 8, and 9, respectively (p=0.0007). Unilobar vs bilobar disease and solitary vs multifocal disease did not stratify OS (p=0.1608, p=0.0917, respectively). The presence of main vs branch vs no PVT was a significant predictor of OS (p=0.0187). Segmental vs lobar radioembolization showed improved OS with median of 80.2 vs 46.7 months, respectively (p=0.0024). Table 2 demonstrates univariate and multivariate analyses. In patients with solitary HCC and no PVT (n=33) dosing of 200Gy or more resulted in a median OS of 82.5 months versus 64.7 months for <200Gy (p=0.0891).
Fig 2.
Overall survival from Y90 (a) by Child-Pugh Score and (b) by lobar or segmental Y90.
Table 2.
| Characteristic | Univariate | Multivariate |
|---|---|---|
|
| ||
| Segmental vs Lobar | 0.357 (95% CI, 0.178–0.714; p=0.004) | 0.365 (95% CI, 0.170–0.787; p=0.010) |
| Increased Age | 0.977 (95% CI, 0.939–1.017; p=0.258) | 0.977 (95% CI, 0.933–1.023; p=0.313) |
| Multifocal vs Solitary | 1.818 (95% CI, 0.898–3.680; p=0.097) | 1.561 (95% CI, 0.673–3.619; p=0.300) |
| Bilobar vs Unilobar | 1.785 (95% CI, 0.785–4.061; p-0.167) | 0.833 (95% CI, 0.292–2.381; p=0.734) |
Note. – Values expressed as Hazard Ratios and 95% Confidence Interval.
Interval Treatment
During follow up, 56.7% (n=38) of Super Survivors were treated with Y90 and no other modality (isolated Y90), 19.4% (n=13) later underwent RFA, and 14.9% (n=10) later received systemic therapy with sorafenib, 4.5% (n=3) later received TACE, 3% (n=2) later received bland embolization, and one patient received sorafenib in combination with Y90. The average number of Y90 treatments was 2.11±1.37 for isolated Y90 (no alternate therapy), 3.69±2.14 for Y90 followed by RFA, 2.8±1.75 for Y90 followed by sorafenib, 2.67±2.08 for Y90 followed by TACE, and 1.5±0.71 for Y90 followed by bland embolization. The maximum and minimum treatment-free-intervals for each group of patients are shown in Table 3. The longest minimum treatment free interval was observed in patients treated with isolated Y90 with a median 35.6 months (95% CI, 23.8–39.4). For patients crossing over to other treatments including RFA, TACE, bland embolization, and sorafenib, median minimum treatment free intervals were 6.3 (95% CI, 3.2–15.9), 3.4 (95% CI, −4.1–11.4), 19.8 (95% CI, −97.1–136.7), and 2.1 months (95% CI, 0.7–6.4), respectively.
Table 3.
Treatment-free-intervals.
| Characteristic | Minimum Treatment-Free Interval | Maximum Treatment-Free Interval | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| No Patients | Median | Mean | 95% CI | Median | Mean | 95% CI | |
| Isolated Y90 | 38 | 35.63 | 31.60 | 23.8–39.4 | 36.82 | 37.92 | 31.3–44.6 |
| Y90−> RFA | 13 | 6.27 | 9.55 | 3.2–15.9 | 20.00 | 24.69 | 17.4–32.0 |
| Y90−> Sorafenib | 10 | 2.05 | 3.54 | 0.7–6.4 | 13.60 | 15.91 | 6.7–25.1 |
| Y90−> TACE | 3 | 3.40 | 3.68 | −4.1–11.4 | 7.73 | 7.97 | −3.7–19.6 |
| Y90−> bland embo | 2 | 19.80 | −97.1–136.7 | 23.55 | −45.7–92.8 | ||
| Y90+Sorafenib | 1 | 2.97 | 2.97 | ||||
Note. – Values expressed as months. CI, confidence interval.
DISCUSSION
Radioembolization is now tailored to the HCC patient’s unique disease state allowing treatment from early to advanced BCLC Stages and has tolerable safety in the setting of hepatic dysfunction [4,16]. These features are reflected in the present study as Super Survivors included the full BCLC A through D and 12% had PVT. While we expected strong survival outcomes in patients with small solitary HCC [6], 40% of Super Survivors had multifocal disease. Comparisons to historical controls are shown in Table 4 with Super Survivors having more favorable baseline characteristics, higher imaging response rates, and decreased toxicity overall.
Table 4.
Comparison to Historical Controls
| Table 4. | ||||
|---|---|---|---|---|
| Super Survivors | Control Data | |||
| Variable | Value (%) | Comparison | Value (%) | Reference |
| Age ≥ 65 y | 72% (48/67) | ↑ | 50% (504/1000) | [4] |
| Cirrhosis | 78% (52/67) | ↓ | 82% (816/1000) | [4] |
| HCV | 31% (21/67) | ↓ | 46% (461/1000) | [4] |
| Alcohol | 21% (14/67) | ↑ | 14% (138/1000) | [4] |
| NASH | 10% (7/67) | ↑ | 6% (60/1000) | [4] |
| Unilobar | 79% (53/67) | ↑ | 64% (639/1000) | [4] |
| Solitary | 60% (40/67) | ↑ | 43% (427/1000) | [4] |
| PVT | 12% (8/67) | ↓ | 27% (270/1000) | [4] |
| Ascites | 10% (7/67) | ↓ | 24% (242/1000) | [4] |
| ALBI | ||||
| 1 | 9% (6/67) | ↑ | 7% (71/1000) | [4] |
| 2 | 75% (50/67) | ↑ | 64% (637/1000) | [4] |
| 3 | 16% (11/67) | ↓ | 29% (292/1000) | [4] |
| Child-Pugh | ||||
| A | 70% (47/67) | ↑ | 51% (506/1000) | [4] |
| B | 28% (19/67) | ↓ | 45% (450/1000) | [4] |
| C | 2% (1/67) | ↓ | 4% (44/1000) | [4] |
| Lab Toxicity Grade 3/4 | ||||
| ↓Albumin | 1% (1/67) | ↓ | 5% (49/966) | [4] |
| ↑Serum Bilirubin | 6% (4/67) | ↓ | 11% (110/966) | [4] |
| Imaging Response | ||||
| WHO | ||||
| 6–9 months | 69% (42/61) | ↑ | 41% (48/116) | [17] |
| 12 months | 74% (44/59) | ↑ | 53% (43/81) | [17] |
| EASL | ||||
| 6–9 months | 83% (51/61) | ↑ | 74% (86/116) | [17] |
| 12 months | 78% (46/59) | ↑ | 67% (54/81) | [17] |
Note. – Values expressed as percent.
BCLC, Barcelona Clinic Liver Cancer; EASL, European Association for the Study of the Liver; ECOG, Eastern Cooperative Oncology Group; HCV, hepatitis C virus; NASH, non-alcoholic steatohepatitis; PVT, portal vein thrombosis; WHO, World Health Organization.
The appropriate clinical endpoint for Y90 in the treatment of HCC for improved OS is an imaging response rather than freedom from progression [17]. This is further emphasized in the present study as ALL Super Survivors had an imaging response (either WHO or EASL) in primary index lesions over the course of follow up. The average number of Y90 treatments was 2.5 corresponding to <0.5 treatments per year of post-treatment survival. While multiple treatments may be required to achieve response, half of patients do not receive more than one Y90 treatment and multiple factors likely contribute: therapeutic response, toxicity, preference for alternative treatment, systemic disease progression, and survival [4].
The overall median TTP following Y90 was 33.7 months yet the median OS for the cohort was 67.5 months. By exclusion criteria, these patients did not receive surgical intervention. Therefore, we theorize this extended survival following progression is due to the successful application of subsequent liver-directed therapies. This is further supported by the minimum treatment-free-interval of 35.6 months for isolated Y90 that mirrors the median TTP of 33.7 months. The application of segmental treatments significantly improved long-term OS in our cohort supporting the concept of radiation segmentectomy for improved preservation of liver parenchyma, especially in the absence of transplantation [11]. Dosing >200Gy was not associated with improved OS or earlier response consistent with the concept of threshold dose [11,18,19]. Y90 dosing has not been significantly linked to imaging response, survival, or toxicity [16,20,21]. Interestingly, Ahmed et al. have suggested use of imaging surrogates based on the targeted hepatic parenchyma within the treated angiosome which may be an area of future interest for monitoring radiation segmentectomy [22].
At baseline, 34/67 (50.7%) patients in our study were outside Milan criteria. Tumors were >5cm on average and 33% of patients had a previous history of cancer other than HCC. In a prospective randomized controlled trial, patients listed for transplantation who were randomized to radioembolization received liver transplantation at a rate of 87% and radioembolization offers comparable post-transplantation outcomes compared to chemoembolization [23,24]. However, an increasing number of patients are receiving radioembolization as destination therapy and, while many centers offer curative surgery, there is an increasing role of radioembolization in the context of reduced access to surgery (i.e., resource-limited environments that cannot offer transplantation), reduced imminent need for surgery (i.e. curative hepatitis C treatments), longer transplant wait times for HCC patients (6 months before MELD 28 with some recommendations this be extended to 9 months) [25–27].
Limitations
Our definition of Super Survivors introduces guaranteed time bias. Given the limited survival of HCC patients, such patients are rare in the absence of curative surgery so an adequate sample of untreated patients for comparison was not available. Since crossover to alternate therapies is a possible confounder, we describe these therapies in detail, including their interval timing. The TTP data was in-line with our previous experience but it should not serve as an estimate for TTP following Y90 in all-comers since patients whose progression resulted in death prior to 3-years were excluded in our cohort by definition. Finally, our study was retrospective in nature with the usual associated limitations.
Conclusions
Long-term survival after intra-arterial therapy with radioembolization is possible in select patients. Super survivors who lived 3-years after radioembolization spanned the BCLC Staging System and had advanced age at the time of treatment yet maintained durable OS after radioembolization that was stratified by the extent of underlying liver disease at baseline. All patients achieved an imaging response. Segmental technique was associated with significantly prolonged survival suggesting a long-term benefit to this liver-sparing approach in patients who do not have surgical intervention.
Supplementary Material
Table S1. Y90 Dosimetry. (95% CI)
Table S2. Laboratory Toxicity.
Table S3. Imaging Response in Primary Index Lesions.
Fig S1: a) 75 year-old woman with Child-Pugh A cirrhosis secondary to acetaminophen toxicity and a 14cm biopsy-proven HCC (arrow) with adjacent satellite lesion (interrupted arrow) who was treated with 110Gy to the right hepatic lobe. b) 3 months imaging demonstrated reduced size and enhancement of the treated dominant right lobe lesion with interval progression of the untreated satellite tumor. c) Treatment with Y90 to this second lesion was accomplished with partial response by EASL criteria. d) Two new lesions were demonstrated at 1.5 years both treated with radioembolization with excellent response. Follow-up imaging at e) 3 years and f) 6 years with maintained treatment response.
Fig S2a: Case 2 a) 79 year-man with Child-Pugh A cirrhosis secondary to alcohol with a 4cm HCC per imaging criteria treated with a single Y90 treatment at 120Gy. Follow-up imaging demonstrated radiation change and left lobar atrophy encompassing the targeted lesion with late development of a new HCC in the untreated right hepatic lobe.
Fig S2b: Case 3 a) 71 year-man with Child-Pugh A cirrhosis secondary to alcohol with a massive 17.8cm biopsy-proven HCC treated with radioembolization a total of six times with response by EASL and WHO criteria. The patient ultimately developed bone metastases and was started on sorafenib 2 years after his first Y90 treatment.
Acknowledgments
Role of Funding: ACG is a Medical Scientist Training Program student (T32GM008152).
The authors thank Carlene del Castillo, Karen Grace, Krystina Salzig, and Melissa Williams for their commitment to patient care and dedication to clinical research.
ABBREVIATIONS
- AASLD
American Association for the Study of Liver Diseases
- ALBI
Albumin-bilirubin
- ALT
ALanine Transaminase
- AST
ASpartate Transaminase
- BCLC
Barcelona Clinic Liver Cancer
- CI
Confidence Intervals
- CR
Complete Response
- ECOG
Eastern Cooperative Oncology Group
- EASL
European Association for the Study of the Liver
- ESL
Extended-Shelf-Life
- HBV
Hepatitis B Virus
- HCC
Hepatocellular Carcinoma
- HCV
Hepatitis C Virus
- KM
Kaplan-Meier
- NASH
Non-Alcoholic SteatoHepatitis
- OS
Overall Survival
- PD
Progressive Disease
- PVT
Portal Vein Thrombosis
- PR
Partial Response
- RFA
RadioFrequency Ablation
- SD
Stable Disease
- TACE
TransArterial ChemoEmbolization
- TTP
Time-To-Progression
- WHO
World Health Organizing
- Y90
Yttrium-90 radioembolization
Footnotes
Conflict of Interest Statement: RJL, RS serve as advisors to BTG. None of the other authors report a conflict of interest.
Ethical Approval Statement: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent Statement: Informed consent was obtained from all individual participants included in the study.
References
- 1.Mazzaferro V, Sposito C, Bhoori S, Romito R, Chiesa C, Morosi C, et al. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: A phase 2 study. Hepatology. 2012;57:1826–37. doi: 10.1002/hep.26014. [DOI] [PubMed] [Google Scholar]
- 2.Kulik LM, Carr BI, Mulcahy MF, Lewandowski RJ, Atassi B, Ryu RK, et al. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology. 2008;47:71–81. doi: 10.1002/hep.21980. [DOI] [PubMed] [Google Scholar]
- 3.Sangro B, Carpanese L, Cianni R, Golfieri R, Gasparini D, Ezziddin S, et al. Survival After Yttrium-90 Resin Microsphere Radioembolization of Hepatocellular Carcinoma Across Barcelona Clinic Liver Cancer Stages: A European Evaluation. Hepatology. 2011;54:868–78. doi: 10.1002/hep.24451. [DOI] [PubMed] [Google Scholar]
- 4.Salem R, Gabr A, Riaz A, Mora R, Ali R, Abecassis M, et al. Institutional Decision to Adopt Y90 as Primary Treatment for HCC Informed by a 1,000-patient 15-year Experience. Hepatology. 2017 doi: 10.1002/hep.29691. [DOI] [PubMed] [Google Scholar]
- 5.Burrel M, Reig M, Forner A, Barrufet M, de Lope CR, Tremosini S, et al. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using Drug Eluting Beads. Implications for clinical practice and trial design. J Hepatol. 2012;56:1330–5. doi: 10.1016/j.jhep.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Vouche M, Habib A, Ward TJ, Kim E, Kulik L, Ganger D, et al. Unresectable solitary hepatocellular carcinoma not amenable to radiofrequency ablation: multicenter radiology-pathology correlation and survival of radiation segmentectomy. Hepatology. 2014;60:192–201. doi: 10.1002/hep.27057. [DOI] [PubMed] [Google Scholar]
- 7.Bruix J, Sherman M American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011:1020–2. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salem R, Lewandowski RJ, Gates VL, Nutting CW, Murthy R, Rose SC, et al. Research reporting standards for radioembolization of hepatic malignancies. J Vasc Interv Radiol. 2011:265–78. doi: 10.1016/j.jvir.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salem R, Lewandowski RJ, Sato KT, Atassi B, Ryu RK, Ibrahim S, et al. Technical aspects of radioembolization with 90Y microspheres. Tech Vasc Interv Radiol. 2007;10:12–29. doi: 10.1053/j.tvir.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Lewandowski RJ, Sato KT, Atassi B, Ryu RK, Nemcek AA, Jr, Kulik L, et al. Radioembolization with 90Y Microspheres: Angiographic and Technical Considerations. Cardiovasc Inter Rad Springer-Verlag. 2007;30:571–92. doi: 10.1007/s00270-007-9064-z. [DOI] [PubMed] [Google Scholar]
- 11.Riaz A, Gates VL, Atassi B, Lewandowski RJ, Mulcahy MF, Ryu RK, et al. Radiation segmentectomy: a novel approach to increase safety and efficacy of radioembolization. Int J Radiat Oncol Biol Phys. 2011;79:163–71. doi: 10.1016/j.ijrobp.2009.10.062. [DOI] [PubMed] [Google Scholar]
- 12.Lewandowski RJ, Minocha J, Memon K, Riaz A. Sustained safety and efficacy of extended-shelf-life 90Y glass microspheres: long-term follow-up in a 134-patient cohort. Eur J Nucl Med Mol Imaging. 2014 doi: 10.1007/s00259-013-2575-8. [DOI] [PubMed] [Google Scholar]
- 13.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid Tumors. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 14.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–30. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 15.Riaz A, Miller FH, Kulik LM, Nikolaidis P, Yaghmai V, Lewandowski RJ, et al. Imaging response in the primary index lesion and clinical outcomes following transarterial locoregional therapy for hepatocellular carcinoma. JAMA. 2010;303:1062–9. doi: 10.1001/jama.2010.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biederman DM, Posham R, Durrani RJ, Titano JJ, Patel RS, Tabori NE, et al. Outcomes of radioembolization for unresectable hepatocellular carcinoma in patients with marginal functional hepatic reserve. Clin Imaging. 2018;47:34–40. doi: 10.1016/j.clinimag.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 17.Riaz A, Gabr A, Abouchaleh N, Ali R, Asadi Al A, Mora R, et al. Radioembolization for hepatocellular carcinoma: Statistical confirmation of improved survival in responders by landmark analyses. Hepatology. 2018;27:1485. doi: 10.1002/hep.29480. [DOI] [PubMed] [Google Scholar]
- 18.Garin E, Lenoir L, Edeline J, Laffont S, Mesbah H, Poree P, et al. Boosted selective internal radiation therapy with 90Y-loaded glass microspheres (B-SIRT) for hepatocellular carcinoma patients: a new personalized promising concept. Eur J Nucl Med Mol Imaging. 2013;40:1057–68. doi: 10.1007/s00259-013-2395-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garin E, Rolland Y, Laffont S, Edeline J. Clinical impact of (99m)Tc-MAA SPECT/CT-based dosimetry in the radioembolization of liver malignancies with (90)Y-loaded microspheres. Eur J Nucl Med Mol Imaging. 2016;43:559–75. doi: 10.1007/s00259-015-3157-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srinivas SM, Natarajan N, Kuroiwa J, Gallagher S, Nasr E, Shah SN, et al. Determination of Radiation Absorbed Dose to Primary Liver Tumors and Normal Liver Tissue Using Post-Radioembolization (90)Y PET. Front Oncol. 2014;4:1–12. doi: 10.3389/fonc.2014.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goin JE, Salem R, Carr BI, Dancey JE, Soulen MC, Geschwind J, et al. Treatment of unresectable hepatocellular carcinoma with intrahepatic yttrium 90 microspheres: Factors associated with liver toxicities. JVIR. 2005;16:205–13. doi: 10.1097/01.rvi.00001142592.89564.f9. [DOI] [PubMed] [Google Scholar]
- 22.Ahmed AF, Samreen N, Grajo JR, Zendejas I, Sistrom CL, Collinsworth A, et al. Angiosomal radiopathologic analysis of transarterial radioembolization for the treatment of hepatocellular carcinoma. Abdom Radiol (NY) 2017 doi: 10.1007/s00261-017-1354-6. [DOI] [PubMed] [Google Scholar]
- 23.Salem R, Gordon AC, Mouli S, Hickey R, Kallini J, Gabr A, et al. Y90 Radioembolization Significantly Prolongs Time to Progression Compared With Chemoembolization in Patients With Hepatocellular Carcinoma. Gastroenterology. 2016;151:1155–1163. e2. doi: 10.1053/j.gastro.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gabr A, Abouchaleh N, Ali R, Vouche M, Atassi R, Memon K, et al. Comparative study of post-transplant outcomes in hepatocellular carcinoma patients treated with chemoembolization or radioembolization. Eur J Radiol. 2017;93:100–6. doi: 10.1016/j.ejrad.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 25.UNOS OPTN MAP. Changes to HCC Criteria for Auto Approval. 2016. pp. 1–21. [Google Scholar]
- 26.Massie AB, Caffo B, Gentry SE, Hall EC, Axelrod DA, Lentine KL, et al. MELD Exceptions and Rates of Waiting List Outcomes. Am J Transplant. 2011;11:2362–71. doi: 10.1111/j.1600-6143.2011.03735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heimbach JK, Hirose R, Stock PG, Schladt DP, Xiong H, Liu J, et al. Delayed hepatocellular carcinoma model for end-stage liver disease exception score improves disparity in access to liver transplant in the United States. Hepatology. 2015;61:1643–50. doi: 10.1002/hep.27704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Y90 Dosimetry. (95% CI)
Table S2. Laboratory Toxicity.
Table S3. Imaging Response in Primary Index Lesions.
Fig S1: a) 75 year-old woman with Child-Pugh A cirrhosis secondary to acetaminophen toxicity and a 14cm biopsy-proven HCC (arrow) with adjacent satellite lesion (interrupted arrow) who was treated with 110Gy to the right hepatic lobe. b) 3 months imaging demonstrated reduced size and enhancement of the treated dominant right lobe lesion with interval progression of the untreated satellite tumor. c) Treatment with Y90 to this second lesion was accomplished with partial response by EASL criteria. d) Two new lesions were demonstrated at 1.5 years both treated with radioembolization with excellent response. Follow-up imaging at e) 3 years and f) 6 years with maintained treatment response.
Fig S2a: Case 2 a) 79 year-man with Child-Pugh A cirrhosis secondary to alcohol with a 4cm HCC per imaging criteria treated with a single Y90 treatment at 120Gy. Follow-up imaging demonstrated radiation change and left lobar atrophy encompassing the targeted lesion with late development of a new HCC in the untreated right hepatic lobe.
Fig S2b: Case 3 a) 71 year-man with Child-Pugh A cirrhosis secondary to alcohol with a massive 17.8cm biopsy-proven HCC treated with radioembolization a total of six times with response by EASL and WHO criteria. The patient ultimately developed bone metastases and was started on sorafenib 2 years after his first Y90 treatment.