Case Report
A 6-year-old, previously healthy Caucasian male was admitted with 10 days of headache, associated with fevers (Tmax 40°C at home) and emesis. Three days prior to admission, he awoke from sleep due to severe headache. His primary care physician (PCP) ordered a non-contrast head Computed Tomography (CT), which revealed mild mucosal thickening of bilateral ethmoid sinuses without intracranial abnormalities. The patient received a dose of intramuscular ceftriaxone and was prescribed a course of per os (PO) cefprozil for treatment of presumed bacterial sinusitis. However, his symptoms worsened and he was referred to the Emergency Department (ED) for further evaluation.
In the ED, he had a fever of 39°C, but other vital signs were normal and physical examination was unremarkable. Laboratory evaluation revealed a normal white blood cell (WBC) count with neutrophilic predominance (WBC 12,690 cells/mm3 [range 4,500-13,500 cells/ mm3] with 72.8% neutrophils), mild hyponatremia (131 mmol/L [range 134-143 mmol/L]), and slightly elevated transaminases (aspartate aminotransferase 84 U/L [range 18-40 U/L], alanine aminotransferase 37 U/L [range 10-30 U/L]). His cerebrospinal fluid (CSF) analysis showed a lymphocytic pleocytosis (286 WBC/uL [range 0-20 WBC/uL] with 75% lymphocytes; 1 RBC/uL), mildly elevated protein (65 mg/dL [range 15-45 mg/dL]), and low glucose level (24 mg/dL [range 40-70mg/dL]). Contrast-enhanced magnetic resonance imaging (MRI) of the brain was normal.
Hospital course
On admission, the patient received empiric treatment with intravenous (IV) doxycycline for possible tick-borne illnesses. CSF, blood, urine cultures obtained on admission showed no growth for 48 hours. Serologic tests for Ehrlichia chaffeensis and Rocky Mountain Spotted Fever came back negative on Hospital Day (HD) 3, leading to discontinuation of IV doxycycline. His clinical status worsened with development of blurry vision and intermittent horizontal nystagmus on HD 4. Non-contrast head CT showed mild communicating hydrocephalus, which prompted a repeat lumbar puncture. He had an elevated opening pressure (33 cmH2O [range < 25 cmH2O]), and the CSF analysis revealed worsening pleocytosis (366 WBC/uL [range 0-20 WBC/uL] with neutrophils 34.7%, lymphocytes 59.2%; 4 RBCs/uL), increased protein (180 mg/uL [range 15-45 mg/dL]), and decreased glucose (4 mg/dl [range 40-70mg/dL]). Broad empiric antimicrobial treatment was started, including PO rifampin, PO isoniazid, PO ethionamide, PO pyrazinamide (for tuberculous meningitis), as well as IV ampicillin, IV ceftriaxone, and IV vancomycin (for bacterial meningitis), and IV liposomal amphotericin B (for fungal meningitis).
The next day, he developed left sixth cranial nerve palsy with fecal incontinence. Contrast-enhanced MRI of the whole spine showed no lesions on his spine, but new leptomeningeal enhancement of the pons, cerebellum, and caudal fourth ventricle was noted. He was transferred to the intensive care unit, and was intubated for placement of external ventricular drains (EVDs) due to increased intracranial pressure. On HD 9, he had acute loss of speech and vision, and repeat contrast-enhanced MRI of the brain revealed worsening hydrocephalus and cerebellar tonsillar herniation with ventriculitis (Figure 1).
Figure 1.
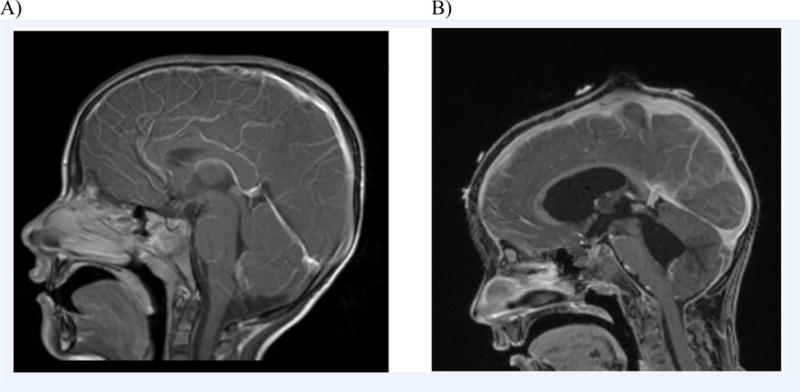
Contrast-enhanced T1 weighted Magnetic Resonance Imaging (MRI) studies of the brain from our patient. A) Normal findings on HD1. B) Hydrocephalus with ventriculitis, cerebellar tonsillar herniation, and leptomeningeal enhancement of the pons, cerebellum, and caudal fourth ventricle on HD9.
With the rapid clinical deterioration despite antimicrobial treatment and negative infectious work up (Table 1), concern for possible amebic meningoencephalitis was raised on HD 11. Initial CSF wet prep was negative, but the specimen was sent for further testing to the Free-Living and Intestinal Ameba Laboratory at the Centers for Disease Control and Prevention (CDC) on HD 12. Within hours of the specimen submission, Balamuthia mandrillaris infection was confirmed by a real-time polymerase chain reaction (PCR) assay. All previous antimicrobials were discontinued, and he began treatment with miltefosine, sulfadiazine, and flucytosine, which were administered via nasogastric tube, as well as IV fluconazole, IV pentamidine, and IV azithromycin, according to the protocol recommended by the CDC. Miltefosine was provided through the CDC’s expanded access investigational new drug protocol. He remained critically ill with marked intracranial pressure spikes, diabetes insipidus, and worsening of cerebellar herniation. He was declared brain-dead on HD 16.
Table 1.
Diagnostic tests performed for evaluation of infectious etiology in our patient (all the test results were negative).
| Cerebrospinal Fluid (CSF) | Blood | Urine |
|---|---|---|
| AFB culture Bacterial culture California Encephalitis virus IgG/IgM Cryptococcus antigen Eastern Equine Encephalitis IgG/IgM Enterovirus PCR Fungal culture Herpes Simplex Virus PCR India Ink LCMV CSF Mycobacterium tuberculosis PCR St. Louis Encephalitis IgG/IgM VDRL West Nile Virus IgG/IgM |
Adenovirus PCR Bartonella henselae/Bartonella quintana IgG/IgM Blood culture California Encephalitis virus IgG/IgM Cryptococcus Antigen Cytomegalovirus IgG/IgM; PCR Eastern Equine Encephalitis IgG/IgM Ehrlichia chaffeensis IgG/IgM Epstein Barr Virus viral capsid antigen IgG/IgM; early antigen IgG; nuclear antigen IgG; PCR Histoplasma antibodies by CF/ID HIV 1 p24 antigen with HIV 1/ 2 antibodies LCMV IgG/IgM Leptospira IgG/IgM Quantiferon TB Gold Rocky Mountain Spotted Fever IgG/IgM RPR St. Louis Encephalitis IgG/IgM West Nile Virus IgG/IgM Western Equine Encephalitis virus IgG/IgM |
Histoplasma galactomannan antigen Urine culture |
Abbreviations: AFB, Acid-fast Bacillus; CF/ID, Complement Fixation/ Immunodiffusion; HIV, Human Immunodeficiency Virus; IgG/IgM, Immunoglobulin G/M; LCMV, Lymphocytic Choriomenigitis Virus; PCR, Polymerase Chain Reaction; RPR, Rapid Plasma Reagin; VDRL, Venereal Disease Research Laboratory test.
Final Diagnosis
Balamuthia mandrillaris meningoencephalitis.
Discussion
Balamuthia mandrillaris is a free-living ameba that exists as motile, infectious trophozoites, and environmentally enduring cysts in soil and water.1 Granulomatous amebic encephalitis (GAE) due to Balamuthia mandrillaris is rare with a case fatality rate of approximately 90%.1 Of the ninety-four cases reported in the United States between 1974 and 2014, one third were children under the age of 13 years.2 It is thought to be transmitted by soil exposure, but this has not been confirmed yet.2 Balamuthia mandrillaris can affect multiple body systems including skin and mucous membranes, but has a predilection for brain tissue where it causes perivascular changes with granuloma formation.3
Balamuthia mandrillaris meningoencephalitis can mimic other CNS infections, especially in the early course of illness, presenting with fever, headache, focal neurologic signs, seizures, or photophobia.2 CSF may show a mild pleocytosis with a lymphocyte predominance, elevated protein and low or low-normal glucose level.2,4 The CSF profile is similar to that seen in viral meningoencephalitis or tuberculous meningitis, which makes a timely diagnosis challenging. Most patients with Balamuthia mandrillaris develop central nervous system (CNS) infections, and initial brain images are typically abnormal with contrast-enhancing lesions with surrounding edema.2 Our patient had a normal MRI at the time of admission, but he later developed hydrocephalus with ventriculitis and cerebella herniation on the follow up MRI.
Currently available diagnostic tools for Balamuthia mandrillaris include wet mount examination of CSF, indirect immunofluorescent staining or immunohistochemistry of brain tissue, serologic studies using indirect immunofluorescent antibody or flow cytometry, and multiplex real-time DNA PCR assay of brain tissue or CSF for the detection of amebic DNA.5 Multiplex real-time DNA PCR assay emerged as the most promising tool with the highest sensitivity and rapid turn-around time.6 It targets the 18S rRNA gene of Acanthamoeba spp., Balamuthia mandrillaris, and Naegleria fowleri, which enables simultaneous detection of all three pathogenic free-living amebae.5 18S rRNA gene analysis of Balamuthia mandrillaris has revealed a single genotype causing human infections worldwide, and there is no cross-reaction between Balamuthia mandrillaris and Acanthamoeba spp.5 The quick turn-around time allows more timely diagnosis and initiation of antimicrobial therapy.5,7
It is important to note that CSF PCR assays are usually negative for Balamuthia mandrillaris, and often times testing of brain or skin tissue is required.8 Our patient was unique in that Balamuthia mandrillaris was detected from the CSF by the PCR assay, without having to test the brain tissue. It remains unclear why it is more difficult to detect the ameba in the CSF, compared to using brain or skin tissue. We postulate that it may be due to its predilection for the brain parenchyma, which leads to the typical ring-enhancing lesions.3,4 Our patient’s family declined autopsy, so we were unable to confirm parenchymal involvement.
Currently, there are no evidence-based treatment guidelines for Balamuthia mandrillaris GAE due to the very low incidence and survival rates.6 As a result, the CDC recommends combination therapy including miltefosine,7 which is now commercially available in the United States. Miltefosine crosses the blood-brain barrier into CSF where it exerts its cysticidal and amebicidal effects by inhibiting the metabolism of phospholipids in cell membranes.9 However, little is known about actual clinical effectiveness of the combination therapy as in vitro studies have conflicting results.9,10 It still remains unclear whether the favorable outcomes were due to individual or synergistic efficacy of the agents.10
Conclusion
Clinicians should have a high index of suspicion for GAE due to Balamuthia mandrillaris when caring for patients with prolonged headache and fever without obvious etiology, particularly when CSF profile appears similar to that of tuberculous meningitis, without risk factors or clear exposure history to persons infected with Mycobacterium tuberculosis.4 Brain tissue is the optimal specimen for diagnosis as CSF test is often negative. Diagnostic modalities, including PCR assays and indirect immunofluorescence techniques, are only available at a few reference laboratories including CDC. If GAE is suspected, clinicians are encouraged to contact CDC for guidance on testing and treatment. Though rare, its early recognition and treatment may improve understanding of efficacy of therapeutic options and improve survival.
Acknowledgments
Funding
The author(s) received no financial support for the research, authorship, and /or publication of this article.
Footnotes
Author’s note
The findings and conclusions herein are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Author Contributions
All authors contributed to the preparation of this manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Perez MT, Bush LM. Balamuthia mandrillaris amebic encephalitis. Curr Infect Dis Rep. 2007;9(4):323–328. doi: 10.1007/s11908-007-0050-z. [DOI] [PubMed] [Google Scholar]
- 2.Cope JPR, Gomez J, Collier S, Moser M, Roy S, Visvesvara GS. Clinical features of Balamuthia mandrillaris Disease in the United States, 1974-2014. Open Forum Infect Dis. 2015;2(Suppl 1) [Google Scholar]
- 3.Baig AM, Khan NA. A proposed cascade of vascular events leading to granulomatous amoebic encephalitis. Microb Pathog. 2015;88:48–51. doi: 10.1016/j.micpath.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Schuster FL, Yagi S, Gavali S, et al. Under the radar: balamuthia amebic encephalitis. Clin Infect Dis. 2009;48(7):879–887. doi: 10.1086/597260. [DOI] [PubMed] [Google Scholar]
- 5.Qvarnstrom Y, Visvesvara GS, Sriram R, da Silva AJ. Multiplex real-time PCR assay for simultaneous detection of Acanthamoeba spp., Balamuthia mandrillaris, and Naegleria fowleri. J Clin Microbiol. 2006;44(10):3589–3595. doi: 10.1128/JCM.00875-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parija SC, Dinoop K, Venugopal H. Management of granulomatous amebic encephalitis: Laboratory diagnosis and treatment. Trop Parasitol. 2015;5(1):23–28. doi: 10.4103/2229-5070.149889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Investigational drug available directly from CDC for the treatment of infections with free-living amebae. MMWR Morb Mortal Wkly Rep. 2013;62(33):666. [PMC free article] [PubMed] [Google Scholar]
- 8.Schuster FL, Honarmand S, Visvesvara GS, Glaser CA. Detection of antibodies against free-living amoebae Balamuthia mandrillaris and Acanthamoeba species in a population of patients with encephalitis. Clin Infect Dis. 2006;42(9):1260–1265. doi: 10.1086/503037. [DOI] [PubMed] [Google Scholar]
- 9.Schuster FL, Guglielmo BJ, Visvesvara GS. In-vitro activity of miltefosine and voriconazole on clinical isolates of free-living amebas: Balamuthia mandrillaris, Acanthamoeba spp., and Naegleria fowleri. J Eukaryot Microbiol. 2006;53(2):121–126. doi: 10.1111/j.1550-7408.2005.00082.x. [DOI] [PubMed] [Google Scholar]
- 10.Ahmad AF, Heaselgrave W, Andrew PW, Kilvington S. The in vitro efficacy of antimicrobial agents against the pathogenic free-living amoeba Balamuthia mandrillaris. J Eukaryot Microbiol. 2013;60(5):539–543. doi: 10.1111/jeu.12062. [DOI] [PubMed] [Google Scholar]


