Summary
We determined the risk factors associated with poor survival in children and adolescents with de novo mature B cell non-Hodgkin lymphoma (B-NHL) who had refractory or relapsed disease during or after the French-American-British mature lymphoma B (FAB/LMB) 96 multi-agent chemotherapy. Among the 1,111 registered on study, 104 patients (9.4%) had refractory disease or disease relapse after first complete remission. Among these 104 patients, 28 (27%) patients had refractory disease and 76 (73%) had relapsed disease. The estimated 1- and 2-year overall survival (OS) (95% confidence interval) was 31.5% (23.3–41.0%) and 22.3% (15.3–31.4%), respectively. Prognostic analysis of OS using a Cox multivariate model showed that factors independently associated with OS included lactate dehydrogenase ≥2 upper normal limit [hazard ratio (HR) = 2.86 (1.57–5.2), P=0.0006]; time to failure (>6 months) [HR = 0·59 (0.36–0.97), P=0.038]; and failure in bone marrow [HR = 2.78 (1.65–4.68), P=0.0001]. New therapeutic strategies are required to significantly reduce refractory disease and disease relapse in patients with newly diagnosed mature B-NHL and, more importantly, there is a critical need to develop novel retrieval approaches in patients with chemotherapy-resistant disease.
Keywords: B-cell non-Hodgkin lymphoma, paediatrics, overall survival, chemotherapy
Introduction
The prognosis for children and adolescents with newly diagnosed mature B cell non-Hodgkin lymphoma (B-NHL) has significantly improved over the past 35 years with the use of short intensive multi-agent chemotherapy. The excellent results of children and adolescents with newly diagnosed B-NHL treated on the French-American-British/mature lymphoma B (FAB/LMB) 96 international trial have previously been reported by our group.(Cairo, et al 2007, Cairo, et al 2012, Gerrard, et al 2008, Gerrard, et al 2013, Patte, et al 2007) The 5-year event-free survival for patients with limited disease (Group A) intermediate risk (Group B) and advanced stage (Group C) was 97±0.5%, 89±1.2% and 79±2.7%, respectively (Cairo, et al 2007, Cairo, et al 2012, Gerrard, et al 2008, Gerrard, et al 2013, Patte, et al 2007). Overall, the results demonstrated that only two cycles of therapy are required for limited stage disease, therapy could be reduced in intensity for intermediate risk disease but that patients with most advanced disease (bone marrow [BM] ± central nervous system [CNS] disease) require full intensity FAB/LMB 96 therapy).(Cairo, et al 2007, Cairo, et al 2012, Gerrard, et al 2008, Gerrard, et al 2013, Patte, et al 2007)
The factors that were significantly associated with refractory disease or disease relapse during or after FAB/LMB 96 in children and adolescents with mature B-NHL have been reported.(Cairo, et al 2007, Patte, et al 2007) In a multivariate analysis, initial lactate dehydrogenase (LDH) level (<2 vs ≥ 2 times upper limit of normal [ULN]), and primary site (BM+/CNS+ and mediastinal large B cell lymphomas) were significantly associated with an increased risk of refractory disease or disease relapse in children and adolescents treated on FAB/LMB 96.(Cairo, et al 2007, Patte, et al 2007)
The retrieval and survival rate in children and adolescents who had refractory disease or disease relapse on or after this type of short intensive multi-agent chemotherapy, however, has steadily decreased, presumably secondary to chemoresistant disease.(Atra, et al 2000, Bowman, et al 1996, Cairo, et al 2007, Cairo, et al 2002, Cairo, et al 2012, Cairo, et al 2003a, Cairo, et al 2003b, Gerrard, et al 2008, Gerrard, et al 2013, Magrath, et al 1996, Patte, et al 2007, Patte, et al 2001, Patte, et al 1986, Patte, et al 1991, Pillon, et al 2011, Reiter, et al 1999, Spreafico, et al 2002, Woessmann, et al 2005) Few studies have analysed the outcome of children and adolescents with mature B-NHL who had refractory disease or disease relapse after identical original upfront chemotherapy.(Anoop, et al 2012, Fujita, et al 2008) Jourdain et al (2015) recently reported the results of children and adolescents with de novo B-NHL who had disease failure after different LMB regimens in three consecutive Societe Francaise d’Oncologie Pediatrique (SFOP) studies and excluded patients with induction failure or refractory disease. We therefore analysed the impact of possible risk factors that were associated with significantly decreased overall survival (OS) in children and adolescents treated only on one identical upfront treatment protocol, FAB/LMB 96, and who had either refractory or relapsed disease during or after study completion.
Methods
The FAB/LMB 96 was a cooperative international study from over 161 paediatric oncology centres within 3 paediatric oncology cooperative groups: Children’s Oncology Group (COG: former Children’s Cancer Group institutions in the United States, Canada, and Australia), the United Kingdom Children’s Cancer Study Group and SFOP (France and some centres in Belgium and the Netherlands). This protocol was approved by all of the local Institutional Review Boards and written informed consent was obtained from parents/legal guardians and patients if over 18 years of age in accordance with the Declaration of Helsinki. This protocol was registered with clinicaltrials.gov (NCT00002757)
Eligibility for the FAB/LMB96 study
Children and adolescents with newly diagnosed mature B-lineage NHL with either Burkitt lymphoma (BL), diffuse large B-cell lymphoma, or Burkitt-like lymphoma (BLL) according to the Revised European-American Lymphoma classification and World Health Organization Classification were eligible.(Harris, et al 1994, Murphy 1980, Swerdlow 2008) Staging was performed as previously described by Murphy (1980). Risk classification was defined as low risk (Group A) with resected stage I and abdominal completely resected Stage II; high risk (Group C) with BM involvement L3 blasts ≥ 25% and/or CNS disease; intermediate risk (Group B) was all others.(Cairo, et al 2007, Cairo, et al 2012, Gerrard, et al 2008) Exclusion criteria included congenital or acquired immunodeficiency, prior malignancy and/or prior chemotherapy.(Cairo, et al 2007, Gerrard, et al 2008, Patte, et al 2007)
Eligibility for the current study
Patients included in the FAB/LMB96 study were primary refractory (never obtained a complete response [CR]) or who relapsed after having achieved CR. Complete remission could be achieved anytime during protocol-derived therapy.
Initial treatment
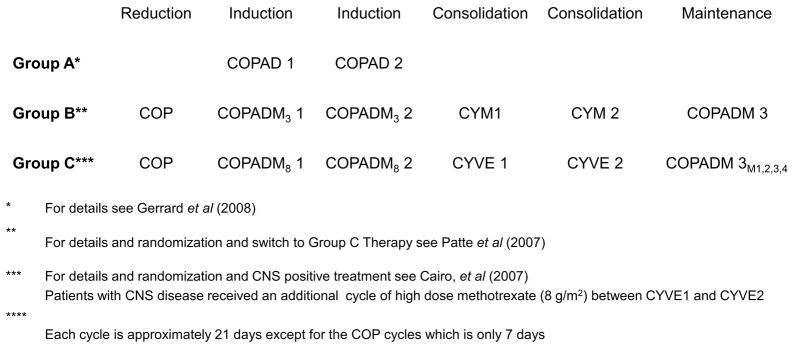
Patients assigned to Group A, B and C therapy were as previously described (Fig 1). (Cairo, et al 2007, Cairo, et al 2012, Gerrard, et al 2008, Gerrard, et al 2013, Patte, et al 2007)
Fig 1.
Experimental design schema of the French-American-British/mature lymphoma B (FAB/LMB) 96 international study. Each of the induction, consolidation and maintenance cycles were approximately 21 days except for the COP reduction cycle which was only 7 days.
CNS: central nervous system; COP: cyclophosphamide, vincristine and prednisone; COPAD: cyclophosphamide, vincristine, prednisone and doxorubicin; COPADM3: fractioned cyclophosphamide, vincristine, prednisone, doxorubicin and high-dose methotrexate 3 g/m2; COPADM8: fractioned cyclophosphamide, vincristine, prednisone, doxorubicin and high-dose methotrexate 8 g/m2; CYM: cytarabine and methotrexate; CYVE: cytarabine and etoposide; M1,2,3,4: maintenance cycle
Group A
Patients received two courses of cyclophosphamide, vincristine, prednisone and doxorubicin (COPAD) without intrathecal chemotherapy. Each cycle of COPAD was approximately 7 days.
Group B
Patients received a 7-day low dose reduction phase, cyclophosphamide, vincristine and prednisone (COP). Induction therapy consisted of two cycles of fractioned cyclophosphamide, vincristine, prednisone, doxorubicin and high-dose methotrexate 3 g/m2 (HD-MTX) (COPADM). Patients then received two consolidation cycles of cytarabine and methotrexate. Patients on the standard arm (B1) received the continuation phase of COPADM. Patients received intrathecal chemotherapy prophylaxis during all phases of the therapy, as previously described.(Patte, et al 2007) Patients with a favourable response to COP prophase were randomised to therapy reduction with 50% cyclophosphamide delivered in the second induction cycles and/or the elimination of continuation therapy in a 4-arm stratified randomisation as previously described.(Patte, et al 2007) Each induction, consolidation and maintenance cycle was approximately 21 days. Patients with less than a 20% response on day 7 of COP and patients with residual disease after cytarabine and methotrexate were given cytabarine with etoposide (CYVE) intensification therapy for two cycles and four cycles of continuation therapy according to Group C therapy (Fig 1). (Cairo, et al 2007)
Group C
Patients received a 7-day reduction course, COP, as described above.(Cairo, et al 2007) Induction therapy consisted of two cycle of COPADM (with HD-MTX 8 g/m2).(Cairo, et al 2007) Consolidation consisted of high-dose and continuous CYVE.(Cairo, et al 2007) Patients with CNS disease did not receive cranial radiation but received additional intrathecal therapy as well as an additional HD-MTX course between consolidation courses, as previously described.(Cairo, et al 2007) The first continuation cycle consisted of COPADM, and three additional chemotherapy cycles followed in the standard arm of therapy. Patients with a ≥ 20% response after COP reduction were randomised to a standard arm or a reduced arm with simultaneous reduction in chemotherapy during the consolidation phase (CYVE) and the elimination of the three continuation courses, as previously described (Fig 1). (Cairo, et al 2007) Each induction, consolidation and maintenance cycle was approximately 21 days, except for the reduction cycle of COP which was only 7 days.
Retrieval therapy
Patients who had primary refractory or relapsed disease on or off this protocol therapy were re-induced and treated according to the discretion of the treating physician and institutional preferences. There was not a uniform approach either to re-induction chemotherapy, use of immunotherapy and/or the use of stem cell transplantation.
Haematopathology
The initial independent and consensus morphology and immunophenotype from diagnostic materials from each case was evaluated by each of the six haematopathologists from the three national cooperative groups to establish a diagnosis using a standard immunophenotyping panel that included the following antibodies: CD20, CD79a, CD3, CD46RO, TDT, CD30and p80, as previously described.(Cairo, et al 2012, Harris, et al 1994, Swerdlow 2008) There was no requirement for central review of patients who had primary refractory or relapsed disease on or off the therapy above.
Statistical methods
Patients were grouped by risk: Group A (limited), Group B (intermediate) and Group C (advanced). The subgroup analysed in this manuscript only consisted of patients who were primary refractory (never obtained a CR) or suffered disease relapse after obtaining a CR. The primary endpoint was OS, which was the time to death from any cause measured from the date of initial treatment failure. We estimated survival probabilities according to the Kaplan-Meier method. The 95% confidence intervals (CI) of survival rates were estimated according to the Rothman method. The prognostic impact on OS of patient characteristics (gender, age), initial disease characteristics (stage, prognosis group, pathological type of B cell lymphoma, primary site, LDH level, BM involvement and CNS involvement) and failure characteristics (time to failure, type of failure, localization of failure, number of sites of failure) was studied by univariate analysis using log-rank test. All factors were also studied by multivariate analysis using Cox model and backward selection based on Wald statistic with removal of variables with p-value > 0.05. Hazard ratios (HR) are presented with their 95 % CI. A significant association was considered at p-value ≤ 0.05. All reported p-value are two-sided. Analyses were done with SAS version 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
Demographics
Among the 1,111 patients entered on the FAB/LMB 96 trial, 126 patients experienced events. Excluding 18 toxic deaths and 4 second malignancies, there were 104 patients (9.4%) who had primary refractory disease or disease relapse after first CR on or off protocol therapy who were the subject of this analysis. The demographics of the 104 patients who had refractory disease or disease relapse is summarised in Table I. Briefly, 28 (27%) never obtained a CR and were considered primary refractory and 76 (73%) were considered to have relapsed disease after having achieved a CR.
Table I.
Patient initial characteristics
| Patients | ||
|---|---|---|
|
| ||
| N | % | |
| Academic group | ||
| CCG | 46 | 44 |
| SFOP | 34 | 33 |
| UKCCSG | 24 | 23 |
| Male | 77 | 74 |
| Female | 27 | 26 |
| Age | ||
| < 15 years | 80 | 77 |
| ≥15 years | 24 | 23 |
| Stage | ||
| I | 1 | 1 |
| II | 4 | 4 |
| III | 58 | 56 |
| IV | 12 | 12 |
| ALL (≥25% in BM) | 29 | 28 |
| Prognosis group | ||
| Group A | 2 | 2 |
| Group B | 66 | 63 |
| Group C CNS negative | 12 | 12 |
| Group C CNS positive | 24 | 23 |
| Histology | ||
| BL/BLL/Burkitt leukaemia | 72 | 69 |
| DLBCL (not PMBL) | 11 | 11 |
| PMBL | 14 | 23 |
| Other NOS | 7 (T cell rich, n=2) | 7 |
| Primary site | ||
| Thorax | 16 | 15 |
| Abdomen/Retroperitoneal | 59 | 57 |
| Head and Neck | 8 | 8 |
| Peripheral Node | 2 | 2 |
| B-ALL | 14 | 13 |
| Other | 5 | 5 |
| LDH | ||
| < 2 NL | 30 | 29 |
| ≥2 NL | 74 | 71 |
| Bone Marrow /Central Nervous System | ||
| BM−/CNS− | 63 | 61 |
| BM+/CNS− | 17 | 16 |
| BM−/CNS+ | 5 | 5 |
| BM+/CNS+ | 19 | 18 |
B-ALL, B cell acute lymphoblastic lymphoma; BL, Burkitt lymphoma; BLL, Burkitt-like lymphoma; BM, bone marrow; CCG, Children’s Cancer Group; CNS, central nervous system; DLBCL, diffuse large B cell lymphoma; LDH, lactate dehydrogenase; NL, normal limit; NOS, not otherwise specified; PMBL, primary mediastinal large B cell lymphoma; SFOP, Societe Francaise d’Oncologie; UKCCSG, United Kingdom Children’s Cancer Study Group.
Treatment failure (primary refractory or relapsed disease) by initial disease stratification
The percentage of patients based on initial FAB risk classification, St. Jude staging classification and primary disease site was determined. The distribution among patients included: 1) risk classification was 2 (2.0%) within Group A, 66 (63%) Group B and 36 (35%) Group C patients; 2) staging classification: I 1 (1%), II 4 (4%), III 58 (56%), IV 12 (12%) and B cell acute lymphoblastic leukaemia (B-ALL) 29 (28%); 3) primary site: abdominal/retroperitoneal 59 (57%), thorax 16 (15%), head and neck 8 (8%), Burkitt leukaemia 14 (13%), other 7 (7%).
Overall survival
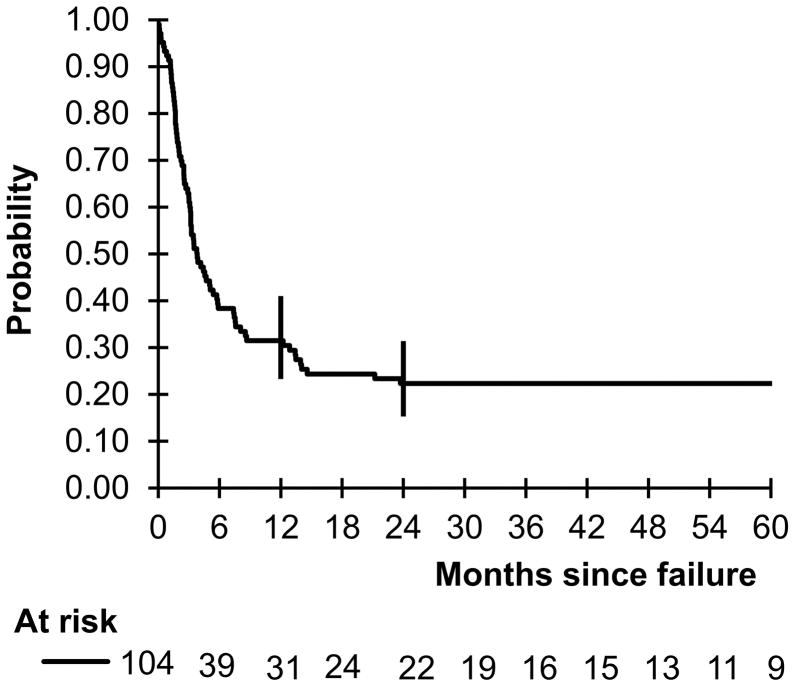
The median follow-up after treatment failure was 4.4 years. Only three alive patients were followed less than two years. Seventy-nine deaths occurred. The probability of 1- and 2-year OS was 31.5% (95% CI 23.3–41.0%) and 23.3% (95% CI 15.3–31.4%), respectively (Fig 2). Failure characteristics are depicted in Table II. In univariate analysis using the log-rank test, patients with LDH ≥ 2 ULN at diagnosis (P = 0.004), time to failure ≤ 6 months (P = 0.002), failure as primary refractory disease (P = 0.047) and failure in the BM (P = 0.003) had significantly worse OS (Table III).
Fig 2.
Probability of overall survival by the Kaplan Meier method of children and adolescents with mature B cell non-Hodgkin lymphoma who had refractory or relapsed disease during or after therapy on the French-American-British/mature lymphoma B (FAB/LMB) 96 international study.
Table II.
Failure characteristics
| Patients | ||
|---|---|---|
|
| ||
| N | % | |
| Duration between diagnosis and treatment failure | ||
| ≤3 months | 17 | 16 |
| > 3 – ≤ 6 months | 48 | 46 |
| > 6 months | 39 | 38 |
| Type of failure | ||
| No CR obtained (refractory) | 28 | 27 |
| Relapse after CR | 76 | 73 |
| Failure at initial localisation | ||
| No | 46 | 44 |
| Yes | 58 | 56 |
| Failure in bone marrow | ||
| No | 78 | 75 |
| Yes | 26 | 25 |
| Failure in central nervous system | ||
| No | 73 | 70 |
| Yes | 31 | 30 |
| Failure in other sites | ||
| No | 64 | 62 |
| Yes | 40 | 38 |
| Number of sites of failure | ||
| 1 | 64 | 62 |
| 2 | 31 | 30 |
| 3 | 7 | 7 |
| 4 | 2 | 2 |
CR, complete response.
Table III.
Univariate prognostic analysis of overall survival
| Univariate analysis | ||
|---|---|---|
|
| ||
| 2-year OS rate | Log-rank p-value | |
| Male | 16.9% | |
| Female | 37.0% | 0.10 |
| Age | ||
| < 15 years | 23.8% | |
| ≥15 years | 17.5% | 0.66 |
| Stage (St. Judes) | ||
| I / II | 40.0% | |
| III | 22.8% | |
| IV / ALL | 20.2% | 0.45 |
| Prognosis group | ||
| Group A | 50.0% | |
| Group B | 25.1% | |
| Group C | 16.7% | 0.25 |
| Histology | ||
| Burkitt / Burkitt like | 19.1% | |
| DLBCL (not PMBL) | 35.1% | |
| PMBL | 28.6% | |
| Other NOS | 28.6% | 0.26 |
| Primary site | ||
| Thorax | 26.9% | |
| Abdomen/Retroperitoneal | 20.7% | |
| B-ALL | 7.1% | |
| Other | 38.9% | 0.058 |
| LDH | ||
| < 2 NL | 41.8% | |
| ≥2 NL | 14.9% | 0.0004 |
| Bone Marrow | ||
| Not involved | 23.4% | |
| Involved | 20.2% | 0.21 |
| Central Nervous System | ||
| Not involved | 24.1% | |
| Involved | 16.7% | 0.27 |
| Time to failure | ||
| ≤ 6 months | 15.4% | |
| > 6 months | 34.5% | 0.002 |
| Type of failure | ||
| No CR (refractory) | 10.7% | |
| Failure after CR | 26.8% | 0.047 |
| Failure at initial localisation | ||
| No | 24.8% | |
| Yes | 20.7% | 0.84 |
| Failure in bone marrow | ||
| No | 26.0% | |
| Yes | 11.5% | 0.003 |
| Failure in CNS | ||
| No | 23.7% | |
| Yes | 19.4% | 0.77 |
| Failure in other sites | ||
| No | 21.9% | |
| Yes | 23.0% | 0.63 |
| Number of sites of failure | ||
| 1 | 27.2% | |
| >1 | 15.0% | 0.14 |
B-ALL, B cell acute lymphoblastic lymphoma; CI, confidence interval; CNS, central nervous system; CR, complete response; DLBCL, diffuse large B cell lymphoma; HR, hazard ratio; LDH, lactate dehydrogenase; NL, normal limit; NOS, not otherwise specified; OS, overall survival; PMBL, primary mediastinal large B cell lymphoma.
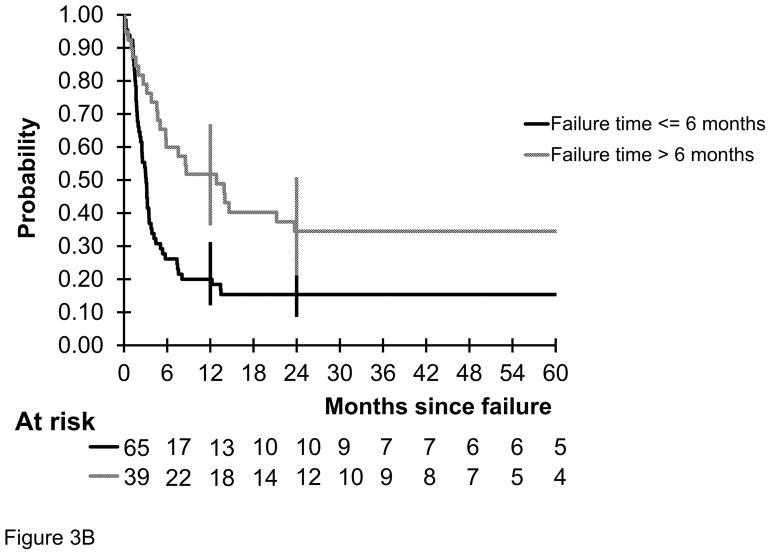
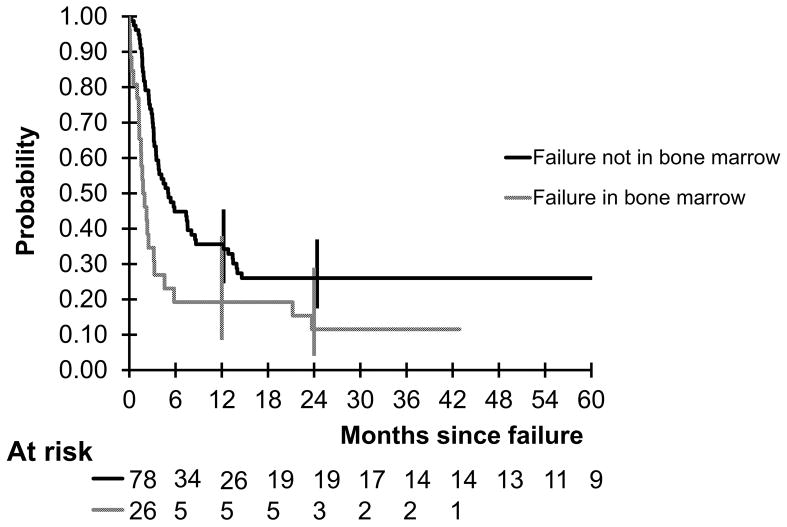
The probability of 2-year OS in children and adolescents with mature B-NHL who had primary refractory disease or disease relapse during or off FAB/LMB 96 with initial LDH of < 2 times ULN versus ≥ 2 times ULN was 41.8% versus 14.9% (P = 0.004) (Fig 3A). The probability of 2-year OS in this same population who did not achieve a CR or failure ≤ vs > 6 months from diagnosis was 15.4% versus 34.5% (P = 0.002) (Fig 3B). The probability in this same population who failed in the BM versus no failure in the BM was 11.5% versus 26%, P = 0.003 (Fig 4).
Fig 3.
Probability of overall survival by the Kaplan Meier method of children and adolescents with mature B cell non-Hodgkin lymphoma who had refractory or relapsed disease during or after therapy on the French-American-British/mature lymphoma B (FAB/LMB) 96 international study (A) stratified by initial lactate dehydrogenase (LDH) < vs ≥ 2 upper normal limits at the time of diagnosis (P < 0.0004). (B) stratified by time to relapse < 3 months ≤ 6 months versus > 6 months from diagnosis (P = 0.002).
Fig 4.
Probability of overall survival by the Kaplan-Meier method of children and adolescents with mature B cell non-Hodgkin lymphoma who had refractory or relapsed disease during or after therapy with failure in the bone marrow (P = 0.0001).
The type of failure (primary refractory disease versus relapse after CR) was strongly associated with the time of failure: in all patients except one who had primary refractory disease, the time of failure was less than 6 months from diagnosis; among patients who relapsed, half of the relapses occurred before 6 months from diagnosis and half after 6 months. Among the patients who relapsed, time to failure was associated with OS. But in patients who had a failure within 6 months from diagnosis, the type of failure (primary refractory disease versus relapse) was not associated with OS.
In the Cox multivariate regression analysis, LDH ≥ 2 times ULN at diagnosis (HR = 2·86 [1.57–5.2], P = 0.0006), time to failure (≥ 6 months) (HR = 0·59 [0.36–0.97]) (P = 0.038), and failure in the BM (HR = 2·78 [1.65–4.68], P = 0.0001) remained significant risk factors for OS after backward selection (Table IV). After taking the time of failure into account, type of failure was not more associated with OS.
Table IV.
Final multivariate analysis of overall survival using Cox model with backward selection
| HR (95% CI) | Wald test p-value | |
|---|---|---|
| Initial LDH | ||
| < 2 NL | 1 | |
| ≥2 NL | 2.86 (1.57–5.20) | 0.0006 |
| Time to failure | ||
| ≤6 months | 1 | |
| > 6 months | 0.59 (0.36–0.97) | 0.038 |
| Failure in bone marrow | ||
| No | 1 | |
| Yes | 2.78 (1.65–4.68) | 0.0001 |
HR, hazard ratio; LDH, lactate dehydrogenase; NL, normal limit.
Discussion
This is the largest analysis performed in a large group of children and adolescents with mature B-NHL who had primary refractory disease or disease relapse during or after initial intensive multi-agent chemotherapy treated uniformly in an international trial. Importantly, these patients were initially treated with a risk-stratified uniform multi-institutional and multiple paediatric cooperative group upfront protocol. Despite retrieval therapy at the discretion of the treating physician, the 1- and 2-year OS was only 31.5% and 22.3%, respectively. Additionally, these data suggest that patients who had refractory or relapsed disease within six months of diagnosis, those with an elevated initial LDH level ≥ 2 times ULN at diagnosis and/or those who failed in the BM have a significantly decreased OS. While initial LDH levels have been identified previously as an independent risk factor for disease failure (Cairo, et al 2002, Cairo, et al 2012, Cairo, et al 2003b, Gerrard, et al 2008, Patte, et al 2007, Patte, et al 2001, Pillon, et al 2004, Reiter, et al 1999), this finding is similar to that of Jourdain et al (2015) who also demonstrated that initial LDH is a significant risk factor for decreased OS in a population who fail on or following initial upfront LMB chemotherapy in three consecutive SFOP studies.
The Children’s Cancer Group previously analysed the OS in 212 children with disseminated large cell lymphoma and focused on the subgroup who relapsed.(Cairo, et al 2002) This retrospective analysis demonstrated a 1-year OS of approximately 31±4.7%.(Cairo, et al 2002) Similarly, 470 children and adolescents with disseminated BL, BLL or Burkitt leukaemia were analysed over a 20-year period.(Bowman, et al 1996) In patients with BL/BLL or B-ALL who relapsed, the 1-year OS was 16%.(Bowman, et al 1996) These results, from an analysis of children and adolescents treated from 1977–1994, are similar to the results of the current study.(Bowman, et al 1996, Cairo, et al 2002) However, the Children’s Cancer Group did not specifically address the prognostic factors associated with OS in those patients who had refractory disease or disease relapse during or after initial mature B-NHL therapy in either of these reports.
FAB risk group classification (Group A vs B vs C) clearly identifies patients with an increased risk of refractory or relapsed disease with the percentage of patients experiencing an adverse event (toxic death, secondary malignancy, refractory or relapsed disease) being 1%, 11% and 21%, respectively. Age was historically considered as a risk factor because adolescents were considered to be at higher risk for failure.(Cairo, et al 2003a, Hochberg, et al 2009) In our analysis, adolescent age at initial presentation did not reach significance as an independent risk factor for OS after refractory disease or disease relapse.(Cairo, et al 2012) Although male gender is predominant in children and adolescent mature B-NHL, there was no significant decreased risk of OS in males versus females in these patients who had refractory disease or disease relapse during or after initial FAB/LMB 96 therapy. Furthermore, the decreased OS in patients with Stage IV disease as a risk factor did not reach significance in the Cox multivariate regression analysis.
One of the most important findings in this study was that the interval from initial diagnosis to refractory disease or disease relapse (less than 6 months) was significantly associated with a decreased OS following initial treatment on FAB/LMB 96. This is similar to the results of the Jourdain et al (2015) The COG demonstrated that in patients with refractory or relapsed lymphoma who achieved a CR/partial response (PR) after reinduction and then underwent cyclophosphamide, carmustine, and etoposide conditioning and autologous stem cell transplantation, those patients whose remission was < 12 vs ≥ 12 months duration had a significantly decreased OS.(Harris, et al 2011) Early haematological relapse appears to denote more resistant disease and commonly results in a significant decrease in OS in children with mature B-NHL as well as those with ALL.(Perkins, et al 2003)
Clearly there is a need to reduce the incidence of refractory or relapsed disease during upfront therapy (especially in group C patients) and to develop more novel and targeted retrieval regimens to circumvent drug resistance in all children and adolescents who have refractory disease or disease relapse on upfront contemporary multi-agent chemotherapy regimens. CD20, the receptor for rituximab, is expressed in more than 98% of all children and adolescents with mature B-NHL.(Perkins, et al 2003) The safety of adding rituximab to children and adolescents with newly diagnosed Stage III/IV mature B-NHL with the B4 and C1 treatment arms of FAB/LMB 96 has been piloted.(Goldman, et al 2013, Goldman, et al 2014, Shiramizu, et al 2011) Reporting for the Berlin-Frankfurt-Munster group, Meinhardt et al (2010) also included the safety and efficacy of rituximab with upfront multi-agent chemotherapy. The benefit of adding rituximab to FAB/LMB 96 therapy in children and adolescents with newly diagnosed high-risk mature B-cell lymphoma/leukaemia was recently reported in the randomised prospective international Inter-B-NHL study.(Minard-Colin, et al 2015) However, the OS in children and adolescents with B-NHL treated with a rituximab-containing chemoimmunotherapy regimen are not significantly better in patients who have disease failure compared to those with chemotherapy only, such as illustrated in this study (Hochberg, et al 2009, Meinhardt, et al 2010).
Numerous regimens, usually incorporating a platinum compound, have been reported to show efficacy in patients with mature B-NHL after disease failure. Gentet et al (1990) and Philip et al (1993) demonstrated the success of CYVE as salvage treatment in refractory mature B-NHL and disease failure. Kobrinsky et al (2001) has reported on the use of dexamethasone, etoposide, cisplatin, cytarabine and L-asparaginase in recurrent/refractory childhood NHL. However, this regimen utilises agents that are currently used today in high-risk mature B-NHL patients as upfront therapy, including corticosteroids, cytarabine and etoposide. Kung et al (1999) and Cairo et al (2001) reported on the use of ifosfamide, carboplatin and etoposide (ICE) for recurrent/refractory childhood NHL. Most recently, Griffin et al (2009) reported a 60% overall response rate (CR + PR) in children and adolescents with mature B-NHL following combination chemoimmunotherapy with rituximab and ICE. Children and adolescents with refractory disease and/or disease relapse mature B-NHL who achieve a CR or PR after reinduction are candidates for a stem cell transplant consolidation. When autologous stem cells can be harvested and are clear of tumour, consolidation with a myeloablative autologous stem cell transplant may be considered.(Bradley and Cairo 2008, Gross, et al 2010, Harris, et al 2011, Kobrinsky, et al 2001) Alternatively, a full intensity or reduced intensity conditioning followed by an allogeneic stem cell transplantation may also be considered in patients with stable or CR/PR after reinduction therapy. (Gross, et al 2010, Satwani, et al 2015)
This retrospective analysis has some limitations. This was a retrospective analysis and was not specifically designed nor powered to address specific risk or prognostic factors that were present at the time of refractory disease or disease relapse and risk of overall long-term OS. However, there are no known genetic and/or molecular factors at disease failure in this population that predict OS after retrieval therapy. Moreover, we do not have information on LDH level at refractory disease or disease relapse, or retrieval or reinduction regimens, nor whether patients received an autologous vs. allogeneic stem cell transplant as consolidation. The majority of these patients did not however receive rituximab in their reinduction therapy as rituximab was not available for children during this time period. Similarly, whether autologous or allogeneic stem cell transplantation was utilised in patients obtaining a CR or PR after retrieval therapy, the results do not indicate an advantage to either stem cell transplantation approach (Pillon, et al 2011). However, the results (OS) were comparable across three paediatric cooperative groups (COG vs SFOP vs UKCCSG) (Table IV). Most importantly, all patients (N=1,111) were treated on the same uniform upfront protocol (FAB/LMB 96), as this represents the largest experience ever reported in children and adolescents with newly diagnosed mature B-NHL.
In summary, these results have demonstrated that initial LDH levels ≥ 2 times ULN, refractory disease or disease relapse less than 6 months from diagnosis and/or failure in the BM are each independently associated with a significant decrease in OS in children and adolescents with mature B-NHL who had refractory or relapsed disease during or after contemporary multi-agent chemotherapy, such as FAB/LMB 96. These results provide critical information to health care providers and families about the chance of survival for children and adolescents with mature B-NHL with refractory or relapsed disease. Future strategies should continue to identify risk factors associated with primary refractory disease or disease relapse, such as tumour cytogenetics or minimal disseminated disease at diagnosis and minimal residual disease following induction therapy, and subsequently design new therapeutic strategies to reduce refractory disease or disease relapse during upfront therapy.(Mussolin, et al 2007, Poirel, et al 2009, Satwani, et al 2015, Shiramizu, et al 2011, Shiramizu, et al 2015) Alternative new retrieval approaches include the identification of new targets, use of new monoclonal antibodies or conjugated antibodies, and novel approaches of cellular and stem cell therapy.(Awasthi, et al 2015, Barth, et al 2013, Cooney-Qualter, et al 2007, Shiramizu, et al 2015) Furthermore, targeting more specific molecular or cellular pathways in children and adolescents with mature B cell lymphoma should be studied in a cooperative setting to improve the long term OS in this poor risk group of patients.(Lee, et al 2017, Pinkerton, et al 2016)
Acknowledgments
The authors would also like to thank the members of the DSMC, Professors Michael Link, Alfred Reiter, David Harrington and Robert Souhami for their diligent oversight of this study. The authors would also like to thank Linda Rahl and Virginia Davenport, RN for their assistance in the administration of this trial and Erin Morris, RN and Virginia Davenport, RN for their editorial assistance in the development of this manuscript, and Richard Sposto, PhD and Jing Fan, MS for statistical support. The authors would also like to thank the other members of the international pathology review panel group (Drs M. Gerrard, K. McCarthy, M. Raphael, M. J. Terrier-Lacombe, M. Lones and A. Wotherspoon) and international cytogenetics review panel (Drs. W. Sanger, N. Heerema, J Swansbury, P Talley, A Bernheim). The authors would finally like to thank all the members and investigators of the Children’s Oncology Group, Société Française d’Oncologie Pédiatrique, and the United Kingdom Children’s Cancer Study Group.
Funding
Research reported in this publication was supported by grants from the Children’s Oncology Group, the National Cancer Institute of the National Institutes of Health under award number U10CA180886 (NCTN Operations Grant); Cancer Research Campaign United Kingdom Children’s Cancer Study Group; Association pour la Recherche Contre le Cancer, La Ligue Nationale Contre le Cancer, Institut-Gustave-Roussy (SFOP), Pediatric Cancer Research Foundation (PCRF) and the St. Baldrick’s Foundation.
Footnotes
Author contribution
MC and CP were primary investigators and RP and SG were principal investigators for FAB/LMB 96 study. MC, AA, SP and CP assisted in the conception of the study and design of the protocol. SP, SG and LH collected the data while MC, AA, RP, SG, CP and SP interpreted the data. AA performed the statistical analysis. MC, AA, SG and CP wrote the manuscript on behalf of the FAB/LMB 96 study group. All authors contributed to the critical review of this manuscript and have approved the final version.
Conflict of interest disclosure
The authors declare no competing interests.
References
- Anoop P, Sankpal S, Stiller C, Tewari S, Lancaster DL, Khabra K, Taj MM. Outcome of childhood relapsed or refractory mature B-cell non-Hodgkin lymphoma and acute lymphoblastic leukemia. Leuk Lymphoma. 2012;53:1882–1888. doi: 10.3109/10428194.2012.677534. [DOI] [PubMed] [Google Scholar]
- Atra A, Imeson JD, Hobson R, Gerrard M, Hann IM, Eden OB, Carter RL, Pinkerton CR. Improved outcome in children with advanced stage B-cell non-Hodgkin’s lymphoma (B-NHL): results of the United Kingdom Children Cancer Study Group (UKCCSG) 9002 protocol. Br J Cancer. 2000;82:1396–1402. doi: 10.1054/bjoc.1999.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasthi A, Ayello J, Van de Ven C, Elmacken M, Sabulski A, Barth MJ, Czuczman MS, Islam H, Klein C, Cairo MS. Obinutuzumab (GA101) compared to rituximab significantly enhances cell death and antibody-dependent cytotoxicity and improves overall survival against CD20(+) rituximab-sensitive/-resistant Burkitt lymphoma (BL) and precursor B-acute lymphoblastic leukaemia (pre-B-ALL): potential targeted therapy in patients with poor risk CD20(+) BL and pre-B-ALL. Br J Haematol. 2015;171:763–775. doi: 10.1111/bjh.13764. [DOI] [PubMed] [Google Scholar]
- Barth MJ, Goldman S, Smith L, Perkins S, Shiramizu B, Gross TG, Harrison L, Sanger W, Geyer MB, Giulino-Roth L, Cairo MS. Rituximab pharmacokinetics in children and adolescents with de novo intermediate and advanced mature B-cell lymphoma/leukaemia: a Children’s Oncology Group report. Br J Haematol. 2013;162:678–683. doi: 10.1111/bjh.12434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman WP, Shuster JJ, Cook B, Griffin T, Behm F, Pullen J, Link M, Head D, Carroll A, Berard C, Murphy S. Improved survival for children with B-cell acute lymphoblastic leukemia and stage IV small noncleaved-cell lymphoma: a pediatric oncology group study. J Clin Oncol. 1996;14:1252–1261. doi: 10.1200/JCO.1996.14.4.1252. [DOI] [PubMed] [Google Scholar]
- Bradley MB, Cairo MS. Stem cell transplantation for pediatric lymphoma: past, present and future. Bone Marrow Transplant. 2008;41:149–158. doi: 10.1038/sj.bmt.1705948. [DOI] [PubMed] [Google Scholar]
- Cairo MS, Shen V, Krailo MD, Bauer M, Miser JS, Sato JK, Blatt J, Blazar BR, Frierdich S, Liu-Mares W, Reaman GH. Prospective randomized trial between two doses of granulocyte colony-stimulating factor after ifosfamide, carboplatin, and etoposide in children with recurrent or refractory solid tumors: a children’s cancer group report. J Pediatr Hematol Oncol. 2001;23:30–38. doi: 10.1097/00043426-200101000-00008. [DOI] [PubMed] [Google Scholar]
- Cairo MS, Krailo MD, Morse M, Hutchinson RJ, Harris RE, Kjeldsberg CR, Kadin ME, Radel E, Steinherz LJ, Morris E, Finlay JL, Meadows AT. Long-term follow-up of short intensive multiagent chemotherapy without high-dose methotrexate (‘Orange’) in children with advanced non-lymphoblastic non-Hodgkin’s lymphoma: a children’s cancer group report. Leukemia. 2002;16:594–600. doi: 10.1038/sj.leu.2402402. [DOI] [PubMed] [Google Scholar]
- Cairo MS, Sposto R, Hoover-Regan M, Meadows AT, Anderson JR, Siegel SE, Kadin ME, Kjeldsberg CR, Wilson JF, Perkins SL, Lones MA, Morris E, Finlay JL. Childhood and adolescent large-cell lymphoma (LCL): a review of the Children’s Cancer Group experience. Am J Hematol. 2003a;72:53–63. doi: 10.1002/ajh.10262. [DOI] [PubMed] [Google Scholar]
- Cairo MS, Sposto R, Perkins SL, Meadows AT, Hoover-Regan ML, Anderson JR, Siegel SE, Lones MA, Tedeschi-Blok N, Kadin ME, Kjeldsberg CR, Wilson JF, Sanger W, Morris E, Krailo MD, Finlay JL. Burkitt’s and Burkitt-like lymphoma in children and adolescents: a review of the Children’s Cancer Group experience. Br J Haematol. 2003b;120:660–670. doi: 10.1046/j.1365-2141.2003.04134.x. [DOI] [PubMed] [Google Scholar]
- Cairo MS, Gerrard M, Sposto R, Auperin A, Pinkerton CR, Michon J, Weston C, Perkins SL, Raphael M, McCarthy K, Patte C. Results of a randomized international study of high-risk central nervous system B non-Hodgkin lymphoma and B acute lymphoblastic leukemia in children and adolescents. Blood. 2007;109:2736–2743. doi: 10.1182/blood-2006-07-036665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairo MS, Sposto R, Gerrard M, Auperin A, Goldman SC, Harrison L, Pinkerton R, Raphael M, McCarthy K, Perkins SL, Patte C. Advanced stage, increased lactate dehydrogenase, and primary site, but not adolescent age (>/= 15 years), are associated with an increased risk of treatment failure in children and adolescents with mature B-cell non-Hodgkin’s lymphoma: results of the FAB LMB 96 study. J Clin Oncol. 2012;30:387–393. doi: 10.1200/JCO.2010.33.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney-Qualter E, Krailo M, Angiolillo A, Fawwaz RA, Wiseman G, Harrison L, Kohl V, Adamson PC, Ayello J, vande Ven C, Perkins SL, Cairo MS Children’s Oncology G. A phase I study of 90yttrium-ibritumomab-tiuxetan in children and adolescents with relapsed/refractory CD20-positive non-Hodgkin’s lymphoma: a Children’s Oncology Group study. Clin Cancer Res. 2007;13:5652s–5660s. doi: 10.1158/1078-0432.CCR-07-1060. [DOI] [PubMed] [Google Scholar]
- Fujita N, Mori T, Mitsui T, Inada H, Horibe K, Tsurusawa M Lymphoma Committee of the Japanese Pediatric Leukemia/Lymphoma Study Group. The role of hematopoietic stem cell transplantation with relapsed or primary refractory childhood B-cell non-Hodgkin lymphoma and mature B-cell leukemia: a retrospective analysis of enrolled cases in Japan. Pediatr Blood Cancer. 2008;51:188–192. doi: 10.1002/pbc.21585. [DOI] [PubMed] [Google Scholar]
- Gentet JC, Patte C, Quintana E, Bergeron C, Rubie H, Pein F, Demaille MC, Philip T, Raybaud C. Phase II study of cytarabine and etoposide in children with refractory or relapsed non-Hodgkin’s lymphoma: a study of the French Society of Pediatric Oncology. J Clin Oncol. 1990;8:661–665. doi: 10.1200/JCO.1990.8.4.661. [DOI] [PubMed] [Google Scholar]
- Gerrard M, Cairo MS, Weston C, Auperin A, Pinkerton R, Lambilliote A, Sposto R, McCarthy K, Lacombe MJ, Perkins SL, Patte C. Excellent survival following two courses of COPAD chemotherapy in children and adolescents with resected localized B-cell non-Hodgkin’s lymphoma: results of the FAB/LMB 96 international study. Br J Haematol. 2008;141:840–847. doi: 10.1111/j.1365-2141.2008.07144.x. [DOI] [PubMed] [Google Scholar]
- Gerrard M, Waxman IM, Sposto R, Auperin A, Perkins SL, Goldman S, Harrison L, Pinkerton R, McCarthy K, Raphael M, Patte C, Cairo MS French-American-British/Lymphome Malins de Burkitt 96 International Study Committee. Outcome and pathologic classification of children and adolescents with mediastinal large B-cell lymphoma treated with FAB/LMB96 mature B-NHL therapy. Blood. 2013;121:278–285. doi: 10.1182/blood-2012-04-422709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman S, Smith L, Anderson JR, Perkins S, Harrison L, Geyer MB, Gross TG, Weinstein H, Bergeron S, Shiramizu B, Sanger W, Barth M, Zhi J, Cairo MS. Rituximab and FAB/LMB 96 chemotherapy in children with Stage III/IV B-cell non-Hodgkin lymphoma: a Children’s Oncology Group report. Leukemia. 2013;27:1174–1177. doi: 10.1038/leu.2012.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman S, Smith L, Galardy P, Perkins SL, Frazer JK, Sanger W, Anderson JR, Gross TG, Weinstein H, Harrison L, Shiramizu B, Barth M, Cairo MS. Rituximab with chemotherapy in children and adolescents with central nervous system and/or bone marrow-positive Burkitt lymphoma/leukaemia: a Children’s Oncology Group Report. Br J Haematol. 2014;167:394–401. doi: 10.1111/bjh.13040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin TC, Weitzman S, Weinstein H, Chang M, Cairo M, Hutchison R, Shiramizu B, Wiley J, Woods D, Barnich M, Gross TG Children’s Oncology G. A study of rituximab and ifosfamide, carboplatin, and etoposide chemotherapy in children with recurrent/refractory B-cell (CD20+) non-Hodgkin lymphoma and mature B-cell acute lymphoblastic leukemia: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2009;52:177–181. doi: 10.1002/pbc.21753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross TG, Hale GA, He W, Camitta BM, Sanders JE, Cairo MS, Hayashi RJ, Termuhlen AM, Zhang MJ, Davies SM, Eapen M. Hematopoietic stem cell transplantation for refractory or recurrent non-Hodgkin lymphoma in children and adolescents. Biol Blood Marrow Transplant. 2010;16:223–230. doi: 10.1016/j.bbmt.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, Delsol G, De Wolf-Peeters C, Falini B, Gatter KC, Grogan TM, Isaacson PC, Knowles DM, Mason DY, Muller-Hermelink H. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84:1361–1392. [PubMed] [Google Scholar]
- Harris RE, Termuhlen AM, Smith LM, Lynch J, Henry MM, Perkins SL, Gross TG, Warkentin P, Vlachos A, Harrison L, Cairo MS. Autologous peripheral blood stem cell transplantation in children with refractory or relapsed lymphoma: results of Children’s Oncology Group study A5962. Biol Blood Marrow Transplant. 2011;17:249–258. doi: 10.1016/j.bbmt.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg J, Waxman IM, Kelly KM, Morris E, Cairo MS. Adolescent non-Hodgkin lymphoma and Hodgkin lymphoma: state of the science. Br J Haematol. 2009;144:24–40. doi: 10.1111/j.1365-2141.2008.07393.x. [DOI] [PubMed] [Google Scholar]
- Jourdain A, Auperin A, Minard-Colin V, Aladjidi N, Zsiros J, Coze C, Gandemer V, Bertrand Y, Leverger G, Bergeron C, Michon J, Patte C. Outcome of and prognostic factors for relapse in children and adolescents with mature B-cell lymphoma and leukemia treated in three consecutive prospective “Lymphomes Malins B” protocols. A Societe Francaise des Cancers de l’Enfant study. Haematologica. 2015;100:810–817. doi: 10.3324/haematol.2014.121434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobrinsky NL, Sposto R, Shah NR, Anderson JR, DeLaat C, Morse M, Warkentin P, Gilchrist GS, Cohen MD, Shina D, Meadows AT. Outcomes of treatment of children and adolescents with recurrent non-Hodgkin’s lymphoma and Hodgkin’s disease with dexamethasone, etoposide, cisplatin, cytarabine, and l-asparaginase, maintenance chemotherapy, and transplantation: Children’s Cancer Group Study CCG-5912. J Clin Oncol. 2001;19:2390–2396. doi: 10.1200/JCO.2001.19.9.2390. [DOI] [PubMed] [Google Scholar]
- Kung FH, Harris MB, Krischer JP. Ifosfamide/carboplatin/etoposide (ICE), an effective salvaging therapy for recurrent malignant non-Hodgkin lymphoma of childhood: a Pediatric Oncology Group phase II study. Med Pediatr Oncol. 1999;32:225–226. doi: 10.1002/(sici)1096-911x(199903)32:3<225::aid-mpo12>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Lee S, Day NS, Miles RR, Perkins SL, Lim MS, Ayello J, van de Ven C, Harrison L, El-Mallawany NK, Goldman S, Cairo MS. Comparative genomic expression signatures of signal transduction pathways and targets in paediatric Burkitt lymphoma: a Children’s Oncology Group report. Br J Haematol. 2017;177:601–611. doi: 10.1111/bjh.14604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrath I, Adde M, Shad A, Venzon D, Seibel N, Gootenberg J, Neely J, Arndt C, Nieder M, Jaffe E, Wittes RA, Horak ID. Adults and children with small non-cleaved-cell lymphoma have a similar excellent outcome when treated with the same chemotherapy regimen. J Clin Oncol. 1996;14:925–934. doi: 10.1200/JCO.1996.14.3.925. [DOI] [PubMed] [Google Scholar]
- Meinhardt A, Burkhardt B, Zimmermann M, Borkhardt A, Kontny U, Klingebiel T, Berthold F, Janka-Schaub G, Klein C, Kabickova E, Klapper W, Attarbaschi A, Schrappe M, Reiter A, Berlin-Frankfurt-Munster g. Phase II window study on rituximab in newly diagnosed pediatric mature B-cell non-Hodgkin’s lymphoma and Burkitt leukemia. J Clin Oncol. 2010;28:3115–3121. doi: 10.1200/JCO.2009.26.6791. [DOI] [PubMed] [Google Scholar]
- Minard-Colin V, Brugieres L, Reiter A, Cairo MS, Gross TG, Woessmann W, Burkhardt B, Sandlund JT, Williams D, Pillon M, Horibe K, Auperin A, Le Deley MC, Zimmerman M, Perkins SL, Raphael M, Lamant L, Klapper W, Mussolin L, Poirel HA, Macintyre E, Damm-Welk C, Rosolen A, Patte C. Non-Hodgkin Lymphoma in Children and Adolescents: Progress Through Effective Collaboration, Current Knowledge, and Challenges Ahead. J Clin Oncol. 2015;33:2963–2974. doi: 10.1200/JCO.2014.59.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SB. Classification, staging and end results of treatment of childhood non-Hodgkin’s lymphomas: dissimilarities from lymphomas in adults. Semin Oncol. 1980;7:332–339. [PubMed] [Google Scholar]
- Mussolin L, Pillon M, Conter V, Piglione M, Lo Nigro L, Pierani P, Micalizzi C, Buffardi S, Basso G, Zanesco L, Rosolen A. Prognostic role of minimal residual disease in mature B-cell acute lymphoblastic leukemia of childhood. J Clin Oncol. 2007;25:5254–5261. doi: 10.1200/JCO.2007.11.3159. [DOI] [PubMed] [Google Scholar]
- Patte C, Philip T, Rodary C, Bernard A, Zucker JM, Bernard JL, Robert A, Rialland X, Benz-Lemoine E, Demeocq F, Boyle C, Lemerle J. Improved survival rate in children with stage III and IV B cell non-Hodgkin’s lymphoma and leukemia using multi-agent chemotherapy: results of a study of 114 children from the French Pediatric Oncology Society. J Clin Oncol. 1986;4:1219–1226. doi: 10.1200/JCO.1986.4.8.1219. [DOI] [PubMed] [Google Scholar]
- Patte C, Philip T, Rodary C, Zucker JM, Behrendt H, Gentet JC, Lamagnere JP, Otten J, Dufillot D, Pein F, Caillou B, Lemerle J. High survival rate in advanced-stage B-cell lymphomas and leukemias without CNS involvement with a short intensive polychemotherapy: results from the French Pediatric Oncology Society of a randomized trial of 216 children. J Clin Oncol. 1991;9:123–132. doi: 10.1200/JCO.1991.9.1.123. [DOI] [PubMed] [Google Scholar]
- Patte C, Auperin A, Michon J, Behrendt H, Leverger G, Frappaz D, Lutz P, Coze C, Perel Y, Raphael M, Terrier-Lacombe MJ Societe Francaise d’Oncologie Pediatrique. The Societe Francaise d’Oncologie Pediatrique LMB89 protocol: highly effective multiagent chemotherapy tailored to the tumor burden and initial response in 561 unselected children with B-cell lymphomas and L3 leukemia. Blood. 2001;97:3370–3379. doi: 10.1182/blood.v97.11.3370. [DOI] [PubMed] [Google Scholar]
- Patte C, Auperin A, Gerrard M, Michon J, Pinkerton R, Sposto R, Weston C, Raphael M, Perkins SL, McCarthy K, Cairo MS. Results of the randomized international FAB/LMB96 trial for intermediate risk B-cell non-Hodgkin lymphoma in children and adolescents: it is possible to reduce treatment for the early responding patients. Blood. 2007;109:2773–2780. doi: 10.1182/blood-2006-07-036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins SL, Lones MA, Davenport V, Cairo MS. B-Cell non-Hodgkin’s lymphoma in children and adolescents: surface antigen expression and clinical implications for future targeted bioimmune therapy: a children’s cancer group report. Clin Adv Hematol Oncol. 2003;1:314–317. [PubMed] [Google Scholar]
- Philip T, Hartmann O, Pinkerton R, Zucker JM, Gentet JC, Lamagnere JP, Berhendt H, Perel Y, Otten J, Lutz P, Rodary C, Caillou B, Bayle C, Chauvin F, Patte C. Curability of relapsed childhood B-cell non-Hodgkin’s lymphoma after intensive first line therapy: a report from the Societe Francaise d’Oncologie Pediatrique. Blood. 1993;81:2003–2006. [PubMed] [Google Scholar]
- Pillon M, Arico M, Basso G, Locatelli F, Citterio M, Micalizzi C, Testi AM, Barisone E, Nardi M, Lombardi A, Rondelli R, Rosolen A. Long-term results of AIEOP-8805 protocol for acute B-cell lymphoblastic leukemia of childhood. Pediatr Blood Cancer. 2011;56:544–550. doi: 10.1002/pbc.22787. [DOI] [PubMed] [Google Scholar]
- Pillon M, Di Tullio MT, Garaventa A, Cesaro S, Putti MC, Favre C, Lippi A, Surico G, Di Cataldo A, D’Amore E, Zanesco L, Rosolen A. Long-term results of the first Italian Association of Pediatric Hematology and Oncology protocol for the treatment of pediatric B-cell non-Hodgkin lymphoma (AIEOP LNH92) Cancer. 2004;101:385–394. doi: 10.1002/cncr.20382. [DOI] [PubMed] [Google Scholar]
- Pinkerton R, Cairo MS, Cotter FE. Childhood, adolescent and young adult non-Hodgkin lymphoma: state of the science. Br J Haematol. 2016;173:503–504. doi: 10.1111/bjh.14091. [DOI] [PubMed] [Google Scholar]
- Poirel HA, Cairo MS, Heerema NA, Swansbury J, Auperin A, Launay E, Sanger WG, Talley P, Perkins SL, Raphael M, McCarthy K, Sposto R, Gerrard M, Bernheim A, Patte C. Specific cytogenetic abnormalities are associated with a significantly inferior outcome in children and adolescents with mature B-cell non-Hodgkin’s lymphoma: results of the FAB/LMB 96 international study. Leukemia. 2009;23:323–331. doi: 10.1038/leu.2008.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter A, Schrappe M, Tiemann M, Ludwig WD, Yakisan E, Zimmermann M, Mann G, Chott A, Ebell W, Klingebiel T, Graf N, Kremens B, Muller-Weihrich S, Pluss HJ, Zintl F, Henze G, Riehm H. Improved treatment results in childhood B-cell neoplasms with tailored intensification of therapy: A report of the Berlin-Frankfurt-Munster Group Trial NHL-BFM 90. Blood. 1999;94:3294–3306. [PubMed] [Google Scholar]
- Satwani P, Jin Z, Martin PL, Bhatia M, Garvin JH, George D, Chaudhury S, Talano J, Morris E, Harrison L, Sosna J, Peterson M, Militano O, Foley S, Kurtzberg J, Cairo MS. Sequential myeloablative autologous stem cell transplantation and reduced intensity allogeneic hematopoietic cell transplantation is safe and feasible in children, adolescents and young adults with poor-risk refractory or recurrent Hodgkin and non-Hodgkin lymphoma. Leukemia. 2015;29:448–455. doi: 10.1038/leu.2014.194. [DOI] [PubMed] [Google Scholar]
- Shiramizu B, Goldman S, Kusao I, Agsalda M, Lynch J, Smith L, Harrison L, Morris E, Gross TG, Sanger W, Perkins S, Cairo MS. Minimal disease assessment in the treatment of children and adolescents with intermediate-risk (Stage III/IV) B-cell non-Hodgkin lymphoma: a children’s oncology group report. Br J Haematol. 2011;153:758–763. doi: 10.1111/j.1365-2141.2011.08681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiramizu B, Goldman S, Smith L, Agsalda-Garcia M, Galardy P, Perkins SL, Frazer JK, Sanger W, Anderson JR, Gross TG, Weinstein H, Harrison L, Barth MJ, Mussolin L, Cairo MS. Impact of persistent minimal residual disease post-consolidation therapy in children and adolescents with advanced Burkitt leukaemia: a Children’s Oncology Group Pilot Study Report. Br J Haematol. 2015;170:367–371. doi: 10.1111/bjh.13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreafico F, Massimino M, Luksch R, Casanova M, Cefalo GS, Collini P, Ferrari A, Polastri D, Terenziani M, Gasparini M, Fossati-Bellani F. Intensive, very short-term chemotherapy for advanced Burkitt’s lymphoma in children. J Clin Oncol. 2002;20:2783–2788. doi: 10.1200/JCO.2002.08.088. [DOI] [PubMed] [Google Scholar]
- Swerdlow SH, Campo E, Harris NL, Pileri SA, Stein H, Thiele J, Vardiman JW. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; Lyon, France: 2008. [Google Scholar]
- Woessmann W, Seidemann K, Mann G, Zimmermann M, Burkhardt B, Oschlies I, Ludwig WD, Klingebiel T, Graf N, Gruhn B, Juergens H, Niggli F, Parwaresch R, Gadner H, Riehm H, Schrappe M, Reiter A BFM Group. The impact of the methotrexate administration schedule and dose in the treatment of children and adolescents with B-cell neoplasms: a report of the BFM Group Study NHL-BFM95. Blood. 2005;105:948–958. doi: 10.1182/blood-2004-03-0973. [DOI] [PubMed] [Google Scholar]