Abstract
Social cognition develops in the context of reciprocal social interaction. However, most neuroimaging studies of mentalizing have used noninteractive tasks that may fail to capture important aspects of real‐world mentalizing. In adults, social‐interactive context modulates activity in regions linked to social cognition and reward, but few interactive studies have been done with children. The current fMRI study examines children aged 8–12 using a novel paradigm in which children believed they were interacting online with a peer. We compared mental and non‐mental state reasoning about a live partner (Peer) versus a story character (Character), testing the effects of mentalizing and social interaction in a 2 × 2 design. Mental versus Non‐Mental reasoning engaged regions identified in prior mentalizing studies, including the temporoparietal junction, superior temporal sulcus, and dorsomedial prefrontal cortex. Moreover, peer interaction, even in conditions without explicit mentalizing demands, activated many of the same mentalizing regions. Peer interaction also activated areas outside the traditional mentalizing network, including the reward system. Our results demonstrate that social interaction engages multiple neural systems during middle childhood and contribute further evidence that social‐interactive paradigms are needed to fully capture how the brain supports social processing in the real world.
Keywords: fMRI, mentalizing, middle childhood, social interaction, social reward, theory of mind
1. INTRODUCTION
Social interaction shapes our daily experiences, personalities, and wellbeing throughout the lifespan, yet its biological mechanisms are underexplored. Mentalizing—the process of attributing mental states to others, also known as theory of mind—is necessary for successful social interactions, and thus there has been considerable effort in the last two decades to explicate its neural bases. Functional magnetic resonance imaging (fMRI) studies have identified several regions that consistently show greater activation during tasks that require mental state reasoning, including the temporoparietal junction (TPJ), superior temporal sulcus (STS), anterior temporal lobes (ATL), dorsomedial prefrontal cortex (dMPFC), inferior frontal gyrus (IFG), posterior cingulate cortex (PCC), and precuneus (meta‐analyses: Mar, 2011; Molenberghs, Johnson, Henry, & Mattingley, 2016; Schurz, Radua, Aichhorn, Richlan, & Perner, 2014). However, this “mentalizing network” has been characterized mainly by noninteractive tasks that use artificial stimuli such as photographs of faces, animated shapes, or stories about fictional characters. This lack of engagement with a live social partner is a crucial limitation in light of recent work suggesting that participating in social interaction profoundly alters social‐cognitive processes (reviewed in Schilbach et al., 2013). Conversely, extant studies on the effect of social interaction on brain function lack the experimental controls needed to directly examine whether and how brain activity differs when mentalizing occurs within social interaction versus observation (“offline”).
Prior neuroimaging research has shown that components of social interaction activate regions within the mentalizing network. For instance, dMPFC and STS have been found in studies examining communicative intent via eye gaze or gestures directed at the participant versus a third party (Ciaramidaro, Becchio, Colle, Bara, & Walter, 2014; Kampe, Frith, & Frith, 2003; Redcay, Velnoskey, & Rowe, 2016; Schilbach et al., 2006; cf. Calder et al., 2002; Conty, N'diaye, Tijus, & George, 2007). Furthermore, joint attention, in which two people coordinate attention to a shared target, compared with solo attention activates posterior STS, TPJ, and dMPFC (Caruana, Brock, & Woolgar, 2015; Redcay et al., 2010; Redcay, Kleiner, & Saxe, 2012; Schilbach et al., 2010). Recent work from our group has shown a similar effect even when participants do not engage in reciprocal interaction. Simply hearing short spoken vignettes with no explicit social‐cognitive demands activated left TPJ and right dMPFC more when the participants believed the speech was live than when they knew it was prerecorded (Rice & Redcay, 2016), suggesting that the mere presence of a potential social partner is sufficient to automatically engage the mentalizing network.
A compelling interpretation of these findings is that each task, though not requiring overt mental state reasoning, nevertheless evoked spontaneous mentalizing. However, the validity of this reverse inference is threatened by the apparent heterogeneity of function of the brain regions in question, particularly the TPJ (Corbetta, Patel, & Shulman, 2008; Lee & McCarthy, 2016; Schuwerk, Schurz, Müller, Rupprecht, & Sommer, 2017), STS (Redcay, 2008), and dMPFC (Isoda & Noritake, 2013), all of which have been linked to domain‐general processes in addition to social cognition. Moreover, it remains unclear whether regions engaged in offline mentalizing are precisely the same as those recruited during social interaction. Given the evidence of functional segregation within regions broadly implicated in social cognition (e.g., Gilbert et al., 2007; Krall et al., 2015; Mars et al., 2012), we cannot rule out the possibility that adjacent areas are differentially involved in social interaction versus offline mentalizing, and such distinctions may be obscured when comparing activation across samples and task designs. The gap in our understanding of how the brain's mentalizing system is affected by interactive context can only be bridged by paradigms that manipulate both social interaction and mentalizing demands within the same task and participants.
One commonly used paradigm does incorporate both elements within the same task: a strategic game in which participants play against a supposedly human partner and must ascribe mental states to their opponents to predict their next move (e.g., Gallagher, Jack, Roepstorff, & Frith, 2002). Human conditions are contrasted with conditions in which responses are computer generated; thus, the computer conditions are neither socially interactive nor do they contain explicit mentalizing demands. Although such tasks suggest that social‐interactive and offline mentalizing involve similar regions (e.g., Coricelli & Nagel, 2009; Gallagher et al., 2002; Kircher et al., 2009; McCabe, Houser, Ryan, Smith, & Trouard, 2001), they cannot directly speak to any differences between types of mentalizing because they conflate social interaction and mentalizing within the same condition. Identifying the role of mentalizing regions in social interaction more broadly necessitates closely matched conditions contrasting mental and non‐mental reasoning within both the social interaction and an offline control task.
Furthermore, mentalizing during social interaction may involve brain systems beyond the mentalizing network. In line with evidence that social interactions are inherently rewarding (Chevallier, Kohls, Troiani, Brodkin, & Schultz, 2012), Redcay et al. (2010) found greater activation of the reward system (including ventral striatum and amygdala) when participants interacted with an experimenter through a live video feed versus watching a recording of the same interaction. Other studies have also shown that reward‐related regions respond to social‐interactive context, such as gaze‐based interactions (Pfeiffer et al., 2014), initiating joint attention (Schilbach et al., 2010), and considering whether to share information with others (Baek, Scholz, O'Donnell, & Falk, 2017). Paradigms that elicit mentalizing while simultaneously capturing the motivational processes that likely differ between interactive and noninteractive contexts will provide a more holistic understanding of how we perceive other minds in real time.
Previous neuroimaging work in this area has also focused overwhelmingly on adults. Middle childhood (roughly, ages 7 to 13) is particularly understudied, despite evidence of significant social and neurocognitive development in this age range. Peer interactions become more complex (Bigelow, 1977; Farmer et al., 2015; Feiring & Lewis, 1991), and this increasing sophistication in social behavior may be accompanied by advances in social cognition (reviewed in Miller, 2009; Devine & Hughes, 2013). There is also evidence that across middle childhood, the TPJ becomes increasingly selective for representing mental states as opposed to more general social information, as revealed by an offline story‐based task (Gweon, Dodell‐Feder, Bedny, & Saxe, 2012). Still, as in the adult literature, neuroimaging studies on the effect of social interaction on social cognition in middle childhood are scarce. In one such study, similar to the aforementioned study in adults (Rice & Redcay, 2016), perceived live versus recorded speech engaged the TPJ and precuneus in children aged 7–13 (Rice, Moraczewski, & Redcay, 2016). In a separate experiment in a similar age group, receiving feedback from a peer after sharing information about oneself activated social‐cognitive and reward regions, and the magnitude of the social‐interactive effect in social‐cognitive regions increased with age (Warnell, Sadikova, & Redcay, 2018). However, as discussed above, because these tasks lacked explicit mentalizing demands, we cannot definitively infer that mentalizing (and not some other computation relevant to social processing) occurred during social‐interactive conditions, nor can we directly compare activation patterns associated with social‐interactive versus offline mentalizing.
This study is the first (to our knowledge) to employ a 2 × 2 factorial design in which the effects of social context and mentalizing can be simultaneously examined. Inside the MRI scanner, children aged 8–12 engaged in a social prediction task in which they believed they were interacting with a peer in another laboratory (Peer condition) and answering questions about a fictional character (Character condition). Across Peer and Character conditions, half the trials required the children to use mental state information when making predictions (Mental condition), while the other half did not (Non‐Mental condition).
We hypothesized that regions of the traditional mentalizing network would be activated by the Mental versus Non‐Mental contrast regardless of social‐interactive context. We further hypothesized that mentalizing regions would be activated more in Peer than in Character conditions, suggestive of spontaneous mentalizing during social interaction regardless of explicit task demands, as in our previous studies (Rice et al., 2016; Rice & Redcay, 2016). Further, through conjunction analysis, we determined the extent to which engagement in social interaction recruited the same neural resources as mentalizing did in the offline task.
The 2 × 2 factorial design also allowed us to assess whether there is an interaction effect between social interaction (Peer vs. Character) and explicit mentalizing demands (Mental vs. Non‐Mental), though we considered several possible hypotheses. One possibility is that mentalizing regions show a greater difference in activation between Mental and Non‐Mental conditions in the Peer as opposed to Character conditions, with the Peer Mental condition showing the greatest activation, which would suggest an additive effect of social interaction and explicit mentalizing demands. On the other hand, there may be less difference in activation of mentalizing regions between the two Peer conditions relative to the Character conditions. In other words, while we expect certain regions to show significantly more activation in Character Mental than in Character Non‐Mental conditions, the Peer conditions might elicit a similar amount of activation in these regions regardless of whether the task contains explicit mentalizing demands, again suggesting that engaging with a social partner is sufficient to induce spontaneous mentalizing.
Beyond mentalizing regions, we predicted that the Peer versus Character contrast would activate reward regions such as the striatum and orbitofrontal cortex (OFC), in line with previous social‐interactive experiments (Pfeiffer et al., 2014; Warnell et al., 2018). Last, we examined whether our results would replicate previous findings that social‐cognitive regions become increasingly specialized for mentalizing (Gweon et al., 2012) and social interaction (Warnell et al., 2018) across middle childhood. Altogether, the present study aims to capture the neural effects of social interaction during a dynamic yet understudied period of social development.
2. MATERIALS AND METHODS
2.1. Participants
Children were recruited using a database of families in the Washington, DC, metropolitan area. Exclusionary criteria were any MRI contraindications, diagnosis of neurological or psychiatric disorders, or first‐degree relatives with autism or schizophrenia. All participants were full‐term, native English speakers. Thirty‐five typically developing children aged 8–12 years participated in the study. Seven children were excluded from data analysis—two for excessive motion in the scanner, one due to a technical error during scanning, three for not believing the live illusion, and one who scored in the “moderate” range on the Social Responsiveness Scale, indicating clinically significant deficits in social interaction (Constantino & Todd, 2003)—leaving a final sample of 28 children (14 females; mean age = 10.41 years, SD = 1.46 years, range = 8.18–12.98 years). We obtained informed assent from all participants and informed consent from their parents or guardians. All procedures were approved by the University of Maryland Institutional Review Board.
2.2. Task procedures
2.2.1. Creating the live illusion
Before the scan, children were told they would be interacting (“chatting”) with a peer in a different laboratory who would also be undergoing an MRI scan. During a demonstration of the chat (see Supporting Information), children learned they would chat with their partners only half the time; for the other half, they would answer questions provided by a computer about a fictional character of the same gender and age as the participant. Participants were then shown photos of two children (and had their own photo taken to enhance the live illusion), both matched to the participant's age and gender, and were told to choose one to be their chat partner (Supporting Information Figure S1). Photos were selected from the NIMH Child Emotional Faces Pictures Set (smiling, direct gaze only; Egger et al., 2011), as well as from Getty Images (http://www.gettyimages.com) and Google Images search to attain racial and ethnic diversity reflective of our participant population.
2.2.2. fMRI task design
In the scanner, children played the role of the “guesser” in a social prediction game. In each trial they received a one‐sentence hint about either their chat partner or a fictional character in a story (see Supporting Information for examples), then answered either “Which will I/she/he pick?” (Mental) or “Which of these match?” (Non‐Mental) by choosing via button‐press between two choices. Each trial was divided into two phases: “Guess” (8 s), including the hint and choice periods, and “Feedback” (2 s), in which participants learned whether their choices matched those of the chat partner or the computer (Figure 1). The task contained 96 trials. In 48 trials, the hints described mental states such as knowledge, beliefs, desires, preferences, and emotions (Mental). The other 48 hints described facts or situations about the peer or character but made no reference to mental states (Non‐Mental). Furthermore, 48 trials (24 Mental, 24 Non‐Mental) were presented in the first‐person (Peer) and the other 48 in the third‐person perspective (Character), yielding four conditions: Peer Mental, Peer Non‐Mental, Character Mental, and Character Non‐Mental. Individual trials were counterbalanced across participants between Peer and Character conditions. Throughout each trial, either the chat partner's name (Peer) or the word “Computer” (Character) was displayed at the top of the screen.
Figure 1.
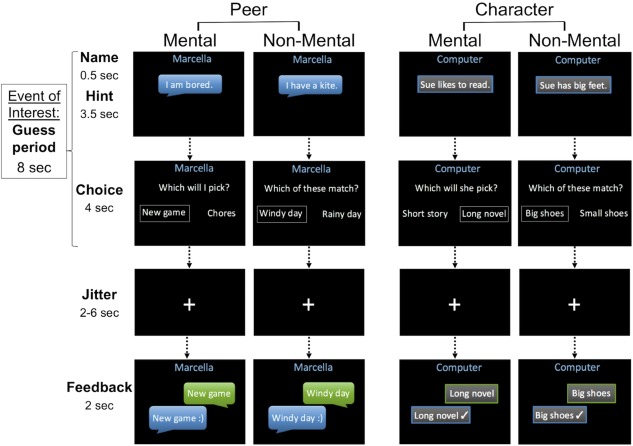
The interactive mentalizing task. Children completed 24 trials of each condition (Peer Mental, Character Mental, Peer Non‐Mental, Character Non‐Mental) in an event‐related design. Mental trials required reasoning about mental states, while Non‐Mental trials did not. In the Peer trials, children believed they were interacting with a child being scanned in another laboratory, whereas in Character trials, they believed they were answering questions about a fictional character provided by a computer. All trials had predetermined peer or computer responses. A smiley face (Peer) or check mark (Character) in the Feedback period indicated a match between the child's response and the peer or computer response [Color figure can be viewed at http://wileyonlinelibrary.com]
2.2.3. Stimuli presentation
The task was presented using PsychoPy (Peirce, 2009) in four runs of 24 trials (24 trials per condition total). Guess and Feedback periods were separated by a fixation cross presented for a jittered 2–6 s, centered around 3.5 s and distributed exponentially. Trials were separated by a fixation cross with the same jittered parameters. Trial distribution and inter‐stimulus/trial intervals were optimized using Design Explorer (Moraczewski et al., unpublished software), which minimizes collinearity between events in the design matrix. The resulting matrix was submitted to AFNI's 1d_tool program (Cox, 1996) to confirm that correlations between regressors of interest were minimal. A fixation cross was presented for 10 s at the beginning and 15 s at the end of each run. To maintain the live illusion, the chat partner's photo appeared at the end of every run.
2.2.4. Posttest questionnaire
After the scan, participants answered a series of questions in which they rated on a scale of 1–5 their preference for and attention to the live partner versus the computer. The posttest also probed participants' belief in the live illusion (see Supporting Information). Three participants who expressed disbelief in the live illusion during the posttest or debriefing were excluded from analysis.
2.3. Image acquisition and preprocessing
fMRI data were acquired at the Maryland Neuroimaging Center on a 3.0 Tesla scanner with a 32‐channel head coil (MAGNETOM Trio Tim System, Siemens Medical Solutions). Four runs of the task were acquired using multiband‐accelerated echo‐planar imaging (66 interleaved axial slices, multiband factor = 6, voxel size = 2.19 × 2.19 × 2.20 mm, repetition time = 1,250 ms, echo time = 39.4 ms, flip angle = 90°, pixel matrix = 96 × 96) followed by a structural scan (3D T1 magnetization‐prepared rapid gradient‐echo sequence, 192 contiguous sagittal slices, voxel size = 0.45 × 0.45 × 0.90 mm, repetition time = 1,900 ms, echo time = 2.32 ms, flip angle = 9°, pixel matrix = 512 × 512). Data were preprocessed using AFNI (Cox, 1996). Functional scans were slice‐time corrected. The structural scan was aligned to the first volume of a functional run and normalized to the Haskins pediatric template (nonlinear; Molfese, Glen, Mesite, Pugh, & Cox, 2015) using a 12‐parameter affine transformation, which was then applied to all functional volumes. Finally, functional data were spatially smoothed with a 5‐mm full width half‐maximum Gaussian kernel and intensity normalized to a mean of 100 per voxel.
Time points for which framewise displacement (FD) of two consecutive volumes exceeded 1 mm were censored in subsequent analyses, and runs were excluded if 10% or more of the volumes would be censored or if mean FD was 0.50 mm or greater. Two participants with fewer than three usable runs were excluded from analyses, leaving a final sample of 20 children with four runs and eight with three runs.
2.4. Data analysis
fMRI data were analyzed in AFNI using general linear models. At the first level, events of interest (Guess periods for Peer Mental, Peer Non‐Mental, Character Mental, Character Non‐Mental conditions) were convolved with the canonical hemodynamic response function using a duration modulated response function (AFNI's dmBlock). Guess and Feedback were modeled as separate events, with only the Guess periods analyzed as events of interest, as they were designed to capture the mentalizing processes relevant to the current study. To exclude task‐irrelevant cognition that might have occurred between the participant's response and the end of the response window, duration modulation was performed based on the reaction time (RT) at each Guess event, such that each modeled Guess period only lasted until the child responded. Regressors of no interest included the four Feedback conditions, the six motion parameters (x, y, z, roll, pitch, and yaw) and their derivatives, time points censored due to FD >1 mm, and polynomial terms (constant, linear, quadratic, and cubic) to model baseline and scanner drift.
At the second level, whole‐brain comparisons between the four conditions were generated using mixed‐effects multilevel analysis (3dMEMA Chen, Saad, Nath, Beauchamp, & Cox, 2012) to model within‐ and between‐subject variability. In addition to the main effect of mentalizing ([Peer Mental + Character Mental] vs. [Peer Non‐Mental + Character Non‐Mental]), the main effect of social interaction ([Peer Mental + Peer Non‐Mental] vs. [Character Mental + Character Non‐Mental]), and their interaction, we conducted pairwise comparisons to isolate the effect of mentalizing in the offline (Character Mental vs. Character Non‐Mental) and social‐interactive (Peer Mental vs. Peer Non‐Mental) contexts separately, as well as the effect of social interaction within Mental and Non‐Mental conditions respectively (Peer Mental vs. Character Mental; Peer Non‐Mental vs. Character Non‐Mental). Each model included age and mean FD as covariates. The same Haskins pediatric template used to normalize the data was resampled to match the functional data and then used as a structural mask (i.e., only voxels within this mask were analyzed). Contrast maps were first thresholded at p < .005 (two‐tailed), then cluster corrected at alpha = 0.05 (k = 86, bi‐sided, second nearest‐neighbor). The cluster‐size threshold was determined by averaging individual participants' non‐Gaussian spatial autocorrelation function parameters and inputting these values (a = 0.51, b = 2.91, c = 7.26) to 3dClustSim according to recent recommendations (Cox, Chen, Glen, Reynolds, & Taylor, 2017).
To determine regions active during both offline mentalizing and social interaction without explicit mentalizing demands, we performed a conjunction analysis by multiplying the binarized, corrected group maps for the Character Mental > Character Non‐Mental and Peer Non‐Mental > Character Non‐Mental contrasts to identify voxels significant for both contrasts (Nichols, Brett, Andersson, Wager, & Poline, 2005).
Analysis of behavioral performance and regions of interest (ROIs) were conducted in R Core Team (2016). RT in seconds and accuracy (percent correct responses) were each entered into a two‐way repeated measures ANOVA to determine the main effects of mentalizing (Mental vs. Non‐Mental) and social interaction (Peer vs. Character), and their interaction. Significant results were followed up with paired t tests. Posttest questionnaire data were analyzed using Wilcoxon signed rank tests to compare ordinal ratings between Peer and Character conditions.
As a post hoc exploration of activation within the mentalizing network during social interaction, we used the Character Mental > Character Non‐Mental contrast to define “offline mentalizing” ROIs, then extracted individual beta values for each condition versus baseline. To examine the relationship between age and activation of mentalizing regions, we extracted individual beta values for each condition versus baseline from ROIs defined by the Mental > Non‐Mental contrast (without age as a covariate), then created difference scores for Mental versus Non‐Mental and Peer versus Character conditions, respectively. We conducted partial correlations between these scores and age, controlling for mean FD, which was significantly correlated with age (r = −0.38, p < .05). For the ROI analyses, p‐values were corrected for multiple comparisons using Holm's sequentially rejective Bonferroni test, which is more powerful than the classical Bonferroni test (Holm, 1979).
3. RESULTS
3.1. Behavioral
Overall in‐scanner performance was high (mean accuracy = 91% correct, SD = 7%; mean RT = 2.04 s, SD = 0.27 s). A repeated measures ANOVA indicated a significant main effect of social interaction (Peer vs. Character) on RT (F[1, 27] = 11.61, p < .005; Figure 2a); a paired t test revealed that children responded more quickly in Peer than in Character conditions (mean difference = 0.07 s, t[55] = 3.74, p < .001). The main effect of mentalizing (Mental vs. Non‐Mental) and the interaction term were not statistically significant for RT (p > .05). No statistically significant effects were found for accuracy.
Figure 2.
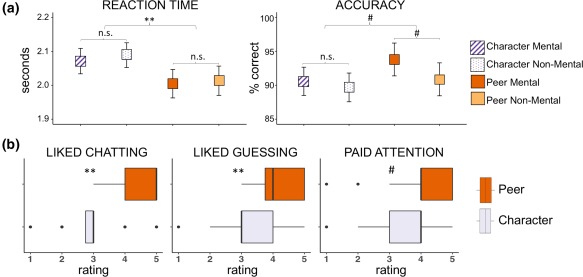
Behavioral results. (a). In‐scanner performance by condition. Mean values are plotted for RT (seconds) and accuracy (% correct) for each of the four conditions. Repeated measures ANOVA indicated a significant main effect of social interaction on RT such that children responded more quickly on Peer than Character trials. Error bars represent 95% CIs. #p < .1; **p < .005. (b) Posttest questionnaire. For Peer and Character conditions separately, children rated on a Likert‐type scale of 1–5 how much they enjoyed interacting with their partners (Peer) and answering questions from the computer (Character), how much they liked guessing what their partners would pick (Peer) and what came next in the story (Character), and how much they paid attention when interacting with their partners (Peer) and when answering questions from the computer (Character). Wilcoxon signed rank tests were used to compare ratings between Peer and Character conditions. #p < .1; **p < .005 [Color figure can be viewed at http://wileyonlinelibrary.com]
Posttest questionnaires indicated a general preference for Peer over Character conditions (Figure 2b). Specifically, participants gave significantly higher ratings (on an ordinal scale of 1–5) for how much they liked interacting with their partners versus answering questions from the computer (median Peer = 5, median Character = 3, p < .001) and how much they liked guessing what their partners would pick versus guessing what would come next in the story (median Peer = 4, median Character = 3, p < .005). There was a trend of children reporting that they paid more attention during Peer than Character conditions (median Peer = 4, median Character = 4, p = .05).
3.2. Neuroimaging
3.2.1. Effect of mentalizing
Whole‐brain analyses revealed a main effect of mentalizing ([Peer Mental + Character Mental] vs. [Peer Non‐Mental + Character Non‐Mental]) in several regions identified in previous mentalizing studies, including right dMPFC, left TPJ, and bilateral STS and ATL (Figure 3, Table 1). A similar pattern of activation emerged for the pairwise comparison of Character Mental versus Character Non‐Mental, albeit in smaller clusters and without TPJ or dMPFC (Figure 4, Table 1). In contrast, no regions were significantly more active for Peer Mental than Peer Non‐Mental.
Figure 3.
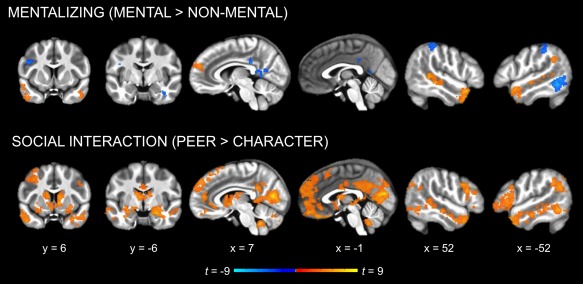
Whole‐brain analysis of the main effects of mentalizing and social interaction (cluster corrected p < .05). Mentalizing (Mental vs. Non‐Mental) activated regions previously identified in the mentalizing literature (dMPFC, TPJ, STS, and ATL). Social interaction (Peer vs. Character) activated similar regions, as well as additional cortical midline regions and subcortical structures associated with reward (e.g., amygdala, striatum) [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 1.
Whole‐brain results for main effects of mentalizing, social interaction, the interaction term, and pairwise comparisons between the four conditions
| Region | Side | Peak t | βa | Cluster k | MNI coordinates | |||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Main Effect of Mentalizing | ||||||||
| Mental > Non‐Mental | ||||||||
| ATL | R | 6.25 | 0.37 | 414 | 52 | 18 | −20 | |
| STS | R | 6.96 | 0.27 | 407 | 54 | −40 | 2 | |
| ATL | L | 5.61 | 0.24 | 375 | −50 | 10 | −18 | |
| Lateral OFC/insulab | L | 4.90 | 0.23 | −39 | 18 | −18 | ||
| STS | L | 5.19 | 0.15 | 147 | −58 | −23 | −2 | |
| dMPFC | R | 4.81 | 0.23 | 140 | 5 | 56 | 21 | |
| TPJ | L | 5.29 | 0.18 | 109 | −56 | −48 | 29 | |
| Non‐Mental > Mental | ||||||||
| Inferior parietal cortex | L | 6.97 | 0.25 | 747 | −24 | −71 | 47 | |
| Inferior temporal gyrus | L | 7.63 | 0.28 | 585 | −54 | −54 | −9 | |
| Fusiform gyrus | L | 7.26 | 0.33 | 390 | −28 | −30 | −20 | |
| Inferior parietal cortex | R | 5.04 | 0.22 | 295 | 33 | −71 | 45 | |
| PCC | R | 5.31 | 0.27 | 273 | 12 | −52 | 9 | |
| Fusiform gyrus | R | 5.66 | 0.22 | 256 | 35 | −24 | −23 | |
| Lateral OFC | L | 6.73 | 0.22 | 191 | −33 | 37 | −11 | |
| PCC | L | 6.67 | 0.20 | 167 | −5 | −54 | 11 | |
| SMG/inferior parietal cortex | R | 5.53 | 0.13 | 157 | 46 | −38 | 45 | |
| SMG/postcentral gyrus | L | 4.86 | 0.17 | 114 | −54 | −33 | 45 | |
| PCC | L | 4.75 | 0.15 | 91 | 8 | −33 | 29 | |
| IFGtri | R | 5.33 | 0.30 | 87 | 50 | 43 | 14 | |
| IFGoper | L | 5.43 | 0.19 | 86 | −44 | 6 | 27 | |
| Main Effect of Social Interaction | ||||||||
| Character > Peer | ||||||||
| Pericalcarine/cuneus | R | 9.14 | 0.42 | 19,206 | 10 | −73 | 11 | |
| Putamen/AMY/hippocampusb | L | 8.82 | 0.19 | −31 | −19 | −9 | ||
| dMPFC/ACCb | L | 7.83 | 0.20 | −12 | 39 | 18 | ||
| ATLb | R | 7.32 | 0.34 | 48 | 16 | −29 | ||
| Caudate/putamenb | R | 6.96 | 0.21 | 10 | 5 | −1 | ||
| Lateral OFC/insulab | L | 6.84 | 0.31 | −41 | 17 | −16 | ||
| Precuneusb | L | 6.80 | 0.26 | −12 | −48 | 25 | ||
| AMY/putamen/insulab | R | 6.76 | 0.19 | 25 | −7 | −20 | ||
| Caudateb | L | 6.74 | 0.15 | −12 | 17 | 10 | ||
| Caudateb | R | 6.71 | 0.16 | 10 | 9 | 12 | ||
| PCC/LGb | R | 6.52 | 0.30 | 18 | −46 | −2 | ||
| Thalamusb | L | 6.51 | 0.35 | −6 | −4 | 9 | ||
| Inferior temporal gyrusb | L | 6.37 | 0.21 | −50 | −56 | −9 | ||
| Inferior parietal cortexb | R | 6.29 | 0.24 | 33 | −73 | 38 | ||
| IFGoper b | R | 6.24 | 0.25 | 52 | 12 | 32 | ||
| PHGb | R | 6.20 | 0.22 | 18 | −37 | −13 | ||
| dMPFCb | R | 6.01 | 0.30 | 4 | 45 | 11 | ||
| Caudate/ventral striatumb | L | 5.90 | 0.21 | −5 | 10 | 0 | ||
| Lateral occipital cortexb | R | 5.70 | 0.19 | 40 | −77 | −3 | ||
| Middle temporal gyrusb | R | 5.65 | 0.19 | 59 | −17 | −18 | ||
| Medial OFCb | R | 5.45 | 0.32 | 4 | 44 | −15 | ||
| STSb | R | 5.32 | 0.25 | 46 | −38 | 2 | ||
| Superior frontal gyrusb | R | 5.11 | 0.15 | 14 | 35 | 50 | ||
| Inferior parietal cortexb | R | 4.89 | 0.23 | 50 | −67 | 34 | ||
| Cuneusb | L | 4.82 | 0.64 | −1 | −79 | 38 | ||
| Lateral OFCb | R | 4.48 | 0.19 | 42 | 26 | −4 | ||
| Inferior parietal cortex/TPJb | R | 4.36 | 0.27 | 61 | −56 | 18 | ||
| Inferior parietal cortex/TPJ | L | 8.16 | 0.38 | 1,572 | −37 | −71 | 40 | |
| Cerebellum | R | 6.49 | 0.25 | 915 | 20 | −73 | −32 | |
| Cerebellum | L | 5.26 | 0.24 | 368 | −16 | −92 | −30 | |
| IFGoper | R | 6.24 | 0.25 | 310 | 52 | 12 | 32 | |
| Cerebellum | R | 6.93 | 0.26 | 260 | 3 | −51 | −39 | |
| Lateral OFC | R | 4.48 | 0.19 | 148 | 42 | 26 | −4 | |
| Character > Peer | ||||||||
| Lateral occipital cortex | L | 5.44 | 0.19 | 123 | −14 | −98 | 4 | |
| Interaction Effect (Mentalizing x Social Interaction) | ||||||||
| (Peer Mental > Peer Non‐Mental) > (Character Mental > Character Non‐Mental) | ||||||||
| None | ||||||||
| Effect of Character Mental vs. Character Non‐Mental | ||||||||
| Character Mental > Character Non‐Mental | ||||||||
| STS | R | 7.11 | 0.18 | 368 | 54 | −40 | 2 | |
| ATL | L | 5.79 | 0.17 | 357 | −56 | 4 | −13 | |
| Lateral OFC/insulab | L | 5.27 | 0.18 | −41 | 20 | −20 | ||
| ATL | R | 7.49 | 0.21 | 241 | 52 | 14 | −20 | |
| STS | L | 5.27 | 0.10 | 99 | −58 | −23 | −2 | |
| IFGtri | R | 5.21 | 0.16 | 93 | 52 | 28 | 0 | |
| Character Non‐Mental > Character Mental | ||||||||
| Inferior temporal gyrus | L | 4.58 | 0.14 | 159 | −54 | −54 | −9 | |
| Effect of Peer Mental vs. Peer Non‐Mental | ||||||||
| Peer Mental > Peer Non‐Mental | ||||||||
| None | ||||||||
| Peer Non‐Mental > Peer Mental | ||||||||
| Inferior parietal cortex | L | 5.77 | 0.14 | 561 | −29 | −62 | 45 | |
| Inferior temporal gyrus | L | 5.60 | 0.17 | 421 | −56 | −56 | −9 | |
| Fusiform gyrus | L | 7.12 | 0.17 | 379 | −28 | −38 | −14 | |
| PCC | L | 4.42 | 0.09 | 180 | −9 | −48 | 13 | |
| Middle frontal gyrus | L | 6.38 | 0.14 | 160 | −27 | 17 | 52 | |
| Postcentral gyrus | L | 4.77 | 0.12 | 143 | −29 | −29 | 65 | |
| IFGoper | L | 5.93 | 0.15 | 137 | −44 | 8 | 27 | |
| Inferior temporal gyrus | R | 5.41 | 0.10 | 128 | 59 | −34 | −20 | |
| Fusiform gyrus | R | 5.50 | 0.16 | 124 | 33 | −24 | −23 | |
| Lateral OFC | L | 4.78 | 0.16 | 101 | −31 | 33 | −11 | |
| Inferior parietal cortex | L | 4.46 | 0.08 | 96 | −44 | −50 | 50 | |
| IFGtri | L | 6.10 | 0.13 | 95 | −41 | 37 | 14 | |
| Effect of Peer Non‐Mental vs. Character Non‐Mental | ||||||||
| Peer Non‐Mental > Character Non‐Mental | ||||||||
| dMPFC | L | 8.40 | 0.16 | 2,946 | −12 | 56 | 30 | |
| dMPFC/ACCb | L | 5.59 | 0.12 | −12 | 39 | 21 | ||
| ACC/medial OFCb | L | 7.49 | 0.14 | −5 | 33 | −2 | ||
| PHG/Fusiform gyrus /PCC/LG | L | 7.43 | 0.11 | 2,808 | −20 | −34 | −14 | |
| LG/cuneus/pericalcarineb | R | 6.89 | 0.25 | 8 | −71 | 11 | ||
| LGb | R | 4.81 | 0.13 | 20 | −48 | −2 | ||
| PCCb | L | 4.74 | 0.14 | −9 | −48 | 25 | ||
| Lateral OFC | L | 6.55 | 0.31 | 2,212 | −35 | 22 | −25 | |
| IFGtri b | L | 6.20 | 0.14 | −39 | 25 | 3 | ||
| ATLb | L | 5.94 | 0.27 | −50 | 16 | −24 | ||
| Insula/medial OFC/AMYb | L | 5.20 | 0.13 | −26 | 5 | −13 | ||
| Inferior parietal cortex/TPJ | L | 6.00 | 0.17 | 1,123 | −41 | −64 | 27 | |
| Inferior parietal cortex/TPJb | L | 5.34 | 0.09 | −39 | −50 | 31 | ||
| ATL | R | 7.12 | 0.26 | 713 | 50 | 18 | −29 | |
| Lateral OFCb | R | 6.15 | 0.13 | 25 | 12 | −16 | ||
| AMYb | R | 5.66 | 0.11 | 29 | −5 | −22 | ||
| Caudate | L | 5.44 | 0.12 | 575 | −12 | 16 | 9 | |
| Thalamusb | L | 4.75 | 0.13 | −6 | −3 | 6 | ||
| Caudateb | R | 4.53 | 0.08 | 8 | 8 | 13 | ||
| Ventral striatumb | L | 4.24 | 0.11 | −7 | 8 | −11 | ||
| Ventral striatumb | R | 3.91 | 0.13 | 5 | 8 | −9 | ||
| IFGoper | R | 5.57 | 0.13 | 487 | 46 | 14 | 23 | |
| Cerebellum | R | 5.28 | 0.14 | 394 | 20 | −75 | −32 | |
| Lateral occipital cortex | R | 5.60 | 0.10 | 367 | 31 | −77 | 9 | |
| Middle frontal gyrus | L | 5.03 | 0.10 | 285 | −29 | 4 | 45 | |
| Middle temporal gyrus | L | 5.23 | 0.17 | 273 | −58 | −23 | −16 | |
| STS | R | 5.46 | 0.14 | 229 | 54 | −38 | 2 | |
| Cerebellum | L | 6.19 | 0.11 | 216 | −11 | −71 | −30 | |
| Middle temporal gyrus | L | 4.64 | 0.13 | 208 | −63 | −50 | 2 | |
| Inferior temporal gyrusb | L | 3.74 | 0.10 | −47 | −53 | −8 | ||
| IFGorb | R | 5.96 | 0.17 | 205 | 44 | 24 | −2 | |
| Inferior parietal cortex/TPJ | R | 5.16 | 0.14 | 183 | 44 | −56 | 27 | |
| PHG | R | 5.35 | 0.14 | 154 | 18 | −38 | −14 | |
| Fusiform gyrusb | R | 4.27 | 0.09 | 35 | −38 | −18 | ||
| Cerebellum | R | 4.58 | 0.15 | 87 | 8 | −51 | −39 | |
| Character Non‐Mental > Peer Non‐Mental | ||||||||
| None | ||||||||
| Effect of Peer Mental vs. Character Mental | ||||||||
| Peer Mental > Character Mental | ||||||||
| Pericalcarine/cuneus/LG | R | 6.87 | 0.13 | 1,654 | 16 | −71 | 11 | |
| PCCb | R | 5.04 | 0.13 | 14 | −38 | −2 | ||
| Hippocampusb | L | 5.76 | 0.16 | −14 | −36 | −5 | ||
| dMPFC | L | 5.70 | 0.15 | 700 | −7 | 62 | 14 | |
| Cerebellum | R | 6.15 | 0.10 | 444 | 20 | −73 | −30 | |
| Inferior parietal cortex/TPJ | L | 5.41 | 0.15 | 426 | −37 | −71 | 40 | |
| PCC/precuneus | L | 5.80 | 0.15 | 362 | −12 | −46 | 25 | |
| Inferior/middle temporal gyrus | L | 4.59 | 0.11 | 272 | −50 | −54 | −9 | |
| AMY/putamen/hippocampus | R | 5.87 | 0.14 | 231 | 20 | −9 | −9 | |
| Hippocampus/AMY/ventral DC | L | 5.34 | 0.15 | 168 | −26 | −19 | −9 | |
| Cerebellum | R | 7.13 | 0.13 | 147 | 3 | −55 | −41 | |
| Cuneus/precuneus | R | 4.41 | 0.29 | 138 | 1 | −77 | 31 | |
| Cerebellum | L | 4.67 | 0.13 | 119 | −26 | −75 | −37 | |
| Character Mental > Peer Mental | ||||||||
| Paracentral gyrus | R | 4.87 | 0.07 | 150 | 5 | −31 | 63 | |
| Lateral occipital cortex | L | 5.95 | 0.16 | 146 | −9 | −104 | −3 | |
β coefficient at the peak t value.
Sub‐peaks within clusters.
ACC = anterior cingulate cortex; AMY = amygdala; dMPFC = dorsomedial prefrontal cortex; IFGoper = inferior frontal gyrus (pars opercularis); IFGorb = inferior frontal gyrus (pars orbitalis); IFGtri = inferior frontal gyrus (pars triangularis); LG = lingual gyrus; OFC = orbitofrontal cortex; PCC = posterior cingulate cortex; PHG = parahippocampal gyrus; SMG = supramarginal gyrus; TPJ = temporoparietal junction; Ventral DC = Ventral diencephalon.
Figure 4.
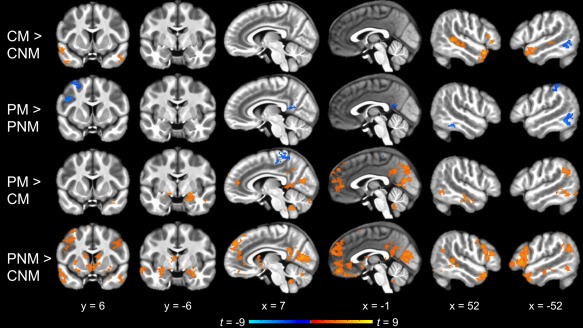
Whole‐brain pairwise comparisons between the four conditions (cluster corrected p < .05). Offline mentalizing (Character Mental vs. Character Non‐Mental) elicited a pattern of activation similar to the main effect of mentalizing (Figure 3). In contrast, no regions were significantly more active for Peer Mental than Peer Non‐Mental. Social interaction without explicit mentalizing demands (Peer Non‐Mental vs. Character Non‐Mental) recruited similar regions as in the main effect of social interaction (Figure 3), whereas a smaller subset of these regions was more active for mentalizing within social interaction than offline mentalizing (Peer Mental vs. Character Mental). CM, Character Mental; CNM, Character Non‐Mental; PM, Peer Mental; PNM, Peer Non‐Mental [Color figure can be viewed at http://wileyonlinelibrary.com]
3.2.2. Effect of social interaction
A test of the main effect of social interaction ([Peer Mental + Peer Non‐Mental] vs. [Character Mental + Character Non‐Mental]) revealed extensive activation, including anterior and posterior midline regions (dMPFC, medial OFC, anterior cingulate cortex, PCC, precuneus); bilateral IFG and lateral OFC; bilateral insula; bilateral STS and ATL; bilateral inferior parietal cortex extending into TPJ; medial occipital regions (cuneus, pericalcarine, and lingual cortex) extending into the fusiform gyri, parahippocampal gyri, and hippocampus; bilateral middle and left inferior temporal cortex; and subcortical structures (striatum, amygdala, thalamus, and cerebellum; Figure 3, Table 1). Most of the same regions were activated to a lesser extent by the pairwise comparison of Peer Non‐Mental versus Character Non‐Mental (Figure 4, Table 1). The contrast of Peer Mental vs. Character Mental yielded still more limited activation along the same general patterns, with notably less activation in bilateral STS and ATL and no activation in right TPJ or bilateral IFG (Figure 4, Table 1).
3.2.3. Interaction effect
Whole‐brain analysis of the interaction term ([Peer Mental vs. Peer Non‐Mental] vs. [Character Mental vs. Character Non‐Mental]) revealed no significant activation.
3.2.4. Shared regions for mentalizing and social interaction
To examine shared regions for mentalizing and social interaction, we conducted a conjunction analysis to identify voxels that were significantly activated for both Character Mental > Character Non‐Mental (i.e., the offline mentalizing task) and Peer Non‐Mental > Character Non‐Mental (i.e., social interaction with no explicit mentalizing demands). This analysis revealed overlapping activation in bilateral ATL, right posterior STS, left lateral OFC and insula, and right IFG (Figure 5, Table 2).
Figure 5.
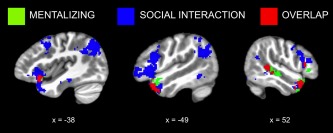
Shared regions for mentalizing and social interaction. Binarized, cluster‐corrected maps for offline mentalizing (Character Mental > Character Non‐Mental; green) and social interaction without mentalizing demands (Peer Non‐Mental > Character Non‐Mental; blue) are shown along with their conjunction (red), which reveals both overlapping and distinct regions of activation [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 2.
Conjunction analysis: Overlapping activation between Character Mental > Character Non‐Mental and Peer Non‐Mental > Character Non‐Mental contrasts
| Region | Side |
Cluster k |
MNI Coordinates | ||
|---|---|---|---|---|---|
| x | y | z | |||
| ATL/lateral OFC/insula | L | 233 | −43 | 15 | −19 |
| ATL | R | 174 | 48 | 15 | −27 |
| Posterior STS | R | 144 | 52 | −37 | 3 |
| IFGoper | R | 29 | 53 | 23 | 13 |
Coordinates are reported for the center of mass of each cluster. Clusters of fewer than 20 voxels are not reported.
IFGoper = inferior frontal gyrus (pars opercularis); OFC = orbitofrontal cortex.
We next examined activation within the offline mentalizing ROIs (Character Mental > Character Non‐Mental; Figure 6). Paired t tests indicated nonsignificant differences between Peer Mental and Peer Non‐Mental conditions in all ROIs except right ATL (mean difference = 0.10, t(27) = 3.60, p < .01 corrected). Comparison between Character Mental and Peer Non‐Mental conditions revealed no significant differences in activation in any regions. Altogether, this ROI analysis suggests that the two Peer conditions elicited similar activation of mentalizing regions regardless of task demands.
Figure 6.
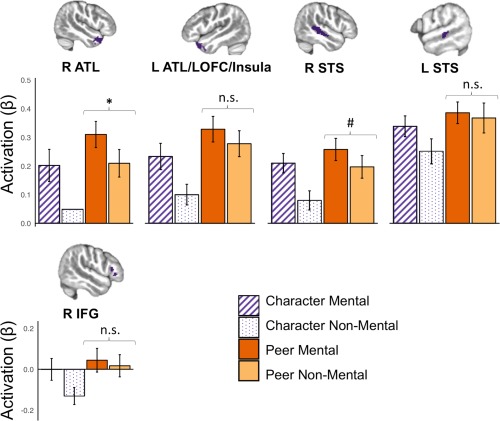
ROI analysis of mentalizing regions during social interaction. ROIs were defined by the Character Mental > Character Non‐Mental contrast. Individual beta values for each condition within each ROI were extracted; average values are plotted with error bars representing 95% CIs. Paired t tests indicated nonsignificant differences between Peer Mental and Peer Non‐Mental in all ROIs except right ATL, as well as nonsignificant differences between Character Mental and Peer Non‐Mental in all ROIs. ATL, anterior temporal lobe; IFG, inferior frontal gyrus; LOFC, lateral orbitofrontal cortex; STS, superior temporal sulcus. * p < .05 corrected; #p < .1 corrected [Color figure can be viewed at http://wileyonlinelibrary.com]
3.2.5. Age effects
Whole‐brain analysis showed no significant effects of age on the Mental versus Non‐Mental contrast. A follow‐up ROI analysis found no significant correlations between age and mentalizing activity within mentalizing ROIs (Mental > Non‐Mental).
Conversely, the whole‐brain Peer versus Character contrast revealed a negative effect of age in many frontal, temporal, insular, and subcortical areas (Supporting Information Table S1, Figure 7a). Analysis of the same mentalizing ROIs as above found that age was significantly (p < .05 corrected) negatively correlated with activation to Peer versus Character conditions in right ATL (r = −0.47), left ATL/lateral OFC/insula (r = −0.51), dMPFC (r = −0.43), right STS (r = −0.59), left STS (r = −0.42), and left TPJ (r = −0.55). However, correlations between age and average activation to Peer and Character conditions, respectively, did not reach significance.
Figure 7.
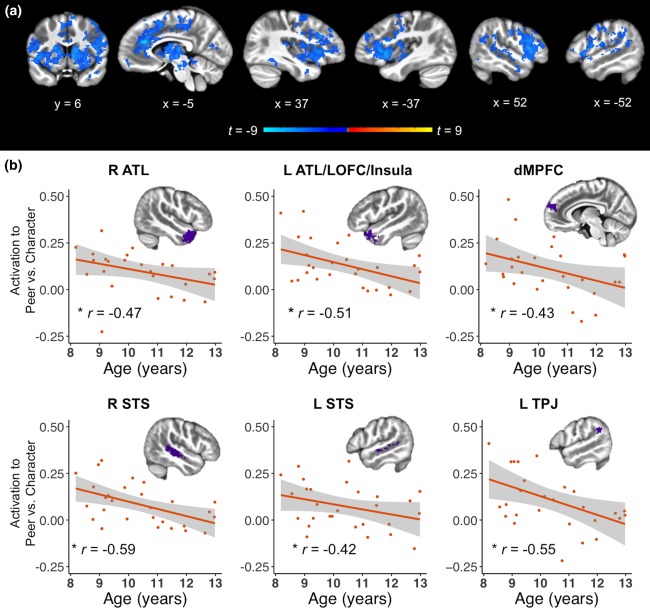
Effects of age on neural response to social interaction. (a) Whole‐brain effects of age on social interaction (Peer vs. Character; cluster corrected p < .05). Differences in activation to Peer versus Character conditions decreased with age in several frontal, temporal, insular, and subcortical areas. (b) Effect of age on mentalizing ROIs. ROIs were defined by the main effect of mentalizing (Mental > Non‐Mental). All regions showed a significant negative correlation between age and difference in activation to Peer versus Character conditions. ATL, anterior temporal lobe; dMPFC, dorsomedial prefrontal cortex; LOFC, lateral orbitofrontal cortex; STS, superior temporal sulcus; TPJ, temporoparietal junction. * p < .05 corrected [Color figure can be viewed at http://wileyonlinelibrary.com]
4. DISCUSSION
This study examined the effect of perceived social interaction on brain activation in the context of a mentalizing task performed by children aged 8–12. By manipulating both social interaction and mentalizing within the same participants, we were able to directly assess shared and distinct neural mechanisms associated with each factor. Social interaction engaged many of the same regions as the offline mentalizing task, even in the absence of explicit mentalizing demands from the task. Moreover, social interaction elicited more extensive activation in some regions associated with mentalizing, as well as regions outside the mentalizing network, including the reward system. These results illuminate an understudied period of development and underscore the need for social‐interactive paradigms to accurately characterize real‐world social processing.
Our hypothesis regarding the main effect of mentalizing was broadly supported. That is, across Peer and Character conditions, the Mental versus Non‐Mental contrast revealed a pattern of activation consistent with the prior literature (Schurz et al., 2014). These results add to the sparse literature on the neural correlates of social cognition in middle childhood by showing that the mentalizing system characterized in adults is generally established by ages 8–12.
Examination of the main effect of social interaction revealed greater activation for Peer versus Character conditions in all major components of the mentalizing network, including anterior and posterior midline and lateral temporal regions. In line with our hypothesis that spontaneous mentalizing occurs during social interaction in the absence of explicit mentalizing demands, a similar activation pattern emerged for the Peer Non‐Mental versus Character Non‐Mental contrast. As a stronger test of this interpretation, we performed a conjunction analysis to identify specific regions activated by both the offline mentalizing task and social interaction without mentalizing demands, which revealed several overlapping areas. Additionally, ROI analyses suggested that offline mentalizing regions were similarly activated by Character Mental, Peer Mental, and Peer Non‐Mental conditions (Figure 6). Furthermore, the whole‐brain contrast of Peer Mental > Peer Non‐Mental revealed no significant activation, consistent with there being comparable recruitment of mentalizing regions in both Peer conditions. Finally, though dMPFC and bilateral TPJ—the regions most consistently activated across previous mentalizing studies (Schurz et al., 2014)—were not significantly activated by our offline mentalizing task (Character Mental > Character Non‐Mental), they were engaged by social interaction (e.g., Peer Non‐Mental > Character Non‐Mental; Figure 4). Together, these findings provide the strongest evidence to date that social interaction induces mentalizing even when the task does not explicitly require it. Additional support for this could come from future studies that link activation of mentalizing regions during social interaction to measures outside the scanner of mentalizing ability or propensity.
Our ROI analysis indicated that for most regions that showed a significant difference between Character Mental and Character Non‐Mental conditions, activation was similar for Peer Mental and Peer Non‐Mental. Based on this, we might have expected mentalizing regions to show a 2 (Peer vs. Character) × 2 (Mental vs. Non‐Mental) interaction effect at the whole‐brain level, but this was not the case, probably due to a lack of statistical power. We also did not find the opposite interaction pattern, that is, a greater difference in activation between Mental and Non‐Mental in Peer versus Character conditions. Such a finding may have indicated an additive effect of social context and explicit mentalizing demands such that activation of certain regions would be greatest in the Peer Mental condition. This effect, if it exists, may be revealed by a future study with a larger sample size, or through analysis of a different set of ROIs than those examined in the current study.
We were also interested in how social interaction modulates the reward network. Taken together, our neuroimaging and behavioral results suggest that participants found Peer conditions more rewarding or motivating than Character conditions. The main effect of social interaction revealed activation in several components of the reward system, including medial OFC, dorsal and ventral striatum, thalamus, and amygdala (Berridge & Kringelbach, 2015; Schultz, 2015). Supporting our interpretation of this activation as reflecting subjective feelings of motivation and reward, participants' responses to the posttest questionnaire indicated greater enjoyment for Peer than Character conditions. Because the Feedback period was modeled as a covariate of no interest, it is unlikely that this activation reflects positive feelings directly resulting from participants learning that their responses matched those of their peers. Rather, our results could be driven by anticipation of such a reward in the Feedback period, hedonic response to the Guess period itself, or both. Additionally, faster responses in Peer than in Character trials may reflect the participants' heightened motivation to interact with their partners. Overall, our results add to extant evidence that social interaction is intrinsically rewarding (Chevallier et al., 2012; Pfeiffer et al., 2014; Schilbach et al., 2010; Warnell et al., 2018).
We also found that social interaction recruits areas outside both the mentalizing and reward networks. Peer more than Character conditions activated large portions of medial occipital cortex, which has been associated with mental imagery (Kosslyn et al., 1999; Kosslyn, Thompson, Klm, & Alpert, 1995), and medial temporal regions linked to memory processes (Eichenbaum, Yonelinas, & Ranganath, 2007). Because participants were shown photos of their partners before the scan and after each run, this activation could result from their recollection of these images during Peer trials. Future studies should explore whether visualization of one's social partner is inherent to social interaction (especially when one's partner is physically remote), as well as how social processing interacts with memory encoding and retrieval.
We found no effect of age on activation of mentalizing regions to Mental versus Non‐Mental conditions. Instead, activation of these regions to Peer versus Character conditions decreased with age. These results are at odds with previous findings that over middle childhood, mentalizing regions become increasingly selective for belief representation (although our age range is narrower and slightly older than that of Gweon et al., 2012, which included ages 5–11) and social interaction (Warnell et al., 2018). However, these findings should be interpreted with caution given the possibility that the current and previous studies of this nature are underpowered to detect what may be subtle between‐subjects effects (e.g., Cremers, Wager, & Yarkoni, 2017). Nevertheless, our analysis of mentalizing ROIs showed a consistent pattern, with differences in activation to Peer versus Character conditions decreasing with age in all ROIs. Though correlations between age and activation to Peer and Character conditions, respectively, did not reach significance, the decreasing difference may have been driven by increasing mentalizing in response to Character but not Peer conditions, which would accord with previous findings of increasing activation of dMPFC to noninteractive social stimuli across middle childhood (Rice et al., 2016) and in adolescence relative to adulthood (reviewed in Blakemore, 2008). It is also possible that our task is more similar to the real‐life peer interactions of younger than older children. Prior research suggests that while younger children's friendships are based around common activities and other superficial aspects, children approaching adolescence increasingly value “empathy, understanding, and self‐disclosure” (Bigelow, 1977)—in other words, a level of intimacy unattainable within the constraints of our paradigm and with an unfamiliar peer. Still, these results warrant further investigation using larger—and ideally, longitudinal—samples to more firmly establish how the social‐interactive brain develops from childhood through adolescence.
Another limitation of our modest sample size is that we were unable to assess gender differences in brain activation related to mentalizing or social interaction. In adults, there is evidence of gender differences in the neural correlates of social cognition, though the direction of effects and the specific brain regions involved vary across studies (Adenzato et al., 2017; Frank, Baron‐Cohen, & Ganzel, 2015; Krach et al., 2009; Veroude, Jolles, Croiset, & Krabbendam, 2014). In middle childhood, some behavioral studies indicate a female advantage for mentalizing (e.g., Devine & Hughes, 2013), which may relate to differential styles of interacting with peers, with girls more likely to form intimate relationships that demand perspective‐taking (Maccoby, 1990, as cited in Devine & Hughs, 2013). Whether these behavioral differences are mirrored by differences in brain activation during social interaction in middle childhood is yet unknown. Also unclear is whether the apparent gender differences pertain to mentalizing ability—which may be captured by offline tasks with explicit mentalizing demands—or the propensity to spontaneously mentalize in the context of a real‐time social interaction. With a larger sample, our interactive mentalizing task may be particularly well‐suited to answering these questions.
In sum, this study provides direct evidence that mentalizing and engagement with a social partner recruit many of the same neural substrates. Furthermore, social interaction elicits activation well beyond these offline mentalizing regions, including the reward system. Beyond advancing our nascent understanding of the social brain in middle childhood, the findings of this and other social‐interactive studies may enable important insights into disorders such as autism spectrum disorder and social anxiety, which are defined by difficulties in real‐world social interactions. Our ability to characterize these difficulties at the neural level hinges on developing an ecologically valid model of how the typical brain functions in the presence of other minds.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information Figure
Supporting Information Table
ACKNOWLEDGEMENTS
The authors wish to thank Ruth Ludlum, Sydney Maniscalco, Tova Rosenthal, Sabine Huber, Alex Mangerian, Zoey Maggid, Hunter Rogoff, Morgan Biggs, Alexandra Hickey, Laura Anderson Kirby, Dustin Moraczewski, and the Maryland Neuroimaging Center staff for their assistance with data collection and analysis. We also thank Dr Peter Carruthers and Dr Donald J. Bolger for their advice on task design and discussion of an early version of the article.
Alkire D, Levitas D, Warnell KR, Redcay E. Social interaction recruits mentalizing and reward systems in middle childhood. Hum Brain Mapp. 2018;39:3928–3942. 10.1002/hbm.24221
Funding information University of Maryland and a grant from the National Institute of Mental Health, Grant/Award Number: R01‐MH107441
REFERENCES
- Adenzato, M. , Brambilla, M. , Manenti, R. , De Lucia, L. , Trojano, L. , Garofalo, S. , … Cotelli, M. (2017). Gender differences in cognitive Theory of Mind revealed by transcranial direct current stimulation on medial prefrontal cortex. Scientific Reports, 7, 41219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek, E. C. , Scholz, C. , O'Donnell, M. B. , & Falk, E. B. (2017). The value of sharing information: a neural account of information transmission. Psychological Science, 28(7), 851–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge, K. C. , & Kringelbach, M. L. (2015). Pleasure systems in the brain. Neuron, 86(3), 646–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigelow, B. J. (1977). Children's friendship expectations: a cognitive‐developmental study. Child Development, 48(1), 246–253. [Google Scholar]
- Blakemore, S.‐J. (2008). The social brain in adolescence. Nature Reviews Neuroscience, 9(4), 267–277. [DOI] [PubMed] [Google Scholar]
- Calder, A. J. , Lawrence, A. D. , Keane, J. , Scott, S. K. , Owen, A. M. , Christoffels, I. , & Young, A. W. (2002). Reading the mind from eye gaze. Neuropsychologia, 40(8), 1129–1138. [DOI] [PubMed] [Google Scholar]
- Caruana, N. , Brock, J. , & Woolgar, A. (2015). A frontotemporoparietal network common to initiating and responding to joint attention bids. NeuroImage, 108, 34–46. [DOI] [PubMed] [Google Scholar]
- Chen, G. , Saad, Z. S. , Nath, A. R. , Beauchamp, M. S. , & Cox, R. W. (2012). FMRI group analysis combining effect estimates and their variances. Neuroimage, 60(1), 747–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier, C. , Kohls, G. , Troiani, V. , Brodkin, E. S. , & Schultz, R. T. (2012). The social motivation theory of autism. Trends in Cognitive Sciences, 16(4), 231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaramidaro, A. , Becchio, C. , Colle, L. , Bara, B. G. , & Walter, H. (2014). Do you mean me? Communicative intentions recruit the mirror and the mentalizing system. Social Cognitive and Affective Neuroscience, 9(7), 909–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino, J. N. , & Todd, R. D. (2003). Autistic traits in the general population. Archives of General Psychiatry, 60(5), 524–530. [DOI] [PubMed] [Google Scholar]
- Conty, L. , N'diaye, K. , Tijus, C. , & George, N. (2007). When eye creates the contact! ERP evidence for early dissociation between direct and averted gaze motion processing. Neuropsychologia, 45(13), 3024–3037. [DOI] [PubMed] [Google Scholar]
- Corbetta, M. , Patel, G. , & Shulman, G. L. (2008). The reorienting system of the human brain: from environment to theory of mind. Neuron, 58(3), 306–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coricelli, G. , & Nagel, R. (2009). Neural correlates of depth of strategic reasoning in medial prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America, 106(23), 9163–9168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, R. W. (1996). AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29(3), 162–173. [DOI] [PubMed] [Google Scholar]
- Cox, R. W. , Chen, G. , Glen, D. R. , Reynolds, R. C. , & Taylor, P. A. (2017). FMRI clustering in AFNI: false‐positive rates redux. Brain Connectivity, 7(3), 152–171. [DOI] [PMC free article] [PubMed]
- Cremers, H. R. , Wager, T. D. , & Yarkoni, T. (2017). The relation between statistical power and inference in fMRI. Plos One, 12(11), e0184923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine, R. T. , & Hughes, C. (2013). Silent Films and Strange Stories: Theory of mind, gender, and social experiences in middle childhood. Child Development, 84(3), 989–1003. [DOI] [PubMed] [Google Scholar]
- Egger, H. L. , Pine, D. S. , Nelson, E. , Leibenluft, E. , Ernst, M. , Towbin, K. E. , & Angold, A. (2011). The NIMH Child Emotional Faces Picture Set (NIMH‐ChEFS): a new set of children's facial emotion stimuli. International Journal of Methods in Psychiatric Research, 20(3), 145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum, H. , Yonelinas, A. P. , & Ranganath, C. (2007). The medial temporal lobe and recognition memory. Annual Review of Neuroscience, 30(1), 123–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer, T. W. , Irvin, M. J. , Motoca, L. M. , Leung, M.‐C. , Hutchins, B. C. , Brooks, D. S. , & Hall, C. M. (2015). Externalizing and internalizing behavior problems, peer affiliations, and bullying involvement across the transition to middle school. Journal of Emotional and Behavioral Disorders, 23(1), 3–16. [Google Scholar]
- Feiring, C. , & Lewis, M. (1991). The transition from middle childhood to early adolescence: Sex differences in the social network and perceived self‐competence. Sex Roles, 24(7–8), 489–509. [Google Scholar]
- Frank, C. K. , Baron‐Cohen, S. , & Ganzel, B. L. (2015). Sex differences in the neural basis of false‐belief and pragmatic language comprehension. NeuroImage, 105, 300–311. [DOI] [PubMed] [Google Scholar]
- Gallagher, H. L. , Jack, A. I. , Roepstorff, A. , & Frith, C. D. (2002). Imaging the intentional stance in a competitive game. NeuroImage, 16(3), 814–821. [DOI] [PubMed] [Google Scholar]
- Gilbert, S. J. , Williamson, I. D. M. , Dumontheil, I. , Simons, J. S. , Frith, C. D. , & Burgess, P. W. (2007). Distinct regions of medial rostral prefrontal cortex supporting social and nonsocial functions. Social Cognitive and Affective Neuroscience, 2(3), 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gweon, H. , Dodell‐Feder, D. , Bedny, M. , & Saxe, R. (2012). Theory of mind performance in children correlates with functional specialization of a brain region for thinking about thoughts. Child Development, 83(6), 1853–1868. [DOI] [PubMed] [Google Scholar]
- Holm, S. (1979). A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics, 6, 65–70. [Google Scholar]
- Isoda, M. , & Noritake, A. (2013). What makes the dorsomedial frontal cortex active during reading the mental states of others? Frontiers in Neuroscience, 7, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampe, K. K. , Frith, C. D. , & Frith, U. (2003). Hey John”: signals conveying communicative intention toward the self activate brain regions associated with “mentalizing,” regardless of modality. The Journal of Neuroscience, 23(12), 5258–5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher, T. , Blümel, I. , Marjoram, D. , Lataster, T. , Krabbendam, L. , Weber, J. , … Krach, S. (2009). Online mentalising investigated with functional MRI. Neuroscience Letters, 454(3), 176–181. [DOI] [PubMed] [Google Scholar]
- Kosslyn, S. M. , Pascual‐Leone, A. , Felician, O. , Camposano, S. , Keenan, J. P. , L, W. … Alpert, (1999). The role of area 17 in visual imagery: convergent evidence from PET and rTMS. Science, 284(5411), 167–170. [DOI] [PubMed] [Google Scholar]
- Kosslyn, S. M. , Thompson, W. L. , Klm, I. J. , & Alpert, N. M. (1995). Topographical representations of mental images in primary visual cortex. Nature, 378(6556), 496–498. [DOI] [PubMed] [Google Scholar]
- Krach, S. , Blümel, I. , Marjoram, D. , Lataster, T. , Krabbendam, L. , Weber, J. , … Kircher, T. (2009). Are women better mindreaders? Sex differences in neural correlates of mentalizing detected with functional MRI. BMC Neuroscience, 10(1), 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krall, S. C. , Rottschy, C. , Oberwelland, E. , Bzdok, D. , Fox, P. T. , Eickhoff, S. B. , … Konrad, K. (2015). The role of the right temporoparietal junction in attention and social interaction as revealed by ALE meta‐analysis. Brain Structure and Function, 220(2), 587–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. M. , & McCarthy, G. (2016). Functional heterogeneity and convergence in the right temporoparietal junction. Cerebral Cortex, 26(3), 1108–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccoby, E. E. (1990). Gender and relationships: A developmental account. American Psychologist, 45(4), 513. [DOI] [PubMed] [Google Scholar]
- Mar, R. A. (2011). The neural bases of social cognition and story comprehension. Annual Review of Psychology, 62(1), 103–134. [DOI] [PubMed] [Google Scholar]
- Mars, R. B. , Sallet, J. , Schüffelgen, U. , Jbabdi, S. , Toni, I. , & Rushworth, M. F. S. (2012). Connectivity‐based subdivisions of the human right “temporoparietal junction area”: evidence for different areas participating in different cortical networks. Cerebral Cortex, 22(8), 1894–1903. [DOI] [PubMed] [Google Scholar]
- McCabe, K. , Houser, D. , Ryan, L. , Smith, V. , & Trouard, T. (2001). A functional imaging study of cooperation in two‐person reciprocal exchange. Proceedings of the National Academy of Sciences of the United States of America, 98(20), 11832–11835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, S. A. (2009). Children's understanding of second‐order mental states. Psychological Bulletin, 135(5), 749–773. [DOI] [PubMed] [Google Scholar]
- Molenberghs, P. , Johnson, H. , Henry, J. D. , & Mattingley, J. B. (2016). Understanding the minds of others: A neuroimaging meta‐analysis. Neuroscience & Biobehavioral Reviews, 65, 276–291. [DOI] [PubMed] [Google Scholar]
- Molfese, P. J. , Glen, D. , Mesite, L. , Pugh, K. R. , & Cox, R. W. (2015, June). The Haskins pediatric brain atlas. Poster session presented at the 21st Annual Meeting of the Organization for Human Brain Mapping, Honolulu, HI.
- Moraczewski, D. , Kinnison, J. , & Pessoa, L. (2016). Design Explorer [Computer software].
- Nichols, T. , Brett, M. , Andersson, J. , Wager, T. , & Poline, J.‐B. (2005). Valid conjunction inference with the minimum statistic. NeuroImage, 25(3), 653–660. [DOI] [PubMed] [Google Scholar]
- Peirce, J. W. (2008). Generating stimuli for neuroscience using PsychoPy. Frontiers in Neuroinformatics, 2, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer, U. J. , Schilbach, L. , Timmermans, B. , Kuzmanovic, B. , Georgescu, A. L. , Bente, G. , & Vogeley, K. (2014). Why we interact: On the functional role of the striatum in the subjective experience of social interaction. NeuroImage, 101, 124–137. [DOI] [PubMed] [Google Scholar]
- R Core Team . (2016). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; Retrieved from https://www.R-project.org/ [Google Scholar]
- Redcay, E. (2008). The superior temporal sulcus performs a common function for social and speech perception: Implications for the emergence of autism. Neuroscience & Biobehavioral Reviews, 32(1), 123–142. [DOI] [PubMed] [Google Scholar]
- Redcay, E. , Dodell‐Feder, D. , Pearrow, M. J. , Mavros, P. L. , Kleiner, M. , Gabrieli, J. D. E. , & Saxe, R. (2010). Live face‐to‐face interaction during fMRI: a new tool for social cognitive neuroscience. NeuroImage, 50(4), 1639–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay, E. , Kleiner, M. , & Saxe, R. (2012). Look at this: the neural correlates of initiating and responding to bids for joint attention. Frontiers in Human Neuroscience, 6, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay, E. , Velnoskey, K. R. , & Rowe, M. L. (2016). Perceived communicative intent in gesture and language modulates the superior temporal sulcus: Shared Neural Systems for Gesture and Language. Human Brain Mapping, 37(10), 3444–3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice, K. , Moraczewski, D. , & Redcay, E. (2016). Perceived live interaction modulates the developing social brain. Social Cognitive and Affective Neuroscience, 11(9), 1354–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice, K. , & Redcay, E. (2016). Interaction matters: A perceived social partner alters the neural processing of human speech. NeuroImage, 129, 480–488. [DOI] [PubMed] [Google Scholar]
- Schilbach, L. , Timmermans, B. , Reddy, V. , Costall, A. , Bente, G. , Schlicht, T. , & Vogeley, K. (2013). Toward a second‐person neuroscience. Behavioral and Brain Sciences, 36(04), 393–414. [DOI] [PubMed] [Google Scholar]
- Schilbach, L. , Wilms, M. , Eickhoff, S. B. , Romanzetti, S. , Tepest, R. , Bente, G. , … Vogeley, K. (2010). Minds made for sharing: initiating joint attention recruits reward‐related neurocircuitry. Journal of Cognitive Neuroscience, 22(12), 2702–2715. [DOI] [PubMed] [Google Scholar]
- Schilbach, L. , Wohlschlaeger, A. M. , Kraemer, N. C. , Newen, A. , Shah, N. J. , Fink, G. R. , & Vogeley, K. (2006). Being with virtual others: Neural correlates of social interaction. Neuropsychologia, 44(5), 718–730. [DOI] [PubMed] [Google Scholar]
- Schultz, W. (2015). Neuronal reward and decision signals: from theories to data. Physiological Reviews, 95(3), 853–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurz, M. , Radua, J. , Aichhorn, M. , Richlan, F. , & Perner, J. (2014). Fractionating theory of mind: A meta‐analysis of functional brain imaging studies. Neuroscience & Biobehavioral Reviews, 42, 9–34. [DOI] [PubMed] [Google Scholar]
- Schuwerk, T. , Schurz, M. , Müller, F. , Rupprecht, R. , & Sommer, M. (2017). The rTPJ's overarching cognitive function in networks for attention and theory of mind. Social Cognitive and Affective Neuroscience, 12(1), 157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veroude, K. , Jolles, J. , Croiset, G. , & Krabbendam, L. (2014). Sex differences in the neural bases of social appraisals. Social Cognitive and Affective Neuroscience, 9(4), 513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnell, K. R. , Sadikova, E. , & Redcay, E. (2018). Let's chat: developmental neural bases of social motivation during real‐time peer interaction. Developmental Science, 21(3), e12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information Figure
Supporting Information Table


