Abstract
Fundamental cellular properties are determined by the repertoire and abundance of proteins displayed on the cell surface. As such, the trafficking mechanisms for establishing and maintaining the surface proteome must be tightly regulated for cells to respond appropriately to extracellular cues, yet plastic enough to adapt to ever-changing environments. Not only are the identity and abundance of surface proteins critical, but in many cases, their regulated spatial positioning within surface nanodomains can greatly impact their function. In the context of neuronal cell biology, surface levels and positioning of ion channels and neurotransmitter receptors play essential roles in establishing important properties, including cellular excitability and synaptic strength. Here we review our current understanding of the trafficking pathways that control the abundance and localization of proteins important for synaptic function and plasticity, as well as recent technological advances that are allowing the field to investigate protein trafficking with increasing spatiotemporal precision.
Keywords: synapse, plasticity, membrane trafficking, dendrite, AMPA receptor, optogenetics
Fundamental cellular properties are determined by the repertoire and abundance of proteins displayed on the cell surface. As such, the trafficking mechanisms for establishing and maintaining the surface proteome must be tightly regulated for cells to respond appropriately to extracellular cues, yet plastic enough to adapt to ever-changing environments. Not only are the identity and abundance of surface proteins critical, but in many cases, their regulated spatial positioning within surface nanodomains can greatly impact their function. In the context of neuronal cell biology, surface levels and positioning of ion channels and neurotransmitter receptors play essential roles in establishing important properties, including cellular excitability and synaptic strength. These properties are subject to potent regulation by neural activity, which directly influences the behavior of cellular trafficking processes. For example, the molecular composition and organization of the postsynaptic plasma membrane is regulated by plasticity-stimuli that trigger long-lasting forms of synaptic plasticity including long-term potentiation, depression (LTP and LTD) and homeostatic scaling. These diverse and important forms of neural plasticity are largely controlled both by mobilization of ion channels and receptors from intracellular stockpiles to the cell surface and regulated positioning of existing surface proteins within functional nanodomains at synaptic sites (Hayashi et al., 2000; Lledo et al., 1998; Luscher et al., 1999; MacGillavry et al., 2013; Nair et al., 2013; M. Park et al., 2004a; Penn et al., 2017; Sinnen et al., 2017; Tang et al., 2016). Given the central importance of protein trafficking for neuronal function and plasticity, it is not surprising this has remained an area of intense investigation. Here we review our current understanding of the trafficking pathways that control the abundance and localization of proteins important for neuronal function and plasticity, as well as recent technological advances that are allowing the field to investigate protein trafficking with increasing spatiotemporal precision. We begin by reviewing our current understanding of the organization and function of the neuronal secretory pathway for trafficking newly synthesized proteins at remote sites in dendrites. We then review recent studies investigating post-secretory trafficking of synaptic proteins through endocytic recycling and lateral diffusion. Finally, we highlight new molecular tools that hold promise for addressing challenging problems in neuronal protein trafficking.
I. Organization of the dendritic secretory pathway for remote protein trafficking
Compared to other cell types, neurons place extreme demands on the protein biosynthetic pathway due to their large size, intricate morphology and requirement for precise temporal control of protein levels within diverse cellular domains and structures (Fig. 1). How the correct proteins are delivered in the appropriate amounts to remote sites in the dendritic arbor has remained a persistent and challenging question in the field. This issue is epitomized by modeling studies that estimate trafficking from the neuronal cell body to specific sites within the dendritic arbor could take several hours to days (Williams et al., 2016), yet diverse forms of protein synthesis-dependent plasticity operate with synapse-level accuracy on significantly shorter timescales (Buffington et al., 2014; Hanus and Schuman, 2013; Mameli et al., 2007; Sutton et al., 2006; Sutton and Schuman, 2006). This problem has been recognized and investigated for decades, with early studies providing a crucial piece of the puzzle by localizing components of the biosynthetic machinery, including polyribosomes, near synaptic sites within proximal and distal dendrites (Bodian, 1965; Steward and Fass, 1983). Later studies confirmed the presence of diverse mRNAs within dendrites supporting a model where local protein synthesis supports a significant fraction of the dendritic proteome (Holt and Schuman, 2013).
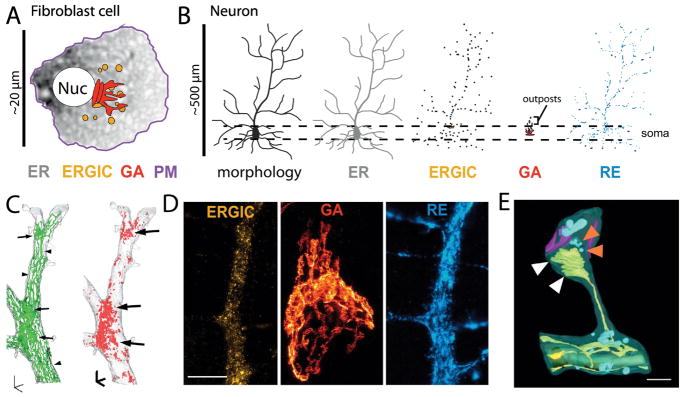
Figure 1. Organization of the secretory network.
(A) Schematic of secretory organelles organization in a fibroblast cell.
(B) Schematic of secretory organelle organization in a neuron.
(C) Three-dimensional reconstructions of serial electron micrographs showing the distribution of the ER (green, left) and ER-bound ribosomes (red, right) in a dendritic segment of a hippocampal CA1 pyramidal neuron. Left: Elongated ER tubules in straight dendritic segments (arrowheads) whereas more complex ER cisternal sheets are found at dendritic branch points (arrows). Right: The density of ER-bound ribosomes at branch points (arrows) corresponds to the sites of ER complexity. Scale bars, 1 μm. Adapted from Cui-Wang et al., (2012) and reprinted with permission from Elsevier, copyright 2012.
(D) Stimulated emission depletion (STED) microscopy images of cultured cortical neurons expressing ERGIC-GFP (left panel) and TfR-mCh (right panel) to label dendritic ERGIC and recycling endosomes respectively. The middle panel shows the soma of a cultured cortical neuron stained with the cis-Golgi marker GM130 (middle panel). Scale bar, 5 μm.
(E) Three-dimensional model of organelles in a dendritic spine (from focused ion beam-scanning electron microscopy [FIB-SEM] image stacks, mouse cerebral cortex). ER/spine apparatus (yellow), endosomes/transport vesicles (light blue), synaptic interface (magenta), mitochondria (green). White arrowheads mark two ER-PM contact sites. Note the presence of endosomes/transport vesicles at the tip of the spine apparatus (orange arrowheads). Scale bar, 400 nm. Adapted from Wu et al., (2017) and reprinted with permission from the National Academy of Sciences.
Organization of the dendritic ER
Intriguingly, many of the mRNAs identified in dendrites encode integral membrane proteins and secreted factors that require specialized processing through the cellular secretory network, which primarily consists of the endoplasmic reticulum (ER) and Golgi apparatus (GA) (Cajigas et al., 2012; Zhong et al., 2006). The endoplasmic reticulum (ER) is broadly distributed throughout the dendritic arbor (Fig. 1B,C) (Spacek and Harris, 1997; Y. Wu et al., 2017). Signal recognition particle (SRP), which recognizes and targets nascent polypeptides and their associated ribosomes to the ER is also present within dendrites (Tiedge and Brosius, 1996). Accordingly, ribosome-associated, or “rough”, ER is observed at remote dendritic sites, confirming decentralized translation of integral membrane and secreted factors (Bodian, 1965; Cui-Wang et al., 2012; Gardiol et al., 1999; Pierce et al., 2000; Tiedge and Brosius, 1996). Following translation, nascent proteins can laterally diffuse within the ER membrane (or in the case of soluble secreted proteins, within the ER lumen) prior to ER exit (Cui-Wang et al., 2012; Fukatsu et al., 2004). Lateral mobility within the ER is an especially important consideration for many neurotransmitter receptors, which may linger in the ER for tens of minutes to hours (Cui-Wang et al., 2012; Greger et al., 2002; Valenzuela et al., 2014). This raises the question of whether these proteins could be locally trafficked to synaptic sites near their birthplace, or if spatial information is blurred or completely lost as they laterally diffuse within the ER, away from their sites of biogenesis. To quantitatively address this issue, Cui-Wang et al. used a combination of photoactivation and photobleaching, to show that specialized regions of ER adopt a complex and convoluted morphology (usually near dendritic branch points) that act as diffusion barriers for nascent proteins within the ER (Fig. 1C). Intriguingly, metabotropic glutamate receptor activation, which stimulates local translation, also increased ER complexity by regulating its dissociation from dendritic microtubules (Cui-Wang et al., 2012). The degree of ER complexity correlated with the density of ER-attached ribosomes (Fig. 1C), further supporting a model where morphological plasticity of ER structure could spatially confine cargoes during synthesis for targeted delivery to nearby synapses. Although no studies have directly investigated the spatial relationship between where a cargo exits the ER and where it appears at the plasma membrane, it is tempting to speculate that local synthesis and forward trafficking of ion channels and receptors could play a role in defining and maintaining subcellular excitability and synaptic properties over spatial scales ranging from dendritic branches to individual synapses (Béïque et al., 2011; M.-C. Lee et al., 2010; Losonczy et al., 2008; Makara et al., 2009). New tools allowing fine spatiotemporal control of ER exit will allow a deeper investigation of this issue (D. Chen et al., 2013).
ER/PM contacts
An intriguing characteristic of ER structure is the presence of contact sites with other organelles, including mitochondria, endosomes and the plasma membrane (PM) (Friedman et al., 2011; Rowland et al., 2014; Y. Wu et al., 2017). While ER/PM contacts have long been appreciated from ultrastructure studies, their functional importance is now emerging with critical roles in calcium signaling (Dittmer et al., 2017; Lees et al., 2017) and lipid exchange (Chung et al., 2015; Schauder et al., 2014; Toulmay and Prinz, 2011). ER/PM junctions may also help anchor protein machinery for protein trafficking. Simultaneous live cell imaging of ER/PM junctions and protein trafficking using total internal reflection microscopy revealed both vesicular delivery to the PM and endocytosis of surface proteins often occurred immediately adjacent to ER/PM junctions (Fox et al., 2013). While these data suggest that ER/PM junctions direct trafficking to specific cellular domains, how or why protein trafficking machinery is associated with these sites remains an open question.
A study by Wu et al. used focused ion beam-scanning electron microscopy to map neuronal ER/organelle contacts (Y. Wu et al., 2017). In agreement with numerous previous studies, they show that the ER extends throughout the dendritic arbor and invades a subset of dendritic spines where, in many cases it forms junctions with the PM in the spine head (Fig. 1E). The significance of these contact sites near synapses remains unclear, but it is tempting to speculate that they could play a role in lipid transfer (Toulmay and Prinz, 2011), synaptic Ca2+ signaling (Dittmer et al., 2017) or even as membrane fusion and endocytosis sites, both of which occur in dendritic spines (Blanpied et al., 2002; Kennedy et al., 2010a). Intriguingly, the amount of ER membrane in spines appears to be regulated by plasticity mechanisms. Expansion of ER within the spine head occurs during activity-triggered spine growth and appears to be regulated by L-type voltage gated Ca2+ channels. Blocking L-VGCCs has no effect on early phases of activity-triggered spine growth, but specifically disrupted ER expansion in potentiated spines (Dittmer et al., 2017). In a subset of spines (typically mature, “mushroom” type spines) the ER network elaborates into a stacked membrane structure resembling the GA, called the spine apparatus. Intriguingly, vesicular structures are often found at the tip of the spine apparatus (Fig. 1E), raising the exciting possibility that the spine apparatus is involved in vesicle biogenesis, which could play a role in forward trafficking of proteins and lipids from the spine ER to the PM at synaptic sites (Y. Wu et al., 2017). While a role for the spine apparatus in anterograde protein trafficking and lipid delivery has been speculated based on its GA-like appearance, no direct evidence for this role currently exists. Alternatively, the spine apparatus could simply act as a barrier to prevent early/recycling endosomes generated from the spine PM from exiting the spine head without playing a direct role in their genesis or function.
Early secretory trafficking
To leave the ER, properly assembled and processed proteins accumulate at specialized ER exit sites (ERES), which have been observed throughout the dendritic arbor (Horton and Ehlers, 2003). Regions of active ER export are called transitional ER (tER). Following assembly and processing in the ER, proteins destined for export concentrate with components of the coat protein II (COPII) complex in tER. The upstream determinant of ERES formation appears to be Sec16, a hydrophobic, multidomain protein (Connerly et al., 2005; Espenshade et al., 1995; Hughes et al., 2009; Ivan et al., 2008; Watson et al., 2006) that peripherally associates with the ER membrane where it scaffolds additional ERES-associated proteins to promote membrane coating and budding.
In non-neuronal cells, the spatial distribution of ERES is relatively constant over time (Hammond and Glick, 2000). Photobleaching studies have revealed that, in contrast to COPII subunits, a steady pool of Sec16 localized to tER sites (Hughes et al., 2009), indicating that Sec16 may be a defining feature of tER (Budnik and Stephens, 2009). In complex cell types like neurons, the location and regulation of ERES may play a major role in targeting integral membrane proteins to specific sites in the dendritic arbor. Although the regulatory pathway for ERES formation and budding is incompletely defined, acute application of GTP-Sar1 induces the formation of ERES throughout the dendritic arbor (Aridor et al., 2004). Thus, Sar1 may normally limit and regulate the formation or function of ERES at pre-existing Sec16-positive locations. Alternatively, ERES may simply be regulated by cargo load (Aridor et al., 1999; Farhan et al., 2008) and could be directly stimulated by changes in local translation or accumulation of nascent cargo in ER by lateral diffusion from somatic pools (Pick et al., 2017).
After exiting the ER in COPII-coated carriers, trafficking proteins first enter the ER-Golgi intermediate compartment (ERGIC) (Presley et al., 1997; Scales et al., 1997). The ERGIC is a diverse assortment of mobile and stationary tubulovesicular carriers (Ben-Tekaya et al., 2005) that sorts proteins for delivery to the GA, or for return to the ER. ERGIC is commonly identified by the mannose-binding protein ERGIC53 (Schweizer et al., 1988) and Rab1 (Sannerud et al., 2006). Although ER-GA trafficking is microtubule dependent, initial delivery to the ERGIC is not (Presley et al., 1997). This may be due to the longer distances necessary for ERGIC-GA delivery and is particularly relevant for the function of ERGIC in neuronal dendrites.
In non-neuronal cells, ERGIC is predominantly localized to the ER-Golgi interface, although a number of studies have also observed ERGIC elements distributed throughout the cellular cortex (reviewed in Saraste et al., 2009). Similarly, ERGIC elements are widely distributed through neuronal dendrites far from the cell body (Hanus et al., 2014; Krijnse-Locker et al., 1995; Sannerud et al., 2006). Work from our own group utilized a novel light-induced ER release strategy to demonstrate that membrane proteins accumulate in punctate dendritic organelles immediately following ER release (D. Chen et al., 2013) (Fig. 2A,B). Subsequent studies revealed these organelles are ERGIC based on the colocalization of dendritic trafficking cargo and ERGIC53, supporting a major trafficking role for ERGIC in decentralized dendritic protein trafficking (Bowen et al., 2017; Hanus et al., 2014).
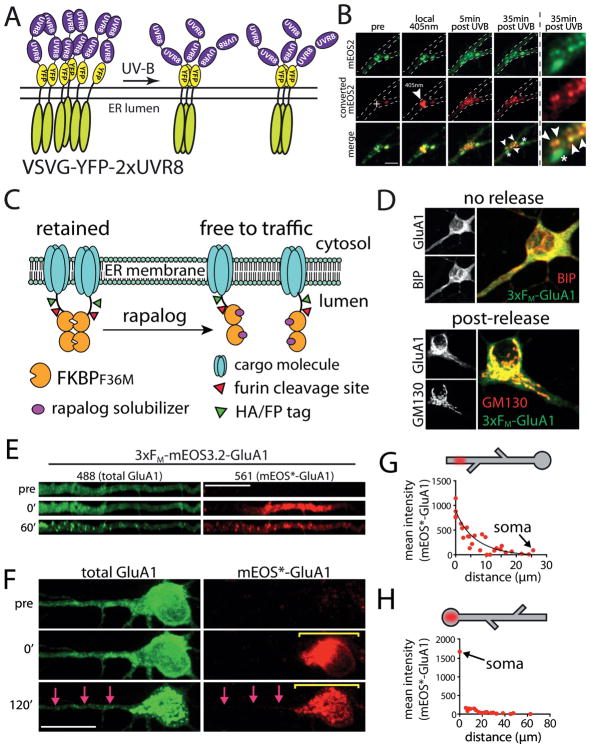
Figure 2. Inducible release systems to study the local dendritic secretory network.
(A) Schematic of the light-inducible release system based on conditional clustering of UVR8. When tandem copies of UVR8 are fused to a target protein (in this case, VSVG) oligomers form and are retained in the ER. Exposure to UV-B triggers dissociation of these clusters, allowing forward trafficking through the secretory pathway. Adapted from Chen et al., (2013) and reprinted with permission from Rockefeller University Press.
(B) Demonstration of the UVR8-dependent ER-retention strategy. ER-retained secretory cargo near dendritic branch points was selectively photoconverted from green to red with local 405-nm illumination (crosshair, middle) and then released with global UV-B. Photoconverted cargo accumulated at nearby secretory organelles after UV-B treatment (arrowheads), supporting a local secretory network in dendrites. Not all puncta contained photoconverted cargo (asterisks), indicating selective trafficking to a subset of secretory organelles. Scale bar, 4 μm. Adapted from Chen et al., (2013) and reprinted with permission from Rockefeller University Press.
(C) Schematic for the chemically-inducible release system based on the self-associating FKBP mutant (FM) and rapalog. FM domains cluster and trap fused cargo molecules in the ER. Addition of the rapalog compound disrupts FM clusters, allowing ER exit and subsequent trafficking.
(D) FM-GluA1 colocalizes with ER marker BIP prior to release and with the GA marker GM130 1 hr following rapalog addition. Adapted from Bowen et al., (2017) and reprinted with permission from eLife.
(E–H) Demonstration of the FM/rapalog inducible release strategy. FM-mEOS-GluA1 was photoconverted from green to red in either the apical dendrite (E) or the soma (F) prior to ER release. Upon ER release, the cargo redistributes to local trafficking organelles. Quantification of the signal distribution relative to the original site of photoconversion is provided for dendritic (G) and somatic (H) photoconversion 1 hr following ER release. Adapted from Bowen et al., (2017) and reprinted with permission from eLife.
Dendritic Golgi outposts
The presence of decentralized GA in neuronal dendrites has been a controversial topic. While punctate GA-derived organelles distinct from somatic GA have been observed (commonly termed “Golgi outposts or GOs”), studies using antibodies against endogenous GA (giantin, GM130, TGN38) generally agree that only a minority (~20%) of dendrites possess Golgi membranes and those that are observed are mainly restricted to the proximal regions of the primary dendrite (Bowen et al., 2017; Horton et al., 2005; Locker et al., 1995; Torre and Steward, 1996). In Drosophila sensory neurons, GOs may be more abundant, with the caveat that GOs are typically visualized with expressed and tagged Golgi proteins (Ye et al., 2007). So far, it remains unknown whether these organelles play a role in forward secretory trafficking, but one potential function of dendritic GOs is to nucleate microtubules to coordinate dendritic branching and morphogenesis (Arthur et al., 2015; Ori-McKenney et al., 2012; Yalgin et al., 2015; Zhou et al., 2014). However, Nguyen et al. observed that in many cases, sparse dendritic GO distribution was inconsistent with the localization of gamma-tubulin, the core microtubule nucleation factor (Nguyen et al., 2014). Furthermore, removing GOs from dendrites by fusing a GA-localized protein domain to a constitutively active kinesin heavy chain had little effect on dendritic microtubule nucleation, arguing against a role for GOs in microtubule dynamics. While the similarly sparse GO distribution in mammalian central neurons seems inconsistent with a major role for microtubule nucleation, it is possible that an unappreciated GA-like organelle in dendrites could serve this role. To this end, Mikhalyava et al. found that an expressed, engineered marker protein (termed “pGolt”) identifies a previously uncharacterized Golgi-like dendritic membrane compartment. These Golgi-like membranes appear to be active mediators of anterograde trafficking based on their accumulation of temperature sensitive vesicular stomatitis viral glycoprotein (VSV-G) and co-immunostaining for a variety of cargoes. pGolt-positive organelles are found in close proximity to ERGIC membranes, as well as endosomal markers such as Rab6, EEA1 and retromer subunits (Mikhaylova et al., 2016). However, given the artificial nature of the reporter, it remains to be determined how closely these dendritic organelles resemble bone fide GA. In light of the discrepancies between expressed GA markers and immunocytochemistry against numerous endogenous GA markers, the function and existence of satellite GA membranes in dendrites remains an intriguing, but unresolved issue.
Despite the sparseness of canonical GA markers, several lines of evidence support anterograde secretory trafficking at remote sites in dendrites. Dendritic protein synthesis is critical for the implementation of diverse forms of synaptic plasticity, a process that likely depends on the synthesis and trafficking of integral membrane proteins through the secretory pathway (Bradshaw et al., 2003; Cracco et al., 2005; Mameli et al., 2007; Pick et al., 2017; Sutton et al., 2006). For example, homeostatic scaling in response to extended blockade of action potentials and NMDA receptors requires local synthesis and trafficking of the AMPA receptor subunit GluA1 (Sutton et al., 2006). Independent approaches for directly visualizing newly synthesized proteins further support dendritic secretory trafficking. For example labelling new proteins using either FLASH/ReASH or timeSTAMP revealed significant synthesis and surface delivery of AMPARs in dendrites that had been mechanically isolated from the soma (Butko et al., 2012; Ju et al., 2004).
How dendritic secretory trafficking could occur in the absence of canonical GA has remained an intriguing and persistent question in cellular neurobiology. Indeed, emerging evidence suggests that some proteins can bypass GA processing in neurons (Hanus et al., 2014; Jeyifous et al., 2009). A recently published study from our own group (Bowen et al., 2017) employed a combination of inducible ER-exit with local photoconversion to track the fate of proteins exiting the ER in different cellular domains (Fig. 2C–H). In agreement with previous studies, we found that dendritic cargoes initially undergo spatially confined delivery to nearby ERGIC membranes (D. Chen et al., 2013; Hanus et al., 2014; Mikhaylova et al., 2016). Following ERGIC trafficking, dendritic cargo accumulated in recycling endosomes (REs) prior to reaching the cell surface. Intriguingly, a significant fraction of forward trafficking from the ER to dendritic REs and the PM occurred even when the GA was disrupted with brefeldin A, further supporting a satellite secretory network that bypasses the somatic GA (Bowen et al., 2017). This observation helps explain how neurons locally deliver new proteins given the paucity of dendritic GA. These experiments also revealed that, in addition to their well-established role in regulating synaptic composition via recycling pathways (see section II below), REs play a critical role in anterograde trafficking from the ER to the dendritic PM. Whether REs receive trafficking cargo directly from ERGIC or intermediate organelle(s) mediate this transfer remains unknown, but Bowen et al. demonstrated a close apposition of ERGIC and RE markers using super-resolution light microscopy consistent with a direct-transfer mechanism (Bowen et al., 2017).
An interesting point to consider is whether dendritic ERGIC or endosomal elements may possess Golgi-like specializations for anterograde trafficking. For example, a key role of the GA is to process post-translational modifications, including refining the glycosylation state of surface receptors and secreted proteins. However, studies using immunocytochemistry to localize glycan-modifying enzymes, including galactosyl transferase and α-mannosidase, found these enzymes largely localize to the soma and proximal apical dendrite, similar to other canonical GA proteins (Krijnse-Locker et al., 1995; Torre and Steward, 1996). Consistent with these early studies, evidence is accumulating for atypical processing of dendritic secretory cargo. Hanus et al. reported that a substantial fraction of surface NMDA, GABA and AMPA receptors display immature N-linked glycans, consistent with a GA-bypass mode of surface trafficking (Hanus et al., 2014). This study helps explain the paradoxical observation that neuronal processes are strongly labeled by lectins that preferentially associate with high-mannose glycans normally restricted to ER-localized proteins. Whether GA-bypass and atypical glycosylation is a fundamental feature of all proteins using the dendritic secretory pathway, or is specific to a subset of neuronal proteins awaits further investigation, but preliminary evidence for cargo specificity comes from Bowen et al., which reported a significant fraction of AMPA receptors can traffic from ERGIC to the PM following GA disruption, while VSV-G and neuroligin 1 could not (Bowen et al., 2017).
Protein sorting and post-Golgi vesicular transport
Effective subcellular targeting of nascent membrane and secreted proteins depends on proper sorting as they traverse the biosynthetic pathway. This process primarily occurs via adaptor protein-mediated sorting of proteins into distinct vesicular carriers at the trans-Golgi network (TGN) that shuttle cargoes to the appropriate cellular domain. Prior to reaching the TGN, different proteins intermingle while traversing the cis-GA as evidenced by early studies using either electron-microscopy to visualize trafficking of apical or basolateral viral proteins (Rindler et al., 1984) or a clever biochemical assay in which a polarized viral enzyme chemically modifies co-trafficking proteins (Fuller et al., 1985). While this is thought to reflect a general principle of GA trafficking, exceptions have been noted. For example, GPI-anchored proteins seem to segregate prior to reaching the GA (Muñiz et al., 2001), although the downstream consequences of this are not known. Furthermore, some Golgi-dependent proteins have altered intra-Golgi trafficking characteristics. For example most, but not all, proteins stall in the GA if cells are incubated at 20 °C during forward trafficking, suggesting distinct trafficking routes within the GA (Farr et al., 2015).
Post-Golgi vesicles originating from the TGN are broadly categorized into the constitutive pathway, present in all cells, and the regulated pathway specific to endocrine, neuronal, neuroendocrine and other secretory cell types. While the molecular profiles of post-Golgi vesicles are poorly defined, there is evidence for distinct subtypes of post-Golgi transport vesicles within the constitutive pathway. For example, cargoes destined for the apical or basolateral domains exit the TGN in distinct carriers (Jacob and Naim, 2001; Keller et al., 2001), consistent with adaptor-specific sorting prior to packaging into transport vesicles. Adaptor mediated TGN sorting is not limited to cargoes destined for different polarized domains. For example, diverse cargoes targeted to the basolateral domain can also segregate into distinct pools of transport vesicles in the TGN. In polarized MDCK cells, the Na+/K+ ATPase takes a different route to the surface than VSV-G and utilizes distinct Rab proteins (Farr et al., 2009). Likewise, in neuronal cells GABAA receptors are delivered through mechanisms distinct from AMPA receptors (Gu et al., 2016). Voltage-gated K+ channels (Kv). Kv2.1 channels, which are targeted to the soma and proximal dendrite, can also be trafficked through a GA-independent mechanism to the axon initial segment. Indeed trafficking pathways for somatic Kv2.1 appear to be distinct from those used by the more distally localized dendritic Kv4.2 channel (Jensen et al., 2014; 2017). For regulated secretion, neuropeptides are sorted into distinct post-Golgi vesicles that mature via sorting and pH-sensitive proteolytic processing, into dense core vesicles (DCVs) (Ailion et al., 2014; Kim et al., 2006). DCV peptides are sorted away from those bound for synaptic-like-vesicles at the TGN in a process regulated by AP-3 and VPS41 (Asensio et al., 2013; Grabner et al., 2006; J. J. Park et al., 2011), although many neurotransmitter vesicles also contain various neuropeptides. In addition to presynaptic localization, DCVs are also transported to dendritic sites where they deliver neuromodulators such as tissue plasminogen activator and brain-derived neurotrophic factor (BDNF) in response to calcium flux (Kolarow et al., 2007; Lochner et al., 2006; Matsuda et al., 2009). DCV docking involves Rab3 and Rab27 (Haynes et al., 2001; Shirakawa et al., 2004; Tsuboi and Fukuda, 2006), indicating this compartment shares some regulatory machinery with neurotransmitter vesicles (Geppert et al., 1994; 1997; Tanaka et al., 2001).
Targeting of post-Golgi carriers to the correct cellular domains can occur through several mechanisms. In neurons, initial polarization of vesicles occurs by exclusion of somatodendritic vesicles from the axon initial segment (AIS) by a myosinVb-dependent “actin filter” (Al-Bassam et al., 2012; Burack et al., 2000; Lewis et al., 2009). Interestingly, the dense actin meshwork at the AIS also maintains PM polarization by anchoring membrane components that restrict diffusion between the axon and soma (Nakada et al., 2003; Winckler et al., 1999). The orientation and specificity of transport is determined by the complement of molecular motors and cargo-motor adaptor proteins that bind to specific vesicular cargoes. For example, KIF5 targets GluA2-containing AMPA receptors to dendrites through an interaction with the scaffold protein GRIP1 (Setou et al., 2002), whereas KIF17 targets NR2B-containing NMDA receptors through an interaction with the scaffold mLin-10 (Setou et al., 2000). For a detailed review on motor proteins and cytoskeleton-dependent trafficking in neurons, see Hirokawa et al., 2010.
The molecular machinery that defines constitutive trafficking from the GA is poorly defined. A number of trafficking factors initially ascribed to post-GA carrier vesicles are also present in the RE compartment. For example, Rab8 was initially identified as a critical mediator of basolateral biosynthetic trafficking in polarized cells (Huber et al., 1993b) and the somatodendritic domain of neurons (Huber et al., 1993a). Subsequently, Rab8 was found to mark REs (Hattula et al., 2006) although on a potentially different subset of endosomes than the canonical RE rab, Rab11 (Roland et al., 2007). Similarly, the yeast homologues of Rab11 were initially identified due to their role in TGN-PM trafficking (Jedd et al., 1997). Although the Rab11a isoform has been associated with the apical recycling pathway in various cell types (Goldenring et al., 1996; X. Wang et al., 2000), Rab11a and Rab11b are both involved in transferrin recycling (Schlierf et al., 2000; Ullrich et al., 1996). In neuronal cells, Rab11a is predominantly localized to the somatodendritic domain, and exhibits more prominent localization to the GA than Rab11b (Schlierf et al., 2000). Experiments demonstrating that REs act as intermediate transport organelles for new cargoes trafficking from TGN to the PM blur the distinction between recycling compartments and post-GA carriers (Ang et al., 2004; Cancino et al., 2007). The proportion of cargoes that depend on RE trafficking for post-GA trafficking is unclear, although the diversity of vesicle sub-types budding from the TGN suggests they traffic a subset of cargoes. Secretory proteins that interact with AP-1B may be preferentially sorted to the RE pathway (Traub and Apodaca, 2003). However, the mechanisms responsible for sorting these cargoes are still unclear as AP-1B may not be required for initial targeting to REs (Cancino et al., 2007). Recently, our lab found that diverse neuronal proteins are forward trafficked through the RE compartment in a process that depends on both Rab11 and Rab8 (Bowen et al., 2017). In agreement with previous studies in heterologous cells, the dendritic RE network participates in biosynthetic trafficking, blurring the distinction between post-Golgi and recycling compartments. In neurons, REs appeared to play a role in both long-range trafficking of cargo that originated in the somatic GA and local trafficking of cargo that was released from the dendritic ER (Bowen et al., 2017). Intriguingly, anterograde secretory cargo populated REs situated within dendritic spines, suggesting that REs could act as conduits for localizing newly synthesized secretory cargoes to specific synaptic sites.
While the fundamental organization of neuronal secretory organelles is becoming increasingly clear, a number of critical questions remain. What is the relationship between ERGIC/Golgi satellite membranes and the endosomal system? What is the function of the spine apparatus? What are the spatial restrictions on synaptic delivery after ER exit of nascent proteins? How does synaptic activity influence anterograde protein delivery and what trafficking steps are affected? What are the molecular determinants that direct cargo to the different trafficking routes in neurons? A more detailed understanding of how new proteins are targeted to synaptic sites within dendrites will require the development of techniques that provide spatial and temporal control of trafficking as well as robust detection of specific pools of trafficking proteins at the plasma membrane. Advances in this direction are discussed in Section III of this review.
II. Post-secretory trafficking
While the secretory network may deliver new proteins to their appropriate cellular domain, often they are not delivered to their precise functional destination. In many cases further refinement of protein localization is required. Here we discuss two principal modes of post-secretory pathway trafficking, lateral diffusion and endocytic trafficking as they relate to synaptic function.
Lateral diffusion
Postsynaptic proteins must diffuse laterally into the postsynaptic membrane following insertion into nearby sites in the PM of dendrites and spines. For example, numerous studies have demonstrated that both inhibitory and excitatory neurotransmitter receptors can move laterally into synaptic sites where they are retained by synaptic scaffolding proteins (Bannai et al., 2009; Bats et al., 2007; Borgdorff and Choquet, 2002; Choquet and Triller, 2013; Dahan et al., 2003; de Luca et al., 2017; Gerrow and Triller, 2014; Hanus et al., 2006; 2004; Opazo et al., 2010; Schnell et al., 2002), or into endocytic zones where they are internalized and sorted for subsequent recycling or degradation (Blanpied et al., 2002; Luscher et al., 1999; Petrini et al., 2009; Steinmetz et al., 2016). Lateral diffusion and activity-dependent trapping appear to dictate the steady state level of receptors within the postsynaptic membrane, and therefore the efficacy or “strength” of the synapse (Borgdorff and Choquet, 2002; Ehlers et al., 2007; Opazo et al., 2010).
While many early studies using quantum dot-based labeling methods support rapid AMPA receptor surface diffusion, with frequent entry and exit from the PSD, a later study suggests that AMPA receptors, once incorporated into the PSD, may be more stable than previously thought (S. H. Lee et al., 2017). This discrepancy could arise from the labeling method utilized. Lee et al. reported that receptors labeled with small (~10 nm) quantum dots or fluorescent dye-labeled streptavidin or antibodies (~4 nm) were almost exclusively localized within the PSD where they displayed limited diffusion. Longitudinal tracking experiments with small quantum dot labels demonstrated that receptors dwelled in the PSD for relatively long (>15 min) time periods. In contrast, larger quantum dots (>20 nm) seemed to preferentially label a more mobile, extrasynaptic pool of AMPA receptors, perhaps because steric hindrance prevents large QD-labeled receptors from entering the synaptic cleft, which is generally estimated to be 15–25 nm in width (Lehre and Danbolt, 1998; Rusakov and Kullmann, 1998; Savtchenko and Rusakov, 2007; Zuber et al., 2005). While this study suggested that few receptors are present outside of the PSD, there is abundant physiological evidence for a substantial pool of extrasynaptic AMPA receptors. Perhaps most compelling is the observation that robust AMPA receptor currents are observed when glutamate is delivered to non-synaptic sites on the dendritic shaft using photolytic uncaging techniques or iontophoresis (Bagal et al., 2005; Makino and Malinow, 2009; Matsuzaki et al., 2004). Furthermore, experimental manipulations that decrease the extrasynaptic pool of receptors or impair their mobility, block LTP, further supporting an extrasynaptic reserve pool of receptors that is important for dynamic regulation of synaptic function (Granger et al., 2013; Penn et al., 2017).
Surface AMPA receptor diffusion may also regulate postsynaptic responses during high frequency trains of glutamate release. Following glutamate binding and activation, AMPA receptors quickly transition into a desensitized state that can last for tens to hundreds of milliseconds (Robert and Howe, 2003; Traynelis et al., 2010). Using an antibody crosslinking strategy, Heine et al. restricted lateral diffusion of surface AMPA receptors, which slowed the recovery of postsynaptic AMPA currents from paired-pulse depression. This observation suggests ongoing replacement of activated/desensitized receptors with laterally diffusing naïve receptors could play a role in shaping postsynaptic responses, especially during periods of high frequency neurotransmitter release (Heine et al., 2008). Surface diffusion of GABAA receptors could also shape inhibitory postsynaptic responses. Activated and desensitized GABAA receptors were observed to laterally diffuse from their sites of activation to adjacent synapses where they exchanged with naïve receptors to dampen inhibitory input (de Luca et al., 2017).
In addition to sculpting postsynaptic responses on short timescales, lateral diffusion of both pre-existing and newly-inserted AMPA receptors plays a major role in LTP (Penn et al., 2017). By acutely crosslinking recombinant or native AMPA receptors, Penn et al. investigated the contribution of lateral diffusion during LTP induced by high frequency stimulation (HFS). Immobilizing pre-existing surface AMPA receptors immediately prior to HFS-induced LTP blocked the potentiation observed immediately following LTP induction. Postsynaptic responses gradually increased in the minutes following LTP induction, presumably as AMPA receptors from intracellular pools were mobilized to the surface and integrated into synaptic sites. Accordingly, LTP was blocked when postsynaptic membrane fusion was inhibited with intracellular tetanus toxin light chain or N-ethylmaleimide. In this scenario, initial potentiation remains but decays back to the baseline value over several minutes suggesting that dendritic membrane trafficking delivers receptors and/or other factors responsible for stabilizing the potentiated state (Lledo et al., 1998). Similar experiments have demonstrated that clostridium neurotoxins also block plasticity at inhibitory synapses, supporting a role for regulated membrane fusion for delivery of GABAA receptors to postsynaptic sites (C. Q. Chiu et al., 2018; Ouardouz and Sastry, 2000). Further investigation will be necessary to determine whether postsynaptic membrane fusion plays a role in plasticity beyond the delivery of additional neurotransmitter receptors to the dendritic surface. We speculate that, in addition to receptor molecules, activity-responsive dendritic organelles deliver a cocktail of factors that could stabilize receptors at synapses and/or induce synapse growth, including cell adhesion proteins (Bemben et al., 2014; Chan et al., 2006; Heisler et al., 2014), soluble factors such as BDNF (Harward et al., 2016), or extracellular remodeling factors such as matrix metalloproteinases (X.-B. Wang et al., 2008).
In a complementary set of experiments from our group, we asked what would happen if we acutely added AMPA receptors to the PSD in the absence of a plasticity stimulus (Sinnen et al., 2017; Zimmerman et al., 2016). Using a novel optogenetic strategy based on light-triggered protein dimerizers (Kennedy et al., 2010b), we rapidly concentrated AMPA receptors in the postsynaptic membrane while simultaneously measuring evoked and spontaneous AMPA receptor currents (Fig. 4A). As expected, evoked AMPA receptor currents grew larger with a time course that matched receptor recruitment to the PSD. Surprisingly, adding high-conductance homomeric GluA1 AMPA receptors to the PSD had no effect on the amplitude of miniature postsynaptic excitatory currents (mEPSCs) but instead increased mEPSC frequency. This observation is consistent with previously described, pre-formed but “silent” synapses containing few or no AMPA receptors that can be functionally activated by receptor recruitment during LTP (Isaac et al., 1995; Liao et al., 1995). While quantal amplitude measured in response to naturally released glutamate was unaltered by receptor recruitment, responses to artificially-delivered glutamate at single spines grew larger. This finding adds support to previous modeling and imaging studies that restrict AMPA receptor activation to PSD nanodomains localized directly apposed to presynaptic neurotransmitter release sites (Franks et al., 2003; MacGillavry et al., 2013; McAllister and Stevens, 2000; Nair et al., 2013; Raghavachari and Lisman, 2004; Sinnen et al., 2017; Tang et al., 2016). Overall, these observations reveal the unexpected resilience of excitatory synaptic transmission, even in the face of acute perturbations in the number of PSD-localized neurotransmitter receptors. Whether the same principles apply at inhibitory synapses remains an open question, but sub-synaptic structures consistent with nano-domain organization of GABAA receptors have been observed (Pennacchietti et al., 2017; Specht et al., 2013).
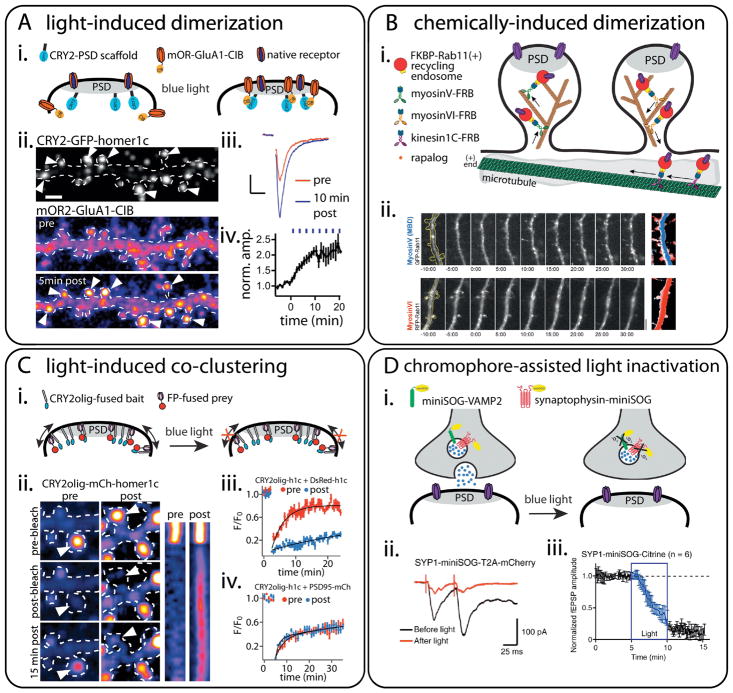
Figure 4. Tools to study trafficking at excitatory synapses.
(A) Light-induced dimerization to tune the abundance of synaptic proteins within the PSD. Adapted from Sinnen et al., (2017); reprinted with permission from Elsevier, copyright 2017. (i) Schematic of the light-dependent recruitment of CIB-fused AMPA receptors to a CRY2-fused PSD scaffold. (ii) Images of live hippocampal neurons expressing CRY2-GFP-homer1c (top panel) and mOrange2-GluA1-CIB before (pre) and after (post) blue light exposure. Note the robust synaptic accumulation of GluA1-CIB (arrowheads). Scale bar, 2 μm. (iii) Averaged (30 sweeps) dark (red) and post-light (blue) traces from a representative experiment measuring whole-cell evoked EPSC amplitudes from a CA1 pyramidal neuron from a hippocampal organotypic slice expressing CRY2-PSD95FingR and mCh-GluA1-CIB. Scale bar, 20 pA/20 ms. (iv) Kinetics of EPSC potentiation averaged from 11 different cells (±SEM).
(B) Chemically-induced dimerization to control endosomal transport in dendritic spines. Adapted from Esteves da Silva et al., (2015) and reprinted with permission from Elsevier. (i) Schematic of the inducible recruitment of KIF1C, myosinV, or myosinVI motors to Rab11-positive recycling endosomes (REs) using rapalog-induced heterodimerization of FRB and FKBP. KIF1C and MyosinV recruitment to Rab11-positive REs causes translocation of recycling endosomes from dendritic shafts to spines. MyosinVI recruitment causes the translocation of REs from spines to shafts. (ii) Stills from time-lapse recordings showing translocation of Rab11-positive REs upon rapalog-induced dimerization at time 0:00. Right: Overlay of sequential binarized frames is color coded for time from blue to white (−10:00–0:00) and white to red (0:00–30:00). Timescale is min:s. Scale bar, 5 μm.
(C) Light-induced co-clustering (LINC) combined with FRAP (LINC-FRAP) to examine protein interactions within the PSD. Adapted from Taslimi et al., (2014); reprinted with permission from Nature publishing group, copyright 2014. (i) Schematic describing LINC-FRAP to visualize synaptic protein interaction dynamics. Light-induced clustering of a CRY2olig-fused ‘bait’ protein restricts the mobility of an interacting FP-fused ‘prey’ protein. Co-clustering of the prey with the bait occurs only if the two proteins interact. (ii–iv) Proof-of-principle demonstrations of LINC-FRAP. (i) The mobility of CRY2olig-mCh-homer1c is decreased at the indicated synapses (arrowheads) after light-induced clustering. (ii) LINC-FRAP quantification before and after blue light. The mobility of DsRed-homer1c is severely limited after light-induced clustering of CRY2olig-homer1c. (iii). LINC-FRAP quantification before and after blue light. Clustering does not affect the mobile fraction of PSD95, indicating that clustering one PSD resident protein does not restrict exchange of all PSD proteins.
(D) Chromophore-assisted light inactivation (CALI) of synaptic proteins to inhibit neurotransmitter release. Adapted from Lin et al., (2013) and reprinted with permission from Elsevier, copyright 2013. (i) Schematic of the CALI-based synaptic inactivation system with either miniSOG-fused Vesicular Associated Membrane Protein 2 (VAMP2) or Synaptophysin (Syp). Light-induced singlet oxygen (1O2) production following focal irradiation permits spatiotemporal disruption of miniSOG-fused and neighboring proteins. (ii) Representative electrically-evoked EPSCs recorded in CA1 neurons from Syp-miniSOG-T2A-mCherry-expressing organotypical hippocampal slices before (black) and 5 min after (red) blue light exposure (~80% reduction). (iii) Quantification of the light-induced inhibition of field EPSP recorded from Syp-miniSOG-Citrine-expressing hippocampal organotypic slices (~85% depression).
Endocytic trafficking
At some point in their lifecycle, all surface proteins are subject to internalization into endocytic organelles. Generally, internalized cargo molecules are first delivered to early endosomal compartments before committing to one of several trafficking routes (Grant and Donaldson, 2009): (1) direct return to the plasma membrane, (2) indirect return to the plasma membrane through REs, (3) sorting for lysosomal degradation as early endosomes mature into late endosomes (Dunn et al., 1989), or (4) retrograde transport to the TGN. For many polarized cells, an additional trafficking route called transcytosis can occur where endocytosed cargo is transferred from the basolateral (somatodendritic) domain to the apical (axonal) domain or vice versa (Wisco et al., 2003).
Clathrin coated pits and early endosomes determine the post-endocytic fate of cargo molecules through what is likely a combination of passive, geometry-based sorting (e.g., the partitioning of cargo destined for reuse into tubular extensions that bud off from the early endosome) (Dunn et al., 1989; Mayor et al., 1993) and active sorting which requires the recognition of a cargo-sorting signal by a coat complex (Gage et al., 2001; Hsu et al., 2012; Lakadamyali et al., 2006; Puthenveedu and Von Zastrow, 2006; Tanowitz and Von Zastrow, 2003). In addition to coat and endocytic adaptor proteins, many other molecular components are required for the formation and fission of transport carriers from the early endosome, and it is the collective identity of these components that determine the destination of a given transport carrier within the endomembrane system. Similarly, fusion of a transport carrier with its target compartment involves coordinated action by specific trafficking factors that include SNARE proteins, tethering proteins, cytoskeletal components, and associated regulatory proteins such as Rab and Arf GTPases. Notably, each organelle is associated with a specific set of Rabs, which function as intracellular traffic controllers to ensure the accurate and expeditious flow of cargo transport through the endocytic pathway. Importantly, all organelles in the endocytic pathway are linked through the continuous flux of cargo-containing transport carriers and are therefore highly dynamic structures. It is precisely this contiguity within the endomembrane system that confers its marked ability to meet the homeostatic demands of the cell while simultaneously presenting challenges for the researchers who wish to study membrane recycling. The following sections will discuss our current understanding of the structure and function of the dendritic endocytic network in neurons, while highlighting some recent technological developments that have helped overcome some of the challenges in studying the endocytic pathway.
Organization of intracellular trafficking organelles in neuronal dendrites
Endocytic and recycling organelles
An extensive endosomal network in neuronal dendrites was revealed in early studies using pulse-chase labeling techniques, live-cell imaging, and serial section EM (Parton et al., 1992). By altering both incubation times and chase periods using a combination of fluid-phase and receptor-bound markers such as HRP, ovalbumin-gold and labeled transferrin, Parton et al. identified early and late endocytic compartments in rat hippocampal dendrites based on differences in morphology, cellular distribution, and labeling kinetics. Tubular structures that corresponded to early endosomes and REs were found to extend to the distal regions of dendrites, whereas larger multivesicular body-like structures that corresponded to late endosomes and lysosomes showed a more centralized distribution within the soma and proximal dendrite (Parton et al., 1992). Subsequent EM studies have expanded upon the characterization of the endocytic network in neuronal dendrites by distinguishing multiple different classes of endosomal compartments based on their distinctive morphologies and their spatial relationship to synaptic sites (Cooney et al., 2002; Spacek and Harris, 1997).
Together with data from immunofluorescence and immuno-EM studies, these ultrastructural analyses provided evidence for local endocytic organelles in spine heads (Blanpied et al., 2002; Cooney et al., 2002; Rácz et al., 2004; Spacek and Harris, 1997). The presence of coated pits and vesicles in spines in combination with the existence of endocytic zones localized adjacent to the PSD (Blanpied et al., 2002) raises the question of whether local trafficking of synaptic membrane lipids and proteins plays a role in mediating synaptic function and plasticity. In support, Toni et al. revealed striking changes in spine morphology and coated vesicle formation 30 min following hippocampal LTP using an EM method to visualize stimulated synapses based on their calcium load (Toni et al., 2001). Toni et al. found an increased proportion of synapses with segmented PSDs following LTP and that these synapses very often contained coated vesicles (the proportion of synapses with coated vesicles was three-to-five times higher than that in any other synapse type) (Toni et al., 2001). Likewise, a different study showed an increase in endosome content at early times following LTP induction (M. Park et al., 2006a). Indeed, dendritic spines have since been found to harbor a local endosomal network that could play roles modifying synaptic connectivity during plasticity and sustaining synaptic function under basal conditions (Brown et al., 2007; Kapitein et al., 2010; Kennedy et al., 2010a; M. Park et al., 2006a; 2004a; Patterson et al., 2010). In particular, REs may play a key role in synaptic membrane remodeling given their ability to respond to changes in synaptic activity (Hiester et al., 2017; M. Park et al., 2004b; 2006b). Following LTP-inducing stimuli, REs rapidly fuse with the plasma membrane in dendrites and spines, presumably to facilitate the synaptic delivery of key synaptic molecules (Brigidi et al., 2015; Keith et al., 2012; Kennedy et al., 2010a; M. Park et al., 2006a; 2004a; Patterson et al., 2010; Woolfrey et al., 2015). Spine-localized REs are small, relatively stable structures that range in size from ~50 to 500 nm in diameter, whereas shaft-localized REs are typically larger (1–4 μm in length) and highly mobile. Long-range bidirectional transport of REs in dendrites is microtubule-based, which is faster than the actin-based motility generally associated with RE transport in spines (Esteves da Silva et al., 2015); however, there have been reports of a stationary subpopulation of shaft-localized REs whose function is currently unknown (Prekeris et al., 1999; Schwenk et al., 2016). In addition to mediating trafficking of recycled proteins, new proteins on their initial voyage to the PM also utilize the dendritic RE network, including spine REs (Bowen et al., 2017). Whether these organelles are a distinct subset of REs or if anterograde traffic merges with REs that are already participating in ongoing recycling remains to be seen but it should be considered that blocking RE function will not only impact the recycling pools of proteins but also newly synthesized proteins making their first trip to the PM (Bowen et al., 2017).
Dendritic lysosomes
Although lysosomes are not part of the endocytic pathway, it is important to consider the dendritic organization and emerging role of these organelles in neuronal functions such as dendritic calcium signaling (Padamsey et al., 2016) and local degradation of synaptic proteins (Goo et al., 2017). Using two-photon single-spine glutamate uncaging, Goo et al. demonstrated activity-dependent stalling of mobile lysosomes at the base of activated spines where they could play a role in cargo trafficking/degradation, or participating in Ca2+ signaling (Goo et al., 2017). Lysosomes are not only localized to the base of spines, but in some cases they are present in spine heads. While this occurs in only a sparse subset of spines it is tempting to speculate they could play a direct role in synaptic function or plasticity. For example, Padamsey et al. found that lysosome fusion and cathepsin B release for extracellular matrix remodeling was necessary for maintaining activity-induced spine growth (Padamsey et al., 2016).
Endocytic transport of synaptic proteins
Endocytic recycling of synaptic proteins and lipids enable rapid remodeling of the postsynaptic membrane following plasticity. The swift removal or delivery of synaptic proteins relies on the coordinated movement of endosomes and endocytic transport vesicles along polarized cytoskeletal networks in dendrites and spines (Esteves da Silva et al., 2015; Goo et al., 2017; Hendricks et al., 2010; McVicker et al., 2016; Prekeris et al., 1999). Transport directionality and cargo specificity are mediated by actin-based and microtubule-based motor proteins and cargo-motor adaptors. Broadly, local surface delivery of recycled proteins to excitatory spine synapses involve the anterograde transport of endosomes/transport vesicles by plus-end-directed myosin motors along the polarized actin filaments in spines (Correia et al., 2008; Lisé et al., 2006; Yoshimura et al., 2006). Conversely, removal of surface proteins at excitatory spine synapses generally involve retrograde transport of endosomes/endocytic vesicles by the uniquely minus-end-directed motor, myosinVI (Osterweil et al., 2005). General trafficking rules for endocytic transport at shaft-localized excitatory and inhibitory synapses are not as straightforward. Microtubules predominate in dendritic shafts; however, the cellular cortex subjacent to the PM is rich in F-actin, actin-binding proteins, and myosin motors. Thus, both microtubule-based and actin-based transport mechanisms have been reported, both alone and in coordination, to mediate the endocytic transport of inhibitory synaptic proteins (Heisler et al., 2011; Maas et al., 2006; Twelvetrees et al., 2010). Furthermore, mixed microtubule orientations in proximal dendrites result in the unpredictable movements of endosomes/transport vesicles containing endocytic cargo from shaft synapses.
Given the redundant roles of many kinesin, dynein, and myosin motors in endocytic transport, cargo-motor adaptor proteins play a critical role in mediating specificity of receptor synapse removal and delivery. For example, dynein is involved in the retrograde transport of both glycine receptors (GlyRs) and GABAA receptors away from inhibitory synapses; however, cargo specificity is conferred through the direct binding of dynein light chain to the GlyR β subunit binding protein gephryin (Maas et al., 2006) or the GABAAR α1 subunit binding protein muskelin (Heisler et al., 2011). These cargo-motor adaptor proteins link specific receptors to distinct actin-based or myosin-based motors and thus regulate the types of cargo that are trafficked to and from inhibitory and excitatory synapses. For example, the reinsertion of GABAA receptors into the inhibitory postsynaptic membrane is mediated by a motor protein complex comprised of KIF5 and the adaptor huntingtin-associated protein 1 (HAP1) (Twelvetrees et al., 2010), whereas the reinsertion of AMPA receptors into excitatory spine synapses involve myosinVb and the endosomal adaptor Rab11-FIP2 (Z. Wang et al., 2008a).
Many studies have manipulated motor protein-based transport processes to better understand the role of endocytic transport in neuronal function. Acute chemical-genetic manipulations of myosin-based RE transport support the idea that endocytic recycling of AMPA receptors play a critical role in activity-dependent synaptic potentiation (M. Park et al., 2004a; Z. Wang et al., 2008b). Wang et al. showed that hippocampal LTP was abrogated in engineered mice that harbor a mutant version of myosinVb that can be specifically inhibited with an orthologous ADP analog (Z. Wang et al., 2008b). A more recent study by Esteves da Silva et al. employed a chemically induced dimerization system to recruit KIF1c, myosinV or myosinVI to Rab11-positive REs to control RE recruitment to or removal from spines (Esteves da Silva et al., 2015) (Fig. 4B). While they observed no immediate effect on dendritic spine morphology, removal of REs from spines decreased synaptic AMPA receptor and PSD95 levels at synapses (Esteves da Silva et al., 2015). In addition to myosinVb, myosin VI, and Rab11 function, several additional factors have been shown to be involved in the endocytic trafficking of AMPA receptors. In brief, AMPA receptor endocytosis has been shown to require the function of RE-associated activity-regulated cytoskeleton associated protein (Arc/Arg3.1) and its interaction with endocytic machinery components adaptor protein complex-2 (AP2), endophilin, and dynamin (Shepherd et al., 2006; Wall and Corrêa, 2017). AMPA receptor endocytic recycling and surface delivery have been shown to require the function of protein kinase A (PKA; Ehlers, 2000), Rab8 (Brown et al., 2007), Ras (Patterson et al., 2010; Zhu et al., 2002), palmitoylated A-kinase anchoring protein 79/150 (AKAP79/150; Keith et al., 2012), retromer (Choy et al., 2014; Temkin et al., 2017; Varandas et al., 2016), GRASP1 (S.-L. Chiu et al., 2017; Hoogenraad and van der Sluijs, 2010), and the Type I PDZ domain-containing protein sorting nexin 27 (SNX27; Loo et al., 2014).
III. New technology for studying protein trafficking
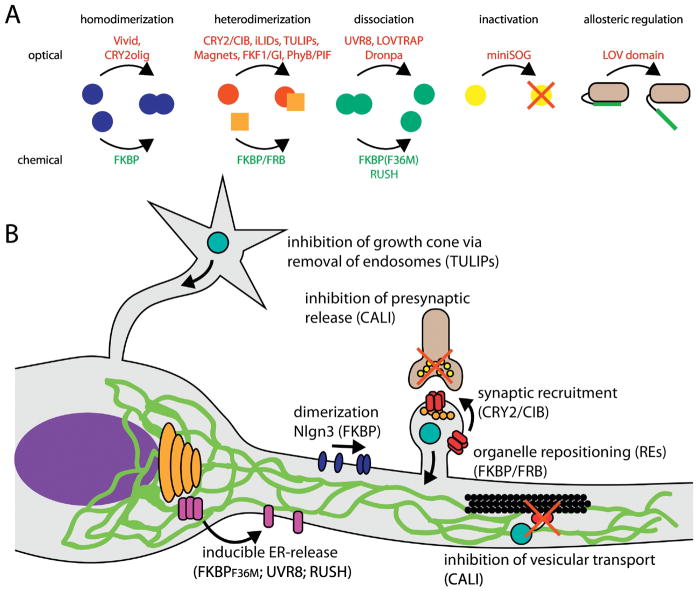
There has been an intense effort to engineer new tools for studying cellular protein trafficking ranging from new imaging hardware to innovative molecular tools for labeling and precisely manipulating protein localization and function (Fig. 3). Here we review new approaches that have already been used, or show great promise for investigating protein trafficking.
Figure 3. Overview of the strategies for chemically and optically controlling trafficking in neurons.
(A) Conditional chemical and optical modules for controlling the function or subcellular localization of proteins and organelles.
(B) Examples of the cellular processes that can be targeted with the indicated tools.
Super resolution imaging
A fundamental constraint on visualizing trafficking organelles in live cells is their small size (often below the diffraction limit of light) and rapid movements. There remains a major and largely unmet challenge for super resolved imaging approaches that can generate multi-channel images on a rapid (ideally millisecond) timescale, using light intensities that do not lead to photodamage in live cells. Perhaps the super-resolution technique considered most amenable to longitudinal live-cell imaging is structured illumination microscopy (SIM), yet conventional, or linear, SIM fails to achieve the sub-100 nm resolution or the imaging frame rate required for many dynamic cellular processes. Recently developed approaches such as high-numerical aperture (NA) TIRF-SIM and patterned-activation nonlinear SIM (PA NL-SIM) can achieve higher resolution than linear SIM without compromising imaging speed and light dose (Li et al., 2015), and are promising methodologies for visualizing both inter-organellar transport carriers and distinct cargo subdomains within endosomal membranes. One major limitation, however, should be considered before using any TIRF-based approach to image cultured neurons. The glial layer that exists between the neurons and the glass coverslip significantly reduces the already restricted penetration depth of 50–200 nm.
Recent developments in light-sheet microscopy also hold great promise for super resolution imaging live tissue in three dimensions with minimal photodamage. Because the excitation energy is only delivered to the imaging plane through a separate objective positioned perpendicular to the imaging objective, photodamage to the surrounding tissue and bleaching of fluorophores outside of the imaging plane are greatly reduced (Huisken et al., 2004). While early light-sheet microscopy used Gaussian light sheets that were too thick to achieve high spatial resolution for imaging subcellular dynamics, further innovations have refined this technique by shaping the light sheet into a non-diffracting Bessel beam (Planchon et al., 2011). Introducing optical interference patterns (lattice light sheet), has led to the ability for long-term, multi-color, 3 dimensional super resolution imaging with minimal photodamage (B.-C. Chen et al., 2014).
A more accessible means for achieving live-cell super-resolution involves the application of an algorithm to an image sequence acquired by conventional widefield, confocal, and TIRF microscopy (Gustafsson et al., 2016). This computational approach, called super-resolution radial fluctuation (SRRF), generates a super-resolution image based on the radial symmetry of the imaged fluorophores, not their individual localizations, in order to achieve sub-100 nm lateral resolution (Gustafsson et al., 2016). While still nascent, this approach holds promise for super resolution imaging of dynamic cellular processes near the plasma membrane, but still lacks temporal resolution since fluctuation analysis requires acquisition of tens to hundreds of individual images to generate one super-resolved image. Other techniques, based on single molecule localizations are also showing great improvements, especially for high resolution, multi-color imaging in three-dimensional space, and have recently been reviewed in depth elsewhere (Nicovich et al., 2017; Sahl et al., 2017).
Another promising approach is to combine super-resolution light microscopy (which offers the ability to localize molecules) with electron microscopy (which offers unparalleled visualization of cellular structure) (Perkovic et al., 2014; Watanabe et al., 2011). Advances in correlated light-electron microscopy (CLEM) hold promise for interrogating the molecular organization of cellular structures. While sample processing for EM precludes visualizing dynamic processes in live cells, some special exceptions deserve discussion. For example, Watanabe et al. combined optogenetic activation of synaptic vesicle fusion with rapid high-pressure freezing at different time intervals following the stimulus to precisely define where and when neurotransmitter vesicle exocytosis and compensatory endocytosis occur in presynaptic terminals (Watanabe et al., 2013). Super-resolution CLEM holds great promise for future studies investigating how exocytosis and endocytosis are coordinated by superimposing molecular localizations of active zone and endocytic zone proteins onto dynamic membrane structures that have been frozen in the act of fusion and/or endocytosis. Thus, visualizing the molecular ultrastructure of dynamic processes is becoming increasingly accessible, especially for processes that can be precisely triggered with a time-locked stimulus.
Selectively labeling newly synthesized proteins
The coupling of protein-labeling strategies with fluorescent microscopy techniques allows for the real-time observation of the activity, mobility, conformation, and subcellular localization of proteins in cells. Detection of a target protein is achieved through the direct or indirect covalent attachment of a biological fluorophore, enzyme-substrate reporter, radioactive isotope, quantum dot, biotinylated probe, or synthetic organic dye. The use of protein-labeling strategies for studying anterograde trafficking in live cells presents numerous new challenges that include selectively labeling newly synthesized proteins and minimally perturbing endogenous trafficking mechanisms with the labeling method.
A genetically encoded labeling strategy called TimeSTAMP2 allows for selective visualization of newly synthesized proteins fused to an engineered fluorophore which contains the NS3 protease domain of hepatitis C virus (HCV), flanked by its own protease recognition sites. Constitutive protease-mediated fragmentation of the fluorophore occurs until a cell-permeable HCV NS3 protease inhibitor is applied. Upon protease inhibition, new copies of the fusion protein will include an intact fluorophore, thereby allowing selective detection of protein that was synthesized following inhibitor application. Butko et al. utilized this approach to determine where and when synaptic activity induced accumulation of new synaptic proteins (Butko et al., 2012). Using hippocampal neurons grown in microfluidic devices, they visualized local activity-dependent translation of PSD95 by coupling their drug-controlled labeling strategy with local application of BDNF or the mGluR agonist DHPG.
In addition to intrinsically fluorescent proteins, new approaches have been developed for targeting a synthetic dye to a relatively short peptide sequence engineered into a target protein. This approach has advantages over traditional fluorescent protein labeling because it allows users flexibility in dye characteristics (color, pH sensitivity, cell permeability etc.). Furthermore, most inorganic dyes have favorable characteristics for bioimaging (e.g. high quantum yield and better photostability). Early approaches used the biarsenical dyes ReAsH-EDT2 and FlAsH-EDT2, which fluoresce red and green, respectively, upon binding to a small motif containing four cysteine residues. This approach was used to investigate trafficking of newly synthesized AMPA receptors by inserting the tetracysteine (TC) motif into the intracellular C-termini of GluA1 and GluA2. This allowed optical “pulse-chase” experiments by sequentially labeling expressed receptors in hippocampal cultured neurons with different colored fluorescent dyes (Ju et al., 2004). Applying FlAsH-EDT2 6–8 hours after ReAsH-EDT2 application selectively labeled the receptors that were synthesized within the chase period since preexisting receptors had already been labeled with the first dye. This approach revealed local synthesis and trafficking of these receptors, even in dendrites that had been mechanically isolated from the cell body (Ju et al., 2004). Subsequent strategies for inorganic dye targeting to genetically encoded tags have become widely adopted, including SNAP and HALO tags, which can be covalently modified with an ever-increasing palette of fluorescent dyes (Encell et al., 2012; Grimm et al., 2017; Keppler et al., 2003; Los et al., 2008).
An alternative strategy for labeling newly synthesized proteins involves the targeting of multiple fluorescent antibody-fusion proteins to a tandem array of epitopes called SunTag (Tanenbaum et al., 2014). For single-molecule imaging of nascent proteins, a SunTag-fused mRNA of interest is co-expressed with GFP-fused single-chain variable fragment (scFv) antibodies. As the SunTag is translated, multiple epitopes are made available for GFP-scFv binding. To limit GFP-scFv sequestration by the translation product, a degron sequence is fused to the C terminus of SunTag. The resulting multivalent fluorescent amplification of nascent protein enables real-time imaging of translation dynamics in live neurons and in vivo (C. Wang et al., 2016; B. Wu et al., 2016; Yan et al., 2016).
While the above approaches require genetically modifying target proteins, which could interfere with localization/function, a different approach for labeling the entire pool of newly synthesized endogenous proteins is to couple fluorescent non-canonical amino acid tags (FUNCAT) (Dieterich et al., 2010). While this approach is useful for biochemical approaches to characterize the entire pool of newly synthesized proteins, it can be combined with antibody labeling and proximity ligation assay (PLA) to selectively visualize new pools of specific proteins (provided that a reliable antibody exists for the protein of interest) (Tom Dieck et al., 2015). However, this PLA-based strategy is not amenable to live-cell imaging for dynamic cellular processes.
Inducible protein trafficking systems for investigating secretory trafficking routes
A powerful approach for investigating protein trafficking through the secretory pathway has been to sequester newly synthesized proteins in the ER and induce their release while visualizing them in real time in live cells. Classic experiments utilized a temperature sensitive mutant of VSV-G (VSV-Gts045) which does not exit the ER at or above ~39 °C (Lodish and Weiss, 1979). Reducing the temperature triggers synchronous release of VSV-G ts045 from the ER. Using fluorescently tagged versions of VSV-Gts045 allowed researchers to directly visualize a secretory cargo as it made its journey from the ER to the PM (Lippincott-Schwartz et al., 1989; Presley et al., 1997). Later, more sophisticated approaches for conditionally releasing cargoes from the ER with chemicals or light were developed (Boncompain et al., 2012; D. Chen et al., 2013; Rivera et al., 2000). These approaches are advantageous in that nearly any protein can be targeted for inducible release, not just proteins for which a temperature-sensitive trafficking mutation is available. Generally speaking, these approaches involve fusing domains onto a target protein to trap it in the ER, for example by anchoring it to another ER-retained protein or by the formation of protein clusters that are too large and immobile for ER export (i.e. conditional aggregation domain or CAD). The first described CAD was a self-associating FK506-binding protein (FKBP) mutant that harbored a single point mutation (F36M) in the ligand binding site (Rivera et al., 2000; Rollins et al., 2000). Proteins fused to multiple, tandem copies of this CAD are trapped in the ER as the CADs self-associate. Following addition of a cell-permeable, biologically inert rapamycin analog, CAD multimers rapidly dissociate, allowing ER exit and progression through the secretory pathway (Fig. 2C). A consensus cleavage site for the GA-resident protease furin can be placed between the protein of interest and the CAD repeats so they are removed in the GA, preventing them from reassociating, or interfering with downstream trafficking. This strategy has been heavily utilized in the study of protein trafficking in a wide variety of cell types, including neurons (Al-Bassam et al., 2012; Bowen et al., 2017; Jaiswal et al., 2009; Rivera et al., 2000; Thuenauer et al., 2014; Valenzuela et al., 2014) (Fig. 2C–H).
A similar inducible release strategy, “retention using selective hooks” (RUSH), exploits the competition between streptavidin-binding protein (SBP) and biotin for strong, but non-covalent binding to streptavidin (Boncompain et al., 2012). Streptavidin, fused to a “hook” protein that promotes anchoring to a specific organelle, reversibly interacts with SBP fused to a “reporter” protein of interest. Targeting to different organelles in the secretory pathway is possible based on the identity of the hook protein (e.g. KDEL signal sequence for ER retention). The synchronous release of the SBP-reporter fusion protein from the target organelle is initiated by adding biotin, which disrupts the SBP/streptavidin interaction. This approach was adopted by Evans et al. to study the secretory trafficking of the kainate receptor subunit GluK2 in cultured hippocampal neurons under basal and stimulated conditions (Evans et al., 2017).
Approaches for the optical control of protein secretion have been developed, with the ultimate goal of using focal illumination to locally release proteins from the ER within specific cellular domains (e.g. axons, soma or dendrites) to investigate how the location of ER release impacts the downstream trafficking itinerary of diverse protein cargoes. The first light-controlled inducible release system used the Arabidopsis photoreceptor UVR8 as the optogenetic actuator module for protein secretion (D. Chen et al., 2013) (Fig. 2A–B). UVR8 forms constitutive homodimers that are dissociated upon UV-B (280 to 315 nm) excitation (Christie et al., 2012; Rizzini et al., 2011). Chen et al. exploited this property to control secretion in cultured fibroblasts and hippocampal neurons using a CAD-based strategy where tandem copies of UVR8 were fused to VSV-G (Fig. 2A). In this case, light quickly dissolved ER-localized clusters of VSV-G, allowing forward trafficking to proceed. This system has subsequently been implemented for chemokine release in vivo (Sarris et al., 2016), but given the short wavelength requirements, the approach has been difficult to implement for precise local release using focal excitation. Thus, while multiple inducible ER-release strategies exist, due to technical limitations, none have proven entirely satisfactory addressing the spatiotemporal relationship between ER release and surface insertion in large, morphologically complex cells like neurons.
Other approaches to manipulate protein trafficking and localization
Chemical dimerization
Chemical control of protein-protein interactions to control cellular function has proven to be an invaluable tool for studying diverse cellular processes. Early approaches utilized a small molecule consisting of two FK506 moieties joined by a chemical linker (FK1012). As FK506 tightly binds FKBP one could use this compound to conditionally homo-dimerize any protein fused to FKBP (Spencer et al., 1993). Subsequent iterations enabled rapamycin-triggered heterodimerization between FKBP and its binding partner FRB (J. Chen et al., 1995; Ho et al., 1996) and engineered selective, orthologous FKBP/FRB protein domains and ligands overcame potential off-target effects of FK506 and rapamycin (Clackson et al., 1998). In neurons, such systems have been used to conditionally anchor organelles to motor proteins for inducible manipulation of their subcellular distribution (Esteves da Silva et al., 2015; Kapitein et al., 2010) (Fig. 4B). The FKBP/FRB system has also been used to probe the functional significance of neuroligin dimerization (Shipman and Nicoll, 2012), and to inducibly sequester proteins from their functional sites for acute loss of function (Robinson et al., 2010).
Light-induced protein dimerization
Building on previous chemical-genetic strategies, numerous new approaches have been engineered for controlling protein interactions with light. Given the major advantage in spatiotemporal precision over chemical-genetic strategies (not to mention the advantage of not having to purchase costly small molecule reagents), these tools are being broadly adopted for controlling diverse cellular processes and biochemistry. Early studies used the plant photoreceptors phytochromeB, cryptochrome2 (CRY2) or FKF1 along with their cognate light-dependent binding partners PIF3/6, CIB1 or gigantea, respectively, to drive light-triggered protein dimerization (Kennedy et al., 2010b; Levskaya et al., 2009; Yazawa et al., 2009). The self-associating CRY2 photoreceptor has also been used for light-controlled protein homo-oligomerization (Bugaj et al., 2013; H. Park et al., 2017; Taslimi et al., 2014) (Fig. 4C). Later dimerizer systems, largely based on the photosensitive LOV domain from Avena sativa phototropin were developed, taking advantage of the robust photo-induced structural change this photoreceptor undergoes. Numerous approaches have exploited this property by fusing peptide sequences to the LOV domain C-terminus. In darkness, C-terminal peptides are largely inaccessible to potential binding partners. Upon photoexcitation, the helix unwinds, effectively “uncaging” the C-terminal peptide, allowing access to different binding partners (Harper et al., 2003; Lungu et al., 2012; Mills et al., 2012; Spiltoir et al., 2016; Strickland et al., 2008; Yi et al., 2014). To generate a light actuated “reverse dimerizer” (i.e. a dimerizer pair that interacts in the dark but is dissociated by light), Wang et al. screened a library of a randomly mutagenized small protein domain (Z domain from protein A) and discovered variants that tightly bound to the Avena sativa LOV2 domain in the dark, but dissociated upon photoexcitation (H. Wang et al., 2016). In addition to their use in light-triggered protein interaction or dissociation tools, LOV domains can be genetically inserted into strategic locations within proteins for allosteric control of protein function with light (J. Lee et al., 2008; Y. I. Wu et al., 2009). Promising efforts to develop sequence- and structure-based algorithms for predicting where LOV domain insertion will be most effective for allosteric control of key signaling enzymes hold promise for streamlining the engineering of opto-alleles of diverse signaling proteins (Dagliyan et al., 2016).
Conditional protein inactivation with light
Chromophore-assisted light inactivation (CALI) of protein function has emerged as a powerful optical technique for manipulating protein function and trafficking. For some chromophores, light absorption generates abundant reactive oxygen species (ROS), such as singlet oxygen and superoxide, that can oxidize nearby electron-dense amino acids (e.g. cysteine, histidine, methionine, tryptophan, tyrosine), often rendering proteins inactive. While the basic CALI approach was described many decades ago (Jay, 1988), the approach has experienced a resurgence in cell biology with the advent of genetically-encoded fluorescent proteins and chemical-genetic targeting technologies (e.g. SNAP and Halo tags) as CALI reagents instead of dye-conjugated antibodies, which can be difficult to introduce into cells (Bulina et al., 2006; Keppler and Ellenberg, 2009; Takemoto et al., 2011; 2013; Tour et al., 2003). Many synthetic dyes (e.g. malachite green, fluorescein, eosin, FlAsH, ReAsH) are effective chemical photosensitizers with high ROS yields. Furthermore, chemical photosensitizers can now be targeted to specific protein modules and cellular domains through the use of SNAP-tag, HaloTag, or TC motifs (Keppler and Ellenberg, 2009; Poskanzer et al., 2003; Takemoto et al., 2011; Tour et al., 2003). Completely genetically encoded sensitizers have also been engineered and are advantageous because they do not require the addition of an exogenous chromophore. For example, an engineered fluorescent flavoprotein called miniSOG (mini-singlet oxygen generator) is a small (106 amino acid), genetically-encoded photosensitizer with a ROS quantum yield almost two times higher than previous genetically-encoded photosensitizers such as KillerRed and SuperNova (Lin et al., 2013; Shu et al., 2011; Takemoto et al., 2013). MiniSOG is considerably smaller than the genetically encoded HaloTag (297 amino acids) or SNAP-tag (182 amino acids), and is the first photosensitizer engineered from a LOV-based fluorescent protein (Buckley et al., 2015). With an inactivation radius of ~70 nm, light-induced singlet oxygen production following focal irradiation of miniSOG permits spatiotemporal disruption of miniSOG-fused and neighboring proteins. A recent study demonstrating the utility of CALI in manipulating protein trafficking used miniSOG to elucidate the effect of acutely inactivating CaMKII (UNC-43) and kinesin-1 (UNC-116) on the endosomal transport of AMPA receptors (GLR-1) in C. elegans (Hoerndli et al., 2015). Likewise, Lin et al. used miniSOG fused to the vesicular protein synaptophysin (Syp) to disrupt synaptic neurotransmitter release (Lin et al., 2013) (Fig. 4D). This study highlighted potential off-target effects of CALI-based approaches as illumination of miniSOG localized to synaptic vesicles or the axonal plasma membrane paradoxically increased spontaneous miniature synaptic transmission while at the same time potently disrupting evoked neurotransmission.
To circumvent potential off-target effects of CALI-based approaches, more refined approaches for light-induced loss of function are being developed. For example the light-induced conformational change of the LOV domain has been used to conditionally present inhibitory peptides against CaMKII and PKA (Murakoshi et al., 2017; Yi et al., 2014). While this approach requires a characterized inhibitory peptide, direct allosteric control of enzyme function through strategic LOV domain insertion is also a promising avenue for acute, light activated loss of function (Dagliyan et al., 2016).
Spatial and temporal control of second messengers
Precisely timed delivery of second messengers has been a powerful approach for unraveling how specific signaling events influence cellular physiology. Early, but still widely used approaches rely on second messengers chemically modified with photo-labile “caging” groups that can be rapidly removed by photo-excitation in the UV spectrum (or in many cases, by multiphoton absorption of energy in the near infrared spectrum). Here we review efforts toward engineering entirely genetically-encoded tools for local control of cellular second messengers, which could hold future promise for investigating how specific signaling events influence protein function, localization and trafficking.
Cyclic nucleotides play critical roles in a myriad of neurobiological functions ranging from neuronal differentiation and growth to neuronal excitability and synaptic plasticity through their ability to stimulate signaling enzymes and channels. The visual signal transduction cascade mediated by mammalian cone and rod opsins was an early example of light-regulated signaling through hydrolysis of cGMP by light-gated G-protein activation of phosphodiesterase enzymes. Cyclic nucleotide modifying enzymes that are directly regulated by light have also been discovered. For example a photoactivatable adenylyl cyclase (euPAC) was identified as a sensor for phototaxis in the flagellate Euglena gracilis (Iseki et al., 2002). euPAC is a tetramer of two large (>100 kDa) FAD-binding subunits (PACα and PACβ) that each contain two blue-light sensing BLUF domains followed by AC catalytic domains. While euPAC and its flavoprotein PACα have been used to study cAMP-dependent processes in neurons (Nagahama et al., 2007; Schröder-Lang et al., 2007; Weissenberger et al., 2011), smaller blue light-activated PACs with less dark activity and a greater dynamic range have since been identified, such as bPAC isolated from the soil bacterium Beggiatoa (Stierl et al., 2011) and the LOV-domain-containing mPAC from the cyanobacterium Microcoleus chthonoplastes (Raffelberg et al., 2013). To optically manipulate cAMP levels in vivo, Ryu et al. engineered a PAC that is activated by light in the near-infrared window (NIRW) of the spectrum (~680–880 nm) by fusing the photosensory module of the Rhodobacter sphaeroides bacteriophytochrome BphG1 to the bacterial AC domain CyaB1 (Ryu et al., 2014). In addition to greater tissue penetration depth, expressing NIRW light-activated AC (IlaC) in cholinergic neurons of C. elegans allowed manipulation of behavior without activating a photoavoidance response or inducing phototoxicity associated with prolonged exposure to blue light. Apart from using photoactivatable cyclases, another strategy to control cAMP and cGMP levels is to employ a light-activated phosphodiesterase (LAPD) that hydrolyzes cyclic nucleotides upon absorption of red light (~650–700 nm) (Gasser et al., 2014). In LAPD, human phosphodiesterase 2A is fused to the photosensory module of the Deinococcus radiodurans bacterial phytochrome that can be photoconverted between a red light-absorbing (Pr) basal state and a far-red light-absorbing (Pfr) “active” state upon photo-isomerization of the covalently bound biliverdin chromophore.
Diacylglycerol is a lipid second messenger known to induce the membrane translocation of C1-domain-containing proteins including protein kinase C (PKC) and DAG kinases as well as activate certain transient receptor potential (TRP) cation channels. To engineer a photoswitchable DAG (PhoDAG), Frank et al. exploited the light-dependent cis/trans isomerization of a previously identified azobenzene derivative that mimicked arachidonic acid in its cis configuration (Frank et al., 2016). Conversion between the cis and trans forms is induced by UV-A and blue light irradiation, respectively, making PhoDAG an effective toggle switch for modulating DAG signaling. PhoDAGs have been used to optically control Munc13 membrane translocation in HeLa cells, synaptic transmission at the C. elegans neuromuscular junction, and Trpc2 channel activation in acute slices of the mouse vomerosonasal organ (Frank et al., 2016; Leinders-Zufall et al., 2017). Combined with calcium imaging, local PhoDAG activation has been demonstrated as a useful tool in the spatial mapping of DAG-sensitive calcium-permeable ion channels localized in discrete subcellular compartments, such as in the dendritic knobs of mouse vomerosonasal sensory neurons (Leinders-Zufall et al., 2017). These examples highlight our increasing ability to manipulate second messengers and their promise for understanding signaling events for regulated protein trafficking in complex cells like neurons.
Conclusions
The protein trafficking field has benefited from major advances in live-cell imaging, protein engineering and new optical techniques that allow high-resolution tagging and manipulation of target proteins in live cells. However, several major challenges remain, including our limited ability to detect distinct protein pools in live cells as they disperse during trafficking; our limited capacity for super resolution imaging rapid, dynamic processes in live cells and our current dependence on genetically fused protein tags for visualizing most proteins in live cells. Ongoing advances in imaging hardware and analysis, optogenetic tools for manipulating proteins and second messengers, genetically encoded intrabodies and new approaches for genetically targeting endogenous proteins through gene editing are quickly surmounting these challenges, allowing the field to move forward with increasing sophistication.
Highlights.
Here we review how proteins traffic in neurons, with an emphasis on synaptic proteins whose regulated trafficking are required for fundamental forms of plasticity
We also review diverse chemical and light-based tools to study protein trafficking with greater spatial and temporal precision, which will allow the field to approach difficult and persistent questions.
Acknowledgments
We apologize to our colleagues whose work we could not cite because of space limitations. A.M.B. is supported by the National Science Foundation (DGE-1553798) and the Howard Hughes Medical Institute Gilliam Fellowship. A.B.B. is supported by the National Institutes of Health (NS092421). Work in the lab of M.J.K. is supported by the National Institute of Neurological Disorders and Stroke (NS082271), National Institute on Aging (AG058005), the Pew Charitable Trusts, and the Boettcher Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ailion M, Hannemann M, Dalton S, Pappas A, Watanabe S, Hegermann J, Liu Q, Han HF, Gu M, Goulding MQ, Sasidharan N, Schuske K, Hullett P, Eimer S, Jorgensen EM. Two Rab2 interactors regulate dense-core vesicle maturation. Neuron. 2014;82:167–180. doi: 10.1016/j.neuron.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Bassam S, Xu M, Wandless TJ, Arnold DB. Differential trafficking of transport vesicles contributes to the localization of dendritic proteins. CellReports. 2012;2:89–100. doi: 10.1016/j.celrep.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang AL, Taguchi T, Francis S, Fölsch H, Murrells LJ, Pypaert M, Warren G, Mellman I. Recycling endosomes can serve as intermediates during transport from the Golgi to the plasma membrane of MDCK cells. The Journal of Cell Biology. 2004;167:531–543. doi: 10.1083/jcb.200408165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aridor M, Bannykh SI, Rowe T, Balch WE. Cargo can modulate COPII vesicle formation from the endoplasmic reticulum. J Biol Chem. 1999;274:4389–4399. doi: 10.1074/jbc.274.7.4389. [DOI] [PubMed] [Google Scholar]
- Aridor M, Guzik AK, Bielli A, Fish KN. Endoplasmic reticulum export site formation and function in dendrites. J Neurosci. 2004;24:3770–3776. doi: 10.1523/JNEUROSCI.4775-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur AL, Yang SZ, Abellaneda AM, Wildonger J. Dendrite arborization requires the dynein cofactor NudE. J Cell Sci. 2015;128:2191–2201. doi: 10.1242/jcs.170316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asensio CS, Sirkis DW, Maas JW, Jr, Egami K, To TL, Brodsky FM, Shu X, Cheng Y, Edwards RH. Self-Assembly of VPS41 Promotes Sorting Required for Biogenesis of the Regulated Secretory Pathway. Dev Cell. 2013;27:425–437. doi: 10.1016/j.devcel.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagal AA, Kao JPY, Tang CM, Thompson SM. Long-term potentiation of exogenous glutamate responses at single dendritic spines. Proc Natl Acad Sci USA. 2005;102:14434–14439. doi: 10.1073/pnas.0501956102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannai H, Lévi S, Schweizer C, Inoue T, Launey T, Racine V, Sibarita JB, Mikoshiba K, Triller A. Activity-dependent tuning of inhibitory neurotransmission based on GABAAR diffusion dynamics. Neuron. 2009;62:670–682. doi: 10.1016/j.neuron.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Bats C, Groc L, Choquet D. The interaction between Stargazin and PSD-95 regulates AMPA receptor surface trafficking. Neuron. 2007;53:719–734. doi: 10.1016/j.neuron.2007.01.030. [DOI] [PubMed] [Google Scholar]
- Bemben MA, Shipman SL, Hirai T, Herring BE, Li Y, Badger JD, Nicoll RA, Diamond JS, Roche KW. CaMKII phosphorylation of neuroligin-1 regulates excitatory synapses. Nature Publishing Group. 2014;17:56–64. doi: 10.1038/nn.3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Tekaya H, Miura K, Pepperkok R, Hauri HP. Live imaging of bidirectional traffic from the ERGIC. J Cell Sci. 2005;118:357–367. doi: 10.1242/jcs.01615. [DOI] [PubMed] [Google Scholar]
- Béïque JC, Na Y, Kuhl D, Worley PF, Huganir RL. Arc-dependent synapse-specific homeostatic plasticity. Proc Natl Acad Sci USA. 2011;108:816–821. doi: 10.1073/pnas.1017914108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpied TA, Scott DB, Ehlers MD. Dynamics and regulation of clathrin coats at specialized endocytic zones of dendrites and spines. Neuron. 2002;36:435–449. doi: 10.1016/s0896-6273(02)00979-0. [DOI] [PubMed] [Google Scholar]
- Bodian D. A suggestive relationship of nerve cell RNA with specific synaptic sites. Proc Natl Acad Sci USA. 1965;53:418–425. doi: 10.1073/pnas.53.2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boncompain G, Divoux S, Gareil N, de Forges H, Lescure A, Latreche L, Mercanti V, Jollivet F, Raposo G, Perez F. Synchronization of secretory protein traffic in populations of cells. Nat Meth. 2012;9:493–498. doi: 10.1038/nmeth.1928. [DOI] [PubMed] [Google Scholar]
- Borgdorff AJ, Choquet D. Regulation of AMPA receptor lateral movements. Nature. 2002;417:649–653. doi: 10.1038/nature00780. [DOI] [PubMed] [Google Scholar]
- Bowen AB, Bourke AM, Hiester BG, Hanus C, Kennedy MJ. Golgi-independent secretory trafficking through recycling endosomes in neuronal dendrites and spines. eLife. 2017;6:89. doi: 10.7554/eLife.27362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw KD, Emptage NJ, Bliss TVP. A role for dendritic protein synthesis in hippocampal late LTP. Eur J Neurosci. 2003;18:3150–3152. doi: 10.1111/j.1460-9568.2003.03054.x. [DOI] [PubMed] [Google Scholar]
- Brigidi GS, Santyr B, Shimell J, Jovellar B, Bamji SX. Activity-regulated trafficking of the palmitoyl-acyl transferase DHHC5. Nature Communications. 2015;6:1–17. doi: 10.1038/ncomms9200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TC, Correia SS, Petrok CN, Esteban JA. Functional compartmentalization of endosomal trafficking for the synaptic delivery of AMPA receptors during long-term potentiation. J Neurosci. 2007;27:13311–13315. doi: 10.1523/JNEUROSCI.4258-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley AM, Petersen J, Roe AJ, Douce GR, Christie JM. LOV-based reporters for fluorescence imaging. Curr Opin Chem Biol. 2015;27:39–45. doi: 10.1016/j.cbpa.2015.05.011. [DOI] [PubMed] [Google Scholar]
- Budnik A, Stephens DJ. ER exit sites--localization and control of COPII vesicle formation. FEBS Lett. 2009;583:3796–3803. doi: 10.1016/j.febslet.2009.10.038. [DOI] [PubMed] [Google Scholar]
- Buffington SA, Huang W, Costa-Mattioli M. Translational control in synaptic plasticity and cognitive dysfunction. Annu Rev Neurosci. 2014;37:17–38. doi: 10.1146/annurev-neuro-071013-014100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugaj LJ, Choksi AT, Mesuda CK, Kane RS, Schaffer DV. Optogenetic protein clustering and signaling activation in mammalian cells. Nat Meth. 2013;10:249–252. doi: 10.1038/nmeth.2360. [DOI] [PubMed] [Google Scholar]
- Bulina ME, Lukyanov KA, Britanova OV, Onichtchouk D, Lukyanov S, Chudakov DM. Chromophore-assisted light inactivation (CALI) using the phototoxic fluorescent protein KillerRed. Nat Protoc. 2006;1:947–953. doi: 10.1038/nprot.2006.89. [DOI] [PubMed] [Google Scholar]
- Burack MA, Silverman MA, Banker G. The role of selective transport in neuronal protein sorting. Neuron. 2000;26:465–472. doi: 10.1016/s0896-6273(00)81178-2. [DOI] [PubMed] [Google Scholar]
- Butko MT, Yang J, Geng Y, Kim HJ, Jeon NL, Shu X, Mackey MR, Ellisman MH, Tsien RY, Lin MZ. Fluorescent and photo-oxidizing TimeSTAMP tags track protein fates in light and electron microscopy. Nature Publishing Group. 2012;15:1742–1751. doi: 10.1038/nn.3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajigas IJ, Tushev G, Will TJ, Tom Dieck S, Fuerst N, Schuman EM. The local transcriptome in the synaptic neuropil revealed by deep sequencing and high-resolution imaging. Neuron. 2012;74:453–466. doi: 10.1016/j.neuron.2012.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancino J, Torrealba C, Soza A, Yuseff MI, Gravotta D, Henklein P, Rodriguez-Boulan E, González A. Antibody to AP1B adaptor blocks biosynthetic and recycling routes of basolateral proteins at recycling endosomes. Mol Biol Cell. 2007;18:4872–4884. doi: 10.1091/mbc.E07-06-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CS, Weeber EJ, Zong L, Fuchs E, Sweatt JD, Davis RL. Beta 1-integrins are required for hippocampal AMPA receptor-dependent synaptic transmission, synaptic plasticity, and working memory. J Neurosci. 2006;26:223–232. doi: 10.1523/JNEUROSCI.4110-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BC, Legant WR, Wang K, Shao L, Milkie DE, Davidson MW, Janetopoulos C, Wu XS, Hammer JA, Liu Z, English BP, Mimori-Kiyosue Y, Romero DP, Ritter AT, Lippincott-Schwartz J, Fritz-Laylin L, Mullins RD, Mitchell DM, Bembenek JN, Reymann AC, Böhme R, Grill SW, Wang JT, Seydoux G, Tulu US, Kiehart DP, Betzig E. Lattice light-sheet microscopy: imaging molecules to embryos at high spatiotemporal resolution. Science. 2014;346:1257998. doi: 10.1126/science.1257998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Gibson ES, Kennedy MJ. A light-triggered protein secretion system. The Journal of Cell Biology. 2013;201:631–640. doi: 10.1083/jcb.201210119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zheng XF, Brown EJ, Schreiber SL. Identification of an 11-kDa FKBP12-rapamycin-binding domain within the 289-kDa FKBP12-rapamycin-associated protein and characterization of a critical serine residue. Proc Natl Acad Sci USA. 1995;92:4947–4951. doi: 10.1073/pnas.92.11.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CQ, Martenson JS, Yamazaki M, Natsume R, Sakimura K, Tomita S, Tavalin SJ, Higley MJ. Input-Specific NMDAR-Dependent Potentiation of Dendritic GABAergic Inhibition. Neuron. 2018;97:368–377e3. doi: 10.1016/j.neuron.2017.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu SL, Diering GH, Ye B, Takamiya K, Chen CM, Jiang Y, Niranjan T, Schwartz CE, Wang T, Huganir RL. GRASP1 Regulates Synaptic Plasticity and Learning through Endosomal Recycling of AMPA Receptors. Neuron. 2017;93:1405–1419e8. doi: 10.1016/j.neuron.2017.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choquet D, Triller A. The dynamic synapse. Neuron. 2013;80:691–703. doi: 10.1016/j.neuron.2013.10.013. [DOI] [PubMed] [Google Scholar]
- Choy RWY, Park M, Temkin P, Herring BE, Marley A, Nicoll RA, von Zastrow M. Retromer mediates a discrete route of local membrane delivery to dendrites. Neuron. 2014;82:55–62. doi: 10.1016/j.neuron.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie JM, Arvai AS, Baxter KJ, Heilmann M, Pratt AJ, O’Hara A, Kelly SM, Hothorn M, Smith BO, Hitomi K, Jenkins GI, Getzoff ED. Plant UVR8 photoreceptor senses UV-B by tryptophan-mediated disruption of cross-dimer salt bridges. Science. 2012;335:1492–1496. doi: 10.1126/science.1218091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J, Torta F, Masai K, Lucast L, Czapla H, Tanner LB, Narayanaswamy P, Wenk MR, Nakatsu F, De Camilli P. INTRACELLULAR TRANSPORT. PI4P/phosphatidylserine countertransport at ORP5- and ORP8-mediated ER-plasma membrane contacts. Science. 2015;349:428–432. doi: 10.1126/science.aab1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clackson T, Yang W, Rozamus LW, Hatada M, Amara JF, Rollins CT, Stevenson LF, Magari SR, Wood SA, Courage NL, Lu X, Cerasoli F, Gilman M, Holt DA. Redesigning an FKBP-ligand interface to generate chemical dimerizers with novel specificity. Proc Natl Acad Sci USA. 1998;95:10437–10442. doi: 10.1073/pnas.95.18.10437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connerly PL, Esaki M, Montegna EA, Strongin DE, Levi S, Soderholm J, Glick BS. Sec16 is a determinant of transitional ER organization. Curr Biol. 2005;15:1439–1447. doi: 10.1016/j.cub.2005.06.065. [DOI] [PubMed] [Google Scholar]
- Cooney JR, Hurlburt JL, Selig DK, Harris KM, Fiala JC. Endosomal compartments serve multiple hippocampal dendritic spines from a widespread rather than a local store of recycling membrane. J Neurosci. 2002;22:2215–2224. doi: 10.1523/JNEUROSCI.22-06-02215.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia SS, Bassani S, Brown TC, Lisé MF, Backos DS, El-Husseini A, Passafaro M, Esteban JA. Motor protein-dependent transport of AMPA receptors into spines during long-term potentiation. Nature Neuroscience. 2008;11:457–466. doi: 10.1038/nn2063. [DOI] [PubMed] [Google Scholar]
- Cracco JB, Serrano P, Moskowitz SI, Bergold PJ, Sacktor TC. Protein synthesis-dependent LTP in isolated dendrites of CA1 pyramidal cells. Hippocampus. 2005;15:551–556. doi: 10.1002/hipo.20078. [DOI] [PubMed] [Google Scholar]
- Cui-Wang T, Hanus C, Cui T, Helton T, Bourne J, Watson D, Harris KM, Ehlers MD. Local zones of endoplasmic reticulum complexity confine cargo in neuronal dendrites. Cell. 2012;148:309–321. doi: 10.1016/j.cell.2011.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagliyan O, Tarnawski M, Chu PH, Shirvanyants D, Schlichting I, Dokholyan NV, Hahn KM. Engineering extrinsic disorder to control protein activity in living cells. Science. 2016;354:1441–1444. doi: 10.1126/science.aah3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan M, Lévi S, Luccardini C, Rostaing P, Riveau B, Triller A. Diffusion dynamics of glycine receptors revealed by single-quantum dot tracking. Science. 2003;302:442–445. doi: 10.1126/science.1088525. [DOI] [PubMed] [Google Scholar]
- de Luca E, Ravasenga T, Petrini EM, Polenghi A, Nieus T, Guazzi S, Barberis A. Inter-Synaptic Lateral Diffusion of GABAA Receptors Shapes Inhibitory Synaptic Currents. Neuron. 2017;95:63–69e5. doi: 10.1016/j.neuron.2017.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterich DC, Hodas JJL, Gouzer G, Shadrin IY, Ngo JT, Triller A, Tirrell DA, Schuman EM. In situ visualization and dynamics of newly synthesized proteins in rat hippocampal neurons. Nature Publishing Group. 2010;13:897–905. doi: 10.1038/nn.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmer PJ, Wild AR, Dell’Acqua ML, Sather WA. STIM1 Ca2+ Sensor Control of L-type Ca2+-Channel-Dependent Dendritic Spine Structural Plasticity and Nuclear Signaling. CellReports. 2017;19:321–334. doi: 10.1016/j.celrep.2017.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KW, McGraw TE, Maxfield FR. Iterative fractionation of recycling receptors from lysosomally destined ligands in an early sorting endosome. The Journal of Cell Biology. 1989;109:3303–3314. doi: 10.1083/jcb.109.6.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MD. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron. 2000;28:511–525. doi: 10.1016/s0896-6273(00)00129-x. [DOI] [PubMed] [Google Scholar]
- Ehlers MD, Heine M, Groc L, Lee MC, Choquet D. Diffusional trapping of GluR1 AMPA receptors by input-specific synaptic activity. Neuron. 2007;54:447–460. doi: 10.1016/j.neuron.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encell LP, Friedman Ohana R, Zimmerman K, Otto P, Vidugiris G, Wood MG, Los GV, McDougall MG, Zimprich C, Karassina N, Learish RD, Hurst R, Hartnett J, Wheeler S, Stecha P, English J, Zhao K, Mendez J, Benink HA, Murphy N, Daniels DL, Slater MR, Urh M, Darzins A, Klaubert DH, Bulleit RF, Wood KV. Development of a dehalogenase-based protein fusion tag capable of rapid, selective and covalent attachment to customizable ligands. Curr Chem Genomics. 2012;6:55–71. doi: 10.2174/1875397301206010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espenshade P, Gimeno RE, Holzmacher E, Teung P, Kaiser CA. Yeast SEC16 gene encodes a multidomain vesicle coat protein that interacts with Sec23p. The Journal of Cell Biology. 1995;131:311–324. doi: 10.1083/jcb.131.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteves da Silva M, Adrian M, Schätzle P, Lipka J, Watanabe T, Cho S, Futai K, Wierenga CJ, Kapitein LC, Hoogenraad CC. Positioning of AMPA Receptor-Containing Endosomes Regulates Synapse Architecture. CellReports. 2015;13:933–943. doi: 10.1016/j.celrep.2015.09.062. [DOI] [PubMed] [Google Scholar]
- Evans AJ, Gurung S, Wilkinson KA, Stephens DJ, Henley JM. Assembly, Secretory Pathway Trafficking, and Surface Delivery of Kainate Receptors Is Regulated by Neuronal Activity. CellReports. 2017;19:2613–2626. doi: 10.1016/j.celrep.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhan H, Weiss M, Tani K, Kaufman RJ, Hauri HP. Adaptation of endoplasmic reticulum exit sites to acute and chronic increases in cargo load. EMBO J. 2008;27:2043–2054. doi: 10.1038/emboj.2008.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr GA, Hull M, Mellman I, Caplan MJ. Membrane proteins follow multiple pathways to the basolateral cell surface in polarized epithelial cells. The Journal of Cell Biology. 2009;186:269–282. doi: 10.1083/jcb.200901021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr GA, Hull M, Stoops EH, Bateson R, Caplan MJ. Dual pulse-chase microscopy reveals early divergence in the biosynthetic trafficking of the Na, K-ATPase and E-cadherin. Mol Biol Cell. 2015;26:4401–4411. doi: 10.1091/mbc.E14-09-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PD, Haberkorn CJ, Weigel AV, Higgins JL, Akin EJ, Kennedy MJ, Krapf D, Tamkun MM. Plasma membrane domains enriched in cortical endoplasmic reticulum function as membrane protein trafficking hubs. Mol Biol Cell. 2013;24:2703–2713. doi: 10.1091/mbc.E12-12-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank JA, Yushchenko DA, Hodson DJ, Lipstein N, Nagpal J, Rutter GA, Rhee JS, Gottschalk A, Brose N, Schultz C, Trauner D. Photoswitchable diacylglycerols enable optical control of protein kinase C. Nat Chem Biol. 2016;12:755–762. doi: 10.1038/nchembio.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks KM, Stevens CF, Sejnowski TJ. Independent sources of quantal variability at single glutamatergic synapses. J Neurosci. 2003;23:3186–3195. doi: 10.1523/JNEUROSCI.23-08-03186.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JR, Lackner LL, West M, DiBenedetto JR, Nunnari J, Voeltz GK. ER tubules mark sites of mitochondrial division. Science. 2011;334:358–362. doi: 10.1126/science.1207385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukatsu K, Bannai H, Zhang S, Nakamura H, Inoue T, Mikoshiba K. Lateral diffusion of inositol 1,4,5-trisphosphate receptor type 1 is regulated by actin filaments and 4.1N in neuronal dendrites. J Biol Chem. 2004;279:48976–48982. doi: 10.1074/jbc.M408364200. [DOI] [PubMed] [Google Scholar]
- Fuller SD, Bravo R, Simons K. An enzymatic assay reveals that proteins destined for the apical or basolateral domains of an epithelial cell line share the same late Golgi compartments. EMBO J. 1985;4:297–307. doi: 10.1002/j.1460-2075.1985.tb03629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage RM, Kim KA, Cao TT, von Zastrow M. A transplantable sorting signal that is sufficient to mediate rapid recycling of G protein-coupled receptors. J Biol Chem. 2001;276:44712–44720. doi: 10.1074/jbc.M107417200. [DOI] [PubMed] [Google Scholar]
- Gardiol A, Racca C, Triller A. Dendritic and postsynaptic protein synthetic machinery. Journal of Neuroscience. 1999;19:168–179. doi: 10.1523/JNEUROSCI.19-01-00168.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser C, Taiber S, Yeh CM, Wittig CH, Hegemann P, Ryu S, Wunder F, Möglich A. Engineering of a red-light-activated human cAMP/cGMP-specific phosphodiesterase. Proc Natl Acad Sci USA. 2014;111:8803–8808. doi: 10.1073/pnas.1321600111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geppert M, Bolshakov VY, Siegelbaum SA, Takei K, De Camilli P, Hammer RE, Südhof TC. The role of Rab3A in neurotransmitter release. Nature. 1994;369:493–497. doi: 10.1038/369493a0. [DOI] [PubMed] [Google Scholar]
- Geppert M, Goda Y, Stevens CF, Südhof TC. The small GTP-binding protein Rab3A regulates a late step in synaptic vesicle fusion. Nature. 1997;387:810–814. doi: 10.1038/42954. [DOI] [PubMed] [Google Scholar]
- Gerrow K, Triller A. GABAA receptor subunit composition and competition at synapses are tuned by GABAB receptor activity. Mol Cell Neurosci. 2014;60:97–107. doi: 10.1016/j.mcn.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Goldenring JR, Smith J, Vaughan HD, Cameron P, Hawkins W, Navarre J. Rab11 is an apically located small GTP-binding protein in epithelial tissues. Am J Physiol. 1996;270:G515–25. doi: 10.1152/ajpgi.1996.270.3.G515. [DOI] [PubMed] [Google Scholar]
- Goo MS, Sancho L, Slepak N, Boassa D, Deerinck TJ, Ellisman MH, Bloodgood BL, Patrick GN. Activity-dependent trafficking of lysosomes in dendrites and dendritic spines. The Journal of Cell Biology. 2017;216:2499–2513. doi: 10.1083/jcb.201704068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabner CP, Price SD, Lysakowski A, Cahill AL, Fox AP. Regulation of large dense-core vesicle volume and neurotransmitter content mediated by adaptor protein 3. Proc Natl Acad Sci USA. 2006;103:10035–10040. doi: 10.1073/pnas.0509844103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger AJ, Shi Y, Lu W, Cerpas M, Nicoll RA. LTP requires a reserve pool of glutamate receptors independent of subunit type. Nature. 2013;493:495–500. doi: 10.1038/nature11775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10:597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greger IH, Khatri L, Ziff EB. RNA Editing at Arg607 Controls AMPA Receptor Exit from the Endoplasmic Reticulum. Neuron. 2002;34:759–772. doi: 10.1016/S0896-6273(02)00693-1. [DOI] [PubMed] [Google Scholar]
- Grimm JB, Muthusamy AK, Liang Y, Brown TA, Lemon WC, Patel R, Lu R, Macklin JJ, Keller PJ, Ji N, Lavis LD. A general method to fine-tune fluorophores for live-cell and in vivo imaging. Nat Meth. 2017;14:987–994. doi: 10.1038/nmeth.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Chiu S-L, Liu B, Wu P-H, Delannoy M, Lin D-T, Wirtz D, Huganir RL. Differential vesicular sorting of AMPA and GABAA receptors. Proc Natl Acad Sci USA. 2016 doi: 10.1073/pnas.1525726113. 201525726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson N, Culley S, Ashdown G, Owen DM, Pereira PM, Henriques R. Fast live-cell conventional fluorophore nanoscopy with ImageJ through super-resolution radial fluctuations. Nature Communications. 2016;7:12471. doi: 10.1038/ncomms12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond AT, Glick BS. Dynamics of transitional endoplasmic reticulum sites in vertebrate cells. Mol Biol Cell. 2000;11:3013–3030. doi: 10.1091/mbc.11.9.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanus C, Ehrensperger MV, Triller A. Activity-dependent movements of postsynaptic scaffolds at inhibitory synapses. J Neurosci. 2006;26:4586–4595. doi: 10.1523/JNEUROSCI.5123-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanus C, Kochen L, Tom Dieck S, Racine V, Sibarita JB, Schuman EM, Ehlers MD. Synaptic control of secretory trafficking in dendrites. CellReports. 2014;7:1771–1778. doi: 10.1016/j.celrep.2014.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanus C, Schuman EM. Proteostasis in complex dendrites. Nat Rev Neurosci. 2013;14:638–648. doi: 10.1038/nrn3546. [DOI] [PubMed] [Google Scholar]
- Hanus C, Vannier C, Triller A. Intracellular association of glycine receptor with gephyrin increases its plasma membrane accumulation rate. J Neurosci. 2004;24:1119–1128. doi: 10.1523/JNEUROSCI.4380-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper SM, Neil LC, Gardner KH. Structural basis of a phototropin light switch. Science. 2003;301:1541–1544. doi: 10.1126/science.1086810. [DOI] [PubMed] [Google Scholar]
- Harward SC, Hedrick NG, Hall CE, Parra-Bueno P, Milner TA, Pan E, Laviv T, Hempstead BL, Yasuda R, McNamara JO. Autocrine BDNF–TrkB signalling within a single dendritic spine. Nature. 2016;538:99–103. doi: 10.1038/nature19766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattula K, Furuhjelm J, Tikkanen J, Tanhuanpää K, Laakkonen P, Peränen J. Characterization of the Rab8-specific membrane traffic route linked to protrusion formation. J Cell Sci. 2006;119:4866–4877. doi: 10.1242/jcs.03275. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- Haynes LP, Evans GJ, Morgan A, Burgoyne RD. A direct inhibitory role for the Rab3-specific effector, Noc2, in Ca2+-regulated exocytosis in neuroendocrine cells. J Biol Chem. 2001;276:9726–9732. doi: 10.1074/jbc.M006959200. [DOI] [PubMed] [Google Scholar]
- Heine M, Groc L, Frischknecht R, Béïque JC, Lounis B, Rumbaugh G, Huganir RL, Cognet L, Choquet D. Surface mobility of postsynaptic AMPARs tunes synaptic transmission. Science. 2008;320:201–205. doi: 10.1126/science.1152089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler FF, Lee HK, Gromova KV, Pechmann Y, Schurek B, Ruschkies L, Schroeder M, Schweizer M, Kneussel M. GRIP1 interlinks N-cadherin and AMPA receptors at vesicles to promote combined cargo transport into dendrites. Proc Natl Acad Sci USA. 2014;111:5030–5035. doi: 10.1073/pnas.1304301111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler FF, Loebrich S, Pechmann Y, Maier N, Zivkovic AR, Tokito M, Hausrat TJ, Schweizer M, Bähring R, Holzbaur ELF, Schmitz D, Kneussel M. Muskelin regulates actin filament- and microtubule-based GABA(A) receptor transport in neurons. Neuron. 2011;70:66–81. doi: 10.1016/j.neuron.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks AG, Perlson E, Ross JL, Schroeder HW, Tokito M, Holzbaur ELF. Motor coordination via a tug-of-war mechanism drives bidirectional vesicle transport. Curr Biol. 2010;20:697–702. doi: 10.1016/j.cub.2010.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiester BG, Bourke AM, Sinnen BL, Cook SG, Gibson ES, Smith KR, Kennedy MJ. L-Type Voltage-Gated Ca2+ Channels Regulate Synaptic-Activity-Triggered Recycling Endosome Fusion in Neuronal Dendrites. CellReports. 2017;21:2134–2146. doi: 10.1016/j.celrep.2017.10.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N, Niwa S, Tanaka Y. Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron. 2010;68:610–638. doi: 10.1016/j.neuron.2010.09.039. [DOI] [PubMed] [Google Scholar]
- Ho SN, Biggar SR, Spencer DM, Schreiber SL, Crabtree GR. Dimeric ligands define a role for transcriptional activation domains in reinitiation. Nature. 1996;382:822–826. doi: 10.1038/382822a0. [DOI] [PubMed] [Google Scholar]
- Hoerndli FJ, Wang R, Mellem JE, Kallarackal A, Brockie PJ, Thacker C, Madsen DM, Maricq AV. Neuronal Activity and CaMKII Regulate Kinesin-Mediated Transport of Synaptic AMPARs. Neuron. 2015;86:457–474. doi: 10.1016/j.neuron.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt CE, Schuman EM. The central dogma decentralized: new perspectives on RNA function and local translation in neurons. Neuron. 2013;80:648–657. doi: 10.1016/j.neuron.2013.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogenraad CC, van der Sluijs P. GRASP-1 regulates endocytic receptor recycling and synaptic plasticity. Commun Integr Biol. 2010;3:433–435. doi: 10.4161/cib.3.5.12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton AC, Ehlers MD. Dual modes of endoplasmic reticulum-to-Golgi transport in dendrites revealed by live-cell imaging. J Neurosci. 2003;23:6188–6199. doi: 10.1523/JNEUROSCI.23-15-06188.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton AC, Rácz B, Monson EE, Lin AL, Weinberg RJ, Ehlers MD. Polarized secretory trafficking directs cargo for asymmetric dendrite growth and morphogenesis. Neuron. 2005;48:757–771. doi: 10.1016/j.neuron.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Hsu VW, Bai M, Li J. Getting active: protein sorting in endocytic recycling. Nat Rev Mol Cell Biol. 2012;13:323–328. doi: 10.1038/nrm3332. [DOI] [PubMed] [Google Scholar]
- Huber LA, de Hoop MJ, Dupree P, Zerial M, Simons K, Dotti C. Protein transport to the dendritic plasma membrane of cultured neurons is regulated by rab8p. The Journal of Cell Biology. 1993a;123:47–55. doi: 10.1083/jcb.123.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber LA, Pimplikar S, Parton RG, Virta H, Zerial M, Simons K. Rab8, a small GTPase involved in vesicular traffic between the TGN and the basolateral plasma membrane. The Journal of Cell Biology. 1993b;123:35–45. doi: 10.1083/jcb.123.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes H, Budnik A, Schmidt K, Palmer KJ, Mantell J, Noakes C, Johnson A, Carter DA, Verkade P, Watson P, Stephens DJ. Organisation of human ER-exit sites: requirements for the localisation of Sec16 to transitional ER. J Cell Sci. 2009;122:2924–2934. doi: 10.1242/jcs.044032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisken J, Swoger J, Del Bene F, Wittbrodt J, Stelzer EHK. Optical sectioning deep inside live embryos by selective plane illumination microscopy. Science. 2004;305:1007–1009. doi: 10.1126/science.1100035. [DOI] [PubMed] [Google Scholar]
- Isaac JT, Nicoll RA, Malenka RC. Evidence for silent synapses: implications for the expression of LTP. Neuron. 1995;15:427–434. doi: 10.1016/0896-6273(95)90046-2. [DOI] [PubMed] [Google Scholar]
- Iseki M, Matsunaga S, Murakami A, Ohno K, Shiga K, Yoshida K, Sugai M, Takahashi T, Hori T, Watanabe M. A blue-light-activated adenylyl cyclase mediates photoavoidance in Euglena gracilis. Nature. 2002;415:1047–1051. doi: 10.1038/4151047a. [DOI] [PubMed] [Google Scholar]
- Ivan V, de Voer G, Xanthakis D, Spoorendonk KM, Kondylis V, Rabouille C. Drosophila Sec16 mediates the biogenesis of tER sites upstream of Sar1 through an arginine-rich motif. Mol Biol Cell. 2008;19:4352–4365. doi: 10.1091/mbc.E08-03-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob R, Naim HY. Apical membrane proteins are transported in distinct vesicular carriers. Curr Biol. 2001;11:1444–1450. doi: 10.1016/s0960-9822(01)00446-8. [DOI] [PubMed] [Google Scholar]
- Jaiswal JK, Rivera VM, Simon SM. Exocytosis of post-Golgi vesicles is regulated by components of the endocytic machinery. Cell. 2009;137:1308–1319. doi: 10.1016/j.cell.2009.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay DG. Selective destruction of protein function by chromophore-assisted laser inactivation. Proc Natl Acad Sci USA. 1988;85:5454–5458. doi: 10.1073/pnas.85.15.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedd G, Mulholland J, Segev N. Two new Ypt GTPases are required for exit from the yeast trans-Golgi compartment. The Journal of Cell Biology. 1997;137:563–580. doi: 10.1083/jcb.137.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen CS, Watanabe S, Rasmussen HB, Schmitt N, Olesen SP, Frost NA, Blanpied TA, Misonou H. Specific sorting and post-Golgi trafficking of dendritic potassium channels in living neurons. J Biol Chem. 2014;289:10566–10581. doi: 10.1074/jbc.M113.534495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen CS, Watanabe S, Stas JI, Klaphaak J, Yamane A, Schmitt N, Olesen SP, Trimmer JS, Rasmussen HB, Misonou H. Trafficking of Kv2.1 Channels to the Axon Initial Segment by a Novel Nonconventional Secretory Pathway. J Neurosci. 2017;37:11523–11536. doi: 10.1523/JNEUROSCI.3510-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyifous O, Waites CL, Specht CG, Fujisawa S, Schubert M, Lin EI, Marshall J, Aoki C, de Silva T, Montgomery JM, Garner CC, Green WN. SAP97 and CASK mediate sorting of NMDA receptors through a previously unknown secretory pathway. Nature Publishing Group. 2009;12:1011–1019. doi: 10.1038/nn.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju W, Morishita W, Tsui J, Gaietta G, Deerinck TJ, Adams SR, Garner CC, Tsien RY, Ellisman MH, Malenka RC. Activity-dependent regulation of dendritic synthesis and trafficking of AMPA receptors. Nature Neuroscience. 2004;7:244–253. doi: 10.1038/nn1189. [DOI] [PubMed] [Google Scholar]
- Kapitein LC, Schlager MA, van der Zwan WA, Wulf PS, Keijzer N, Hoogenraad CC. Probing intracellular motor protein activity using an inducible cargo trafficking assay. Biophys J. 2010;99:2143–2152. doi: 10.1016/j.bpj.2010.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith DJ, Sanderson JL, Gibson ES, Woolfrey KM, Robertson HR, Olszewski K, Kang R, El-Husseini A, Dell’Acqua ML. Palmitoylation of A-kinase anchoring protein 79/150 regulates dendritic endosomal targeting and synaptic plasticity mechanisms. J Neurosci. 2012;32:7119–7136. doi: 10.1523/JNEUROSCI.0784-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller P, Toomre D, Díaz E, White J, Simons K. Multicolour imaging of post-Golgi sorting and trafficking in live cells. Nat Cell Biol. 2001;3:140–149. doi: 10.1038/35055042. [DOI] [PubMed] [Google Scholar]
- Kennedy MJ, Davison IG, Robinson CG, Ehlers MD. Syntaxin-4 Defines a Domain for Activity-Dependent Exocytosis in Dendritic Spines. Cell. 2010a;141:524–535. doi: 10.1016/j.cell.2010.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MJ, Hughes RM, Peteya LA, Schwartz JW, Ehlers MD, Tucker CL. Rapid blue-light-mediated induction of protein interactions in living cells. Nat Meth. 2010b;7:973–975. doi: 10.1038/nmeth.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppler A, Ellenberg J. Chromophore-assisted laser inactivation of alpha- and gamma-tubulin SNAP-tag fusion proteins inside living cells. ACS Chem Biol. 2009;4:127–138. doi: 10.1021/cb800298u. [DOI] [PubMed] [Google Scholar]
- Keppler A, Gendreizig S, Gronemeyer T, Pick H, Vogel H, Johnsson K. A general method for the covalent labeling of fusion proteins with small molecules in vivo. Nat Biotechnol. 2003;21:86–89. doi: 10.1038/nbt765. [DOI] [PubMed] [Google Scholar]
- Kim T, Gondré-Lewis MC, Arnaoutova I, Loh YP. Dense-core secretory granule biogenesis. Physiology (Bethesda) 2006;21:124–133. doi: 10.1152/physiol.00043.2005. [DOI] [PubMed] [Google Scholar]
- Kolarow R, Brigadski T, Lessmann V. Postsynaptic secretion of BDNF and NT-3 from hippocampal neurons depends on calcium calmodulin kinase II signaling and proceeds via delayed fusion pore opening. J Neurosci. 2007;27:10350–10364. doi: 10.1523/JNEUROSCI.0692-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krijnse-Locker J, Parton RG, Fuller SD, Griffiths G, Dotti CG. The organization of the endoplasmic reticulum and the intermediate compartment in cultured rat hippocampal neurons. Mol Biol Cell. 1995;6:1315–1332. doi: 10.1091/mbc.6.10.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakadamyali M, Rust MJ, Zhuang X. Ligands for clathrin-mediated endocytosis are differentially sorted into distinct populations of early endosomes. Cell. 2006;124:997–1009. doi: 10.1016/j.cell.2005.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Natarajan M, Nashine VC, Socolich M, Vo T, Russ WP, Benkovic SJ, Ranganathan R. Surface sites for engineering allosteric control in proteins. Science. 2008;322:438–442. doi: 10.1126/science.1159052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MC, Yasuda R, Ehlers MD. Metaplasticity at single glutamatergic synapses. Neuron. 2010;66:859–870. doi: 10.1016/j.neuron.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Jin C, Cai E, Ge P, Ishitsuka Y, Teng KW, de Thomaz AA, Nall D, Baday M, Jeyifous O, Demonte D, Dundas CM, Park S, Delgado JY, Green WN, Selvin PR. Super-resolution imaging of synaptic and Extra-synaptic AMPA receptors with different-sized fluorescent probes. eLife. 2017;6:461. doi: 10.7554/eLife.27744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees JA, Messa M, Sun EW, Wheeler H, Torta F, Wenk MR, De Camilli P, Reinisch KM. Lipid transport by TMEM24 at ER–plasma membrane contacts regulates pulsatile insulin secretion. Science. 2017;355:eaah6171–16. doi: 10.1126/science.aah6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehre KP, Danbolt NC. The number of glutamate transporter subtype molecules at glutamatergic synapses: chemical and stereological quantification in young adult rat brain. Journal of Neuroscience. 1998;18:8751–8757. doi: 10.1523/JNEUROSCI.18-21-08751.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinders-Zufall T, Storch U, Bleymehl K, Mederos Y, Schnitzler M, Frank JA, Konrad DB, Trauner D, Gudermann T, Zufall F. PhoDAGs Enable Optical Control of Diacylglycerol-Sensitive Transient Receptor Potential Channels. Cell Chem Biol. 2017 doi: 10.1016/j.chembiol.2017.11.008. [DOI] [PubMed] [Google Scholar]
- Levskaya A, Weiner OD, Lim WA, Voigt CA. Spatiotemporal control of cell signalling using a light-switchable protein interaction. Nature. 2009;461:997–1001. doi: 10.1038/nature08446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TL, Mao T, Svoboda K, Arnold DB. Myosin-dependent targeting of transmembrane proteins to neuronal dendrites. Nature Publishing Group. 2009;12:568–576. doi: 10.1038/nn.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Shao L, Chen B-C, Zhang X, Zhang M, Moses B, Milkie DE, Beach JR, Hammer JA, Pasham M, Kirchhausen T, Baird MA, Davidson MW, Xu P, Betzig E. ADVANCED IMAGING. Extended-resolution structured illumination imaging of endocytic and cytoskeletal dynamics. Science. 2015;349:aab3500–aab3500. doi: 10.1126/science.aab3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Hessler NA, Malinow R. Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature. 1995;375:400–404. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- Lin JY, Sann SB, Zhou K, Nabavi S, Proulx CD, Malinow R, Jin Y, Tsien RY. Optogenetic inhibition of synaptic release with chromophore-assisted light inactivation (CALI) Neuron. 2013;79:241–253. doi: 10.1016/j.neuron.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Yuan LC, Bonifacino JS, Klausner RD. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell. 1989;56:801–813. doi: 10.1016/0092-8674(89)90685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisé MF, Wong TP, Trinh A, Hines RM, Liu L, Kang R, Hines DJ, Lu J, Goldenring JR, Wang YT, El-Husseini A. Involvement of myosin Vb in glutamate receptor trafficking. J Biol Chem. 2006;281:3669–3678. doi: 10.1074/jbc.M511725200. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Zhang X, Südhof TC, Malenka RC, Nicoll RA. Postsynaptic membrane fusion and long-term potentiation. Science. 1998;279:399–403. doi: 10.1126/science.279.5349.399. [DOI] [PubMed] [Google Scholar]
- Lochner JE, Honigman LS, Grant WF, Gessford SK, Hansen AB, Silverman MA, Scalettar BA. Activity-dependent release of tissue plasminogen activator from the dendritic spines of hippocampal neurons revealed by live-cell imaging. J Neurobiol. 2006;66:564–577. doi: 10.1002/neu.20250. [DOI] [PubMed] [Google Scholar]
- Locker JK, Opstelten DJE, Ericsson M, Horzinek MC, Rottier PJM. Oligomerization of a trans-Golgi/trans-Golgi Network Retained Protein Occurs in the Golgi Complex and May Be Part of Its Retention. J Biol Chem. 1995;270:8815–8821. doi: 10.1074/jbc.270.15.8815. [DOI] [PubMed] [Google Scholar]
- Lodish HF, Weiss RA. Selective isolation of mutants of vesicular stomatitis virus defective in production of the viral glycoprotein. J Virol. 1979;30:177–189. doi: 10.1128/jvi.30.1.177-189.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo LS, Tang N, Al-Haddawi M, Dawe GS, Hong W. A role for sorting nexin 27 in AMPA receptor trafficking. Nature Communications. 2014;5:3176. doi: 10.1038/ncomms4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Los GV, Encell LP, McDougall MG, Hartzell DD, Karassina N, Zimprich C, Wood MG, Learish R, Ohana RF, Urh M, Simpson D, Mendez J, Zimmerman K, Otto P, Vidugiris G, Zhu J, Darzins A, Klaubert DH, Bulleit RF, Wood KV. HaloTag: a novel protein labeling technology for cell imaging and protein analysis. ACS Chem Biol. 2008;3:373–382. doi: 10.1021/cb800025k. [DOI] [PubMed] [Google Scholar]
- Losonczy A, Makara JK, Magee JC. Compartmentalized dendritic plasticity and input feature storage in neurons. Nature. 2008;452:436–441. doi: 10.1038/nature06725. [DOI] [PubMed] [Google Scholar]
- Lungu OI, Hallett RA, Choi EJ, Aiken MJ, Hahn KM, Kuhlman B. Designing photoswitchable peptides using the AsLOV2 domain. Chem Biol. 2012;19:507–517. doi: 10.1016/j.chembiol.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Xia H, Beattie EC, Carroll RC, von Zastrow M, Malenka RC, Nicoll RA. Role of AMPA receptor cycling in synaptic transmission and plasticity. Neuron. 1999;24:649–658. doi: 10.1016/s0896-6273(00)81119-8. [DOI] [PubMed] [Google Scholar]
- Maas C, Tagnaouti N, Loebrich S, Behrend B, Lappe-Siefke C, Kneussel M. Neuronal cotransport of glycine receptor and the scaffold protein gephyrin. The Journal of Cell Biology. 2006;172:441–451. doi: 10.1083/jcb.200506066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGillavry HD, Song Y, Raghavachari S, Blanpied TA. Nanoscale scaffolding domains within the postsynaptic density concentrate synaptic AMPA receptors. Neuron. 2013;78:615–622. doi: 10.1016/j.neuron.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makara JK, Losonczy A, Wen Q, Magee JC. Experience-dependent compartmentalized dendritic plasticity in rat hippocampal CA1 pyramidal neurons. Nature Neuroscience. 2009;12:1485–1487. doi: 10.1038/nn.2428. [DOI] [PubMed] [Google Scholar]
- Makino H, Malinow R. AMPA receptor incorporation into synapses during LTP: the role of lateral movement and exocytosis. Neuron. 2009;64:381–390. doi: 10.1016/j.neuron.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameli M, Balland B, Luján R, Lüscher C. Rapid synthesis and synaptic insertion of GluR2 for mGluR-LTD in the ventral tegmental area. Science. 2007;317:530–533. doi: 10.1126/science.1142365. [DOI] [PubMed] [Google Scholar]
- Matsuda N, Lu H, Fukata Y, Noritake J, Gao H, Mukherjee S, Nemoto T, Fukata M, Poo MM. Differential activity-dependent secretion of brain-derived neurotrophic factor from axon and dendrite. J Neurosci. 2009;29:14185–14198. doi: 10.1523/JNEUROSCI.1863-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GCR, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor S, Presley JF, Maxfield FR. Sorting of membrane components from endosomes and subsequent recycling to the cell surface occurs by a bulk flow process. The Journal of Cell Biology. 1993;121:1257–1269. doi: 10.1083/jcb.121.6.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister AK, Stevens CF. Nonsaturation of AMPA and NMDA receptors at hippocampal synapses. Proc Natl Acad Sci USA. 2000;97:6173–6178. doi: 10.1073/pnas.100126497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVicker DP, Awe AM, Richters KE, Wilson RL, Cowdrey DA, Hu X, Chapman ER, Dent EW. Transport of a kinesin-cargo pair along microtubules into dendritic spines undergoing synaptic plasticity. Nature Communications. 2016;7:12741. doi: 10.1038/ncomms12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhaylova M, Bera S, Kobler O, Frischknecht R, Kreutz MR. A Dendritic Golgi Satellite between ERGIC and Retromer. CellReports. 2016;14:189–199. doi: 10.1016/j.celrep.2015.12.024. [DOI] [PubMed] [Google Scholar]
- Mills E, Chen X, Pham E, Wong S, Truong K. Engineering a photoactivated caspase-7 for rapid induction of apoptosis. ACS Synth Biol. 2012;1:75–82. doi: 10.1021/sb200008j. [DOI] [PubMed] [Google Scholar]
- Muñiz M, Morsomme P, Riezman H. Protein sorting upon exit from the endoplasmic reticulum. Cell. 2001;104:313–320. doi: 10.1016/s0092-8674(01)00215-x. [DOI] [PubMed] [Google Scholar]
- Murakoshi H, Shin ME, Parra-Bueno P, Szatmari EM, Shibata ACE, Yasuda R. Kinetics of Endogenous CaMKII Required for Synaptic Plasticity Revealed by Optogenetic Kinase Inhibitor. Neuron. 2017;94:690. doi: 10.1016/j.neuron.2017.04.027. [DOI] [PubMed] [Google Scholar]
- Nagahama T, Suzuki T, Yoshikawa S, Iseki M. Functional transplant of photoactivated adenylyl cyclase (PAC) into Aplysia sensory neurons. Neurosci Res. 2007;59:81–88. doi: 10.1016/j.neures.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Nair D, Hosy E, Petersen JD, Constals A, Giannone G, Choquet D, Sibarita JB. Super-resolution imaging reveals that AMPA receptors inside synapses are dynamically organized in nanodomains regulated by PSD95. J Neurosci. 2013;33:13204–13224. doi: 10.1523/JNEUROSCI.2381-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada C, Ritchie K, Oba Y, Nakamura M, Hotta Y, Iino R, Kasai RS, Yamaguchi K, Fujiwara T, Kusumi A. Accumulation of anchored proteins forms membrane diffusion barriers during neuronal polarization. Nat Cell Biol. 2003;5:626–632. doi: 10.1038/ncb1009. [DOI] [PubMed] [Google Scholar]
- Nguyen MM, McCracken CJ, Milner ES, Goetschius DJ, Weiner AT, Long MK, Michael NL, Munro S, Rolls MM. Γ-tubulin controls neuronal microtubule polarity independently of Golgi outposts. Mol Biol Cell. 2014;25:2039–2050. doi: 10.1091/mbc.E13-09-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicovich PR, Owen DM, Gaus K. Turning single-molecule localization microscopy into a quantitative bioanalytical tool. Nat Protoc. 2017;12:453–460. doi: 10.1038/nprot.2016.166. [DOI] [PubMed] [Google Scholar]
- Opazo P, Labrecque S, Tigaret CM, Frouin A, Wiseman PW, De Koninck P, Choquet D. CaMKII triggers the diffusional trapping of surface AMPARs through phosphorylation of stargazin. Neuron. 2010;67:239–252. doi: 10.1016/j.neuron.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Ori-McKenney KM, Jan LY, Jan YN. Golgi outposts shape dendrite morphology by functioning as sites of acentrosomal microtubule nucleation in neurons. Neuron. 2012;76:921–930. doi: 10.1016/j.neuron.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterweil E, Wells DG, Mooseker MS. A role for myosin VI in postsynaptic structure and glutamate receptor endocytosis. The Journal of Cell Biology. 2005;168:329–338. doi: 10.1083/jcb.200410091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouardouz M, Sastry BR. Mechanisms underlying LTP of inhibitory synaptic transmission in the deep cerebellar nuclei. J Neurophysiol. 2000;84:1414–1421. doi: 10.1152/jn.2000.84.3.1414. [DOI] [PubMed] [Google Scholar]
- Padamsey Z, McGuinness L, Bardo SJ, Reinhart M, Tong R, Hedegaard A, Hart ML, Emptage NJ. Activity-Dependent Exocytosis of Lysosomes Regulates the Structural Plasticity of Dendritic Spines. Neuron. 2016 doi: 10.1016/j.neuron.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Kim NY, Lee S, Kim N, Kim J, Heo WD. Optogenetic protein clustering through fluorescent protein tagging and extension of CRY2. Nature Communications. 2017;8:30. doi: 10.1038/s41467-017-00060-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JJ, Gondré-Lewis MC, Eiden LE, Loh YP. A distinct trans-Golgi network subcompartment for sorting of synaptic and granule proteins in neurons and neuroendocrine cells. J Cell Sci. 2011;124:735–744. doi: 10.1242/jcs.076372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, Penick EC, Edwards JG, Kauer JA, Ehlers MD. Recycling endosomes supply AMPA receptors for LTP. Science. 2004a;305:1972–1975. doi: 10.1126/science.1102026. [DOI] [PubMed] [Google Scholar]
- Park M, Penick EC, Edwards JG, Kauer JA, Ehlers MD. Recycling endosomes supply AMPA receptors for LTP. Science. 2004b;305:1972–1975. doi: 10.1126/science.1102026. [DOI] [PubMed] [Google Scholar]
- Park M, Salgado JM, Ostroff L, Helton TD, Robinson CG, Harris KM, Ehlers MD. Plasticity-induced growth of dendritic spines by exocytic trafficking from recycling endosomes. Neuron. 2006a;52:817–830. doi: 10.1016/j.neuron.2006.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, Salgado JM, Ostroff L, Helton TD, Robinson CG, Harris KM, Ehlers MD. Plasticity-induced growth of dendritic spines by exocytic trafficking from recycling endosomes. Neuron. 2006b;52:817–830. doi: 10.1016/j.neuron.2006.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton RG, Simons K, Dotti CG. Axonal and dendritic endocytic pathways in cultured neurons. The Journal of Cell Biology. 1992;119:123–137. doi: 10.1083/jcb.119.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson MA, Szatmari EM, Yasuda R. AMPA receptors are exocytosed in stimulated spines and adjacent dendrites in a Ras-ERK-dependent manner during long-term potentiation. Proc Natl Acad Sci USA. 2010;107:15951–15956. doi: 10.1073/pnas.0913875107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn AC, Zhang CL, Georges F, Royer L, Breillat C, Hosy E, Petersen JD, Humeau Y, Choquet D. Hippocampal LTP and contextual learning require surface diffusion of AMPA receptors. Nature. 2017;549:384–388. doi: 10.1038/nature23658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennacchietti F, Vascon S, Nieus T, Rosillo C, Das S, Tyagarajan SK, Diaspro A, Del Bue A, Petrini EM, Barberis A, Cella Zanacchi F. Nanoscale Molecular Reorganization of the Inhibitory Postsynaptic Density Is a Determinant of GABAergic Synaptic Potentiation. J Neurosci. 2017;37:1747–1756. doi: 10.1523/JNEUROSCI.0514-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkovic M, Kunz M, Endesfelder U, Bunse S, Wigge C, Yu Z, Hodirnau VV, Scheffer MP, Seybert A, Malkusch S, Schuman EM, Heilemann M, Frangakis AS. Correlative light- and electron microscopy with chemical tags. J Struct Biol. 2014;186:205–213. doi: 10.1016/j.jsb.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrini EM, Lu J, Cognet L, Lounis B, Ehlers MD, Choquet D. Endocytic Trafficking and Recycling Maintain a Pool of Mobile Surface AMPA Receptors Required for Synaptic Potentiation. Neuron. 2009;63:92–105. doi: 10.1016/j.neuron.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick JE, Khatri L, Sathler MF, Ziff EB. mGluR long-term depression regulates GluA2 association with COPII vesicles and exit from the endoplasmic reticulum. EMBO J. 2017;36:232–244. doi: 10.15252/embj.201694526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce JP, van Leyen K, McCarthy JB. Translocation machinery for synthesis of integral membrane and secretory proteins in dendritic spines. Nature Neuroscience. 2000;3:311–313. doi: 10.1038/73868. [DOI] [PubMed] [Google Scholar]
- Planchon TA, Gao L, Milkie DE, Davidson MW, Galbraith JA, Galbraith CG, Betzig E. Rapid three-dimensional isotropic imaging of living cells using Bessel beam plane illumination. Nat Meth. 2011;8:417–423. doi: 10.1038/nmeth.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poskanzer KE, Marek KW, Sweeney ST, Davis GW. Synaptotagmin I is necessary for compensatory synaptic vesicle endocytosis in vivo. Nature. 2003;426:559–563. doi: 10.1038/nature02184. [DOI] [PubMed] [Google Scholar]
- Prekeris R, Foletti DL, Scheller RH. Dynamics of tubulovesicular recycling endosomes in hippocampal neurons. Journal of Neuroscience. 1999;19:10324–10337. doi: 10.1523/JNEUROSCI.19-23-10324.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presley JF, Cole NB, Schroer TA, Hirschberg K, Zaal KJ, Lippincott-Schwartz J. ER-to-Golgi transport visualized in living cells. Nature. 1997;389:81–85. doi: 10.1038/38001. [DOI] [PubMed] [Google Scholar]
- Puthenveedu MA, von Zastrow M. Cargo regulates clathrin-coated pit dynamics. Cell. 2006;127:113–124. doi: 10.1016/j.cell.2006.08.035. [DOI] [PubMed] [Google Scholar]
- Raffelberg S, Wang L, Gao S, Losi A, Gärtner W, Nagel G. A LOV-domain-mediated blue-light-activated adenylate (adenylyl) cyclase from the cyanobacterium Microcoleus chthonoplastes PCC 7420. Biochem J. 2013;455:359–365. doi: 10.1042/BJ20130637. [DOI] [PubMed] [Google Scholar]
- Raghavachari S, Lisman JE. Properties of quantal transmission at CA1 synapses. J Neurophysiol. 2004;92:2456–2467. doi: 10.1152/jn.00258.2004. [DOI] [PubMed] [Google Scholar]
- Rácz B, Blanpied TA, Ehlers MD, Weinberg RJ. Lateral organization of endocytic machinery in dendritic spines. Nature Neuroscience. 2004;7:917–918. doi: 10.1038/nn1303. [DOI] [PubMed] [Google Scholar]
- Rindler MJ, Ivanov IE, Plesken H, Rodriguez-Boulan E, Sabatini DD. Viral glycoproteins destined for apical or basolateral plasma membrane domains traverse the same Golgi apparatus during their intracellular transport in doubly infected Madin-Darby canine kidney cells. The Journal of Cell Biology. 1984;98:1304–1319. doi: 10.1083/jcb.98.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera VM, Wang X, Wardwell S, Courage NL, Volchuk A, Keenan T, Holt DA, Gilman M, Orci L, Cerasoli F, Rothman JE, Clackson T. Regulation of protein secretion through controlled aggregation in the endoplasmic reticulum. Science. 2000;287:826–830. doi: 10.1126/science.287.5454.826. [DOI] [PubMed] [Google Scholar]
- Rizzini L, Favory JJ, Cloix C, Faggionato D, O’Hara A, Kaiserli E, Baumeister R, Schäfer E, Nagy F, Jenkins GI, Ulm R. Perception of UV-B by the Arabidopsis UVR8 protein. Science. 2011;332:103–106. doi: 10.1126/science.1200660. [DOI] [PubMed] [Google Scholar]
- Robert A, Howe JR. How AMPA receptor desensitization depends on receptor occupancy. J Neurosci. 2003;23:847–858. doi: 10.1523/JNEUROSCI.23-03-00847.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MS, Sahlender DA, Foster SD. Rapid inactivation of proteins by rapamycin-induced rerouting to mitochondria. Dev Cell. 2010;18:324–331. doi: 10.1016/j.devcel.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland JT, Kenworthy AK, Peränen J, Caplan S, Goldenring JR. Myosin Vb interacts with Rab8a on a tubular network containing EHD1 and EHD3. Mol Biol Cell. 2007;18:2828–2837. doi: 10.1091/mbc.E07-02-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins CT, Rivera VM, Woolfson DN, Keenan T, Hatada M, Adams SE, Andrade LJ, Yaeger D, van Schravendijk MR, Holt DA, Gilman M, Clackson T. A ligand-reversible dimerization system for controlling protein-protein interactions. Proc Natl Acad Sci USA. 2000;97:7096–7101. doi: 10.1073/pnas.100101997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland AA, Chitwood PJ, Phillips MJ, Voeltz GK. ER contact sites define the position and timing of endosome fission. Cell. 2014;159:1027–1041. doi: 10.1016/j.cell.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusakov DA, Kullmann DM. Extrasynaptic glutamate diffusion in the hippocampus: ultrastructural constraints, uptake, and receptor activation. Journal of Neuroscience. 1998;18:3158–3170. doi: 10.1523/JNEUROSCI.18-09-03158.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu MH, Kang IH, Nelson MD, Jensen TM, Lyuksyutova AI, Siltberg-Liberles J, Raizen DM, Gomelsky M. Engineering adenylate cyclases regulated by near-infrared window light. Proc Natl Acad Sci USA. 2014;111:10167–10172. doi: 10.1073/pnas.1324301111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahl SJ, Hell SW, Jakobs S. Fluorescence nanoscopy in cell biology. Nat Rev Mol Cell Biol. 2017;18:685–701. doi: 10.1038/nrm.2017.71. [DOI] [PubMed] [Google Scholar]
- Sannerud R, Marie M, Nizak C, Dale HA, Pernet-Gallay K, Perez F, Goud B, Saraste J. Rab1 defines a novel pathway connecting the pre-Golgi intermediate compartment with the cell periphery. Mol Biol Cell. 2006;17:1514–1526. doi: 10.1091/mbc.E05-08-0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste J, Dale HA, Bazzocco S, Marie M. Emerging new roles of the pre-Golgi intermediate compartment in biosynthetic-secretory trafficking. FEBS Lett. 2009;583:3804–3810. doi: 10.1016/j.febslet.2009.10.084. [DOI] [PubMed] [Google Scholar]
- Sarris M, Olekhnovitch R, Bousso P. Manipulating leukocyte interactions in vivo through optogenetic chemokine release. Blood. 2016;127:e35–41. doi: 10.1182/blood-2015-11-684852. [DOI] [PubMed] [Google Scholar]
- Savtchenko LP, Rusakov DA. The optimal height of the synaptic cleft. Proc Natl Acad Sci USA. 2007;104:1823–1828. doi: 10.1073/pnas.0606636104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scales SJ, Pepperkok R, Kreis TE. Visualization of ER-to-Golgi transport in living cells reveals a sequential mode of action for COPII and COPI. Cell. 1997;90:1137–1148. doi: 10.1016/s0092-8674(00)80379-7. [DOI] [PubMed] [Google Scholar]
- Schauder CM, Wu X, Saheki Y, Narayanaswamy P, Torta F, Wenk MR, De Camilli P, Reinisch KM. Structure of a lipid-bound extended synaptotagmin indicates a role in lipid transfer. Nature. 2014;510:552–555. doi: 10.1038/nature13269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlierf B, Fey GH, Hauber J, Hocke GM, Rosorius O. Rab11b is essential for recycling of transferrin to the plasma membrane. Exp Cell Res. 2000;259:257–265. doi: 10.1006/excr.2000.4947. [DOI] [PubMed] [Google Scholar]
- Schnell E, Sizemore M, Karimzadegan S, Chen L, Bredt DS, Nicoll RA. Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc Natl Acad Sci USA. 2002;99:13902–13907. doi: 10.1073/pnas.172511199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder-Lang S, Schwärzel M, Seifert R, Strünker T, Kateriya S, Looser J, Watanabe M, Kaupp UB, Hegemann P, Nagel G. Fast manipulation of cellular cAMP level by light in vivo. Nat Meth. 2007;4:39–42. doi: 10.1038/nmeth975. [DOI] [PubMed] [Google Scholar]
- Schweizer A, Fransen JA, Bächi T, Ginsel L, Hauri HP. Identification, by a monoclonal antibody, of a 53-kD protein associated with a tubulo-vesicular compartment at the cis-side of the Golgi apparatus. The Journal of Cell Biology. 1988;107:1643–1653. doi: 10.1083/jcb.107.5.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenk BM, Hartmann H, Serdaroglu A, Schludi MH, Hornburg D, Meissner F, Orozco D, Colombo A, Tahirovic S, Michaelsen M, Schreiber F, Haupt S, Peitz M, Brüstle O, Küpper C, Klopstock T, Otto M, Ludolph AC, Arzberger T, Kuhn PH, Edbauer D. TDP-43 loss of function inhibits endosomal trafficking and alters trophic signaling in neurons. EMBO J. 2016;35:2350–2370. doi: 10.15252/embj.201694221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setou M, Nakagawa T, Seog DH, Hirokawa N. Kinesin superfamily motor protein KIF17 and mLin-10 in NMDA receptor-containing vesicle transport. Science. 2000;288:1796–1802. doi: 10.1126/science.288.5472.1796. [DOI] [PubMed] [Google Scholar]
- Setou M, Seog DH, Tanaka Y, Kanai Y, Takei Y, Kawagishi M, Hirokawa N. Glutamate-receptor-interacting protein GRIP1 directly steers kinesin to dendrites. Nature. 2002;417:83–87. doi: 10.1038/nature743. [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Rumbaugh G, Wu J, Chowdhury S, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron. 2006;52:475–484. doi: 10.1016/j.neuron.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipman SL, Nicoll RA. Dimerization of postsynaptic neuroligin drives synaptic assembly via transsynaptic clustering of neurexin. Proc Natl Acad Sci USA. 2012;109:19432–19437. doi: 10.1073/pnas.1217633109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa R, Higashi T, Tabuchi A, Yoshioka A, Nishioka H, Fukuda M, Kita T, Horiuchi H. Munc13-4 is a GTP-Rab27-binding protein regulating dense core granule secretion in platelets. J Biol Chem. 2004;279:10730–10737. doi: 10.1074/jbc.M309426200. [DOI] [PubMed] [Google Scholar]
- Shu X, Lev-Ram V, Deerinck TJ, Qi Y, Ramko EB, Davidson MW, Jin Y, Ellisman MH, Tsien RY. A genetically encoded tag for correlated light and electron microscopy of intact cells, tissues, and organisms. PLoS Biol. 2011;9:e1001041. doi: 10.1371/journal.pbio.1001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnen BL, Bowen AB, Forte JS, Hiester BG, Crosby KC, Gibson ES, Dell’Acqua ML, Kennedy MJ. Optogenetic Control of Synaptic Composition and Function. Neuron. 2017;93:646–660e5. doi: 10.1016/j.neuron.2016.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spacek J, Harris KM. Three-dimensional organization of smooth endoplasmic reticulum in hippocampal CA1 dendrites and dendritic spines of the immature and mature rat. Journal of Neuroscience. 1997;17:190–203. doi: 10.1523/JNEUROSCI.17-01-00190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specht CG, Izeddin I, Rodriguez PC, El Beheiry M, Rostaing P, Darzacq X, Dahan M, Triller A. Quantitative nanoscopy of inhibitory synapses: counting gephyrin molecules and receptor binding sites. Neuron. 2013;79:308–321. doi: 10.1016/j.neuron.2013.05.013. [DOI] [PubMed] [Google Scholar]
- Spencer DM, Wandless TJ, Schreiber SL, Crabtree GR. Controlling signal transduction with synthetic ligands. Science. 1993;262:1019–1024. doi: 10.1126/science.7694365. [DOI] [PubMed] [Google Scholar]
- Spiltoir JI, Strickland D, Glotzer M, Tucker CL. Optical Control of Peroxisomal Trafficking. ACS Synth Biol. 2016;5:554–560. doi: 10.1021/acssynbio.5b00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz CC, Tatavarty V, Sugino K, Shima Y, Joseph A, Lin H, Rutlin M, Lambo M, Hempel CM, Okaty BW, Paradis S, Nelson SB, Turrigiano GG. Upregulation of μ3A Drives Homeostatic Plasticity by Rerouting AMPAR into the Recycling Endosomal Pathway. Cell Reports. 2016;16:2711–2722. doi: 10.1016/j.celrep.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Fass B. Polyribosomes associated with dendritic spines in the denervated dentate gyrus: evidence for local regulation of protein synthesis during reinnervation. Prog Brain Res. 1983;58:131–136. doi: 10.1016/S0079-6123(08)60013-8. [DOI] [PubMed] [Google Scholar]
- Stierl M, Stumpf P, Udwari D, Gueta R, Hagedorn R, Losi A, Gärtner W, Petereit L, Efetova M, Schwärzel M, Oertner TG, Nagel G, Hegemann P. Light modulation of cellular cAMP by a small bacterial photoactivated adenylyl cyclase, bPAC, of the soil bacterium Beggiatoa. J Biol Chem. 2011;286:1181–1188. doi: 10.1074/jbc.M110.185496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland D, Moffat K, Sosnick TR. Light-activated DNA binding in a designed allosteric protein. Proc Natl Acad Sci USA. 2008;105:10709–10714. doi: 10.1073/pnas.0709610105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton MA, Ito HT, Cressy P, Kempf C, Woo JC, Schuman EM. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell. 2006;125:785–799. doi: 10.1016/j.cell.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Schuman EM. Dendritic protein synthesis, synaptic plasticity, and memory. Cell. 2006;127:49–58. doi: 10.1016/j.cell.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Takemoto K, Matsuda T, McDougall M, Klaubert DH, Hasegawa A, Los GV, Wood KV, Miyawaki A, Nagai T. Chromophore-assisted light inactivation of HaloTag fusion proteins labeled with eosin in living cells. ACS Chem Biol. 2011;6:401–406. doi: 10.1021/cb100431e. [DOI] [PubMed] [Google Scholar]
- Takemoto K, Matsuda T, Sakai N, Fu D, Noda M, Uchiyama S, Kotera I, Arai Y, Horiuchi M, Fukui K, Ayabe T, Inagaki F, Suzuki H, Nagai T. SuperNova, a monomeric photosensitizing fluorescent protein for chromophore-assisted light inactivation. Sci Rep. 2013;3:2629. doi: 10.1038/srep02629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Miyoshi J, Ishizaki H, Togawa A, Ohnishi K, Endo K, Matsubara K, Mizoguchi A, Nagano T, Sato M, Sasaki T, Takai Y. Role of Rab3 GDP/GTP exchange protein in synaptic vesicle trafficking at the mouse neuromuscular junction. Mol Biol Cell. 2001;12:1421–1430. doi: 10.1091/mbc.12.5.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanenbaum ME, Gilbert LA, Qi LS, Weissman JS, Vale RD. A protein-tagging system for signal amplification in gene expression and fluorescence imaging. Cell. 2014;159:635–646. doi: 10.1016/j.cell.2014.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang AH, Chen H, Li TP, Metzbower SR, MacGillavry HD, Blanpied TA. A trans-synaptic nanocolumn aligns neurotransmitter release to receptors. Nature. 2016;536:210–214. doi: 10.1038/nature19058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanowitz M, von Zastrow M. A novel endocytic recycling signal that distinguishes the membrane trafficking of naturally occurring opioid receptors. J Biol Chem. 2003;278:45978–45986. doi: 10.1074/jbc.M304504200. [DOI] [PubMed] [Google Scholar]
- Taslimi A, Vrana JD, Chen D, Borinskaya S, Mayer BJ, Kennedy MJ, Tucker CL. An optimized optogenetic clustering tool for probing protein interaction and function. Nature Communications. 2014;5:4925. doi: 10.1038/ncomms5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temkin P, Morishita W, Goswami D, Arendt K, Chen L, Malenka R. The Retromer Supports AMPA Receptor Trafficking During LTP. Neuron. 2017;94:74–82e5. doi: 10.1016/j.neuron.2017.03.020. [DOI] [PubMed] [Google Scholar]
- Thuenauer R, Hsu YC, Carvajal-Gonzalez JM, Deborde S, Chuang JZ, Römer W, Sonnleitner A, Rodriguez-Boulan E, Sung CH. Four-dimensional live imaging of apical biosynthetic trafficking reveals a post-Golgi sorting role of apical endosomal intermediates. Proc Natl Acad Sci USA. 2014;111:4127–4132. doi: 10.1073/pnas.1304168111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedge H, Brosius J. Translational machinery in dendrites of hippocampal neurons in culture. Journal of Neuroscience. 1996;16:7171–7181. doi: 10.1523/JNEUROSCI.16-22-07171.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tom Dieck S, Kochen L, Hanus C, Heumüller M, Bartnik I, Nassim-Assir B, Merk K, Mosler T, Garg S, Bunse S, Tirrell DA, Schuman EM. Direct visualization of newly synthesized target proteins in situ. Nat Meth. 2015;12:411–414. doi: 10.1038/nmeth.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni N, Buchs PA, Nikonenko I, Povilaitite P, Parisi L, Muller D. Remodeling of synaptic membranes after induction of long-term potentiation. J Neurosci. 2001;21:6245–6251. doi: 10.1523/JNEUROSCI.21-16-06245.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre ER, Steward O. Protein synthesis within dendrites: glycosylation of newly synthesized proteins in dendrites of hippocampal neurons in culture. Journal of Neuroscience. 1996;16:5967–5978. doi: 10.1523/JNEUROSCI.16-19-05967.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulmay A, Prinz WA. Lipid transfer and signaling at organelle contact sites: the tip of the iceberg. Curr Opin Cell Biol. 2011;23:458–463. doi: 10.1016/j.ceb.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tour O, Meijer RM, Zacharias DA, Adams SR, Tsien RY. Genetically targeted chromophore-assisted light inactivation. Nat Biotechnol. 2003;21:1505–1508. doi: 10.1038/nbt914. [DOI] [PubMed] [Google Scholar]
- Traub LM, Apodaca G. AP-1B: polarized sorting at the endosome. Nat Cell Biol. 2003;5:1045–1047. doi: 10.1038/ncb1203-1045. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi T, Fukuda M. Rab3A and Rab27A cooperatively regulate the docking step of dense-core vesicle exocytosis in PC12 cells. J Cell Sci. 2006;119:2196–2203. doi: 10.1242/jcs.02962. [DOI] [PubMed] [Google Scholar]
- Twelvetrees AE, Yuen EY, Arancibia-Carcamo IL, MacAskill AF, Rostaing P, Lumb MJ, Humbert S, Triller A, Saudou F, Yan Z, Kittler JT. Delivery of GABAARs to synapses is mediated by HAP1-KIF5 and disrupted by mutant huntingtin. Neuron. 2010;65:53–65. doi: 10.1016/j.neuron.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich O, Reinsch S, Urbé S, Zerial M, Parton RG. Rab11 regulates recycling through the pericentriolar recycling endosome. The Journal of Cell Biology. 1996;135:913–924. doi: 10.1083/jcb.135.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela JI, Jaureguiberry-Bravo M, Salas DA, Ramírez OA, Cornejo VH, Lu HE, Blanpied TA, Couve A. Transport along the dendritic endoplasmic reticulum mediates the trafficking of GABAB receptors. J Cell Sci. 2014;127:3382–3395. doi: 10.1242/jcs.151092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varandas KC, Irannejad R, von Zastrow M. Retromer Endosome Exit Domains Serve Multiple Trafficking Destinations and Regulate Local G Protein Activation by GPCRs. Curr Biol. 2016;26:3129–3142. doi: 10.1016/j.cub.2016.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall MJ, Corrêa SAL. The mechanistic link between Arc/Arg3.1 expression and AMPA receptor endocytosis. Seminars in Cell and Developmental Biology. 2017 doi: 10.1016/j.semcdb.2017.09.005. [DOI] [PubMed] [Google Scholar]
- Wang C, Han B, Zhou R, Zhuang X. Real-Time Imaging of Translation on Single mRNA Transcripts in Live Cells. Cell. 2016;165:990–1001. doi: 10.1016/j.cell.2016.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Vilela M, Winkler A, Tarnawski M, Schlichting I, Yumerefendi H, Kuhlman B, Liu R, Danuser G, Hahn KM. LOVTRAP: an optogenetic system for photoinduced protein dissociation. Nat Meth. 2016;13:755–758. doi: 10.1038/nmeth.3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Kumar R, Navarre J, Casanova JE, Goldenring JR. Regulation of vesicle trafficking in madin-darby canine kidney cells by Rab11a and Rab25. J Biol Chem. 2000;275:29138–29146. doi: 10.1074/jbc.M004410200. [DOI] [PubMed] [Google Scholar]
- Wang XB, Bozdagi O, Nikitczuk JS, Zhai ZW, Zhou Q, Huntley GW. Extracellular proteolysis by matrix metalloproteinase-9 drives dendritic spine enlargement and long-term potentiation coordinately. Proc Natl Acad Sci USA. 2008;105:19520–19525. doi: 10.1073/pnas.0807248105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Edwards JG, Riley N, Provance DW, Jr, Karcher R, Li XD, Davison IG, Ikebe M, Mercer JA, Kauer JA, Ehlers MD. Myosin Vb Mobilizes Recycling Endosomes and AMPA Receptors for Postsynaptic Plasticity. Cell. 2008a;135:535–548. doi: 10.1016/j.cell.2008.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Edwards JG, Riley N, Provance DW, Jr, Karcher R, Li XD, Davison IG, Ikebe M, Mercer JA, Kauer JA, Ehlers MD. Myosin Vb Mobilizes Recycling Endosomes and AMPA Receptors for Postsynaptic Plasticity. Cell. 2008b;135:535–548. doi: 10.1016/j.cell.2008.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Punge A, Hollopeter G, Willig KI, Hobson RJ, Davis MW, Hell SW, Jorgensen EM. Protein localization in electron micrographs using fluorescence nanoscopy. Nat Meth. 2011;8:80–84. doi: 10.1038/nmeth.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Rost BR, Camacho-Pérez M, Davis MW, Söhl-Kielczynski B, Rosenmund C, Jorgensen EM. Ultrafast endocytosis at mouse hippocampal synapses. Nature. 2013;504:242–247. doi: 10.1038/nature12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson P, Townley AK, Koka P, Palmer KJ, Stephens DJ. Sec16 defines endoplasmic reticulum exit sites and is required for secretory cargo export in mammalian cells. Traffic. 2006;7:1678–1687. doi: 10.1111/j.1600-0854.2006.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenberger S, Schultheis C, Liewald JF, Erbguth K, Nagel G, Gottschalk A. PACα--an optogenetic tool for in vivo manipulation of cellular cAMP levels, neurotransmitter release, and behavior in Caenorhabditis elegans. J Neurochem. 2011;116:616–625. doi: 10.1111/j.1471-4159.2010.07148.x. [DOI] [PubMed] [Google Scholar]
- Williams AH, O’Donnell C, Sejnowski TJ, O’Leary T. Dendritic trafficking faces physiologically critical speed-precision tradeoffs. eLife. 2016;5:489. doi: 10.7554/eLife.20556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winckler B, Forscher P, Mellman I. A diffusion barrier maintains distribution of membrane proteins in polarized neurons. Nature. 1999;397:698–701. doi: 10.1038/17806. [DOI] [PubMed] [Google Scholar]
- Wisco D, Anderson ED, Chang MC, Norden C, Boiko T, Fölsch H, Winckler B. Uncovering multiple axonal targeting pathways in hippocampal neurons. The Journal of Cell Biology. 2003;162:1317–1328. doi: 10.1083/jcb.200307069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolfrey KM, Sanderson JL, Dell’Acqua ML. The palmitoyl acyltransferase DHHC2 regulates recycling endosome exocytosis and synaptic potentiation through palmitoylation of AKAP79/150. J Neurosci. 2015;35:442–456. doi: 10.1523/JNEUROSCI.2243-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, Eliscovich C, Yoon YJ, Singer RH. Translation dynamics of single mRNAs in live cells and neurons. Science. 2016;352:1430–1435. doi: 10.1126/science.aaf1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Whiteus C, Xu CS, Hayworth KJ, Weinberg RJ, Hess HF, De Camilli Pietro. Contacts between the endoplasmic reticulum and other membranes in neurons. Proc Natl Acad Sci USA. 2017;114:E4859–E4867. doi: 10.1073/pnas.1701078114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YI, Frey D, Lungu OI, Jaehrig A, Schlichting I, Kuhlman B, Hahn KM. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature. 2009;461:104–108. doi: 10.1038/nature08241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalgin C, Ebrahimi S, Delandre C, Yoong LF, Akimoto S, Tran H, Amikura R, Spokony R, Torben-Nielsen B, White KP, Moore AW. Centrosomin represses dendrite branching by orienting microtubule nucleation. Nature Publishing Group. 2015;18:1437–1445. doi: 10.1038/nn.4099. [DOI] [PubMed] [Google Scholar]
- Yan X, Hoek TA, Vale RD, Tanenbaum ME. Dynamics of Translation of Single mRNA Molecules In Vivo. Cell. 2016;165:976–989. doi: 10.1016/j.cell.2016.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazawa M, Sadaghiani AM, Hsueh B, Dolmetsch RE. Induction of protein-protein interactions in live cells using light. Nat Biotechnol. 2009;27:941–945. doi: 10.1038/nbt.1569. [DOI] [PubMed] [Google Scholar]
- Ye B, Zhang Y, Song W, Younger SH, Jan LY, Jan YN. Growing dendrites and axons differ in their reliance on the secretory pathway. Cell. 2007;130:717–729. doi: 10.1016/j.cell.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi JJ, Wang H, Vilela M, Danuser G, Hahn KM. Manipulation of endogenous kinase activity in living cells using photoswitchable inhibitory peptides. ACS Synth Biol. 2014;3:788–795. doi: 10.1021/sb5001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A, Fujii R, Watanabe Y, Okabe S, Fukui K, Takumi T. Myosin-Va facilitates the accumulation of mRNA/protein complex in dendritic spines. Curr Biol. 2006;16:2345–2351. doi: 10.1016/j.cub.2006.10.024. [DOI] [PubMed] [Google Scholar]
- Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, Cahalan MD. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Zhang T, Bloch LM. Dendritic mRNAs encode diversified functionalities in hippocampal pyramidal neurons. BMC Neurosci. 2006;7:17. doi: 10.1186/1471-2202-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Chang J, Wang X, Savelieff MG, Zhao Y, Ke S, Ye B. GM130 is required for compartmental organization of dendritic golgi outposts. Curr Biol. 2014;24:1227–1233. doi: 10.1016/j.cub.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JJ, Qin Y, Zhao M, Van Aelst L, Malinow R. Ras and Rap control AMPA receptor trafficking during synaptic plasticity. Cell. 2002;110:443–455. doi: 10.1016/s0092-8674(02)00897-8. [DOI] [PubMed] [Google Scholar]
- Zimmerman SP, Hallett RA, Bourke AM, Bear JE, Kennedy MJ, Kuhlman B. Tuning the Binding Affinities and Reversion Kinetics of a Light Inducible Dimer Allows Control of Transmembrane Protein Localization. Biochemistry. 2016;55:5264–5271. doi: 10.1021/acs.biochem.6b00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber B, Nikonenko I, Klauser P, Muller D, Dubochet J. The mammalian central nervous synaptic cleft contains a high density of periodically organized complexes. Proc Natl Acad Sci USA. 2005;102:19192–19197. doi: 10.1073/pnas.0509527102. [DOI] [PMC free article] [PubMed] [Google Scholar]