SUMMARY
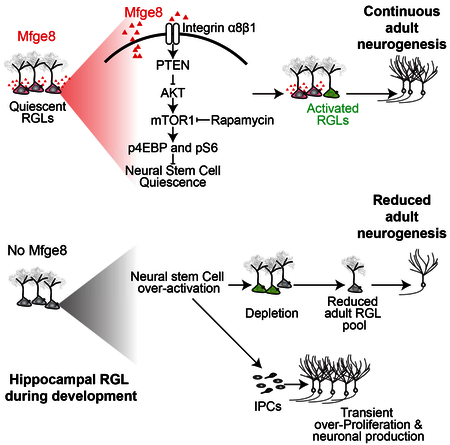
Adult neurogenesis, arising from quiescent radial-glia like neural stem cells (RGLs), occurs throughout life in the dentate gyrus. How neural stem cells are maintained throughout development to sustain adult mammalian neurogenesis is not well understood. Here we show that Milk Fat Globule-EGF 8 (Mfge8), a known phagocytosis factor, is highly enriched in quiescent RGLs in the dentate gyrus. Mfge8 null mice exhibit decreased adult dentate neurogenesis, and furthermore, adult RGL-specific deletion of Mfge8 leads to RGL overactivation and depletion. Similarly, loss of Mfge8 promotes RGL activation in the early postnatal dentate gyrus, resulting in a decreased number of label-retaining RGLs in adulthood. Mechanistically, loss of Mfge8 elevates mTOR1 signaling in RGLs, inhibition of which by rapamycin returns RGLs to quiescence. Together, our study identifies a neural stem cell-enriched niche factor that maintains quiescence and prevents developmental exhaustion of neural stem cells to sustain continuous neurogenesis in the adult mammalian brain.
ETOC BLURB
Zhou et al. identify Mfge8, traditionally known for its role in phagocytosis, as a neural stem cell-enriched niche factor that maintains the neural stem cell pool in the dentate gyrus during early postnatal development and in adulthood by promoting neural stem cell quiescence.
INTRODUCTION
Neurogenesis persists throughout life in the subgranular zone (SGZ) in the dentate gyrus of the hippocampus and the subventricular zone (SVZ) of the lateral ventricles (Gage, 2000). In the adult hippocampus, quiescent radial glia-like neural stem cells (RGLs) continuously give rise to newborn dentate granule neurons and astrocytes (Ming and Song, 2011). Accumulative evidence has demonstrated critical roles of new neurons in the adult hippocampus in regulating neural plasticity as well as cognitive and affective behaviors, whereas deficits in adult hippocampal neurogenesis have been implicated in various brain disorders (Anacker and Hen, 2017; Christian et al., 2014). Therefore, understanding how the pool of adult neural stem cells is regulated during development and maintained in adulthood has implications for brain plasticity and regenerative medicine.
Both extrinsic environmental signals and intrinsic signaling pathways regulate the sequential process of neurogenesis in adult SGZ and SVZ, ranging from quiescent neural stem cell activation and fate specification, to new neuron development and integration (Bond et al., 2015). Multiple lines of evidence suggest that RGL quiescence is a highly regulated state and is critical to maintain continuous neurogenesis in the adult brain. First, single-cell transcriptome analyses have revealed high expression levels of many signaling pathway receptors and intracellular mediators during quiescence, which become down-regulated upon RGL activation (Llorens-Bobadilla et al., 2015; Shin et al., 2015). Second, dysregulation of cytoplasmic signaling pathways, such as FoxO (Paik et al., 2009; Renault et al., 2009) and PTEN (Bonaguidi et al., 2011), activates quiescent RGLs. Third, activation of quiescent RGLs can lead to their depletion in both adult SGZ and SVZ (Calzolari et al., 2015; Encinas et al., 2011; Mira et al., 2010; Seib et al., 2013). Understanding how adult neural stem cell quiescence is regulated remains an important cornerstone in the field and may have implications for understanding other somatic stem cells in various tissues. Among niche factors known to regulate adult RGL quiescence, almost all of them are paracrine factors, including neurotransmitters and peptides released from axon terminals (Berg et al., 2013), the Wnt inhibitor sFRP3 released from mature granule cells (Jang et al., 2013), Notch ligand DLL1 from newborn neurons (Kawaguchi et al., 2013), and growth factors NT-3 and VEGF released from endothelial cells (Delgado et al., 2014). Much less is known about whether RGL quiescence is also regulated by neural stem cell-derived factors.
Quiescence has also been suggested to be essential for establishing the adult neural stem cell pool during development. Neural stem cells that will populate the adult SVZ are set aside and remain quiescent during development (Fuentealba et al., 2015; Furutachi et al., 2015). Importantly, activation of these quiescent or slowly dividing populations during development, by deletion of either the cyclin-dependent kinase inhibitor p57 (Furutachi et al., 2015) or VCAM1 (Hu et al., 2017), reduces the pool of adult SVZ neural stem cells. The niche mechanism that regulates the adult neural stem cell pool during development is completely unknown.
Our recent single-cell transcriptomic analysis has provided insight into the molecular signature of quiescent RGLs in the adult mouse dentate gyrus (Shin et al., 2015). We noted that the transcript of milk-fat globule-EGF factor 8 (Mfge8, also known as SED1 or lactadherin) is highly enriched during the quiescent state compared to the activated state of RGLs (Figure 1A). Mfge8 is traditionally known to play a critical role in phagocytosis (Raymond et al., 2009). Phagocytes secrete Mfge8, which binds to ‘eat-me’ signals secreted by dying cells, such as exposed phosphatidylserine. Mfge8 then binds to integrin receptors αvβ3 or αvβ5 expressed on phagocytes to promote engulfment of apoptotic cells. In the nervous system, activated microglia or astrocytes during neuroinflammation or ischemic injury secrete Mfge8 and phagocytose dying cells and fragments (Fricker et al., 2012; Mills et al., 2015; Neher et al., 2013). Here we investigated the physiological role and mechanism of Mfge8 in regulating early postnatal and adult neurogenesis.
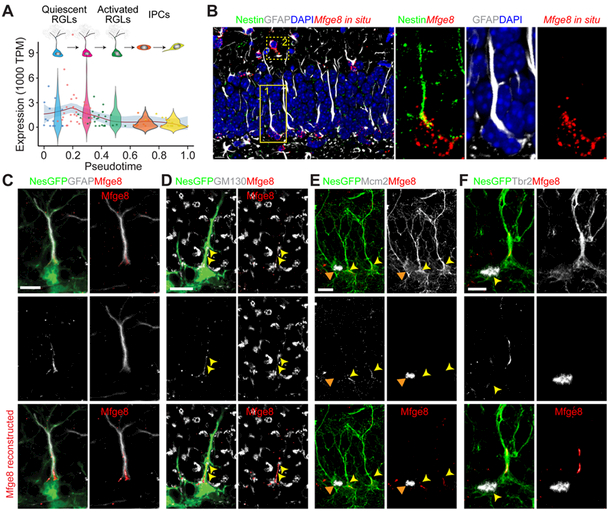
Figure 1. Mfge8 is enriched in quiescent adult mouse hippocampal neural stem cells.
(A) Enrichment of Mfge8 mRNA in quiescent RGLs. Shown is an expression profile of Mfge8 from single-cell RNA-seq analysis of Nestin-CFP marked neural progenitors in adult mouse dentate gyrus (Shin et al., 2015). Each data point represents the expression level of Mfge8 of a single cell along a pseudotime trajectory from quiescent RGLs to activated RGLs to proliferating IPCs. Data points are fitted with local polynomial regression fitting (red line) with 95% confidence intervals (gray area). The superimposed Violin plots represent a summary of expression from different stages along the neurogenesis trajectory.
(B) Sample confocal images of Mfge8 fluorescent in-situ hybridization, and Nestin and GFAP immunostaining in the adult mouse dentate gyrus. Note the presence of Mfge8 mRNA in Nestin+GFAP+ RGLs (an example in box 1 with solid line) and in Nestin−GFAP+ astrocytes (an example in box 2 with dashed line). The region in box 1 is shown at a higher magnification (right panels). Scale bar: 10 μm.
(C-F) Sample confocal images of immunostaining for Mfge8 and GFAP (C), Golgi marker GM130 (D; arrow points to Mfge8 and GM130 colocalization at the base of RGL radial fiber), proliferating cell marker Mcm2 (E; yellow arrowheads point to Mcm2− RGLs and orange arrows point to a Mcm2+ RGL; See Movie S1), and an intermediate progenitor cell (IPC) marker Tbr2 (F; arrow points to a Tbr2+ cell), in Nestin-GFP+ cells in adult mouse dentate gyrus. Bottom panel shows 3D volume-rendered Mfge8 immunostaining. Scale bars: 10 μm. Note the presence of Mfge8 in GM130+ Golgi compartment in Mcm2−Nestin-GFP+GFAP+ quiescent RGLs, but not in Tbr2+ IPCs.
RESULTS
Mfge8 is Enriched in Quiescent RGLs in Adult Mouse Dentate Gyrus
Our recent single-cell RNA-sequencing analysis suggested an enrichment of Mfge8 mRNA in quiescent, but not active neural progenitors in the adult mouse dentate gyrus (Figure 1A) (Shin et al., 2015). To validate this result, we performed in situ hybridization in combination with immunohistology. Indeed, Mfge8 mRNA was present in Nestin+GFAP+ RGLs and Nestin-GFAP+ astrocytes in the adult dentate gyrus (Figure 1B). Using Nestin-GFP transgenic mice (Encinas et al., 2006) to label adult RGLs and validated anti-Mfge8 antibodies (Figure S1A), we detected Mfge8 protein at the base of radial fibers in most Nestin-GFP+GFAP+ RGLs (94.7 ± 3.3%; n = 3 mice), and in particular at the GM130+ Golgi apparatus (Figure 1C-D). Consistent with single-cell RNA-sequencing results (Figure 1A), few proliferating Mcm2+ RGLs (16.2 ± 3.5%; n = 3; Figure 1E and Movie S1) or Tbr2+ intermediate progenitor cells (IPCs; 6.1 ± 1.1%; n = 3; Figure 1F) expressed Mfge8 protein. Mfge8 protein was also present in s100β+ astrocytes (95.3 ± 4.5%; n = 3; Figure S1B), but was not detectable in NeuN+ mature granule cells, Iba1+ microglia, or Olig2+ oligodendrocyte precursors (n = 3; Figure S1C-E). Together, these results revealed highly enriched expression of Mfge8 in quiescent RGLs and astrocytes in the adult dentate gyrus.
Mfge8 Deletion Leads to Reduced Adult Hippocampal Neurogenesis
To explore the potential function of Mfge8 in the adult hippocampal neurogenesis, we first examined Mfge8 homozygous germline knockout mice (KO) and their wild-type (WT) littermates (Hanayama et al., 2004). We injected bromodeoxyuridine (BrdU) into P60 mice and collected tissue 2 hours later (Figure 2A). Quantification showed reduced densities of BrdU+Nestin+GFAP+ RGLs, BrdU+Tbr2+ IPCs, and BrdU+DCX+ neuroblasts (NBs) in KO mice compared to WT littermates (Figure 2B-C). We also examined proliferating cells independent of BrdU labeling with Mcm2 immunostaining and found similar results (Figure 2D-E). To examine the production of adult-born neurons, we injected P60 animals with BrdU five times at 12-hour intervals, and collected tissue 30 days later (Figure 2F). We found a significant decrease in the density of BrdU+NeuN+ adult-born neurons in KO mice (Figure 2G-H). These results showed that Mfge8 is required to maintain a proper level of adult hippocampal neurogenesis.
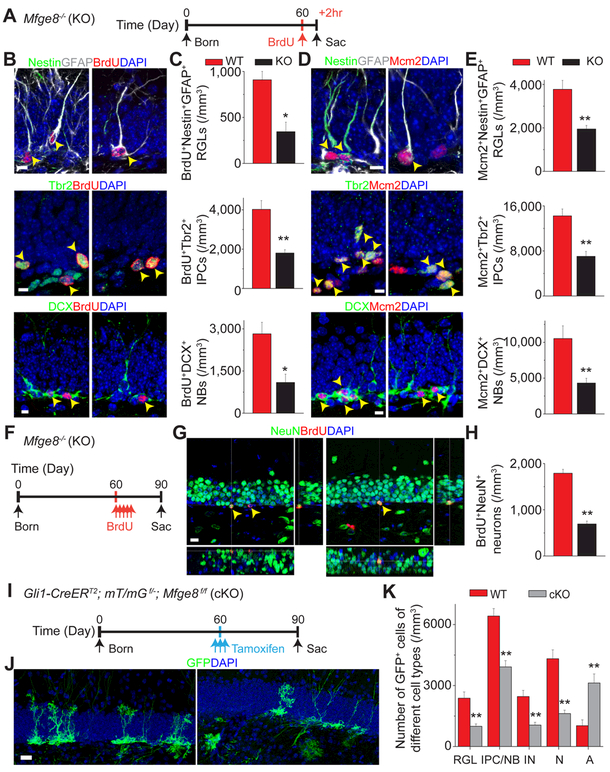
Figure 2. Mfge8 deletion leads to decreased adult hippocampal neurogenesis.
(A-E) Reduced densities of proliferating RGLs, IPCs, and neuroblasts (NBs) in the dentate gyrus of P60 Mfge8 KO mice. Shown in (A) is a schematic diagram of experimental design of a 2-hour BrdU pulse-chase in P60 mice. Also shown are sample confocal images (B, D) and quantification (C, E) of Nestin+GFAP+ RGLs, Tbr2+ IPCs, and DCX+ NBs that were BrdU+ or Mcm2+. Arrows point to BrdU+ or Mcm2+ marker+ cells. Scale bars: 10 μm. Values represent mean ± SEM (n = 5 for C and E; ** P < 0.001; * P < 0.01; unpaired Student’s t-test).
(F-H) Reduced generation of adult-born neurons in the dentate gyrus of Mfge8 KO mice. Shown in (F) is a schematic diagram of the experimental design of 30 day BrdU pulse-chase in P60 mice. Also shown are sample confocal images (G) and quantification (H) of adult-born BrdU+NeuN+ dentate granule neurons. Scale bar: 10 μm. Values represent mean ± SEM (n = 5; ** P < 0.001; unpaired Student’s t-test).
(I-K) Reduced RGL maintenance and neurogenesis, but increased astrocyte production upon conditional Mfge8 deletion specifically in adult RGLs. Shown in (I) is a schematic diagram of experimental design of 30 day chase post tamoxifen induction of P60 mice. Also shown are sample confocal images (J) and quantification of composition of GFP+ cells in the dentate gyrus 1 month after induction (K). Scale bar: 10 μm. Values represent mean ± SEM (n = 5; ** P < 0.001; unpaired Student’s t-test). IN: immature neuron; N: mature neuron; A: astrocyte.
Given that Mfge8 is expressed by both RGLs and astrocytes in the adult dentate gyrus, we next examined the specific contribution of Mfge8 from RGLs on adult neurogenesis. We generated the Gli-CreERT2::mT/mGf/+::Mfge8f/f (cKO) model by crossing Mfge8f/f conditional allele with the Gli1-CreERT2 driver, which specifically targets RGLs within the adult mouse dentate gyrus (Ahn and Joyner, 2005; Sun et al., 2015a), and with the mT/mGf/+ reporter (Muzumdar et al., 2007). We injected P60 mice with multiple doses of tamoxifen for analysis 30 days later (Figure 2I). Quantification showed a significant decrease in the density of GFP+ RGLs in the adult dentate gyrus of cKO mice (Figure 2J-K). In parallel, we observed a decrease in the number of GFP+ neuronal progeny, but an increase in GFP+ astrocytes (Figure 2K). These results suggest that adult RGL-derived Mfge8 is required to maintain proper RGL numbers and adult neurogenesis levels, and to prevent RGL depletion via differentiation into astrocytes.
Mfge8 Promotes RGL Quiescence in the Adult and Early Postnatal Dentate Gyrus
Activation of quiescent RGLs can lead to their depletion via differentiation into astrocytes in the adult dentate gyrus (Bonaguidi et al., 2011; Encinas et al., 2011). To determine whether RGLs were overactivated, we performed short-term fate-mapping upon adult RGL-specific Mfge8 deletion. We injected tamoxifen into P60 cKO mice and analyzed 3 days later (Figure 3A). Quantification of Mcm2+ GFP+ RGLs among all GFP+ RGLs showed a significant increase in cKO mice (Figure 3B-C). Thus, Mfge8 functions to promote adult dentate RGL quiescence.
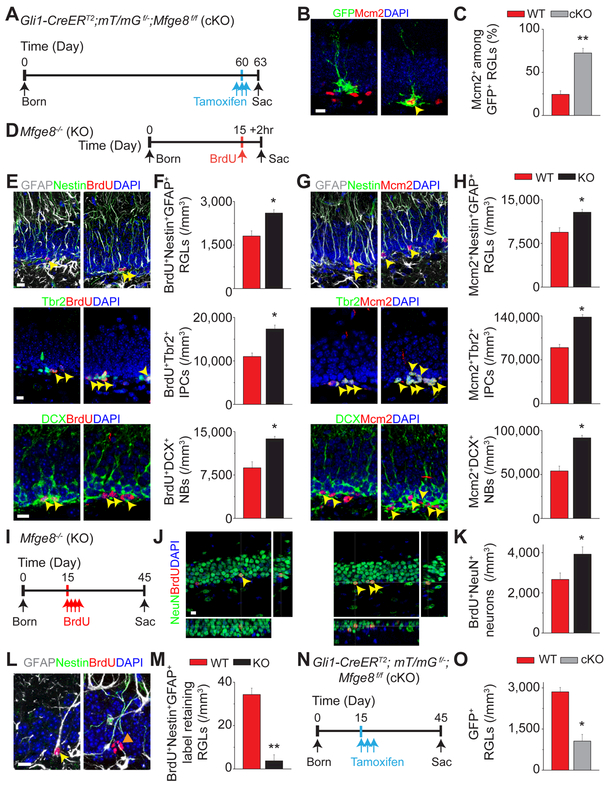
Figure 3. Mfge8 suppresses RGL activation and prevents developmental exhaustion of RGLs in the dentate gyrus.
(A-C) Conditional deletion of Mfge8 in adult RGLs leads to their activation. Shown in (A) is a schematic diagram of experimental design. Shown in (B) are sample confocal images. Arrowhead points to a Mcm2+GFP+ RGL. Scale bar: 10 μm. Shown in (C) are quantifications. Values represent mean ± SEM (n = 4; ** P < 0.001; unpaired Student’s t-test).
(D-H) Lack of Mfge8 leads to an increased number of proliferating RGLs, IPCs and NBs in P15 dentate gyrus. Shown in (D) is a schematic diagram of experimental design of a 2-hour BrdU pulse-chase in P15 mice. Also shown are sample confocal images (E, G) and quantifications (F, H) of Nestin+GFAP+ RGLs, Tbr2+ IPCs, and DCX+ NBs that were BrdU+or Mcm2+. Scale bars: 10 μm. Arrowheads point to BrdU+ or Mcm2+ and marker+ cells. Values represent mean ± SEM (n = 4 for E and H; * P < 0.01; unpaired Student’s t-test).
(I-M) Loss of Mfge8 leads to increased neurogenesis in early postnatal dentate gyrus, but reduced number of RGLs in the adult dentate gyrus. Shown in (I) is a schematic diagram of the experimental design of 30 day BrdU pulse-chase in P15 mice. Also shown are sample confocal images (J, L) and quantification of BrdU+NeuN+ early postnatally born granule neurons (K) and BrdU+Nestin+GFAP+ label-retaining RGLs (M) 30 days after BrdU injection. Yellow arrows point to BrdU+NeuN+ neurons (J) or BrdU+Nestin+GFAP+ label-retaining RGL (L). An orange arrowhead points to a BrdU+Nestin− GFAP− cell (L). Scale bars: 10 μm. Values represent mean ± SEM (n = 5 for K and M; * P < 0.01; ** P < 0.001; unpaired student’s t-test).
(N-O) Conditional deletion of Mfge8 in RGLs at P15 leads to a decreased number of RGLs at P45. Shown are a schematic diagram of experimental design (N) and quantification (O). Values represent mean ± SEM (n = 4; * P < 0.01; unpaired Student’s t-test).
Also see Figure S2.
This result of increased RGL activation in the adult RGL-specific cKO model is in contrast to the decreased density of dividing RGLs in the adult dentate gyrus of the germline KO model (Figure 2B-E). One possibility is that the loss of Mfge8 leads to premature activation and depletion of RGLs during early postnatal development, resulting in a decreased total number of RGLs, which in turn leads to a reduced number of proliferating RGLs and a decreased level of adult dentate neurogenesis (Figure S2A).
We directly tested this hypothesis by analyzing early postnatal dentate neurogenesis. Immunohistological analyses showed that Mfge8 was expressed in most Mcm2− quiescent RGLs from P3 to adult (Figure S2B). We pulsed WT and KO mice at P15 with BrdU and analyzed 2 hours later (Figure 3D). Quantification showed significantly increased densities of BrdU+Nestin+GFAP+ RGLs, BrdU+Tbr2+ IPCs, and BrdU+DCX+ NBs in KO mice (Figure 3E-F). Analysis of proliferating cells based on Mcm2 expression showed similar results (Figure 3G-H). To examine the long-term consequences, we pulsed animals with multiple BrdU injections at P15 for analysis 30 days later (Figure 3I). Consistent with increased RGL activation and increased numbers of IPCs and NBs at P15, we found an increased density of BrdU+NeuN+ mature neurons in KO mice (Figure 3J-K). Importantly, the density of BrdU+Nestin+GFAP+ labeling-retaining RGLs was significantly reduced in KO mice (Figure 3L-M), indicating a reduced pool of adult RGLs in the absence of Mfge8. Similarly, conditional deletion of Mfge8 with Gli-CreERT2 at P15 led to a reduced number of GFP+ RGLs examined 30 days later (Figure 3N-O). At the population level, KO mice exhibited a slight increase of Nestin+GFAP+ RGLs and significant increase of Tbr2+ IPCs, and BrdU+ or Mcm2+ proliferating progenitors at P15, in contrast to significant decreases in these cell populations at P60 (Figure S2D-F).
Together, these results support the model that RGL-derived Mfge8 promotes RGL quiescence and maintenance in early postnatal stages to sustain a proper level of dentate neurogenesis into adulthood.
Mfge8 Promotes RGL Quiescence via Suppression of mTOR1 Pathway
We next examined how Mfge8 maintains RGL quiescence. Integrin αvβ3/5 is the best known Mfge8 receptor for phagocytosis (Nagata et al., 2010) (Figure S3A). However, immunohistological analysis showed that integrin β5 does not appear to be expressed by RGLs, but instead is colocalized almost exclusively with Iba1+ microglia in the adult dentate gyrus (Figure S3B, D). This result raised the question of whether Mfge8-dependent regulation of RGLs involves microglia. Quantitative analyses showed no detectable differences in the densities of Iba1+Itgb5+ microglia or Iba1+CD68+ activated microglia between the WT and KO adult dentate gyrus (Figure S3D-E). Thus, microglia activity does not appear to be affected by the loss of Mfge8 in the dentate gyrus.
Integrin receptor α8β1 was recently identified as a non-canonical Mfge8 receptor in regulating gastrointestinal motility (Figure S3A) (Khalifeh-Soltani et al., 2016). Integrin β1 is known to be expressed in neural progenitor cells in the adult SVZ and SGZ (Brooker et al., 2016; Shen et al., 2008). Indeed, immunohistological analysis showed expression of both integrin α8 and β1 by Nestin-GFP+ RGLs in the P15 and P60 dentate gyrus (Figure 4A and S3C). We next used an in vitro adult neural progenitor cell culture model (Ma et al., 2008) to examine the role of integrin β1 in regulating neural stem cell proliferation. We first confirmed that Mfge8 protein is released into the culture medium by adult neural progenitors (Figure 4B). Addition of recombinant Mfge8 protein reduced proliferation of adult neural progenitors (Figure 4C-E and S3F). Importantly, addition of functional blocking antibodies for integrin β1 (Goh et al., 2008), but not integrin αv, abolished the effect of Mfge8 on suppression of adult neural progenitor proliferation (Figure 4C-E).
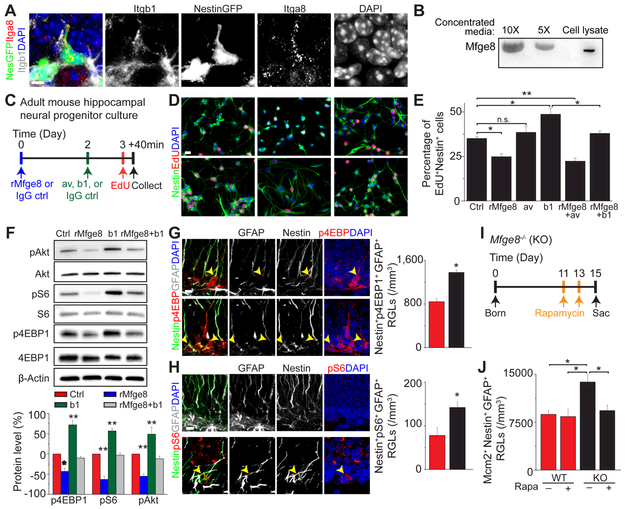
Figure 4. Mfge8 promotes RGL quiescence by suppressing mTOR1 signaling.
(A) Sample confocal images of immunostaining for Mfge8, integrin receptor α8β1 in Nestin-GFP+ RGLs in the P15 dentate gyrus. Scale bar: 10 μm.
(B) Release of Mfge8 protein into the culture medium of adult neural progenitors. Shown is a sample Western blot of concentrated culture medium (10x, 5x) and whole cell lysate.
(C-E) Mfge8 suppresses proliferation of adult neural progenitors. Shown are a schematic diagram of the experimental design (C), sample confocal images (D; scale bar: 20 μm) and quantifications (E) of adult mouse dentate gyrus-derived neural progenitors cultured in the presence or absence of rMfge8 (0.5 μg/ml), anti-αv integrin antibodies (av; 0.625 μg/ml), or anti-β1 integrin antibodies (b1; 0.625 μg/ml). Values represent mean ± SEM (n = 4; ** P < 0.001; * P < 0.01; n.s.: not significant; one-way ANOVA).
(F) Mfge8 suppresses mTOR1 signaling in adult neural progenitors. Adult neural progenitors were similarly treated as in (C) and then subject to Western blot analysis. Shown are sample Western blot images (top panels) and quantifications (bottom panel). Values represent mean ± SEM (n = 4; ** P < 0.001; * P < 0.01; one-way ANOVA).
(G-H) Increased mTOR1 signaling in RGLs in Mfge8 KO mice. Shown are sample confocal images and quantifications of numbers of p4EBP1+ (G) and pS6+ (H) Nestin+GFAP+ RGLs in P15 mice. Arrowheads point to p4EBP1+ or pS6+ Nestin+GFAP+ RGLs. Scale bar: 10 μm. Values represent mean ± SEM (n = 4; * P < 0.01; unpaired Student’s t-test).
(I-J) Rapamycin treatment rescues RGL overactivation in the P15 dentate gyrus of Mfge8 KO mice. Shown in (I) is a schematic diagram of the experimental design. Rapamycin (20 mg/kg body weight) or vehicle was i.p. injected. Shown in (J) is the quantification of BrdU+Nestin+GFAP+ RGLs. Values represent mean ± SEM (n = 4; * P < 0.01; one-way ANOVA).
Previous studies in the gastrointestinal system have shown that Mfge8 can either upregulate (Khalifeh-Soltani et al., 2014) or downregulate (Khalifeh-Soltani et al., 2016) the intracellular PTEN-PI3K-Akt pathway by binding to integrin receptors αvβ3/5 or α8β1, respectively (Figure S3A). One major target of the PTEN-Akt pathway is mTOR1, which is known to be required for neural stem cell proliferation (Ka et al., 2014; Sato et al., 2010). We found that in cultured adult neural progenitors, exogenous Mfge8 reduced levels of phosphorylated AKT (pAKT), phosphorylated 4EBP1 protein (p4EBP1), and phosphorylated S6 protein (pS6) (Figure 4F-G). Such effects were blocked by anti-integrin β1 antibodies, but not by anti-αv antibodies (Figure 4F-G). Immunohistological analyses also showed a significant increase in densities of p4EBP1+ and pS6+ GFAP+ RGLs in KO mice at P15 (Figure 4G-H) and in the cKO model in the adult dentate gyrus (Figure S3G-H). These results suggest that Mfge8 serves as a suppressor of mTOR1 activation in neural stem cells (Figure S4A).
To assess the functional role of the mTOR1 pathway in Mfge8-dependent regulation of RGL quiescence, we pharmacologically inhibited mTOR1 activation with rapamycin injections at P11 and P13 and performed analysis at P15 (Figure 4I). Immunohistological analysis confirmed the effectiveness of rapamycin treatment on reducing p4EBP1 and pS6 expression (Figure S4B-C). Quantification of Mcm2+ Nestin+GFAP+ RGLs in the dentate gyrus showed that, while rapamycin had no effect on WT animals, it completely abolished elevated RGL activation in Mfge8 KO mice (Figure 4J). Similarly, rapamycin treatment abolished Mfge8 deletion-induced adult RGL activation in the cKO model (Figure S4D-F).
DISCUSSION
How the neural stem cell pool is maintained throughout early development to ensure a sufficient number of adult neural stem cells to support life-long neurogenesis is not well understood (Berg et al., 2018). Here we identified Mfge8 as a stem cell-enriched niche factor that maintains the adult neural stem cell pool during early postnatal development and in adulthood by promoting RGL quiescence (Figure S2A). In addition to a large number of previously identified paracrine factors released by other niche cell types, our finding illustrates the complexity of neurogenesis regulation that is also modulated by a stem cell-derived factor in vivo. While Mfge8 is best known to mediate phagocytosis, here we identified a non-canonical role of Mfge8 signaling in maintaining neural stem cell quiescence by suppressing mTOR1 activation (Figure S4A). Interestingly, recent transcriptome analyses have also found enrichment of Mfge8 in different stem cell populations, such as neural stem cells in the embryonic cortex (Pollen et al., 2015; Yuzwa et al., 2017; Zeisel et al., 2015), developing and adult dentate gyrus (Hochgerner et al., 2018), adult SVZ (Dulken et al., 2017), hair follicle stem cells (Lay et al., 2016), and pluripotent stem cells (Yan et al., 2013). These findings raise the possibility that Mfge8 may play a general role as an autocrine signal for various types of stem cells.
Mfge8 is traditionally known to be involved in phagocytosis by phagocytes and microglia (Raymond et al., 2009). In the adult dentate gyrus, however, Mfge8 is selectively expressed by quiescent RGLs and astrocytes, but not by RGL progeny or microglia. Through adult RGL-specific deletion, we provide evidence that the RGL population regulates its own quiescence and maintenance via Mfge8. One advantage of autocrine regulation is the spatial proximity of signals for fine tuning of stem cell behavior. Given diffusion limits of secreted molecules in the adult mammalian brain, especially when their receptors are in proximity for efficient trapping, effects could be spatially restricted. Astrocytes are known to modulate adult dentate neurogenesis via secreted factors (Lie et al., 2005; Ma et al., 2005; Song et al., 2002) and it will be interesting to examine whether astrocyte-derived Mfge8 also regulates RGL quiescence when genetic tools become available in the future. Since astrocytes are born during later postnatal stages (Figure S2C), it is likely that RGL-derived Mfge8 plays a more dominant role in regulating RGL quiescence during early postnatal development. Our study also identifies the signaling mechanism whereby Mfge8 promotes RGL quiescence (Figure S4A). Our results are consistent with previous findings that loss of integrin β1 depletes RGLs, promotes astrocyte differentiation, and reduces neurogenesis in the adult dentate gyrus (Brooker et al., 2016). Notably, genetic deletion of Ilk (integrin-linked kinase) or Pten also leads to quiescent RGL activation in the adult dentate gyrus (Bonaguidi et al., 2011; Porcheri et al., 2014). Together, these findings identify the PTEN-Akt-mTOR1 pathway as a hub that integrates stem cell-activation signals to regulate RGL quiescence and maintenance.
How the adult neural stem cell pool is regulated during development is not well understood. Recent studies have suggested a critical role for quiescence in preserving the somatic stem cell pool during development. For example, the adult muscle stem cell pool is established during puberty by sex hormone-dependent regulation of Notch signaling that converts cycling juvenile stem cells to a quiescent stem cell population (Kim et al., 2016). For epidermal stem cells, NFATc1 (Horsley et al., 2008) and Lrig1 (Jensen et al., 2009) play a critical role in governing the quiescence of a reservoir of stem cells throughout hair follicle morphogenesis, which is retained during adulthood. In the central nervous system, activation of the reserve neural stem cells during development by deletion of p57 or VCAM1 reduces their numbers in the adult SVZ (Furutachi et al., 2015; Hu et al., 2017). Similarly, deletion of FoxOs leads to increased early postnatal hippocampal neurogenesis, but a reduced number of RGLs and decreased neurogenesis in the adult hippocampus (Paik et al., 2009; Renault et al., 2009), a phenotype very similar to Mfge8 KO mice. Our study identifies a stem-cell enriched niche mechanism that maintains neural stem cell quiescence to sustain neurogenesis in the adult hippocampus. Our finding also highlights the complexity of prospective regulatory mechanisms to maintain a viable stem cell pool over the lifespan, involving both paracrine and autocrine signaling from a stem cell-derived factor.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact Hongjun Song (shongjun@pennmedicine.upenn.edu). There are no restrictions on any data or materials presented in this paper.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Animals
All animal procedures used in this study were performed in accordance with the protocol approved by the Institutional Animal Care and Use Committee of Johns Hopkins University School of Medicine and University of Pennsylvania. All mice in the study were backcrossed to the C57BL/6 background for at least six generations. Animals were housed in a 14-hour light/10-hour dark cycle and had free access to food and water. Housing and husbandry conditions followed standard settings. Both 15-day-old mice (postnatal) and 2-month-old mice (adult) were used in this study. For immunohistology, C57Bl6/J wild-type mice and Nestin-GFP transgenic mice (Encinas et al., 2006) were used. For knockout and rapamycin rescue analysis, mice used in different groups were housed as littermates: C57Bl6/J WT mice, and Mfge8−/−(KO). For conditional knockout analyses, Gli-CreERT2; Mfge8f/f; mT/mGf/−, and Gli1-CreERT2; mT/mGf/− were generated by crossing Gli1-CreERT2 driver (Ahn and Joyner, 2005) with mT/mGf/− reporter (Muzumdar et al., 2007) and Mfge8f/f mice (Atabai lab), where applicable. Primers used for mouse genotyping were listed in Table S1.
METHODS DETAILS
BrdU administration
For analysis of cell proliferation in the dentate gyrus, P15 or P60 mice were injected with BrdU (200 mg/kg body weight, i.p.) and analyzed 2 hours or 30 days later. Tissue processing and quantification of BrdU+ cells within the SGZ and granule cell layer were carried out as previously described (Jang et al., 2013).
Tamoxifen administration
Tamoxifen (66.67 mg/ml, Sigma, T5648) was prepared in a 5:1 corn oil (Sigma) to ethanol mixture (Sun et al., 2015a; Sun et al., 2015b). A dose of 375 mg/kg body weight was intraperitoneally injected into P15 or P60 male and female mice every 12 hours for 3 times. Mice were analyzed 3 or 30 days post-tamoxifen injection.
Rapamycin administration
Rapamycin (LC Laboratories) was first dissolved in ethanol at a stock concentration of 25 mg/ml and was further diluted with a final concentration of 1 mg/ml in 4% ethanol, 5% Tween 80, and 5% PEG 400 as previously described (Kim et al., 2009). Rapamycin was intraperitoneally delivered twice to mice at 20 mg/kg body weight at P11 and P13, analyzed at P15, or injected at P60 and P62 and analyzed at P64.
Tissue processing, immunostaining, and confocal imaging
Animals were perfused with cold 4% paraformaldehyde [wt/vol; in 0.1 M phosphate buffer (PB), pH 7.4], and cryoprotected with 30% sucrose (wt/vol). Serial 40-μm-thick coronal brain sections were cut on a frozen sliding microtome (Leica; SM2010R) for immunohistology as previously described (Sun et al., 2015a; Sun et al., 2015b). Every 6th section was taken for immunohistology throughout the anterior to posterior axis of the dentate gyrus. Antibodies were diluted in TBS (Tris buffered saline) with 0.1% Triton X-100 and 3% (vol/vol) donkey serum. Sections were incubated with primary antibodies at 4 °C overnight. The following primary antibodies were used: BrdU (Novus; rat; 1:300), CD68 (Bio-Rad; rat; 1:300), DCX (Santa Cruz, goat, 1:100), GFAP (Millipore, mouse, rabbit, 1:1,000), GFP (Aves Labs, chicken, 1:500), GM130 (BD Biosciences, mouse, 1:300), Iba1 (WAKO; rabbit; 1:500), integrin α8 (R&D Systems; goat; 1:100), integrin β1 (Millipore; rat; 1:300), integrin β5 (R&D Systems; sheep; 1:500), Ki67 (Leica; rabbit; 1:500; BD Bioscience; mouse; 1:500), Mcm2 (aka BM28; BD Biosciences; mouse; 1:300), Mfge8 (MBL; Armenian Hamster; 1:500; R&D Systems; Mouse; 1:100; Santa Cruz; mouse; 1:300), Nestin (Santa Cruz; Goat; 1:250; Aves Labs; chicken; 1:500), NeuN (Millipore; mouse; 1:500; and Alexa Fluor 488 conjugated), Oligo2 (guinea pig; 1:500); p4EBP1 (Cell Signaling; rabbit; 1:500), pS6 (Ser-235/236; Cell Signaling; rabbit; 1:500), S-100b (Sigma; rabbit; 1:300); and Tbr2 (Abcam; rabbit; 1:250).
The mT-labeled cells and non-specific blood vessel labeling were removed, and Mcm2 and NeuN antigens were retrieved by incubating brain sections in 1 X target retrieval solution (DAKO) at 92.5 °C for 10 min, followed by a 20-min cooling to room temperature. BrdU antigen was retrieved by incubating brain sections in 2N HCl for 1 hour in room temperature, followed by 0.1M boric acid neutralization for 30 min. Cy2-, Cy3- or Cy5-conjugated secondary antibodies (Jackson ImmunoResearch; 1:500) to appropriate species were incubated at room temperature for 2 hours. Stained sections were imaged at 40x on a Zeiss LSM 710 or 800 confocal microscope (Carl Zeiss).
In-situ hybridization
In-situ hybridization was performed on PFA-fixed brain sections (40 μm thickness) as described previously (Ma et al., 2009; Mills et al., 2015). Fluorescein-conjugated antisense riboprobe for Mfge8 mRNA was generated by in vitro transcription. Hybridization of the riboprobes on sections was performed at 65 °C overnight and followed by wa shing once with 5x SSC and 1% SDS, then twice with 0.2x SSC for 1 hour each at 72 °C. The sections were incubated with anti-fluorescein peroxidase (Roche) at 4 °C overnight, and were visu alized using Cy3-conjugated Tyramide Signal Amplification system (Perkin-Elmer) at room temperature. The in-situ hybridization was followed by immunostaining for Nestin (goat anti-Nestin, Santa Cruz, 1:500), and GFAP (Millipore, mouse, rabbit, 1: 1,000) incubated at 4 °C overnight and followed by incubation of Cy2- and Cy5-conjugated secondary antibodies (Jackson ImmunoResearch; 1:500) for 2 hours at room temperature.
Adult neural progenitor culture and analyses
Adult mouse hippocampal neural progenitor cells were derived from adult mouse dentate gyrus as previously described (Ma et al., 2008; Song et al., 2002). To determine whether Mfge8 was secreted by adult neural progenitors in culture, cells were cultured to reach 75% confluency and media were collected and concentrated 5-10 times by centrifugation at 4°C with Amicon Ultra-4 Centrifugal Filter Units (Millipore, 10,000 NMWL) at 4,000 × g. Samples were processed for Western blot analysis to detect Mfge8 protein at ~50 KD.
To examine neural progenitor proliferation, adult neural progenitors were incubated with exogenous recombinant Mfge8 (R&D Systems) for 2 days. Blocking antibodies against integrin αv (BioLegend; 0.625 μg/ml) or β1 (BD Biosciences; 0.625 μg/ml), or IgG control (BD Biosciences; 0.625 μg/ml) were added for 24 hours before an EdU (Thermo Fisher Scientific, 10 μM) pulse for 40 min followed by PFA fixation for immunostaining, imaging and quantification. EdU was detected by Click-iT Imaging Kit (Invitrogen), in combination with immunostaining for Nestin (goat anti-Nestin, Santa Cruz, 1:500).
Biochemical analyses were performed as previously described (Kim et al., 2009). Adult neural progenitor culture was lysed in cold RIPA buffer containing 50 mM Tris HCl (pH 7.5), 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS supplemented with protease and phosphatase inhibitor cocktail (Cell Signaling; Sigma) at 4°C for 10 min, followed by sonication on ice for 30 sec for 3 times. Protein concentration was determined by Bradford assay (Bio-Rad). Equal amounts of protein were loaded to be separated by SDS-PAGE on 4-20% resolving gels (Bio-Rad) and transblotted onto PVDF membranes (Bio-Rad). Membranes were incubated in primary antibodies at a 1:1,000 dilution at 4 °C overnight against 4EBP1 (Cell Signaling; rabbit), p4EBP1 (Cell Signaling; rabbit), S6 (Cell Signaling; mouse), pS6 (Cell Signaling; rabbit), β-actin (Sigma; mouse), Mfge8 (R&D Systems; mouse; Santa Cruz; mouse), Akt (Cell Signaling; mouse), and pAkt (Cell Signaling; rabbit), followed by a secondary HRP-conjugated antibody (Cell Signaling; rabbit; Santa Cruz; mouse). For evaluation of total 4EBP1, S6, and Akt, we stripped and reprobed membranes that had been blotted for phospho-versions of these proteins.
Image processing and data analyses
All confocal images were blindly acquired between experimental and control groups under the same laser power and gain, and were analyzed with Imaris 7.2, 7.6, 9.0 software (BitPlane), or ImageJ (NIH) without adjusting image brightness or contrast, as previously described (Sun et al., 2015a; Sun et al., 2015b). The Spots module in Imaris was used to digitize cell-nucleus locations in the 3D space and to code cell type classifications according to distinct morphological and molecular markers. The Clipping Plane module in Imaris was used to estimate the local SGZ plane for 2D clone projections. For illustrative purposes, some cells and features were volume-rendered using the Surface module in Imaris (Figure 1C-F; bottom panels). A confirmed RGL needed to satisfy the following criteria, (1) located in the SGZ of the dentate gyrus; (2) have a distinct Nestin+GFAP+ radial process; and (3) have a DAPI+ nuclei that is largely wrapped around by GFAP immunostaining signal in the same focal plane (Bonaguidi et al., 2011; Song et al., 2012). A cell nucleus located within the granule cell layer and having overlapped immunostaining signals of DAPI, BrdU/Mcm2, and Tbr2 or NeuN in the same focal plane, was defined as a BrdU+/Mcm2+ intermediate progenitor cell or dentate granule neuron, respectively. A BrdU+/Mcm2+ DCX+ neuroblast should be located in the granule cell layer and have a DAPI+ and BrdU+/Mcm2+ nuclei that is largely wrapped around by DCX immunostaining signal in the same focal plane.
QUANTIFICATION AND STATISTICAL ANALYSIS
The studies were blinded during data collection and quantification. Data in figure panels reflect several independent experiments performed on different days. No data were excluded. An estimate of variation within each group of data is indicated using standard error of the mean (SEM). Statistical analysis was performed with one-way ANOVA (with Tukey post hoc test), or one-tailed unpaired Student’s t-test, as indicated in the text and figures. All statistical analyses were performed in Origin software (OriginLab).
Supplementary Material
Shown is a confocal image of immunostaining for DAPI (blue), GFP (green), Mfge8 (red), and Mcm2 (white) in a Nestin-GFP reporter mouse. Note there is one Mcm2+GFP+ activated RGL (the furthest left) that was Mfge8−. There are three Mcm2−GFP+Mfge8+ quiescent RGLs (right). Also shown is a surface rendering of Mfge8 immunostaining (red).
HIGHLIGHTS.
Mfge8 is enriched in quiescent neural stem cells
Mfge8 deletion depletes neural stem cells and decreases adult neurogenesis
Mfge8 promotes early postnatal and adult neural stem cell quiescence
Mfge8 regulates neural stem cell quiescence via mTOR1 signaling
ACKNOWLEDGEMENTS:
We thank J. Nathans, members of Ming and Song laboratories for discussion, J. Schnoll and K.M. Christian for comments, and Y. Cai, L. Liu, and D. Johnson for technical support. This work was supported by grants from the National Institutes of Health (P01NS097206 and R37NS047344 to H.S., R35NS097370 and R01MH105128 to G-l.M., and R01DK11098 and R01HL136377 to K.A.). Y. Zhou was partially supported by a fellowship from the American Heart Association.
Footnotes
DECLARATION OF INTERESTS: The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahn S, and Joyner AL (2005). In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature 437, 894–897. [DOI] [PubMed] [Google Scholar]
- Anacker C, and Hen R (2017). Adult hippocampal neurogenesis and cognitive flexibility - linking memory and mood. Nat Rev Neurosci 18, 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg DA, Belnoue L, Song H, and Simon A (2013). Neurotransmitter-mediated control of neurogenesis in the adult vertebrate brain. Development 140, 2548–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg DA, Bond AM, Ming GL, and Song H (2018). Radial glial cells in the adult dentate gyrus: what are they and where do they come from? F1000Res 7, 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaguidi MA, Wheeler MA, Shapiro JS, Stadel RP, Sun GJ, Ming GL, and Song H (2011). In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell 145, 1142–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond AM, Ming G.-l., and Song H (2015). Adult mammalian neural stem cells and neurogenesis: Five decades later. Cell Stem Cell 17, 385–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker SM, Bond AM, Peng CY, and Kessler JA (2016). beta1-integrin restricts astrocytic differentiation of adult hippocampal neural stem cells. Glia 64, 1235–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzolari F, Michel J, Baumgart EV, Theis F, Gotz M, and Ninkovic J (2015). Fast clonal expansion and limited neural stem cell self-renewal in the adult subependymal zone. Nat Neurosci 18, 490–492. [DOI] [PubMed] [Google Scholar]
- Christian KM, Song H, and Ming GL (2014). Functions and dysfunctions of adult hippocampal neurogenesis. Annual review of neuroscience 37, 243–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado AC, Ferron SR, Vicente D, Porlan E, Perez-Villalba A, Trujillo CM, D'Ocon P, and Farinas I (2014). Endothelial NT-3 delivered by vasculature and CSF promotes quiescence of subependymal neural stem cells through nitric oxide induction. Neuron 83, 572–585. [DOI] [PubMed] [Google Scholar]
- Dulken BW, Leeman DS, Boutet SC, Hebestreit K, and Brunet A (2017). Single-Cell Transcriptomic Analysis Defines Heterogeneity and Transcriptional Dynamics in the Adult Neural Stem Cell Lineage. Cell Rep 18, 777–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinas JM, Michurina TV, Peunova N, Park JH, Tordo J, Peterson DA, Fishell G, Koulakov A, and Enikolopov G (2011). Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell 8, 566–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinas JM, Vaahtokari A, and Enikolopov G (2006). Fluoxetine targets early progenitor cells in the adult brain. Proc Natl Acad Sci U S A 103, 8233–8238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker M, Neher JJ, Zhao JW, Thery C, Tolkovsky AM, and Brown GC (2012). MFG-E8 mediates primary phagocytosis of viable neurons during neuroinflammation. J Neurosci 32, 2657–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentealba LC, Rompani SB, Parraguez JI, Obernier K, Romero R, Cepko CL, and Alvarez-Buylla A (2015). Embryonic Origin of Postnatal Neural Stem Cells. Cell 161, 1644–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furutachi S, Miya H, Watanabe T, Kawai H, Yamasaki N, Harada Y, Imayoshi I, Nelson M, Nakayama KI, Hirabayashi Y, et al. (2015). Slowly dividing neural progenitors are an embryonic origin of adult neural stem cells. Nat Neurosci 18, 657–665. [DOI] [PubMed] [Google Scholar]
- Gage FH (2000). Mammalian neural stem cells. Science 287, 1433–1438. [DOI] [PubMed] [Google Scholar]
- Goh EL, Young JK, Kuwako K, Tessier-Lavigne M, He Z, Griffin JW, and Ming GL (2008). beta1-integrin mediates myelin-associated glycoprotein signaling in neuronal growth cones. Molecular brain 1, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanayama R, Tanaka M, Miyasaka K, Aozasa K, Koike M, Uchiyama Y, and Nagata S (2004). Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science 304, 1147–1150. [DOI] [PubMed] [Google Scholar]
- Hochgerner H, Zeisel A, Lonnerberg P, and Linnarsson S (2018). Conserved properties of dentate gyrus neurogenesis across postnatal development revealed by single-cell RNA sequencing. Nat Neurosci 21, 290–299. [DOI] [PubMed] [Google Scholar]
- Horsley V, Aliprantis AO, Polak L, Glimcher LH, and Fuchs E (2008). NFATc1 balances quiescence and proliferation of skin stem cells. Cell 132, 299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XL, Chen G, Zhang S, Zheng J, Wu J, Bai QR, Wang Y, Li J, Wang H, Feng H, et al. (2017). Persistent Expression of VCAM1 in Radial Glial Cells Is Required for the Embryonic Origin of Postnatal Neural Stem Cells. Neuron 95, 309–325 e306. [DOI] [PubMed] [Google Scholar]
- Jang MH, Bonaguidi MA, Kitabatake Y, Sun J, Song J, Kang E, Jun H, Zhong C, Su Y, Guo JU, et al. (2013). Secreted frizzled-related protein 3 regulates activity-dependent adult hippocampal neurogenesis. Cell Stem Cell 12, 215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KB, Collins CA, Nascimento E, Tan DW, Frye M, Itami S, and Watt FM (2009). Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell stem cell 4, 427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ka M, Condorelli G, Woodgett JR, and Kim WY (2014). mTOR regulates brain morphogenesis by mediating GSK3 signaling. Development 141, 4076–4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi D, Furutachi S, Kawai H, Hozumi K, and Gotoh Y (2013). Dll1 maintains quiescence of adult neural stem cells and segregates asymmetrically during mitosis. Nature communications 4, 1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalifeh-Soltani A, Ha A, Podolsky MJ, McCarthy DA, McKleroy W, Azary S, Sakuma S, Tharp KM, Wu N, Yokosaki Y, et al. (2016). alpha8beta1 integrin regulates nutrient absorption through an Mfge8-PTEN dependent mechanism. eLife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalifeh-Soltani A, McKleroy W, Sakuma S, Cheung YY, Tharp K, Qiu Y, Turner SM, Chawla A, Stahl A, and Atabai K (2014). Mfge8 promotes obesity by mediating the uptake of dietary fats and serum fatty acids. Nature medicine 20, 175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Han GC, Seo JY, Park I, Park W, Jeong HW, Lee SH, Bae SH, Seong J, Yum MK, et al. (2016). Sex hormones establish a reserve pool of adult muscle stem cells. Nat Cell Biol 18, 930–940. [DOI] [PubMed] [Google Scholar]
- Kim JY, Duan X, Liu CY, Jang MH, Guo JU, Pow-anpongkul N, Kang E, Song H, and Ming GL (2009). DISC1 regulates new neuron development in the adult brain via modulation of AKT-mTOR signaling through KIAA1212. Neuron 63, 761–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lay K, Kume T, and Fuchs E (2016). FOXC1 maintains the hair follicle stem cell niche and governs stem cell quiescence to preserve long-term tissue-regenerating potential. Proceedings of the National Academy of Sciences of the United States of America 113, E1506–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie DC, Colamarino SA, Song HJ, Desire L, Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR, et al. (2005). Wnt signalling regulates adult hippocampal neurogenesis. Nature 437, 1370–1375. [DOI] [PubMed] [Google Scholar]
- Llorens-Bobadilla E, Zhao S, Baser A, Saiz-Castro G, Zwadlo K, and Martin-Villalba A (2015). Single-Cell Transcriptomics Reveals a Population of Dormant Neural Stem Cells that Become Activated upon Brain Injury. Cell Stem Cell 17, 329–340. [DOI] [PubMed] [Google Scholar]
- Ma DK, Chiang CH, Ponnusamy K, Ming GL, and Song H (2008). G9a and Jhdm2a regulate embryonic stem cell fusion-induced reprogramming of adult neural stem cells. Stem cells 26, 2131–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma DK, Jang MH, Guo JU, Kitabatake Y, Chang ML, Pow-Anpongkul N, Flavell RA, Lu B, Ming GL, and Song H (2009). Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science 323, 1074–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma DK, Ming GL, and Song H (2005). Glial influences on neural stem cell development: cellular niches for adult neurogenesis. Curr Opin Neurobiol 15, 514–520. [DOI] [PubMed] [Google Scholar]
- Mills EA, Davis CH, Bushong EA, Boassa D, Kim KY, Ellisman MH, and Marsh-Armstrong N (2015). Astrocytes phagocytose focal dystrophies from shortening myelin segments in the optic nerve of Xenopus laevis at metamorphosis. Proc Natl Acad Sci U S A 112, 10509–10514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming GL, and Song H (2011). Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70, 687–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mira H, Andreu Z, Suh H, Lie DC, Jessberger S, Consiglio A, San Emeterio J, Hortiguela R, Marques-Torrejon MA, Nakashima K, et al. (2010). Signaling through BMPR-IA regulates quiescence and long-term activity of neural stem cells in the adult hippocampus. Cell Stem Cell 7, 78–89. [DOI] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, and Luo L (2007). A global double-fluorescent Cre reporter mouse. Genesis 45, 593–605. [DOI] [PubMed] [Google Scholar]
- Nagata S, Hanayama R, and Kawane K (2010). Autoimmunity and the clearance of dead cells. Cell 140, 619–630. [DOI] [PubMed] [Google Scholar]
- Neher JJ, Emmrich JV, Fricker M, Mander PK, Thery C, and Brown GC (2013). Phagocytosis executes delayed neuronal death after focal brain ischemia. Proc Natl Acad Sci U S A 110, E4098–4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik JH, Ding Z, Narurkar R, Ramkissoon S, Muller F, Kamoun WS, Chae SS, Zheng H, Ying H, Mahoney J, et al. (2009). FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell Stem Cell 5, 540–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollen AA, Nowakowski TJ, Chen J, Retallack H, Sandoval-Espinosa C, Nicholas CR, Shuga J, Liu SJ, Oldham MC, Diaz A, et al. (2015). Molecular Identity of Human Outer Radial Glia during Cortical Development. Cell 163, 55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcheri C, Suter U, and Jessberger S (2014). Dissecting integrin-dependent regulation of neural stem cell proliferation in the adult brain. J Neurosci 34, 5222–5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond A, Ensslin MA, and Shur BD (2009). SED1/MFG-E8: a bi-motif protein that orchestrates diverse cellular interactions. J Cell Biochem 106, 957–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault VM, Rafalski VA, Morgan AA, Salih DA, Brett JO, Webb AE, Villeda SA, Thekkat PU, Guillerey C, Denko NC, et al. (2009). FoxO3 regulates neural stem cell homeostasis. Cell Stem Cell 5, 527–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A, Sunayama J, Matsuda K, Tachibana K, Sakurada K, Tomiyama A, Kayama T, and Kitanaka C (2010). Regulation of neural stem/progenitor cell maintenance by PI3K and mTOR. Neurosci Lett 470, 115–120. [DOI] [PubMed] [Google Scholar]
- Seib DR, Corsini NS, Ellwanger K, Plaas C, Mateos A, Pitzer C, Niehrs C, Celikel T, and Martin-Villalba A (2013). Loss of Dickkopf-1 restores neurogenesis in old age and counteracts cognitive decline. Cell stem cell 12, 204–214. [DOI] [PubMed] [Google Scholar]
- Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, Roysam B, and Temple S (2008). Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell 3, 289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J, Berg DA, Zhu Y, Shin JY, Song J, Bonaguidi MA, Enikolopov G, Nauen DW, Christian KM, Ming GL, et al. (2015). Single-Cell RNA-Seq with Waterfall Reveals Molecular Cascades underlying Adult Neurogenesis. Cell Stem Cell 17, 360–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Stevens CF, and Gage FH (2002). Astroglia induce neurogenesis from adult neural stem cells. Nature 417, 39–44. [DOI] [PubMed] [Google Scholar]
- Song J, Zhong C, Bonaguidi MA, Sun GJ, Hsu D, Gu Y, Meletis K, Huang ZJ, Ge S, Enikolopov G, et al. (2012). Neuronal circuitry mechanism regulating adult quiescent neural stem-.cell fate decision. Nature 489, 150–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun GJ, Zhou Y, Ito S, Bonaguidi MA, Stein-O’Brien G, Kawasaki NK, Modak N, Zhu Y, Ming GL, and Song H (2015a). Latent tri-lineage potential of adult hippocampal neural stem cells revealed by Nf1 inactivation. Nat Neurosci 18, 1722–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun GJ, Zhou Y, Stadel RP, Moss J, Yong JH, Ito S, Kawasaki NK, Phan AT, Oh JH, Modak N, et al. (2015b). Tangential migration of neuronal precursors of glutamatergic neurons in the adult mammalian brain. Proceedings of the National Academy of Sciences of the United States of America 112, 9484–9489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Yang M, Guo H, Yang L, Wu J, Li R, Liu P, Lian Y, Zheng X, Yan J, et al. (2013). Single-cell RNA-Seq profiling of human preimplantation embryos and embryonic stem cells. Nat Struct Mol Biol 20, 1131–1139. [DOI] [PubMed] [Google Scholar]
- Yuzwa SA, Borrett MJ, Innes BT, Voronova A, Ketela T, Kaplan DR, Bader GD, and Miller FD (2017). Developmental Emergence of Adult Neural Stem Cells as Revealed by Single-Cell Transcriptional Profiling. Cell Rep 21, 3970–3986. [DOI] [PubMed] [Google Scholar]
- Zeisel A, Munoz-Manchado AB, Codeluppi S, Lonnerberg P, La Manno G, Jureus A, Marques S, Munguba H, He L, Betsholtz C, et al. (2015). Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science 347, 1138–1142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Shown is a confocal image of immunostaining for DAPI (blue), GFP (green), Mfge8 (red), and Mcm2 (white) in a Nestin-GFP reporter mouse. Note there is one Mcm2+GFP+ activated RGL (the furthest left) that was Mfge8−. There are three Mcm2−GFP+Mfge8+ quiescent RGLs (right). Also shown is a surface rendering of Mfge8 immunostaining (red).