Abstract
Immune checkpoint inhibitors and monoclonal antibodies re-invigorate cancer immunotherapy. However, these immunotherapies only benefit a subset of patients. We previously reported that ALDHhigh tumor cells were highly enriched for cancer stem cells (CSCs), and ALDHhigh CSC lysate-pulsed dendritic cell (CSC-DC) vaccine was shown to induce CSC-specific CTLs. In this study, we investigated the CSC targeting effect of the CSC-DC vaccine combined with a dual blockade of PD-L1 and CTLA-4 in B16-F10 murine melanoma tumor model. Our data showed that animals treated with the dual blockade of PD-L1 and CTLA-4 and CSC-DC vaccine conferred significantly more tumor regression than the CSC-DC vaccine alone. Importantly, the triple combination treatment dramatically eliminated ALDHhigh CSCs in vivo. We observed that CSC-DC vaccine in combination with anti-PD-L1 and anti-CTLA-4 administration resulted in ~1.7-fold fewer PD-1+CD8+ T cells and ~2.5-fold fewer CTLA-4+CD8+ T cells than the populations observed following the CSC-DC vaccination alone. Moreover, significant antitumor effects and dramatically eliminated ALDHhigh CSCs following the triple combination treatment were accompanied by significantly enhanced T cell expansion, suppressed TGF-β secretion, enhanced IFN-γ secretion, and significantly enhanced host specific CD8+ T cell response against CSCs. Collectively, these data demonstrated that administration of a-PD-L1 and a-CTLA-4 combined with CSC-DC vaccine may represent an effective immunotherapeutic strategy for cancer patients in clinical.
Keywords: cancer stem cells, vaccine, PD-L1, CTLA-4, immunotherapy
Introduction
A particular subpopulation of cancer cells characterized by self-renewing ability and the capacity to initiate, replenish and expand human tumors, has been reported as cancer stem cells (CSCs) 1–3. Cancer stem cells have been isolated from different human solid tumors including melanoma 1, 4. The frequency of human melanoma cancer stem cells can reach up to 27% 4. Based on the cancer stem cell theory, targeting cancer stem cells should impair tumor growth and decrease the risk of tumor relapse and progression. It has been established that cancer stem cells express several tumor-associated antigens (TAA) that can be recognized by cytotoxic T lymphocytes (CTLs) 5, 6. We previously demonstrated that ALDHhigh murine squamous carcinoma SCC7 and D5 melanoma cells are highly enriched for cancer stem cells, and the induction of CTLs to cancer stem cells has been observed using ALDHhigh CSC lysate-pulsed dendritic cell (CSC-DC) vaccination7. Recently, accumulating studies have demonstrated that CSC-DC can induce anti-CSC humoral as well as cellular immune responses, resulting in efficient antitumor immunity8–11. These studies have clearly suggested that the potential efficacy of CSC-DC vaccine in targeting cancer stem cells is reliable. However, in general, the effectiveness of cancer vaccines has been less robust than initially expected 12. A recent meta-analysis showed objective response rates of 8.5% in 1205 advanced melanoma patients treated with DC vaccination 13.
One of the reasons for the lack of efficacy of cancer vaccines is that suppression immune checkpoints (e.g. CTLA-4, PD-1, PD-L1, and others) inhibit vaccine-induced CTL responses14. In the past few years, the successful use of checkpoint inhibitor (CPI) blockade has re-invigorated the field of immunotherapy in cancer. CTLA-4 blockade with ipilimumab represented the first checkpoint inhibition approved by the FDA for the treatment of metastatic melanoma 15. More recently, applications of the inhibitors of PD-1 and its ligand (PD-L1) by monoclonal antibodies nivolumab and pembrolizumab (anti-PD-1) and atezolizumab (anti-PD-L1) have achieved great success 16. The prevailing guidelines recommend either anti-PD-1 monotherapy or nivolumab and ipilimumab combination therapy as the standard first-line treatment for unresectable metastatic melanoma based on two randomized trials 17, 18. However, while some patients exhibited partial or even complete responses to CPI therapy, many patients remained unresponsive 19. The lack of a response in 40–80% of the patients not benefiting from CPIs are partly related to the characters of tumor-infiltrating lymphocytes (TILs) 20. Obviously, both cancer vaccines and CPIs have shortcomings by themselves. Theoretically, cancer vaccines may generate tumor-specific T cells, which could be enhanced by CPIs that counteract the immune inhibitory mechanisms. To test this hypothesis, we focused on targeting cancer stem cells using the CSC-DC vaccine in this study while simultaneously blocked CTLA-4 and PD-L1 to investigate the effects of the combined cancer immunotherapy utilizing the B16–F10 murine melanoma model.
Materials and Methods
Cell lines and mice
B16-F10 melanoma cells were obtained from the ATCC (catalog CRL-6475, Shanghai, China) and maintained in RPMI 1640 (Gibco, Invitrogen, USA) containing 10% fetal bovine serum (FBS) (Gibco, Invitrogen, USA), 100 U/ml penicillin and 100 μg/mL streptomycin (Sigma-Aldrich China, Beijing, China). Cells were cultured at 37°C with 95% humidity and 5% CO2. Six to eight week-old C57BL/6 (B6) female mice were purchased from Wuhan University Animal Center (Wuhan, China) and maintained under specific pathogen-free conditions in Huazhong University of Science and Technology Animal Facilities. All experiments were approved by the Institutional Animal Care and Use Committee, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, and conducted under appropriate supervision.
ALDEFLUOR assay
It was demonstrated that ALDH was involved in maintaining the subpopulation of cancer stem-like cells in B16–F10 melanoma cells21. It has been established that the Aldefluor® kit (Stem Cell Technologies) can be used to isolate stem cells with high ALDH activity in many cancer types. In melanoma, we7 and Luo Y, et al22 reported that the sorted population with high ALDH activity was enriched for melanoma stem cells. The ALDEFLUOR kit (Stem Cell Technologies, Vancouver, Canada) was used to isolate cancer stem cells expressing high levels of ALDH from the B16–F10 melanoma cells. For the assay, briefly, cells were suspended in Aldefluor® assay buffer containing BODIPY-aminoacetaldehyde and incubated at 37°C for 30 minutes. Control samples were incubated with the buffer containing 50mM diethylaminobenzaldehyde (DEAB), an ALDH inhibitor. Cell sorting was conducted using a FACSAria flow cytometer with FACSDiva software (BD Immunocytometry). The Aldefluor® staining was detected using the FITC channel. To prevent cross-contamination between ALDHhigh and ALDHlow cells, sorting gates of these 2 populations were set up at least one log apart. The purity of sorted populations was re-analyzed using ALDHhigh and ALDHlow cells and was shown to be greater than 95% ALDHhigh and ALDHlow respectively.
Preparation of ALDHhigh cancer stem cell lysate-pulsed DC vaccination
Tumor cell lysates of ALDHhigh B16–F10 cells and bone marrow-derived murine DCs were prepared as we previously described 7. Briefly, tumor cell lysates of unsorted and ALDHhigh B16–F10 cells were frozen and thawed 3 times to make cell lysate. Bone marrow-derived DCs were cultured in complete medium supplemented with 20 ng/mL IL-4 and 20 ng/mL GM-CSF on day 0. Fresh medium supplemented with 20 ng/mL GM-CSF was added every two days. On day 10, DCs were harvested by dispenser and enriched by Opti-Prep density gradient medium. The lysate of ALDHhigh cells was added to DCs at a 1:3 cell equivalent ratio. The DCs were then incubated at 37°C for 24 h with 5% CO2. After incubation, CSC-DC was used as a vaccine as specified in the subsequent experiments.
Tumor model
B6 mice were inoculated subcutaneously (s.c.) with 0.5 million B16-F10 cells on day 0. The animals were divided into 6 groups (n=5). One group of mice was administered with PBS; one group of mice was administered with unsorted tumor cell lysate-DC vaccine (H-DC), and four groups of mice were administered with CSC-DC vaccine 3 days after B16-F10 cell inoculation. The vaccination was repeated on days 6 and 9. Each mouse was inoculated s.c. with 1 million DCs per vaccine. In the CSC-DC vaccination groups, 100 μg anti-CTLA-4 (clone 9D9, Bio X Cell, West Lebanon, NH) and/or anti-PD-L1 (clone10F.9G2, Bio X Cell, West Lebanon, NH) or control rat immunoglobulin (IgG) were administered intraperitoneally, on days 3, 6, and 9 following the B16-F10 cell inoculation. Tumor size were recorded three times per week by measuring the width and length diameters of the tumors.
Immunophenotyping of the splenocytes
Spleens were harvested from the treated animals. Splenocytes were prepared by mashing the spleens with 70-μm strainers. Red blood cells were lysed with red blood cell lysis buffer. CD3+ T cells were sorted by fluorescence-activated cell sorting (FACS). To identify the immunophenotypies of the T splenocytes, sorted CD3+ T cells were directly stained with antibodies for phenotype characterization by flow cytometry. PE-conjugated anti-CTLA-4 and PE-conjugated anti-PD-1 were used in conjunction with FITC-conjugated anti-CD8. All antibodies were obtained from eBioscience (San Diego, California, USA).
T cell activation
CD3+T splenocytes harvested from the treated animals were activated by anti-CD3/anti-CD28 followed by IL-2 expansion as we previously described7, 11. T cell proliferation was detected by a WST-8 Cell Counting Kit-8 (Beyotime C0037, Wuhan, China) according to the manufacturer’s protocol. Briefly, a 100 μl activated T cell suspension from each group was transferred into a 96-well plate. Next, 20 μl CCK-8 solution was added to each well for a 2-h incubation period at 37°C. The absorbance was then measured using a microplate reader (Bio-Rad, CA, USA) at 450 nm with a reference wavelength of 650 nm. Each sample was performed in triplicate.
Cytokine production
Cytokines produced by activated/expended splenic T cells into the supernatants of cell culture were detected by ELISA. Commercially available ELISA kits specific for mouse IFN-γ, TGF-β, and IL-10 (all from R&D Systems, MN, USA) were used as indicated by the manufacturer’s protocols.
CTL cytotoxicity
CTLs were generated from the splenocytes harvested from the treated animals by anti-CD3/anti-CD28 activation and IL-2 expansion as described above. CTL-mediated cancer stem cell cytotoxicity was tested using the lactate dehydrogenase (LDH) Release Assay (CytoTox 96 Non-Radioactive Cytotoxicity Assay, Promega, Madison, WI) according to the manufacturer’s protocol. Briefly, the control and experimental wells were set up in a round 96-well culture plate. In the experimental wells, effector cells (CTLs) and target cells (ALDHhigh B16-F10 cells) with different ratios were incubated at 37°C for 4 hours. Then, LDH measurements were performed according to the protocol. The reality of cytotoxicity was calculated as follows:
Statistical analysis
Statistical analyses were performed using Graph Pad Prism 5.0 software (GraphPad Software Inc., San Diego, CA). Data were evaluated by unpaired Student’s t-test or one-way analysis of variance. The statistical significance was set at p <0.05.
Results
1. CSC-DC vaccination combined with immune checkpoint blockades promoted tumor eradication
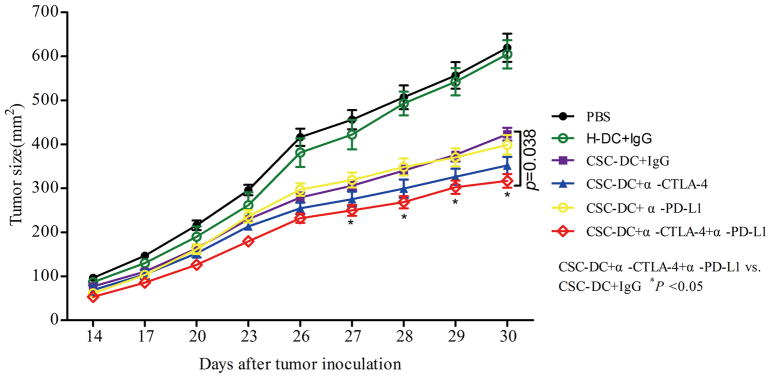
We previously demonstrated that CSC-DC vaccines could induce more effective antitumor immunity than H-DCs 7. In this setting, we tested whether simultaneous blockade of PD-L1 and/or CTLA-4 with CSC-DC vaccine could induce stronger antitumor immunity than CSC-DC vaccine alone. As shown in Fig.1, compared with PBS and H-DC-treated mice, CSC-DC vaccination significantly inhibited tumor growth, which corroborated our previous observations7. While we failed to observe the combination of either anti-PD-L1 or anti-CTLA-4 with CSC-DC vaccinations to induce more significant tumor regression than CSC-DC vaccination alone, we did find that the triple combination (CSC-DC vaccination/anti-PD-L1/anti-CTLA-4) treatment induced far more significant tumor regression than CSC-DC vaccination alone (p=0.038). These experiments clearly indicated that simultaneous PD-L1 and CTLA-4 blockades could significantly boost the antitumor immune response induced by CSC-DC vaccination in the B16-F10 melanoma model.
Fig. 1.
Inhibition of tumor growth by CSC-DC vaccination combined with immune checkpoints blockade. B6 mice were inoculated subcutaneously with 0.5 million B16-F10 cells on day 0 and treated with H-DC or CSC-DC vaccine on day 3. The vaccination was repeated on days 6 and 9. Anti-CTLA-4, anti-PD-L1, or control IgG were administered intraperitoneally in some of the groups as indicated on days 3, 6, and 9 following tumor inoculation. Tumor size in each group of mice treated as indicated (n=5). CSC-DC vaccination, ALDHhigh CSC lysate-pulsed dendritic cell vaccination. H-DC vaccination, unsorted tumor cell lysate-pulsed dendritic cell vaccination.
2. CSC-DC vaccination combined with immune checkpoint blockades enhanced the elimination of ALDHhigh CSCs in vivo
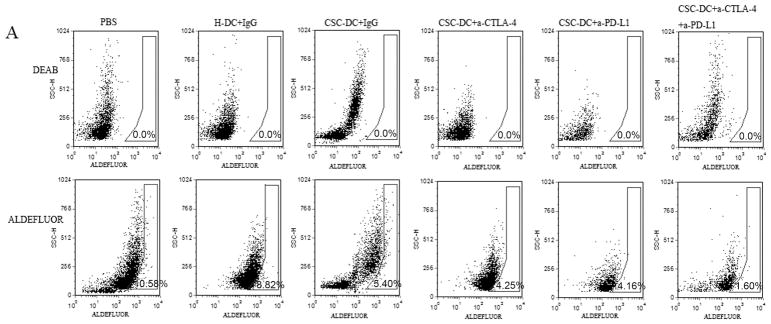
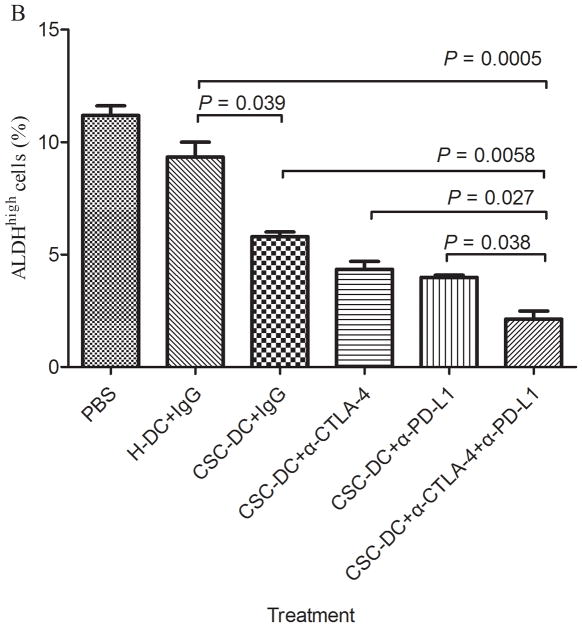
To confirm that cancer stem cells were effectively targeted by CSC-DC vaccination combined with PD-L1/CTLA-4 blockades, we determined the number of ALDHhigh CSCs in the residual tumors after treatments. The results are shown in Fig. 2. The CSC-DC vaccination-treated mice contained (5.8±0.4)% ALDHhigh cells, which was significantly less than the number in the H-DC-treated mice (9.3±0.5)% and the PBS-treated mice (11.2±0.7)%. The combination treatment of CSC-DC vaccinations with anti-PD-L1 or anti-CTLA-4 or the triple combination treatment significantly reduced the number of ALDHhigh cells in the residual tumor of the treated mice to (3.9±0.2)%, (4.4±0.6)%, and (2.1±0.6)%, respectively. Of note, the dual blockade of PD-L1 and CTLA-4 combined with CSC-DC vaccinations dramatically eliminated the ALDHhigh cells in vivo compared with CSC-DC (p=0.0058) or CSC-DC combined with anti-PD-L1 (p=0.0038) or CSC-DC combined with anti-CTLA-4 (p=0.0027), respectively.
Fig. 2.
CSC-DC vaccination combined with immune checkpoints blockade significantly enhanced the elimination of ALDHhigh CSCs. A, FACS plots showing the percentage of ALDHhigh CSCs in the residual tumors of mice subjected to treatments as indicated. Single-cell residual tumor suspensions were prepared from tumors on day 30 and stained for ALDEFLUOR. B, The percentage and statistical analyses of ALDHhigh CSCs in residual tumors of mice treated as indicated. Each column represents the mean±SE of three independent experiments performed.
3. CSC-DC vaccination combined with immune checkpoints blockade enhanced the proliferation of functional CD8+ T cells
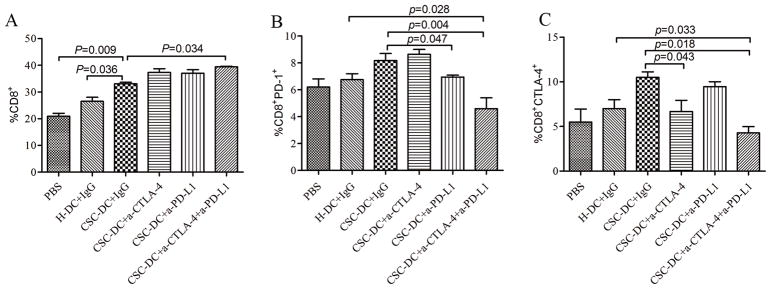
To understand the effects of the CSC-DC vaccine combined with anti-PD-L1/anti-CTLA-4 in vivo, we analyzed the immunological characteristics of T cells from spleens in each treated group of mice at the end of the experiment (day 30). CSC-DC vaccination induced a preferential expansion of CD8+ T cells (~35% of the splenocytes ), which was higher than that of the PBS group and the H-DC vaccination group (p<0.05). Importantly, while the CD8+ T cells of mice treated with the CSC-DC vaccine in combination with anti-PD-L1 or anti-CTLA-4 respectively showed a similar percentage of CD8+ T cells in the spleen of mice as those vaccinated with CSC-DC alone (Fig. 3A), the CD8+ T cells of mice treated with the CSC-DC vaccine in combination with both anti-PD-L1 and anti-CTLA-4 revealed a significant (p=0.034) higher percentage of CD8+ T cells than those in the spleen of mice vaccinated with CSC-DC alone.
Fig. 3.
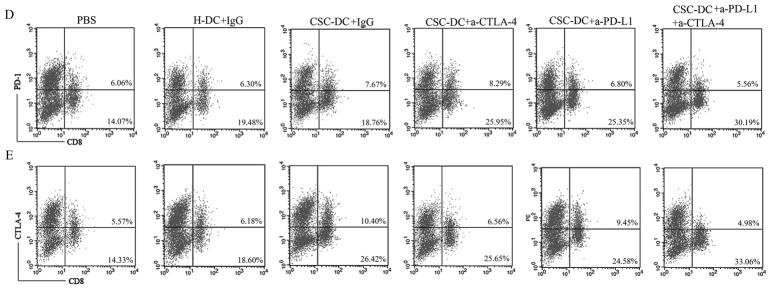
CSC-DC vaccination combined with immune checkpoints blockade changed the phenotype of CD8+ T cells. A, B, C: The percentage of CD8+ T cells (A), PD-1+CD8+ T cells (B) and CTLA-4+CD8+ T cells (C) in the spleens harvested from mice treated with PBS, H-DC vaccines, CSC-DC vaccines alone or in combination with anti-CTLA-4 and/or anti-PD-L1 as indicated. Each column represents the mean±SE of three independent experiments performed. D, E: FACS plots representing PD-1+CD8+ T cell (D) and CTLA-4+CD8+ T (E) populations in the spleens of mice treated as indicated. Single-cell suspensions were prepared from spleens on day 30 after tumor challenge and stained for immune checkpoints, PD-1 and CTLA-4.
A number of studies have indicated that melanoma induces T cell exhaustion in the tumor microenvironment 23, 24. It is now well established that higher and sustained expression of inhibitory receptors (such as CTLA-4, PD-1) is a hallmark of exhausted T cells 25. To determine the exhausted T cells in the CSC-DC vaccine treatment, CTLA-4+CD8+ T cell and PD-1+CD8+ T cell populations were evaluated in the spleens of each treated group of mice as indicated. This analysis revealed that compared with the populations observed in the H-DC vaccination group, CSC-DC vaccination induced ~1.2-fold (8.18% vs. 6.75%) more PD-1+CD8+ T cells (Fig.3B 3D) and ~1.5-fold (10.50% vs. 7.00%) more CTLA-4+CD8+T cells (Fig. 3C, 3E). It was interesting that CSC-DC vaccination combined with anti-PD-L1 or anti-CTLA-4 respectively resulted in lower PD-1+CD8+ T cells (p=0.047) and CTLA-4+CD8+ T cells (p=0.043) than CSC-DC vaccination alone. More importantly, triple combination treatment resulted in ~2.5-fold (4.27% vs. 10.50%) significantly (p=0.018) fewer CTLA-4+CD8+T cells than the populations observed in CSC-DC vaccination alone (Fig. 3C, 3E). Similarly, the triple combination treatment resulted in ~1.7-fold (4.60% vs. 8.18 %) significantly (p=0.004) fewer PD-1+CD8+T cells than the populations observed in CSC-DC vaccination alone (Fig. 3B, 3D).
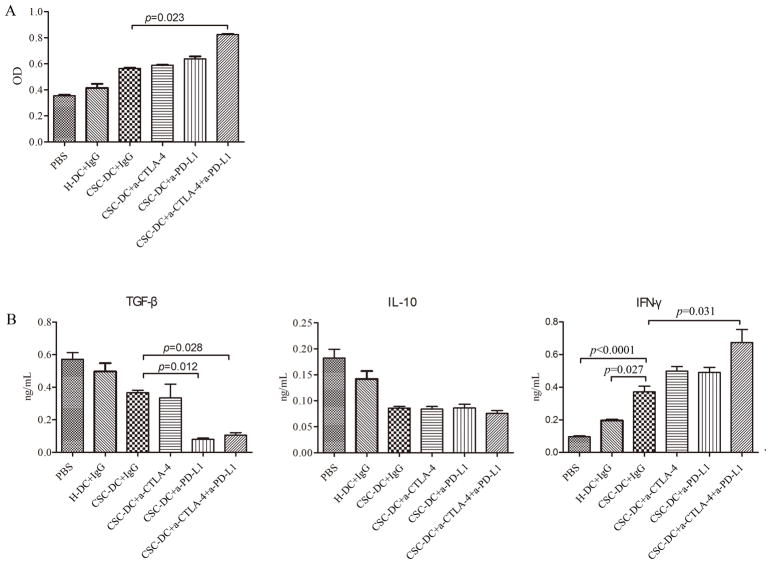
We also performed T cell proliferation assay in vitro. The T cell proliferation was then analyzed by a WST-8 Cell Counting Kit-8. In this assay, the triple combination treatment induced a significant (p=0.023) increase in the proliferation of T cells compared with the proliferation observed in CSC-DC vaccine alone group (Fig. 4A), even though CSC-DC vaccination combined with anti-PD-L1 or anti-CTLA-4 respectively resulted in a minimum increase in T cell proliferation compared to CSC-DC vaccination alone.
Fig. 4.
CSC-DC vaccination combined with immune checkpoints blockade significantly enhanced the proliferation of T cells and IFNγ production, but significantly reduced the production of TGF-β. A, The proliferation of splenic T cells from mice treated with PBS, H-DC vaccines, and CSC-DC vaccines alone or in combination with anti-CTLA-4 and/or anti-PD-L1. Splenic T cells were obtained on days 30 after tumor challenge. CD3+ T cells were sorted and activated in vitro with anti-CD3/anti-CD28 followed by expansion with IL-2. The T cell proliferation was then analyzed using the WST-8 Cell Counting Kit-8. B, The function of the T cells was characterized by measuring their IFN-γ, TGF-β, and IL-10 production. Each column represents the mean±SE of three independent experiments performed.
The immune activity of the T cell proliferation was characterized by measuring IFN-γ, TGF-β, and IL-10 cytokines produced by these activated and expended splenic T cells into the culture supernatants. As showed in Fig. 4B, TGF-β secretion was suppressed following CSC-DC vaccination and was further suppressed after the triple combination treatment (p=0.028). Furthermore, IL-10 secretion was also decreased following CSC-DC vaccination and remained decreased after the combination treatments. In contrast, IFN-γ secretion was significantly augmented following CSC-DC vaccination and was further elevated after the triple combination treatment (p=0.031). Together, these data suggested that the immune checkpoints PD-L1 and CTLA-4 blockades may reverse the exhausted T cells induced by CSC-DC vaccine.
4. CSC-DC vaccination combined with immune checkpoints blockade enhanced host CSC-specific CTL activity
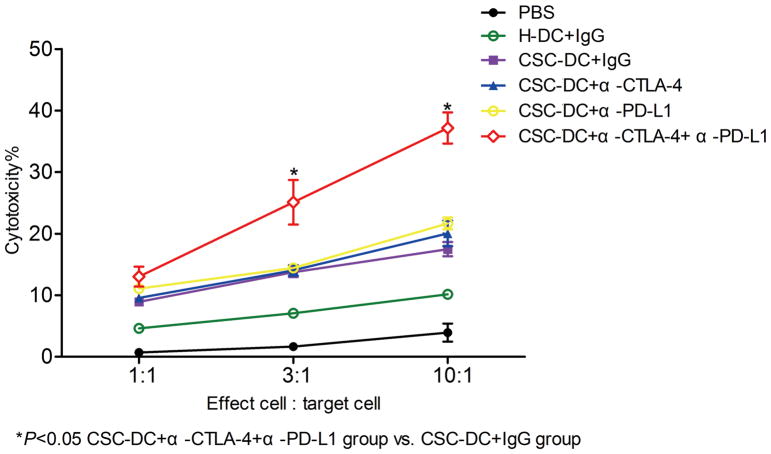
We further examined the ability of CSC-DC vaccination combined with anti-PD-L1/anti-CTLA-4 to induce CSC-specific CTL activity in vitro. Cytotoxic T cells from spleens of treated mice were generated as described above. Target cells were sorted B16-F10 ALDHhigh cells. As in Fig. 5, CTLs from the triple combination treatment group mediated significantly greater cytotoxicity against the B16-F10 ALDHhigh cells at high E:T ratios (3:1 and 10:1) compared with the CTLs generated from PBS, H-DC, CSC-DC, and CSC-DC combined with anti-PD-L1 or anti-CTLA-4 (p<0.05). We previously demonstrated that CSC-DC vaccine induced more effective antitumor immunity than H-DC 7. In the present study, we observed that CTLs generated from mice subjected to CSC-DC vaccination induced stronger cytotoxicity than CTLs generated from the H-DC vaccination group, which corroborated our previous observation 7. While CTLs generated from the splenocytes of mice subjected to CSC-DC vaccination combined with anti-PD-L1 or anti-CTLA-4 respectively failed to enhance the cytotoxicity compared with CTLs generated from the CSC-DC vaccination alone group (Fig. 5), CSC-DC vaccination plus PD-L1 as well as CTLA-4 blockades triple combination treatment conferred significantly stronger host CTL responses against ALDHhigh CSCs than the CSC-DC vaccination alone or its combination with a single immune checkpoint e.g. PD-L1 or CTLA-4 blockade.
Fig. 5.
CSC-DC vaccination combined with immune checkpoints blockade significantly enhanced host CSC-specific CTL activity. The procedure for generating cytotoxic T cells from the spleens was described in the Methods section. Target cells were sorted B16-F10 ALDHhigh cells. CTLs and target cells were incubated at ratios of 1:1, 3:1, and 10:1 as indicated. The cytotoxicity mediated by CTLs was measured by an LDH release assay.
Discussion
Various melanoma vaccines have been explored26, 27. However, limited success has been achieved, particularly in the metastatic setting. With further understanding of the melanoma tumor microenvironment (TME), it is becoming clear that immune checkpoints play an important role in T cell exhaustion in TME. For example, CTLA-4 engagement suppresses T cell activation by blocking T cell costimulation, whereas binding of PD-1 with its ligands PD-L1 and PD-L2 inhibits T cell activity by promoting anergy, death, or exhaustion in tumor28, 29. The recent successes of CPIs e.g. anti-CTLA-4 and anti-PD-1 are promising. However, a significant number of the patients are not benefiting from CPIs, which makes it necessary to pursue treatment together with other strategies, such as a combination of cancer vaccines with CPIs 20.
Targeting cancer stem cells may impair tumor growth and decrease the risk of tumor relapse and progression1, 30. We focused on CSCs as a target of cancer immunotherapy to achieve more efficient anti-tumor responses. In this study, we illustrated that blockade of both PD-L1 and CTLA-4 pathways significantly enhanced CSC-DC vaccine-induced host immune responses against CSCs in vivo. Elucidating the mechanism underlying these effects indicated that such combination therapy enhanced the CD8+ T cell population and reversed T cell exhaustion, ultimately enhancing host CSC-specific CTL activity.
Several studies have demonstrated successful use of cancer vaccines in combination with two CPIs (α-CTLA-4 and α-PD-L1/α-PD-1) 31–35. However, our study provides the first evidence that cancer vaccines in combination with CPIs could reduce the number of CSCs due to the use of a CSC-targeted vaccine in melanoma. In our previously study, we demonstrated that ALDHhigh tumor cells were highly enriched in CSCs, and ALDHhigh CSC-DC vaccines could induce more effective antitumor immunity than DCs pulsed with the lysate of unsorted tumor cells (H-DC) or sorted ALDHlow cells. In addition, we showed in several reports that CSC-DC vaccine-conferred antitumor immunity was associated with CSC-DC vaccine-induced anti-ALDHhigh CSCs vs. ALDHlow non-CSCs7, 8, 11. The present study showed that α-PD-L1 or α-CTLA-4 alone failed to enhance the antitumor effects of the CSC-DC vaccines in melanoma. In contrast, we reported that murine squamous carcinoma SCC7 CSC-DC vaccine limited local tumor recurrence and spontaneous pulmonary metastasis, with increased host survival, which was further accentuated by simultaneous PD-L1 blockade11. These studies suggested that the efficacy of cancer vaccine in combination with CPIs is tumor model dependent, and is also dependent on the dosages of the CPIs tested.
Curran, M A et al. demonstrated that the combination of αCTLA-4 and αPD-1 is more than twice as effective in promoting the rejection of B16 melanoma as either alone or in conjunction with Fvax vaccines, and that addition of αPD-L1 elevated the rate of tumor-free survival from this triple combination treatment 34. On the other hand, the duration time after tumor inoculation may affect the observation of antitumor effects. Our results indicated that the longer after tumor inoculation, the stronger the antitumor effects were observed. The observation time of our study was 30 days after tumor inoculation, and the survival rate was not observed, which was a limitation of this study.
It has been established that T cells are exhausted after long-term exposure to the tumor antigens in melanoma 36. The B7/CTLA-4 and PD-1/PD-L1 pathways mediate T cell exhaustion by antagonizing activation signaling pathways 25. Furthermore, the PD-1 and CTLA-4 pathways operate at different stages of the immune responses 37. Spurred by the impressive effects of the combination of CTLA-4 and PD-1 blockade in melanoma 38, a large number of immunotherapy combinations, such as combining different CPIs or CPIs with a therapeutic cancer vaccine, are now being clinically evaluated. We combined α-CTLA-4 and α-PD-L1 due to the following considerations: First, the CTLA-4-blockade induces a proliferative signature predominantly in a subset of transitional memory T cells 39, while the PD-L1-blockade decreases the number and/or the suppressive activity of regulatory T cells and rescues the activity of effector T cells40. Second, the PD-L1-blockade decreases the percentages of the highly immunosuppressive myeloid-derived suppressor cells 41. Third, PD-L1 mRNA and protein can be upregulated by cytokines produced by TILs, such as IFN-γ 42. Fourth, melanoma-PD-1:PD-L1 interactions promote tumor growth 43, and PD-L1 expression is an independent, poor prognostic factor for malignant melanoma 44. In this study, combined α-CTLA-4/α-PD-L1 and CSC-DC vaccine have shown synergistic antitumor effects by targeting cancer stem cells.
Elucidating the mechanism underlying the anti-tumor effects in the present study indicated that combined α-CTLA-4/α-PD-L1 and CSC-DC vaccine enhanced the number of circulating functional CD8+T cells. Studies have shown that exhausted T cells upregulate IRs, including PD-1 and CTLA-4 23. We investigated whether our treatment had the ability to restore the exhausted T cells. We checked the phenotype profiles of T cells from the splenocytes. The data showed that the expressions of PD-1 and CTLA-4 on CD8+T cells were dramatically down-regulated in mice treated with the triple combination therapy. Additional data showed that the triple combination therapy induced the robust expansion of CD3+ T cells and enhanced the production of several immunoinhibitory cytokines, such as IFN-γ. We also found that combined α-CTLA-4/α-PD-L1 and CSC-DC vaccine-induced CTLs significantly killed the ALDHhigh CSCs compared with the CTLs induced by H-DC, or by CSC-DC combined with either α-CTLA-4 or α-PD-L1 alone. These results indicate that this triple combination therapy confers strong CTL activity that specifically target CSCs. In addition, exhausted T cells are characterized by a progressive loss of their functional capacities to proliferate, produce cytokines, and lyse upon chronic antigen exposure25. From this point of view, this triple combination therapy reversed T cell functions, which provides certain novel insights into the mechanisms of this combination therapy. The limitation of elucidating the mechanism underlying this combination therapy in the present study was that we didn’t illuminate the affection of the absolute number of CD8+ T cells. It will take a lot of time and effort to illuminate this issue. However, in our next experiment, we plant to quantify the tumor-infiltrating lymphocyte density both in the tumor parenchyma and at the invasive tumor margin at the endpoint.
In conclusion, our work suggests that administration of a-PD-L1 and a-CTLA-4 with CSC-DC vaccine could reverse T cell functions and induce more effective T cell activation, proliferation, as well as the function of CTLs targeting CSCs. Our study provides the scientific basis for a clinical trial that would involve the combination of CSC-DC vaccine and simultaneous PD-L1 and CTLA-4 blockades for sustained tumor control in patients with cancer.
Acknowledgments
The financial supports of this work are received from the National Nature Sciences Foundation of China (No. 81301954, No.81500639) and Hubei provincial health and family planning scientific research project (No. 2015060101010043). This work was also partly supported by the Gillson Longenbaugh Foundation and the University of Michigan MICHRGrant(UL1TR000433). We appreciate the English Language Service (American Journal Experts) who edit this manuscript.
Footnotes
Conflict of Interest
No conflict of interest exits in the submission of this manuscript.
References
- 1.Murphy GF, Wilson BJ, Girouard SD, et al. Stem cells and targeted approaches to melanoma cure. Mol Aspects Med. 2014;39:33–49. doi: 10.1016/j.mam.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clarke MF, Dick JE, Dirks PB, et al. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 3.Croker AK, Allan AL. Cancer stem cells: implications for the progression and treatment of metastatic disease. J Cell Mol Med. 2008;12:374–390. doi: 10.1111/j.1582-4934.2007.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quintana E, Shackleton M, Sabel MS, et al. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirohashi Y, Torigoe T, Inoda S, et al. Cytotoxic T lymphocytes: Sniping cancer stem cells. Oncoimmunology. 2012;1:123–125. doi: 10.4161/onci.1.1.18075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saijo H, Hirohashi Y, Torigoe T, et al. Cytotoxic T lymphocytes: the future of cancer stem cell eradication? Immunotherapy-Uk. 2013;5:549–551. doi: 10.2217/imt.13.44. [DOI] [PubMed] [Google Scholar]
- 7.Ning N, Pan Q, Zheng F, et al. Cancer Stem Cell Vaccination Confers Significant Antitumor Immunity. Cancer Res. 2012;72:1853–1864. doi: 10.1158/0008-5472.CAN-11-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu L, Tao H, Chang AE, et al. Cancer stem cell vaccine inhibits metastases of primary tumors and induces humoral immune responses against cancer stem cells. Oncoimmunology. 2015;4:e990767. doi: 10.4161/2162402X.2014.990767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan Q, Li Q, Liu S, et al. Concise Review: Targeting Cancer Stem Cells Using Immunologic Approaches. Stem Cells. 2015;33:2085–2092. doi: 10.1002/stem.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dashti A, Ebrahimi M, Hadjati J, et al. Dendritic cell based immunotherapy using tumor stem cells mediates potent antitumor immune responses. Cancer Lett. 2016;374:175–185. doi: 10.1016/j.canlet.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 11.Hu Y, Lu L, Xia Y, et al. Therapeutic Efficacy of Cancer Stem Cell Vaccines in the Adjuvant Setting. Cancer Res. 2016;76:4661–4672. doi: 10.1158/0008-5472.CAN-15-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kroemer G, Zitvogel L, Galluzzi L. Victories and deceptions in tumor immunology: Stimuvax(R) Oncoimmunology. 2013;2:e23687. doi: 10.4161/onci.23687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anguille S, Smits EL, Lion E, et al. Clinical use of dendritic cells for cancer therapy. Lancet Oncol. 2014;15:e257–e267. doi: 10.1016/S1470-2045(13)70585-0. [DOI] [PubMed] [Google Scholar]
- 14.Dillman RO. Is there a role for therapeutic cancer vaccines in the age of checkpoint inhibitors? Hum Vaccin Immunother. 2016:1–5. doi: 10.1080/21645515.2016.1244149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma W, Gilligan BM, Yuan J, et al. Current status and perspectives in translational biomarker research for PD-1/PD-L1 immune checkpoint blockade therapy. J Hematol Oncol. 2016;9:47. doi: 10.1186/s13045-016-0277-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larkin J, Hodi FS, Wolchok JD. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373:1270–1271. doi: 10.1056/NEJMc1509660. [DOI] [PubMed] [Google Scholar]
- 18.Coit DG, Thompson JA, Algazi A, et al. NCCN Guidelines Insights: Melanoma, Version 3.2016. J Natl Compr Canc Netw. 2016;14:945–958. doi: 10.6004/jnccn.2016.0101. [DOI] [PubMed] [Google Scholar]
- 19.Bu X, Mahoney KM, Freeman GJ. Learning from PD-1 Resistance: New Combination Strategies. Trends Mol Med. 2016;22:448–451. doi: 10.1016/j.molmed.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vreeland TJ, Clifton GT, Herbert GS, et al. Gaining ground on a cure through synergy: combining checkpoint inhibitors with cancer vaccines. Expert Rev Clin Immunol. 2016;12:1347–1357. doi: 10.1080/1744666X.2016.1202114. [DOI] [PubMed] [Google Scholar]
- 21.Contador-Troca M, Alvarez-Barrientos A, Merino JM, et al. Dioxin receptor regulates aldehyde dehydrogenase to block melanoma tumorigenesis and metastasis. Mol Cancer. 2015;14:148. doi: 10.1186/s12943-015-0419-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo Y, Dallaglio K, Chen Y, et al. ALDH1A isozymes are markers of human melanoma stem cells and potential therapeutic targets. Stem Cells. 2012;30:2100–2113. doi: 10.1002/stem.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fourcade J, Zarour HM. Strategies to reverse melanoma-induced T-cell dysfunction. Clin Dermatol. 2013;31:251–256. doi: 10.1016/j.clindermatol.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schatton T, Schutte U, Frank NY, et al. Modulation of T-Cell Activation by Malignant Melanoma Initiating Cells. Cancer Res. 2010;70:697–708. doi: 10.1158/0008-5472.CAN-09-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15:486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Lint S, Wilgenhof S, Heirman C, et al. Optimized dendritic cell-based immunotherapy for melanoma: the TriMix-formula. Cancer Immunol Immunother. 2014;63:959–967. doi: 10.1007/s00262-014-1558-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Rosa F, Ridolfi L, Ridolfi R, et al. Vaccination with autologous dendritic cells loaded with autologous tumor lysate or homogenate combined with immunomodulating radiotherapy and/or preleukapheresis IFN-alpha in patients with metastatic melanoma: a randomised “proof-of-principle” phase II study. J Transl Med. 2014;12:209. doi: 10.1186/1479-5876-12-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferris R. PD-1 targeting in cancer immunotherapy. Cancer-Am Cancer Soc. 2013;119:E1–E3. doi: 10.1002/cncr.27832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao F, He X, Sun J, et al. Cancer stem cell vaccine expressing ESAT-6-gpi and IL-21 inhibits melanoma growth and metastases. Am J Transl Res. 2015;7:1870–1882. [PMC free article] [PubMed] [Google Scholar]
- 31.Ali OA, Lewin SA, Dranoff G, et al. Vaccines Combined with Immune Checkpoint Antibodies Promote Cytotoxic T-cell Activity and Tumor Eradication. Cancer Immunol Res. 2016;4:95–100. doi: 10.1158/2326-6066.CIR-14-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lussier DM, Johnson JL, Hingorani P, et al. Combination immunotherapy with alpha-CTLA-4 and alpha-PD-L1 antibody blockade prevents immune escape and leads to complete control of metastatic osteosarcoma. J Immunother Cancer. 2015;3:21. doi: 10.1186/s40425-015-0067-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duraiswamy J, Kaluza KM, Freeman GJ, et al. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res. 2013;73:3591–3603. doi: 10.1158/0008-5472.CAN-12-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Curran MA, Montalvo W, Yagita H, et al. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107:4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hailemichael Y, Woods A, Fu T, et al. Cancer vaccine formulation dictates synergy with CTLA-4 and PD-L1 checkpoint blockade therapy. J Clin Invest. 2018;128:1338–1354. doi: 10.1172/JCI93303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fourcade J, Sun Z, Benallaoua M, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Das R, Verma R, Sznol M, et al. Combination therapy with anti-CTLA-4 and anti-PD-1 leads to distinct immunologic changes in vivo. J Immunol. 2015;194:950–959. doi: 10.4049/jimmunol.1401686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valsecchi ME. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373:1270. doi: 10.1056/NEJMc1509660. [DOI] [PubMed] [Google Scholar]
- 39.Fu J, Malm IJ, Kadayakkara DK, et al. Preclinical evidence that PD1 blockade cooperates with cancer vaccine TEGVAX to elicit regression of established tumors. Cancer Res. 2014;74:4042–4052. doi: 10.1158/0008-5472.CAN-13-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blackburn SD, Shin H, Freeman GJ, et al. Selective expansion of a subset of exhausted CD8 T cells by alphaPD-L1 blockade. Proc Natl Acad Sci U S A. 2008;105:15016–15021. doi: 10.1073/pnas.0801497105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dong Y, Sun Q, Zhang X. PD-1 and its ligands are important immune checkpoints in cancer. Oncotarget. 2017;8:2171–2186. doi: 10.18632/oncotarget.13895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abiko K, Matsumura N, Hamanishi J, et al. IFN-gamma from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br J Cancer. 2015;112:1501–1509. doi: 10.1038/bjc.2015.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kleffel S, Posch C, Barthel SR, et al. Melanoma Cell-Intrinsic PD-1 Receptor Functions Promote Tumor Growth. Cell. 2015;162:1242–1256. doi: 10.1016/j.cell.2015.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hino R, Kabashima K, Kato Y, et al. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer-Am Cancer Soc. 2010;116:1757–1766. doi: 10.1002/cncr.24899. [DOI] [PubMed] [Google Scholar]