Abstract
Neuroinflammation plays an important role in the pathogenesis of various neurodegenerative diseases including Alzheimer’s disease (AD). Suppressor of cytokine signaling 3 (SOCS3) is an anti-inflammatory molecule that suppresses cytokine signaling and inflammatory gene expression in different cells including microglia. However, pathways through which SOCS3 could be upregulated are poorly described. Cinnamic acid is a metabolite of cinnamon, a natural compound that is being widely used all over the world as a spice or flavoring agent. This study delineates the importance of cinnamic acid for the upregulation of SOCS3 in microglia. Cinnamic acid upregulated the expression of SOCS3 mRNA and protein in mouse BV-2 microglial cells in dose- and time-dependent manner. Accordingly, cinnamic acid also increased the level of SOCS3 and suppressed the expression of inducible nitric oxide synthase and proinflammatory cytokines (TNFα, IL-1β and IL-6) in LPS-stimulated BV-2 microglial cells. Similar to BV-2 microglial cells, cinnamic acid also increased the expression of SOCS3 in primary mouse microglia and astrocytes. Presence of cAMP response element in the promoter of socs3 gene, activation of cAMP response element binding (CREB) by cinnamic acid, abrogation of cinnamic acid-mediated upregulation of SOCS3 by siRNA knockdown of CREB, and the recruitment of CREB to the socs3 gene promoter by cinnamic acid suggest that cinnamic acid increases the expression of SOCS3 by CREB. These studies suggest that cinnamic acid upregulates SOCS3 via CREB pathway, which may be of importance in neuroinflammatory and neurodegenerative disorders.
Keywords: Cinnamic acid, Glial cells, SOCS3, CREB, Neuroinflammation
Introduction
Neurodegenerative disorders are characterized by the loss of neurons of the motor, sensory or cognitive systems (1–6). One of the hallmarks of these diseases is neuroinflammation associated with the elevation of different proinflammatory molecules (2). Microglia are sensing cells of the CNS that respond quickly to various inflammatory and degenerative insults to express different proinflammatory mediators (cytokines, chemokines, enzymes, adhesion molecules, etc.) (7). Among these proinflammatory mediators, cytokines are very crucial for initiating as well as aggravating inflammatory response as cytokines themselves can induce other inflammatory mediators (8).
Fortunately, to tackle these proinflammatory cytokines we have been endowed with different anti-inflammatory molecules. Suppressor of Cytokine Signaling (SOCS) are a group of anti-inflammatory molecules that are known to induce the negative feedback for inhibiting cytokine-transduced signaling pathways (6). There are many partners of SOCS family like SOCS1, SOCS2, SOCS3, SOCS4, SOCS5, SOCS6, and SOCS7 (9). Each SOCS protein has a central SH2 domain and a carboxy-terminal 40-amino-acid region termed as the SOCS box. Moreover, SOCS3 also harbors a kinase inhibitory region in their N-terminal region. Recent studies have shown that SOCS3 not only inhibits the IL-6 signaling pathway via suppression of STAT3 but also functions as an agonist of the cAMP mediated pathway (10). Therefore, increase in SOCS3 is an important step to ameliorate and/or balance cytokine-mediated inflammation and identification of compounds for the upregulation of SOCS3 is an important avenue of research.
Cinnamon is a widely-used spice and flavoring material for food throughout the world. Ayurvedic and ancient medicines also used cinnamon as a traditional medicine for the treatment of variety of human disorders. The major constituent of cinnamon is cinnamaldehyde, which is oxidized to cinnamic acid. Here, we show that cinnamic acid is capable of upregulating SOCS3 and suppressing the expression of proinflammatory molecules in microglia. Furthermore, we demonstrate that cinnamic acid used CREB for the upregulation of SOCS3. Our study suggests that cinnamic acid may find its use as an anti-inflammatory agent via upregulation of SOCS3.
Materials and Methods
Reagents
DMEM/F-12 50/50 1x, Hank’s balanced salt solution (HBSS) and 0.05% trypsin were purchased from Mediatech (Washington, DC). Fetal bovine serum (FBS) was obtained from Atlas Biologicals (Fort Collins, CO). Antibiotic-antimycotic mixture and cinnamic acid were obtained from Sigma.
Isolation of Primary Mouse Astrocytes and Microglia
Microglia were isolated from mixed glial cultures according to the procedure of Giulian and Baker (11) with modifications as previously described by us (12–14). Briefly, on day 9, the mixed glial cultures were washed thrice with Dulbecco’s modified Eagle’s medium/F-12 and shaken at 240 rpm for 2 h at 37°C on a rotary shaker. The floating cells were washed, plated onto tissue culture dishes or flasks and incubated at 37 °C for 1 h. More than ninety-six percent of these cells were found to be positive for Mac-1. On day 11, mixed glial cells were washed and shaken again on a rotary shaker at 180 rpm for 18 h. Floating cells were removed and adherent cells were trypsinized and plated onto new dishes for experiments. More than ninety-eight percent of cells were positive for GFAP, a marker of astrocytes.
Mouse BV-2 microglial cells (kind gift from Virginia Bocchini of University of Perugia) were also cultured as described above.
Mass spectrometric analysis
A JEOL GCMate II (JEOL USA, Peabody MA) gas chromatograph/mass spectrometer was used for GC-MS as described earlier (12). Briefly, the gas chromatography column was a fused silica capillary column with a nonpolar 5% phenyl 95% dimethylpolysiloxane phase (Agilent HP-5ms), 30 meters long, 0.25 mm internal diameter, 0.25 um film thickness. The carrier gas was Helium (99.9995% Research Grade) run through a STG triple filter (Restek Corp.) at a constant flow rate of 1.1 ml/min. The injector was held at 275 Deg C and was fitted with an Agilent 4mm ID single taper split liner containing deactivated glass wool. The spectrometer was operated in full scan EI mode (+Ve) at 70eV scanning from m/z 10 to m/z 850 using a linear magnet scan.
Reverse Transcriptase-Coupled Polymerase Chain Reaction (RT-PCR)
Total RNA was isolated from BV2 microglial cells, mouse primary microglia and mouse primary astrocytes using RNA-Easy Qiagen (Valencia, CA) kit following manufacturer’s protocol. Semi-quantitative RT-PCR was performed as described by us (15,16) using oligo (dT) 12–18 as primer and moloney murine leukemia virus reverse transcriptase (MMLV-RT, Invitrogen) in a 20μl reaction mixture. The resulting cDNA was appropriately amplified using Promega Master Mix (Madison, WI) and the following primers (Invitrogen) for murine genes:
| SOCS3: | Sense: 5′-CGCCTCAAGACCTTCAGCTC-3′ |
| Antisense: 5′-CTGATCCAGGAACTCCCGAA-3′ | |
| iNOS: | Sense: 5′-CCC TTC CGA AGT TTC TGG CAG CAG C-3′ |
| Antisense: 5′-GGC TGT CAG AGC CTC GTG GCT TTG G-3′ | |
| IL-1β: | Sense: 5′-ATG GCA ACT GTT CCT GAA CTC AAC T-3′ |
| Antisense: 5′-CAG GAC AGG TAT AGA TTC TTT CCT TT-3′ | |
| TNF-α: | Sense: 5′-TTC TGT CTA CTG AAC TTC GGG GTG ATC GGT CC-3′ |
| Antisense: 5′-GTA TGA GAT AGC AAA TCG GCT GAC GGT GTG GG-3′ | |
| IL-6: Sense: | 5′-TGG AGT CAC AGA AGG AGT GGC TAA G-3′ |
| Antisense: 5′-TCT GAC CAC AGT GAG GAA TGT CCA C-3′ | |
| GAPDH: | Sense: 5′-GGTGAAGGTCGGAGTCAACG-3′ |
| Antisense: 5′-GTGAAGACGCCA GTG GACTC-3′ |
Amplified products were electrophoreses on 2% agars (Invitrogen) gels and visualized by anthodium bromide (Invitrogen) staining. Glyceraldehyde-3-phosphate dehydrogenate (GAPDH) was run as a loading control to ascertain that an equivalent amount of cDNA was synthesized from each sample (17).
Quantitative Real-Time PCR
Real-time PCR was performed using the ABI Prism 7700 sequence detection system (Applied Bios stems, Foster City, CA) using SYBR® Green Real-Time PCR Master Mix (Thermo Fisher Scientific NY, USA). The mRNA expression of targeted genes was normalized to GAPDH mRNA (18,19).
Immunoassaying
Immunocytochemistry was performed as described earlier (20). Briefly, 8-well chamber slides containing BV-2 microglial cells, mouse primary microglia or astrocytes cultured to 70–80% confluence were fixed with chilled methanol (Fisher Scientific, Waltham, MA) overnight, followed by two brief rinses with filtered PBS. Samples were blocked with 2% BSA (Fisher Scientific) in PBS containing Tween 20 (Sigma) and Triton X-100 (Sigma) for 30 min and incubated at room temperature under shaking conditions for 2 hr in PBS containing the following anti-mouse primary antibodies: SOCS3 (1:200; Santa Cruz Biotech, Santa Cruz, CA), phospho-CREB (1:600; Cell Signaling, Beverly, MA), total CREB (1:600; Cell Signaling), GFAP, (1:100; Santa Cruz), and IBA1 (1:500; Cedarlane Laboratories, Burlington, NC). After four 15 min washes in filtered PBS, slides were incubated with Cy2 and Cy5-labeled secondary antibodies (all 1:200; Jackson ImmunoResearch, West Grove, PA) for 1 hr under shaking conditions. Following four 15 minute washes with filtered PBS, cells were incubated for 4–5 min with 4′,6-diamidino-2-phenylindole (DAPI, 1:10,000; Sigma). The samples were run in an ethanol and Xylene (Fisher) gradient, mounted and visualized under BX-41 Olympus fluorescence microscope (21–23).
Transfection
Cells were transfected with siRNAs using RNAiMax Reagent (Invitrogen) according to manufacturer’s protocol as described before (6). Briefly, cells plated in 12-well plates were transfected with 0.5–1.0 μg/well of control or CREB siRNA (Cell Signaling) complexed with RNAi Max reagent in serum-free DMEM/F-12 containing L-glutamine (Invitrogen). After 5 hr, 20% FBS was added at a volume of 1:1 with the transfection media and cells were further incubated for 36 hrs (24).
Immunoblotting
Western blotting was performed as described earlier (12,23,25) with some modifications. Briefly, cells were scraped with SDS sample buffer, electrophoreses on NuPAGE® Novex® 4–12% Bis-Tris gels (Invitrogen) and proteins transferred onto a nitrocellulose membrane (Bio-Rad). The membrane was then washed for 15 min in PBS plus Tween 20 (PBST) and blocked for 1 hr in Licor blocking buffer (Li-COR, Lincoln, NE). Next, membranes were incubated overnight at 4°C with the following primary antibodies: SOCS3 (1:500, Abcam, Cambridge, MA), phospho-CREB, total CREB (1:600; Cell Signaling), β-actin (1:800; Abcam, Cambridge, MA). The next day, membranes were washed in PBST for 1 hr, incubated in secondary antibodies against primary antibody hosts (all 1:10,000; Jackson ImmunoResearch) for 1 hr at room temperature, washed for one more hour and visualized under the Odyssey® Infrared Imaging System (Li-COR, Lincoln, NE) (26).
Densitometric Analysis
Protein blots were analyzed using ImageJ (NIH, Bethesda, MD) and bands were normalized to their respective β-actin loading controls. Data are representative of the average fold change with respect to control for three independent experiments.
Chromatin Immunoprecipitation (ChIP) Assay
ChIP assays were performed as described before (6,27). Briefly, cells were stimulated by 300 μM cinnamic acid for 1 h followed by fixing with formaldehyde (1.42% final volume) and quenching with 125 mM glycine. The cells were pelleted and lysed in IP buffer containing 150 mM NaCl, 50 mM Tris-HCl (pH 7.5), 5 mM EDTA, Nonidet P-40 (0.5% v/v), Triton X-100 (1.0% v/v) and different protease and phosphatase inhibitors (10 μg/ml leupeptin, 0.5 mM phenylmethylsulfonyl fluoride or PMSF, 30 mM p-nitrophenyl phosphate, 10 mM sodium fluoride, 0.1 mM sodium orthovanadate, 0.1 mM Na2MoO4, and 10 mM β-glycerophosphate). After one wash with 1.0 ml of IP buffer, the pellet was resuspended in 1 ml of IP buffer (containing all inhibitors), and sonicated and sheared chromatin was split into two fractions (one to be used as input). The remaining fraction was incubated overnight under rotation at 4 °C with 5–7 μg of anti-CREB Abs or normal IgG followed by incubation with protein G-agars (Santa Cruz Biotechnology) for 2 h at 4 °C under rotation. Beads were then washed five times with cold IP buffer, and a total of 100 μl of 10% Chelex (10 g/100 ml H2O) was added directly to the washed protein G beads and vortexed. After 10 min of boiling, the Chelex/protein G bead suspension was allowed to cool to room temperature. Proteinase K (100 μg/ml) was then added, and the beads were incubated for 30 min at 55 °C while shaking, followed by another round of boiling for 10 min. The suspension was centrifuged, and the supernatant was collected. The Chelex/protein G beads fraction was vortexed with another 100 μl of water, centrifuged again, and the first and the second supernatants combined. Eluate was used directly as a template in PCR. The following primers were used to amplify fragments flanking the CRE-binding element in the mouse socs3 promoter:
Sense: 5′-TGGGCGGATGAGCTGAGGCCAGGTAGC-3′
Antisense: 5′-AGGTCCTCATGTACAGTTCCAAGCATCC-3′
The PCRs were repeated by using varying cycle numbers and different amounts of templates to ensure that results were in the linear range of PCR.
Electrophoretic mobility shift assay (EMSA)
DNA-binding activities of CREB was analyzed by non-radioactive electrophoretic mobility shift assay (EMSA) (8,24). After treatment, cells were washed with HBSS, scraped into 1.5 mL tubes and centrifuged in 4°C for 5 min at 500 rpm. The supernatant was aspirated and the pellet was resuspended in a membrane lysis buffer comprised of (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) (HEPES, pH 8.0), MgCl2, KCl, dithiothreitol (DTT) and protease/phosphatase inhibitors (Sigma), vortexed and centrifuged in 4°C at 720 × g for 5 minutes. Again, the supernatant was aspirated and the pellet was resuspended in a high salt, nuclear envelope lysis buffer comprised of HEPES (pH 8.0), MgCl2, glycerol, NaCl, ethylene diaminetetraacetic acid (EDTA), DTT and protease/phosphatase inhibitors, rotated vigorously in 4°C for 15 min and centrifuged in 4°C at 13,000 rpm for 15 minutes. The resultant supernatant was complexes with a cocktail of binding buffer (Tris-HCl, KCl, EDTA, DTT, 10x TGE, glycerol and Triton-X 100), custom designed fluorescent CREB-specific probes (Li-Cor Biosciences), and salmon sperm DNA (Invitrogen) for 15 min at room temperature and electrophoreses on custom-cast 6% polyacrylamide-TGE gels in 1X TGE for 2 hrs. The shift was visualized under the Odyssey® Infrared Imaging System (Li-Cor) (24,28).
Statistics
Values are expressed as means ± SD of at least three independent experiments. Statistical analyses for differences were performed via Student’s T-test. This criterion for statistical significance was p < 0.05.
Results
Upregulation of SOCS3 by cinnamic acid in mouse BV-2 microglial cells
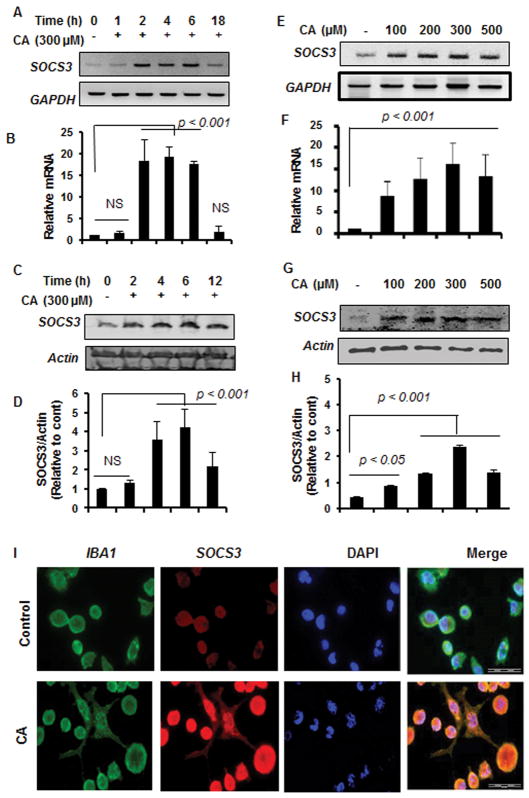
According to the National Institute of Standards and Technology, mass spectrum (electron ionization) of pure trans-cinnamic acid exhibits a total of 75 peaks including m/z 147, 103, 51, etc. Cinnamic acid purchased from Sigma also exhibited these peaks upon GC-MS analysis (Supplemental Fig. 1) indicating that it is truly cinnamic acid. To examine whether cinnamic acid could upregulate SOCS3 in glial cells, microglial cells were treated with cinnamic acid with different time periods. Our RT-PCR (Fig. 1A) and real-time PCR (Fig. 1B) data show that cinnamic acid markedly upregulated the expression of SOCS3 mRNA in microglial cells in a time-dependent manner, exhibiting maximum SOCS3 mRNA expression at 2 h to 6 h of stimulation that decreased at 18 h (Fig. 1A–B). Consistently, Western blot analysis also showed that cinnamic acid was capable of increasing the level of SOCS3 protein in a time-dependent manner (Fig. 1C–D). Next, we examined the dose-dependent effect on SOCS3. Although cinnamic acid at a dose of 100 μM, was capable of upregulating the expression of SOCS3 mRNA significantly in microglial cells, maximum upregulation of SOCS3 mRNA was observed at 300 μM cinnamic acid (Fig. 1E–F). Western blot of cinnamic acid-treated microglial cells also supports this conclusion (Fig. 1G–H). To further confirm this finding, after cinnamic acid treatment, microglial cells were double-labeled with Iba-1 and SOCS3. It is clearly evident from Figure 1I that cinnamic acid markedly stimulated the level of SOCS3 in microglia.
Figure 1. Upregulation of SOCS3 by cinnamic acid in mouse BV-2 microglial cells.
Cells were treated with 300 μM cinnamic acid (CA) for different time points (1, 2, 4, 6, and 18 h) in serum-free DMEM/F12 followed by monitoring the mRNA expression of SOCS3 by semi-quantitative RT-PCR (A) and real-time PCR (B). The protein level of SOCS3 was monitored by Western blot (C). Actin was run as a loading control. Bands were scanned and values (SOCS3/actin) presented as relative to control (D). Results are mean ± SD of at least three independent experiments. NS, not significant. Cells were treated with different doses of CA for 2 h followed by monitoring the mRNA expression of SOCS3 by semi-quantitative RT-PCR (E) and real-time PCR (F). The protein level of SOCS3 after 6 h of CA treatment was monitored by Western blot (G). Bands were scanned and values (SOCS3/actin) presented as relative to control (H). Cells were treated with 300 μM CA for 6 h under the same culture conditions and were double-labeled for SOCS3 and Iba1 (I). DAPI was used to stain nuclei. Results represent three independent experiments.
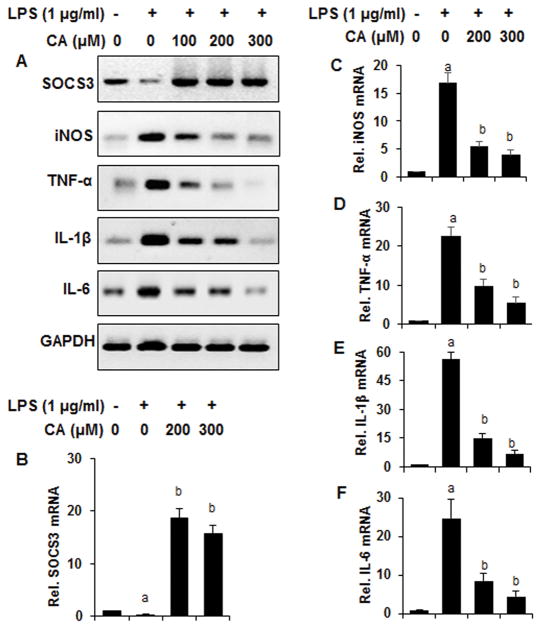
Since cinnamic acid upregulated SOCS3 in unstimulated microglial cells, we examined if cinnamic acid was also capable of doing so and suppressing proinflammatory molecules in activated microglial cells. Therefore, we monitored the effect of cinnamic acid on the expression of SOCS3 and different proinflammatory molecules in bacterial lipopolysaccharide (LPS)-stimulated BV-2 microglial cells. Interestingly, LPS stimulation decreased the expression of SOCS3 and cinnamic acid markedly increased the expression of SOCS3 in LPS-stimulated microglial cells (Fig. 2A–B). As expected, LPS challenge induced the expression of inducible nitric oxide synthase (iNOS) (Fig. 2A & 2C) and different proinflammatory cytokines (TNFα, IL-1β and IL-6) (Fig. 2A & 2D–F) in microglial cells. However, cinnamic acid dose-dependently inhibited the expression of iNOS, TNFα, IL-1β, and IL-6 in LPS-stimulated microglia (Fig. 2A & 2C–F).
Figure 2. Cinnamic acid attenuates LPS-induced expression of proinflammatory molecules while upregulating SOCS3 in mouse BV-2 microglial cells.
Cells pretreated with different concentrations of cinnamic acid (CA) for 2 h were stimulated with LPS (1 μg/ml) under serum-free conditions for 4 h followed by monitoring the mRNA expression of SOCS3, iNOS, TNFα, IL-1β, and IL-6 by semi-quantitative RT-PCR (A) and real-time PCR (B, SOCS3; C, iNOS; D, TNFα; E, IL-1β; F, IL-6). Results are mean ± SD of at least three independent experiments. ap < 0.001 vs control; bp < 0.001 vs LPS.
Cinnamic acid upregulates SOCS3 in primary mouse microglia and astrocytes
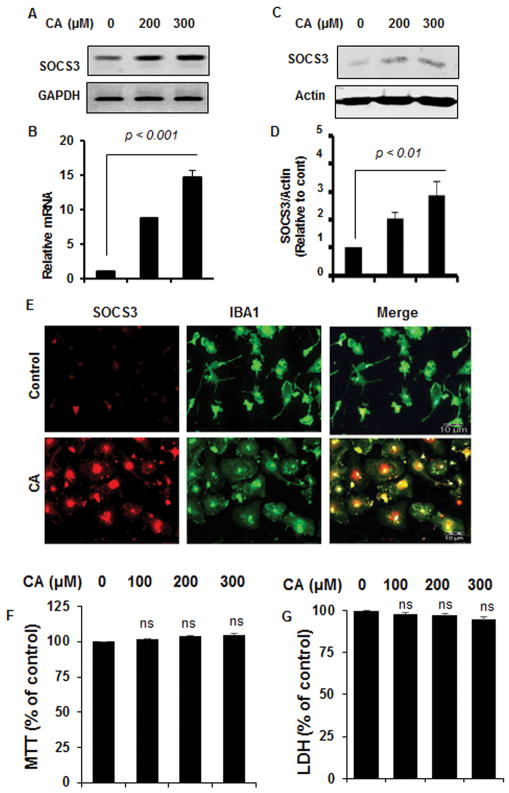
Next, we examine if cinnamic acid was capable of upregulating SOCS3 in primary microglia. Therefore, primary microglia isolated from mouse pups were treated with different doses of cinnamic acid. Similar to that seen in mouse BV-2 microglial cells, cinnamic acid strongly induced the expression of SOCS3 mRNA (Fig. 3A–B) and protein (Fig. 3C–D) in primary mouse microglia. Double-label immunofluorescence of Iba-1 and SOCS3 (Fig. 3E) also indicates marked upregulation SOCS3 in primary microglia. MTT (Fig. 3F) and LDH (Fig. 3G) assays showed that cinnamic acid did not induce any death response in primary microglia at doses (100 to 300 μM) tested.
Figure 3. Upregulation of SOCS3 by cinnamic acid (CA) in primary mouse microglia.
Primary microglia were treated with different concentrations of CA for 2 h under serum-free condition followed by monitoring the mRNA expression of SOCS3 by semi-quantitative RT-PCR (A) and real-time PCR (B). After 6 h of treatment with CA, the protein level of SOCS3 was monitored by Western blot (C). Actin was run as a loading control. Bands were scanned and values (SOCS3/actin) presented as relative to control (D). Results are mean ± SD of at least three independent experiments. Primary microglia were treated with 300 μM CA for 6 h under the same culture conditions followed by double-labeling for SOCS3 and Iba1 (E). Results represent three independent experiments. Primary microglia were treated with different concentrations of CA for 24 h followed by monitoring cell survival by MTT (F) and LDH (G). Results are mean ± SD of at least three independent experiments. NS, not significant.
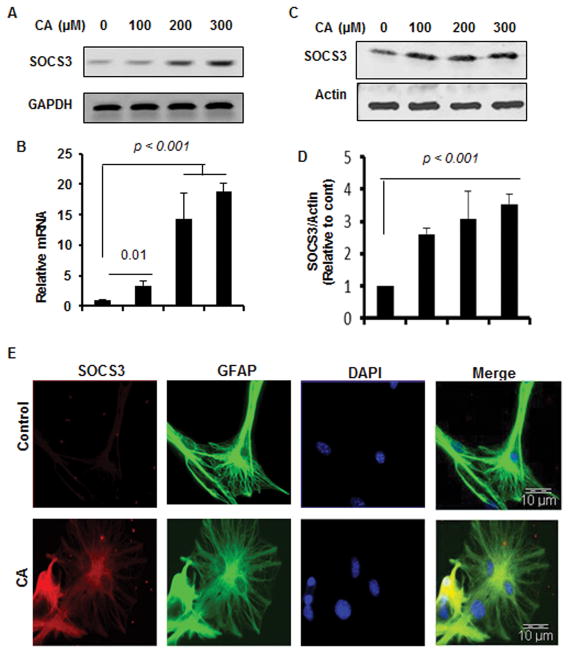
Although microglia are more inflammatory than astrocytes, astrocytes are the major cell type in the CNS and therefore, astroglial activation is also an important pathological event in neurodegenerative disorders. Semi-quantitative RT-PCR (Fig. 4A), real-time PCR (Fig. 4B), Western blot analysis (Fig. 4C–D), and double-label immunofluorescence for GFAP and SOCS3 (Fig. 4E) also indicate upregulation of SOCS3 by cinnamic acid in astrocytes.
Figure 4. Cinnamic acid (CA) upregulates SOCS3 in primary mouse astrocytes.
Primary astrocytes were treated with different concentrations of CA for 2 h under serum-free condition followed by monitoring the mRNA expression of SOCS3 by semi-quantitative RT-PCR (A) and real-time PCR (B). After 6 h of treatment with CA, the protein level of SOCS3 was monitored by Western blot (C). Actin was run as a loading control. Bands were scanned and values (SOCS3/actin) presented as relative to control (D). Results are mean ± SD of at least three independent experiments. Primary astrocytes were treated with 300 μM CA for 6 h under the same culture conditions followed by double-labeling for SOCS3 and GFAP (E). DAPI was used to stain nuclei. Results represent three independent experiments.
Involvement of CREB in cinnamic acid-mediated upregulation of SOCS3 in microglial cells
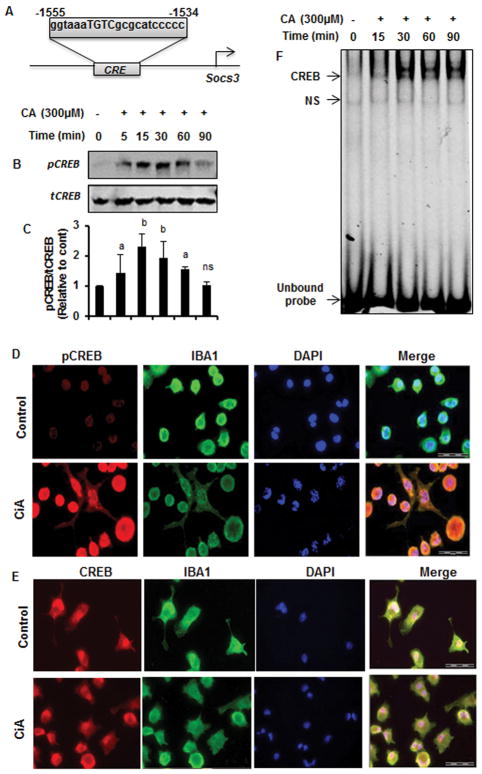
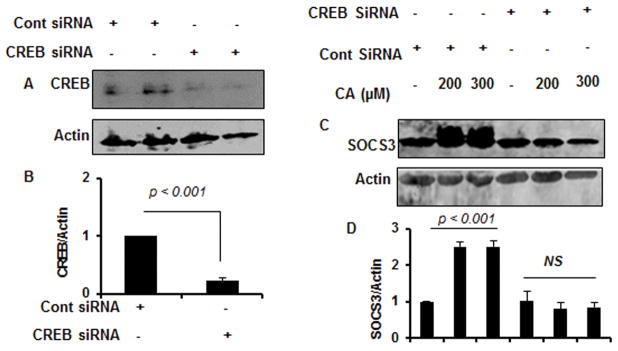
Next, we investigated mechanisms by which cinnamic acid induced the expression of SOCS3 in microglial cells. Since upon MatInspector analysis of the Socs3 gene promoter, we found the presence of a consensus cAMP response element (CRE) (Fig. 5A), we examined the role of CREB in cinnamic acid-mediated upregulation of SOCS3. At first, we examined if cinnamic acid was capable of inducing the activation of CREB in microglial cells. As evident from Figure 5B, cinnamic acid treatment rapidly induced the phosphorylation of CREB with maximum phosphorylation observed at 15 min. However, phosphorylation of CREB decreased afterwards becoming almost normal at 90 min of stimulation (Fig. 5B). In contrast, cinnamic acid was unable to increase the level of total CREB (Fig. 5B). Quantification of phospho-CREB and total CREB also confirmed these finding (Fig. 5C). Double-label immunofluorescence of microglia with Iba1 and either phospho-CREB (Fig. 5D) or total CREB (Fig. 5E) also shows that cinnamic acid increases phospho-CREB, but not total CREB, in microglial cells. To further confirm the activation of CREB, we monitored the DNA-binding activity of CREB by electrophoretic mobility shift assay (EMSA), which also shows marked increase in DNA-binding activity by cinnamic acid treatment (Fig. 5F). This gel shift assay detected a specific band in response to cinnamic acid that was not found in untreated control cells (Fig. 5F). The induction of CREB activation was visible as early as 15 min and maximum at 30 min of cinnamic acid stimulation (Fig. 5F). Next, to investigate the role of CREB in cinnamic acid-mediated expression of SOCS3, we employed CREB siRNA. Decrease in CREB protein expression in microglial cells by CREB siRNA, but not control siRNA, indicates the specificity of CREB siRNA (Fig. 6A–B). Stimulation of SOCS3 protein expression by cinnamic acid in control siRNA-transfected microglia, but not CREB siRNA-transfected microglia (Fig. 6C–D), suggest that cinnamic acid increases the expression of SOCS3 in microglial cells via CREB.
Figure 5. Activation of CREB by cinnamic acid (CA) in mouse BV-2 microglial cells.
(A) Position of CRE in the Socs3 gene promoter. Cells were treated with 300 μM CA for different minutes under serum-free conditions followed by monitoring the level of phospho-CREB (pCREB) by Western blot (B). Graph represents densitometric analysis of pCREB protein levels normalized to total CREB (loading control) (C). Results are mean ± SD of three independent experiments. ap < 0.05 vs control; bp < 0.01 vs control. Cells treated with 300 μM CA for 30 mins under serum-free condition were double-labeled for pCREB & Iba1 (D) and total CREB & Iba1 (E). DAPI was used to stain nuclei. Cells were treated with 300uM of CA under serum-free condition for 15, 30, 60, and 90 min followed by monitoring the DNA-binding activity of CREB by EMSA (F). Results represent three independent experiments. Upper, middle and bottom arrows indicate CREB DNA-binding, non-specific (NS) DNA-binding and unbound probe, respectively.
Figure 6. Effect of siRNA knockdown of CREB on cinnamic acid-mediated up regulation of SOCS3 in mouse BV-2 microglial cells.
Cells were transfected with either control or CREB siRNA. Forty-eight h after transfection, level of CREB was monitored by Western blot (A). Graph represents densitometric analysis of CREB protein levels normalized to β-Actin (loading control) (B). Cells were transfected with either control or CREB siRNA. Forty-eight h after transfection, cells were treated with 200 and 300 μM CA for 6 h under serum-free condition followed by monitoring the level of SOCS3 by Western Blot (C). Graph represents densitometric analysis of SOCS3 protein levels normalized to β-Actin (loading control) (D). Results are mean ± SD of three independent experiments. NS, not significant.
Cinnamic acid stimulates the recruitment of CREB to the Socs3 promoter
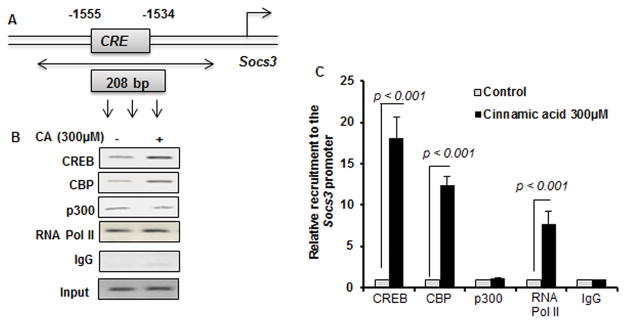
CREB is a transcription factor and since siRNA knockdown of CREB suppressed the level of SOCS3, we investigated whether CREB was directly involved in the transcription of the Socs3 gene in cinnamic acid-treated microglial cells. Therefore, we examined the recruitment of CREB to the Socs3 gene promoter (Fig. 7A) by ChIP assay. After immunoprecipitation of cinnamic acid-treated microglial chromatin fragments using antibodies against CREB, we were also able to amplify 208 bp fragments from the Socs3 promoter (Fig. 7A–B). CREB-binding protein (CBP), a histone acetyl transferase, is known to play an important role in multiple CREB-mediated transcriptional activities. Therefore, we investigated if CBP was also involved in cinnamic acid-induced transcription of the Socs3 gene. As evident from PCR (Fig. 7B) and real-time PCR (Fig. 7C), cinnamic acid treatment increased the recruitment of CBP to the Socs3 gene promoter. In contrast, cinnamic acid was unable to recruit another histone acetyl transferase p300 to the CRE of Socs3 promoter (Fig. 7B–C), suggesting that p300 was not involved in cinnamic acid-mediated transcription of Socs3 in microglia. Consistent to the recruitment of CREB and CBP to the Socs3 gene promoter, cinnamic acid was able to recruit RNA polymerase to the promoter of Socs3 gene (Fig. 7B–C). These results are specific as no amplification product was observed in any of the immuno-precipitates obtained with control IgG (Fig. 7B–C).
Figure 7. Cinnamic acid (CA) treatment induces the recruitment of CREB to the socs3 gene promoter in mouse BV-2 microglial cells.
(A) DNA sequence of the Socs3 promoter region containing the CRE with positions of the primers used for the ChIP analysis. Cells were stimulated with 300 μM CA for 1h under serum-free condition. The recruitment of CREB to the Socs3 promoter was monitored by ChIP PCR analysis (B) and ChIP real-time PCR (C). Normal IgG was used as control. Results are mean ± SD of three independent experiments.
Discussion
Chronic inflammation is becoming an important pathological hallmark of different neurodegenerative disorders including AD (29–31). Although microglia, prototypical CNS macrophages, play a pivotal role in immune surveillance, phagocytosis and neuroprotection (32–34), persistent activation and recruitment is detrimental (35–37). It has been shown that prolonged microglial activation results in elevated production of various proinflammatory cytokines, known to contribute to the degeneration of neurons (38,39). Therefore, understanding mechanism by which the healthy CNS can be protected from inflammatory attacks is an important area of research. Studies have shown that SOCS3 might have an important inhibitory effect against cytokine-induced immune and inflammatory responses (10,40–42). In addition to inhibiting IL-6 signaling via suppression of STAT3 activation, SOCS3 can also function as a positive regulator for the ERK-MAPK pathway, an inhibitor of the NF-κB pathway, and may act as an antagonist to the cAMP-mediated signaling (10,43,44). Accordingly, Socs3 gene disruption leads to embryonic lethality in mice at 12–16 days with severe eryrthrocytosis (45). SOCS3 deficient (in hemopoetic and endothelial cell) mice also exhibit IL-1 dependent arthritis that could be suppressed by over-expression of SOCS3. Again, intracellular administration of cell penetrating SOCS3 could inhibit the cytokine-mediated acute inflammatory response (46). According to Qin et al (47), SOCS3 is also capable of inhibiting chemokine-mediated chemotaxis in T-cells by binding to chemokine receptor. Therefore upregulation of SOCS3 is beneficial for different neurodegenerative and neuroinflammatory diseases.
Cinnamon, the brown bark of cinnamon tree, has already been being used for centuries throughout the world as either spice or flavoring agent. Moreover, medieval health practitioners used to treat a variety of disorders including arthritis, common cold, coughing, hoarseness, sore throats, etc. by cinnamon. Upon ingestion, cinnamaldehyde present in Cinnamon is converted into cinnamic acid by oxidation. Here, we delineate for the first time that cinnamic acid is capable of upregulating SOCS3 mRNA and protein in mouse BV-2 microglial cells and primary mouse microglia and astrocytes. Consistent to the anti-inflammatory property of SOCS3, cinnamic acid also suppressed the expression of iNOS and different proinflammatory cytokines (TNFα, IL-1β and IL-6) in LPS-stimulated microglia. These studies provide a potentially important mechanism to reduce the pathogenic effects of cytokine signaling and attenuate glial activation in the CNS by cinnamic acid.
Mechanisms by which the Socs3 gene is upregulated are poorly understood. Earlier we delineated that gemfibrozil, a lipid-lowering drug, upregulated SOCS3 in astrocytes via KLF4 (6). However, cinnamic acid remained unable to activate KLF4 in astrocytes (data not shown). Therefore, we analyzed the Socs3 promotor using the Genomatix Software Suite and found one cAMP response element (CRE) between 1534 and 1555 base pairs upstream of the Socs3 open reading frame, prompting us to investigate whether CREB was required for cinnamic acid-mediated upregulation of SOCS3. Activation of CREB by cinnamic acid alone, abrogation of cinnamic acid-mediated SOCS3 upregulation by siRNA knockdown of CREB and recruitment of CREB to the promoter of Socs3 by cinnamic acid suggest that cinnamic acid employs CREB for the transcription of Socs3. Being histone acetyl transferases, both p300 and CBP interact with numerous transcription factors and ultimately facilitates the transcription of target genes. In this case, cinnamic acid induced the recruitment of CBP, but not p300, to the CRE of Socs3, indicating the specificity of the effect. However, mechanism by which cinnamic acid induces the activation of CREB in microglial cells is currently unknown. Since in many cases, cAMP-dependent protein kinase A (PKA) phosphorylates CREB to function upstream of phospho-CREB dimerization and nuclear translocation (48,49), it is possible that PKA is involved in cinnamic acid-mediated activation of CREB.
Although we have demonstrated beneficial effect of whole cinnamon powder in animal models of multiple sclerosis (50), Parkinson’s disease (51) and Alzheimer’s disease (52), some brands of cinnamon (e.g. Cinnamonun cassia) contains coumarin, a hepatotoxic molecule. Therefore, obtaining the right kind of cinnamon free of coumarin is important for long-term uptake, which is sometimes a challenge. Moreover, whole cinnamon powder contains numerous compounds and some of these compounds [styrene; benzene, 1,1′-(2-butene-1,4-diyl)bis-; benzene, 1,1′-(1,2-cyclobutanediyl)bis-; 4-phenylbutyl chloride; (2,3-diphenylcyclopropyl)methyl phenyl sulfoxide] (53) may not be beneficial. Therefore, identification of the active ingredient of cinnamon capable of recapitulating beneficial effects of cinnamon is an important step. Our results suggest that cinnamic acid is one such candidate that may defend the CNS against neuroinflammatory and neurodegenerative disorders via upregulation of SOCS3.
In summary, we have delineated that cinnamic acid upregulates SOCS3, a potent anti-inflammatory molecule, in microglia and astroglia via CREB-CBP pathway. Although the in vitro situation of mouse microglia and astrocytes in culture and its treatment with cinnamic acid may not be similar to in vivo situation of these glial cells in the brain of patients with neurodegenerative disorders, our results points out cinnamic acid as a possible agent to increase the defense mechanism against inflammatory insults via upregulation of SOCS3.
Supplementary Material
Acknowledgments
This study was supported by a merit award from the Department of Veteran Affairs (1I01BX003033) and a grant (AT006681) from National Institutes of Health.
References
- 1.Tansey MG, McCoy MK, Frank-Cannon TC. Neuroinflammatory mechanisms in Parkinson’s disease: potential environmental triggers, pathways, and targets for early therapeutic intervention. Exp Neurol. 2007;208:1–25. doi: 10.1016/j.expneurol.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frank-Cannon TC, Alto LT, McAlpine FE, Tansey MG. Does neuroinflammation fan the flame in neurodegenerative diseases? Mol Neurodegener. 2009;4:47. doi: 10.1186/1750-1326-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin JB. Molecular basis of the neurodegenerative disorders. N Engl J Med. 1999;340:1970–1980. doi: 10.1056/NEJM199906243402507. [DOI] [PubMed] [Google Scholar]
- 4.Cummings JL, Vinters HV, Cole GM, Khachaturian ZS. Alzheimer’s disease: etiologies, pathophysiology, cognitive reserve, and treatment opportunities. Neurology. 1998;51:S2–17. doi: 10.1212/wnl.51.1_suppl_1.s2. discussion S65–17. [DOI] [PubMed] [Google Scholar]
- 5.Jana A, Pahan K. Fibrillar amyloid-beta-activated human astroglia kill primary human neurons via neutral sphingomyelinase: implications for Alzheimer’s disease. J Neurosci. 30:12676–12689. doi: 10.1523/JNEUROSCI.1243-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghosh A, Pahan K. Gemfibrozil, a lipid-lowering drug, induces suppressor of cytokine signaling 3 in glial cells: implications for neurodegenerative disorders. J Biol Chem. 2012;287:27189–27203. doi: 10.1074/jbc.M112.346932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim YS, Joh TH. Microglia, major player in the brain inflammation: their roles in the pathogenesis of Parkinson’s disease. Exp Mol Med. 2006;38:333–347. doi: 10.1038/emm.2006.40. [DOI] [PubMed] [Google Scholar]
- 8.Jana M, Palencia CA, Pahan K. Fibrillar amyloid-beta peptides activate microglia via TLR2: implications for Alzheimer’s disease. J Immunol. 2008;181:7254–7262. doi: 10.4049/jimmunol.181.10.7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krebs DL, Hilton DJ. SOCS proteins: negative regulators of cytokine signaling. Stem Cells. 2001;19:378–387. doi: 10.1634/stemcells.19-5-378. [DOI] [PubMed] [Google Scholar]
- 10.Baker BJ, Akhtar LN, Benveniste EN. SOCS1 and SOCS3 in the control of CNS immunity. Trends Immunol. 2009;30:392–400. doi: 10.1016/j.it.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giulian D, Baker TJ. Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci. 1986;6:2163–2178. doi: 10.1523/JNEUROSCI.06-08-02163.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Modi KK, Jana M, Mondal S, Pahan K. Sodium Benzoate, a Metabolite of Cinnamon and a Food Additive, Upregulates Ciliary Neurotrophic Factor in Astrocytes and Oligodendrocytes. Neurochem Res. 2015;40:2333–2347. doi: 10.1007/s11064-015-1723-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roy A, Jana M, Kundu M, Corbett GT, Rangaswamy SB, Mishra RK, Luan CH, Gonzalez FJ, Pahan K. HMG-CoA Reductase Inhibitors Bind to PPARalpha to Upregulate Neurotrophin Expression in the Brain and Improve Memory in Mice. Cell Metab. 2015;22:253–265. doi: 10.1016/j.cmet.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roy A, Fung YK, Liu X, Pahan K. Up-regulation of microglial CD11b expression by nitric oxide. The Journal of biological chemistry. 2006;281:14971–14980. doi: 10.1074/jbc.M600236200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jana M, Pahan K. Redox regulation of cytokine-mediated inhibition of myelin gene expression in human primary oligodendrocytes. Free Radic Biol Med. 2005;39:823–831. doi: 10.1016/j.freeradbiomed.2005.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jana M, Mondal S, Jana A, Pahan K. Interleukin-12 (IL-12), but not IL-23, induces the expression of IL-7 in microglia and macrophages: implications for multiple sclerosis. Immunology. 141:549–563. doi: 10.1111/imm.12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mondal S, Roy A, Pahan K. Functional blocking monoclonal antibodies against IL-12p40 homodimer inhibit adoptive transfer of experimental allergic encephalomyelitis. J Immunol. 2009;182:5013–5023. doi: 10.4049/jimmunol.0801734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khasnavis S, Pahan K. Sodium benzoate, a metabolite of cinnamon and a food additive, upregulates neuroprotective Parkinson disease protein DJ-1 in astrocytes and neurons. J Neuroimmune Pharmacol. 7:424–435. doi: 10.1007/s11481-011-9286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brahmachari S, Jana A, Pahan K. Sodium benzoate, a metabolite of cinnamon and a food additive, reduces microglial and astroglial inflammatory responses. J Immunol. 2009;183:5917–5927. doi: 10.4049/jimmunol.0803336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saha RN, Jana M, Pahan K. MAPK p38 regulates transcriptional activity of NF-kappaB in primary human astrocytes via acetylation of p65. J Immunol. 2007;179:7101–7109. doi: 10.4049/jimmunol.179.10.7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dasgupta S, Jana M, Zhou Y, Fung YK, Ghosh S, Pahan K. Antineuroinflammatory effect of NF-kappaB essential modifier-binding domain peptides in the adoptive transfer model of experimental allergic encephalomyelitis. J Immunol. 2004;173:1344–1354. doi: 10.4049/jimmunol.173.2.1344. [DOI] [PubMed] [Google Scholar]
- 22.Corbett GT, Roy A, Pahan K. Sodium phenylbutyrate enhances astrocytic neurotrophin synthesis via protein kinase C (PKC)-mediated activation of cAMP-response element-binding protein (CREB): implications for Alzheimer disease therapy. J Biol Chem. 288:8299–8312. doi: 10.1074/jbc.M112.426536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saha RN, Liu X, Pahan K. Up-regulation of BDNF in astrocytes by TNF-alpha: a case for the neuroprotective role of cytokine. J Neuroimmune Pharmacol. 2006;1:212–222. doi: 10.1007/s11481-006-9020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khasnavis S, Jana A, Roy A, Mazumder M, Bhushan B, Wood T, Ghosh S, Watson R, Pahan K. Suppression of nuclear factor-kappaB activation and inflammation in microglia by physically modified saline. J Biol Chem. 287:29529–29542. doi: 10.1074/jbc.M111.338012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Modi KK, Rangasamy SB, Dasarathi S, Roy A, Pahan K. Cinnamon Converts Poor Learning Mice to Good Learners: Implications for Memory Improvement. J Neuroimmune Pharmacol. 2016;11:693–707. doi: 10.1007/s11481-016-9693-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corbett GT, Roy A, Pahan K. Gemfibrozil, a lipid-lowering drug, upregulates IL-1 receptor antagonist in mouse cortical neurons: implications for neuronal self-defense. J Immunol. 189:1002–1013. doi: 10.4049/jimmunol.1102624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghosh A, Jana M, Modi K, Gonzalez FJ, Sims KB, Berry-Kravis E, Pahan K. Activation of peroxisome proliferator-activated receptor alpha induces lysosomal biogenesis in brain cells: implications for lysosomal storage disorders. J Biol Chem. 2015;290:10309–10324. doi: 10.1074/jbc.M114.610659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Auch CJ, Saha RN, Sheikh FG, Liu X, Jacobs BL, Pahan K. Role of protein kinase R in double-stranded RNA-induced expression of nitric oxide synthase in human astroglia. FEBS Lett. 2004;563:223–228. doi: 10.1016/S0014-5793(04)00302-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Streit WJ. Microglia and Alzheimer’s disease pathogenesis. J Neurosci Res. 2004;77:1–8. doi: 10.1002/jnr.20093. [DOI] [PubMed] [Google Scholar]
- 30.Giulian D. Microglia and the immune pathology of Alzheimer disease. Am J Hum Genet. 1999;65:13–18. doi: 10.1086/302477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O’Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aloisi F. Immune function of microglia. Glia. 2001;36:165–179. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- 33.Streit WJ. Microglial senescence: does the brain’s immune system have an expiration date? Trends Neurosci. 2006;29:506–510. doi: 10.1016/j.tins.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Rock RB, Gekker G, Hu S, Sheng WS, Cheeran M, Lokensgard JR, Peterson PK. Role of microglia in central nervous system infections. Clin Microbiol Rev. 2004;17:942–964. doi: 10.1128/CMR.17.4.942-964.2004. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gonzalez-Scarano F, Baltuch G. Microglia as mediators of inflammatory and degenerative diseases. Annu Rev Neurosci. 1999;22:219–240. doi: 10.1146/annurev.neuro.22.1.219. [DOI] [PubMed] [Google Scholar]
- 36.Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 37.Ling EA, Wong WC. The origin and nature of ramified and amoeboid microglia: a historical review and current concepts. Glia. 1993;7:9–18. doi: 10.1002/glia.440070105. [DOI] [PubMed] [Google Scholar]
- 38.Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nat Rev Neurosci. 2001;2:734–744. doi: 10.1038/35094583. [DOI] [PubMed] [Google Scholar]
- 39.Simi A, Lerouet D, Pinteaux E, Brough D. Mechanisms of regulation for interleukin-1beta in neurodegenerative disease. Neuropharmacology. 2007;52:1563–1569. doi: 10.1016/j.neuropharm.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 40.Alexander WS. Suppressors of cytokine signalling (SOCS) in the immune system. Nat Rev Immunol. 2002;2:410–416. doi: 10.1038/nri818. [DOI] [PubMed] [Google Scholar]
- 41.Chen XP, Losman JA, Rothman P. SOCS proteins, regulators of intracellular signaling. Immunity. 2000;13:287–290. doi: 10.1016/s1074-7613(00)00028-5. [DOI] [PubMed] [Google Scholar]
- 42.Qin H, Roberts KL, Niyongere SA, Cong Y, Elson CO, Benveniste EN. Molecular mechanism of lipopolysaccharide-induced SOCS-3 gene expression in macrophages and microglia. J Immunol. 2007;179:5966–5976. doi: 10.4049/jimmunol.179.9.5966. [DOI] [PubMed] [Google Scholar]
- 43.Dominguez E, Mauborgne A, Mallet J, Desclaux M, Pohl M. SOCS3-mediated blockade of JAK/STAT3 signaling pathway reveals its major contribution to spinal cord neuroinflammation and mechanical allodynia after peripheral nerve injury. J Neurosci. 2010;30:5754–5766. doi: 10.1523/JNEUROSCI.5007-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 2007;7:454–465. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- 45.Takahashi Y, Carpino N, Cross JC, Torres M, Parganas E, Ihle JN. SOCS3: an essential regulator of LIF receptor signaling in trophoblast giant cell differentiation. EMBO J. 2003;22:372–384. doi: 10.1093/emboj/cdg057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wong PK, Egan PJ, Croker BA, O’Donnell K, Sims NA, Drake S, Kiu H, McManus EJ, Alexander WS, Roberts AW, Wicks IP. SOCS-3 negatively regulates innate and adaptive immune mechanisms in acute IL-1-dependent inflammatory arthritis. J Clin Invest. 2006;116:1571–1581. doi: 10.1172/JCI25660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qin H, Niyongere SA, Lee SJ, Baker BJ, Benveniste EN. Expression and functional significance of SOCS-1 and SOCS-3 in astrocytes. J Immunol. 2008;181:3167–3176. doi: 10.4049/jimmunol.181.5.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sands WA, Palmer TM. Regulating gene transcription in response to cyclic AMP elevation. Cell Signal. 2008;20:460–466. doi: 10.1016/j.cellsig.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 49.Waltereit R, Weller M. Signaling from cAMP/PKA to MAPK and synaptic plasticity. Mol Neurobiol. 2003;27:99–106. doi: 10.1385/MN:27:1:99. [DOI] [PubMed] [Google Scholar]
- 50.Mondal S, Pahan K. Cinnamon ameliorates experimental allergic encephalomyelitis in mice via regulatory T cells: implications for multiple sclerosis therapy. PLoS One. 2015;10:e0116566. doi: 10.1371/journal.pone.0116566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khasnavis S, Pahan K. Cinnamon treatment upregulates neuroprotective proteins Parkin and DJ-1 and protects dopaminergic neurons in a mouse model of Parkinson’s disease. J Neuroimmune Pharmacol. 2014;9:569–581. doi: 10.1007/s11481-014-9552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Modi KK, Roy A, Brahmachari S, Rangasamy SB, Pahan K. Cinnamon and Its Metabolite Sodium Benzoate Attenuate the Activation of p21rac and Protect Memory and Learning in an Animal Model of Alzheimer’s Disease. PLoS One. 2015;10:e0130398. doi: 10.1371/journal.pone.0130398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jana A, Modi KK, Roy A, Anderson JA, van Breemen RB, Pahan K. Up-regulation of neurotrophic factors by cinnamon and its metabolite sodium benzoate: therapeutic implications for neurodegenerative disorders. J Neuroimmune Pharmacol. 2013;8:739–755. doi: 10.1007/s11481-013-9447-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.