Abstract
Ankyrins are broadly expressed adaptors that organize diverse membrane proteins into specialized domains and link them to the sub-membranous cytoskeleton. In neurons, ankyrins are known to have essential roles in organizing the axon initial segment and nodes of Ranvier. However, recent studies have revealed novel functions for ankyrins at synapses, where they organize and stabilize neurotransmitter receptors, modulate dendritic spine morphology and control adhesion to the presynaptic site. Ankyrin genes have also been highly associated with a range of neurodevelopmental and psychiatric diseases, including bipolar disorder, schizophrenia and autism, which all demonstrate overlap in their genetics, mechanisms and phenotypes. This review discusses the novel synaptic functions of ankyrin proteins in neurons, and places these exciting findings in the context of ANK genes as key neuropsychiatric disorder risk-factors.
Introduction
Ankyrins are a broadly expressed multi-gene family of scaffolding adaptor proteins that have the common function of linking a variety of membrane proteins to the spectrin-based sub-membranous cytoskeleton (Figure 1A). As they primarily organize and stabilize protein networks, they are considered ‘master-organizers’ of membrane-associated protein complexes, and form membrane microdomains throughout the plasma membrane of many different cell types, thereby fulfilling numerous crucial cell biological roles including organization of transverse tubules in cardiomyocytes (Mohler et al., 2003; Mohler et al., 2004) and assembly of the epithelial lateral membrane (Kizhatil and Bennett, 2004; Kizhatil et al., 2007). In neurons, ankyrins play a significant role in organizing the axon initial segment (AIS), the proximal region of the axon which is responsible for neuron firing, as well as the nodes of Ranvier (NoR), sites of action potential regeneration in myelinated axons (for a review see (Nelson and Jenkins, 2017)). In addition, recent studies have revealed novel functions for ankyrins at synapses, specialized micron-sized contact-sites between neurons, which require precise organization and regulation to control synaptic transmission. Within excitatory synapses, ankyrins have been shown to arrange and stabilize neurotransmitter receptors, modulate dendritic spine morphology and control adhesion to the presynaptic site. Ankyrin genes have also been highly associated with a range of neurodevelopmental and psychiatric diseases, including bipolar disorder, schizophrenia and autism, all of which demonstrate overlap in their genetics, mechanisms and disease phenotypes. These significant links with human disease and the broad conserved functions of the ankyrin protein family across cell biology has ignited much interest in the potential synaptic functions of ankyrins. Here we collate the most recent work concerning ankyrin function at a variety of synapses, and place it in the context of what is known about ankyrin proteins in other cell-types, and discuss how ankyrins are linked to psychiatric disease, in particular, bipolar disorder.
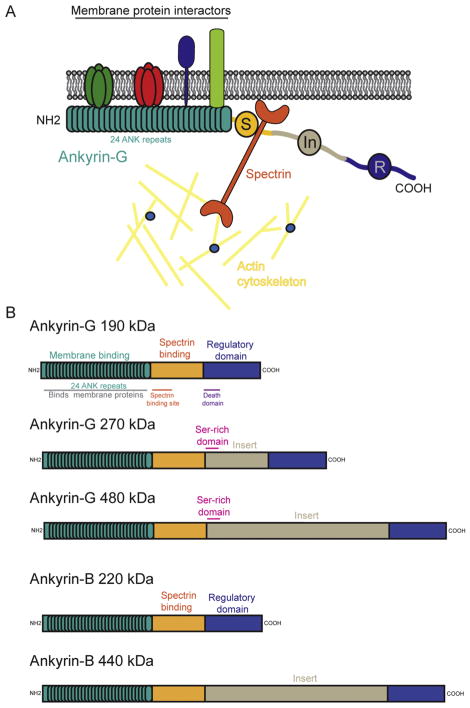
Figure 1. Ankyrins: function and domain structure.
A. Ankyrins have a conserved function across many cell types; to link membrane proteins to the submembranous actin-spectrin cytoskeleton.
B. The domain structure of ankyrin-G and ankyrin-B, the main ankyrins to have characterized functions at synapses. The domain structure is conserved across all ankyrins. 24 ankyrin repeats form the membrane binding domain which is the binding site for membrane targets that are organized by ankyrins. Ankyrins interact with the actin-spectrin cytoskeleton via the spectrin-binding region (S), and the c-terminal regulatory domain is responsible for modulating the interactions of the membrane binding domain. Larger ankyrin isoforms have a neuronal specific insert. Ankyrin-G 270/480 kDa harbor a serine-rich domain which is responsible for targeting these isoforms to the AIS.
Ankyrin proteins
The ankyrin protein family
Vertebrate ankyrins comprise of three family members: ankyrin-R, ankyrin-B and ankyrin-G (encoded by ANK1, ANK2 and ANK3 respectively). The first isoform to be characterized was Ankyrin-R, a scaffolding protein necessary for erythrocyte shape and function, and in addition to its expression in the heart, exhibits a restricted distribution in striated muscle and some neurons in the central nervous system (CNS) (Lambert and Bennett, 1993). Ankyrin-B has a varied expression profile, and is found in tissues including the brain, thymus, heart and skeletal muscle (Cunha and Mohler, 2008; Kunimoto, 1995; Kunimoto et al., 1991; Tse et al., 1991). Ankyrin-B plays a critical role in the heart where it is responsible for the correct localization of ion channels and transporters that are essential for proper calcium signaling and heart function (Mohler et al., 2003). Indeed, a loss-of-function mutation in ankyrin-B, leading to reduced expression, causes type 4 Long QT syndrome, a heart rhythm disorder characterized by erratic heartbeats and lethal cardiac arrhythmias (Mohler et al., 2003). Ankyrin-B is also commonly coexpressed with ankyrin-G in many cell types, including neurons, although they appear to have distinct functions (Abdi et al., 2006; Galiano et al., 2012; Lorenzo et al., 2014; Mohler et al., 2002). In neurons, the 440kDa isoform of ankyrin-B resides in unmyelinated axons where it is though to contribute to an intracellular barrier via interactions with αII and βII spectrin, and thereby limit ankyrin-G localization to the proximal axon (Galiano et al., 2012). The 220kDa variant of ankyrin-B, which is more prominent later in brain development, is shown to be important for long-range axonal transport by coupling the dynein-dynactin motor complex to organelle cargoes such as synaptic vesicles and mitochondria (Lorenzo et al., 2014). Interestingly, knock-out of ankyrin-B causes no AIS abnormalities, suggesting that the roles of the different isoforms of ankyrin-B in the axon are complicated, and require further study(Lorenzo et al., 2014). Compared to its function in the axon little is known about ankyrin-B at mammalian synapses.
Multiple isoforms of ankyrin-G are encoded by ANK3, and they are broadly expressed in almost all tissues including the brain, epithelium, kidney and muscle (Devarajan et al., 1996; Kordeli et al., 1995; Peters et al., 1995; Thevananther et al., 1998). Most of the work concerning the myriad functions of ankyrin proteins in the brain have focused on ankyrin-G, therefore this review will focus on these studies with the view of presenting potential models for the roles of ankyrins at synapses, which might also be attributed to ankyrin-B. Multiple isoforms of ankyrin-G exist in neurons, however the 190, 270 and 480kDa isoforms are the most prominent in brain ((Zhang and Bennett, 1998) Figure 1B). The giant 270/480kDa isoforms have well-documented roles as membrane protein and synapse organizers at the AIS and NoR (Leterrier and Dargent, 2014; Nelson and Jenkins, 2017), as well as the 480kDa ankyrin-G having an important role for maintaining GABAergic synapses (Tseng et al., 2015). The role of the 190kDa isoform in neurons is less well characterized, however, evidence now suggests that 190kDa ankyrin-G is an important component of rodent post-synaptic densities and a crucial player in synaptic plasticity (Nanavati et al., 2011; Smith et al., 2014). In addition to the large forms of ankyrin-G, there are also smaller isoforms (110, 116 and 120 kDa), which are localized to the golgi apparatus, endosomes and late endosomes in non-neuronal cells (Devarajan et al., 1996; Hoock et al., 1997; Ignatiuk et al., 2006). Little is known about these isoforms in neurons, however, and combined with the function of ankyrin-B in axonal trafficking described above, these smaller trafficking isoforms indicate that ankyrins (both –B and –G) may play important roles in protein trafficking in neurons, and potentially at synaptic sites.
Ankyrin protein topology
All ankyrin proteins share a common molecular organization with essentially three key functional domains: membrane binding, spectrin binding and regulatory domains (Figure 1B). Ankyrins interact with membrane proteins through their membrane-binding domain which is comprised of 24 N-terminal ANK repeats that are folded into a super-helical solenoid structure (Bennett and Lorenzo, 2013). This region binds an array of highly diverse membrane targets including ion channels, transporters, adhesion molecules, signaling proteins and cytoskeletal elements (Cunha and Mohler, 2009), and provides a platform at the plasma membrane at which these proteins can be arranged. Importantly, the membrane binding regions of all three main ankyrin-G transcripts harbor a cysteine residue (C70), which can be palmitoylated, a process that is required for ankyrin-G association with the membrane, appropriate cellular localization and function (He et al., 2014; He et al., 2012). Palmitoylation of synaptic proteins such as PSD-95 and AMPARs is a key regulator of synaptic function and plasticity (Fukata and Fukata, 2010). It will therefore be intriguing to assess how palmitoylation might regulate the localization and function of ankyrin-G at glutamatergic synapses.
The ankyrin-associated membrane-protein complex interacts with the spectrin/actin cytoskeleton via the spectrin binding domain, through the 160 amino acid ZU5 motif (Mohler et al., 2004; Wang et al., 2012). This motif has conserved sites, such as DAR999 and S2417, which are essential for spectrin binding in the smaller and larger isoforms of ankyrin-G respectively (Jenkins et al., 2015; Kizhatil et al., 2007). Interestingly, mutation of DAR999 in ankyrin-G-190 reduces its synaptic targeting and impairs dendritic spine structure, underlining the importance of this interaction for glutamatergic synapse stability (Smith et al., 2014).
The C-terminal regulatory domain is comprised of a highly conserved ‘death domain’ and unstructured stretch of 300 amino acids, which modulates interactions with the membrane-binding and spectrin-binding domains (Davis et al., 1992; Hall and Bennett, 1987). Indeed, ankyrins can adopt an autoinhibited conformation to modulate their binding to target membrane-proteins (Abdi et al., 2006; Wang et al., 2014). A recent study has elucidated the autoinhibition mechanisms of ankyrin-B and ankyrin-G: both contain three differing autoinhibition segments within their tail regions, which interact with different sites within their membrane-binding domains (Chen et al., 2017). This therefore enables both -B and -G isoforms to autoregulate in differing ways, resulting in diversity of binding partners and hence cellular localization.
Ankyrins at the neuromuscular junction (NMJ)
The earliest studies of ankyrins at synapses were conducted in mammalian skeletal muscle NMJ, and revealed the primary characteristics of ankyrin function: as an organizer of channels and other proteins in specific membrane domains. Studies of the perijunctional membrane of mammalian NMJ showed that ankyrin-G was localized with spectrin in postsynaptic specializations, which were enriched with voltage-gated sodium channels, (Bailey et al., 2003; Flucher and Daniels, 1989; Kordeli et al., 1998; Wood and Slater, 1998). Further insight into the function of ankyrins at synapses was provided by studies of the Drosophila NMJ. At the presynaptic site of the NMJ a novel giant brain-specific ankyrin2 was identified, the loss of which caused synapse disassembly and retraction, disruption of neuronal excitability and NMJ morphology (Koch et al., 2008; Pielage et al., 2008). Giant ankyrin2 directly binds and organizes microtubules, thereby contributing to the stability of the presynaptic site and active zones. These studies were the first to describe the organization of ankyrin as a lattice structure and propose the property of this lattice as a linker between synaptic membrane proteins and spectrin, and therefore the underlying microtubule cytoskeleton. In addition, inactivating mutations in ankyrin2 significantly affected NMJ stability, reducing synaptic terminal size by disintegrating the synaptic cytoskeleton and disassembling active zones (Koch et al., 2008).
Ankyrin-dependent mechanisms at the Drosophila NMJ also exist to stabilize synapses during development. For instance, ankyrin2 phosphorylation by CK2 promotes presynaptic organization and stability by linking synaptic cell adhesion molecules to microtubules, therefore providing a novel mechanism to actively control the development and longevity of synapses (Bulat et al., 2014). Another study shows that interactions between ankyrin2 and the L1-type cell adhesion molecule, neuroglian, are important in controlling the balance of synapse growth and stability at the Drosophila NMJ, as well as coordinating pre- and post-synaptic development in the giant fiber CNS synapse (Enneking et al., 2013). Together, the work on ankyrin proteins at the Drosophila NMJ shows that ankyrins form a lattice structure that functions to organize and stabilize the pre-synaptic cytoskeleton through interactions with spectrin, which is also important for pre- and post-synaptic development.
Ankyrins at the mammalian inhibitory synapse
Axon initial segment (Axo-axonal connections)
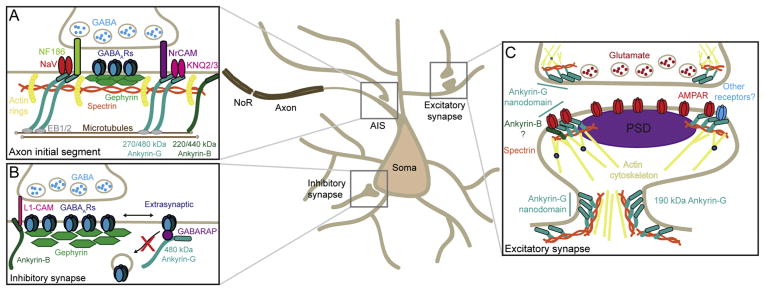
The AIS is a specialized neuronal membrane domain found at the most proximal region of the axon and the site of inhibitory axo-axonal synapses. It harbors high densities of voltage-gated ion channels, scaffolding molecules and cytoskeletal components, and is primarily responsible for action potential initiation and axonal polarity (Nelson and Jenkins, 2017; Rasband, 2010). The 270/480kDa isoforms of ankyrins are highly expressed at the AIS and are considered ‘master organizers’ of this essential structure due to the dependence of multiple AIS-associated proteins on the presence of ankyrin for their clustering (including neurofascin-186 (NF-186), β1V-spectrin and numerous ion channels (Nelson and Jenkins, 2017; Rasband, 2010)). In addition to the organization of the AIS, ankyrins and their binding partners play a key role in the targeting of GABAergic interneuron presynaptic inputs onto the AIS of excitatory neurons (Figure 2A). GABAergic innervation in this region is essential for controlling the excitability, firing frequency and input-output relationship of neurons in numerous brain regions including hippocampus, cortex and cerebellum (Klausberger and Somogyi, 2008; Kole and Stuart, 2012). GABAergic synapses in the CNS are composed of GABAA receptor (GABAAR) clusters, which are organized by the scaffolding protein gephyrin ((Smith and Kittler, 2010), Figure 2B): this is also the case for GABAAR clusters at the AIS (Muir and Kittler, 2014). AIS GABAARs are predominantly composed of the α2 subunit, which directly binds to gephyrin (Tretter and Moss, 2008), and gephyrin in turn also indirectly associates with NF-186, which is responsible for targeting inhibitory presynaptic boutons to the AIS (Burkarth et al., 2007). Therefore, although they are not thought to directly interact with the inhibitory PSD at the AIS, ankyrins do anchor NF-186 and are therefore likely in close proximity to gephyrin and GABAAR clusters, and important for their overall stability.
Figure 2. Ankyrin functions at synapses.
A. Ankyrins at axo-axonic synapses at the axon initial segment (AIS). Larger isoforms of ankyrin-G and ankyrin-B are found in myelinated and unmyelinated axons respectively. Ankyrin-G scaffolds ion channels and cell adhesion molecules, the latter of which recruit presynaptic terminals of interneurons to the AIS.
B. Ankyrins at somato-dendritic inhibitory synapses. The giant 480 kDa isoform of ankyrin-G is localized to the extrasynaptic regions of somato-dendritic inhibitory synapses. Here it also functions to stabilize the synapse through an interaction with the GABAAR associated protein (GABARAP), whereby it opposes the endocytosis of extrasynaptic GABAARs. Ankyrin-B is also thought to contribute to stability of perisomatic and proximal dendritic inhibitory synapses through L1-CAM.
C. Ankyrins at the dendritic spine. Ankyrin-G forms nanodomains at perisynaptic regions and in the spine neck, where it interacts with spectrin/actin cytoskeleton to modulate spine head and neck dimensions. Ankyrin-G interacts with AMPA receptors (AMPARs) and promotes receptor stability at the synaptic site. Ankyrin-G is also localized at the presynaptic terminals of glutamatergic synapses of CNS neurons, and of the neuromuscular junction.
The importance of ankyrin-G at the AIS was originally demonstrated in cerebellar Purkinje cells, which are innervated at the AIS by inhibitory basket cell axons, forming ‘pinceau synapses’. Knock-out of all ank3 isoforms in mice caused disruption of the subcellular gradient of NF-186 which exists along the AIS-soma axis (Ango et al., 2004; Zhou et al., 1998). NF-186 is imperative for correct localization of inhibitory synapses originating from basket cell axons and in the absence of ankyrin, these synapses are mislocalized, underlying the importance of ankyrins in correct inhibitory synapse clustering at the AIS (Ango et al., 2004; Buttermore et al., 2012). Ankyrin-G has a similar function at the AIS of cortical excitatory neurons, which receive presynaptic inputs from chandelier cells, forming GABAergic cartridge synapses. The organization of these synapses is highly dependent on the presence of ankyrin proteins (Guan and Maness, 2010; Inda et al., 2006; Zhu et al., 2017). In vivo, conditional knock-out of ankyrin-G in cortical pyramidal neurons later in development reveals a dramatic loss of AIS cartridge synapses, leading to elevated cortical activity and behavioral phenotypes that are reminiscent of bipolar disorder (Zhu et al., 2017). In addition, a study of the AIS in monkey prefrontal cortex during development suggests that this ankyrin-G-dependent organization of the AIS changes over time, where ankyrin-G and βIV-spectrin define an early postnatal developmental stage of synapse formation and stabilization at the AIS (Cruz et al., 2009). Combined, these studies suggest a common mechanism by which ankyrin-G organizes adhesion molecules that that subsequently contribute to the subcellular synaptic organization of inhibitory synapses at the AIS and is essential for both the formation and maintenance of these synapses, and thereby correct excitability of the neuron.
Ankyrin-G at somatodendritic inhibitory synapses
GABAergic synapses target not only the AIS, but also the soma and dendrites of neurons (Megias et al., 2001). Until recently, the functions of ankyrin-G have been thought to be restricted to the AIS and NoR in neurons. However, recent studies have suggested important roles for ankyrins at the synapses of neuronal dendrites (Smith et al., 2014; Tseng et al., 2015). Tseng and colleagues recently showed that the giant 480kDa isoform of ankyrin-G is present at inhibitory synapses in the somatodendritic compartment of pyramidal neurons. Here, ankyrin-G forms micron-scale domains which associate with extrasynaptic GABAARs, suggesting it is not directly associated with the inhibitory PSD ((Tseng et al., 2015), Figure 2B). Functionally, this pool of ankyrin-G is thought to contribute to the stability of GABAergic synapses through opposition of GABAAR endocytosis, via an interaction with GABARAP, a GABAAR-associated protein (Fig 2B; Tseng 2015). Interestingly, this paper also suggests that the ankyrin-G-GABARAP interaction might be important for targeting inhibitory synapses to the AIS, pointing to potential common mechanisms of GABAergic synapse stability in both membrane regions. Another group also suggested ankyrin-B may contribute to the development and stability of perisomatic and proximal dendritic inhibitory synapses in the mouse cingulate cortex, possibly through an interaction with L1-CAM (Guan and Maness, 2010). Clearly further work is now required to assess how the interactions between these different proteins lead to the stability of inhibitory synapses outside of the AIS and therefore maintain inhibition.
Ankyrins at the PSD of the mammalian glutamatergic synapse
In addition to its functions at somatodendritic inhibitory synapses, it has now become clear that ankyrins also serve to facilitate synaptic function and plasticity. More than a decade ago, proteomic analyses detected all three ankyrins in rodent PSD preparations (Collins et al., 2006; Jordan et al., 2004; Peng et al., 2004). Later analysis of the hippocampal PSD proteome after treatment with the mood stabilizing drugs lithium and valproate showed increased levels of the 190kDa ankyrin-G in synapses (Nanavati et al., 2011), thus linking ankyrin-G and excitatory synapses to psychiatric disease. More recently, our own laboratory used a range of techniques to show that the 190kDa isoform of ankyrin-G is integral for maintenance of spine morphology and glutamatergic synaptic transmission (Smith et al., 2014), and the first to demonstrate a function for ankyrin-G in neurons outside its canonical functions at the AIS and NoR.
A role for Ankyrin-G 190kDa isoform in synapse maintenance
The longer isoforms of ankyrin-G have well-defined functions at the AIS and NoR; however, 190 kDa ankyrin-G has a key role in the maintenance and function of mature cortical synapses (Figure 2C). RNAi knock-down of all ankyrin-G isoforms in cultured cortical neurons causes reduced dendritic spine area, mEPSC amplitude and AMPAR clustering, indicating that ankyrin-G is important for maintaining the strength of excitatory synapses (Smith et al., 2014). Interestingly, these effects were rescued with an RNAi-resistant form of the 190kDa isoform of ankyrin-G (ankyrin-G-190) but not the longer, 270kDa isoform (ankyrin-G-270), suggesting that ankyrin-G-190 may be mediating these effects. In addition, confocal and super-resolution imaging of ankyrin-G-190 tagged with GFP showed enrichment of this isoform at postsynaptic sites within mature spines, compared with ankyrin-G-270, which was restricted to the AIS. Together, the ability of ankyrin-G-190 isoform to rescue the phenotypes elicited by ankyrin-G RNAi, and the opposing effects that overexpression of this isoform has on spine morphology suggests that ankyrin-G-190 is present in spines and is important for the maintenance of glutamatergic synapse morphology and efficacy.
Ankyrin-G forms nanoscale clusters in dendritic spines
Confocal imaging demonstrated the colocalization of endogenous ankyrin-G with glutamatergic synaptic markers, however higher resolution imaging was required to uncover nanoscale subsynaptic detail that would point to potential functions for ankyrin-G. The advent of super-resolution microscopy methodologies has facilitated imaging beyond the diffraction limit of light, providing unprecedented insight into the nanoscale architecture of synapses (reviewed in detail in (MacGillavry and Hoogenraad, 2015; Tonnesen and Nagerl, 2013)). Indeed, several recent papers have utilized these methods to show the nanoscale organization of AMPARs, PSD95, actin, adhesion molecules and CamKII (Frost et al., 2010; Lu et al., 2014; MacGillavry et al., 2013; Smith et al., 2017; Tang et al., 2016). Smith et al. used the versatile super-resolution method Structured Illumination Microscopy (SIM), to image ankyrin-G in dendritic spines, with a cell fill to visualize spine morphology and either immunolabeled PSD95 or GluA1 subunit-containing AMPARs. These imaging experiments revealed that, in contrast a classical scaffold, ankyrin-G forms distinct condensed clusters of ~130nm in diameter, that are organized perisynaptically around the PSD, and within the spine neck ((Smith et al., 2014), Figure 2C).
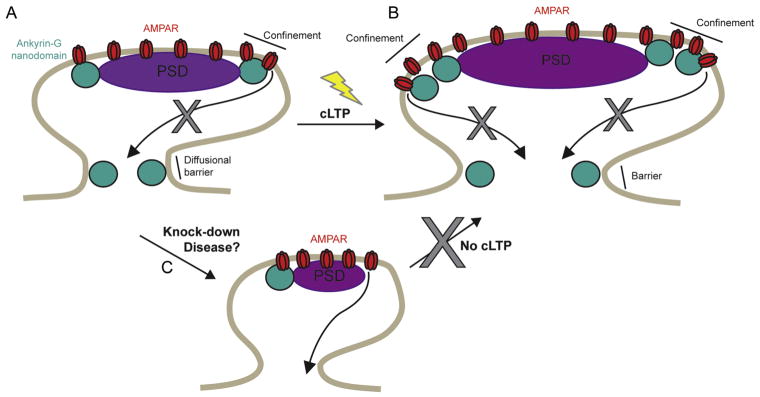
What are the functions of perisynaptic and spine neck pools of ankyrin-G? As described above, knock-down studies show that ankyrin-G is required for the stability and maintenance of spines and AMPAR-mediated currents by stabilizing AMPARs in the spine. We propose that ankyrin-G performs this task in two ways: (1) as a perisynaptic scaffold and (2) by contributing to a diffusional barrier in the spine neck (Figure 3A). The perisynaptic pool of ankyrin-G may be important in the organization of the distinct perisynaptic region which boarders the PSD. This perisynaptic region is thought to be compositionally different from the PSD: metabotropic glutamate receptors (mGluRs, (Lopez-Bendito et al., 2002)), dopamine D1 receptors (Ladepeche et al., 2013) and the endocytic machinery (Blanpied et al., 2002) are thought to localize to this region. Moreover, the perisynaptic region exhibits a highly dynamic pool of continuously polymerizing F-actin (Frost et al., 2010), which contributes to the continual morphological distortion of the PSD and the anchoring of AMPARs. Functionally, the perisynaptic region is thought to control the exchange between synaptic and extrasynaptic glutamate receptors and provide a reservoir of ‘back-up’ glutamate receptors, poised to move into the PSD to potentiate synaptic transmission (Tardin et al., 2003). The position of ankyrin-G in this region, its role as a scaffold linking membrane proteins to the cytoskeleton and the observation that ankyrin-G complexes with GluA1 from brain extracts, suggests that it may act as a scaffold for perisynaptic AMPARs and other membrane proteins. Therefore, ankyrin-G likely stabilizes this perisynaptic region and ensures an abundance of extra AMPA receptors are readily available. In addition, the direct links between ankyrin-G and the spectrin-actin cytoskeleton at the perisynaptic region and in the spine head as a whole would add structural stability to the spine and therefore promote AMPAR retention at the synapse.
Figure 3. A role for ankyrin-G in synaptic plasticity.
A. In dendritic spines ankyrin-G functions to confine AMPA receptors (AMPARs) and stabilize them at the PSD. B. During chemical LTP stimuli, more ankyrin-G moves into the spine where it further stabilizes the PSD and AMPARS, and likely contributes to structural enlargement and stability of the spine through its interactions with the actin-spectrin cytoskeleton. C. Knock-down of ankyrin-G causes decreased spine area and glutamatergic synaptic transmission, and makes spines unable to undergo cLTP-dependent spine enlargement.
The second mechanism by which ankyrin-G contributes to spine and AMPAR stability is likely through its localization to the spine neck, an essential structure that underlies the biochemical and electrical compartmentalization of excitatory synaptic signals (Tonnesen and Nagerl, 2016). The spine neck functions as a diffusional barrier that restricts molecular exchange between the spine head and dendritic shaft, thereby compartmentalizing synaptic signaling events (Ashby et al., 2006; Kusters et al., 2013; Simon et al., 2014). This is supported by simultaneous analysis of diffusive cytosolic proteins in and out of the spine showing that spine neck width is a significant determinant of the ability of proteins to pass through the spine neck (Takasaki and Sabatini, 2014; Tonnesen et al., 2014). Importantly, spine neck geometry changes with neuronal activity (Bloodgood and Sabatini, 2005; Grunditz et al., 2008; Smith et al., 2014; Takasaki and Sabatini, 2014; Tonnesen et al., 2014). Indeed, remarkable live-cell super-resolution imaging of spines in hippocampal slices revealed that spine neck diameter increases during LTP induced by glutamate-uncaging at the spine head, with important implications for neck resistance and diffusional recovery of the spine (Tonnesen et al., 2014).
Despite its importance in neuronal function, the molecular mechanisms that control spine neck morphology and function remain undefined. To address this dearth of mechanistic knowledge, multiple groups have employed super-resolution microscopy and electron microscopy to aid studies of the spine neck molecular composition. Photoactivated Localization Microscopy (PALM) has been used to visualize dynamically polymerizing F-actin (Frost et al., 2010), immunogold-EM has been utilized to localize septin-7 and βIII-spectrin to the neck region (Efimova et al., 2017; Ewers et al., 2014), and synaptopodin was recently shown to localize with F-actin in the neck (Wang et al., 2016). Using SIM imaging, ankyrin-G nanodomains were observed in the neck of 74% of spines in addition to its presence in the spine head (Smith et al., 2014). Spines with ankyrin-G in the neck were larger contained a higher abundance of AMPARs, but not PSD95, suggesting that neck ankyrin-G plays a role in modulating the synaptic localization of dynamic, diffusive proteins rather than the relatively static PSD itself. Further, the overexpression of ankyrin-G-190 in the spine neck in addition to the head also was associated with larger spine heads and wider spine necks in mature spines (Smith et al., 2014), suggesting that ankyrin-G may play a direct role in modulating spine neck morphology. This is likely due to association with F-actin in this region via an interaction with β-spectrin, which is also abundant in the spine neck (Efimova et al., 2017) and is essential for synaptic function (Nestor et al., 2011). More work is now required, with the assistance of super-resolution microscopy modalities, to understand how proteins that are found in the spine neck work in concert to regulate spine dimensions and generate a diffusional barrier.
Together, in dendritic spines, ankyrin-G contributes to maintaining the synaptic AMPAR complement by their stabilization at the perisynaptic region where they can be readily added to the PSD to potentiate synaptic function. Neck ankyrin-G also likely contributes to a diffusional barrier in the spine neck (akin its function at the AIS), limiting the mobility of AMPARs (and presumably other membrane proteins), preventing their movement out of the spine and thereby maintaining their presence in the spine head.
Ankyrin-G contributes to LTP-dependent spine enlargement
In addition to its importance in the maintenance of glutamatergic synapses, ankyrin-G is essential for spine plasticity during LTP. Knock-down of ankyrin-G in cortical neurons impairs the ability of spines to undergo enlargement during a chemical LTP (cLTP) protocol (Figure 3 (Smith et al., 2014)). Moreover, SIM imaging shows that during cLTP, ankyrin-G nanodomains accumulate in spines and demonstrate greater overlap with AMPARs during LTP (Smith et al., 2014). Considering that overexpression of ankyrin-G in spines causes increased spine area, it is likely that ankyrin-G accumulation in spines during LTP plays a key role in spine enlargement during synaptic plasticity. During this early stage of LTP, actin undergoes rapid polymerization, CamKII is activated, and specific proteins such as AMPARs and actin remodeling proteins are trafficked into the spine head (Sala and Segal, 2014). This, combined with the location of ankyrin-G nanodomains at the spine neck and at the interface of the PSD and the actin cytoskeleton, regulatory sites for synaptic plasticity (Cingolani and Goda, 2008), points to a key role for ankyrin-G in the modulation of spine LTP. The scaffolding role of ankyrin-G also makes it a major candidate for providing more anchoring platforms for multiple membrane proteins including ion channels and NMDARs, which are enriched in the spine during plasticity and are essential for LTP induction and expression.
These studies in cultured cortical neurons suggest an in vivo role for ankyrin-G in learning and memory. Support for this comes from analysis of hippocampal lysates from mice that have undergone a learning task: significantly more 190 kDa ankyrin-G was found in the membrane and cytoskeletal fractions of trained mice compared with control mice, indicating that a physiological learning paradigm can cause relocation of a pool of ankyrin-G in vivo (Smith et al., 2014). This is also corroborated by a study using Drosophila showing that knockdown of ankyrin2 results in reduced synapse size and number, and behavioral deficits in short-term memory (Iqbal et al., 2013). Further work will now be required to fully determine the role(s) of synaptic ankyrin-G in synaptic plasticity and in learning and memory in vivo.
Roles of ankyrins in neurodevelopmental and psychiatric disease
As a key organizer of multiple types of synapses, disruption of ankyrin function and expression might be expected to have detrimental effects on numerous brain functions. Accordingly, the human ANK3 gene is associated with a variety of neuropsychiatric and cognitive disorders including bipolar disorder (BD), schizophrenia, autism spectrum disorder (ASD), intellectual disability (ID) and ADHD (Ferreira et al., 2008; Schulze et al., 2009; Scott et al., 2009; Smith et al., 2009). The role that alterations in ANK3 play in the pathogenesis of these disorders remains elusive, however, disrupted synaptic function (either at the AIS, inhibitory or excitatory synapses) is a likely mechanism due to the convergence of synaptic pathology as an underlying mechanism for these disorders (Forrest et al., 2018; Penzes et al., 2011). Indeed, function of ankyrin-G as a synaptic scaffold, anchoring AMPA receptors, GABAARs and a plethora of ion channels at important neuronal contact sites, suggests that its disruption would cause myriad alterations to the excitatory/inhibitory balance in neurons and circuits, which is likely to lead to alterations in information processing, cognition and behavior (Gao and Penzes, 2015; Yizhar et al., 2011). Indeed, the fact that mutations and polymorphisms in ANK3 link this gene to a wide range of mental disorders, point to both how critical ankyrin-G is to neuronal function, and how disruption in its expression can lead to a variety of overlapping behavioral and cognitive characteristics
Multiple independent genome wide association studies (GWAS) have strongly linked ANK3 to bipolar disorder (Baum et al., 2008; Ferreira et al., 2008; Schulze et al., 2009; Tesli et al., 2011), making it one of the leading BD risk genes. These association studies have been replicated in a variety of different types and sizes of populations, thereby strongly supporting the association between BD and ANK3 (Muhleisen et al., 2014; Roby, 2017; Scott et al., 2009). Furthermore, genome sequencing has revealed BD-associated rare variants in ANK3 (Ament et al., 2015; Hughes et al., 2016), although how they affect ankyrin-G expression and function remains to be determined. The causality of BD has a large genetic component, with heritability estimated at 59–93% (Kieseppa et al., 2004; Lichtenstein et al., 2009; McGuffin et al., 2003), however the pathogenesis of BD is poorly understood, exemplified by the continued use of the mood-stabilizer, lithium, to treat BD patients. Interestingly, the expression of the 190kDa isoform in rat hippocampal PSDs increases after treatment with lithium, underscoring the relevance of ankyrin-G function in BD brains (Nanavati et al., 2011).
Although, primarily thought of as a BD-associated gene, ANK3 is also genetically linked to multiple other neuropsychiatric disorders. GWAS and meta-analysis have identified ANK3 as an important schizophrenia risk factor (Nie et al., 2015; Schizophrenia Psychiatric Genome-Wide Association Study, 2011)). Moreover, analysis of post-mortem brain tissue from schizophrenic patients reveal a 15–19% loss of ankyrin-G expression in the AIS of pyramidal neurons in the superficial cortex compared to controls (Cruz et al., 2009). Rare pathogenic mutations in ANK3 have been identified in patients with ID/ADHD (Bonnet-Brilhault et al., 2016; Iqbal et al., 2013; Kloth et al., 2017) and ASD (Bi et al., 2012; Shi et al., 2013). Inactivating mutations in the ANK3 gene result in severe cognitive deficit. A balanced translocation, that disrupts expression of all ANK3 isoforms, was identified in a patient with cognitive deficits, ADHD and ASD (Iqbal et al., 2013). Further, the same study reported the first familial mutation in ANK3, identified in a family with autosomal recessive ID and severe behavioral problems (Iqbal et al., 2013). This mutation produced a premature stop codon in the 480 kDa ankyrin-G isoform, leading to severely reduced expression. Together, the identification of ANK3 disruption in patients with these disorders supports the functions of ankyrin-G at synapses and the importance of ankyrin-G in cognitive function in humans.
Modeling BD with Ankyrin-G KO mice
Several mouse models of ankyrin-G depletion have been generated and characterized, showing a set of psychiatric-related behaviors consistent with ANK3 involvement in neuropsychiatric disorders and the functions of ankyrin-G at synaptic sites. Ablation of brain-specific Ank3 isoforms in the cerebellum causes early onset ataxia, due to impaired action potential firing at the AIS of purkinje cells in the cerebellum (Zhou et al., 1998). In comparison, heterozygous knock out of brain-specific Ank3 isoforms in mice results in altered mood related behaviors such as reduced anxiety and increased reward motivation. Interestingly, the behavioral traits of Ank3+/− mice transitioned to depression-related features after chronic stress, a trigger of mood episodes in BD (Leussis et al., 2013). In addition, viral-mediated RNA interference of Ank3 expression in the hippocampal dentate gyrus also induced decreased anxiety-related behaviors and increased activity, which were attenuated by chronic treatment with the mood stabilizer lithium (Leussis et al., 2013). This finding was replicated in another study (Gottschalk et al., 2017), in which the authors used proteomics of Ank3+/− mice treated with lithium to analyze changes in protein levels with the view of determining mechanism of action for this drug. This revealed that axonal transport and the kinesin family of motor proteins may be important for the function of lithium in Ank3+/− mice, in concert with changes to the glutamatergic signaling system. Further evidence linking ankyrin-G function to BD-like behavior was recently provided through study of a conditional Ank3 KO mouse, where Ank3 expression was reduced in pyramidal neurons of the adult forebrain (Zhu et al., 2017). This resulted in loss of pyramidal neuron AIS voltage-gated sodium and potassium channels, loss of GABAergic cartridge synapses, and increased c-fos expression suggesting increased neuronal activity due to disinhibition. Importantly, these mice showed behavioral phenotypes reminiscent of aspects of human mania, ameliorated by lithium and valproate (Zhu et al., 2017). These behavioral deficits have been further replicated further in heterozygous KO mice that display elevated anxiety, depression and cognitive impairment, combined with reduced size of various brain regions including hippocampus and motor cortex (Liu et al., 2017; van der Werf et al., 2017).
Given the important role of synapse dysfunction in these neuropsychiatric disorders, it is therefore tempting to speculate that at least some of these phenotypes are caused by ankyrin-mediated dysfunction of synapses, alterations to E/I balance and disrupted circuitry in critical forebrain regions. However, additional work will be required to determine the underlying cellular mechanisms, and which isoforms of ankyrin-G, at which types of synapses contribute to specific behavioral phenotypes.
Conclusions and future challenges
The study of ankyrins at dendritic synapses is still in its infancy, however, common mechanisms of ankyrin function at all types of synapses have emerged, providing essential maintenance of synapse structure and function. It is clear that ankyrins form micro/nanodomains in specific regions of the NMJ, and at excitatory and inhibitory PSDs. Furthermore, a membrane protein-ankyrin-spectrin complex forms a lattice under the plasma membrane, where it can both anchor synaptic membrane proteins, in addition to intracellular binding-partners. Although there is now an outline for ankyrin function at synapses outside of the AIS, there are significant gaps in our knowledge leading to many unanswered questions. For instance, what other glutamatergic and GABAergic synaptic proteins does ankyrin-G complex with? Similarly to at the AIS, it is likely that ankyrin-G interacts with numerous receptors, channels, adhesion proteins and signaling molecules in dendritic spines and at somatodendritic inhibitory synapses. Identification of these binding-partners will provide us with a greater understanding of the role that ankyrin-G plays at these synapses and its involvement in mechanisms that mediate synaptic function and plasticity.
Are other ankyrins present at CNS synapses? Proteomic analysis suggests that all three ankyrin proteins (-R, -B and -G) are expressed in glutamatergic PSDs (Collins et al., 2006; Jordan et al., 2004; Peng et al., 2004), suggesting that in addition to ankyrin-G, ankyrin-R and ankyrin-B may also have important functions in dendritic spines. Determining cell-type and synapse-type abundance of these ankyrins will be paramount in understanding how these proteins work together to maintain synapse strength and contribute to synaptic plasticity. Additionally, little is known about ankyrin function at glutamatergic or GABAergic presynaptic terminals. At the NMJ, ankyrin-B functions to create a lattice network around which the presynaptic machinery can be organized (Koch et al., 2008; Pielage et al., 2008). Further, ankyrin-G localizes with the excitatory and inhibitory presynaptic marker, bassoon (Smith et al., 2014), therefore it is possible that ankyrins may have a similar functions at mammalian CNS synapses, making them essential for presynaptic function and therefore synaptic transmission. Finally, a role for ankyrins in protein trafficking described in non-neuronal cells (Devarajan et al., 1996; Hoock et al., 1997) and axonal transport (Barry et al., 2014; Lorenzo et al., 2014) also opens up new avenues of research, and intriguing possibilities for ankyrin function in trafficking of key proteins to and from the synapse. These represent just a handful of potential interesting future directions with the aim of fully understanding how ankyrins contribute to synapse function and plasticity. Importantly, future work focused on revealing the mechanisms of ankyrin function at synapses will likely provide insight into novel therapy targets for neuropsychiatric disorders.
Highlights.
Ankyrin proteins link membrane proteins to the submembranous actin cytoskeleton
Ankyrins organize synapses, in addition to their canonical roles in the axon
Dendritic spines rely on ankyrin-G for proper stability and synaptic plasticity
Synaptic functions of ankyrins may contribute to neuropsychiatric diseases
Acknowledgments
This work was supported by NINDS R01 MH107182 (PP) and a NARSAD Young Investigator grant (KRS). We thank Katlin Hahm for comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdi KM, Mohler PJ, Davis JQ, Bennett V. Isoform specificity of ankyrin-B: a site in the divergent C-terminal domain is required for intramolecular association. The Journal of biological chemistry. 2006;281:5741–5749. doi: 10.1074/jbc.M506697200. [DOI] [PubMed] [Google Scholar]
- Ament SA, Szelinger S, Glusman G, Ashworth J, Hou L, Akula N, Shekhtman T, Badner JA, Brunkow ME, Mauldin DE, et al. Rare variants in neuronal excitability genes influence risk for bipolar disorder. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:3576–3581. doi: 10.1073/pnas.1424958112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ango F, di Cristo G, Higashiyama H, Bennett V, Wu P, Huang ZJ. Ankyrin-based subcellular gradient of neurofascin, an immunoglobulin family protein, directs GABAergic innervation at purkinje axon initial segment. Cell. 2004;119:257–272. doi: 10.1016/j.cell.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Ashby MC, Maier SR, Nishimune A, Henley JM. Lateral diffusion drives constitutive exchange of AMPA receptors at dendritic spines and is regulated by spine morphology. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:7046–7055. doi: 10.1523/JNEUROSCI.1235-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey SJ, Stocksley MA, Buckel A, Young C, Slater CR. Voltage-gated sodium channels and ankyrinG occupy a different postsynaptic domain from acetylcholine receptors from an early stage of neuromuscular junction maturation in rats. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:2102–2111. doi: 10.1523/JNEUROSCI.23-06-02102.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry J, Gu Y, Jukkola P, O’Neill B, Gu H, Mohler PJ, Rajamani KT, Gu C. Ankyrin-G directly binds to kinesin-1 to transport voltage-gated Na+ channels into axons. Dev Cell. 2014;28:117–131. doi: 10.1016/j.devcel.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum AE, Hamshere M, Green E, Cichon S, Rietschel M, Noethen MM, Craddock N, McMahon FJ. Meta-analysis of two genome-wide association studies of bipolar disorder reveals important points of agreement. Molecular psychiatry. 2008;13:466–467. doi: 10.1038/mp.2008.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett V, Lorenzo DN. Spectrin- and ankyrin-based membrane domains and the evolution of vertebrates. Curr Top Membr. 2013;72:1–37. doi: 10.1016/B978-0-12-417027-8.00001-5. [DOI] [PubMed] [Google Scholar]
- Bi C, Wu J, Jiang T, Liu Q, Cai W, Yu P, Cai T, Zhao M, Jiang YH, Sun ZS. Mutations of ANK3 identified by exome sequencing are associated with autism susceptibility. Human mutation. 2012;33:1635–1638. doi: 10.1002/humu.22174. [DOI] [PubMed] [Google Scholar]
- Blanpied TA, Scott DB, Ehlers MD. Dynamics and regulation of clathrin coats at specialized endocytic zones of dendrites and spines. Neuron. 2002;36:435–449. doi: 10.1016/s0896-6273(02)00979-0. [DOI] [PubMed] [Google Scholar]
- Bloodgood BL, Sabatini BL. Neuronal activity regulates diffusion across the neck of dendritic spines. Science. 2005;310:866–869. doi: 10.1126/science.1114816. [DOI] [PubMed] [Google Scholar]
- Bonnet-Brilhault F, Alirol S, Blanc R, Bazaud S, Marouillat S, Thepault RA, Andres CR, Lemonnier E, Barthelemy C, Raynaud M, et al. GABA/Glutamate synaptic pathways targeted by integrative genomic and electrophysiological explorations distinguish autism from intellectual disability. Molecular psychiatry. 2016;21:411–418. doi: 10.1038/mp.2015.75. [DOI] [PubMed] [Google Scholar]
- Bulat V, Rast M, Pielage J. Presynaptic CK2 promotes synapse organization and stability by targeting Ankyrin2. The Journal of cell biology. 2014;204:77–94. doi: 10.1083/jcb.201305134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkarth N, Kriebel M, Kranz EU, Volkmer H. Neurofascin regulates the formation of gephyrin clusters and their subsequent translocation to the axon hillock of hippocampal neurons. Molecular and cellular neurosciences. 2007;36:59–70. doi: 10.1016/j.mcn.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Buttermore ED, Piochon C, Wallace ML, Philpot BD, Hansel C, Bhat MA. Pinceau organization in the cerebellum requires distinct functions of neurofascin in Purkinje and basket neurons during postnatal development. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:4724–4742. doi: 10.1523/JNEUROSCI.5602-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Li J, Wang C, Wei Z, Zhang M. Autoinhibition of ankyrin-B/G membrane target bindings by intrinsically disordered segments from the tail regions. Elife. 2017;6 doi: 10.7554/eLife.29150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cingolani LA, Goda Y. Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nature reviews Neuroscience. 2008;9:344–356. doi: 10.1038/nrn2373. [DOI] [PubMed] [Google Scholar]
- Collins MO, Husi H, Yu L, Brandon JM, Anderson CN, Blackstock WP, Choudhary JS, Grant SG. Molecular characterization and comparison of the components and multiprotein complexes in the postsynaptic proteome. Journal of neurochemistry. 2006;97(Suppl 1):16–23. doi: 10.1111/j.1471-4159.2005.03507.x. [DOI] [PubMed] [Google Scholar]
- Cruz DA, Lovallo EM, Stockton S, Rasband M, Lewis DA. Postnatal development of synaptic structure proteins in pyramidal neuron axon initial segments in monkey prefrontal cortex. The Journal of comparative neurology. 2009;514:353–367. doi: 10.1002/cne.22006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha SR, Mohler PJ. Obscurin targets ankyrin-B and protein phosphatase 2A to the cardiac M-line. The Journal of biological chemistry. 2008;283:31968–31980. doi: 10.1074/jbc.M806050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha SR, Mohler PJ. Ankyrin protein networks in membrane formation and stabilization. Journal of cellular and molecular medicine. 2009;13:4364–4376. doi: 10.1111/j.1582-4934.2009.00943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis LH, Davis JQ, Bennett V. Ankyrin regulation: an alternatively spliced segment of the regulatory domain functions as an intramolecular modulator. The Journal of biological chemistry. 1992;267:18966–18972. [PubMed] [Google Scholar]
- Devarajan P, Stabach PR, Mann AS, Ardito T, Kashgarian M, Morrow JS. Identification of a small cytoplasmic ankyrin (AnkG119) in the kidney and muscle that binds beta I sigma spectrin and associates with the Golgi apparatus. The Journal of cell biology. 1996;133:819–830. doi: 10.1083/jcb.133.4.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efimova N, Korobova F, Stankewich MC, Moberly AH, Stolz DB, Wang J, Kashina A, Ma M, Svitkina T. betaIII Spectrin Is Necessary for Formation of the Constricted Neck of Dendritic Spines and Regulation of Synaptic Activity in Neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2017;37:6442–6459. doi: 10.1523/JNEUROSCI.3520-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enneking EM, Kudumala SR, Moreno E, Stephan R, Boerner J, Godenschwege TA, Pielage J. Transsynaptic coordination of synaptic growth, function, and stability by the L1-type CAM Neuroglian. PLoS Biol. 2013;11:e1001537. doi: 10.1371/journal.pbio.1001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewers H, Tada T, Petersen JD, Racz B, Sheng M, Choquet D. A Septin-Dependent Diffusion Barrier at Dendritic Spine Necks. PloS one. 2014;9:e113916. doi: 10.1371/journal.pone.0113916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira MA, O’Donovan MC, Meng YA, Jones IR, Ruderfer DM, Jones L, Fan J, Kirov G, Perlis RH, Green EK, et al. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nature genetics. 2008;40:1056–1058. doi: 10.1038/ng.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flucher BE, Daniels MP. Distribution of Na+ channels and ankyrin in neuromuscular junctions is complementary to that of acetylcholine receptors and the 43 kd protein. Neuron. 1989;3:163–175. doi: 10.1016/0896-6273(89)90029-9. [DOI] [PubMed] [Google Scholar]
- Forrest MP, Parnell E, Penzes P. Dendritic structural plasticity and neuropsychiatric disease. Nature reviews Neuroscience. 2018;19:215–234. doi: 10.1038/nrn.2018.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost NA, Shroff H, Kong H, Betzig E, Blanpied TA. Single-molecule discrimination of discrete perisynaptic and distributed sites of actin filament assembly within dendritic spines. Neuron. 2010;67:86–99. doi: 10.1016/j.neuron.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata Y, Fukata M. Protein palmitoylation in neuronal development and synaptic plasticity. Nature reviews Neuroscience. 2010;11:161–175. doi: 10.1038/nrn2788. [DOI] [PubMed] [Google Scholar]
- Galiano MR, Jha S, Ho TS, Zhang C, Ogawa Y, Chang KJ, Stankewich MC, Mohler PJ, Rasband MN. A distal axonal cytoskeleton forms an intra-axonal boundary that controls axon initial segment assembly. Cell. 2012;149:1125–1139. doi: 10.1016/j.cell.2012.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R, Penzes P. Common mechanisms of excitatory and inhibitory imbalance in schizophrenia and autism spectrum disorders. Curr Mol Med. 2015;15:146–167. doi: 10.2174/1566524015666150303003028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk MG, Leussis MP, Ruland T, Gjeluci K, Petryshen TL, Bahn S. Lithium reverses behavioral and axonal transport-related changes associated with ANK3 bipolar disorder gene disruption. Eur Neuropsychopharmacol. 2017;27:274–288. doi: 10.1016/j.euroneuro.2017.01.001. [DOI] [PubMed] [Google Scholar]
- Grunditz A, Holbro N, Tian L, Zuo Y, Oertner TG. Spine neck plasticity controls postsynaptic calcium signals through electrical compartmentalization. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:13457–13466. doi: 10.1523/JNEUROSCI.2702-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan H, Maness PF. Perisomatic GABAergic innervation in prefrontal cortex is regulated by ankyrin interaction with the L1 cell adhesion molecule. Cereb Cortex. 2010;20:2684–2693. doi: 10.1093/cercor/bhq016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall TG, Bennett V. Regulatory domains of erythrocyte ankyrin. The Journal of biological chemistry. 1987;262:10537–10545. [PubMed] [Google Scholar]
- He M, Abdi KM, Bennett V. Ankyrin-G palmitoylation and betaII-spectrin binding to phosphoinositide lipids drive lateral membrane assembly. The Journal of cell biology. 2014;206:273–288. doi: 10.1083/jcb.201401016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Jenkins P, Bennett V. Cysteine 70 of ankyrin-G is S-palmitoylated and is required for function of ankyrin-G in membrane domain assembly. The Journal of biological chemistry. 2012;287:43995–44005. doi: 10.1074/jbc.M112.417501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoock TC, Peters LL, Lux SE. Isoforms of ankyrin-3 that lack the NH2-terminal repeats associate with mouse macrophage lysosomes. The Journal of cell biology. 1997;136:1059–1070. doi: 10.1083/jcb.136.5.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes T, Hansson L, Sonderby IE, Athanasiu L, Zuber V, Tesli M, Song J, Hultman CM, Bergen SE, Landen M, et al. A Loss-of-Function Variant in a Minor Isoform of ANK3 Protects Against Bipolar Disorder and Schizophrenia. Biological psychiatry. 2016;80:323–330. doi: 10.1016/j.biopsych.2015.09.021. [DOI] [PubMed] [Google Scholar]
- Ignatiuk A, Quickfall JP, Hawrysh AD, Chamberlain MD, Anderson DH. The smaller isoforms of ankyrin 3 bind to the p85 subunit of phosphatidylinositol 3′-kinase and enhance platelet-derived growth factor receptor down-regulation. The Journal of biological chemistry. 2006;281:5956–5964. doi: 10.1074/jbc.M510032200. [DOI] [PubMed] [Google Scholar]
- Inda MM, Munoz J, Coullin P, Fauvet D, Danglot G, Tunon T, Bernheim A, Castresana JS. High promoter hypermethylation frequency of p14/ARF in supratentorial PNET but not in medulloblastoma. Histopathology. 2006;48:5 79–587. doi: 10.1111/j.1365-2559.2006.02374.x. [DOI] [PubMed] [Google Scholar]
- Iqbal Z, Vandeweyer G, van der Voet M, Waryah AM, Zahoor MY, Besseling JA, Roca LT, Vulto-van Silfhout AT, Nijhof B, Kramer JM, et al. Homozygous and heterozygous disruptions of ANK3: at the crossroads of neurodevelopmental and psychiatric disorders. Human molecular genetics. 2013;22:1960–1970. doi: 10.1093/hmg/ddt043. [DOI] [PubMed] [Google Scholar]
- Jenkins PM, Kim N, Jones SL, Tseng WC, Svitkina TM, Yin HH, Bennett V. Giant ankyrin-G: a critical innovation in vertebrate evolution of fast and integrated neuronal signaling. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:957–964. doi: 10.1073/pnas.1416544112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan BA, Fernholz BD, Boussac M, Xu C, Grigorean G, Ziff EB, Neubert TA. Identification and verification of novel rodent postsynaptic density proteins. Molecular & cellular proteomics: MCP. 2004;3:857–871. doi: 10.1074/mcp.M400045-MCP200. [DOI] [PubMed] [Google Scholar]
- Kieseppa T, Partonen T, Haukka J, Kaprio J, Lonnqvist J. High concordance of bipolar I disorder in a nationwide sample of twins. Am J Psychiatry. 2004;161:1814–1821. doi: 10.1176/ajp.161.10.1814. [DOI] [PubMed] [Google Scholar]
- Kizhatil K, Bennett V. Lateral membrane biogenesis in human bronchial epithelial cells requires 190-kDa ankyrin-G. The Journal of biological chemistry. 2004;279:16706–16714. doi: 10.1074/jbc.M314296200. [DOI] [PubMed] [Google Scholar]
- Kizhatil K, Yoon W, Mohler PJ, Davis LH, Hoffman JA, Bennett V. Ankyrin-G and beta2-spectrin collaborate in biogenesis of lateral membrane of human bronchial epithelial cells. The Journal of biological chemistry. 2007;282:2029–2037. doi: 10.1074/jbc.M608921200. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloth K, Denecke J, Hempel M, Johannsen J, Strom TM, Kubisch C, Lessel D. First de novo ANK3 nonsense mutation in a boy with intellectual disability, speech impairment and autistic features. Eur J Med Genet. 2017;60:494–498. doi: 10.1016/j.ejmg.2017.07.001. [DOI] [PubMed] [Google Scholar]
- Koch I, Schwarz H, Beuchle D, Goellner B, Langegger M, Aberle H. Drosophila ankyrin 2 is required for synaptic stability. Neuron. 2008;58:210–222. doi: 10.1016/j.neuron.2008.03.019. [DOI] [PubMed] [Google Scholar]
- Kole MH, Stuart GJ. Signal processing in the axon initial segment. Neuron. 2012;73:235–247. doi: 10.1016/j.neuron.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Kordeli E, Lambert S, Bennett V. AnkyrinG. A new ankyrin gene with neural-specific isoforms localized at the axonal initial segment and node of Ranvier. The Journal of biological chemistry. 1995;270:2352–2359. doi: 10.1074/jbc.270.5.2352. [DOI] [PubMed] [Google Scholar]
- Kordeli E, Ludosky MA, Deprette C, Frappier T, Cartaud J. AnkyrinG is associated with the postsynaptic membrane and the sarcoplasmic reticulum in the skeletal muscle fiber. Journal of cell science. 1998;111(Pt 15):2197–2207. doi: 10.1242/jcs.111.15.2197. [DOI] [PubMed] [Google Scholar]
- Kunimoto M. A neuron-specific isoform of brain ankyrin, 440-kD ankyrinB, is targeted to the axons of rat cerebellar neurons. The Journal of cell biology. 1995;131:1821–1829. doi: 10.1083/jcb.131.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunimoto M, Otto E, Bennett V. A new 440-kD isoform is the major ankyrin in neonatal rat brain. The Journal of cell biology. 1991;115:1319–1331. doi: 10.1083/jcb.115.5.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusters R, Kapitein LC, Hoogenraad CC, Storm C. Shape-induced asymmetric diffusion in dendritic spines allows efficient synaptic AMPA receptor trapping. Biophysical journal. 2013;105:2743–2750. doi: 10.1016/j.bpj.2013.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladepeche L, Dupuis JP, Bouchet D, Doudnikoff E, Yang L, Campagne Y, Bezard E, Hosy E, Groc L. Single-molecule imaging of the functional crosstalk between surface NMDA and dopamine D1 receptors. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:18005–18010. doi: 10.1073/pnas.1310145110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert S, Bennett V. From anemia to cerebellar dysfunction. A review of the ankyrin gene family. Eur J Biochem. 1993;211:1–6. doi: 10.1111/j.1432-1033.1993.tb19863.x. [DOI] [PubMed] [Google Scholar]
- Leterrier C, Dargent B. No Pasaran! Role of the axon initial segment in the regulation of protein transport and the maintenance of axonal identity. Semin Cell Dev Biol. 2014;27:44–51. doi: 10.1016/j.semcdb.2013.11.001. [DOI] [PubMed] [Google Scholar]
- Leussis MP, Berry-Scott EM, Saito M, Jhuang H, de Haan G, Alkan O, Luce CJ, Madison JM, Sklar P, Serre T, et al. The ANK3 bipolar disorder gene regulates psychiatric-related behaviors that are modulated by lithium and stress. Biological psychiatry. 2013;73:683–690. doi: 10.1016/j.biopsych.2012.10.016. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Yip BH, Bjork C, Pawitan Y, Cannon TD, Sullivan PF, Hultman CM. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–239. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Zhang L, Wu J, Sui X, Xu Y, Huang L, Han Y, Zhu H, Li Y, Sun X, Qin C. AnkG hemizygous mice present cognitive impairment and elevated anxiety/depressive-like traits associated with decreased expression of GABA receptors and postsynaptic density protein. Exp Brain Res. 2017;235:3375–3390. doi: 10.1007/s00221-017-5056-7. [DOI] [PubMed] [Google Scholar]
- Lopez-Bendito G, Shigemoto R, Fairen A, Lujan R. Differential distribution of group I metabotropic glutamate receptors during rat cortical development. Cereb Cortex. 2002;12:625–638. doi: 10.1093/cercor/12.6.625. [DOI] [PubMed] [Google Scholar]
- Lorenzo DN, Badea A, Davis J, Hostettler J, He J, Zhong G, Zhuang X, Bennett V. A PIK3C3-ankyrin-B-dynactin pathway promotes axonal growth and multiorganelle transport. The Journal of cell biology. 2014;207:735–752. doi: 10.1083/jcb.201407063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu HE, MacGillavry HD, Frost NA, Blanpied TA. Multiple Spatial and Kinetic Subpopulations of CaMKII in Spines and Dendrites as Resolved by Single-Molecule Tracking PALM. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2014;34:7600–7610. doi: 10.1523/JNEUROSCI.4364-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGillavry HD, Hoogenraad CC. The internal architecture of dendritic spines revealed by super-resolution imaging: What did we learn so far? Exp Cell Res. 2015;335:180–186. doi: 10.1016/j.yexcr.2015.02.024. [DOI] [PubMed] [Google Scholar]
- MacGillavry HD, Song Y, Raghavachari S, Blanpied TA. Nanoscale scaffolding domains within the postsynaptic density concentrate synaptic AMPA receptors. Neuron. 2013;78:615–622. doi: 10.1016/j.neuron.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuffin P, Rijsdijk F, Andrew M, Sham P, Katz R, Cardno A. The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Archives of general psychiatry. 2003;60:497–502. doi: 10.1001/archpsyc.60.5.497. [DOI] [PubMed] [Google Scholar]
- Megias M, Emri Z, Freund TF, Gulyas AI. Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience. 2001;102:527–540. doi: 10.1016/s0306-4522(00)00496-6. [DOI] [PubMed] [Google Scholar]
- Mohler PJ, Gramolini AO, Bennett V. The ankyrin-B C-terminal domain determines activity of ankyrin-B/G chimeras in rescue of abnormal inositol 1,4,5-trisphosphate and ryanodine receptor distribution in ankyrin-B (−/−) neonatal cardiomyocytes. The Journal of biological chemistry. 2002;277:10599–10607. doi: 10.1074/jbc.M110958200. [DOI] [PubMed] [Google Scholar]
- Mohler PJ, Schott JJ, Gramolini AO, Dilly KW, Guatimosim S, du Bell WH, Song LS, Haurogne K, Kyndt F, Ali ME, et al. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature. 2003;421:634–639. doi: 10.1038/nature01335. [DOI] [PubMed] [Google Scholar]
- Mohler PJ, Yoon W, Bennett V. Ankyrin-B targets beta2-spectrin to an intracellular compartment in neonatal cardiomyocytes. The Journal of biological chemistry. 2004;279:40185–40193. doi: 10.1074/jbc.M406018200. [DOI] [PubMed] [Google Scholar]
- Muhleisen TW, Leber M, Schulze TG, Strohmaier J, Degenhardt F, Treutlein J, Mattheisen M, Forstner AJ, Schumacher J, Breuer R, et al. Genome-wide association study reveals two new risk loci for bipolar disorder. Nat Commun. 2014;5:3339. doi: 10.1038/ncomms4339. [DOI] [PubMed] [Google Scholar]
- Muir J, Kittler JT. Plasticity of GABAA receptor diffusion dynamics at the axon initial segment. Front Cell Neurosci. 2014;8:151. doi: 10.3389/fncel.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanavati D, Austin DR, Catapano LA, Luckenbaugh DA, Dosemeci A, Manji HK, Chen G, Markey SP. The effects of chronic treatment with mood stabilizers on the rat hippocampal post-synaptic density proteome. Journal of neurochemistry. 2011;119:617–629. doi: 10.1111/j.1471-4159.2011.07424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AD, Jenkins PM. Axonal Membranes and Their Domains: Assembly and Function of the Axon Initial Segment and Node of Ranvier. Front Cell Neurosci. 2017;11:136. doi: 10.3389/fncel.2017.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor MW, Cai X, Stone MR, Bloch RJ, Thompson SM. The actin binding domain of betaI-spectrin regulates the morphological and functional dynamics of dendritic spines. PloS one. 2011;6:e16197. doi: 10.1371/journal.pone.0016197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie F, Wang X, Zhao P, Yang H, Zhu W, Zhao Y, Chen B, Valenzuela RK, Zhang R, Gallitano AL, Ma J. Genetic analysis of SNPs in CACNA1C and ANK3 gene with schizophrenia: A comprehensive meta-analysis. American journal of medical genetics Part B, Neuropsychiatric genetics: the official publication of the International Society of Psychiatric Genetics. 2015;168:637–648. doi: 10.1002/ajmg.b.32348. [DOI] [PubMed] [Google Scholar]
- Peng J, Kim MJ, Cheng D, Duong DM, Gygi SP, Sheng M. Semiquantitative proteomic analysis of rat forebrain postsynaptic density fractions by mass spectrometry. The Journal of biological chemistry. 2004;279:21003–21011. doi: 10.1074/jbc.M400103200. [DOI] [PubMed] [Google Scholar]
- Penzes P, Cahill ME, Jones KA, VanLeeuwen JE, Woolfrey KM. Dendritic spine pathology in neuropsychiatric disorders. Nature neuroscience. 2011;14:285–293. doi: 10.1038/nn.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters LL, John KM, Lu FM, Eicher EM, Higgins A, Yialamas M, Turtzo LC, Otsuka AJ, Lux SE. Ank3 (epithelial ankyrin), a widely distributed new member of the ankyrin gene family and the major ankyrin in kidney, is expressed in alternatively spliced forms, including forms that lack the repeat domain. The Journal of cell biology. 1995;130:313–330. doi: 10.1083/jcb.130.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pielage J, Cheng L, Fetter RD, Carlton PM, Sedat JW, Davis GW. A presynaptic giant ankyrin stabilizes the NMJ through regulation of presynaptic microtubules and transsynaptic cell adhesion. Neuron. 2008;58:195–209. doi: 10.1016/j.neuron.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband MN. The axon initial segment and the maintenance of neuronal polarity. Nature reviews Neuroscience. 2010;11:552–562. doi: 10.1038/nrn2852. [DOI] [PubMed] [Google Scholar]
- Roby Y. ANK3 gene polymorphisms and bipolar disorder: a meta-analysis. Psychiatr Genet. 2017;27:225–235. doi: 10.1097/YPG.0000000000000186. [DOI] [PubMed] [Google Scholar]
- Sala C, Segal M. Dendritic spines: the locus of structural and functional plasticity. Physiological reviews. 2014;94:141–188. doi: 10.1152/physrev.00012.2013. [DOI] [PubMed] [Google Scholar]
- Schizophrenia Psychiatric Genome-Wide Association Study C. Genome-wide association study identifies five new schizophrenia loci. Nature genetics. 2011;43:969–976. doi: 10.1038/ng.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze TG, Detera-Wadleigh SD, Akula N, Gupta A, Kassem L, Steele J, Pearl J, Strohmaier J, Breuer R, Schwarz M, et al. Two variants in Ankyrin 3 (ANK3) are independent genetic risk factors for bipolar disorder. Molecular psychiatry. 2009;14:487–491. doi: 10.1038/mp.2008.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott LJ, Muglia P, Kong XQ, Guan W, Flickinger M, Upmanyu R, Tozzi F, Li JZ, Burmeister M, Absher D, et al. Genome-wide association and meta-analysis of bipolar disorder in individuals of European ancestry. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:7501–7506. doi: 10.1073/pnas.0813386106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Zhang X, Golhar R, Otieno FG, He M, Hou C, Kim C, Keating B, Lyon GJ, Wang K, Hakonarson H. Whole-genome sequencing in an autism multiplex family. Molecular autism. 2013;4:8. doi: 10.1186/2040-2392-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon CM, Hepburn I, Chen W, De Schutter E. The role of dendritic spine morphology in the compartmentalization and delivery of surface receptors. Journal of computational neuroscience. 2014;36:483–497. doi: 10.1007/s10827-013-0482-4. [DOI] [PubMed] [Google Scholar]
- Smith EN, Bloss CS, Badner JA, Barrett T, Belmonte PL, Berrettini W, Byerley W, Coryell W, Craig D, Edenberg HJ, et al. Genome-wide association study of bipolar disorder in European American and African American individuals. Molecular psychiatry. 2009;14:755–763. doi: 10.1038/mp.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR, Jones KA, Kopeikina KJ, Burette AC, Copits BA, Yoon S, Forrest MP, Fawcett-Patel JM, Hanley JG, Weinberg RJ, et al. Cadherin-10 Maintains Excitatory/Inhibitory Ratio through Interactions with Synaptic Proteins. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2017;37:11127–11139. doi: 10.1523/JNEUROSCI.1153-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR, Kittler JT. The cell biology of synaptic inhibition in health and disease. Current opinion in neurobiology. 2010;20:550–556. doi: 10.1016/j.conb.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Smith KR, Kopeikina KJ, Fawcett-Patel JM, Leaderbrand K, Gao R, Schurmann B, Myczek K, Radulovic J, Swanson GT, Penzes P. Psychiatric risk factor ANK3/ankyrin-G nanodomains regulate the structure and function of glutamatergic synapses. Neuron. 2014;84:399–415. doi: 10.1016/j.neuron.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasaki K, Sabatini BL. Super-resolution 2-photon microscopy reveals that the morphology of each dendritic spine correlates with diffusive but not synaptic properties. Frontiers in neuroanatomy. 2014;8:29. doi: 10.3389/fnana.2014.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang AH, Chen H, Li TP, Metzbower SR, MacGillavry HD, Blanpied TA. A trans-synaptic nanocolumn aligns neurotransmitter release to receptors. Nature. 2016;536:210–214. doi: 10.1038/nature19058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardin C, Cognet L, Bats C, Lounis B, Choquet D. Direct imaging of lateral movements of AMPA receptors inside synapses. EMBO J. 2003;22:4656–4665. doi: 10.1093/emboj/cdg463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesli M, Koefoed P, Athanasiu L, Mattingsdal M, Gustafsson O, Agartz I, Rimol LM, Brown A, Wirgenes KV, Smorr LL, et al. Association analysis of ANK3 gene variants in nordic bipolar disorder and schizophrenia case-control samples. American journal of medical genetics Part B, Neuropsychiatric genetics: the official publication of the International Society of Psychiatric Genetics. 2011;156B:969–974. doi: 10.1002/ajmg.b.31244. [DOI] [PubMed] [Google Scholar]
- Thevananther S, Kolli AH, Devarajan P. Identification of a novel ankyrin isoform (AnkG190) in kidney and lung that associates with the plasma membrane and binds alpha-Na, K-ATPase. The Journal of biological chemistry. 1998;273:23952–23958. doi: 10.1074/jbc.273.37.23952. [DOI] [PubMed] [Google Scholar]
- Tonnesen J, Katona G, Rozsa B, Nagerl UV. Spine neck plasticity regulates compartmentalization of synapses. Nature neuroscience. 2014;17:678–685. doi: 10.1038/nn.3682. [DOI] [PubMed] [Google Scholar]
- Tonnesen J, Nagerl UV. Superresolution imaging for neuroscience. Experimental neurology. 2013;242:33–40. doi: 10.1016/j.expneurol.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Tonnesen J, Nagerl UV. Dendritic Spines as Tunable Regulators of Synaptic Signals. Front Psychiatry. 2016;7:101. doi: 10.3389/fpsyt.2016.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter V, Moss SJ. GABA(A) Receptor Dynamics and Constructing GABAergic Synapses. In Front Mol Neurosci. 2008:7. doi: 10.3389/neuro.02.007.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse WT, Menninger JC, Yang-Feng TL, Francke U, Sahr KE, Lux SE, Ward DC, Forget BG. Isolation and chromosomal localization of a novel nonerythroid ankyrin gene. Genomics. 1991;10:858–866. doi: 10.1016/0888-7543(91)90173-c. [DOI] [PubMed] [Google Scholar]
- Tseng WC, Jenkins PM, Tanaka M, Mooney R, Bennett V. Giant ankyrin-G stabilizes somatodendritic GABAergic synapses through opposing endocytosis of GABAA receptors. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:1214–1219. doi: 10.1073/pnas.1417989112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Werf IM, Van Dam D, Missault S, Yalcin B, De Deyn PP, Vandeweyer G, Kooy RF. Behavioural characterization of AnkyrinG deficient mice, a model for ANK3 related disorders. Behav Brain Res. 2017;328:218–226. doi: 10.1016/j.bbr.2017.04.014. [DOI] [PubMed] [Google Scholar]
- Wang C, Wei Z, Chen K, Ye F, Yu C, Bennett V, Zhang M. Structural basis of diverse membrane target recognitions by ankyrins. Elife. 2014:3. doi: 10.7554/eLife.04353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Yu C, Ye F, Wei Z, Zhang M. Structure of the ZU5-ZU5-UPA-DD tandem of ankyrin-B reveals interaction surfaces necessary for ankyrin function. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:4822–4827. doi: 10.1073/pnas.1200613109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Dumoulin A, Renner M, Triller A, Specht CG. The Role of Synaptopodin in Membrane Protein Diffusion in the Dendritic Spine Neck. PloS one. 2016;11:e0148310. doi: 10.1371/journal.pone.0148310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SJ, Slater CR. beta-Spectrin is colocalized with both voltage-gated sodium channels and ankyrinG at the adult rat neuromuscular junction. The Journal of cell biology. 1998;140:675–684. doi: 10.1083/jcb.140.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Bennett V. Restriction of 480/270-kD ankyrin G to axon proximal segments requires multiple ankyrin G-specific domains. The Journal of cell biology. 1998;142:1571–1581. doi: 10.1083/jcb.142.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Lambert S, Malen PL, Carpenter S, Boland LM, Bennett V. AnkyrinG is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. The Journal of cell biology. 1998;143:1295–1304. doi: 10.1083/jcb.143.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Cordner ZA, Xiong J, Chiu CT, Artola A, Zuo Y, Nelson AD, Kim TY, Zaika N, Woolums BM, et al. Genetic disruption of ankyrin-G in adult mouse forebrain causes cortical synapse alteration and behavior reminiscent of bipolar disorder. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:10479–10484. doi: 10.1073/pnas.1700689114. [DOI] [PMC free article] [PubMed] [Google Scholar]