Introduction
One of the most remarkable aspects of the brain is its ability to modify its function in response to its own activity. This capacity makes possible fundamental brain activities, such as learning and memory and is thought to depend upon activity-dependent changes in the excitability of neurons in brain circuits. These activity-dependent changes may take place by a variety of mechanisms, such as Hebbian or homeostatic plasticity, but for excitatory glutamatergic synapses, a major component of regulation has been ascribed to changes in the abundance of glutamate receptors, specifically AMPA receptors, in the postsynaptic membrane (Chater and Goda, 2014). This recognition has motivated studies of AMPA receptor trafficking at the synapse. Most studies have considered steps that take place proximal to the synapse, including plasma membrane insertion and removal of receptors, receptor association with scaffolding proteins, and receptor recycling through endosomal pools (Greger et al., 2017; Henley and Wilkinson, 2016; Hirling, 2009). In this review, we consider another equally fundamental, but not extensively considered trafficking step, endoplasmic reticulum (ER) processing and especially exit of receptors from the ER. The contribution of ER trafficking is significant for several reasons. AMPA receptor subunit composition, which strongly influences receptor function, is determined during receptor assembly in the ER, and the ER is also often the site for protein-synthesis-dependent forms of control (Penn and Greger, 2009). The ER is a major site of release of Ca2+ (Berridge, 2002). The ER is the start site for synaptic trafficking, and the ER membrane is, in some synapses, proximal to the synaptic membrane (Hayashi et al., 2009; Saheki and De Camilli, 2017; Wu et al., 2017) making it a ready source of receptors for synaptic transport. Also, as we shall discuss, specific regulatory mechanisms, in particular mGluR-LTD, are firmly associated with AMPA receptor trafficking from the ER (Pick et al., 2017).
Subunit-specific trafficking
The AMPA receptor subunits, GluA1-4, plus their splice variants (Greger et al., 2017), are distinguished by their cytoplasmic C-terminal domains (CTD), which fall into two groups: the “long tails” (~80 aa), GluA1 and GluA4, and the “short tails” (~50 aa), Glua2 and GluA3 (Henley and Wilkinson, 2016; Herguedas et al., 2013). The CTDs are attachment sites for proteins that traffick and anchor the receptors at different membranes, including the synaptic membrane. Thus, receptors in the two CTD groups will obey different trafficking and anchorage rules (See Box).
GluA1 synaptic trafficking
GluA1 insertion at the synapse requires neuronal activity and a multi-step process. GluA1 enters the plasma membrane at extrasynaptic sites (Boehm et al., 2006; Makino and Malinow, 2009; Oh et al., 2006) where activity-dependent phosphorylation of serine 845 in the GluA1 CTD by either PKA (cAMP-regulated kinase) (Roche et al., 1996) or cGKII (cGMP-regulated kinase) (Serulle et al., 2007) blocks endocytosis and stabilizes an extrasynaptic GluA1 pool (Ehlers, 2000; Lee et al., 2003; Man et al., 2007).
GluA1 subsequently enters the synapse by rapid, random-walk diffusion followed by PKC phosphorylation on serine 818 which gates synaptic entry (Boehm et al., 2006).
Dephosphorylation of GluA1 by the Ca2+-regulated phosphatase, calcineurin, releases GluA1 to endocytose (Boehm et al., 2006).
GluA1 traffics in a complex with the MAGUK scaffold, SAP97, which in turn binds A Kinase anchoring protein, AKAP-79, which associates with PKA, PKC and calcineurin (Dell'Acqua et al., 2006). Thus, GluA1 traffics to the synapse associated with the scaffold and enzymes that control its synaptic entry, localization and removal.
GluA2 synaptic trafficking
GluA2 traffics to synapses previously populated with AMPA receptors in a constitutive and direct manner (Shi et al., 2001).
GluA2 binds through its CTD to the related multi-PDZ synaptic scaffolds, GRIP1 and ABP (Dong et al., 1997; Srivastava et al., 1998), which bind to other synaptic protein matrix components. GRIP binds to GRASPs (Ye et al., 2007) and ABP to the NPRAP/delta catenin protein, which binds to the intracellular domain of the cadherins cell adhesion molecules (Silverman et al., 2007), stabilizing GluA2 at the synapse.
GluA2 also binds PICK1, which binds the activated form of PKC (Dev et al., 1999; Xia et al., 1999). Because phosphorylation of GluA2 by PKC on serine 880 of the GluA2 CTD releases GluA2 from the GRIP1 and ABP scaffold anchorages (Hanley, 2008; Lu and Ziff, 2005; Perez et al., 2001), PKC guided by PICK1 to GluA2 could phosphorylate serine 880, initiating GluA2 release from GRIP1/ABP and the synapse.
Recent studies have illustrated different roles for different homotypic and heterotypic AMPA receptors. AMPA receptors comprise a combination of GluA1-GluA4 subunits in a tetrameric conformation. Most AMPA receptors contain the GluA2 subunit, which renders the receptor impermeable to Ca2+ (Herguedas et al., 2013; Hume et al., 1991). GluA2 subunits form complexes with either GluA1 or GluA3 subunits to form the receptor tetramer. While inclusion of GluA2 makes the receptor impermeable to Ca2+, GluA2-lacking AMPA receptors, such as GluA1 homomers, are Ca2+-permeable (Lee, 2012; Man, 2011). In organotypic hippocampal slices, GluA1-containing AMPA receptors are thought to be inserted into synapses in an activity-dependent manner, while GluA2/3 AMPA receptors are thought to be constitutively inserted (Shi et al., 2001). Additionally, GluA2-lacking, Ca2+-permeable AMPA receptors traffic under different conditions than GluA2-containing AMPA receptors (Isaac et al., 2007; Passafaro et al., 2001). While much is known about the rules governing insertion and removal of these various types of AMPA receptors at the plasma membrane, less is known about the origins of the different subtypes of AMPA receptors and the associated intracellular trafficking and regulatory processes.
AMPA receptor trafficking plays a crucial role in various forms of synaptic plasticity including long-term depression (LTD). Activation of Group I metabotropic glutamate receptors (mGluRs) induces long-term depression (LTD) via a reduction of synaptic strength (Luscher and Huber, 2010). A common mechanism of mGluR-LTD is the net reduction of synaptic AMPA receptors. An alternate mechanism of mGluR-LTD, well described in the ventral tegmental area, is an exchange of higher-conducting, Ca2+-permeable AMPA receptors with lower-conducting, GluA2-containing, AMPA receptors (Mameli et al., 2007). This form of mGluR-LTD has been observed in other brain regions as well including the striatum, and cerebellum (Luscher and Huber, 2010). A recent study examining cultured medium spiny neurons has demonstrated that this mGluR-LTD mechanism involves a novel regulatory step: the exit of GluA2 from the ER induced by the release of Ca2+ from internal ER stores (Pick et al., 2017). We will use this model of mGluR-LTD as a means of exploring the dynamics of AMPA receptor assembly, exit from the ER, and trafficking in the early secretory pathway.
Differences between GluA2 and GluA1 exit from the ER
The dependence of synaptic trafficking of AMPA receptors on ER export is not well studied. Differences in ER localization and trafficking dynamics of GluA1 and GluA2 may contribute to the regulation of ER exit of different types of AMPA receptors. In the hippocampus, GluA1 is evenly distributed in the dendritic ER and quickly exits to the plasma membrane (Lu et al., 2014) via association with SAP97 (Sans et al., 2001). It is inserted into the synapse in a dual-step process, first entering the plasma membrane and then trafficking laterally through the plasma membrane to the synapse (Makino and Malinow, 2009; Passafaro et al., 2001). One study, also looking at hippocampal neurons, examined plasmid expressed GFP-tagged GluA1, and demonstrated that as GluA1 exits the ER, it traffics via the conventional secretory pathway from the somatic, rather than the dendritic, ER, to the plasma membrane (Jeyifous et al., 2009) where it then moves laterally to the synapse. On the other hand, studies of GluA2 in hippocampal neurons demonstrate that GluA2 accumulates in the ER (Greger et al., 2002), is found to reside in puncta associated with internal membranes along the dendrite (Perestenko and Henley, 2003), and may be targeted directly to the synaptic membrane (Beretta et al., 2005; Passafaro et al., 2001). Cultured hippocampal and medium spiny neurons demonstrate that GluA2 exit from the ER is dependent on Ca2+ release via IP3 and ryanodine receptors both basally and after mGluR-LTD (Lu et al., 2014; Pick et al., 2017). While some studies distinguish between ER and surface AMPA receptors based on their sensitivity to Endoglycosidase H (Endo H), it is important to note that several recent studies of pyramidal neurons have identified a pool of surface proteins, including AMPAR receptors, that remain sensitive to Endo H even in the plasma membrane (Bowen et al., 2017; Hanus et al., 2016). Nonetheless, the ER exit trafficking behaviors of heteromers of GluA1 and GluA2 have not been extensively studied.
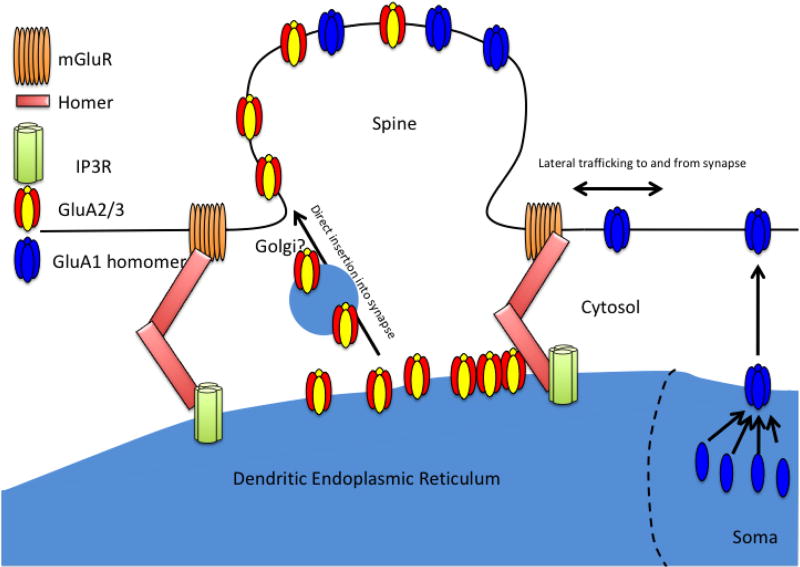
In cerebellar Purkinje cells the dendritic ER can penetrate into spines so as to be proximal to the synapse, and this proximity is required for mGluR-LTD (Miyata et al., 2000). Indeed, the linker protein, Homer, forms dimers that link cytoplasmic domains of Group 1 mGluRs, which are synaptic, and IP3Rs, which are localized in the ER (Tu et al., 1998). This suggests that particularly in the cerebellum, two proteins that participate in the mGluR-LTD mechanism, Group 1 mGluRs and IP3Rs, may be proximal in complexes that bring the ER adjacent to the synapse, making possible release from the ER and immediate synaptic insertion of GluA2 upon mGluR activation. In addition, recent studies of pyramidal neurons have illustrated that some AMPA receptors can bypass the Golgi while trafficking to the plasma membrane (Bowen et al., 2017; Hanus et al., 2016). If so, the direct insertion of a GluA2-containing AMPA receptor from the ER to the synapse may be direct and rapid (Figure 1). Such positioning could increase the speed, efficiency and selectivity of the mGluR-LTD mechanism.
Figure 1.
The dendritic ER is proximal to the plasma membrane due to Homer dimers that link mGluRs at the spines with IP3Rs in the ER. This could enable the insertion of a population of GluA2/3 AMPA receptors directly into the synapse especially upon stimulation of mGluR-LTD. GluA1 homomers are assembled away from the synapse at the somatic ER and inserted into the plasma membrane where they subsequently traffic into the synapse.
Protein translation during mGluR-LTD
mGluR-LTD requires new protein synthesis (Huber et al., 2000). Pick et al. have shown in medium spiny neurons that new translation is required for incorporation of AMPA receptors into COPII vesicles exiting the ER after mGluR activation (Pick et al., 2017). The identity of the required newly-translated protein(s) is not known, however, GluA2 and/or GluA3 are candidates (Mameli et al., 2007). Protein synthesis may be required for other steps in mGluR-LTD as well, such as the endocytosis of GluA1. Expression of the ARC protein, which contributes to GluA1 endocytosis, is induced translationally during mGluR-LTD (Wilkerson et al., 2017), and a number of additional proteins that are induced have been identified in hippocampal neurons, including oligophrenin, which mediates AMPA receptor endocytosis (Di Prisco et al., 2014; Nakano-Kobayashi et al., 2009; Postiglione et al., 2011).
Regulation of protein synthesis during the early stages of the mGluR-LTD mechanism also involves the fragile X mental retardation protein, FMRP, a multifunctional RNA binding protein that contributes to dendrite genesis, mRNA transport, and control of translation (Ashley et al., 1993; Maurin et al., 2014). Group I mGluR stimulation induces FMRP synthesis and degradation (Antar et al., 2004; Bassell and Warren, 2008; Hou et al., 2006; Weiler et al., 1997). FMRP in turn is thought to function as a negative regulator of translation that, prior to mGluR stimulation, limits the production of one or more proteins that contribute to the mGluR-LTD mechanism. Because FMRP binds to GluA1 and GluA2 mRNA and PSD95 (a synaptic scaffold protein) mRNA (Muddashetty et al., 2007), FMRP may control translation of AMPA receptor subunits and of PSD95 during mGluR-LTD, perhaps regulating a translational mechanism for GluA2-containing AMPA receptor formation. Another Fragile X Related Protein, FXR1P, has also been reported to bind GluA2 mRNA and to control GluA2 translation in the hippocampus (Cook et al., 2014). Thus, several different factors involved in mGluR-induced translation may contribute to the subunit composition of AMPA receptors.
The role of AMPA receptor assembly
The dependence of trafficking mechanisms on subunit composition raises the question of how receptor subunit composition is established. The facts that: 1) mGluR-LTD relies on export of GluA2-containing AMPA receptors from the ER, and 2) the ER is the site of assembly of AMPA receptors, suggest that receptor assembly is regulated during mGluR-LTD. Assembly is thought to be a multi-step process involving different subunit domains at each step. AMPA receptor subunits consist of four domains: the amino terminal domain (NTD), the ligand binding domain (LBD), the transmembrane domains (TMD, M1–M4), which form the membrane spanning regions, and the CTD (Greger et al., 2017). The NTD, at the N terminus, is the first region to be translated. The NTDs have nanomolar affinity for one another (Rossmann et al., 2011), and the interaction of the NTD of one monomer with the NTD of another monomer initiates dimer assembly (Ayalon and Stern-Bach, 2001; Herguedas et al., 2013). It has been suggested that dimerization occurs as these nascent subunit chains emerge from the polysome (while the remainder is still translating and folding) (Greger et al., 2017). The next step in the assembly involves the LBDs of the two subunits interacting in a transient cis conformation. Two dimers then interact to form unstable proto-tetramers (reviewed by (Gan et al., 2015), in which monomers continue to interact with their cis partner. Following this, the M4 region of one subunit is proposed to wrap around M1–M3 regions of the adjacent subunit to stabilize the tetramer. At the same time, the cis interactions of the LBDs of dimers of the proto-tetramer rearrange to form a trans LBD conformation. Thus, the LBDs most likely make their final association during tetramerization, as a result of the domain swap (Gan et al., 2015; Greger et al., 2017; Herguedas et al., 2013).
The NTD interactions are subunit-specific and could favor assembly of particular hetero-tetramers (Herguedas et al., 2013; Rossmann et al., 2011). The availability of a heteromeric partner, determined by rate of synthesis, protein folding and local distribution including rates of diffusion in the ER membrane, are also factors in the assembly of specific, functional AMPA receptors (Greger et al., 2017).
The mGluR-LTD mechanism in medium spiny neurons promotes the export of GluA2-containing AMPA receptors from the ER, and although not fully established, the receptors that traffic from the ER may lack GluR1, suggesting that they are GluA2/3 heteromers (Pick et al., 2017). GluA2 homomers are not thought to be present in physiological conditions in any cell type (Lu et al., 2009). Thus, any assembly step for a GluA2-containing receptor that contributes to mGluR-LTD likely involves GluA3. In these GluA2/3 heteromers, the GluA2 could come from several sources. The GluA2 could be drawn from the substantial pre-existing, basal-state pool of monomeric and dimeric GluA2 found in the ER (Greger et al., 2002). It was proposed that the presence of this population ensured that GluA2 was incorporated into the vast majority of newly formed heterotetramers (Greger et al., 2002). Alternatively, newly translated GluA2 could be incorporated into the tetramers, putative GluA2/3 heteromers, that traffic synaptically upon stimulation of mGluRs.
Chaperones interacting with AMPA receptors
Upon translation, membrane protein polypeptides are inserted into the ER where they interact with protein chaperones that assist secondary folding and facilitate assembly of larger complexes (Hebert and Molinari, 2007). Two well-characterized chaperones, BiP and calnexin, are involved in the early processing of AMPA receptors. In hippocampal neurons, BiP and calnexin co-precipitate and co-localize with AMPA receptor subunits throughout the neuron (Rubio and Wenthold, 1999). This co-localization is observed in proximal and distal dendrites, including puncta thought to be spines, which supports the notion of local protein synthesis of AMPA receptors (Rubio and Wenthold, 1999). Only a subpopulation of AMPA receptors is associated with BiP and calnexin (Fukata et al., 2005; Rubio and Wenthold, 1999), likely indicating that BiP and calnexin bind to an immature form of AMPA receptor subunits during receptor assembly (Fukata et al., 2005). It is possible that the binding of these chaperones is responsible for maintaining the pool of immature GluA2 in the ER (Greger et al., 2002), thus regulating the assembly and ER exit of GluA2-containing AMPA receptors. Additional proteins that associate transiently and selectively with AMPA receptors in the ER and that are likely to act in an early step in AMPA receptor biogenesis, FRRS1l and CPT1c, have been recently reported (Sivalingam and Kumar, 2015).
In addition to its role as an ER chaperone, there is evidence from studies of cerebellar granule neurons that BiP, which regulates ER exit of many proteins, can play a role in AMPA receptor exit from the ER. The unfolded protein response (UPR) is known to increase BiP levels. This increase in BiP correlates with increased surface levels of GluA1 (Vandenberghe et al., 2005), suggesting that ER chaperones may play additional roles in regulating AMPA receptor exit from the ER.
Regulation of GluA2 ER exit and COPII association
Newly assembled proteins in the ER accumulate at ER exit sites (ERES). Here, proteins are loaded into COPII vesicles, which transport the proteins out of the ER (Budnik and Stephens, 2009). During export, a series of proteins is sequentially recruited to ER exit sites to drive the formation of COPII vesicles (D'Arcangelo et al., 2013). Activation of the GTPase, Sar1, leads to the insertion of its N-terminal amphipathic helix into the ER membrane, deforming it. This deformation sequentially recruits the Sec23/Sec24 pre-budding complex followed by the Sec13/Sec31 complex to the deformed membrane imposing curvature and ultimately fission of the vesicle from the ER. Two characteristic features of ERES that enable COPII vesicle generation are the presence of Sec16 and an enrichment of acidic lipids, in particular phophatidylinositol 4-phosphate (PI4P) (Blumental-Perry et al., 2006; Farhan et al., 2008; Klinkenberg et al., 2014). Evidence of GluA2 accumulation in the ER (Greger et al., 2002) and interaction with COPII proteins, specifically Sec23 (Pick et al., 2017) suggest that AMPA receptors traffic via the COPII secretory pathway. Nonetheless, little is known about AMPA receptor accumulation at the ERES and interaction with COPII vesicles.
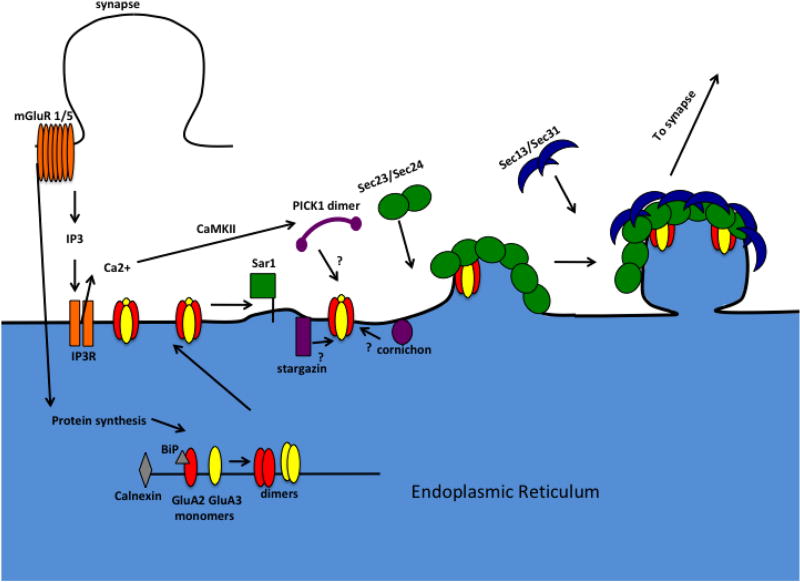
Several proteins known to play a role in AMPA receptor trafficking such as cornichons, TARPs, and PICK1, also interact with negatively charged membranes including phosphatidylinositols (PIPs) that are enriched at ERES (Jin et al., 2006; Sumioka et al., 2010). These proteins could mediate the interaction between AMPA receptors and COPII vesicles, regulating their exit from the ER (Figure 2).
Figure 2.
mGluR-LTD activates mGluR channels which leads to initiation of protein synthesis and IP3R activation. Protein synthesis leads to accumulation of GluA2 at ERES, while ER Ca2+ release leads to CaMKII and PICK1 activation that drives GluA2 containing AMPA receptors (red and yellow) out of the ER via COPII vesicles (green and blue). Accumulation at the ER may involve various AMPA receptor associated proteins (purple) such as stargazin, cornichons, and PICK1.
Cornichons are a group of AMPA receptor-interacting proteins that are involved in AMPA receptor trafficking (Brockie et al., 2013; Harmel et al., 2012; Schwenk et al., 2009). They can also act as cargo adaptors to bring cargo proteins into COPII vesicles. For instance, the yeast homologue of the cornichon family, erv14, binds to both cargo (yor-1) and a protein in the COPII vesicle (Sec24) (Pagant et al., 2015). In addition, cornichons act as cargo receptors for TGF-alpha proteins (Bokel et al., 2006; Castro et al., 2007). In C. elegans, cornichons negatively regulate AMPA receptor exit from the ER, and eliminating cornichons increases the number of surface AMPA receptors (Brockie et al., 2013). Cornichons are found in the ER and shuttle cargo from the ER to the Golgi, and AMPA receptor binding to cornichons enables surface expression of cornichons (Brockie et al., 2013; Harmel et al., 2012). This evidence suggests that cornichons play a role in ER exit of AMPA receptors, though the precise role is not well understood.
Another group of auxiliary proteins that may contribute to AMPA receptor accumulation at the ERES is the TARPs (Transmembrane AMPA receptor Regulating Proteins). One well-studied TARP is stargazin, which contributes to AMPA receptor exit from the ER (Tomita et al., 2003). Stargazin knockout mice have reduced AMPA receptor surface expression in the cerebellum and increased accumulation in the ER compared with heterozygous mice, suggesting that stargazin contributes to the ER exit of AMPA receptors (Tomita et al., 2003). Moreover, in cerebellar granule cells, activation of the UPR compensates for the absence of stargazin in stargazin knockout mice, which promotes ER exit of AMPA receptors (Vandenberghe et al., 2005). In fact, UPR induction itself increases the number of COPII sites facilitating ER exit (Farhan et al., 2008). Interestingly, in cerebellar granule cells, the stargazin Cterminus binds to PIPs (Sumioka et al., 2010). These studies suggest a role for stargazin in AMPA receptor delivery to ERES.
A third GluA2-interacting protein is the PDZ domain-containing protein, PICK1. PICK1 dimerizes though a coiled-coil structure formed by a BAR domain, and it possesses Ca2+ binding sites (Hanley, 2008). The BAR domain dimer associates with curved membranes, and it can also induce membrane curvature upon membrane binding (Peter et al., 2004). In hippocampal neurons, PICK1 is also known to interact with PIP sites (Jin et al., 2006) and play a role in ER exit (Greger et al., 2002; Lu et al., 2014). Finally, PICK1-GluA2 interactions are involved in constitutive exit from the ER via RAB39B (Mignogna et al., 2015). These studies suggest a significant role for several proteins in delivering AMPA receptors to ERES and COPII vesicles. Further studies are required to understand the precise function and conditions that each of these proteins play in AMPA receptor exit from the ER.
Dendritic ER in plasticity and activity
While conventional protein entry into the early secretory pathway occurs in the somatic ER, an extensive network of ER tubules and sheets in dendrites and spines suggests secretory trafficking in dendrites (Cui-Wang et al., 2012; Hanus and Ehlers, 2016). In hippocampal neurons both ERES and COPII subunits have been detected at proximal and distal dendritic sites, and many of these sites are stable over time (Aridor et al., 2004). Dendritic branch points and spines have a complex network of ER, ribosomes, and ERES that regulates protein mobility (Cui-Wang et al., 2012). This enables neurons to modulate aspects of the dendritic arbor with speed and specificity.
The dendritic ER has an important role in mGluR-LTD. mGluR-LTD in hippocampal neurons leads to increased ER exit of NMDARs in dendrites (Aridor et al., 2004) and enhances the complexity of the ER near spines (Cui-Wang et al., 2012). Also in hippocampal neurons, mGluR-LTD leads to the release of ER Ca2+ through IP3 receptors, and spines containing ER protrusions undergo mGluR-LTD (Holbro et al., 2009). While in cerebellar Purkinje cells, preventing the dendritic ER from extending into spines blocks the induction of mGluR-LTD (Miyata et al., 2000). These studies indicate a role for the ER in spines, and Ca2+ release from that ER is required for mGluR-LTD, however the function of this Ca2+ release is not clear.
Recent work from both hippocampal and medium spiny neurons suggests that ER Ca2+ release triggers a signaling cascade that mediates the exit of GluA2 from the ER (Lu et al., 2014; Pick et al., 2017). Upon mGluR-LTD induction, inhibiting ER Ca2+ release in medium spiny neurons prevents GluA2-containing COPII vesicles from exiting the ER and the appearance of GluA2 at the plasma membrane. This block of ER Ca2+ does not prevent the interaction between GluA2 and the COPII protein, Sec23, suggesting that the role for Ca2+ release in mGluR-LTD is subsequent to the GluA2-Sec23 interaction (Pick et al., 2017). In related studies in pyramidal neurons, the basal rate of export of GluA2 from the ER was diminished by mutations of the GluA2 CTD that selectively blocked binding of PICK1 (Greger et al., 2002; Lu et al., 2014).
Studies of the basal transport of GluA2 from the ER in hippocampal neurons showed that ER export depends on the Ca2+ regulated kinase, CaMKII (Lu et al., 2014). Furthermore, PICK1 forms a complex with the activated form of CaMKII (Lu et al., 2014). This suggests that Ca2+ release from the ER may activate CaMKII, enabling it to bind to PICK1, a step that could target CaMKII to GluA2 in GluA2-PICK1 complexes. This signaling cascade may contribute to GluA2 exit from the ER under mGluR-LTD.
Conclusion
While much is known about AMPA receptor insertion and removal at the plasma membrane, the dynamics underlying AMPA receptor trafficking in the ER are under-appreciated. Several different steps regulate AMPA receptor assembly and trafficking in the early secretory pathway which have a direct effect on synaptic AMPA receptor expression and plasticity. These early processes have been implicated in various mental illnesses including impaired mental cognition (Mignogna et al., 2015) and Parkinson’s (Cho et al., 2014). In addition, cue-induced cocaine craving results from insertion of Ca2+ permeable AMPA receptors at synapses of the nucleus accumbens, following withdrawal from cocaine self-administration (Conrad et al., 2008). Because Type 1 mGluR stimulation reverses this accumulation (McCutcheon et al., 2011), driven by events in AMPA receptor trafficking from the ER as reviewed here, enhancement of ER trafficking of GluA2 to replace synaptic GluA1 may be a basis for therapeutic intervention for cocaine addiction. Understanding these regulatory steps will be crucial to developing new therapeutic targets. Furthermore, important distinctions between different types of AMPA receptors are emerging and need to be better understood.
Highlights.
AMPA receptor trafficking in the early stages of the secretory pathway contributes to synaptic plasticity
Differences in AMPA receptor subunit localization and dynamics in the ER contribute to different trafficking patterns.
Steps in the ER include receptor assembly in the ER, subunit-specific interactions in heterotetramer formation, protein synthesis, and incorporation of receptors into COPII vesicles for ER export.
Special features of the dendritic ER proximal to synapses may facilitate receptor transport to synapses
The mechanism of mGluR LTD may utilize regulation of receptor trafficking in the early secretory pathway
Acknowledgments
We thank Kara Zang (NYU) and Ingo Greger (MRC LMB) for helpful critical suggestions concerning the manuscript. This manuscript was supported by NIH grants 5R01MH067229 (EBZ) and T32 DA007254 (JEP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antar LN, Afroz R, Dictenberg JB, Carroll RC, Bassell GJ. Metabotropic glutamate receptor activation regulates fragile X mental retardation protein and FMR1 mRNA localization differentially in dendrites and at synapses. J Neurosci. 2004;24:2648–2655. doi: 10.1523/JNEUROSCI.0099-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aridor M, Guzik AK, Bielli A, Fish KN. Endoplasmic reticulum export site formation and function in dendrites. J Neurosci. 2004;24:3770–3776. doi: 10.1523/JNEUROSCI.4775-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley CT, Jr, Wilkinson KD, Reines D, Warren ST. FMR1 protein: conserved RNP family domains and selective RNA binding. Science. 1993;262:563–566. doi: 10.1126/science.7692601. [DOI] [PubMed] [Google Scholar]
- Ayalon G, Stern-Bach Y. Functional assembly of AMPA and kainate receptors is mediated by several discrete protein-protein interactions. Neuron. 2001;31:103–113. doi: 10.1016/s0896-6273(01)00333-6. [DOI] [PubMed] [Google Scholar]
- Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60:201–214. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beretta F, Sala C, Saglietti L, Hirling H, Sheng M, Passafaro M. NSF interaction is important for direct insertion of GluR2 at synaptic sites. Mol Cell Neurosci. 2005;28:650–660. doi: 10.1016/j.mcn.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium. 2002;32:235–249. doi: 10.1016/s0143416002001823. [DOI] [PubMed] [Google Scholar]
- Blumental-Perry A, Haney CJ, Weixel KM, Watkins SC, Weisz OA, Aridor M. Phosphatidylinositol 4-phosphate formation at ER exit sites regulates ER export. Dev Cell. 2006;11:671–682. doi: 10.1016/j.devcel.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Boehm J, Kang MG, Johnson RC, Esteban J, Huganir RL, Malinow R. Synaptic incorporation of AMPA receptors during LTP is controlled by a PKC phosphorylation site on GluR1. Neuron. 2006;51:213–225. doi: 10.1016/j.neuron.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Bokel C, Dass S, Wilsch-Brauninger M, Roth S. Drosophila Cornichon acts as cargo receptor for ER export of the TGFalpha-like growth factor Gurken. Development. 2006;133:459–470. doi: 10.1242/dev.02219. [DOI] [PubMed] [Google Scholar]
- Bowen AB, Bourke AM, Hiester BG, Hanus C, Kennedy MJ. Golgi-independent secretory trafficking through recycling endosomes in neuronal dendrites and spines. Elife. 2017;6 doi: 10.7554/eLife.27362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockie PJ, Jensen M, Mellem JE, Jensen E, Yamasaki T, Wang R, Maxfield D, Thacker C, Hoerndli F, Dunn PJ, et al. Cornichons control ER export of AMPA receptors to regulate synaptic excitability. Neuron. 2013;80:129–142. doi: 10.1016/j.neuron.2013.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budnik A, Stephens DJ. ER exit sites--localization and control of COPII vesicle formation. FEBS Lett. 2009;583:3796–3803. doi: 10.1016/j.febslet.2009.10.038. [DOI] [PubMed] [Google Scholar]
- Castro CP, Piscopo D, Nakagawa T, Derynck R. Cornichon regulates transport and secretion of TGFalpha-related proteins in metazoan cells. J Cell Sci. 2007;120:2454–2466. doi: 10.1242/jcs.004200. [DOI] [PubMed] [Google Scholar]
- Chater TE, Goda Y. The role of AMPA receptors in postsynaptic mechanisms of synaptic plasticity. Front Cell Neurosci. 2014;8:401. doi: 10.3389/fncel.2014.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HJ, Yu J, Xie C, Rudrabhatla P, Chen X, Wu J, Parisiadou L, Liu G, Sun L, Ma B, et al. Leucine-rich repeat kinase 2 regulates Sec16A at ER exit sites to allow ER-Golgi export. EMBO J. 2014;33:2314–2331. doi: 10.15252/embj.201487807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook D, Nuro E, Jones EV, Altimimi HF, Farmer WT, Gandin V, Hanna E, Zong R, Barbon A, Nelson DL, et al. FXR1P limits long-term memory, long-lasting synaptic potentiation, and de novo GluA2 translation. Cell Rep. 2014;9:1402–1416. doi: 10.1016/j.celrep.2014.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui-Wang T, Hanus C, Cui T, Helton T, Bourne J, Watson D, Harris KM, Ehlers MD. Local zones of endoplasmic reticulum complexity confine cargo in neuronal dendrites. Cell. 2012;148:309–321. doi: 10.1016/j.cell.2011.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Arcangelo JG, Stahmer KR, Miller EA. Vesicle-mediated export from the ER: COPII coat function and regulation. Biochim Biophys Acta. 2013;1833:2464–2472. doi: 10.1016/j.bbamcr.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Acqua ML, Smith KE, Gorski JA, Horne EA, Gibson ES, Gomez LL. Regulation of neuronal PKA signaling through AKAP targeting dynamics. Eur J Cell Biol. 2006;85:627–633. doi: 10.1016/j.ejcb.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Dev KK, Nishimune A, Henley JM, Nakanishi S. The protein kinase C alpha binding protein PICK1 interacts with short but not long form alternative splice variants of AMPA receptor subunits. Neuropharmacology. 1999;38:635–644. doi: 10.1016/s0028-3908(98)00230-5. [DOI] [PubMed] [Google Scholar]
- Di Prisco GV, Huang W, Buffington SA, Hsu CC, Bonnen PE, Placzek AN, Sidrauski C, Krnjevic K, Kaufman RJ, Walter P, et al. Translational control of mGluR-dependent long-term depression and object-place learning by eIF2alpha. Nat Neurosci. 2014;17:1073–1082. doi: 10.1038/nn.3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, O'Brien RJ, Fung ET, Lanahan AA, Worley PF, Huganir RL. GRIP: a synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature. 1997;386:279–284. doi: 10.1038/386279a0. [DOI] [PubMed] [Google Scholar]
- Ehlers MD. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron. 2000;28:511–525. doi: 10.1016/s0896-6273(00)00129-x. [DOI] [PubMed] [Google Scholar]
- Farhan H, Weiss M, Tani K, Kaufman RJ, Hauri HP. Adaptation of endoplasmic reticulum exit sites to acute and chronic increases in cargo load. EMBO J. 2008;27:2043–2054. doi: 10.1038/emboj.2008.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata Y, Tzingounis AV, Trinidad JC, Fukata M, Burlingame AL, Nicoll RA, Bredt DS. Molecular constituents of neuronal AMPA receptors. J Cell Biol. 2005;169:399–404. doi: 10.1083/jcb.200501121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan Q, Salussolia CL, Wollmuth LP. Assembly of AMPA receptors: mechanisms and regulation. J Physiol. 2015;593:39–48. doi: 10.1113/jphysiol.2014.273755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greger IH, Khatri L, Ziff EB. RNA editing at arg607 controls AMPA receptor exit from the endoplasmic reticulum. Neuron. 2002;34:759–772. doi: 10.1016/s0896-6273(02)00693-1. [DOI] [PubMed] [Google Scholar]
- Greger IH, Watson JF, Cull-Candy SG. Structural and Functional Architecture of AMPA-Type Glutamate Receptors and Their Auxiliary Proteins. Neuron. 2017;94:713–730. doi: 10.1016/j.neuron.2017.04.009. [DOI] [PubMed] [Google Scholar]
- Hanley JG. PICK1: a multi-talented modulator of AMPA receptor trafficking. Pharmacol Ther. 2008;118:152–160. doi: 10.1016/j.pharmthera.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Hanus C, Ehlers MD. Specialization of biosynthetic membrane trafficking for neuronal form and function. Curr Opin Neurobiol. 2016;39:8–16. doi: 10.1016/j.conb.2016.03.004. [DOI] [PubMed] [Google Scholar]
- Hanus C, Geptin H, Tushev G, Garg S, Alvarez-Castelao B, Sambandan S, Kochen L, Hafner AS, Langer JD, Schuman EM. Unconventional secretory processing diversifies neuronal ion channel properties. Elife. 2016;5 doi: 10.7554/eLife.20609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmel N, Cokic B, Zolles G, Berkefeld H, Mauric V, Fakler B, Stein V, Klocker N. AMPA receptors commandeer an ancient cargo exporter for use as an auxiliary subunit for signaling. PLoS One. 2012;7:e30681. doi: 10.1371/journal.pone.0030681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi MK, Tang C, Verpelli C, Narayanan R, Stearns MH, Xu RM, Li H, Sala C, Hayashi Y. The postsynaptic density proteins Homer and Shank form a polymeric network structure. Cell. 2009;137:159–171. doi: 10.1016/j.cell.2009.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert DN, Molinari M. In and out of the ER: protein folding, quality control, degradation, and related human diseases. Physiol Rev. 2007;87:1377–1408. doi: 10.1152/physrev.00050.2006. [DOI] [PubMed] [Google Scholar]
- Henley JM, Wilkinson KA. Synaptic AMPA receptor composition in development, plasticity and disease. Nat Rev Neurosci. 2016;17:337–350. doi: 10.1038/nrn.2016.37. [DOI] [PubMed] [Google Scholar]
- Herguedas B, Krieger J, Greger IH. Receptor heteromeric assembly-how it works and why it matters: the case of ionotropic glutamate receptors. Prog Mol Biol Transl Sci. 2013;117:361–386. doi: 10.1016/B978-0-12-386931-9.00013-1. [DOI] [PubMed] [Google Scholar]
- Hirling H. Endosomal trafficking of AMPA-type glutamate receptors. Neuroscience. 2009;158:36–44. doi: 10.1016/j.neuroscience.2008.02.057. [DOI] [PubMed] [Google Scholar]
- Holbro N, Grunditz A, Oertner TG. Differential distribution of endoplasmic reticulum controls metabotropic signaling and plasticity at hippocampal synapses. Proc Natl Acad Sci U S A. 2009;106:15055–15060. doi: 10.1073/pnas.0905110106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Antion MD, Hu D, Spencer CM, Paylor R, Klann E. Dynamic translational and proteasomal regulation of fragile X mental retardation protein controls mGluR-dependent long-term depression. Neuron. 2006;51:441–454. doi: 10.1016/j.neuron.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288:1254–1257. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- Hume RI, Dingledine R, Heinemann SF. Identification of a site in glutamate receptor subunits that controls calcium permeability. Science. 1991;253:1028–1031. doi: 10.1126/science.1653450. [DOI] [PubMed] [Google Scholar]
- Isaac JT, Ashby MC, McBain CJ. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron. 2007;54:859–871. doi: 10.1016/j.neuron.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Jeyifous O, Waites CL, Specht CG, Fujisawa S, Schubert M, Lin EI, Marshall J, Aoki C, de Silva T, Montgomery JM, et al. SAP97 and CASK mediate sorting of NMDA receptors through a previously unknown secretory pathway. Nat Neurosci. 2009;12:1011–1019. doi: 10.1038/nn.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W, Ge WP, Xu J, Cao M, Peng L, Yung W, Liao D, Duan S, Zhang M, Xia J. Lipid binding regulates synaptic targeting of PICK1, AMPA receptor trafficking, and synaptic plasticity. J Neurosci. 2006;26:2380–2390. doi: 10.1523/JNEUROSCI.3503-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinkenberg D, Long KR, Shome K, Watkins SC, Aridor M. A cascade of ER exit site assembly that is regulated by p125A and lipid signals. J Cell Sci. 2014;127:1765–1778. doi: 10.1242/jcs.138784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK. Ca-permeable AMPA receptors in homeostatic synaptic plasticity. Front Mol Neurosci. 2012;5:17. doi: 10.3389/fnmol.2012.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Takamiya K, Han JS, Man H, Kim CH, Rumbaugh G, Yu S, Ding L, He C, Petralia RS, et al. Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell. 2003;112:631–643. doi: 10.1016/s0092-8674(03)00122-3. [DOI] [PubMed] [Google Scholar]
- Lu W, Khatri L, Ziff EB. Trafficking of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPA) receptor subunit GluA2 from the endoplasmic reticulum is stimulated by a complex containing Ca2+/calmodulin-activated kinase II (CaMKII) and PICK1 protein and by release of Ca2+ from internal stores. J Biol Chem. 2014;289:19218–19230. doi: 10.1074/jbc.M113.511246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Shi Y, Jackson AC, Bjorgan K, During MJ, Sprengel R, Seeburg PH, Nicoll RA. Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron. 2009;62:254–268. doi: 10.1016/j.neuron.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Ziff EB. PICK1 interacts with ABP/GRIP to regulate AMPA receptor trafficking. Neuron. 2005;47:407–421. doi: 10.1016/j.neuron.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Luscher C, Huber KM. Group 1 mGluR-dependent synaptic long-term depression: mechanisms and implications for circuitry and disease. Neuron. 2010;65:445–459. doi: 10.1016/j.neuron.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino H, Malinow R. AMPA receptor incorporation into synapses during LTP: the role of lateral movement and exocytosis. Neuron. 2009;64:381–390. doi: 10.1016/j.neuron.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameli M, Balland B, Lujan R, Luscher C. Rapid synthesis and synaptic insertion of GluR2 for mGluR-LTD in the ventral tegmental area. Science. 2007;317:530–533. doi: 10.1126/science.1142365. [DOI] [PubMed] [Google Scholar]
- Man HY. GluA2-lacking, calcium-permeable AMPA receptors--inducers of plasticity? Curr Opin Neurobiol. 2011;21:291–298. doi: 10.1016/j.conb.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man HY, Sekine-Aizawa Y, Huganir RL. Regulation of {alpha}-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking through PKA phosphorylation of the Glu receptor 1 subunit. Proc Natl Acad Sci U S A. 2007;104:3579–3584. doi: 10.1073/pnas.0611698104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurin T, Zongaro S, Bardoni B. Fragile X Syndrome: from molecular pathology to therapy. Neurosci Biobehav Rev. 2014;46(Pt 2):242–255. doi: 10.1016/j.neubiorev.2014.01.006. [DOI] [PubMed] [Google Scholar]
- McCutcheon JE, Loweth JA, Ford KA, Marinelli M, Wolf ME, Tseng KY. Group I mGluR activation reverses cocaine-induced accumulation of calcium-permeable AMPA receptors in nucleus accumbens synapses via a protein kinase C-dependent mechanism. J Neurosci. 2011;31:14536–14541. doi: 10.1523/JNEUROSCI.3625-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignogna ML, Giannandrea M, Gurgone A, Fanelli F, Raimondi F, Mapelli L, Bassani S, Fang H, Van Anken E, Alessio M, et al. The intellectual disability protein RAB39B selectively regulates GluA2 trafficking to determine synaptic AMPAR composition. Nat Commun. 2015;6:6504. doi: 10.1038/ncomms7504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata M, Finch EA, Khiroug L, Hashimoto K, Hayasaka S, Oda SI, Inouye M, Takagishi Y, Augustine GJ, Kano M. Local calcium release in dendritic spines required for long-term synaptic depression. Neuron. 2000;28:233–244. doi: 10.1016/s0896-6273(00)00099-4. [DOI] [PubMed] [Google Scholar]
- Muddashetty RS, Kelic S, Gross C, Xu M, Bassell GJ. Dysregulated metabotropic glutamate receptor-dependent translation of AMPA receptor and postsynaptic density-95 mRNAs at synapses in a mouse model of fragile X syndrome. J Neurosci. 2007;27:5338–5348. doi: 10.1523/JNEUROSCI.0937-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano-Kobayashi A, Kasri NN, Newey SE, Van Aelst L. The Rho-linked mental retardation protein OPHN1 controls synaptic vesicle endocytosis via endophilin A1. Curr Biol. 2009;19:1133–1139. doi: 10.1016/j.cub.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MC, Derkach VA, Guire ES, Soderling TR. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J Biol Chem. 2006;281:752–758. doi: 10.1074/jbc.M509677200. [DOI] [PubMed] [Google Scholar]
- Pagant S, Wu A, Edwards S, Diehl F, Miller EA. Sec24 is a coincidence detector that simultaneously binds two signals to drive ER export. Curr Biol. 2015;25:403–412. doi: 10.1016/j.cub.2014.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passafaro M, Piech V, Sheng M. Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat Neurosci. 2001;4:917–926. doi: 10.1038/nn0901-917. [DOI] [PubMed] [Google Scholar]
- Penn AC, Greger IH. Sculpting AMPA receptor formation and function by alternative RNA processing. RNA Biol. 2009;6:517–521. doi: 10.4161/rna.6.5.9552. [DOI] [PubMed] [Google Scholar]
- Perestenko PV, Henley JM. Characterization of the intracellular transport of GluR1 and GluR2 alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor subunits in hippocampal neurons. J Biol Chem. 2003;278:43525–43532. doi: 10.1074/jbc.M306206200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez JL, Khatri L, Chang C, Srivastava S, Osten P, Ziff EB. PICK1 targets activated protein kinase Calpha to AMPA receptor clusters in spines of hippocampal neurons and reduces surface levels of the AMPA-type glutamate receptor subunit 2. J Neurosci. 2001;21:5417–5428. doi: 10.1523/JNEUROSCI.21-15-05417.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJ, Evans PR, McMahon HT. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science. 2004;303:495–499. doi: 10.1126/science.1092586. [DOI] [PubMed] [Google Scholar]
- Pick JE, Khatri L, Sathler MF, Ziff EB. mGluR long-term depression regulates GluA2 association with COPII vesicles and exit from the endoplasmic reticulum. EMBO J. 2017;36:232–244. doi: 10.15252/embj.201694526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postiglione MP, Juschke C, Xie Y, Haas GA, Charalambous C, Knoblich JA. Mouse inscuteable induces apical-basal spindle orientation to facilitate intermediate progenitor generation in the developing neocortex. Neuron. 2011;72:269–284. doi: 10.1016/j.neuron.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche KW, O'Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179–1188. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- Rossmann M, Sukumaran M, Penn AC, Veprintsev DB, Babu MM, Greger IH. Subunit-selective N-terminal domain associations organize the formation of AMPA receptor heteromers. EMBO J. 2011;30:959–971. doi: 10.1038/emboj.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio ME, Wenthold RJ. Calnexin and the immunoglobulin binding protein (BiP) coimmunoprecipitate with AMPA receptors. J Neurochem. 1999;73:942–948. doi: 10.1046/j.1471-4159.1999.0730942.x. [DOI] [PubMed] [Google Scholar]
- Saheki Y, De Camilli P. Endoplasmic Reticulum-Plasma Membrane Contact Sites. Annu Rev Biochem. 2017;86:659–684. doi: 10.1146/annurev-biochem-061516-044932. [DOI] [PubMed] [Google Scholar]
- Sans N, Racca C, Petralia RS, Wang YX, McCallum J, Wenthold RJ. Synapse-associated protein 97 selectively associates with a subset of AMPA receptors early in their biosynthetic pathway. J Neurosci. 2001;21:7506–7516. doi: 10.1523/JNEUROSCI.21-19-07506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenk J, Harmel N, Zolles G, Bildl W, Kulik A, Heimrich B, Chisaka O, Jonas P, Schulte U, Fakler B, et al. Functional proteomics identify cornichon proteins as auxiliary subunits of AMPA receptors. Science. 2009;323:1313–1319. doi: 10.1126/science.1167852. [DOI] [PubMed] [Google Scholar]
- Serulle Y, Zhang S, Ninan I, Puzzo D, McCarthy M, Khatri L, Arancio O, Ziff EB. A GluR1-cGKII interaction regulates AMPA receptor trafficking. Neuron. 2007;56:670–688. doi: 10.1016/j.neuron.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105:331–343. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- Silverman JB, Restituito S, Lu W, Lee-Edwards L, Khatri L, Ziff EB. Synaptic anchorage of AMPA receptors by cadherins through neural plakophilin-related arm protein AMPA receptor-binding protein complexes. J Neurosci. 2007;27:8505–8516. doi: 10.1523/JNEUROSCI.1395-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivalingam J, Kumar A. Spinal Tuberculosis Resembling Neoplastic Lesions on MRI. J Clin Diagn Res. 2015;9 doi: 10.7860/JCDR/2015/14030.6719. TC01-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, Osten P, Vilim FS, Khatri L, Inman G, States B, Daly C, DeSouza S, Abagyan R, Valtschanoff JG, et al. Novel anchorage of GluR2/3 to the postsynaptic density by the AMPA receptor-binding protein ABP. Neuron. 1998;21:581–591. doi: 10.1016/s0896-6273(00)80568-1. [DOI] [PubMed] [Google Scholar]
- Sumioka A, Yan D, Tomita S. TARP phosphorylation regulates synaptic AMPA receptors through lipid bilayers. Neuron. 2010;66:755–767. doi: 10.1016/j.neuron.2010.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S, Chen L, Kawasaki Y, Petralia RS, Wenthold RJ, Nicoll RA, Bredt DS. Functional studies and distribution define a family of transmembrane AMPA receptor regulatory proteins. J Cell Biol. 2003;161:805–816. doi: 10.1083/jcb.200212116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Yuan JP, Lanahan AA, Leoffert K, Li M, Linden DJ, Worley PF. Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron. 1998;21:717–726. doi: 10.1016/s0896-6273(00)80589-9. [DOI] [PubMed] [Google Scholar]
- Vandenberghe W, Nicoll RA, Bredt DS. Interaction with the unfolded protein response reveals a role for stargazin in biosynthetic AMPA receptor transport. J Neurosci. 2005;25:1095–1102. doi: 10.1523/JNEUROSCI.3568-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler IJ, Irwin SA, Klintsova AY, Spencer CM, Brazelton AD, Miyashiro K, Comery TA, Patel B, Eberwine J, Greenough WT. Fragile X mental retardation protein is translated near synapses in response to neurotransmitter activation. Proc Natl Acad Sci U S A. 1997;94:5395–5400. doi: 10.1073/pnas.94.10.5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson JR, Albanesi JP, Huber KM. Roles for Arc in metabotropic glutamate receptor-dependent LTD and synapse elimination: Implications in health and disease. Semin Cell Dev Biol. 2017 doi: 10.1016/j.semcdb.2017.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Whiteus C, Xu CS, Hayworth KJ, Weinberg RJ, Hess HF, De Camilli P. Contacts between the endoplasmic reticulum and other membranes in neurons. Proc Natl Acad Sci U S A. 2017;114:E4859–E4867. doi: 10.1073/pnas.1701078114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Zhang X, Staudinger J, Huganir RL. Clustering of AMPA receptors by the synaptic PDZ domain-containing protein PICK1. Neuron. 1999;22:179–187. doi: 10.1016/s0896-6273(00)80689-3. [DOI] [PubMed] [Google Scholar]
- Ye B, Yu WP, Thomas GM, Huganir RL. GRASP-1 is a neuronal scaffold protein for the JNK signaling pathway. FEBS Lett. 2007;581:4403–4410. doi: 10.1016/j.febslet.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]