Abstract
A rapid change in the lifestyle has witnessed poor health with the increased incidences of numerous diseases in the recent years, and ultimately increasing the demand of nutritious foods containing phytochemicals. A wide range of phytochemicals (secondary metabolites) is being synthesized in plants, which influence the human health upon consumption as dietary component. Recently, a number of the technologies (conventional and non-conventional methods) have been standardized by the different researchers for the extraction of these phytochemicals depending upon the raw material. However, selection of extraction method for commercial use depends upon various factors such as extraction efficiency, time required, and cost of operation. Considering these factors, microbial maceration is one of the viable approaches which is easy to handle, cost-effective, energy efficient, less hazardous and having high extraction rate. Recently, researchers have utilized this technique for the maceration of different plant-based substrates (such as legumes, cereals, pulses, fruits and vegetables) and their respective wastes for the efficient extraction of numerous phytochemicals with increased efficiency. However, scale up studies and analysis of toxic compounds produced by microbes are still a lacking field and need to be explored further by the researchers and industrialists to bring it into reality. Therefore, the present review aims to document the recent findings related to microbial maceration in a crisp way to provide the complete information to the readers.
Keywords: Microbial maceration, Phytochemical extraction, Bioactive compounds
Introduction
The history of utilizing plants for humankind (nutritional and medicinal purposes) is as old as the beginning of the human era. Plants synthesize a wide variety of secondary metabolites (phytochemicals) besides the primary metabolites. Phytochemicals are nonnutritive chemicals that are produced by the plant for protecting themselves from insect infestation and microbial attack and having the tendency to protect the humans from various diseases such as heart diseases, cancer, and many other chronic diseases. Phytochemicals are natural bioactive compounds found in fruits and vegetables that work together with many other components in promoting good health in many ways. In addition, they can be used as nutraceuticals having beneficial health effects for the treatment of various diseases (Bravo and Mateos 2008). Nowadays, researchers are looking towards the potential benefits of phytochemicals as an alternate to synthetic substances, which are mostly used in pharmaceutical, food and cosmetic industries (Joshi et al. 2012). Many functional foods produced with bioactive compounds are available in the market that provide health benefits beyond fulfilling the basic need of energy and nutrition (Šaponjac et al. 2016). In the early age, Maceration and fermentation technology was used to improve the nutritional properties (digestibility and bioactivity), shelf life, organoleptic quality characteristics of food and for extraction of the active compounds which can be used for the production of value-added food. With the base of these themes, research took a shift for the extraction of phytochemicals using microbial maceration from numerous agro-based raw materials (whole or waste).
Several classes of phytochemicals which include phenolics, antioxidants, pigments, alkaloids compounds have the ability to possess numerous health benefits such as antimicrobial, antidiarrhoeal (Cowan 1999), and anthelmintic (Mute et al. 2009; Sharma et al. 2009, 2010). A complete detail of the phytochemicals is given in Table 1 with their mode of actions.
Table 1.
Activity and mode of action of phytochemical
| Phytochemicals | Activity/ mode of action | References |
|---|---|---|
| Quinones | It shows antimicrobial activity that binds to the adhesions, form complex with the cell wall and enzymes get inactivated | Cowan (1999) |
| Flavanoids | It shows antimicrobial activity that forms complex with the cell wall and binds to the adhesions | Cowan (1999), Kumar et al. (2010) |
| It has antidiarrheal activity that prevents the release of autacoids and prostaglandins, also prevent the contraction which is caused by the spasmogens, it also normalize the water transport across the mucosal cells, it prevent the release acetylcholine from gastrointestinal tract | ||
| Polyphenols and tannins | It show the antimicrobial activity that binds to the adhesions, inhibit the enzymes, form complex with the cell wall, and disrupt the cell membrane | Cowan (1999) |
| Mute et al. (2009) | ||
| It also possess the antidiarrheal activity that makes mucosa present in the intestine more resistant and also reduce secretion, it normalize the water transport system across the mucosal cells and also reduce the intestinal transit, it also block the binding of enterotoxin to GM which results in enterotoxin-induced diarrhea and show astringent action | Sharma et al. (2009), Kumar et al. (2010) | |
| It also has anthelmintic activity that it forms protein complexes that help in increasing digestible protein in rumen, it decrease the gastrointestinal metabolism | ||
| Terpenoids and essential oils | It shows the antimicrobial activity that disrupts the membrane system | Cowan (1999) |
| It also shows the antidiarrheal activity that prevents the release of prostaglandins and autacoids | ||
| Alkaloids | It shows the antimicrobial activity that interchelates the cell wall of parasites | Cowan (1999), Mute et al. (2009) |
| It also shows the antidiarrheal activity that prevents the release of prostaglandins and autacoids | Kumar et al. (2010) | |
| It shows the anthelmintic activity that helps in synthesis of protein by generating nitrate, it suppress the transfer of sugar to intestine, it also acts on central nervous system causing paralysis | Sharma et al. (2009) | |
| Polypeptides and lectins | It shows the antiviral activity by forming the disulfide bridges it block the viral adsorption | Wang et al. (2010) |
| Glycosides | It shows the antidiarrheal activity that prevents the release of prostaglandins and autacoids | Kumar et al. (2010) |
| Steroids | It shows the antidiarrheal activity that increases the transport of sodium and water to the intestinal | Maniyar et al. (2010) |
| Saponins | It shows the antidiarrheal activity that prevents the histamine to release | Maniyar et al. (2010) |
| It shows the anticancer activity that shows the membrane permeabilizing properties | Wang et al. (2010) | |
| It shows the anthelmintic activity teguments disintegrate | ||
| Coumarins | It shows the antiviral activity that interacts with the DNA | Wang et al. (2010) |
Extraction of phytochemicals/bioactive compounds
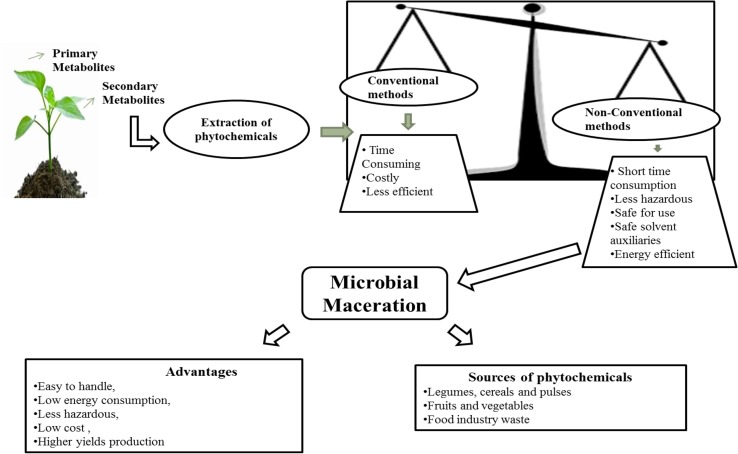
With urbanization, globalization and economic development, a rapid change in the dietary lifestyle has been observed since last few years leading to increase in the incidence of poor health, which is being reflected by increased incidences of numerous diseases (obesity, diabetes, cardiovascular disease, stroke, hypertension, and some types of cancer) (Jnawali et al. 2016). Because of this, the demand of the health and nutraceutical foods is increasing day by day as the consumer are becoming more health conscious. Many attempts have been made by researchers and industrialists to fulfill the demand of consumers by enriching or supplementing the foods with phytochemicals (in crude or pure form). For the extraction of these phytochemicals from different sources, a wide range of physical, chemical and biological techniques have been explored by various researchers depending upon the nature of raw material. Conventional extraction techniques include ecofriendly extraction, hydro-distillation, solvent maceration, soxhlet extraction (Azmir et al. 2013; Jansirani et al. 2014; Kushwaha et al. 2017), whereas non-conventional extraction techniques include microwave-assisted extraction, supercritical fluid extraction, pressurized liquid extraction, microbial maceration, enzymatic maceration, pulsed-electric field extraction, ultrasound-assisted extraction (Corrales et al. 2008; Azmir et al. 2013; Lenucci et al. 2015).
The efficiency of conventional techniques depends on the solvents, but it is also necessary to consider environmental safety and toxicity before selecting the solvent for the extraction process (Cowan 1999). Beside this, conventional methods have many disadvantages, i.e., time consuming, costly and less efficient as compared to non-conventional methods (Wang and Weller 2006). Whereas, numerous advantages are being possessed by the non-conventional methods including short time consumption, less hazardous, safe to use, energy efficient, lesser use of non-renewable resources, reduced derivatives production, prevention of the degradation of final product (Azmir et al. 2013). The extraction efficiency of these methods (conventional and non-conventional) may vary according to their capability and no doubt the substrate, especially for the waste management (Kushwaha et al. 2017). Cost of operation is one of the crucial factors influencing the extraction method selection. This situation is raising the demand of the low-cost technologies; and microbial maceration can be an acceptable option as it is very easy to handle, require low energy consumption, less hazardous, low-cost and having higher production (Singhania et al. 2009). A complete overview of the conventional and non-conventional extraction techniques is given in Fig. 1.
Fig. 1.
An overview of the conventional and non-conventional extraction techniques
Microbial maceration
Maceration is the process of softening the tissue and breaking them into pieces using liquids. During maceration, the tissue gets soften and the compound present inside the tissue gets leached out into the liquid (extract). The extract thus obtained contains many of the metabolites such as phenols, terpenes, flavonoids, and pigments (Azwanida 2015). This technique has been used by many of the researchers for making wine from the various fruits where the compounds are leached out into the must (Joshi et al. 2009). Recently, researchers have exploited the microbial maceration technique using different microbes for the extraction of various bioactive compounds such as phenolics, flavonoids, antioxidants, tannins, and saponins from the numerous substrates, i.e., fruits, vegetables, legumes, cereals and pulses as well as agro-industrial waste. The advantage of using microbes is to extract compound from different sources and its simplicity for getting high yields, very easy to handle, low-cost, and low energy consumption (Demain and Fang 2000).
In microbial maceration, different input factors (such as time, temperature, humidity, concentration of the inoculum and other conditions) are playing important role to determine the efficiency of the process. Moreover, maintaining microbial maceration conditions is necessary for the efficient growth of the microbes to macerate and ferment different sources and enhancing the extraction efficiency. Different microbes and their respective temperature required for the maceration of food materials are enlisted in Table 2.
Table 2.
Different microbes and temperature required for the maceration
| Microorganism | Species | Temperature (°C) | References |
|---|---|---|---|
| Bacteria | Lactobacillus acidophilus, Lactobacillus brevis, Lactobacillus bulgaricus, Lactobacillus johnsonii, Lactobacillus casei, Lactobacillus fermentum, Lactobacillus lactis, Lactobacillus plantarum, Lactobacillus johnsonii, Lactobacillus paracasei, Lactobacillus reuteri, Lactobacillus rossiae, Lactobacillus zeae, Lactococcus lactis, Bifidobacterium animalis, Bifidobacterium infantis, Streptococcus thermophilus, and Weissella paramesenteroides | 22–37 | Frias et al. (2005), Othman et al. (2009), Hur et al. (2014), Gan et al. (2017) |
| Fungi | Bacillus subtilis, Bacillus cereus, Bacillus thuringiensis, Aspergillus oryzae, Aspergillus niger, Aspergillus awamori, Aspergillus sojae, Agrocybe cylindracea, Cordyceps militaris, Coprinus cinereus, Grifola frondosa, Ganoderma austral, Ganoderma lucidum, Lentinus edodes, Monascus ruber, Rhizopus microsporus, Rhizopus oligosporus, Rhizopus oryzae, Thamnidium elegans | 22–30 | Fernandez-Orozco et al. (2007), Bhanja et al. (2009), Cai et al. (2011) |
| Yeast | Cryptococcus flavus, Issatchenkia orientalis, Saccharomyces cerevisiae, Saccharomyces boulardii, Cryptococcus sp.S-2, Rhodotorula glutinis | 20–30 | Hur et al. (2014), Escuder et al. (2013) |
| Kumar et al. (2015), Gan al. (2017) |
Crop-specific extraction of phytochemicals using microbial maceration
Legumes, cereals, and pulses
Legumes, cereals, and pulses are playing very important role in human diet and possess many health benefits (Saleh et al. 2013). Moreover, recent studies reported that the microbial maceration of legumes, cereals, and pulses resulted in extraction of higher amount of phytochemicals, which possess numerous biological functions such as antioxidant activity and anticancer effects (Azmir et al. 2013). Recently, different researchers have reported that microbial maceration significantly enhanced the soluble total phenolic content of cheonggukjang soybean, soybean, black soybean, chickpea, cowpea, bran, black soybean, pea, common bean, kidney beans, wheat koji, buckwheat, barley, wheat, rye, Avena sativa and lotus seeds (Zheng and Shetty 2000; Duenas et al. 2005; Katina et al. 2007; Bhanja et al. 2008, 2009; Dordevic et al. 2010; Hu et al. 2010; Cho et al. 2011; Xiao et al. 2015; Starzyńska et al. 2014; Wang et al. 2014; Limon et al. 2015). Many microbes are able to produce enzymes, which can degrade the cell wall matrix and can release the bound phenolics (Huynh et al. 2014). Both the bound and free phenolics contribute for the increased yields of the total phenolic content. However, many studies show that microbial maceration not only increase the yield of the phenolic content but also reduce the yield in some sources such as soybean (Lee et al. 2008), which is due to the degradation of some phenols during the maceration process.
Recent studies have witnessed an increase in the antioxidant activity (free radical-scavenging, reducing and metal-chelating effects), flavonoids and anthocyanin content of legumes, pulses and cereals extract because of the microbial maceration as compared to the control samples (Table 3). It has been reported that black soybean, small runner bean, small rice bean, lentil, speckled kidney bean, mottled cowpea, black cow gram, cowpeas, black bean koji, rye, wheat, barley, buckwheat, cheonggukjang soybean, brown soybean, Moringa oleifera seeds, black soybeans, and black soybean show high antioxidant activity, flavonoids and anthocyanin as compared to the control (Lee et al. 2008; Dordevic et al. 2010; Juan and Chou 2010; Hu et al. 2010; Cho et al. 2011; Shin et al. 2014; Gan et al. 2016). In some cases, such as the microbial maceration of yellow soybean, black soybean, small runner bean, lentil may lead to the decrease in antioxidant activity (Gan et al. 2016).
Table 3.
Effect of microbial maceration on extraction of phytochemicals from legumes, cereals and pulses
| Source/product | Microorganism | Time and temperature | Phenols | Control samples | Macerated samples | References |
|---|---|---|---|---|---|---|
| Phenolic | ||||||
| Cheonggukjang Soybean | B. subtilis CS90 | 60 h at 37 °C | Gallic acid | 306.40 (mg/kg) | 1062.5 (mg/kg) | Cho et al. (2011) |
| 48 h at 37 °C | Protocatechuic acid | 4.42 (mg/kg) | 4.56 (mg/kg) | |||
| 36 h at 37 °C | p-Coumaric acid | 0.16 (mg/kg) | 0.17 (mg/kg) | |||
| Red beans | B. subtilis | 120 h 30 °C | Total phenolic | ND | 22.58 (mg/g) | Chung et al. (2002) |
| Jatropha curcas | Rhizopus oryzae | 8 h at 37 °C | Total phenolic | ND | 1.17% | Oseni and Akindahunsi (2011) |
| Tannins | ND | 0.76% | ||||
| Soybean | Monascus (MFS-31499) | 24 h at 25 °C | Total phenol | 5.82 ± 0.04 (mg/g) | 0.35 ± 0.05 (mg/g) | Lee et al. (2008) |
| Monascus (MFS-31527) | Total phenol | 5.82 ± 0.04 (mg/g) | 6.05 ± 0.02 (mg/g) | |||
| Wheat koji | Aspergillus oryzae | 96 h at 30 °C | Phenolic | 7.226 (µmol/g) | 158.912 (µmol/g) | Bhanja et al. (2009) |
| Wheat koji | Aspergillus awamori | 120 h at 30 °C | Phenolic | 7.226 (µmol/g) | 124.176 (µmol/g) | Bhanja et al. (2009) |
| Moringa oleifera seeds | Natural fermentation | 72 h at 25 °C | Tannins | ND | 146.67 (mg/100 g) | Ijarotimi et al. (2013) |
| Phenolics | ND | 23.00 (mg/100 g) | ||||
| Saponins | ND | 7.5 (mg/100 g) | ||||
| Terpenoids | ND | 25.0 (mg/100 g) | ||||
| Soybeans | Aspergillus oryzae | 120 h at 30 °C | Phenolics | ND | 56.2 (mg/g) | Wardhani et al. (2010) |
| Buckwheat | Lactobacillus rhamnosus | 24 h at 37 °C | Total phenolic | 50.7 ± 0.04 (mg/g) | 59.4 ± 0.06 (mg/g) | Dordevic et al. (2010) |
| Saccharomyces cerevisiae | 24 h at 30 °C | Total phenolic | 50.7 ± 0.04 (mg/g) | 53.2 ± 0.02 (mg/g) | ||
| Barley | Lactobacillus rhamnosus | 24 h at 37 °C | Total phenolic | 16.4 ± 0.04 (mg/g) | 20.1 ± 0.08 (mg/g) | |
| Saccharomyces cerevisiae | 24 h at 30 °C | Total phenolic | 16.4 ± 0.04 (mg/g) | 18.5 ± 0.09 (mg/g) | ||
| Wheat | Lactobacillus rhamnosus | 24 h at 37 °C | Total phenolic | 16.2 ± 0.07 (mg/g) | 20.7 ± 0.06b (mg/g) | Dordevic et al. (2010) |
| Saccharomyces cerevisiae | 24 h at 30 °C | Total phenolic | 16.2 ± 0.07 (mg/g) | 18.4 ± 0.08 (mg/g) | ||
| Rye | Lactobacillus rhamnosus | 24 h at 37 °C | Total phenolic | 13.2 ± 0.06 (mg/g) | 18.4 ± 0.06 (mg/g) | Dordevic et al. (2010) |
| Saccharomyces cerevisiae | 24 h at 30 °C | Total phenolic | 13.2 ± 0.06 (mg/g) | 16.2 ± 0.04 (mg/g) | ||
| Black soybeans | B. subtilis | 18 h at 40 °C | Total phenolic | 6.04 0.33 (mg/g) | 12.44 0.41 (mg/g) | Juan and Chou (2010) |
| Avena sativa L. | A. oryzae var. effuses | 3 days at 25 °C | Chlorogenic acid | 63.9 ± 5.34 (mg/100 g) | 163.8 ± 2.72 (mg/100 g) | Cai et al. (2011) |
| Caffeic acid | 116.6 ± 2.34 (mg/100 g) | 385.7 ± 4.57 (mg/100 g) | ||||
| p-Coumaric acid | 55.1 ± 1.23 (mg/100 g) | 119.0 ± 5.69 (mg/100 g) | ||||
| Ferulic acid | 89.0 ± 0.84 (mg/100 g) | 793.8 ± 6.85 (mg/100 g) | ||||
| Avena sativa L. | A. oryzae | 3 days at 25 °C | Chlorogenic acid | 63.9 ± 5.34 (mg/100 g) | 138.4 ± 0.76 (mg/100 g) | Cai et al. (2011) |
| Caffeic acid | 116.6 ± 2.34 (mg/100 g) | 319.6 ± 0.72 (mg/100 g) | ||||
| p-Coumaric acid | 55.1 ± 1.23 (mg/100 g) | 98.5 ± 3.35 (mg/100 g) | ||||
| Ferulic acid | 89.0 ± 0.84 (mg/100 g) | 493.1 ± 5.36 (mg/100 g) | ||||
| Avena sativa L. | A. niger | 3 days at 25 °C | Caffeic acid | 116.6 ± 2.34 (mg/100 g) | 160.6 ± 3.21 (mg/100 g) | Cai et al. (2011) |
| p-Coumaric acid | 55.1 ± 1.23 (mg/100 g) | 104.9 ± 4.78 (mg/100 g) | ||||
| Ferulic acid | 89.0 ± 0.84 (mg/100 g) | 87.2 ± 4.12 (mg/100 g) | ||||
| Chickpea | Cordyceps militaris SN-18 | 8 days at 25 °C | Total phenolic contents | 6.07 ± 0.19 (mg/g) | 10.53 ± 0.02 (mg/g) | Xiao et al. (2015) |
| Total saponin contents | 5.61 ± 0.19 (mg/g) | 6.82 ± 0.19 (mg/g) | ||||
| Cowpeas | Lactobacillus plantarum ATCC 14917 | 48 h at 37 °C | Vanillic acid | 2.51 ± 0.87 (mg/g) | 4.44 ± 1.00 (mg/g) | Duenas et al. (2005) |
| Quercetin | ND | 22.02 ± 0.40 (mg/g) | ||||
| trans-Ferulic acid | 1.60 ± 0.07 (mg/g) | 4.10 ± 0.14 (mg/g) | ||||
| cis-Ferulic acid | 1.24 ± 0.09 (mg/g) | 0.36 ± 0.02 (mg/g) | ||||
| Bran | Baker’s yeast | 20 h at 35 °C | Total phenolic | ND | 383 (mg/100 g) | Katina et al. (2007) |
| 13 h at 27.5 °C | Ferulic acid | ND | 30 (mg/100 g) | |||
| Black soybean | Bacillus natto | 48 h at 37 °C | Total phenolic | 614.82 ± 13.12 (µg/ g) | 668.41 ± 31.26 (µg/ g) | Hu et al. (2010) |
| Pea | Trichoderma viride IF-26 | 5 days at 25 °C | Total phenolics | 0.633 ± 90.054 (mg/g) | 0.717 ± 90.078 (mg/g) | Zheng and Shetty (2000) |
| Trichoderma harzianum ATCC 24274 | 5 days at 25 °C | Total phenolics | 0.633 ± 90.054 (mg/g) | 0.746 ± 90.044 (mg/g) | ||
| Trichoderma pseudokoningii ATCC 26801 | 5 days at 25 °C | Total phenolics | 0.633 ± 90.054 (mg/g) | 0.738 ± 90.047 (mg/g) | ||
| Common bean | Lactobacillus plantarum DSM 20174 | 18 h at 30 °C | Total phenolics | ND | 1.61 (mg/g) | Starzyńska-Janiszewska (2014) |
| R. microspores var. chinensis | 18 h at 30 °C | Total phenolics | ND | 1.69 (mg/g) | ||
| Kidney beans | Bacillus subtilis | 96 h at 30 °C | Total phenolic | 15.89 ± 0.56 (mg/g) | 35.93 ± 0.69 (mg/g) | Limón et al. (2015) |
| Kidney beans | Lactobacillus plantarum | 48 h at 37 °C | Total phenolic | 20.68 ± 1.04 (mg/g) | 21.96 ± 0.54 (mg/g) | |
| Adlay | B. subtilis | 24 h at 37 °C | Total phenolic | 8.58 ± 0.62 (mg/g) | 13.29 ± 1.67 (mg/g) | Wang et al. (2014) |
| Lactobacillus plantarum | 24 h at 37 °C | Total phenolic | 8.58 ± 0.62 (mg/g) | 11.91 ± 1.94 (mg/g) | ||
| Chestnut | B. subtilis | 24 h at 37 °C | Total phenolic | 12.95 ± 0.57 (mg/g) | 15.28 ± 1.85 (mg/g) | Wang et al. (2014) |
| Lactobacillus plantarum | 24 h at 37 °C | Total phenolic | 17.52 ± 1.67 (mg/g) | 28.67 ± 2.95 (mg/g) | ||
| Lotus seed | B. subtilis | 24 h at 37 °C | Total phenolic | 17.52 ± 1.67 (mg/g) | 28.67 ± 2.95 (mg/g) | Wang et al. (2014) |
| Lactobacillus plantarum | 24 h at 37 °C | Total phenolic | 17.52 ± 1.67 (mg/g) | 24.62 ± 3.54 (mg/g) | ||
| Walnut | B. subtilis | 24 h at 37 °C | Total phenolic | 22.80 ± 4.23 (mg/g) | 33.89 ± 3.84 (mg/g) | Wang et al. (2014) |
| Lactobacillus plantarum | 24 h at 37 °C | Total phenolic | 22.80 ± 4.23 (mg/g) | 28.61 ± 4.24 (mg/g) | ||
| Antioxidant activity | ||||||
| Soybeans | Aspergillus oryzae | 120 h at 30 °C | DPPH | ND | 81.6% | Wardhani et al. (2010) |
| Buckwheat (Fagopyrum esculentum) | Lactobacillus rhamnosus | 24 h at 37 °C | FRAP | 49.43 ± 0.49 (nmol/mg) | 51.54 ± 0.65 (nmol/mg) | Dordevic et al. (2010) |
| Saccharomyces cerevisiae | 24 h at 30 °C | FRAP | 49.43 ± 0.49 (nmol/mg) | 49.76 ± 0.62 (nmol/mg) | ||
| Barley (Hordeum vulgare) | Lactobacillus rhamnosus | 24 h at 37 °C | FRAP | 15.56 ± 0.67 (nmol/mg) | 20.0 ± 0.54 (nmol/mg) | |
| Saccharomyces cerevisiae | 24 h at 30 °C | FRAP | 15.56 ± 0.67 (nmol/mg) | 19.83 ± 0.51 (nmol/mg) | ||
| Wheat (Triticum durum) | Lactobacillus rhamnosus | 24 h at 37 °C | FRAP | 12.15 ± 0.60 (nmol/mg) | 15.11 ± 0.57 (nmol/mg) | Dordevic et al. (2010) |
| Saccharomyces cerevisiae | 24 h at 30 °C | Antioxidant: DPPH |
ND | > 200 (µg/ml) | ||
| 24 h at 30 °C | FRAP | 12.15 ± 0.60 (nmol/mg) | 12.25 ± 0.62 (nmol/mg) | |||
| Rye (Secale cereal) | Lactobacillus rhamnosus | 24 h at 37 °C | FRAP | 8.94 ± 0.86 (nmol/mg) | 13.94 ± 0.91 (nmol/mg) | Dordevic et al. (2010) |
| Saccharomyces cerevisiae | 24 h at 30 °C | FRAP | 8.94 ± 0.86 (nmol/mg) | 10.68 ± 0.83 (nmol/mg) | ||
| Black bean koji | Rhizopus sp. | 3 days at 30 °C | DPPH radical-scavenging | 1.95 ± 0.01 | 2.11 ± 0.12 (mg/ml) | Lee et al. (2008) |
| Fe2+-chelating ability | 2.68 ± 0.09 (mg/ml) | 3.11 ± 0.85 (mg/ml) | ||||
| Cowpeas | Lactobacillus plantarum ATCC 14917 | 48 h at 37 °C | Antioxidant activity | ND | 8.89 ± 0.02 (mg/g) | Duenas et al. (2005) |
| Black cow gram | Lactobacillus paracasei 279 | 48 h at 37 °C | FRAP | 20.0 ± 1.14 (µg/g) | 23.6 ± 1.10 (µg/g) | Gan et al. (2016) |
| ABTS | 16.5 ± 0.94 (µg/g) | 16.6 ± 0.45 (µg/g) | ||||
| Lactobacillus plantarum WCFS1 | 48 h at 37 °C | FRAP | 20.0 ± 1.14 (µg/g) | 25.1 ± 0.72 (µg/g) | ||
| ABTS | 16.5 ± 0.94 (µg/g) | 17.3 ± 0.85 (µg/g) | ||||
| Mottled cowpea | Lactobacillus paracasei 279 | 48 h at 37 °C | FRAP | 32.1 ± 1.13 (µg/g) | 48.7 ± 2.30 (µg/g) | Gan et al. (2016) |
| ABTS | 29.2 ± 0.74 (µg/g) | 35.0 ± 35.0 (µg/g) | ||||
| Lactobacillus plantarum WCFS1 | 48 h at 37 °C | FRAP | 32.1 ± 1.13 (µg/g) | 47.9 ± 2.56 (µg/g) | ||
| ABTS | 29.2 ± 0.74 (µg/g) | 34.9 ± 0.80 (µg/g) | ||||
| Speckled kidney bean | Lactobacillus paracasei 279 | 48 h at 37 °C | FRAP | 17.7 ± 0.49 (µg/g) | 30.0 ± 0.40 (µg/g) | Gan et al. (2016) |
| ABTS | 18.0 ± 1.08 (µg/g) | 28.2 ± 0.94 (µg/g) | ||||
| Lactobacillus plantarum WCFS1 | 48 h at 37 °C | FRAP | 17.7 ± 0.49 (µg/g) | 28.1 ± 0.30 (µg/g) | ||
| ABTS | 18.0 ± 1.08 (µg/g) | 26.7 ± 0.72 (µg/g) | ||||
| Lentil | Lactobacillus paracasei 279 | 48 h at 37 °C | FRAP | 17.5 ± 0.37 (µg/g) | 20.6 ± 0.80 (µg/g) | Gan et al. (2016) |
| ABTS | 17.3 ± 0.31 (µg/g) | 16.7 ± 0.44 (µg/g) | ||||
| Lactobacillus plantarum WCFS1 | 48 h at 37 °C | FRAP | 17.5 ± 0.37 (µg/g) | 19.7 ± 0.26 (µg/g) | ||
| ABTS | 17.3 ± 0.31 (µg/g) | 16.2 ± 0.48 (µg/g) | ||||
| Small rice bean | Lactobacillus paracasei 279 | 48 h at 37 °C | FRAP | 25.9 ± 0.26 (µg/g) | 34.0 ± 1.02 (µg/g) | Gan et al. (2016) |
| ABTS | 24.6 ± 1.27 (µg/g) | 29.1 ± 0.92 (µg/g) | ||||
| Lactobacillus plantarum WCFS1 | 48 h at 37 °C | FRAP | 25.9 ± 0.26 (µg/g) | 34.5 ± 0.79 (µg/g) | ||
| ABTS | 24.6 ± 1.27 (µg/g) | 29.7 ± 0.94 (µg/g) | ||||
| Small runner bean | Lactobacillus paracasei 279 | 48 h at 37 °C | FRAP | 31.8 ± 1.27 (µg/g) | 24.0 ± 1.18 (µg/g) | Gan et al. (2016) |
| ABTS | 25.4 ± 0.80 (µg/g) | 24.4 ± 1.58 (µg/g) | ||||
| Lactobacillus plantarum WCFS1 | 48 h at 37 °C | FRAP | 1.27 (µg/g) | 28.0 ± 0.27 (µg/g) | ||
| ABTS | 25.4 ± 0.80 (µg/g) | 25.2 ± 0.66 (µg/g) | ||||
| Black soybean | Lactobacillus paracasei 279 | 48 h at 37 °C | FRAP | 21.2 ± 0.59 (µg/g) | 22.7 ± 0.29 (µg/g) | Gan et al. (2016) |
| ABTS | 18.0 ± 0.57 (µg/g) | 15.4 ± 0.75 (µg/g) | ||||
| Lactobacillus plantarum WCFS1 | 48 h at 37 °C | FRAP | 21.2 ± 0.59 (µg/g) | 24.1 ± 0.5 (µg/g) | ||
| ABTS | 18.0 ± 0.57 (µg/g) | 15.8 ± 0.68 (µg/g) | ||||
| Yellow soybean | Lactobacillus paracasei 279 | 8 h at 37 °C | FRAP | 9.20 ± 0.17 (µg/g) | 8.00 ± 0.40 (µg/g) | Gan et al. (2016) |
| ABTS | 10.8 ± 0.33 (µg/g) | 5.22 ± 0.21 (µg/g) | ||||
| Lactobacillus plantarum WCFS1 | 48 h at 37 °C | FRAP | 9.20 ± 0.17 (µg/g) | 7.30 ± 0.08 (µg/g) | ||
| ABTS | 10.8 ± 0.33 (µg/g) | 5.49 ± 0.13 (µg/g) | ||||
| Flavonoids | ||||||
| Cheonggukjang Soybean | B. subtilis CS90 | 60 h at 37 °C | Catechin | 6.64 (mg/kg) | 48.60 (mg/kg) | Cho et al. (2011) |
| Epicatechin | 12.37 (mg/kg) | 54.1 (mg/kg) | ||||
| Cheonggukjang Soybean | B. subtilis CS90 | 36 h at 37 °C | Daidzein | 0.00 | 372.28 (mg/kg) | Cho et al. (2011) |
| 60 h at 37 °C | Genistein | 0.00 | 25.62 (mg/kg) | |||
| 36 h at 37 °C | Acetyl daizin | 0.00 (mg/kg) | 335.99 (mg/kg) | |||
| 24 h at 37 °C | Acetyl glycitin | 172.50d (mg/kg) | 187.24 (mg/kg) | |||
| 24 h at 37 °C | Malonylglycitin | 61.10 (mg/kg) | 62.81 (mg/kg) | |||
| Brown Soybean | B. subtilis | 48 h at 37 °C | Daidzein | 3.7 ± 0.08 (µg/g) | 156.5 (µg/g) | Shin et al. (2014) |
| Glycitein | 12.5 ± 0.11a (µg/g) | 10.2 (µg/g) | ||||
| Genistein | ND | 2.5 (µg/g) | ||||
| Soybean | Rhizopus oligosporus | 32.06 h at 29.39 °C | Daidzin | ND | 1284.14 (µg/g) | Yaakob et al. (2011) |
| 48 h at 35 °C | Daidzein | ND | 1663.85 (µg/g) | |||
| Moringa oleifera seeds | Anaerobic fermentation | 72 h at 25 °C | Flavanoids | ND | 5.00 (mg/100 g) | Ijarotimi et al. (2013) |
| Black soybeans | B. subtilis | 18 h at 40 °C | Total flavanoids | 0.89 ± 0.10 (mg/g) | 1.89 ± 0.17 (mg/g) | Juan and Chou (2010) |
| Black soybean | Bacillus natto | 48 h at 37 °C | Genistein | 132 ± 12 (µg/g) | 186 ± 10 (µg/g) | Hu et al. (2010) |
| Daidzein | 160 ± 20 (µg/g) | 238 ± 16 (µg/g) | ||||
| Pigment | ||||||
| Red beans | B. subtilis | 120 h at 30 °C | Anthocyanin | ND | 1.00 (µmol/g) | Chung et al. (2002) |
| Black soybean | Bacillus natto | 48 h at 37 °C | Anthocyanin | 0.52 ± 0.10 (µg/g) | 1.28 ± 0.14 (µg/g) | Hu et al. (2010) |
ND not detected
Fruits and vegetables
It was reported that fruits and vegetables contain high amount of phytochemicals, nutrients and dietary fibers and many more compounds, which are essential for the human nutrition (Boeing et al. 2012). Epidemiological studies narrated that long-term consumption of fruits and vegetables reduces the risk of cancer and many other chronic diseases especially because of phytochemicals (Batra and Sharma 2013). Many industries are utilizing these phytochemicals for the production of value-added products after extracting them from fruits and vegetables with the help of microbial maceration technique (Boeing et al. 2012). It has been reported that maceration significantly increased the soluble total phenolics, antioxidant and flavonoids content in Citrus sinensis, cabernet sauvignon grapes, tempranillo grapes, kiwifruit, green olive, varicoloured olives, black olives, Brassica pekinensis Skeels (Mayen et al. 1995; Sun et al. 2009; Othman et al. 2009; Escudero et al. 2013; Li et al. 2013) (Table 4). In addition, there is an increase in antioxidant activity of Citrus sinensis; Basella rubra (Escudero et al. 2013; Kumar et al. 2015), flavonoids in cabernet sauvignon grapes, black mulberry (Mayén et al. 1995; Pérez-Gregorio et al. 2011), pigment composition in Citrus sinensis, cabernet sauvignon grapes, tempranillo grapes, black mulberry (Mayén et al. 1995; Pérez-Gregorio et al. 2011; Escudero et al. 2013) as compared to the control samples (Table 4).
Table 4.
Effect of microbial maceration on extraction of phytochemicals from fruits and vegetables
| Sources/products | Microorganism | Time and temperature | Phenols | Control samples | Macerated samples | References |
|---|---|---|---|---|---|---|
| Fruits | ||||||
| Phenolic | ||||||
| Citrus sinensis L. | Saccharomycetaceae var. Pichia kluyveri | 1 day at 20 °C | Total phenolics | 793 ± 0.5 (mg/l) | 801 ± 7.3 (mg/l) | Escudero-López et al. (2013) |
| Cabernet Sauvignon grapes | Saccharomyces cerevisiae | 8 days at 25 °C | Quercetin | < 0.001 (mg/l) | 0.666 ± 0.144 (mg/l) | Mayén et al. (1995) |
| 44 days at 25 °C | Gallic acid | 0.162 ± 0.024 (mg/l) | 11.1 ± 0.666 (mg/ml) | |||
| Kiwifruit | S. cerevisiae (RA17) | 25 ± 1 °C 2 weeks | Total phenolics | 298 ± 11 (mg/l) | 305 ± 15 (mg/l) | Li et al. (2013) |
| S. cerevisiae (RC212) | 25 ± 1 °C 2 weeks | Total phenolics | 298 ± 11 (mg/l) | 317 ± 10 (mg/l) | ||
| Tempranillo grapes | Saccharomyces cerevisiae | 44 day at 25 °C | Gallic acid | 0.258 ± 0.039 (mg/l) | 6.51 ± 0.774 (mg/l) | |
| Antioxidant activity | ||||||
| Basella rubra | Saccharomyces cerevisiae | 6 h at 30 °C | DPPH scavenging activity | 1.9 mg/ml | 2.4 µg/ml | Kumar et al. (2015) |
| Citrus sinensis L. var. Navel late | Saccharomycetaceae var. Pichia kluyveri | 9 days at 20 °C | ORAC | 6044 µM | 9355 ± 678 (µM) | Escudero-López et al. (2013) |
| 1 days at 20 °C | FRAP | 10.3 mM | 10.9 ± 0.4 (mM) | |||
| Flavonoids | ||||||
| Cabernet Sauvignon grapes | Saccharomyces cerevisiae | 14 h at 25 °C | Catechin | 0.180 ± 0.030 (mg/l) | 86.1 ± 9.00 (mg/l) | Mayén et al. (1995) |
| Black mulberry | Saccharomyces cerevisiae | 24 h at 18 °C | Flavanols | 62 ± 7 (mg/kg) | 65 ± 1 (mg/kg) | Pérez-Gregorio et al. (2011) |
| Pigments | ||||||
| Citrus sinensis L. var. Navel late | Saccharomycetaceae var. Pichia kluyveri | 13 day at 20 °C | Total carotenoids | 5.8 (mg/l) | 6.5 ± 0.2 (mg/l) | Escudero-López et al. (2013) |
| Cabernet Sauvignon grapes | Saccharomyces cerevisiae | 3 day at 25 °C | Z-Anthocyans | 42.6 + 7.07 (u.a) | 238 ± 34.8 (mg/l) | Mayén et al. (1995) |
| Tempranillo grapes | Saccharomyces cerevisiae | 5 day at 25 °C | Z-Anthocyans | 42.6 + 7.07 (u.a) | 239 + 18.27 (u.a.) | |
| Black mulberry | Saccharomyces cerevisiae | 24 h at 18 °C | Cyanidin 3-glucoside | 2048 ± 146 (mg/kg) | 2084 ± 15 (mg/kg) | Pérez-Gregorio et al. (2011) |
| Vegetables | ||||||
| Phenols | ||||||
| Brassica pekinensis Skeels | Lactobacillus plantarum | 2 days at 25 °C | Total phenolic | 3.18 ± 0.24 (µg /mg) | 4.38 ± 0.02 (µg /mg) | Sun et al. (2009) |
| Green olive | Lactobacillus plantarum | 8 days at 25 °C | Total phenolic | 1556 ± 46.7(mg/100 g) | 1204 ± 36.8 (mg/l) | Othman et al. (2009) |
| Varicoloured olives | Lactobacillus plantarum | 8 days at 25 °C | Total phenolic | 384 ± 16.6 (mg/100 g) | 461 ± 11.3 (mg/100 g) | |
| Varicoloured olives | Lactobacillus plantarum | 8 days at 25 °C | Total phenolic | 652 ± 30.2 (mg/l) | 1065 ± 27.1 (mg/l) | |
| Black olives | Lactobacillus plantarum | 8 days at 25 °C | Total phenolic | 311 ± 9.84 (mg/100 g) | 403 ± 17.8 (mg/100 g) | |
| Black olives | Lactobacillus plantarum | 8 days at 25 °C | Total phenolic | 311 ± 9.84 (mg/l) | 1060 ± 57.7 (mg/l) | |
| Antioxidant activity | ||||||
| Brassica pekinensis Skeels | Lactobacillus plantarum | 2 days at 25 °C | DPPH radical-scavenging activity | 33.21 ± 0.47 (mg/ml) | 42.18 ± 5.39 (mg/ml) | Sun et al. (2009) |
ND not detected
Food industry waste
Nowadays, food processing industry has been recognized as a sunrise sector in terms of production, consumption, export and growth prospects and no doubt in the generation of waste materials too (Joshi et al. 2012). The waste obtained from fruit processing industry is extremely diverse due to the use of wide variety of fruits and vegetables, the broad range of processes and the multiplicity of the product. These wastes are novel, natural and economic sources of numerous phytochemicals (Joshi et al. 2012). In recent years, researchers are showing more interest in agro-industrial waste, for their effective utilization as whole or as extracted components (that is the fiber or phytochemicals) in food products to enhance the health effects and phytochemicals potential (Joshi et al. 2012). It has been found that agro-industrial waste contains numerous phytochemicals, especially phenolics, antioxidant, flavonoids and anthocyanin. It has been reported that microbial maceration in apple pomace, green tea waste, mango seed kernel, olive mill, palm kernel cake, peat moss, tamarind, citrus peel, mango peel increased the yields of phenolic content (Zheng and Shetty 2000; Vattem and Shetty 2002, 2003; Gupta et al. 2013; Ajila et al. 2012; El-Fouly et al. 2012; Mannepula et al. 2015) (Table 5). Some scientists have reported that the yield of phenolic content is getting reduced in apple pomace. Phenols are water-soluble compound and get leach out with water while extracting juice from the apple (Joshi et al. 2009). Microbial maceration also increased the yield of flavonoids in Sambucus ebulus L. berry pomace, brewers’ spent grain, citrus peel, mango peel, mango raspuri peel (g/100 ml), mango badami peel, totapuri peel as per reported by (Gupta et al. 2013; Mannepula et al. 2015; Dulf et al. 2015). An increase in the anthocyanin of Sambucus nigra L. berry pomace and antioxidant activity of brewer’s spent grain as compared to the control samples have also been reported by Gupta et al. (2013) and Dulf et al. (2015) (Table 5).
Table 5.
Effect of microbial maceration on extraction of phytochemicals from food industry waste
| Sources/products | Microorganism | Time and temperature | Phenols | Control samples | Macerated samples | References |
|---|---|---|---|---|---|---|
| Phenolic | ||||||
| Apple pomace | Phanerochaete chrysosporium | 7 days at 37 °C | Total phenolics | 15.53 (mg/g) | 29.28 (mg/g) | Ajila et al. (2012) |
| Cranberry pomace | Lentinus edodes | 15 days at 28 °C | Ellagic acid | ND | 350 µg/g | Vattem and Shetty (2003) |
| 10 days at 28 °C | Total phenolic | ND | 118 (mg/10 g) | |||
| Cranberry pomace | Rhizopus oligosporus | 14 days at 28 °C | Ellagic acid | ND | 330 (mg/g) | Vattem and Shetty (2002) |
| Total phenolic | ND | 120 (mg/10 g) | ||||
| Apple pomace | Trichoderma viride IF-26 | 5 days at 25 °C | Total phenolics | 0.633 ± 0.054 (mg/l) | 0.289 ± 0.005(mg/ml) | Zheng and Shetty (2000) |
| Trichoderma harzianum ATCC 24274 | 5 days at 25 °C | Total phenolics | 0.633 ± 0.054 (mg/l) | 0.303 ± 0.013 (mg/ml) | ||
| Trichoderma pseudokoningii ATCC 26801 | 5 days at 25 °C | Total phenolics | 0.633 ± 0.054 (mg/l) | 0.383 ± 0.012 (mg/ml) | ||
| Brewers’ spent grain | Lactobacillus plantarum ATCC 8014 | 19 h at 37 °C | Phenolic | ND | 268.6 (mg/ ml) | Gupta et al. (2013) |
| Apple bagasse | A. niger AUMC 4301 | 3 days | Gallic acid | 0.50 (mg/ml) | 1.96 (mg/ml) | El-Fouly et al. (2012) |
| Green tea waste | 3 days | Gallic acid | 2.51 (mg/ml) | 3.95 (mg/ml) | ||
| Mango seed kernel | 3 days | Gallic acid | 9.60 (mg/ml) | 10.6 (mg/ml) | ||
| Olive mill | 12 days | Gallic acid | 0.00 (mg/ml) | 0.43 (mg/ml) | ||
| Palm kernel cake | 3 days | Gallic acid | 0.30 (mg/ml) | 0.46 (mg/ml) | ||
| Peat moss | 3 days | Gallic acid | 0.00 (mg/ml) | 0.31 (mg/ml) | ||
| Tamarind | 3 days | Gallic acid | 0.00 (mg/ml) | 0.45 (mg/ml) | ||
| Citrus peel | Rhizopus oryzae NCIM 1009 | 35 °C | Total phenolics | ND | 9.0–44.4 (mg/g) | Mannepula et al. (2015) |
| Mango peel | Total phenolics | ND | 26.3 (mg /g) | |||
| Antioxidant activity | ||||||
| Brewers’ spent grain | Lactobacillus plantarum ATCC 8014 | 19 h at 37 °C | FRAP | ND | 33.7 (mg /ml) | Gupta et al. (2013) |
| Flavonoids | ||||||
| Sambucus nigra L. berry pomace | A. niger | 3 h at 25 °C | Quercetin 3-rutinoside | 40.25 ± 2.10 (mg/100 g) | 45.50 ± 1.90(mg/100 g) | Dulf et al. (2015) |
| Sambucus ebulus L. berry pomace | A. niger | 3 h at 25 °C | Quercetin 3-rutinoside | 12.80 ± 0.65 (mg/100 g) | 13.01 ± 0.65 (mg/100 g) | Dulf et al. (2015) |
| Quercetin 3-glucoside | 9.85 ± 0.45 (mg/100 g) | 10.69 ± 0.45 (mg/100 g) | ||||
| Brewers’ spent grain | Lactobacillus plantarum ATCC 8014 | 19 h at 37 °C | Quercetin | ND | 135 mg/ml | Gupta et al. (2013) |
| Citrus peel | Rhizopus oryzae NCIM 1009 | 35 °C | Total flavonoids | ND | 0.2 to 3.25 (mg /g) | Mannepula et al. (2015) |
| Mango peel | Total flavonoids | ND | 0.48 (mg /g) | |||
| Mango Raspuri peel | Rhizopus oryzae NCIM 1009 | 35 °C | Kaempferol | ND | 10.29 (µg/g) | Mannepula et al. (2015) |
| Quercetin | ND | 56.83 (µg/g) | ||||
| Mango Badami peel | Rhizopus oryzae NCIM 1009 | 35 °C | Kaempferol | ND | 52.73 (µg/g) | |
| Quercetin | ND | 18.58 (µg/g) | ||||
| Totapuri peel | Rhizopus oryzae NCIM 1009 | 35 °C | Total flavanoids | ND | 48 (µg/g) | |
| Pigments | ||||||
| Sambucus nigra L. berry pomace | A. niger | 3 h at 25 °C | Cyanidin 3-sambubioside-5-glucoside | 44.94 ± 2.50 (mg/100 g) | 46.88 ± 2.20 (mg/100 g) | Dulf et al. (2015) |
| Cyanidin 3-sambubioside | 4.46 ± 0.15 (mg/100 g) | 4.62 ± 0.18 (mg/100 g) | ||||
| Cyanidin 3,5-diglucoside | 18.70 ± 1.10 (mg/100 g) | 23.04 ± 1.18 (mg/100 g) | ||||
| Sambucus ebulus L. berry pomace |
A. niger | 3 h at 25 °C | Cyanidin 3-sambubioside-5-glucoside | 28.90 ± 1.40 (mg/100 g) | 29.61 ± 1.62 (mg/100 g) | Dulf et al. (2015) |
| Cyanidin 3,5-diglucoside | 13.71 ± 0.72 (mg/100 g) | 13.75 ± 0.75 (mg/100 g) | ||||
ND not detected
Future prospect and conclusions
The demand for healthy foods having phytochemicals is increasing in the industry with every passing day. Microbial maceration has proved to be a successful cost-effective technique in terms of extraction of phytochemicals without being hazardous. The use of agro-industrial waste as a substrate for microbial growth has reduced pollution caused by the waste, has made the process cheaper and easier due to the availability of the substrate, therefore rendering this method as a cleaner technique. More research is required to improve the understanding of extraction mechanism of microbes and scale up of the novel extraction system for their industrial application. Only few reports are available until date for the extraction of active compounds from the agro-industrial waste and still some part is untouched, which needs to be explored in the coming era. Toxicity caused by microbes needs to be considered while standardizing the extraction process and conditions. In nutshell, this technique can be advantageous in the near future for the development and supplementation of value added products.
Acknowledgements
The authors want to thank Lovely Professional University, Phagwara (Punjab), India for providing appropriate infrastructure and financial support.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest regarding publication of this paper.
References
- Ajila CM, Gassara F, Brar SK, Verma M, Tyagi RD, Valero JR. Polyphenolic antioxidant mobilization in apple pomace by different methods of solid-state fermentation and evaluation of its antioxidant activity. Food Bioproc Tech. 2012;5(7):2697–2707. doi: 10.1007/s11947-011-0582-y. [DOI] [Google Scholar]
- Azmir J, Zaidul IS, Rahman MM, Sharif KM, Mohamed A, Sahena F, Jahurul MH, Ghafoor K, Norulaini NA, Omar AK. Techniques for extraction of bioactive compounds from plant materials: a review. J Food Eng. 2013;117(4):426–436. doi: 10.1016/j.jfoodeng.2013.01.014. [DOI] [Google Scholar]
- Azwanida NN. A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med Aromat Plants. 2015;4(196):2167–0412. [Google Scholar]
- Batra P, Sharma AK. Anti-cancer potential of flavonoids: recent trends and future perspectives. 3 Biotech. 2013;3(6):439–59. doi: 10.1007/s13205-013-0117-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhanja T, Rout S, Banerjee R, Bhattacharyya BC. Studies on the performance of a new bioreactor for improving antioxidant potential of rice. LWT-Food Sci Technol. 2008;41(8):1459–1465. doi: 10.1016/j.lwt.2007.08.015. [DOI] [Google Scholar]
- Bhanja T, Kumari A, Banerjee R. Enrichment of phenolics and free radical scavenging property of wheat koji prepared with two filamentous fungi. Bioresour Technol. 2009;100(11):2861–2866. doi: 10.1016/j.biortech.2008.12.055. [DOI] [PubMed] [Google Scholar]
- Boeing H, Bechthold A, Bub A, Ellinger S, Haller D, Kroke A, Leschik-Bonnet E, Müller MJ, Oberritter H, Schulze M, Stehle P. Critical review: vegetables and fruit in the prevention of chronic diseases. Eur J Nutr. 2012;51(6):637–663. doi: 10.1007/s00394-012-0380-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo L, Mateos R. Analysis of flavonoids in functional foods and nutraceuticals. Methods Anal Funct Foods Nutraceuticals. 2008;17:147–206. [Google Scholar]
- Cai S, Wang O, Wu W, Zhu S, Zhou F, Ji B, Gao F, Zhang D, Liu J, Cheng Q. Comparative study of the effects of solid-state fermentation with three filamentous fungi on the total phenolics content (TPC), flavonoids, and antioxidant activities of subfractions from oats (Avena sativa L.) J Agric Food Chem. 2011;60:507–513. doi: 10.1021/jf204163a. [DOI] [PubMed] [Google Scholar]
- Cho KM, Lee JH, Yun HD, Ahn BY, Kim H, Seo WT. Changes of phytochemical constituents (isoflavones, flavanols, and phenolic acids) during cheonggukjang soybeans fermentation using potential probiotics Bacillus subtilis CS90. J Food Compost Anal. 2011;24(3):402–410. doi: 10.1016/j.jfca.2010.12.015. [DOI] [Google Scholar]
- Chung YC, Chang CT, Chao WW, Lin CF, Chou ST. Antioxidative activity and safety of the 50 ethanolic extract from red bean fermented by Bacillus subtilis IMR-NK1. Agric Food Chem. 2002;50(8):2454–2458. doi: 10.1021/jf011369q. [DOI] [PubMed] [Google Scholar]
- Corrales M, Toepfl S, Butz P, Knorr D, Tauscher B. Extraction of anthocyanins from grape by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields: a comparison. Innov Food Sci Emerg Technol. 2008;9(1):85–91. doi: 10.1016/j.ifset.2007.06.002. [DOI] [Google Scholar]
- Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12(4):564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demain AL, Fang A. The natural functions of secondary metabolites. History of modern biotechnology I. Berlin, Heidelberg: Springer; 2000. [DOI] [PubMed] [Google Scholar]
- Dordević TM, Šiler-Marinković SS, Dimitrijević-Branković SI. Effect of fermentation on antioxidant properties of some cereals and pseudo cereals. Food Chem. 2010;119(3):957–963. doi: 10.1016/j.foodchem.2009.07.049. [DOI] [Google Scholar]
- Dueñas M, Fernández D, Hernández T, Estrella I, Muñoz R. Bioactive phenolic compounds of cowpeas (Vigna sinensis L.) modifications by fermentation with natural microflora and with Lactobacillus plantarum ATCC 14917. J Sci Food Agric. 2005;85(2):297–304. doi: 10.1002/jsfa.1924. [DOI] [Google Scholar]
- Dulf FV, Vodnar DC, Dulf EH, Toşa MI. Total phenolic contents, antioxidant activities, and lipid fractions from berry pomaces obtained by solid-state fermentation of two Sambucus species with Aspergillus niger. J Agric Food Chem. 2015;63(13):3489–3500. doi: 10.1021/acs.jafc.5b00520. [DOI] [PubMed] [Google Scholar]
- El-Fouly MZ, El-Awamry Z, Shahin AA, El-Bialy HA, Naeem E, El-Saeed GE. Gallic acid formation from gallotannins-rich agricultural wastes using Aspergillus niger AUMC 4301 or its tannase enzyme. Ar J Nucl Sci Appl. 2012;45(2):489–496. [Google Scholar]
- Escudero-López B, Cerrillo I, Herrero-Martín G, Hornero-Méndez D, Gil-Izquierdo A, Medina S, Ferreres F, Berná G, Martín F, Fernández-Pachón MS. Fermented orange juice: source of higher carotenoid and flavanone contents. J Agric Food Chem. 2013;61(37):8773–8782. doi: 10.1021/jf401240p. [DOI] [PubMed] [Google Scholar]
- Fernandez-Orozco R, Frias J, Muñoz R, Zielinski H, Piskula MK, Kozlowska H, Vidal-Valverde C. Fermentation as a bio-process to obtain functional soybean flours. J Agric Food Chem. 2007;55(22):8972–8979. doi: 10.1021/jf071823b. [DOI] [PubMed] [Google Scholar]
- Frias J, Miranda ML, Doblado R, Vidal-Valverde C. Effect of germination and fermentation on the antioxidant vitamin content and antioxidant capacity of Lupinus albus L. var. multolupa. Food Chem. 2005;92(2):211–220. doi: 10.1016/j.foodchem.2004.06.049. [DOI] [Google Scholar]
- Gan RY, Shah NP, Wang MF, Lui WY, Corke H. Fermentation alters antioxidant capacity and polyphenol distribution in selected edible legumes. Int J Food Sci Tech. 2016;51(4):875–884. doi: 10.1111/ijfs.13062. [DOI] [Google Scholar]
- Gan R-Y, Li H-B, Gunaratne A, Sui Z-Q, Corke H. Effects of fermented edible seeds and their products on human health: bioactive components and bioactivities. Compr Rev Food Sci Food Saf. 2017;16(3):489–531. doi: 10.1111/1541-4337.12257. [DOI] [PubMed] [Google Scholar]
- Gupta S, Jaiswal AK, Abu-Ghannam N. Optimization of fermentation conditions for the utilization of brewing waste to develop a nutraceutical rich liquid product. Ind Crops Prod. 2013;44:272–282. doi: 10.1016/j.indcrop.2012.11.015. [DOI] [Google Scholar]
- Hu Y, Ge C, Yuan W, Zhu R, Zhang W, Du L, Xue J. Characterization of fermented black soybean natto inoculated with Bacillus natto during fermentation. J Sci Food Agric. 2010;7:194–202. doi: 10.1002/jsfa.3947. [DOI] [PubMed] [Google Scholar]
- Hur SJ, Lee SY, Kim YC, Choi I, Kim GB. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014;160:346–356. doi: 10.1016/j.foodchem.2014.03.112. [DOI] [PubMed] [Google Scholar]
- Huynh NT, Van Camp J, Smagghe G, Raes K. Improved release and metabolism of flavonoids by steered fermentation processes: a review. Int J Mol Sci. 2014;15(11):19369–19388. doi: 10.3390/ijms151119369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijarotimi OS, Fagbemi TN, Osundahunsi OF. Comparative study of nutritional profiles and phytochemical components of raw, blanched and fermented flour from the leaves of Moringa oleifera lam. Malays J Nutr. 2013;19(3):371–382. [Google Scholar]
- Jansirani D, Saradha R, Salomideborani N, Selvapriyadharshini Comparative evaluation of various extraction methods of curcuminoids from Curcuma longa. J Chem Pharma Sci. 2014;4:286–288. [Google Scholar]
- Jnawali P, Kumar V, Tanwar B. Celiac disease: overview and considerations for development of gluten-free foods. Food Sci Hum Wellness. 2016;5(4):169–176. doi: 10.1016/j.fshw.2016.09.003. [DOI] [Google Scholar]
- Joshi VK, Rana N, Devi MP. Technology for utilization of apple pomace: a waste from apple juice processing industry. Indian Food Indus. 2009;28(4):19–28. [Google Scholar]
- Joshi VK, Kumar A, Kumar V. Antimicrobial, antioxidant and phyto-chemicals from fruit and vegetable wastes: a review. Int J Food Ferment Technol. 2012;2(2):123–136. [Google Scholar]
- Juan MY, Chou CC. Enhancement of antioxidant activity, total phenolic and flavonoid content of black soybeans by solid state fermentation with Bacillus subtilis BCRC 14715. Food microbial. 2010;27(5):586–591. doi: 10.1016/j.fm.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Katina K, Laitila A, Juvonen R, Liukkonen KH, Kariluoto S, Piironen V, Landberg R, Åman P, Poutanen K. Bran fermentation as a means to enhance technological properties and bioactivity of rye. Food Microbiol. 2007;24(2):175–186. doi: 10.1016/j.fm.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Kumar R, Sharma RJ, Bairwa K, Roy RK, Kumar A. Pharmacological review on natural antidiarrhoel agents. Der Pharma Chem. 2010;2(2):66–93. [Google Scholar]
- Kumar SS, Manoj P, Shetty NP, Prakash M, Giridhar P. Characterization of major betalain pigments-gomphrenin, betanin and isobetanin from Basella rubra L. fruit and evaluation of efficacy as a natural colourant in product (ice cream) development. J Food Sci Technol. 2015;52(8):4994–5002. doi: 10.1007/s13197-014-1527-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushwaha R, Kumar V, Vyas G, Kaur J. Optimization of different variable for eco-friendly extraction of betalains and phytochemicals from beetroot pomace. Waste Biomass Valoriz. 2017 doi: 10.1007/s12649-017-9953-6. [DOI] [Google Scholar]
- Lee YL, Yang JH, Mau JL. Antioxidant properties of water extracts from Monascus fermented soybeans. Food Chem. 2008;106(3):1128–1137. doi: 10.1016/j.foodchem.2007.07.047. [DOI] [Google Scholar]
- Lenucci MS, De Caroli M, Marrese PP, Iurlaro A, Rescio L, Böhm V, Dalessandro G, Piro G. Enzyme-aided extraction of lycopene from high-pigment tomato cultivars by supercritical carbon dioxide. Food Chem. 2015;170:193–202. doi: 10.1016/j.foodchem.2014.08.081. [DOI] [PubMed] [Google Scholar]
- Li Y, Ma R, Xu Z, Wang J, Chen T, Chen F, Wang Z. Identification and quantification of anthocyanins in Kyoho grape juice-making pomace, Cabernet Sauvignon grape winemaking pomace and their fresh skin. J Sci Food Agri. 2013;93(6):1404–1411. doi: 10.1002/jsfa.5907. [DOI] [PubMed] [Google Scholar]
- Limón RI, Peñas E, Torino MI, Martínez-Villaluenga C, Dueñas M, Frias J. Fermentation enhances the content of bioactive compounds in kidney bean extracts. Food Chem. 2015;172:343–352. doi: 10.1016/j.foodchem.2014.09.084. [DOI] [PubMed] [Google Scholar]
- Maniyar Y, Bhixavatimath P, Agashikar NV. Antidiarrheal activity of flowers of Ixora Coccinea Linn. in rats. J Ayurveda Integr Med. 2010;1(4):287–291. doi: 10.4103/0975-9476.74422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannepula S, Bathal VK, Obulam VS. A comparative study on utilisation of citrus and mango peels for lactic acid production and optimisation by Rhizopus oryzae in submerged fermentation. Eur J Biotechnol Biosci. 2015;3:18–26. [Google Scholar]
- Mayén M, Mérida J, Medina M. Flavonoid and non-flavonoid compounds during fermentation and post-fermentation standing of musts from Cabernet Sauvignon and Tempranillo grapes. AJEV. 1995;46(2):255–261. [Google Scholar]
- Mute VM, Keta A, Patel KS, Mirchandani D, Parth C. Anthelmintic effect of Tamarind indica linn leaves juice extract on Pheretimaposthuma. Int J Pharm Res Dev. 2009;7:1–6. [Google Scholar]
- Oseni OA, Akindahunsi AA. Some phytochemical properties and effect of fermentation on the seed of Jatropha curcas L. Am J Food Technol. 2011;6(2):158–165. doi: 10.3923/ajft.2011.158.165. [DOI] [Google Scholar]
- Othman NB, Roblain D, Chammen N, Thonart P, Hamdi M. Antioxidant phenolic compounds loss during the fermentation of Chétoui olives. Food Chem. 2009;116(3):662–669. doi: 10.1016/j.foodchem.2009.02.084. [DOI] [Google Scholar]
- Pérez-Gregorio MR, Regueiro J, Alonso-González E, Pastrana-Castro LM, Simal-Gándara J. Influence of alcoholic fermentation process on antioxidant activity and phenolic levels from mulberries (Morus nigra L.) LWT Food Sci Technol. 2011;44(8):1793–1801. doi: 10.1016/j.lwt.2011.03.007. [DOI] [Google Scholar]
- Saleh AS, Zhang Q, Chen J, Shen Q. Millet grains: nutritional quality, processing, and potential health benefits. Compr Rev Food Sci Food Saf. 2013;12(3):281–295. doi: 10.1111/1541-4337.12012. [DOI] [Google Scholar]
- Šaponjac VT, Čanadanović-Brunet J, Ćetković G, Djilas S. Detection of bioactive compounds in plants and food products. In: Nedović V, Raspor P, Lević J, Šaponjac VT, Barbosa-Cánovas GV, editors. Emerging and traditional technologies for safe, healthy and quality food. Switzerland: Springer International Publishing; 2016. pp. 81–109. [Google Scholar]
- Sharma S, Joshi VK, Abrol G. An overview on strawberry [Fragaria × ananassa (Weston) Duchesne ex Rozier] wine production technology, composition, maturation and quality evaluation. Nat Prod Radiance. 2009;8(4):356–365. [Google Scholar]
- Sharma US, Sharma UK, Abhishek S, Niranjan S, Singh PJ. In vitro anthelmintic activity of Murraya koenigii Linn. leaves extracts. Int J Pharma Bio Sci. 2010;1(3):PS71. [Google Scholar]
- Shin EC, Lee JH, Hwang CE, Lee BW, Kim HT, Ko JM, Baek IY, Shin JH, Nam SH, Seo WT, Cho KM. Enhancement of total phenolic and isoflavone-aglycone contents and antioxidant activities during Cheonggukjang fermentation of brown soybeans by the potential probiotic Bacillus subtilis CSY191. Food Sci Biotechnol. 2014;23(2):531–538. doi: 10.1007/s10068-014-0073-9. [DOI] [Google Scholar]
- Singhania RR, Patel AK, Soccol CR, Pandey A. Recent advances in solid-state fermentation. Biochem Eng J. 2009;44:13–18. doi: 10.1016/j.bej.2008.10.019. [DOI] [Google Scholar]
- Starzyńska-Janiszewska A, Stodolak B, Mickowska B. Effect of controlled lactic acid fermentation on selected bioactive and nutritional parameters of tempeh obtained from unhulled common bean (Phaseolus vulgaris) seeds. J Sci Food Agric. 2014;94(2):359–366. doi: 10.1002/jsfa.6385. [DOI] [PubMed] [Google Scholar]
- Sun YP, Chou CC, Yu RC. Antioxidant activity of lactic-fermented Chinese cabbage. Food Chem. 2009;115(3):912–917. doi: 10.1016/j.foodchem.2008.12.097. [DOI] [Google Scholar]
- Vattem DA, Shetty K. Solid-state production of phenolic antioxidants from cranberry pomace by Rhizopus oligosporus. Food Biotechnol. 2002;16(3):189–210. doi: 10.1081/FBT-120016667. [DOI] [Google Scholar]
- Vattem DA, Shetty K. Ellagic acid production and phenolic antioxidant activity in cranberry pomace (Vaccinium macrocarpon) mediated by Lentinus edodes using a solid-state system. Process Biochem. 2003;39(3):367–379. doi: 10.1016/S0032-9592(03)00089-X. [DOI] [Google Scholar]
- Wang L, Weller CL. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci Technol. 2006;17(6):300–312. doi: 10.1016/j.tifs.2005.12.004. [DOI] [Google Scholar]
- Wang GX, Han J, Zhao LW, Jiang DX, Liu YT, Liu XL. Anthelmintic activity of steroidal saponins from Paris polyphylla. Phytomed. 2010;17(14):1102–1105. doi: 10.1016/j.phymed.2010.04.012. [DOI] [PubMed] [Google Scholar]
- Wang CY, Wu SJ, Shyu YT. Antioxidant properties of certain cereals as affected by food-grade bacteria fermentation. J Biosci Bioeng. 2014;117(4):449–456. doi: 10.1016/j.jbiosc.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Wardhani DH, Vázquez JA, Pandiella SS. Optimisation of antioxidants extraction from soybeans fermented by Aspergillus oryzae. Food Chem. 2010;118(3):731–739. doi: 10.1016/j.foodchem.2009.05.057. [DOI] [Google Scholar]
- Xiao Y, Xing G, Rui X, Li W, Chen X, Jiang M, Dong M. Effect of solid-state fermentation with Cordyceps militaris SN-18 on physicochemical and functional properties of chickpea (Cicer arietinum L.) flour. LWT-Food SciTechnol. 2015;63(2):1317–1324. doi: 10.1016/j.lwt.2015.04.046. [DOI] [Google Scholar]
- Yaakob H, Malek RA, Misson M, Jalil MF, Sarmidi MR, Aziz R. Optimization of isoflavone production from fermented soybean using response surface methodology. Food Sci Biotechnol. 2011;20(6):1525–1531. doi: 10.1007/s10068-011-0211-6. [DOI] [Google Scholar]
- Zheng Z, Shetty K. Enhancement of pea (Pisum sativum) seedling vigour and associated phenolic content by extracts of apple pomace fermented with Trichoderma spp. Process Biochem. 2000;36(1–2):79–84. doi: 10.1016/S0032-9592(00)00183-7. [DOI] [Google Scholar]