Abstract
During bacterial infections, damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs) activate immune cells. Here, we investigated whether plasma and sputum levels of High Mobility Group Box 1 (HMGB1), a prototypic DAMP, are associated with disease severity and aetiology in community-acquired pneumonia (CAP). In addition, in patients with pneumococcal CAP, the impact of the level of sputum lytA DNA load, a PAMP, was investigated. We studied patients hospitalised for bacterial CAP (n = 111), and samples were collected at admission. HMGB1 was determined by enzyme-linked immunosorbent assays, and pneumococcal lytA DNA load was determined by quantitative polymerase chain reaction. Plasma and sputum HMGB1 levels did not correlate to disease severity (pneumonia severity index or presence of sepsis), but high sputum HMGB1 level was correlated to pneumococcal aetiology (p = 0.002). In pneumococcal pneumonia, high sputum lytA DNA load was associated with respiratory failure (low PaO2/FiO2 ratio; p = 0.019), and high sputum HMGB1 level was associated with bacteraemia (p = 0.006). To conclude, high sputum HMGB1 was not associated with severe disease, but with pneumococcal bacteraemia, indicating a potential role for HMGB1 in bacterial dissemination. High sputum lytA was associated with severe disease.
Introduction
High Mobility Group Box 1 (HMGB1) is a nuclear protein that can be actively released by immune cells1 or passively released by dead or injured cells2. Both infections and injury can cause HMGB1 release3,4. Extracellular HMGB1 is a prototypical damage-associated molecular pattern (DAMP). DAMPs are endogenous substances that activate the innate immune cells via pattern recognition receptors (PRRs), resulting in inflammation. However, during bacterial infections, PRRs are at the same time activated by pathogen-associated molecular patterns (PAMPs), i.e., microbial components3. It is still unclear how DAMPs and PAMPs co-vary and what their relative contributions are as triggers for inflammation in bacterial infections.
The role of HMGB1 in patients with various bacterial infections is not established. In sepsis, the most severe consequence of bacterial infection, some studies have found a correlation between high levels and disease severity5, while others have reported elevated levels without correlation to severity6,7. Sepsis can be caused by many different types of infections, of which pneumonia is the most common type8. Wang et al.9 studied plasma HMGB1 levels in 90 patients with suspected community-acquired pneumonia (CAP) and found that it correlated to disease severity, with higher levels in higher pneumonia severity index (PSI) risk classes. Meanwhile, in a study by Angus et al.10, HMGB1 levels in plasma were similar in CAP patients with or without sepsis. The conflicting results in these studies motivate further studies.
To our knowledge, no previous studies have taken bacterial aetiology into account when studying HMGB1 in CAP. Since bacterial aetiology is important for the clinical course, pathogenesis and severity11, we hypothesise that aetiology can be an important factor for HMGB1 levels in CAP.
For patients with severe infection, it has been proposed that HMGB1 release predominantly occurs locally, at the site of infection12. Levels of HMGB1 in lower respiratory secretions have been correlated to disease severity in chronic obstructive pulmonary disease (COPD)13, asthma14, cystic fibrosis15, and acute lung injury in Legionella pneumophila pneumonia16, as well as to low oxygenation index in patients with pneumonia caused by Pneumocystis jirovecii17. Thus, HMGB1 levels at the site of infection may best reflect the severity of disease in bacterial infections. Streptococcus pneumoniae is the most common aetiology of CAP in all severity classes18,19. To our knowledge there are no studies on HMGB1 in respiratory secretions from patients with pneumococcal pneumonia.
HMGB1 has been shown to increase the expression of the pro-inflammatory cytokine interleukin 8 (IL-8) in human bronchial epithelial cells20. We hypothesise that sputum HMGB1 and plasma HMGB1 levels correlate with plasma IL-8 in patients with bacterial CAP.
To improve our understanding of the role of HMGB1 for inflammation and disease severity in CAP and to compare HMGB1 levels between different aetiologies, we analysed local (sputum) and systemic (plasma) HMGB1 levels in a cohort of well-characterised patients with bacterial CAP. In a sub-analysis of pneumococcal CAP, sputum HMGB1 (a DAMP) was analysed in relation to sputum lytA DNA load (a PAMP).
Material and Methods
Patients
A total of 235 patients hospitalised for CAP were enrolled in a prospective study19 during a 2.5-year period (November 1999–April 2002). The criteria for CAP were acute illness, radiological signs of pulmonary consolidation, and at least two of five of the following signs or symptoms: fever of >38 °C, dyspnoea, cough, pleuritic chest pain, or abnormal lung auscultation. Patients were excluded from the study if they had been hospitalised for any reason during the preceding month.
Clinical samples and microbiological methods
Samples from blood, sputum, nasopharynx, and urine were collected at admission. Blood cultures were collected in all study patients and were analysed with a Bactec non-radiometric blood culture system (Becton Dickinson, USA). Sputum samples with >5 neutrophils per squamous epithelial cell were considered to be representative of the lower respiratory tract and were subjected to semi-quantitative culturing on blood agar and haematin agar, with incubation in carbon dioxide for 24–48 h21. Bacterial pathogens were identified according to standard microbiological methods22. The detection limit for a positive sputum culture was 105 colony forming units/mL.
Polymerase chain reaction (PCR) for Mycoplasma pneumoniae and Chlamydophila pneumoniae were performed on sputum and nasopharyngeal secretions23, and detected DNA was considered diagnostic.
The BinaxNOW Streptococcus pneumoniae and BinaxNOW Legionella pneumophila urinary antigen tests (Alere Inc, USA) were used on non-concentrated urine according to the instructions of the manufacturer, and a positive test was considered diagnostic.
Serology on paired serum samples (acute and convalescent serum, collected at admission and after approximately 4 weeks) was run for detection of Chlamydophila species. We used a complement fixation technique, with serology titres of 1:10, 1:20, 1:40, 1:80, 1:160, and 1:320. A >4-fold titre increase between paired sera was considered diagnostic.
Severity classification
The scoring system of Fine et al.24 was used for calculating PSI scores. Patients were classified as having severe CAP according to the American Thoracic Society/Infectious Diseases Society of America (ATS/IDSA) criteria25, i.e., patients with ≥1 major criteria (invasive mechanical ventilation or septic shock with the need for vasopressors) or ≥3 minor criteria (respiratory rate ≥30 breaths/min, PaO2/FiO2ratio ≤250, multilobar infiltrates, confusion/disorientation, uraemia (blood urea nitrogen level ≥20 mg/dL), leukopenia (white blood cell count <4000 cells/mm3), thrombocytopenia (platelet count <100,000 cells/mm3), hypothermia (core temperature, <36 °C), or hypotension requiring aggressive fluid resuscitation). The Sequential Organ Failure Assessment (SOFA) score was calculated at admission. CAP patients with an increase in SOFA score of ≥2 points compared to baseline levels were considered to have sepsis, according to the Sepsis-3 definition26.
We prospectively collected data on respiratory rate, oxygen saturation, and administration of oxygen. In the calculation of PaO2/FiO2 ratio, PaO2 was derived from oxygen saturation, according to an oxyhaemoglobin dissociation curve. Data on comorbidities (solid tumour, blood malignancy, liver disease, renal disease, COPD, heart disease, stroke, and diabetes) were collected from the medical records. Mortality data were obtained from the Swedish Population Register.
Blood samples and sputum samples for HMGB1, IL-8, and lytA DNA
Blood samples were collected into endotoxin-free tubes (EndoTube ET; Chromogenix AB, Sweden) at admission. The tubes were centrifuged to separate plasma from red blood cells and were then kept at −20 °C until analysis. Sputum samples were mixed with an equal amount of dithiothreitol (Sputolysin®) and frozen at −70 °C.
HMGB1 analyses
Plasma and sputum samples were analysed with an enzyme linked immunosorbent assay (ELISA) method for HMGB1 (HMGB1 ELISA kit; IBL International), performed in accordance with the manufacturer’s instructions.
Plasma IL-8
Plasma IL-8 levels were measured with an ELISA (the Human CXCL8/IL-8 Quantikine ELISA kit from R&D Systems), performed in accordance with the manufacturer’s instructions.
Quantitative lytA real-time PCR
The sputum samples were subjected to DNA extraction and quantitative lytA PCR, as reported previously27. DNA extraction was carried out with the Mag-NAPure Compact Nucleic Acid Isolation Kit I, using a MagNAPure Compact robot (Roche Diagnostics).
The extracted DNA was subjected to quantitative lytA PCR, using a LightCycler 2.0 system with Light-Cycler software version 4.1 (Roche Diagnostics). Real-time PCR amplification was performed using the lytA forward (CAGCGGTTGAACTGATTGA) and lytA reverse (TGGTTGGTTATTCGTGCAA) primers and the P1 (GAAAACGCTTGATACAGGGAGTT-FL) and P2 (LC Red 640-AGCTGGAATTAAAACGCACGAG-PH) probes, with LightCycler FastStart reaction mix hybridisation probes (Roche Diagnostics, Germany). A quantification step was included to calculate the lytA DNA load, by using standards with known pneumococcal DNA concentrations.
Statistical analysis
The statistical analyses were done using SPSS software. The Mann-Whitney U test was used for comparisons between groups in univariate analyses, and the Pearson correlation was used for evaluation of the linear relationship between two continuous variables. Fisher’s exact test was used for comparison of proportions. A p-value below 0.05 was regarded as significant.
Ethics Statement
The study and all experimental protocols were approved by the Ethics Committee of the Orebro County Council (868–1999). Informed consent was obtained from all participants or their legal guardians. The study was conducted in accordance with the regulations in the Declaration of Helsinki.
Results
Study cohort
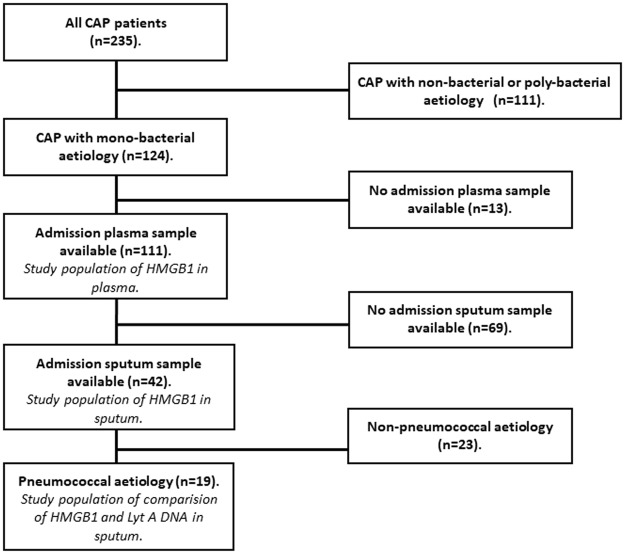
Figure 1 presents a flow chart of the study patients. Of the 235 enrolled patients with CAP, 111 patients had mono-bacterial aetiology19 and a plasma sample collected at admission (Fig. 1). These 111 patients were included in the study, and an analysis of plasma HMGB1 was performed. There were admission sputum samples available from 42 of the 111 study patients, and those were analysed for sputum HMGB1. Pneumococcal aetiology was diagnosed in 19 of 42 patients with available sputum samples. In all 19 patients, quantitative lytA DNA was detected27, and they were included in a subgroup analysis for sputum HMGB1 levels and sputum lytA DNA load.
Figure 1.
Flow chart of the study population of patients with community-acquired pneumonia (CAP).
Table 1 shows patient characteristics of the study population. Sixty-two (56%) of 111 study subjects were females. The median SOFA score was 2 (range, 0–8), the median PSI score was 78 (range, 18–191), 9 patients (8%) were admitted to the ICU department, and 3 patients (3%) died within 30 days of hospital admission.
Table 1.
Characteristics of the patient cohort.
| Patient characteristics | All patients (n = 111) | All patients with an available sputum sample (n = 42) | All patients with an available sputum sample and pneumococcal aetiology (n = 19) |
|---|---|---|---|
| Female gender, n (%) | 62 (56) | 22 (52) | 7 (37) |
| Age, median years (range) | 67 (18–93) | 67 (18–93) | 67 (39–89) |
| Comorbiditya, n (%) | 50 (45) | 22 (52) | 12 (63) |
| Chronic obstructive pulmonary disease, n (%) | 19 (17) | 12 (29) | 8 (42) |
| Smoking, n (%) | 53 (48) | 26 (62) | 16 (84) |
| C-reactive protein, median mg/L (range) | 214 (9–773) | 214 (9–610) | 253 (9–610) |
| Plasma interleukin-8, median pg/mL (range) | 32 (0–2009) | 33 (2–1516) | 46 (2–1516) |
| PaO2/FiO2 ratio, median (range) | 290 (135–555) | 290 (135–458) | 300 (135–405) |
| Severe pneumonia (ATS/IDSA criteria), n (%) | 15 (14) | 5 (12) | 3 (16) |
| Pneumonia Severity Index risk class IV-V, n (%) | 38 (34) | 17 (40) | 8 (42) |
| Sepsis (Sepsis-3 criteria), n (%) | 67 (60) | 29 (69) | 13 (68) |
| Intensive care unit admission, n (%) | 9 (8) | 4 (10) | 2 (11) |
| 30-day mortality, n (%) | 3 (3) | 1 (2) | 0 (0) |
aMalignancy, liver disease, renal disease, chronic obstructive pulmonary disease, heart disease, history of stroke, diabetes.
Pneumococcal aetiology was detected in 63 patients (57%) by blood culture (n = 22), urinary antigen test (n = 20) or sputum culture (n = 21). Haemophilus influenzae aetiology was noted in 16 patients (14%) by blood culture (n = 1) or sputum culture (n = 15). Staphylococcus aureus aetiology was noted in 3 patients (3%) all diagnosed by sputum culture. Mycoplasma pneumoniae aetiology was diagnosed in 25 patients (23%) by PCR on respiratory secretions. Pneumonia with other atypical bacteria was diagnosed in 4 patients (4%), Legionella pneumophila detected in sputum culture (n = 1) or urinary antigen test (n = 1) and Chlamydophila species diagnosed with PCR for Chlamydophila pneumoniae (n = 1) or serology (n = 1).
Local and systemic HMGB1 levels in relation to inflammation and disease severity
The median plasma HMGB1 level in all 111 study subjects was 58 ng/mL (range, 6–320 ng/mL). The median plasma HMGB1 level in the 42 CAP patients who had both sputum and plasma samples available was 56 ng/mL (range, 6–320 ng/mL), and the median sputum HMGB1 level was 9 ng/mL (range, 0–563 ng/mL). There was no correlation between HMBG1 levels in sputum and plasma, r = 0.10 and p = 0.55 (Pearson correlation test).
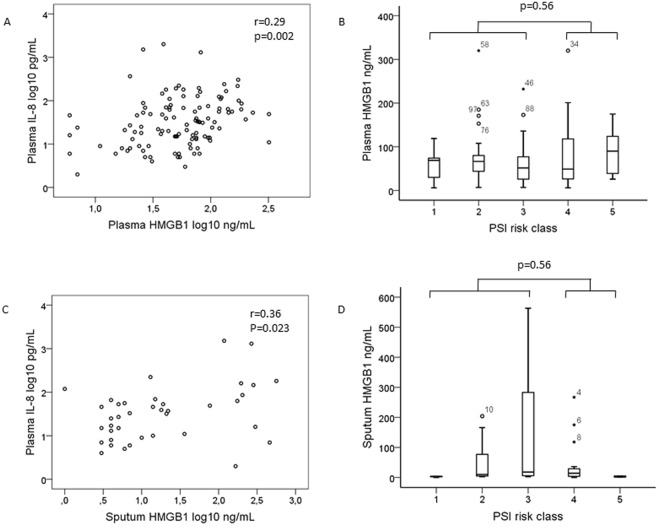
There was a weak but significant positive correlation between the levels of plasma HMGB1 and plasma IL-8, r = 0.29, p = 0.002 (Fig. 2A). The median plasma IL-8 concentration was 32 pg/mL (range 0–2009 pg/mL). Plasma HMGB1 levels were not correlated to disease severity as defined by PSI risk class (Fig. 2B), and plasma HMGB1 levels were similar in patients with and without severe pneumonia according to the ATS/IDSA criteria, 44 ng/mL (range 20–173 ng/mL) vs. 59 ng/mL (range 6–320 ng/mL), p = 0.86, and in patients with and without sepsis, according to the Sepsis-3 criteria26, 58 ng/mL (range 6–320 ng/mL) vs. 59 ng/mL (range 6–320 ng/mL), p = 0.42. There was no correlation between the plasma HMGB1 level and the PaO2/FiO2 ratio, r = −0.02 and p = 0.84 (Pearson correlation test).
Figure 2.
HMGB1 in plasma and sputum in relation to inflammation and disease severity. Correlation between plasma HMGB1 and plasma IL-8 (A) or Pneumonia Severity Index (PSI) risk class I (n = 9), II (n = 34), III (n = 30), IV (n = 32) and V (n = 6) (B) in 111 patients with bacterial community-acquired pneumonia (CAP). Correlation between sputum HMGB1 and plasma IL-8 (C) or PSI risk class I (n = 3), II (n = 9), III (n = 13), IV (n = 15) and V (n = 2) (D) in 42 patients with bacterial CAP. In the box plots, the open circles and stars represent outliers and extreme outliers, with patient study number.
Similarly, sputum HMGB1 levels showed a weak positive correlation to plasma IL-8, r = 0.36, p = 0.023 (Fig. 2C), but no relation to PSI risk class (Fig. 2D). Additionally, the sputum HMGB1 levels were similar in patients with and without severe pneumonia according to the ATS/IDSA criteria, 5 ng/mL (range 0–300 ng/mL) vs. 10 ng/mL (range 0–563 ng/mL), p = 0.65, and in patients with and without sepsis, according to the Sepsis-3 criteria, 10 ng/mL (range 0–563 ng/mL) vs. 7 ng/mL (range 0–461 ng/mL), p = 0.83. There was no correlation between the sputum HMGB1 level and the PaO2/FiO2 ratio, r = −0.01 and p = 0.96 (Pearson correlation test).
HMGB1 levels and aetiology
There was no difference in plasma HMGB1 levels in patients with different bacterial aetiologies (Table 2). However, sputum HMGB1 levels were significantly higher in patient with S. pneumoniae aetiology compared to other bacterial aetiologies, median 118 ng/mL vs. 5 ng/mL, p = 0.002 (Table 2).
Table 2.
Plasma HMGB1 and sputum HMGB1 levels in patients with bacterial community-acquired pneumonia, for different aetiologies.
| Aetiology | Plasma HMGB1 median(range), ng/mL | Number of patients | Sputum HMGB1 median (range), ng/mL | Number of patients |
|---|---|---|---|---|
| Streptococcus pneumoniae | 51 (6–320) | 63 | 118 (3–563)a | 19 |
| Haemophilus influenzae | 53 (20–153) | 16 | 5 (0–15) | 13 |
| Mycoplasma pneumoniae or Chlamydophila species | 69 (6–173) | 27 | 8 (0–77) | 8 |
| Staphylococcus aureus | 124 (60–201) | 3 | 12 (1–22) | 2 |
| Legionella pneumophila | 121 (67–175) | 2 | 0 |
aA statistically significant difference between S. pneumoniae-infected patients and patients with other aetiologies was shown by the Mann-Whitney U test, p = 0.002.
HMGB1 levels and patient factors and comorbidities
Univariate analysis of plasma HMGB1 levels and sputum HMGB1 levels, respectively, showed no significant correlations to gender, age ≥65 years old, smoking status, viral co-infection, bilateral infiltrates or pleural fluid.
Patients with COPD tended to have higher sputum HMGB1 levels than patients without COPD, median 17 ng/mL (range 4–563 ng/mL) vs. median 7 ng/mL (range 0–300 ng/mL), p = 0.080, but plasma HMGB1 levels did not differ between the groups (p = 0.33). HMGB1 levels were not correlated to presence of malignancy, heart disease or diabetes. Since only a few patients had liver disease (n = 2), renal disease (n = 2), or history of stroke (n = 3), HMGB1 correlations were not studied for those comorbidities.
HMGB1 in pneumococcal pneumonia
In 19 patients with pneumococcal CAP, sputum samples were available. Sputum lytA DNA was detected in all 19 patients. The median sputum lytA DNA load was 7.17 log10 copies/mL (range 4.89–8.31 log10 copies/mL), and the median sputum HMGB1 concentration was 118 ng/mL (range 3–563 ng/mL). There was no correlation between the sputum HMGB1 level and the sputum lytA DNA load, r = −0.37 and p = 0.12 (Pearson correlation test).
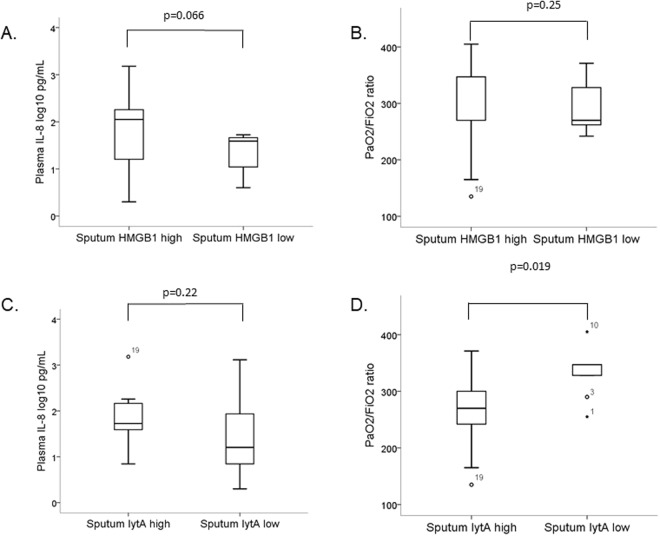
Patients with sputum HMGB1 levels above or equal to the median tended to have higher plasma IL-8 (p = 0.066), but there was no correlation between the sputum HMGB1 level and the PaO2/FiO2 ratio (Fig. 3).
Figure 3.
Plasma IL-8 level and PaO2/FiO2 ratio related to sputum HMGB1 (A,B) and sputum lytA DNA load (C,D) in 19 patients with pneumococcal community-acquired pneumonia. In the box plots, the open circles and stars represent outliers and extreme outliers, with patient study number.
Patients with sputum lytA DNA loads above or equal to the median had significantly lower PaO2/FiO2 ratios (p = 0.019), but there was no correlation between the sputum lytA load and the plasma IL-8 level (Fig. 3).
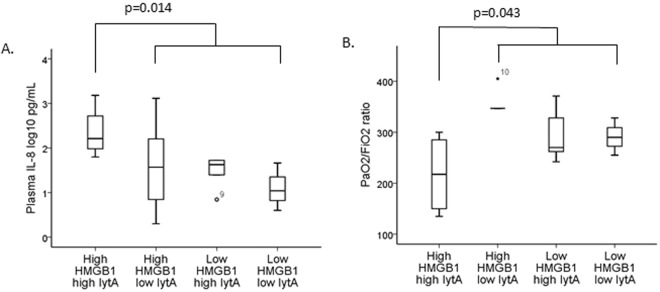
When combining the sputum HMGB1 levels and the sputum lytA DNA load, we found that patients with high sputum HMGB1 levels (above or equal to the median) and high sputum lytA DNA load (above or equal to the median) had significantly higher plasma IL-8 levels (p = 0.014) and significantly lower PaO2/FiO2 ratios (p = 0.043) compared to the remaining patients (Fig. 4).
Figure 4.
Plasma IL-8 level (A) and PaO2/FiO2 ratio (B) in patient groups with different combinations of high and low levels of sputum HMGB1 and sputum lytA DNA load in 19 patients with pneumococcal community-acquired pneumonia. In the box plots, the open circles and stars represent outliers and extreme outliers, with patient study number.
In pneumococcal CAP, patients with pneumococcal bacteraemia had significantly higher sputum HMGB1 levels than patient without pneumococcal bacteraemia, median 232 ng/mL vs. 14 ng/mL, p = 0.005 (Fig. 5). Accordingly, pneumococcal bacteraemia was noted in 8/10 patients with sputum HMGB1 levels above or equal to the median level and in 0/9 of patients with levels lower than the median (p < 0.001). However, there was no difference in median sputum pneumococcal DNA load between patients with and without pneumococcal bacteraemia, 6.74 log10 copies/mL vs 7.17 log10 copies/mL, p = 0.31.
Figure 5.
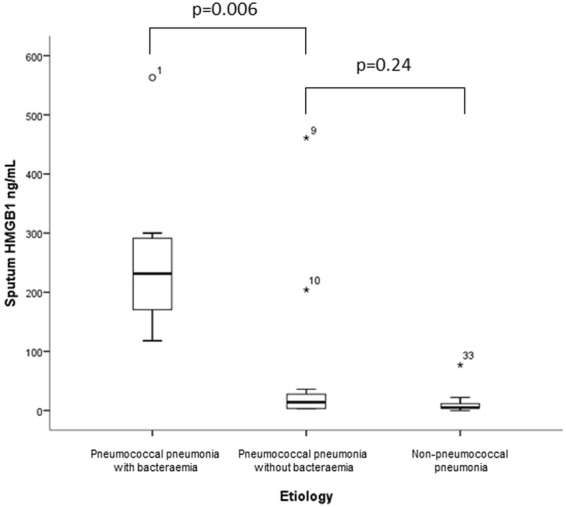
Sputum HMGB1 in patients with bacterial community-acquired pneumonia, comparing the levels of sputum HMGB1 in patients with pneumococcal pneumonia with bacteraemia (n = 8), pneumococcal pneumonia without bacteraemia (n = 11) and non-pneumococcal pneumonia (n = 23). The open circles and stars represent outliers and extreme outliers, with patient study number.
Discussion
This study shows for the first time the relationship between plasma and sputum HMGB1 levels and how they vary with aetiology in patients with bacterial CAP. We found that sputum HMGB1 levels were significantly higher in patients with pneumococcal aetiology compared to other aetiologies. We found no correlation between HMGB1 levels in plasma and sputum, and no correlation between HMGB1 and pneumonia severity.
In pneumococcal CAP, patients with both high HMGB1 levels and high lytA DNA load in sputum had more severe disease, based on significantly lower PaO2/FiO2 ratios, than the remaining patients. However, when looking at the biomarkers separately, only the correlation between the PaO2/FiO2 ratio and the sputum lytA DNA load remained significant. Unexpectedly, in patients with pneumococcal CAP, bacteraemia was more frequent in patients with high sputum HMGB1 levels.
Our working hypothesis was that the release of HMGB1 in the lung, secondary to tissue damage, would yield correlating sputum and plasma HMGB1 levels and be associated with disease severity. However, the results did not support this theory. Rather, the findings suggest that the lung is likely not the main source of plasma HMGB1 during bacterial CAP. Our cohort consisted mainly of CAP patients with non-severe pneumonia. On average, we do not expect them to suffer from extensive tissue damage outside the infected lung. Therefore, we hypothesise that immune activation, rather than tissue damage, is the main source of plasma HMGB1 in bacterial CAP.
Our study showed a weak, but statistically significant, correlation between local sputum and systemic plasma HMGB1 levels and systemic levels of the pro-inflammatory cytokine IL-8 in patients with bacterial CAP. This is in agreement with the fact that extracellular HMGB1 activates innate immune cells and is in line with a previous in vitro study, where HMGB1 was shown to increase the expression of IL-8 in human bronchial epithelial cells20.
We found no correlation between plasma HMGB1 and disease severity in CAP. These results are in agreement with the study by Angus et al.10, in which similar HMGB1 levels in uncomplicated pneumonia and pneumonia with sepsis were found, but conflict with the result of Wang et al.9, who found HMGB1 to be correlated to PSI risk class. It is difficult to compare our study with the Wang study, as their study lacked information about X-ray findings, comorbidity, and aetiological information.
To our knowledge, there are no previous studies on HMGB1 in sputum from patients with pneumonia. As HMGB1 levels in sputum of patients with COPD13, asthma14, and cystic fibrosis15, and HMGB1 levels in bronchoalveolar lavage of patients with L. pneumophila pneumonia16, have been linked to disease severity28, we hypothesised that the level of HMGB1 in sputum would be related to pneumonia severity. However, we found no correlation between HMGB1 levels and severe disease, defined by the PSI score, ATS/IDSA criteria, sepsis (defined by Sepsis-3) or PaO2/FiO2 ratio. In our study, only two patients had L. pneumophila pneumonia and neither of them had a sputum sample available for this study, so we cannot compare our results with the study of Higa et al.16.
High sputum HMGB1 levels were associated with pneumococcal aetiology. However, when comparing patients with pneumococcal CAP with and without bacteraemia, we found that high levels of sputum HMGB1 were primarily seen in patients with pneumococcal bacteraemia. There was no difference in sputum HMGB1 levels between patients with pneumococcal CAP without bacteraemia and patients with non-pneumococcal CAP. Furthermore, there was no correlation between sputum pneumococcal DNA load and bacteraemia. We found this most interesting since our hypothesis was that a high bacterial load in sputum would increase the risk for bacteraemia. There are several plausible explanations for the correlation between high sputum HMGB1 levels and pneumococcal bacteraemia. First, HMGB1 has been shown to increase endothelial permeability29. Second, high concentrations of DAMPs have been linked to reduced immune function4. For instance, in mice with Pseudomonas infection and hypoxic lung damage, treatment with a neutralising anti-HMGB1 monoclonal antibody resulted in an increase in leukocyte phagocytic activity compared with mice receiving control mAb, and this improved phagocytic function was associated with reduced concentrations of airway HMGB130. The correlation between phagocytic activity and concentrations of extracellular HMGB1 was also observed in cultured macrophages. Thus, in a pneumonia lung, high levels of airway HMGB1 may impair bacterial killing. Third, HMGB1 is a marker for tissue damage, and high sputum HMGB1 levels could be secondary to cell and tissue injury in the lungs resulting in impaired mucosal barrier function and thereby facilitating the spread of bacteria from the lungs into the bloodstream.
In the present study, HMGB1 levels were not correlated to the presence of comorbidities, apart from COPD. Patients with COPD tended to have higher levels of sputum HMGB1 than patients without COPD. Accordingly, in induced sputum samples of outpatients, Hou et al.13 found that high sputum HMGB1 levels were significantly correlated to the presence of COPD13.
Both DAMPs and PAMPs activate PRRs to start inflammatory processes. To our knowledge, it has not been clarified which pattern, DAMP or PAMP, is most important for this process in pneumococcal pneumonia31. The present study showed that the combination of a high sputum HMGB1 level and a high sputum lytA DNA load was associated with inflammation (IL-8 elevation) and respiratory failure (reduced PaO2/FiO2 ratio) in patients with pneumococcal CAP (Fig. 4). However, when doing separate analyses of DAMP and PAMP, we found that respiratory failure was driven by the lytA DNA load (Fig. 3), and the PaO2/FiO2 ratio was not at all related to the HMGB1 level in univariate analysis (Fig. 3). These findings are in line with our previous study32, in which a high pneumococcal DNA load in the respiratory tract was correlated to disease severity in pneumococcal CAP.
This study has limitations. Most importantly, the patient cohort is small, and it is a single cohort. Further studies are needed to examine how the combination of PAMPs and DAMPs, locally at the site of infection as well as in circulation, influence disease severity, the inflammatory response and the immunological response in CAP. One major limitation is that the samples were frozen for several years before analysis. We cannot rule out that the sample conditions have affected the median HMGB1 levels. However, the samples were prospectively collected and stored in a structured and systematic way and they were frozen for a similar period of time. Thus, if the freezing time would have affected the samples, we believe that it would have affected the samples in a similar way and that it would not cause a substantial variation within the cohort. It should be noted that there is a considerable variation in plasma HMGB1 levels between different studies. The study by Angus et al.10 shows similar plasma HMGB1 levels to our study, whereas the study by Wang et al.9 shows lower levels. Standardisation of the methods of measuring circulating levels and airway levels of HMGB1 is needed for further research and/or clinical use.
Conclusions
The present study found that high sputum lytA load, but not high sputum HMGB1 level, was associated with more severe disease. However, high sputum HMGB1 level was associated with bacteraemia in pneumococcal pneumonia, indicating a potential role for HMGB1 in bacterial dissemination.
Author Contributions
H.A. and A.N.T. performed the HMGB1 and IL-8 analyses, P.M. performed the lytA analyses, S.A. and K.S. enrolled the patients and collected samples. H.A. wrote the main manuscript and prepared the figures. A.N.T. and K.S. initiated the study design and coordinated the work. All authors read and approved the final manuscript.
Data Availability
The datasets generated and analysed during the current study are available from the corresponding author upon reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wang H, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science (New York, N.Y.) 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 2.Degryse B, et al. The high mobility group (HMG) boxes of the nuclear protein HMG1 induce chemotaxis and cytoskeleton reorganization in rat smooth muscle cells. The Journal of cell biology. 2001;152:1197–1206. doi: 10.1083/jcb.152.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annual review of immunology. 2011;29:139–162. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Timmermans K, Kox M, Scheffer GJ, Pickkers P. Danger in the intensive care unit: damps in critically ill patients. Shock (Augusta, Ga.) 2016;45:108–116. doi: 10.1097/SHK.0000000000000506. [DOI] [PubMed] [Google Scholar]
- 5.Gaini S, Koldkjaer OG, Moller HJ, Pedersen C, Pedersen SS. A comparison of high-mobility group-box 1 protein, lipopolysaccharide-binding protein and procalcitonin in severe community-acquired infections and bacteraemia: a prospective study. Critical care (London, England) 2007;11:R76. doi: 10.1186/cc5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sunden-Cullberg J, et al. Persistent elevation of high mobility group box-1 protein (HMGB1) in patients with severe sepsis and septic shock. Critical care medicine. 2005;33:564–573. doi: 10.1097/01.CCM.0000155991.88802.4D. [DOI] [PubMed] [Google Scholar]
- 7.Narvaez-Rivera RM, Rendon A, Salinas-Carmona MC, Rosas-Taraco AG. Soluble RAGE as a severity marker in community acquired pneumonia associated sepsis. BMC infectious diseases. 2012;12:15. doi: 10.1186/1471-2334-12-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pereira JM, Paiva JA, Rello J. Severe sepsis in community-acquired pneumonia–early recognition and treatment. European journal of internal medicine. 2012;23:412–419. doi: 10.1016/j.ejim.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 9.Wang HL, Tsao SM, Yeh CB, Chou YE, Yang SF. Circulating level of high mobility group box 1 predicts the severity of communityacquired pneumonia: Regulation of inflammatory responses via the cJun Nterminal signaling pathway in macrophages. Molecular medicine reports. 2017;16:2361–2366. doi: 10.3892/mmr.2017.6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Angus DC, et al. Circulating high-mobility group box 1 (HMGB1) concentrations are elevated in both uncomplicated pneumonia and pneumonia with severe sepsis. Critical care medicine. 2007;35:1061–1067. doi: 10.1097/01.CCM.0000259534.68873.2A. [DOI] [PubMed] [Google Scholar]
- 11.Ishiguro T, et al. Etiology and factors contributing to the severity and mortality of community-acquired pneumonia. Internal medicine (Tokyo, Japan) 2013;52:317–324. doi: 10.2169/internalmedicine.52.8830. [DOI] [PubMed] [Google Scholar]
- 12.van Zoelen MA, et al. Systemic and local high mobility group box 1 concentrations during severe infection. Critical care medicine. 2007;35:2799–2804. doi: 10.1097/00003246-200712000-00019. [DOI] [PubMed] [Google Scholar]
- 13.Hou C, et al. High mobility group protein B1 (HMGB1) in Asthma: comparison of patients with chronic obstructive pulmonary disease and healthy controls. Molecular medicine (Cambridge, Mass.) 2011;17:807–815. doi: 10.2119/molmed.2010.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watanabe T, et al. Increased levels of HMGB-1 and endogenous secretory RAGE in induced sputum from asthmatic patients. Respiratory medicine. 2011;105:519–525. doi: 10.1016/j.rmed.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 15.Liou TG, et al. Sputum biomarkers and the prediction of clinical outcomes in patients with cystic fibrosis. PloS one. 2012;7:e42748. doi: 10.1371/journal.pone.0042748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higa F, et al. Clinical evaluation of high mobility group box 1 protein in Legionella pneumophila pneumonia. Journal of infection and chemotherapy: official journal of the Japan Society of Chemotherapy. 2014;20:289–292. doi: 10.1016/j.jiac.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 17.Tasaka S, et al. Cytokine profiles of bronchoalveolar lavage fluid in patients with pneumocystis pneumonia. Microbiology and immunology. 2010;54:425–433. doi: 10.1111/j.1348-0421.2010.00229.x. [DOI] [PubMed] [Google Scholar]
- 18.Woodhead M, et al. Guidelines for the management of adult lower respiratory tract infections–full version. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2011;17(Suppl 6):E1–59. doi: 10.1111/j.1469-0691.2011.03672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stralin K, Olcen P, Tornqvist E, Holmberg H. Definite, probable, and possible bacterial aetiologies of community-acquired pneumonia at different CRB-65 scores. Scandinavian journal of infectious diseases. 2010;42:426–434. doi: 10.3109/00365540903552353. [DOI] [PubMed] [Google Scholar]
- 20.Wu X, et al. The activation of HMGB1 as a progression factor on inflammation response in normal human bronchial epithelial cells through RAGE/JNK/NF-kappaB pathway. Molecular and cellular biochemistry. 2013;380:249–257. doi: 10.1007/s11010-013-1680-0. [DOI] [PubMed] [Google Scholar]
- 21.Stralin K, Holmberg H, Olcen P. Antibody response to the patient’s own Haemophilus influenzae isolate can support the aetiology in lower respiratory tract infections. APMIS: acta pathologica, microbiologica, et immunologica Scandinavica. 2004;112:299–303. doi: 10.1111/j.1600-0463.2004.apm11204-0511.x. [DOI] [PubMed] [Google Scholar]
- 22.Murray, P. R. & American Society for Microbiology. Manual of clinical microbiology. 7th edn, (ASM Press, 1999).
- 23.Stralin K, Tornqvist E, Kaltoft MS, Olcen P, Holmberg H. Etiologic diagnosis of adult bacterial pneumonia by culture and PCR applied to respiratory tract samples. Journal of clinical microbiology. 2006;44:643–645. doi: 10.1128/JCM.44.2.643-645.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fine MJ, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. The New England journal of medicine. 1997;336:243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 25.Mandell LA, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stralin K, et al. Comparison of sputum and nasopharyngeal aspirate samples and of the PCR gene targets lytA and Spn9802 for quantitative PCR for rapid detection of pneumococcal pneumonia. Journal of clinical microbiology. 2014;52:83–89. doi: 10.1128/JCM.01742-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gioia SD, et al. From Genesis to Revelation: The Role of Inflammatory Mediators in Chronic Respiratory Diseases and their Control by Nucleic Acid-based Drugs. Current drug delivery. 2017;14:253–271. doi: 10.2174/1567201813666160824142843. [DOI] [PubMed] [Google Scholar]
- 29.Huang W, et al. HMGB1 increases permeability of the endothelial cell monolayer via RAGE and Src family tyrosine kinase pathways. Inflammation. 2012;35:350–362. doi: 10.1007/s10753-011-9325-5. [DOI] [PubMed] [Google Scholar]
- 30.Patel VS, et al. High Mobility Group Box-1 mediates hyperoxia-induced impairment of Pseudomonas aeruginosa clearance and inflammatory lung injury in mice. American journal of respiratory cell and molecular biology. 2013;48:280–287. doi: 10.1165/rcmb.2012-0279OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hotchkiss RS, et al. Sepsis and septic shock. Nature reviews. Disease primers. 2016;2:16045. doi: 10.1038/nrdp.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alpkvist H, et al. Clinical and Microbiological Factors Associated with High Nasopharyngeal Pneumococcal Density in Patients with Pneumococcal Pneumonia. PloS one. 2015;10:e0140112. doi: 10.1371/journal.pone.0140112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analysed during the current study are available from the corresponding author upon reasonable request.