Abstract
In search of efficient phosphorus resource recovery and pollution remediation should be highly concerned due to the view of phosphorus nonrenewable and eutrophication. This work presented a new insight into conversion of sewage sludge into favorable carbonaceous adsorbent for accelerated removing and recovering phosphorus from aqueous solution, what addressed the issues of phosphorus recovery and pollution remediation as well as sludge disposal. Ca and water hyacinth were evolved to decorate sludge derived carbon. Effect of mass ratio of sludge, water hyacinth and calcium carbonate on the morphologies and adsorption kinetics was investigated. The adsorbents (SW-Ca-112) resulted from sludge in the presence of water hyacinth and CaCO3 in a mass ratio of 1:1:2 had the highest adsorption capacity of 49.50 mg/g P and adsorption rate. Decoration of Ca favored adsorption ability and the presence of water hyacinth accelerated the adsorption rate due to the enhanced porosity. Formation of acicular Ca5(PO4)3OH nanoparticles contributed to the favorable adsorption process. Thus, the contribution of decorated Ca and water hyacinth to the adsorption ability and rate to phosphorus was understand, providing important information on resource utilization of sewage sludge as efficient adsorbent for immobilizing phosphorus from aqueous solution.
Introduction
Phosphorus (P), as one of raw materials in fertilizer production, is urgently demand in these days. However, the P resource is non-renewable1. It is estimated that phosphate rock lasts for only 100 years2. Unfortunately, high level of P was founded in some surface water bodies in China due to discharge of P carried by domestic and industrial sewers. The severe runoff of excessive P triggers eutrophication. Resource of P from P pollution water body follows the point view of resource cycle and sustainable, what is prospective. In the past decades, various adsorbents from carbon to minerals were designed to extract P from aqueous solution3–9. It is promise to design environmental friendly materials from waste.
Sewage sludge, as an inevitable byproduct generated from sewage treatment, is produced in a large amount around the world as the rapid development of urbanization10. Reuse of waste sludge to product is compliant for life cycle assessment studies11. It is reviewed from literatures that sludge substances and its derived carbonaceous were widely applied in removing organic compounds, heavy metals12–14. Carbonization of sludge to produce low-cost sludge carbon has emerged as a sustainable strategy for sewage sludge management15, because this strategy takes the advantages in not only reducing sewage sludge volume but also converting solid waste into adsorbents13,16–19 and catalysts20–24 for environmental pollution remediation. Extraction of P by sewage sludge and its ash was paid more attention25. Recently, Ca-decorated sludge biochar was prepared for phosphorus pollution remediation, in which the sludge biochar could be acted as a carrier and Ca could react with phosphorus by complexation26.
However, the surface area is limited comparing to the activated carbon obtained from coal and agriculture waste due to their high mineral contents27,28. Addition of carbon rich biomass to sludge could address the above issue29. Wu et al. reported that the surface area could be increased from 287 to 591 m2/g, further increased the microporosity from 5% to 48% due to the addition of biomass30.
Water hyacinth (W) is a water weed in several water bodies (e.g., lakers, rivers, canals) around the world31. This plant grows rapidly and can completely cover water bodies, causing difficulty in navigation and depletion of nutrients and dissolved oxygen that are essential for aquatic life. These effects have negative impact on the ecologic environment, human health, and economic development32. Treatment of water hyacinth has become a serious environmental problem in many parts of the world. Therefore, water hyacinth is considered to be a suitable and an easily available biomass33. Considering its high volatile content, utilization of water hyacinth for preparing biochar with loose structure could be considered as one of the most economical and environmental friendly ways for treatment and final disposal of water hyacinth.
In this study, another kind of sewage sludge was resourced as carbonaceous adsorbent for removing and recovering phosphorus, in which it was decorated by Ca, and water hyacinth was added to accelerate its adsorption rate. Herein, water hyacinth was mixed with sewage sludge in varied mass ratio, being carbonized at 800 °C for preparing efficient adsorbent. Batch adsorption experiments were conducted to investigate the adsorption behavior while XRD and SEM techniques were conducted to further understand the adsorption mechanism by morphology and phase analysis. This work gives a new insight into understanding the effect of Ca and water hyacinth decoration on the adsorption capacity and rate to phosphorus from aqueous solution, providing a new view in sludge resource utilization as well as phosphorus removal and recovery.
Results and Discussion
Morphology of the biochar
Figure 1 presented the micro-morphology of the resulted biochars. Obviously, the particles were agglomerated and tight after being carbonized. Interestingly, some small nanoflakes were observed after addition of CaCO3 as shown in Fig. 1(b) comparing to the samples derived from sludge as shown in Fig. 1(a). However, nanoflake was not observed, but many cracks were observed for biochar in the presence of water hyacinth as shown in Fig. 1(c). Amazingly, many cracks and flakes were observed after adding water hyacinth and CaCO3 as shown in Fig. 1(d–f). These cracks fabricated due to the addition of water hyacinth and CaCO3 could increase the porosity of biochar30. The nanoflake was assigned to the Ca derived material. It is different from our previous results that nano-rods were observed in the presence of CaCO326. The difference may be due to the interaction between CaCO3 and sludge, what could be confirmed in the future. Also, we can observe that the nano-flake structure was different from the ratio of sludge to water hyacinth. The nanoflakes were distinct for the resulted samples in a higher mass ratio of hyacinth to sludge as shown in Fig. 1(d). With the decrease in mass ratio of water hyacinth to sludge, the agglomeration of nanoparticle was observed as shown in Fig. 1(e,f). Because volatile content of water hyacinth was 77.47%, a lot of volatile was decomposed and emitted after carbonization. Thus, the decomposition of volatiles leaded to formation of many cracks and increase in porosity. This result suggested that the presence of water hyacinth increased the porosity of biochar, further providing considerable channels favoring phosphorus adsorption.
Figure 1.
SEM morphologies of the sludge derived biochars: (a) sludge char (S), (b) S-Ca-11, (c) S-W-11, (d) SW-Ca-112, (e) SW-Ca-325, (f) SW-Ca-415.
Adsorption behaviors
Figure 2 presented the adsorption amounts of phosphorus as a function of contact time. Clearly, the adsorption capacity of sludge char (S) is very low, which is not higher than 5 mg/g. Interestingly, the adsorption capacity highly increased to almost 50 mg/g in the presence of calcium decoration. Adsorption of phosphorus on S could be neglectful, while the addition of CaCO3 highly favored phosphorus adsorption, meaning that the favorable adsorption abilities of SW-Ca-112 and S-Ca-11 to phosphorus were ascribed to the decorated calcium. It also can be seen that the adsorption capacities were CaCO3 dependent since S-Ca-11 had similar adsorption capacity to SW-Ca-112. Interestingly, the adsorption kinetic process is quite different due to the addition of water hyacinth.
Figure 2.
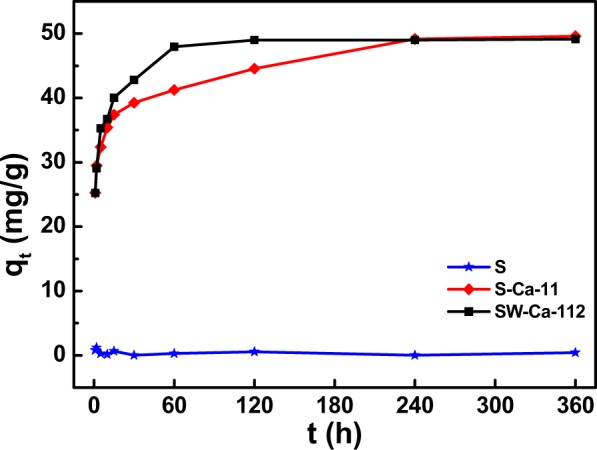
Adsorption capacities of sludge derived char as a function of contact time.
Adsorption kinetic models described in Eqs (2) and (3) were conducted to investigate the kinetic parameters, what were listed in Table 1. Clearly, the pseudo-second-order model fitted well to the adsorption data considering the high correlation coefficients as shown in Table 1. In addition, it can be seen from Table 1 that the adsorption rate of SW-Ca-112 highly increased to 0.0083 in the presence of water hyacinth. It was 2.3 times comparing to that of S-Ca-11 in the absence of water hyacinth, indicating the accelerated adsorption kinetic rate. The increased adsorption rate was agreement with the formed cracks and flakes, suggesting that the cracks and flakes fabricated from decomposition of water hyacinth favored the preferable adsorption rate.
Table 1.
Adsorption kinetic parameters of phosphorus onto sludge derived adsorbents in the presence of water hyacinth and Ca.
| Samples | Pseudo-first-order model | Pseudo-second-order model | ||||
|---|---|---|---|---|---|---|
| qe (mg/g) | k1 (min−1) | R2 | qe (mg/g) | k2 (g/(mg·min)) | R2 | |
| S-Ca-11 | 17.8678 | 0.01148 | 0.8446 | 49.9500 | 0.0036 | 0.9983 |
| SW-Ca-112 | 17.9382 | 0.03094 | 0.9454 | 49.5050 | 0.0083 | 0.9999 |
To further investigate the effect of addition of water hyacinth on the adsorption behavior, mass content of CaCO3 was fixed to 50%, and the adsorption capacities of adsorbents in varied mass ratio of sludge to water hyacinth were presented in Fig. 3. They had the same equilibrium adsorption capacities of about 49.50 mg/g P, but the reaction time to equilibrium was different. SW-Ca-112, SW-Ca-325 had similar equilibrium time of 120 min because the mass ratio of sludge to water hyacinth was similar, while SW-Ca-415 had a longer equilibrium time of 240 min due to the relative low water hyacinth content. Also, as can be seen from Fig. 1(f), the cracks of SW-Ca-415 were not as distinct as those of SW-Ca-112 and SW-Ca-325. Therefore, the shorter time to equilibrium of SW-Ca-112 and SW-Ca-325 than that of SW-Ca-415 was concluded from the fabricated cracks by decomposition of water hyacinth. The presence of water hyacinth highly decreases the time to equilibrium. In addition, it is not hard to understand that the adsorption capacities are determined by the decorated Ca as shown in our previous study34.
Figure 3.
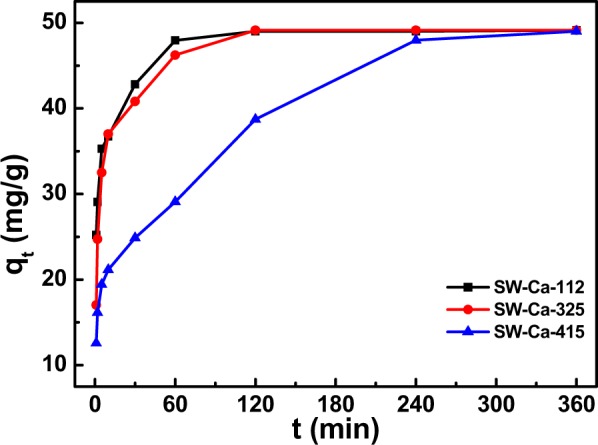
Effect of water hyacinth contents on adsorption capacities as a function of contact time.
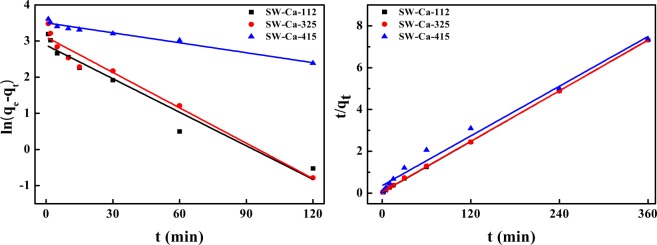
Two widely used pseudo-first-order and pseudo-second-order models were presented to fit the above experimental data to evaluate the adsorption rate, and the nonlinear fitted technique was applied to estimate the kinetic parameters35. However, the nonlinear optimization was not fit for the experimental data adequately (Fig. S1). Linear optimization techniques were applied to estimate the experimental results and corresponding parameters were listed in Fig. 4 and Table 2. Considering the correlation coefficients shown in Table 2, adsorption of P onto SW-Ca-112, SW-Ca-325 fitted well by the pseudo-second-order model. In addition, the fitted equilibrium adsorption capacities of 49.50 and 49.68 mg/g onto SW-Ca-112, SW-Ca-325 were quite agreement with the experimental adsorption capacities. Since the pseudo-second-order model is based on the assumption that adsorption of P onto adsorbent involves the chemical reaction between PO43− and Ca, as reported by Mitrogiannis et al.36, indicating a chemisorptions-dominated process. Also, the adsorption capacity to P is higher than the previous results36–39. Importantly, the fitted adsorption rate calculated from pseudo-second-order model for SW-Ca-112 was higher than that for SW-Ca-325 due to higher content of water hyacinth. In addition, the SW-Ca-415 had quite low adsorption rate due to the low content of water hyacinth. These results were quite agreement with the fabricated cracks of SW-Ca-112, SW-Ca-325 and SW-Ca-415 as shown in Fig. 1. Thus, these results all confirmed that the presence of water hyacinth could accelerate the adsorption rate due to fabrication of favorable cracks.
Figure 4.
Linear fitted adsorption kinetics of phosphorus on the SW-Ca with varied mass ratio: (a) pseudo-first-order model and (b) pseudo-second-order model.
Table 2.
Adsorption kinetic parameters of phosphorus onto SW-Ca derived adsorbents with varied water hyacinth and sludge content.
| Samples | Pseudo-first-order model | Pseudo-second-order model | ||||
|---|---|---|---|---|---|---|
| qe (mg/g) | k1 (min−1) | R2 | qe (mg/g) | k2 (g/(mg·min)) | R2 | |
| SW-Ca-112 | 17.9382 | 0.0309 | 0.9454 | 49.5050 | 0.0083 | 0.9999 |
| SW-Ca-325 | 22.4502 | 0.0326 | 0.9656 | 49.6771 | 0.0062 | 0.9998 |
| SW-Ca-415 | 33.2908 | 0.0092 | 0.9672 | 50.5306 | 0.0011 | 0.9852 |
Figure 5 showed that the adsorption capacity of P depended on the CaCO3 content. Pseudo-first-order and pseudo-second-order models were performed to fit the experiment data. The fitted parameters were listed in Table 3. Clearly, pseudo-second-order model was better than pseudo-first-order model to fit the adsorption experiments as indicated by the favorable correlation coefficients. In addition, the calculated equilibrium adsorption capacities for SW-Ca-112, SW-Ca-221 and SW-Ca-441 were quite agreement with the experimental data. The SW-Ca-441 with the lowest CaCO3 content had the lowest adsorption capacity to P. This result confirmed that the favorable adsorption capacity to P was determined by the chemical reaction of P with Ca. In addition, the adsorption rates calculated from pseudo-second-order model were agreement with the CaCO3 ratio. The higher CaCO3 ratio, the higher adsorption rate was. Thus, it is easy to conclude that the decorated Ca plays a key role in influencing the adsorption capacity.
Figure 5.
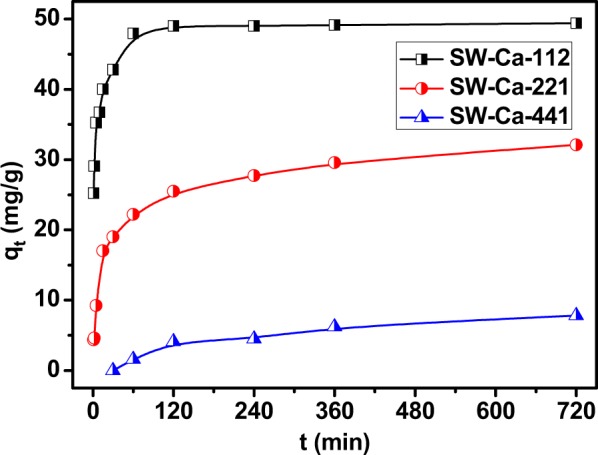
Effect of CaCO3 contents on the P adsorption capacity.
Table 3.
Adsorption kinetic parameters of phosphorus onto SW-Ca derived adsorbents with varied Ca content.
| Samples | Pseudo-first-order model | Pseudo-second-order model | ||||
|---|---|---|---|---|---|---|
| qe (mg/g) | k1 (min−1) | R2 | qe (mg/g) | k2 (g/(mg⋅min)) | R2 | |
| SW-Ca-112 | 18.2910 | 2.0467 | 0.9620 | 49.1642 | 0.7410 | 0.9999 |
| SW-Ca-221 | 23.8566 | 0.6490 | 0.8303 | 33.3222 | 0.07340 | 0.9989 |
| SW-Ca-441 | 10.9412 | 0.4155 | 0.9994 | 10.6168 | 0.0204 | 0.9864 |
Characterization of adsorption products
The products after adsorption of P were characterized by SEM analysis as shown in Fig. 6. Comparing to the samples before adsorption, nanoparticles in acicular was observed on the nanoflakes. Especially, these nanoparticles were well dispersed onto the well dispersed nanoflakes of SW-Ca-112 (as shown in Fig. 6(a)). For the other SW-Ca-325 and SW-Ca-415, nanoparticles were also observed on the surface of nanoflakes. The observed nanoparticles could be ascribed to the crystal products after reaction of P with Ca.
Figure 6.
SEM morphologies of (a) SW-Ca-112, (b) SW-Ca-325 and (c) SW-Ca-415 after adsorption of P.
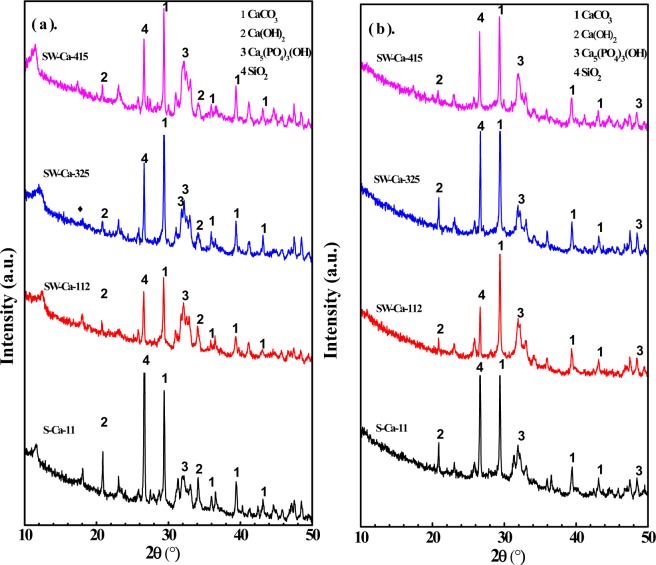
XRD analysis of the adsorbents before and after phosphorus adsorption was conducted in Fig. 7 to understand the phase of the crystal nanoparticles after adsorption. The diffraction peaks assigned to CaCO3 and Ca(OH)2 were observed in the presence of CaCO3. The presented Ca(OH)2 was due to the decomposition of CaCO3 into CaO and CO2, further the CaO can reacted with the water during adsorption to form Ca(OH)226. Besides, diffraction peaks located at 33.2°, being assigned to Ca5(PO4)3OH, were both observed for adsorbents before and after P adsorption. The presence of Ca5(PO4)3OH before P adsorption was due to the initial P content in these sludge derived chars were about 1.17 to 1.71% (Table S2). And Ca5(PO4)3OH crystal was also observed after P adsorption, but mass content of P after adsorption increased to 8.46% (Table 4). The increase in the P content indicated favorable P adsorption, and the Ca5(PO4)3OH crystal was the fate of P after adsorption.
Figure 7.
XRD patterns of the sludge derived biochars in the presence of Ca and water hyacinth (a) before adsorption, (b) after adsorption.
Table 4.
EDS analysis results (wt%) of the sludge derived biochars in the presence of various ratios of sludge to Ca and water hyacinth after P adsorption.
| Sample | C | O | Mg | Al | Si | P | K | Ca | Fe |
|---|---|---|---|---|---|---|---|---|---|
| SW-Ca-112 | 28.84 | 41.01 | 0.66 | 0.68 | 2.49 | 5.91 | 0.26 | 18.9 | 1.25 |
| SW-Ca-415 | 11.52 | 45.51 | 0.6 | 1.41 | 3.03 | 6.93 | 0.28 | 28.73 | 1.98 |
| SW-Ca-325 | 15.57 | 44.82 | 0.62 | 1.04 | 2.64 | 8.46 | 0.4 | 24.58 | 1.88 |
| S-Ca-11 | 14.81 | 44.46 | 0.46 | 1.76 | 4.24 | 7.62 | 0.52 | 23.93 | 2.2 |
Conclusion
Sewage sludge was successfully converted into carbonaceous adsorbent for removing and recovering phosphorus from aqueous solution, in which decorated Ca favored adsorption capacity while added water hyacinth accelerated the adsorption rate. Nanoflakes were observed due to Ca decoration, and carbonization of water hyacinth leaded to the occurrence of cracks, contributing to the increase in adsorption capacity and rate. Pseudo-second-order model fitted well to the adsorption process. Formation of acicular Ca5(PO4)3OH nanoparticles contributed to the favorable adsorption process. Therefore, this work provides a new insight into conversion of sludge and water hyacinth into adsorbent in application of phosphorus pollution remediation.
Materials and Methods
Materials
Water hyacinth (W) was collected from the Pear River in Guangzhou, further being dried and ground into powder less than 100 meshes. The volatile content was 77.47%. Dewatered sewage sludge was collected from Lijiao sewage treatment plant, in Guangzhou, China, and subsequently being dried by an oven set at 105 °C. Table S1 showed that the volatile of dried sludge was 31.47%. C. O, Al, Si, Fe were the main elements in dried sludge. Also, Ca and P were also observed in the initial dried sludge. The ash mass content of the dried sludge is high to 48.54%. Calcium carbonate (CaCO3) and potassium phosphate (K3PO4) were chemical grade purchased from sigma reagent Corporation, USA.
Preparation of adsorbent
Ca-decorated biochar was prepared by carbonization in a programmable tube furnace at 800 °C. Firstly, the sludge was mixed with CaCO3 and water hyacinth in a mass ratio of 1:1, being carbonized at 800 °C in a tube furnace (SKF-210, Hangzhou Lantian Instrument Co., China) and washed by deionized water to remove the residual ions. The obtained sample was named as S-Ca-11 according to the mass ratio of sludge to CaCO3 was 1:1. In addition, the sludge, water hyacinth and CaCO3 were mixed in varied mass ratio to investigate the effect of each component on the adsorption behavior. These samples were prepared as described above. These resulted samples were named as SW-Ca-112, SW-Ca-325, SW-Ca-415, SW-Ca-221, SW-Ca-441 according to the mass ratio of sludge, water hyacinth and CaCO3 were1:1:2, 3:2:5, 4:1:5, 2:2:1, 4:4:1, respectively.
Adsorption experiments
A stock solution containing 1,000 mg/L P was prepared by dissolving K3PO4 in deionized water, and the desired solutions were prepared by diluting the stock solution. In the adsorption experiments, the adsorbent dose was 1.000 g/L. All concentrations are expressed in P-PO43−. All batch adsorption experiments were performed in conical flasks with a plug on a shaker equipped with a thermostat at 200 rpm and at 25 °C.
Adsorption kinetic was investigated by conducting an initial P concentration of 50 mg/L, and the residual sample at each determined time interval was drawn. For each test, the suspension was filtrated through a 0.45 μm cellulose acetate membrane, and the residual P concentration in the filtrate was measured using an ultraviolet spectrophotometer at 700 nm (HITACHI U-2910, Japan). The adsorption amount was calculated as the difference between the initial and residual concentrations.
All experiments were repeated thrice, and the average value was calculated. The adsorption capacities at different time t (qt, mg/g) were calculated as follows:
| 1 |
where C0 and Ct (mg/L) represent the P concentration at initial and t time (min), respectively. V (L) represents the volume of the solution, and m (g) represents the adsorbent mass.
Adsorption kinetic models
To further understand the effect of the addition of Ca and water hyacinth on the adsorption behavior of P, two widely used pseudo-first-order and pseudo-second-order kinetic models were linear fitted as shown below to describe the adsorption kinetics40.
| 2 |
| 3 |
where qt and qe (mg/g) represented the adsorption amounts of phosphorus at t time and equilibrium, respectively. t (min) represents the adsorption time. k1, k2 were the adsorption rate constants of pseudo-first-order model, pseudo-second-order kinetic model, respectively.
Analytical method
The major phase of biochar before and after adsorption of PO43− were characterized through XRD analysis using a D/max 2200 vpc diffractometer (Rigaku Corporation, Japan) with Cu Kα radiation at 40 kV and 30 mA, to investigate their transformation behaviors and fates. Scanning electron microscopy (SEM) images were observed using a JEOL JSM-6330F-mode Field Emission Scanning Electron Microscope (JED-2300).
Electronic supplementary material
Acknowledgements
This research was supported by the National Nature Science Foundations of China (Nos 51508116, 21407155, U1501231), the Nature Science Foundations of Guangdong Province (2016A030310265), the Science and Technology Research Programs of Guangzhou City (201607010311), Guangzhou University’s 2017 training program for young top-notch personnels (BJ201706), the Project of Guangdong Provincial Key Laboratory of radioactive contamination control and resources (2012A061400023), the Hongkong Scholarship (XJ2016037).
Author Contributions
L.J. Kong prepared all the Figures and writes the manuscript, X. Hu, Z. Xie and X. Ren conducted the experiments, D.Y. Chen and J. Long analysis the results, K. Shih, M.H. Su, Z.H. Diao, L. Hou provides suggestion. All authors reviewed the manuscript and agreed to submit the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-31750-6.
References
- 1.Cordell D, Drangert J-O, White S. The story of phosphorus: global food security and food for thought. Global Environ. Change. 2009;19:292–305. doi: 10.1016/j.gloenvcha.2008.10.009. [DOI] [Google Scholar]
- 2.Cooper J, Lombardi R, Boardman D, Carliell-Marquet C. The future distribution and production of global phosphate rock reserves. Resour. Conserv. Recycl. 2011;57:78–86. doi: 10.1016/j.resconrec.2011.09.009. [DOI] [Google Scholar]
- 3.Koilraj P, Sasaki K. Selective removal of phosphate using La-porous carbon composites from aqueous solutions: batch and column studies. Chem. Eng. J. 2017;317:1059–1068. doi: 10.1016/j.cej.2017.02.075. [DOI] [Google Scholar]
- 4.Li R, et al. Enhancing phosphate adsorption by Mg/Al layered double hydroxide functionalized biochar with different Mg/Al ratios. Sci. Total Environ. 2016;559:121–129. doi: 10.1016/j.scitotenv.2016.03.151. [DOI] [PubMed] [Google Scholar]
- 5.Li R, et al. Recovery of phosphate from aqueous solution by magnesium oxide decorated magnetic biochar and its potential as phosphate-based fertilizer substitute. Bioresour. Technol. 2016;215:209–214. doi: 10.1016/j.biortech.2016.02.125. [DOI] [PubMed] [Google Scholar]
- 6.Lim BH, Kim D-J. Selective acidic elution of Ca from sewage sludge ash for phosphorus recovery under pH control. J. Ind. Eng. Chem. 2017;46:62–67. doi: 10.1016/j.jiec.2016.10.016. [DOI] [Google Scholar]
- 7.Liu H, Sun X, Yin C, Hu C. Removal of phosphate by mesoporous ZrO2. J. Hazard. Mater. 2008;151:616–622. doi: 10.1016/j.jhazmat.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Zhou Q, Chen J, Zhang L, Chang N. Phosphate adsorption on hydroxyleironelanthanum doped activated carbon fiber. Chem. Eng. J. 2013;215-216:859–867. doi: 10.1016/j.cej.2012.11.067. [DOI] [Google Scholar]
- 9.Lin J, et al. Effect of calcium ion on phosphate adsorption onto hydrous zirconium oxide. Chem. Eng. J. 2017;309:118–129. doi: 10.1016/j.cej.2016.10.001. [DOI] [Google Scholar]
- 10.Kelessidis A, Stasinakis AS. Comparative study of the methods used for treatment and final disposal of sewage sludge in European countries. Waste Manag. 2012;32:1186–1195. doi: 10.1016/j.wasman.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Pradel M, Aissani L, Villot J, Baudez JC, Laforest V. From waste to added value product: towards a paradigm shift in life cycle assessment applied to wastewater sludge: a review. J. Clean. Prod. 2016;131:60–75. doi: 10.1016/j.jclepro.2016.05.076. [DOI] [Google Scholar]
- 12.Li N, Wei D, Wang S, Hu L, Xu W. Comparative study of the role of extracellular polymeric substances in biosorption of Ni(II) onto aerobic/anaerobic granular sludge. J. Colloid Interf. Sci. 2017;490:754–761. doi: 10.1016/j.jcis.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Li WH, Yue QY, Gao BY, Ma ZH, Li YJ. Preparation and utilization of sludge-based activated carbon for the adsorption of dyes from aqueous solutions. Chem. Eng. J. 2017;171:320–327. doi: 10.1016/j.cej.2011.04.012. [DOI] [Google Scholar]
- 14.Li, J.S. et al. Change in re-use value of incinerated sewage sludge ash due to chemical extraction of phosphorus. Waste Manag. 10.1016/j.wasman.2018.01.007 (2018). [DOI] [PubMed]
- 15.Smith KM, Fowler GD, Pullket S, Graham NJ. Sewage sludge-based adsorbents: A review of their production, properties and use in water treatment applications. Water Res. 2009;43:2569–2594. doi: 10.1016/j.watres.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 16.Jaria G, Calisto V, Gil MV, Otero M, Esteves VI. Removal of fluoxetine from water by adsorbent materials produced from paper mill sludge. J. Colloid Interf. Sci. 2015;448:32–40. doi: 10.1016/j.jcis.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Li WH, Yue QY, Gao BY, Wang XJ, Qi YF. Preparation of sludge-based activated carbon made from paper mill sewage sludge by steam activation for dye wastewater treatment. Desalination. 2011;278:179–185. doi: 10.1016/j.desal.2011.05.020. [DOI] [Google Scholar]
- 18.Xiao B, Dai Q, Yu X, Yu P, Zhai S. Effects of sludge thermal-alkaline pretreatment on cationic red X-GRL adsorption onto pyrolysis biochar of sewage sludge. J. Hazard. Mater. 2017;343:347–355. doi: 10.1016/j.jhazmat.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Chen YD, Ho SH, Wang D, Wei ZS, Chang JS. Lead removal by a magnetic biochar derived from persulfate-ZVI treated sludge together with one-pot pyrolysis. Bioresour. Technol. 2017;247:463–470. doi: 10.1016/j.biortech.2017.09.125. [DOI] [PubMed] [Google Scholar]
- 20.Bandosz TJ, Block K. Municipal sludge-industrial sludge composite desulfurization adsorbents: synergy enhancing the catalytic properties. Environ. Sci. Technol. 2006;40:3378–3383. doi: 10.1021/es052272d. [DOI] [PubMed] [Google Scholar]
- 21.Kong LJ, et al. Conversion of Fe-rich waste sludge into nano-flake Fe-SC hybrid Fenton-like catalyst for degradation of AOII. Environ. Pollut. 2016;216:568–574. doi: 10.1016/j.envpol.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 22.Lebigue CJ, Andriantsiferana C, Krou N, Ayral C, Mohamed E. Application of sludge-based carbonaceous materials in a hybrid water treatment process based on adsorption and catalytic wet air oxidation. J. Environ. Manag. 2010;91:2432–2439. doi: 10.1016/j.jenvman.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 23.Marques RRN, Stüber F, Smith KM, Fabregat A, Bengoa C. Sewage sludge based catalysts for catalytic wet air oxidation of phenol: Preparation, characterisation and catalytic performance. Appl. Catal. B. Environ. 2011;101:306–316. doi: 10.1016/j.apcatb.2010.09.033. [DOI] [Google Scholar]
- 24.Tu YT, Tian SH, Kong LJ, Xiong Y. Co-catalytic effect of sewage sludge-derived char as the support of Fenton-like catalyst. Chem. Eng. J. 2012;185:44–51. doi: 10.1016/j.cej.2012.01.008. [DOI] [Google Scholar]
- 25.Adam C, Peplinski B, Michaelis M, Kley G, Simon FG. Thermochemical treatment of sewage sludge ashes for phosphorus recovery. Waste Manag. 2009;29:1122–1128. doi: 10.1016/j.wasman.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 26.Kong LJ, et al. Nano-rod Ca-decorated sludge derived carbon for removal of phosphorus. Environ. Pollut. 2018;233:698–705. doi: 10.1016/j.envpol.2017.10.099. [DOI] [PubMed] [Google Scholar]
- 27.Arenas E, Chejne F. The effect of the activating agent and temperature on the porosity development of physically activated coal chars. Carbon. 2004;42:2451–2455. doi: 10.1016/j.carbon.2004.04.041. [DOI] [Google Scholar]
- 28.Ioannidou O, Zabaniotou A. Agricultural residues as precursors for activated carbon production-A review. Renew. Sust. Energ. Rev. 2007;11:1966–2005. doi: 10.1016/j.rser.2006.03.013. [DOI] [Google Scholar]
- 29.Li Y, Li Y, Li L, Shi X, Wang Z. Preparation and analysis of activated carbon from sewage sludge and corn stalk. Adv. Pow. Technol. 2016;27:684–691. doi: 10.1016/j.apt.2016.02.029. [DOI] [Google Scholar]
- 30.Wu C, Song M, Jin B, Wu Y, Huang Y. Effect of biomass addition on the surface and adsorption characterization of carbon-based adsorbents from sewage sludge. J. Environ. Sci. 2013;25:405–412. doi: 10.1016/S1001-0742(12)60028-X. [DOI] [PubMed] [Google Scholar]
- 31.Malik. A. Environmental challenge vis a vis opportunity: The case of water hyacinth. Environ. Int. 2007;33:122–138. doi: 10.1016/j.envint.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Santibañez-Aguilar JE, Ponce-Ortega JM, González-Campos JB, Serna-González M, El-Halwagi MM. Synthesis of Distributed Biorefining Networks for the Value-Added Processing of Water Hyacinth. ACS Sustain. Chem. Eng. 2013;1:284-–305. doi: 10.1021/sc300137a. [DOI] [Google Scholar]
- 33.Phothisantikul PP, Tuanpusa R, Nakashima M, Charinpanitkul T, Matsumura Y. Effect of CH3COOH and K2CO3 on Hydrothermal Pretreatment of Water Hyacinth (Eichhorniacrassipes) Ind. Eng. Chem. Res. 2013;52:5009–5015. doi: 10.1021/ie302434w. [DOI] [Google Scholar]
- 34.Wang SD, et al. Adsorption of phosphorus by calcium-flour biochar: Isotherm, kinetic and transformation studies. Chemosphere. 2018;195:666–672. doi: 10.1016/j.chemosphere.2017.12.101. [DOI] [PubMed] [Google Scholar]
- 35.Tran HN, You SJ, Hosseinibandegharaei A, Chao HP. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017;120:88–116. doi: 10.1016/j.watres.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 36.Mitrogiannis D, et al. Removal of phosphate from aqueous solutions by adsorption on to Ca(OH)2 treated natural clinoptilolite. Chem. Eng. J. 2017;320:510–522. doi: 10.1016/j.cej.2017.03.063. [DOI] [Google Scholar]
- 37.Kilpimaa S, Runtti H, Kangas T, Lassi U, Kuokkanen T. Physical activation of carbon residue from biomass gasification: Novel sorbent for the removal of phosphates and nitrates from aqueous solution. J. Ind. Eng. Chem. 2015;21:1354–1364. doi: 10.1016/j.jiec.2014.06.006. [DOI] [Google Scholar]
- 38.Lin J, et al. Effect of calcium ion on phosphate adsorption onto hydrous zirconium oxide. Chem. Eng. J. 2017;309:118–129. doi: 10.1016/j.cej.2016.10.001. [DOI] [Google Scholar]
- 39.Ren Z, Shao L, Zhang G. Adsorption of Phosphate from Aqueous Solution Using an Iron–Zirconium Binary Oxide Sorbent. Water. Air. Soil. Pollut. 2012;223:4221–4231. doi: 10.1007/s11270-012-1186-5. [DOI] [Google Scholar]
- 40.Wang D, Chen N, Yu Y, Hu W, Feng C. Investigation on the adsorption of phosphorus by Fe-loaded ceramic adsorbent. J. Colloid. Interf. Sci. 2016;464:277–284. doi: 10.1016/j.jcis.2015.11.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.