Abstract
Matrix metalloproteinases (MMPs) are a group of zinc-dependent endopeptidases that can breakdown almost all extracellular matrix components. MMP8 and MMP9 have been shown to be associated with breast cancer (BC) risk in European and American populations. However, few studies have focused on the polymorphisms of MMP8 and MMP9 in Chinese Han BC patients. We investigated nine single nucleotide polymorphisms (SNPs) in 571 BC cases and 578 controls to evaluating their association with risk of BC. The frequency of the “A” allele of rs3787268 was significantly lower in BC cases than in controls (P = 0.025). In the genetic model analysis, the minor allele “T” of rs11225394 in MMP8 was associated with increased risk of BC under the recessive model (P = 0.019), and the minor allele “A” of rs3787268 was associated with decreased risk of BC under the dominant model (P = 0.014). Additionally, the haplotype “AGTCA” constructed by rs3740938, rs2012390, rs1940475, rs11225394, and rs11225395 and the haplotype “CCG” constructed by rs3918249, rs3918254 and rs3787268 were associated with increased risk of BC (P < 0.05). Our data showed that polymorphisms of MMP8 and MMP9 may be associated with BC risk in the Chinese Han population.
Introduction
Breast cancer (BC) is the most frequently diagnosed cancer among females, and its incidence and mortality rate have continued to increase in recent years worldwide1. Identification of risk factors and early detection are important to clinical treatment for BC. BC is a multifactorial disease that is influenced by both genetic and environmental factors2. The environmental factors mainly include reproductive patterns, obesity, physical activity3 and some other lifestyle factors. However, hereditary factors account for a higher proportion of the BC burden. Genome-wide association studies (GWAS) have reported several susceptible single nucleotide polymorphisms (SNPs) associated with BC risk including the work of iCOGS4,5 and subsequent replication studies in different populations6–11; however, these results were still not enough to explain the hereditary of BC.
Matrix metalloproteinases (MMPs) are a group of zinc-dependent endopeptidases that can breakdown almost all extracellular matrix components12. MMPs are known to play a crucial role in tumour invasion and metastasis and are also involved in a series of other cellular process13. Previous studies have reported that aberrant MMP expression is associated with risk of several types of cancers14,15 and suggested that serum MMP level could be used as biological markers for early detection of disease16. Decock J et al.17 reported high expression levels of MMP2 and MMP11 as well as interactions of MMP2-MMP10 and MMP8-MMP9 in patients with BC. The enzyme MMP8 could degrade the type I, II and III collagens, while MMP9 degrades type IV and V collagens. MMP8 and MMP9 genes have multiple transcript variants, which have been associated with BC risk in European and American populations, especially advanced stage, lymph node metastasis and poor prognosis18,19. However, the specific pathogenesis is still unclear. Additionally, few previous studies focused on the polymorphisms of MMP8 and MMP9 in Chinese Han BC patients. We intended to identify more SNPs in MMP8 and MMP9 gene that may be associated with BC risk.
The SNPs rs3740938, rs2012390 and rs11225394 were reported to be associated with the risk of osteonecrosis of the femoral head in the Chinese Han population20,21. Rs1940475 may take part in modifying the host response to inflammatory stimuli22. Rs11225395 and rs2274755 were considered as candidate SNPs in previous association studies on bladder cancer and glaucoma23,24. In addition, the haplotype constructed by rs3918249, rs3918254 and rs3787268 in MMP-9 were associated with primary angle-closure glaucoma in a Chinese Han population25. In this case-control study, we selected and genotyped the above nine SNPs in MMP8 and MMP9 to evaluate their association with risk of BC.
Results
A total of 571 BC patients and 578 healthy controls were recruited in the study. The ages of the patient and control groups are described in Table 1. The mean age of the participants was 50.91 years in the case group and 50.21 years in the control group. There was no significant difference in the distribution of age between BC patients and healthy controls (P > 0.05).
Table 1.
Basic characteristic of patients with breast cancer and the control individuals.
| Characteristics | Cases (N = 571) | Controls (N = 578) | P |
|---|---|---|---|
| Age | 0.517 | ||
| Mean ± SD | 50.91 ± 11.23 | 50.21 ± 10.11 |
SD: Standard deviation; P value was calculated by Welch’s t test.
The basic information of the MMP8 and MMP9 polymorphisms (rs3740938, rs2012390, rs1940475, rs11225394, rs11225395, rs3918249, rs2274755, rs3918254 and rs3787268) is shown in Table 2, including gene, band, position, role, alleles and minor allele frequency (MAF). One SNP (rs2274755) was excluded due to significant deviation from Hardy-Weinberg equilibrium (P < 0.05). By comparing the difference of allele frequencies between two groups, we found that the frequency of the “A” allele of rs3787268 was significantly lower in BC cases than in controls (32.2% versus 37.7%), which suggested that the “A” allele of rs3787268 may be associated with a decreased risk of BC [odds ratios (ORs) = 0.822, and 95% confidence intervals (CIs): 0.692–0.975, P = 0.025].
Table 2.
Basic information of candidate SNPs in this study.
| SNP ID | Gene | Band | Position | Role | Alleles A/B | p-HWE | MAF | P a | OR (95% CI) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | |||||||||
| rs3740938 | MMP8 | 11q22.2 | 102587062 | Coding exon | A/G | 0.794 | 0.232 | 0.199 | 0.057 | 1.214 (0.994–1.481) |
| rs2012390 | MMP8 | 11q22.2 | 102590777 | Intron | G/A | 0.349 | 0.264 | 0.231 | 0.063 | 1.197 (0.990–1.447) |
| rs1940475 | MMP8 | 11q22.2 | 102593248 | Coding exon | T/C | 0.713 | 0.380 | 0.343 | 0.068 | 1.172 (0.988–1.390) |
| rs11225394 | MMP8 | 11q22.2 | 102595413 | Intron (boundary) | T/C | 0.065 | 0.118 | 0.115 | 0.813 | 1.031 (0.799–1.332) |
| rs11225395 | MMP8 | 11q22.2 | 102596480 | Promoter | A/G | 0.514 | 0.366 | 0.335 | 0.116 | 1.147 (0.966–1.362) |
| rs3918249 | MMP9 | 20q13.12 | 44638136 | Intron | T/C | 1 | 0.325 | 0.307 | 0.345 | 1.089 (0.913–1.298) |
| rs2274755 | MMP9 | 20q13.12 | 44639692 | Intron (boundary) | T/G | 0.001# | 0.147 | 0.121 | 0.067 | 1.252 (0.984–1.593) |
| rs3918254 | MMP9 | 20q13.12 | 44640391 | Intron (boundary) | T/C | 0.579 | 0.184 | 0.183 | 0.960 | 1.006 (0.814–1.242) |
| rs3787268 | MMP9 | 20q13.12 | 44641731 | Intron variant | A/G | 0.184 | 0.332 | 0.377 | 0.025* | 0.822 (0.692–0.975) |
SNP: single-nucleotide polymorphism, Alleles A/B: Minor/major alleles; MAF, minor allele frequency; OR: odds ratio, CI: confidence interval, HWE: Hardy–Weinberg equilibrium;
#HWE p-value < 0.05 was excluded;
ap values were calculated using two-sided Chi-squared test;
*p < 0.05 indicates statistical significance.
Next, we investigated the association between each SNP and risk of BC based on different genetic models (Table 3). When the sum of AIC and BIC were at the minimum, the model was considered as the best model. Under the best model-recessive model, the minor allele “T” of rs11225394 in MMP8 was associated with an increased risk of BC (OR = 3.94, 95% CI: 1.10–14.07, P = 0.019). Under the best model-dominant model, the minor allele “A” of rs3787268 was associated with decreased risk of BC (OR = 0.74, 95% CI: 0.59–0.94, P = 0.014).
Table 3.
Genotype frequencies of the SNPs and their associations with risk of breast cancer.
| SNP | Model | Genotype | Case | Control | Without adjustment | With adjustment of age | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | AIC | BIC | OR (95% CI) | P | AIC | BIC | |||||
| rs11225394 | Codominant | C/C | 445 (78.5%) | 445 (77.5%) | 1 | 0.033* | 1580.9 | 1596 | 1 | 0.042* | 1576.2 | 1596.4 |
| C/T | 110 (19.4%) | 126 (21.9%) | 0.87 (0.65–1.16) | 0.88 (0.66–1.17) | ||||||||
| T/T | 12 (2.1%) | 3 (0.5%) | 4.00 (1.12–14.26) | 3.83 (1.07–13.71) | ||||||||
| Dominant | C/C | 445 (78.5%) | 445 (77.5%) | 1 | 0.7 | 1585.6 | 1595.6 | 1 | 0.71 | 1580.4 | 1595.5 | |
| C/T-T/T | 122 (21.5%) | 129 (22.5%) | 0.95 (0.71–1.25) | 0.95 (0.72–1.26) | ||||||||
| Recessive | C/C-C/T | 555 (97.9%) | 571 (99.5%) | 1 | 0.015* | 1579.8 | 1589.8 | 1 | 0.019* | 1575 | 1590.1 | |
| T/T | 12 (2.1%) | 3 (0.5%) | 4.12 (1.16–14.65) | 3.94 (1.10–14.07) | ||||||||
| Log-additive | — | — | — | 1.03 (0.80–1.33) | 0.81 | 1585.7 | 1595.7 | 1.03 (0.80–1.33) | 0.81 | 1580.5 | 1595.6 | |
| rs3787268 | Codominant | G/G | 253 (44.3%) | 216 (37.5%) | 1 | 0.058 | 1590.4 | 1605.5 | 1 | 0.043* | 1584.5 | 1604.7 |
| A/G | 257 (45%) | 286 (49.6%) | 0.77 (0.60–0.98) | 0.76 (0.59–0.97) | ||||||||
| A/A | 61 (10.7%) | 74 (12.8%) | 0.70 (0.48–1.03) | 0.69 (0.47–1.01) | ||||||||
| Dominant | G/G | 253 (44.3%) | 216 (37.5%) | 1 | 0.019* | 1588.6 | 1598.6 | 1 | 0.014* | 1582.8 | 1597.9 | |
| A/G-A/A | 318 (55.7%) | 360 (62.5%) | 0.75 (0.60–0.95) | 0.74 (0.59–0.94) | ||||||||
| Recessive | G/G-A/G | 510 (89.3%) | 502 (87.2%) | 1 | 0.26 | 1592.8 | 1602.9 | 1 | 0.22 | 1587.3 | 1602.4 | |
| A/A | 61 (10.7%) | 74 (12.8%) | 0.81 (0.57–1.16) | 0.80 (0.55–1.14) | ||||||||
| Log-additive | — | — | — | 0.82 (0.68–0.97) | 0.022* | 1588.8 | 1598.9 | 0.81 (0.68–0.96) | 0.016* | 1583 | 1598.1 | |
ORs: odds ratios; CI: confidence interval; AIC: Akaike’s Information criterion; BIC: Bayesian Information criterion.
*p value < 0.05 indicates statistical significance.
The association of MMP8 and MMP9 haplotypes with the risk of BC was analysed. Figures 1 and 2 show the linkage disequilibrium (LD) block in MMP8 and MMP9. The association between different haplotypes and BC risk is shown in Table 4. The haplotype “AGTCA” constructed by rs3740938, rs2012390, rs1940475, rs11225394, and rs11225395 was associated with an increased risk of BC after the adjustment (OR = 1.23; 95% CI = 1.00–1.51; P = 0.048). The haplotype “CCG” constructed by rs3918249, rs3918254 and rs3787268 was also associated with an increased risk of BC after the adjustment (OR = 1.37; 95% CI = 1.05–1.77; P = 0.019).
Figure 1.
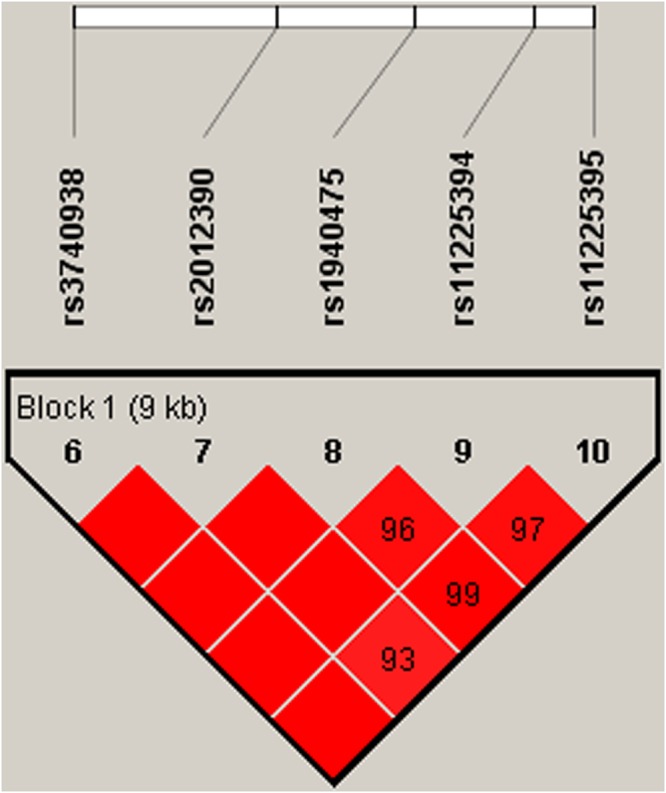
D′ linkage map for the five SNPs in MMP8.
Figure 2.
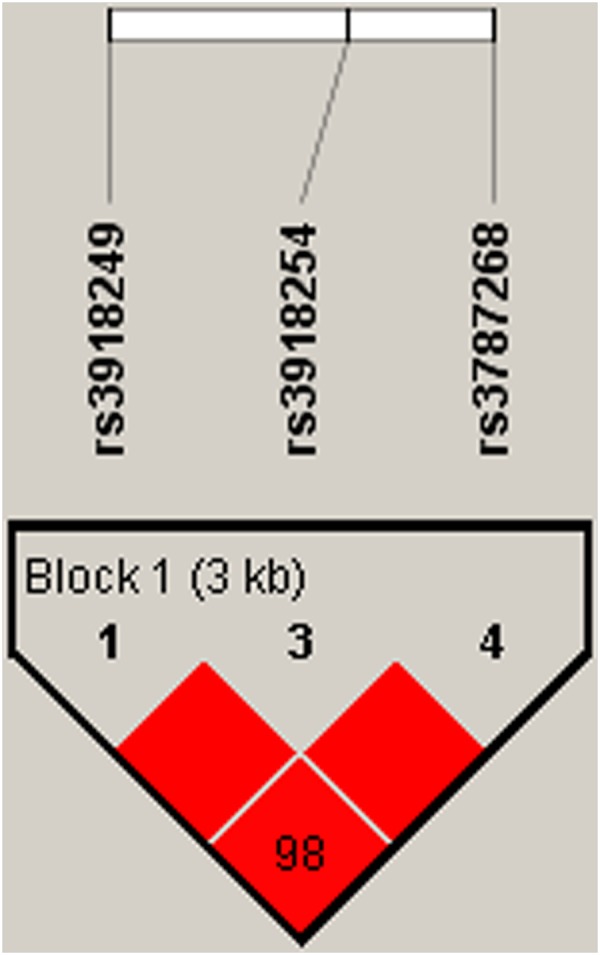
D’ linkage map for the three SNPs in MMP9.
Table 4.
MMP8 and MMP9 haplotype frequencies and the association with the breast cancer risk.
| Blocks | Genes | SNPs | Haplotype | Freq-case | Freq-control | Without adjustment | With adjustment of age | ||
|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | ||||||
| 1 | MMP8 | rs3740938|rs2012390|rs1940475|rs11225394|rs11225395 | GACCG | 0.617 | 0.654 | 1.00 | — | 1.00 | — |
| AGTCA | 0.232 | 0.200 | 1.22 (1.00–1.50) | 0.051 | 1.23 (1.00–1.51) | 0.048* | |||
| GATTA | 0.116 | 0.112 | 1.09 (0.83–1.42) | 0.530 | 1.09 (0.84–1.43) | 0.520 | |||
| GGTCA | 0.018 | 0.022 | 0.88 (0.50–1.55) | 0.660 | 0.90 (0.51–1.59) | 0.710 | |||
| GGTCG | 0.014 | 0.010 | 1.51 (0.71–3.22) | 0.280 | 1.51 (0.71–3.23) | 0.280 | |||
| 2 | MMP9 | rs3918249|rs3918254|rs3787268 | CCA | 0.331 | 0.375 | 1.00 | — | 1.00 | — |
| TCG | 0.325 | 0.305 | 1.21 (0.99–1.48) | 0.064 | 1.22 (0.99–1.49) | 0.060 | |||
| CTG | 0.185 | 0.183 | 1.16 (0.91–1.48) | 0.240 | 1.19 (0.93–1.52) | 0.170 | |||
| CCG | 0.158 | 0.134 | 1.34 (1.04–1.74) | 0.025* | 1.37 (1.05–1.77) | 0.019* | |||
Freq: frequency; ORs: odds ratios; CI: confidence interval; * p value < 0.05 indicates statistical significance.
Finally, we summarized the OR and p-values of candidate SNP from previous studies. The results are listed in Table 5.
Table 5.
The OR and 95% CI of candidate SNPs in previous studies.
| SNPs | Minor/Major allele | OR(95% CI) | P | Disease | Reference |
|---|---|---|---|---|---|
| rs3740938 | A/G | 1.36 (1.02–1.82) | 0.034 | Steroid-induced osteonecrosis of the femoral head | 20 |
| rs2012390 | G/A | 1.55 (1.10–2.21) | 0.013 | ||
| rs11225395 | A/G | 1.34 (1.04–1.73) | 0.023 | ||
| rs11225394 | T/C | 1.44 (1.05–1.96) | 0.023 | Osteonecrosis of the femoral head | 21 |
| rs11225395 | T/C | 0.96 (0.63–1.46) | 0.836 | Bladder cancer | 23 |
| rs2274755 | T/G | 1.67 (1.12–2.50) | 0.021 | Glaucoma | 24 |
| rs3918249 | T/C | 1.041 (0.477–2.269) | 0.010 | Primary angle-closure glaucoma | 25 |
| rs3918254 | T/C | 4.397 (1.455–13.289) | 0.006 | ||
| rs3787268 | A/G | 1.009 (0.764–1.334) | 0.004 |
Discussion
In this study, we investigated the associations between nine candidate SNPs in MMP8 and MMP9 genes and BC risk in a Chinese Han population. We found that rs11225394 in MMP8 and rs3787268 in MMP9 are significantly associated with BC risk. We also found two haplotypes were associated with increased risk of BC: haplotype “AGTCA” constructed by rs3740938, rs2012390, rs1940475, rs11225394, and rs11225395 in MMP8, and haplotype “CCG” constructed by rs3918249, rs3918254 and rs3787268 in MMP9. Our results suggest that the polymorphisms of MMP8 and MMP9 may play an important role in the risk of BC in the Chinese Han population.
MMPs are a family of endopeptidases involved in the degradation of extracellular matrix and basement membrane barriers. As such, they contribute when tumour cells separate from adjacent normal tissues26,27. Several studies have revealed that MMPs are associated with the invasion and metastasis of tumour cells. MMP8 and MMP9 are members of the MMPs family. Plasma MMP8 levels were lower in BC patients than in healthy control individuals, and MMP8 was found to protect against lymph node metastasis in BC patients28. Differential expression of MMP9 was found in BC cells with different degrees of cellular differentiation29. We identified two SNPs in MMP8 and MMP9 that were associated with risk of BC, which further corroborates the association between MMPs and BC risk.
In the present study, we investigated nine SNPs in the MMP8 and MMP9 genes. Among these SNPs, rs3740938, rs2012390, rs11225394, rs2274755 and rs3918254 were identified that have been associated with risk of osteonecrosis of the femoral head in the Chinese Han population20,21,30. We report for the first time that rs11225394 was associated with BC risk. This association needs to be confirmed in a further study with a larger sample size. Rs1940475 was found to have association with community-acquired pneumonia-associated sepsis and inflammatory response in Caucasians22,31. Rs11225395 was associated with bladder cancer risk in a Polish population23. Rs3918249 was demonstrated to be associated with risk for primary angle-closure glaucoma in Chinese Han population25 and risk for asthma in Mexican paediatric patients32. Rs3787268 was also correlated with BC prognosis in previous studies; the “GA” and “AA” genotypes of rs3787268 were significantly associated with 1.52-fold risk of BC in Native American women33. However, in our study, we found that the minor allele “A” of rs3787268 was associated with decreased risk of BC in Chinese women, which is inconsistent with previous result. This inconsistency may be due to the limited sample size and different populations.
Our study has some intrinsic limitations. First, the sample size was relatively small, so the results identified here just provide some pilot data for continued in-depth studies on BC. Second, the occurrence of BC was also influenced by BMI, menarche, marital status, parity, and so on. We failed to collect these specific data, which is a weakness of the study. Additionally, although we identified significant SNPs and haplotypes with risk of BC, this is still not enough to explain the molecular mechanism between MMP and the onset and development of BC.
In conclusion, the present study showed that polymorphisms of MMP8 and MMP9 are associated with risk of BC in a Chinese Han population. Further studies will focus on two directions. One is to demonstrate the association between MMP and BC risk in larger sample sizes and different populations; the other is to investigate the mechanisms or pathways by which MMP influence BC risk.
Materials and Methods
Study participants
A total of 571 BC patients and 578 healthy controls were consecutively recruited between June 2012 and July 2016 at the First Affiliated Hospital of Xi’an Jiaotong University, People’s Republic of China. Patients diagnosed with other types of cancers or who underwent radiotherapy or chemotherapy were excluded. The controls with no history of cancer were recruited from the physical examination centre of our hospital.
SNP selection and genotyping
Nine SNPs (rs3740938, rs2012390, rs1940475, rs11225394, rs11225395, rs3918249, rs2274755, rs3918254 and rs3787268) in MMP8 and MMP9 were selected from previous association studies. DNA extraction and concentrations were done as previously described34. SNP genotyping was performed by the Sequenom MassARRAY RS1000, and the data analyses were completed by Sequenom Type 4.0. The polymerase chain reaction (PCR) primers used for the study are listed in Table 6.
Table 6.
Primers used in this study.
| SNPID | First PCR primer | Second PCR primer | UEP SEQ |
|---|---|---|---|
| rs3740938 | ACGTTGGATGGTCAGTAAGAGGAATCAAAG | ACGTTGGATGTGACATTTGATGCTATCAC | GATGCTATCACCACACT |
| rs2012390 | ACGTTGGATGACTGTTTCTAGGTCACACCC | ACGTTGGATGTCAGGGAGAGGAAGCAATTC | gAAGCAAATGTGAGGAAGAT |
| rs1940475 | ACGTTGGATGTTTGGGTTGAATGTGACGGG | ACGTTGGATGTAAAACCACCACTGTCAGGC | CTCCACAGCGAGGCTTTT |
| rs11225394 | ACGTTGGATGCAATCTCAAACTAATCACCC | ACGTTGGATGTTAGGAAATAGTGTGGGTTG | AGTGTGGGTTGTTTTCTCTT |
| rs11225395 | ACGTTGGATGAGAGCTGCTGCTCCACTATG | ACGTTGGATGGTTTAGAGAGACTGAGCTGG | gCTGAGCTGGGAGCTACTATA |
| rs3918249 | ACGTTGGATGAAGCACTGGTGTCTGGAAAG | ACGTTGGATGGATTACAAGTGTGAGCCGTC | gaaGTCATGCCCAGCAGGGACTA |
| rs2274755 | ACGTTGGATGGGGAGAGAATGAAGGGAATC | ACGTTGGATGTTCGACGATGACGAGTTGTG | gCTGGGCAAGGGCGTCGGT |
| rs3918254 | ACGTTGGATGTCTTCGGCTTCTGCCCGAC | ACGTTGGATGCAATACATGATGAGAGGGCG | CTGGTAGACAGGGTGGA |
| rs3787268 | ACGTTGGATGATCCTGGGCCATAGAGGATG | ACGTTGGATGCTTCCCAAACCACAGGACTT | aCACAGGACTTTCTTCTTCTTCTTTTT |
UEP SEQ, unextended mini‐sequencing primer.
Statistical Analyses
All of the statistical analyses were performed with Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) and the SPSS 21.0 statistical package (SPSS, Chicago, IL, USA). SNP allele frequencies in the control subjects were tested for departure from Hardy–Weinberg Equilibrium (HWE) before analysis. Differences in the distributions of allele frequencies between the cases and controls were evaluated using chi-square tests. Four models (co-dominant, dominant, recessive, and log-additive) were used to assess the association between each genotype and the BC risk35. Akaike’s Information criterion (AIC) and Bayesian Information criterion (BIC) were used to select the best model for each SNP. ORs and 95%CIs were calculated with and without adjustment for age. All p values presented in this study were two sided, and p = 0.05 was considered the cutoff for statistical significance.
Haploview software version 4.2 was used to analyse the association between haplotypes and BC. Linkage disequilibrium (LD) analysis was performed using genotype data from all the subjects. Statistical significance was established when p < 0.05.
Approval
All of the participants provided written informed consent. The Human Research Committee for Approval of Research Involving Human Subjects, the First Affiliated Hospital of Xi’an Jiaotong University, approved the use of human blood samples in this study. All methods were performed in accordance with the relevant guidelines and regulations.
Author Contributions
K.W. and X.Z. conceived and supervised the project. SNP genotyping experiment was conducted by Y.Z., G.L., X.W. and Y.K. Statistical Analyses was conducted by J.Y., Q.Z. and F.X. All the authors contributed to the discussion. K.W. wrote the manuscript with help of J.W.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ferlay J, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International journal of cancer. 2015;136(E359-E386):E359. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Hirotsu Y, et al. Multigene panel analysis identified germline mutations of DNA repair genes in breast and ovarian cancer. Molecular Genetics & Genomic Medicine. 2015;3:459. doi: 10.1002/mgg3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colditz GA, Sellers TA, Trapido E. Epidemiology |[mdash]| identifying the causes and preventability of cancer? Nature Reviews Cancer. 2006;6:75. doi: 10.1038/nrc1784. [DOI] [PubMed] [Google Scholar]
- 4.Michailidou K, et al. Large-scale genotyping identifies 41 new loci associated with breast cancer risk. Nature Genetics. 2013;45:353. doi: 10.1038/ng.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia-Closas M, et al. Genome-wide association studies identify four ER negative-specific breast cancer risk loci. Nature Genetics. 2013;45:1–2. doi: 10.1038/ng.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ren HT, et al. PD-1rs2227982 Polymorphism Is Associated With the Decreased Risk of Breast Cancer in Northwest Chinese Women: A Hospital-Based Observational Study. Medicine. 2016;95:e3760. doi: 10.1097/MD.0000000000003760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xia P, et al. FGFR2 gene polymorphisms are associated with breast cancer risk in the Han Chinese population. American Journal of Cancer Research. 2015;5:1854. [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou L, et al. Association of five single nucleotide polymorphisms at 6q25.1 with breast cancer risk in northwestern China. American Journal of Cancer Research. 2015;5:2467. [PMC free article] [PubMed] [Google Scholar]
- 9.Xia P, et al. Polymorphisms in ESR1 and FLJ43663 are associated with breast cancer risk in the Han population. Tumour Biology the Journal of the International Society for Oncodevelopmental Biology & Medicine. 2014;35:2187–2190. doi: 10.1007/s13277-013-1289-7. [DOI] [PubMed] [Google Scholar]
- 10.Dai ZJ, et al. Association Between Single Nucleotide Polymorphisms in DNA Polymerase Kappa Gene and Breast Cancer Risk in Chinese Han Population: A STROBE-Compliant Observational Study. Medicine. 2016;95:e2466. doi: 10.1097/MD.0000000000002466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xia P, et al. Mitochondrial DNA levels in blood and tissue samples from breast cancer patients of different stages. Asian Pacific Journal of Cancer Prevention Apjcp. 2014;15:1339–1344. doi: 10.7314/APJCP.2014.15.3.1339. [DOI] [PubMed] [Google Scholar]
- 12.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nature Reviews Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 13.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer & Metastasis Reviews. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 14.Fan SH, et al. CERS2 suppresses tumor cell invasion and is associated with decreased V-ATPase and MMP-2/MMP-9 activities in breast cancer. Journal of Cellular Biochemistry. 2015;116:502–513. doi: 10.1002/jcb.24978. [DOI] [PubMed] [Google Scholar]
- 15.Tabouret E, et al. MMP2 and MMP9 serum levels are associated with favorable outcome in patients with inflammatory breast cancer treated with bevacizumab-based neoadjuvant chemotherapy in the BEVERLY-2 study. Oncotarget. 2016;7:18531–18540. doi: 10.18632/oncotarget.7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darlix A, et al. Serum NSE, MMP-9 and HER2 extracellular domain are associated with brain metastases in metastatic breast cancer patients: predictive biomarkers for brain metastases? International journal of cancer. 2016;139:2299–2311. doi: 10.1002/ijc.30290. [DOI] [PubMed] [Google Scholar]
- 17.Decock J, et al. Matrix Metalloproteinase Expression Patterns in Luminal A Type Breast Carcinomas. Disease Markers. 2014;23:189–196. doi: 10.1155/2007/281727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coskun U, et al. Locally advanced breast carcinoma treated with neoadjuvant chemotherapy: are the changes in serum levels of YKL-40, MMP-2 and MMP-9 correlated with tumor response? Neoplasma. 2007;54:348–352. [PubMed] [Google Scholar]
- 19.Dębniak T, et al. Association of MMP8 gene variation with an increased risk of malignant melanoma. Melanoma Research. 2011;21:464–468. doi: 10.1097/CMR.0b013e3283485fdd. [DOI] [PubMed] [Google Scholar]
- 20.Du J, et al. Association between genetic polymorphisms of MMP8 and the risk of steroid-induced osteonecrosis of the femoral head in the population of northern China. Medicine. 2016;95:e4794. doi: 10.1097/MD.0000000000004794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.An F, et al. MMP8 polymorphism is associated with susceptibility to osteonecrosis of the femoral head in a Chinese Han population. Oncotarget. 2017;8:21561–21566. doi: 10.18632/oncotarget.15371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rella JM, Jilma B, Fabry A, Kaynar AM, Mayr FB. MMP-8 Genotypes Influence the Inflammatory Response in Human Endotoxemia. Inflammation. 2014;37:451–456. doi: 10.1007/s10753-013-9758-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wieczorek E, et al. MMP7 and MMP8 genetic polymorphisms in bladder cancer patients. Central European Journal of Urology. 2014;66:405–410. doi: 10.5173/ceju.2013.04.art3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suh, W., Won, H. H. & Kee, C. The Association of Single-Nucleotide Polymorphisms in the MMP-9 Gene with Normal Tension Glaucoma and Primary Open-Angle Glaucoma. Current Eye Research, 1–5 (2017). [DOI] [PubMed]
- 25.Xiao-Jin G, Sheng-Ping H, Ping-Hua L. The association between matrix metalloprotease-9 gene polymorphisms and primary angle-closure glaucoma in a Chinese Han population. International Journal of Ophthalmology. 2014;7:397–402. doi: 10.3980/j.issn.2222-3959.2014.03.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yadav L, et al. Matrix metalloproteinases and cancer - roles in threat and therapy. Asian Pacific Journal of Cancer Prevention Apjcp. 2014;15:1085–1091. doi: 10.7314/APJCP.2014.15.3.1085. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, Jin G, Li J, Zhang L. Association between four MMP-9 polymorphisms and breast cancer risk: a meta-analysis. Breast Care. 2015;6:126–129. doi: 10.12659/MSM.893890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Decock J, et al. Plasma MMP1, MMP8 and MMP13 expression in breast cancer: protective role of MMP8 against lymph node metastasis. Breast Cancer Research. 2008;10:P31. doi: 10.1186/bcr1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yousef EM, Tahir MR, Stpierre Y, Gaboury LA. MMP-9 expression varies according to molecular subtypes of breast cancer. Bmc Cancer. 2014;14:609. doi: 10.1186/1471-2407-14-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen J, et al. MMP-3 and MMP-8 single-nucleotide polymorphisms are related to alcohol-induced osteonecrosis of the femoral head in Chinese males. Oncotarget. 2017;8:25177. doi: 10.18632/oncotarget.15587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaynar A, et al. Matrix Metalloproteinase (MMP)-8 is Critical in a Murine Model of Sepsis as well as in Patients with Community -Acquired Pheumonia (CAP)-Associated Sepsis. American Journal of Respiratory & Critical Care Medicine. 2010;181:A1132–A1132. [Google Scholar]
- 32.Jiménezmorales S, et al. Polymorphisms in metalloproteinase-9 are associated with the risk for asthma in Mexican pediatric patients. Human Immunology. 2013;74:998–1002. doi: 10.1016/j.humimm.2013.04.036. [DOI] [PubMed] [Google Scholar]
- 33.Slattery ML, et al. Matrix Metalloproteinase Genes Are Associated with Breast Cancer Risk and Survival: The Breast Cancer Health Disparities Study. Plos One. 2013;8:e63165. doi: 10.1371/journal.pone.0063165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin, T. et al. IL-1RN gene polymorphisms are associated with breast cancer risk in a Chinese Han population. Journal of Gene Medicine19 (2017). [DOI] [PubMed]
- 35.Thakkinstian A, Mcelduff P, D’Este C, Duffy D, Attia J. A method for meta‐analysis of molecular association studies. Statistics in Medicine. 2005;24:1291. doi: 10.1002/sim.2010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.


