Abstract
More than half of Latino adults living in the USA are expected to develop type 2 diabetes in their lifetime. Despite the growing interest in smartphone use for weight loss and diabetes prevention, relatively few clinical trials have evaluated the efficacy of mobile app-based interventions in Latino populations. The aim of this study was to evaluate the potential efficacy of an in-person weight loss intervention in conjunction with a commercially available Fitbit app in a Latino sample at risk for type 2 diabetes and explore significant predictors associated with weight loss. After the run-in period, 54 self-identified Latinos with body mass index (BMI) > 24.9 kg/m2 were enrolled in an 8-week uncontrolled pilot study, and received a Fitbit Zip, its app, and two in-person weight loss sessions adapted from the Diabetes Prevention Program. Mean age was 45.3 (SD ± 10.8) years, 61.1% were born in the USA, and mean BMI was 31.4 (SD ± 4.1) kg/m2. Participants lost an average of 3.3 (SD ± 3.4) % of their body weight (p < .0005). We also observed statistically significant reductions in hip and waist circumferences, and systolic and diastolic blood pressure (p < .001). After controlling for demographic factors, use of the mobile app weight diary at least twice a week (p = .01) and change in the International Physical Activity Questionnaire score (p = .03) were associated with change in percent body weight. The intervention showed the potential efficacy of this intervention, which should be formally evaluated in a randomized controlled trial.
Keywords: Weight loss, Latino, Digital technology, Mobile app, Clinical trial, Diabetes prevention
Latino adults at risk for type 2 diabetes who participated in an in-person weight loss intervention in conjunction with a commercially available lifestyle app had a moderate amount of weight loss and significant blood pressure reduction.
Implications
Research: The in-person weight loss intervention in conjunction with a commercially available mobile lifestyle app demonstrated the potential efficacy of this intervention, which should be formally evaluated in a randomized controlled trial.
Policy: Given high prevalence of obesity and type 2 diabetes in Latino populations, a cost-effective prevention program such as combining digital technologies and in-person sessions is urgently needed to reduce financial and societal burden.
Practice: This type of the program must be tested and adapted in clinical practice settings to assess its sustainability in the real world.
INTRODUCTION
Being overweight or obese is one of the major risk factors for development of type 2 diabetes. Latinos have the highest prevalence of overweight or obesity (77.1%) when compared with other racial/ethnic groups in the USA [1]. The prevalence of total diabetes (both diagnosed and undiagnosed) among Latinos is approximately 16.9% (12.8% and 4.1%, respectively) for both men and women [2]. Prevalence in different Latino subgroups also differs. Of Latinos, Mexican Americans and Puerto Ricans have the highest rates of type 2 diabetes, 13.8% and 12.0%, respectively [3]. More importantly, more than 50% of Latino adults living in the USA are expected to develop type 2 diabetes in their lifetime [4]. Overall, the total estimated direct and indirect cost of diagnoses of diabetes increased by 41% from 2007 ($174 billion) to 2013 ($245 billion) in the USA [5]. Type 2 diabetes not only imposes an enormous financial burden on society, but also has a significant impact on one’s quality of life.
The Diabetes Prevention Program trial [6] and other large trials [7, 8] have shown that a moderate amount of weight loss (5%–10% of body weight) achieved by reducing total caloric intake and increasing physical activity can prevent or delay the onset of type 2 diabetes. However, these prevention programs involving in-person counseling can be expensive to implement and sustain in clinical or community settings over extended periods of time. The rapid rise of sophisticated digital devices (i.e., mobile applications and wireless activity trackers) provides an opportunity to transform the way clinical researchers deliver weight loss interventions. These digital technologies are now deeply integrated into individual lifestyles and can reach large numbers of adults with obesity at risk for type 2 diabetes. Core weight loss intervention principles, such as personalized goals, feedback, self-monitoring, relapse prevention, and social support, can be implemented using these technologies at relatively low cost. Currently, 75% of Latinos own a smartphone and Latinos who access the Internet on a mobile device have risen from 76% to 80% from 2012 to 2015 [9].
Despite the growing interest in smartphone use for weight loss and diabetes prevention, relatively few clinical trials have evaluated the efficacy of mobile app-based interventions in general and specifically within Latino populations. A recent published systematic review paper reported there were only 10 mobile phone-based weight loss intervention studies [10]. It appears that none of these interventions focused on Latino populations. Given the high prevalence of overweight/obesity and type 2 diabetes in Latino Americans, there is an urgent need to develop emerging weight loss interventions that can be cost-effective and have broad applications in clinical settings. Therefore, the aims of this pre- and post-trial are to: (i) examine the potential efficacy of a commercially available Fitbit app in conjunction with two in-person weight loss counseling session in changes in percent body weight and other metabolic risk factors over the 8-week period, and (ii) explore factors potentially associated with change in percent body weight after controlling for baseline characteristics.
METHODS
Study design
A single group pre- and post-study with 54 Latino adults was conducted and involved screening/baseline, run-in period, eligibility, and midpoint 3-week and final 8-week visits. The study was approved by the University of California, San Francisco, Committee on Human Research prior to participant enrollment, and all participants provided written informed consent.
Intervention
The intervention was adapted from the Diabetes Prevention Program [11, 12] for overweight/obese Latino adults at risk for type 2 diabetes, and consisted of two brief in-person counseling sessions, daily use of Fitbit Zip (3-axis accelerometer) and Fitbit app, and social media (Facebook). The overall goal of the intervention was to achieve 5% body weight loss over 8 weeks, at a rate of 1 to 2 pounds per week, by gradually increasing physical activity and reducing daily total caloric intake. To facilitate this goal, a Fitbit Zip was provided to each participant and worn on the waist to track his/her steps and then a Fitbit app was downloaded onto his/her smartphone at the screening/baseline visit. The Fitbit app is available for iOS and Android free of charge. During the 8-week intervention period, participants were asked to log all food/drinks and calories every day and their weight twice a week (Monday and Friday mornings before breakfast) into the Fitbit app, sync daily steps data that were stored in a Fitbit Zip with the Fitbit app, and interact on Facebook weekly.
The initial in-person intervention session at the eligibility visit focused on increasing physical activity and making small sustainable dietary changes. All participants were instructed on the difference between prediabetes and type 2 diabetes; personal risk for diabetes as determined by the American Diabetes Association Type 2 Diabetes Risk Test [7]; and the complications associated with diabetes. Research staff taught participants about the health benefits of lifestyle change, and instructed participants in increasing physical activity (daily steps) by 20% each week until reaching 12,000 steps/day and engaging in at least a 10-min daily bout of moderate intensity activity. Furthermore, research staff advised participants to consume 5–6 small meals a day, use portion control, increase water intake, eat slowly, limit fat intake to 25% of total caloric intake, and replace sugar-sweetened beverages with unsweetened beverages. Research staff taught basic calorie counting and reading nutrition labels, and provided personalized tips for adjusting diet based on food photos provided in the run-in period. Research staff provided several bottles of non- sugar-sweetened beverages (e.g., non-sweetened tea and soda water) during the eligibility and 3-week visits. Lastly, participants were encouraged to join in a password-protected private study Facebook group to view and/or post suggestions and tips for lifestyle modifications. Research staff monitored this private Facebook group (i.e., posting weekly topics and answering participants’ questions).
The second in-person intervention session at the 3-week follow-up visit focused on reviewing participants’ progress, answering their questions, and introducing the following key concepts: healthy eating out, interpreting social cues, problem solving, talking back to negative thoughts, addressing slips, and social support for weight loss. Weekly follow-up phone calls or e-mails were sent to remind and encourage participants to meet their goals.
Participants
Participants were recruited from September 2014 to May 2016 through online advertisement, letters mailed to the home addresses of Latinos by zip code (as identified by census data), and by posting study flyers in hospitals, local businesses, and community centers in San Francisco, CA. Initial eligibility was assessed by telephone, and final eligibility was confirmed by in-person screening at the screening/baseline visit. Inclusion criteria were as follows: body mass index (BMI) ≥ 25 kg/m2; age ≥ 18 years; self-identifies as Latino; owns a smartphone; and willingness to use an app every day and wear a Fitbit Zip. Exclusion criteria were as follows: self-reported diagnosis of type 1 diabetes, type 2 diabetes with insulin therapy, untreated type 2 diabetes, or other disease associated with disordered glucose metabolism; medical condition or other physical problem necessitating special attention in an exercise or diet program; untreated mental illness; inability to walk 1 mile or 20 min; current participation in a weight-loss program or research study; mild cognitive impairment (screened by the Mini-Cog test) [13]; planning a trip outside the USA during the 10-week study period; planning to have gastric bypass surgery; use of antituberculosis agents, phenytoin, multiclass combination drugs or NRTIS to treat human immunodeficiency virus; being currently pregnant or having given birth in the last 6 months.
Procedures and measures
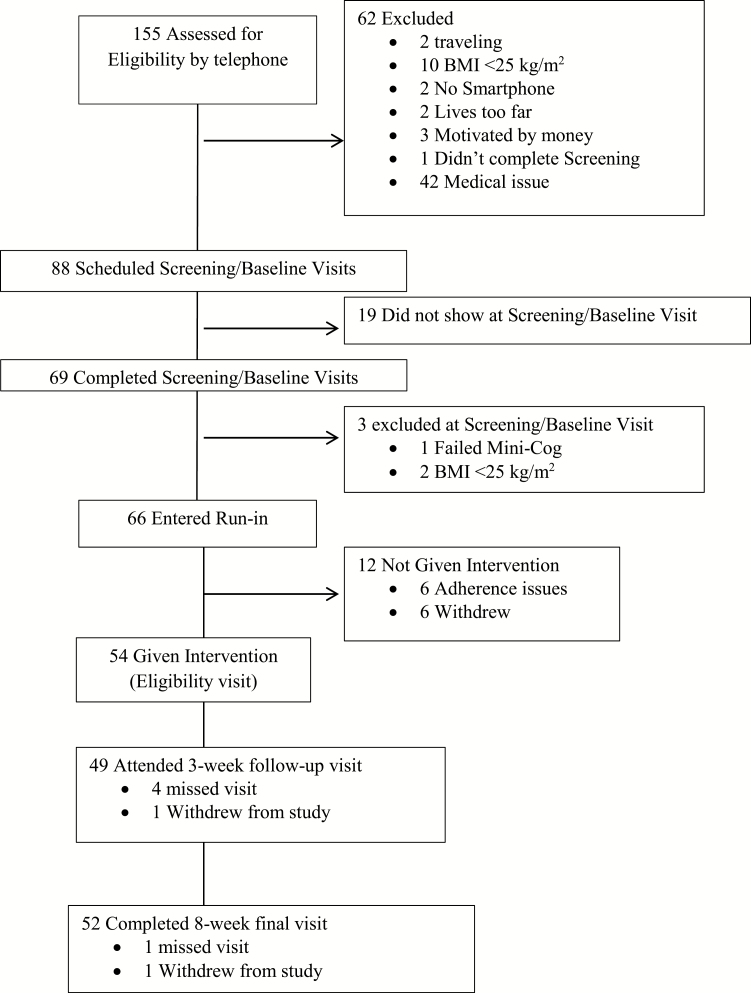
As seen in Fig. 1, a total of 155 men and women were screened for eligibility by telephone, and 69 attended a screening/baseline visit, two of whom were subsequently excluded due to BMI < 25 kg/m2 and one of whom was excluded for cognitive impairment. A total of 66 participants were eligible to begin a 2-week run-in period after completing the screening/baseline visit. Of these, six participants were excluded due to noncompliance (see the run-in criteria below) and six participants decided not to continue in the program during the 2-week run-in period. All remaining eligible participants (n = 54) were given the intervention.
Fig. 1.
Flow diagram: screening, enrollment, and follow-up of study participants
The run-in period had two purposes (criteria for receiving the intervention): (i) to determine if participants were able to comply with the requirements of wearing the Fitbit Zip (at least 70% compliance) and using the app daily; and (ii) to collect baseline average daily steps (physical activity). The research team used the Fitbit Zip and app for this phase of the study. During the 2-week run-in period, all participants were asked to use the app daily and wear a Fitbit Zip on their waist from the time they arose in the morning until the time they retired at night (at least 10 hr/day) every day. Participants were asked to log sugar-sweetened beverages into the app for the entirety of the run-in period and send photos of all food and beverages consumed for the initial 3 days of the run-in period.
Table 1 shows the timeline of the study measures and research office visits. In brief, all baseline data such as sociodemographics, self-reported medical history, and the Short Acculturation Scale for Hispanics [14] were collected at the screening/baseline or eligibility visit before delivering the intervention. Prior to each research office visit, participants received a reminder from research staff. At the 8-week final visit, participants were instructed in continuation and maintenance of lifestyle. Participants were compensated up to a total of $60 in cash for their transportation and their time for study participation.
Table 1.
Timeline of Study Measures and Research Office Visits
| Baseline data collection | Intervention period | ||||
|---|---|---|---|---|---|
| Measures | Screening/ baseline visit | Run-in period | Eligibility visit for interventiona | 3-week follow-up visita | 8-week final visit |
| Sociodemographics and medical history | ✓ | ||||
| Short Acculturation Scale for Hispanics | ✓ | ||||
| Anthropometric measurements | |||||
| Body weight/body mass index | ✓ | ✓ | ✓ | ||
| Height | ✓ | ||||
| Waist and hip circumference | ✓ | ✓ | ✓ | ||
| Blood pressure | ✓ | ✓ | ✓ | ||
| Food and caloric intakeb | |||||
| Modified Beverage Intake Questionnaire (BEVQ-15) | ✓ | ✓ | ✓ | ||
| Fat Intake Screener | ✓ | ✓ | ✓ | ||
| Physical activity | |||||
| International Physical Activity Questionnaire (IPAQ) short version | ✓ | ✓ | ✓ | ||
| Barriers to Being Active Quiz | ✓ | ✓ | ✓ | ||
| Objectively measured daily steps by Fitbit Zip | ✓ | ✓ | ✓ | ||
| Depressive symptoms | |||||
| Center for Epidemiologic Studies Depression Scale (CES-D) | ✓ | ✓ | |||
| Patient Health Questionnaire (PHQ) | ✓ | ✓ | ✓ | ||
| Patient-reported outcomes measurement information system | ✓ | ✓ | ✓ | ||
| (PROMIS) Global Short Form | |||||
aAn in-person intervention was delivered.
bAlthough participants were asked to log all food/drinks and calories every day into the Fitbit app, we did not use those caloric intake data as an outcome.
The primary outcomes were changes in percent body weight and BMI from the screening/baseline to Week 3 follow-up and 8-week final visit. Weight was measured with a Tanita WB-110 digital electronic scale and height was measured by standard stadiometer. Secondary outcomes (hip and waist circumference and resting systolic and diastolic blood pressure) were measured twice at each visit, and the average of the two measurements used for the final analysis. Participants were asked to change into a hospital gown and remove their shoes at each physical assessment. The modified Beverage Intake Questionnaire (BEVQ-15) and the Fat Intake Screener questionnaire were used to assess changes in caloric intake [15, 16]. We added two popular beverage items (aguas frescas and coconut water) among Latino populations to the BEVQ-15. Additional self-reported measures included the International Physical Activity Questionnaire (IPAQ) (short version) [17]; Barriers to Being Active Quiz [18]; PROMIS Global Short Form [19]; and Center for Epidemiologic Studies Depression Scale (CES-D) [20]. Fitbit Zip-measured mean daily steps per week were calculated at the screening/baseline, 3-week follow-up, and 8-week final visits by averaging daily steps over the course of 7 days. Days were excluded when the step count was less than 1,000 steps/day and the Fitbit was worn less than 8 hr/day. Adverse events were assessed at Week 3 and the Week 8 final visit using a checklist and open-ended questions.
Statistical analysis
The sample of 54 participants provided 80% power to detect an average weight loss of ~1 kg, or about 0.9% of body weight, as well as adjusted correlations of 0.39 between weight loss and changes in other factors potentially affected by the intervention. Baseline characteristics of the cohort were described using means and standard deviations or frequencies, as appropriate. Average changes in outcomes observed at screening/baseline, Week 3 follow-up, and Week 8 final visits were estimated using linear mixed models (LMMs), with Wald tests to assess heterogeneity and orthogonal contrasts to assess linear trend. The LMMs for observations assessed at screening/baseline, Week 3 follow-up, and Week 8 final visits used random intercepts and slopes with unstructured covariance matrices to account for within-subject correlation of the repeated measures. In the analyses of percent changes in weight, the baseline observation was omitted from the analysis and the LMM used random intercepts only. All analyses were conducted using Stata version 14 or SPSS version 23.0.
RESULTS
Baseline characteristics
Mean participant age was 45.3 (SD ± 10.8) with a range 24–65 years, 68.5% were female, 68.5% had a bachelor’s or more advanced degree, and 61.1% were born in the USA (see Table 2). Based on the Short Acculturation Scale for Hispanics [14], the subjects are relatively acculturated. All of participants used a smartphone and a computer at least once a week prior to the study enrollment. Mean BMI was 31.4 (SD ± 4.1) with a range of 25.1 to 42.0 kg/m2, and mean random plasma glucose was 97.8 (SD ± 13.6) with a range of 73 to 133 mg/dl. The 54 participants who completed a run-in period and qualified for the intervention did not differ from the 15 unqualified participants with respect to age, education, employment, annual household income, U.S. born, BMI, depressive symptoms, health literacy, and acculturation (p > .05). However, qualified participants were significantly older than unqualified participants (45.3 [SD ± 10.6] vs. 38.3 [SD ± 11.2] years, p = .003).
Table 2.
Baseline Characteristics (n = 54)
| Variables | Mean (SD) or % (n) | |
|---|---|---|
| Age (years) | – | 45.30 ± 10.75 |
| Gender | Men | 31.5 (17) |
| Women | 68.5 (37) | |
| Education | Completed college or graduate school | 68.5 (37) |
| Completed high school or some college education or less than high school | 31.5 (17) | |
| Marital status | Married/cohabitating | 57.4 (31) |
| Single/divorced/widowed | 42.6 (23) | |
| Employment | Employed for paid | 77.8 (42) |
| Unemployed/home maker/disable/others | 22.2 (12) | |
| # of years living in the USA | Born in the USA | 61.1 (33) |
| ≥10 years | 38.9 (21) | |
| Self-reported ethnicity | Mexican | 61.1 (33) |
| Salvadoran | 13.0 (7) | |
| Nicaraguan | 5.6. (3) | |
| Guatemalan | 3.7 (2) | |
| Peruvian | 3.7 (2) | |
| Puerto Rican | 3.7 (2) | |
| Cuban | 1.9 (1) | |
| Columbian | 1.9 (1) | |
| Honduran | 1.9 (1) | |
| Ecuadoran | 1.9 (1) | |
| Panamanian | 1.9 (1) | |
| Living with child(ren) | Yes | 22.2 (12) |
| Drove at least once/week | Yes | 81.5 (44) |
| Past pedometer use | Yes | 55.6 (30) |
| Smoking | Yes | 9.3 (5) |
| Antidepressant/psychiatric medication | Yes | 11.6 (8) |
| Hyperlipidemia medication | Yes | 7.2 (5) |
| Blood pressure medication | Yes | 13.0 (9) |
| Perceived future diabetes | Much less/somewhat less likely | 25.9 (14) |
| Same | 16.7 (9) | |
| Somewhat/more likely | 51.6 (28) | |
| Not applicable (taking oral medication for type 2 diabetes) | 5.6 (3) | |
| Acculturationa | 1.Language subscale | 3.79 ± 0.82 |
| 2.Media subscale | 4.49 ± 0.66 | |
| 3.Social subscale | 3.19 ± 0.57 |
aThe Short Acculturation Scale for Hispanics was used. A potential score ranges from 1 to 5 and a higher score indicates greater acculturation. An average score above 2.99 indicates more acculturated [14].
Fidelity of in-person and app interventions
About 97% (n = 52) of the participant completed an 8-week final visit. About 90.7% of the participants completed the two in-person intervention sessions and the remaining participants completed only one intervention at eligibility visit. The sessions 1 and 2 had a mean duration of 53.0 (SD ± 10.8) with range from 38.0 to 90.0 min and 37.5 (SD ± 8.7) with range from 22.2 to 64.1 min, respectively. Participants were asked to record their weight at least twice a week during the 8-week intervention period. The mean adherence to self-weighing at least twice per week was 49.3 (SD ± 29.9) % (median 47.2% with range from 0% to 100.0%) of the study weeks and at least once per week during 76.7 (SD ± 20.8) % (median 85.2% range from 22.2% to 100.0%). There were no serious adverse events (hospitalization or emergency visits) or deaths associated with the intervention.
Potential efficacy of intervention
Tables 3 and 4 show changes in primary and secondary outcomes over the 8-week intervention period. Overall, participants lost an average of 3.3 (SD ± 3.4) % of their body weight (p < .0005), corresponding to an average change of −2.4 kg (−5.3 lb) (p < .0005), respectively. In addition, we observed statistically significant reductions in hip and waist circumferences (p < .0005) and both systolic (p < .0005) and diastolic (p < .001) blood pressure. Table 4 shows changes in self-reported questionnaires and Fitbit Zip measured daily step outcomes in the 8-week intervention period.
Table 3.
Changes in Primary and Secondary Outcomes over the 8-Week Intervention Period
| n | Baseline | n | 3-week follow-up visit | n | 8-week final visit | Fitted mean change from baseline to 8-week final visit | Trend p | |
|---|---|---|---|---|---|---|---|---|
| Mean (±SD) | Mean (±SD) | Mean (±SD) | Mean (95% CI) | |||||
| Primary outcomes | ||||||||
| Body weight (kg) | 54 | 84.5 (11.8) | 49 | 83.5 (11.2) | 52 | 82.1 (10.8) | −2.8 (−2 to −3.6) | <.0005 |
| BMI (kg/m2) | 54 | 31.4 (4.1) | 49 | 30.9 (3.95) | 52 | 30.4 (3.9) | −1.0 (−0.7 to −1.3) | <.0005 |
| % body weight | 54 | 100.0 (0) | 49 | 98.2 (1.6) | 52 | 96.7 (3.4) | −2.4 (−3.4 to −1.5) | <.0005 |
| Secondary outcomes | ||||||||
| Waist circumference (cm) | 54 | 98.6 (10.3) | 49 | 96.2 (10.3) | 52 | 94.1 (10.4) | −4.6 (−3.5 to −5.7) | <.0005 |
| Hip circumference (cm) | 54 | 109.4 (9.2) | 49 | 107.6 (9.2) | 52 | 105.9 (9.5) | −3.4 (−2.4 to −4.5) | <.0005 |
| Systolic BP (mmHg) | 54 | 122.1 (14.4) | 49 | 117.9 (12.3) | 52 | 117.2 (12.8) | −4.3 (−2.0 to −6.5) | <.0005 |
| Diastolic BP (mmHg) | 54 | 76.6 (9.8) | 49 | 74.4 (10.5) | 52 | 73.5 (10.6) | −2.9 (−1.1 to −4.6) | .001 |
BMI body mass index; BP blood pressure.
Table 4.
Change in Self-Reported and Measured Daily Step Outcomes over the 8-Week Intervention Period
| Baseline | 3-week follow-up visit | 8-week final visit | Heterogeneity p | Trend p | ||||
|---|---|---|---|---|---|---|---|---|
| n | Mean (±SD) | n | Mean (±SD) | n | Mean (±SD) | |||
| IPAQ | ||||||||
| Total MET-min/week | 54 | 2,443.6 (2,285) | 49 | 2,814.3 (2,016.1) | 53 | 3,393.4 (3,020.1) | .01 | .003 |
| Walk MET-min/week | 54 | 939.1 (1,104) | 49 | 1,164.9 (1,007.0) | 53 | 1,254.0 (1,262.0) | .07 | .032 |
| Moderate MET-min/week | 54 | 597.9 (1,021) | 49 | 504.1 (657.2) | 53 | 777.3 (825.1) | .04 | .078 |
| Vigorous MET-min/week | 54 | 906.7 (1,261) | 49 | 1,145.3 (1,106.8) | 53 | 1,362.1 (1,668.6) | .09 | .028 |
| Barriers to being active | 54 | 49 | 53 | |||||
| Total score | 54 | 19.0 (8.9) | 49 | 16.1 (10.1) | 53 | 13.6 (9.3) | <.0005 | <.0005 |
| Lack of time | 54 | 3.8 (2.6) | 49 | 3.3 (2.3) | 53 | 2.8 (2.2) | .002 | <.0005 |
| Influence from others | 54 | 2.8 (2.1) | 49 | 2.5 (2.1) | 53 | 2.2 (1.9) | .12 | .039 |
| Lack of energy | 54 | 3.6 (2.6) | 49 | 3.1 (2.4) | 53 | 3.1 (2.2) | .06 | .09 |
| Lack of willpower | 54 | 5.5 (2.9) | 49 | 3.6 (2.7) | 53 | 2.7 (2.3) | <.0005 | <.0005 |
| Fear of injury | 54 | 0.7 (1.3) | 49 | 1.0 (1.4) | 53 | 0.7 (1.4) | .19 | .99 |
| Lack of skill | 54 | 0.9 (1.3) | 49 | 0.9 (1.7) | 52 | 0.6 (1.4) | .16 | .054 |
| Lack of resources | 54 | 1.7 (1.6) | 49 | 1.7 (1.7) | 52 | 1.6 (1.7) | .76 | .46 |
| Center for Epidemiologic Studies Depression Scale | 54 | 4.4 (4.3) | 0 | 53 | 6.3 (6.1) | .03 | .03 | |
| PHQ-2 (depressive symptoms) Use continuous data |
54 | 0.5 (0.7) | 49 | 0.4 (0.9) | 53 | 0.4 (0.8) | .84 | .56 |
| PROMIS–global physical health | 54 | 12.0 (1.3) | 49 | 11.9 (1.2) | 53 | 11.8 (1.1) | .60 | .32 |
| PROMIS–global mental health | 54 | 10.3 (1.9) | 49 | 10.4 (1.8) | 53 | 10.5 (1.7) | .64 | .35 |
| Fat-related diet habits | 54 | 21.3 (6.0) | 49 | 17.1 (5.9) | 52 | 16.2 (6.0) | <.0005 | <.0005 |
| Total daily SSB (BEVQ-15) | 54 | 49 | 52 | . | ||||
| Total beverage calories | 54 | 264.9 (153.1) | 49 | 241.5 (172.0) | 52 | 227.2 (147.5) | .21 | .11 |
| Total beverage grams | 54 | 1,679.8 (607.2) | 49 | 1,758.6 (779.0) | 52 | 1,678.9 (810.4) | .67 | .87 |
| SSB calories | 54 | 131.9 (120.1) | 49 | 101.2 (101.5) | 52 | 91.3 (91.3) | .004 | .003 |
| SSB grams | 54 | 370.1 (316.0) | 49 | 314.2 (314.2) | 52 | 273.1 (304.7) | .03 | .008 |
| Water | 54 | 27.8 (15.7) | 49 | 32.4 (17.8) | 52 | 31.8 (18.1) | .13 | .16 |
| Daily steps | 54 | 7,899.1 (2,814.7) | 54 | 9,879.7 (3,625.3) | 54 | 9,880.6 (3,634.6) | <.0005 | <.0005 |
IPAQ International Physical Activity Questionnaire; MET metabolic equivalent of task; PHQ Patient Health Questionnaire; PROMIS Patient-Reported Outcomes Measurement Information System; SSB sugar-sweetened beverage; BEVQ-15 modified Beverage Intake Questionnaire.
Predictors of % change in body weight
Table 5 shows the results of an adjusted analysis in predicting changes percent body weight from eligibility to 8-week final visit. After controlling for baseline factors (age, gender, health literacy, and acculturation), the mobile app weight diary usage at least twice a week (p = .01) and change in IPAQ score (p = .03) were significantly associated with change in percent body weight.
Table 5.
Predictors of % Weight Loss over the 8-Week Period (Adjusted Week 3 and Week 8 Visits)
| Predictors | Effect (%) | 95% CI (%) | p-value |
|---|---|---|---|
| Age (per 5 years) | −0.22 | −0.52 to 0.07 | .14 |
| Female sex | 0.82 | −0.57 to 2.21 | .25 |
| Health literacy | −0.09 | −0.49 to 0.31 | .65 |
| Acculturation subscale—language | 0.31 | −0.52 to 1.14 | .47 |
| Acculturation subscale—media | 0.19 | −0.95 to 1.32 | .75 |
| Acculturation subscale—socialization | −0.18 | −1.31 to 0.95 | .76 |
| Mobile app weight loss adherence (twice a week) | −0.26 | −0.46 to −0.06 | .01 |
| Change in IPAQ (total MET min/week) (per 1,000) | −0.22 | −0.42 to −0.02 | .03 |
| Change in total barriers to being physically active | 0.04 | −0.03 to 0.11 | .23 |
| Change in CES-D score | −0.05 | −0.16 to 0.05 | .34 |
| Change in fat-related diet habits | −0.05 | −0.13 to 0.04 | .32 |
| Change in BEVQ-15 (SSB calories per day) (per 100) | 0.08 | −0.21 to 0.36 | .59 |
Age, gender, health literacy, and acculturation were included in the model for face validity.
IPAQ International Physical Activity Questionnaire; MET metabolic equivalent of task; CES-D Center for Epidemiologic Studies Depression Scale; BEVQ-15 modified Beverage Intake Questionnaire; SSB sugar-sweetened beverage.
DISCUSSION
In this analysis of 54 overweight/obese, but otherwise healthy, well-educated smartphone using Latinos who participated in an 8-week uncontrolled pilot study of a commercially available mobile application plus in-person counseling sessions, an average weight loss of 3.3% was observed. In addition, other metabolic risk factors such as systolic and diastolic blood pressure and waist and hip circumferences notably improved over the course of the intervention. Given the high study completion rate (97%), excellent fidelity of the intervention, and potential efficacy of the intervention, the overall findings of this study support the idea that use of a commercially available mobile application plus in-person counseling program is feasible and accepted by this Latino sample, and could be effective. The current guidelines for the Management of Overweight and Obesity in Adults recommend a 5% to 10% of weight loss within the first 6 months of the program [21]. We observed 3% of loss of body weight (−2.4 kg/−5.3 lb) over only an 8-week period, and this was associated with clinically meaningful reductions in some metabolic risk factors, such as both systolic and diastolic blood pressure and waist/hip circumferences in this Latino sample. Consistent with the findings of this current study, a systematic review published in 2014 reported that five out of the seven mobile phone-based weight loss interventions reviewed led 1.6 to 4.5 kg weight loss over a 2- to 4-month intervention period [10]. However, none of the studies had follow-up information regarding weight loss after the initial intervention.
Self-weighing is important part of managing overweight and obesity [21]. In a study of weekend lifestyle patterns on body weight, it was reported that people tend to have higher caloric intake and lower physical activity on weekends compared to weekdays, and these behaviors result in weekly weight gain [22]. Therefore, in this current study, the subjects were specifically instructed to report their body weight by the app at least twice a week, on Fridays and Mondays before breakfast. We hypothesize that the specific self-weighing instructions helped participants to self-regulate their weight over the time. The results of the current study also suggested that adherence to self-weighing at least twice a week was a significant predictor of percent body weight change over the 8-week period. A recent systematic review of self-weighing in weight management concluded that regular self-weighing was associated with more weight loss and not associated with adverse psychological outcomes (e.g., depression, anxiety) [23]. Use of a smartphone app-based weight diary, compared to a paper and pencil diary, provides the opportunity to be able to obtain time-stamped weight entry data and remotely monitor self- weighting adherence. Given the rapid increased use of smartphones in general, and in particular among Latinos, smartphone-based self-weighing app diaries and weight loss interventions are expected to grow exponentially.
In this study, increasing total physical activity was also significantly associated with percent body weight reduction, but reducing intake of total fat and sugar-sweetened beverages by self-report was not shown to be a predictor in this multivariate model. In general, a combination of physical activity increase and caloric intake restriction is a more effective weight loss strategy than caloric intake restriction alone. Physical activity is also important to prevent weight gain after an initial weight loss. According to the 2009 American College of Sports Medicine (ACSM) Position Stand entitled “Appropriate Intervention Strategies for Weight Loss and Prevention of Weight Regain for Adults,” moderate intensity physical activity > 150 min/week resulted in modest weight loss of 2 to 3 kg and 225 to 420 min/week resulted in 5 to 7.5 kg weight loss [24]. In this study, both self-reported and objectively measured physical activity substantially increased over the 8-week period. However, we do not have a clear explanation as to why reductions in total fat intake and sugar-sweetened beverage consumption were not associated with percent body weight change after adjusting other factors. One possible explanation is that the total daily caloric intake was not measured in this study and total fat intake and sugar-sweetened beverage consumption represented only part of their total daily caloric intake. Another possible explanation could be related to accuracy of dietary intake using self-reported questionnaires due to recall bias, social desirability bias, and lack of dietary knowledge [25, 26].
Strengths and limitations
To our knowledge, the Adelgaza Program was the first weight-loss program using a commercial mobile application plus two in-person counseling sessions in Latino populations. We observed a clinically and statistically significant reduction in percent body weight and other metabolic factors over the course of the study. However, several limitations need to be taken into account. First, because there was no randomized control group, we were not able to estimate the efficacy of the intervention, determine the relative effectiveness of its components, or evaluate moderators or mediators of intervention effects. Second, the majority of the sample represented Mexican Americans living in the San Francisco Bay Area, CA, and 69% had bachelor or higher degrees, whereas 2015 census data on educational attainment of Hispanics reveals that only about 20% hold bachelor or higher degrees [27]. Thus, the results may not be generalizable to other Latino and lower educational attainment groups. Lastly, the study duration was relatively short, with a small sample size, so it may not fully reflect the effects of a longer intervention, nor could it determine other factors associated which could be associated with potential benefits.
CONCLUSIONS
Use of a commercially available mobile application plus in-person counseling program appears to be feasible and accepted by the well-educated Latino sample. The intervention was associated with a clinically and statistically significant reduction in body weight and other metabolic risks over the 8-week period, but a randomized controlled trial is warranted.
ACKNOWLEDGMENTS
The authors thank Nadra Lisha, PhD, at University of California (UC), San Francisco, for data cleaning and analysis assistance, and ITO EN Inc. donating non-sugar-sweetened tea products. This project was supported by the CTSI Resource Allocation Program/Academic Senate award grant from the University of California, San Francisco, and by a grant (K24NR015812) from the National Institute of Nursing Research. The study sponsors had no role in the study design; collection, analysis, or interpretation of data; writing the report; or the decision to submit the report for publication. We confirm that the findings reported have not been previously published and that the manuscript is not being simultaneously submitted elsewhere; the paper does not contain any previous reporting of data; the authors have full control of all primary data and that they agree to allow the journal to review their data if requested; the project was approved by the University of California, San Francisco IRB (#IRB Number: 14-13466).
Compliance with Ethical Standards
Conflict of Interest: The authors declare no conflict of interest.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
References
- 1. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schneiderman N, Llabre M, Cowie CC, et al. . Prevalence of diabetes among Hispanics/Latinos from diverse backgrounds: the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Diabetes Care. 2014;37(8):2233–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Estimates of diabetes and its burden in the United States Available at http://www.diabetes.org/assets/pdfs/basics/cdc-statistics-report-2017.pdf. Accessed 22 July 2017.
- 4. Gregg EW, Zhuo X, Cheng YJ, Albright AL, Narayan KM, Thompson TJ. Trends in lifetime risk and years of life lost due to diabetes in the USA, 1985–2011: a modelling study. Lancet Diabetes Endocrinol. 2014;2(11):867–874. [DOI] [PubMed] [Google Scholar]
- 5. American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36(4):1033–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Knowler WC, Barrett-Connor E, Fowler SE, et al. ; Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lindström J, Ilanne-Parikka P, Peltonen M, et al. ; Finnish Diabetes Prevention Study Group Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet. 2006;368(9548):1673–1679. [DOI] [PubMed] [Google Scholar]
- 8. Li G, Zhang P, Wang J, et al. . The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet. 2008;371(9626):1783–1789. [DOI] [PubMed] [Google Scholar]
- 9. Brown A, Lopez G, Lopez MH.. Digital divide narrows for Latinos as more Spanish speakers and immigrants go online. Washington, DC: Pew Research Center; 2016. [Google Scholar]
- 10. Aguilar-Martínez A, Solé-Sedeño JM, Mancebo-Moreno G, Medina FX, Carreras-Collado R, Saigí-Rubió F. Use of mobile phones as a tool for weight loss: a systematic review. J Telemed Telecare. 2014;20(6):339–349. [DOI] [PubMed] [Google Scholar]
- 11. Diabetes Prevention Program Research G. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25(12):2165–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wing R, Gillis B.. The Diabetes Prevention Program’s lifestyle change program. Pittsburgh, PA: University of Pittsburgh, 1996. Available at http://www.bsc.gwu.edu/dpp/lifestyle/DPP_duringcore.pdf. Accessed 1 February 2015. . [Google Scholar]
- 13. Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The mini-cog: a cognitive ‘vital signs’ measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry. 2000 Nov;15(11):1021–1027.. [DOI] [PubMed] [Google Scholar]
- 14. Marin G, Sabogal F, Marin BV, Oterosabogal R, Perezstable EJ. Development of a Short Acculturation Scale for Hispanics. Hispanic J Behav Sci. 1987;9(2):183–205. [Google Scholar]
- 15. Hedrick VE, Savla J, Comber DL, et al. . Development of a brief questionnaire to assess habitual beverage intake (BEVQ-15): sugar-sweetened beverages and total beverage energy intake. J Acad Nutr Diet. 2012;112(6):840–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wakimoto P, Block G, Mandel S, Medina N. Development and reliability of brief dietary assessment tools for Hispanics. Prev Chronic Dis. 2006;3(3):A95. [PMC free article] [PubMed] [Google Scholar]
- 17. International Physical Activity Questionnaire Group. Guidelines for data processing and analysis of the International Physical Activity Questionnaire (IPAQ) - Short Form Available at http://www.institutferran.org/documentos/scoring_short_ipaq_april04.pdf. Accessed 22 July 2017.
- 18. Centers for Disease Control and Prevention. Barriers to Being Active Quiz Available at http://www.cdc.gov/diabetes/ndep/pdfs/8-road-to-health-barriers-quiz-508.pdf. Accessed 22 July 2017.
- 19. Hays RD, Bjorner JB, Revicki DA, Spritzer KL, Cella D. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual Life Res. 2009;18(7):873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 21. Jensen MD, Ryan DH, Apovian CM, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. J Am Coll Cardiol. 2014;63(25 pt B):2985–3023. [DOI] [PubMed] [Google Scholar]
- 22. Racette SB, Weiss EP, Schechtman KB, et al. . Influence of weekend lifestyle patterns on body weight. Obesity (Silver Spring). 2008;16(8):1826–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zheng Y, Klem ML, Sereika SM, Danford CA, Ewing LJ, Burke LE. Self-weighing in weight management: a systematic literature review. Obesity (Silver Spring). 2015;23(2):256–265. [DOI] [PubMed] [Google Scholar]
- 24. Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK; American College of Sports Medicine: American College of Sports Medicine Position Stand Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41(2):459–471. [DOI] [PubMed] [Google Scholar]
- 25. Hébert JR. Social desirability trait: biaser or driver of self-reported dietary intake?J Acad Nutr Diet. 2016;116(12):1895–1898. [DOI] [PubMed] [Google Scholar]
- 26. Di Noia J, Cullen KW, Monica D. Social desirability trait is associated with self-reported vegetable intake among women enrolled in the Special Supplemental Nutrition Program for Women, Infants, and Children. J Acad Nutr Diet. 2016;116(12):1942–1950. [DOI] [PubMed] [Google Scholar]
- 27. Ryan L, Bauman K. Educational attainment in the United States: 2015. Population characteristics. US Census Bureau. 2016;2017(6.29):20–578. [Google Scholar]