Abstract
Background
In 2015, Namibia implemented an Acceleration Plan to address the high burden of HIV (13.0% adult prevalence and 216 311 people living with HIV [PLHIV]) and achieve the UNAIDS 90-90-90 targets by 2020. We provide an update on Namibia’s overall progress toward achieving these targets and estimate the percent reduction in HIV incidence since 2010.
Methods
Data sources include the 2013 Namibia Demographic and Health Survey (2013 NDHS), the national electronic patient monitoring system, and laboratory data from the Namibian Institute of Pathology. These sources were used to estimate (1) the percentage of PLHIV who know their HIV status, (2) the percentage of PLHIV on antiretroviral therapy (ART), (3) the percentage of patients on ART with suppressed viral loads, and (4) the percent reduction in HIV incidence.
Results
In the 2013 NDHS, knowledge of HIV status was higher among HIV-positive women 91.8% (95% confidence interval [CI], 89.4%–93.7%) than HIV-positive men 82.5% (95% CI, 78.1%–86.1%). At the end of 2016, an estimated 88.3% (95% CI, 86.3%–90.1%) of PLHIV knew their status, and 165 939 (76.7%) PLHIV were active on ART. The viral load suppression rate among those on ART was 87%, and it was highest among ≥20-year-olds (90%) and lowest among 15–19-year-olds (68%). HIV incidence has declined by 21% since 2010.
Conclusions
With 76.7% of PLHIV on ART and 87% of those on ART virally suppressed, Namibia is on track to achieve UNAIDS 90-90-90 targets by 2020. Innovative strategies are needed to improve HIV case identification among men and adherence to ART among youth.
Keywords: 90-90-90, Antiretroviral therapy, HIV, HIV testing, HIV viral load, Namibia, retention
In 2014, the Joint United Nations Programme on HIV/AIDS (UNAIDS) issued ambitious global HIV treatment targets to help end the HIV epidemic [1]. The targets called for diagnosing 90% of people living with HIV (PLHIV), placing 90% of those identified on sustainable antiretroviral therapy (ART), and ensuring that 90% of those on ART are virally suppressed by 2020. UNAIDS estimates that HIV incidence would drop by 90% and AIDS-related deaths by over 80% by 2030 if countries were able to achieve these targets [1].
Globally, Namibia is one of the countries most affected by HIV. In 2016, the estimated HIV prevalence among adults (15–49 years) was 13.0%, with an estimated 216 311 (range, 200 000–237 000) individuals living with HIV, including 207 000 adults and 9000 children under 15 years of age. HIV/AIDS continues to be the leading cause of death, and an estimated 3100 people died from AIDS in 2015 [2].
The Government of the Republic of Namibia (GRN), with support from the United States President’s Emergency Plan for AIDS Relief (PEPFAR), has developed an Acceleration Plan to help Namibia meet the UNAIDS targets by 2020. In this manuscript, we provide an update on Namibia’s overall progress toward achieving the 90-90-90 targets and evaluate the impact of this progress on reducing HIV incidence. We also provide a brief summary of the strategies GRN has employed to achieve this progress.
METHODS
For the First 90: Proportion of PLHIV who Know Their HIV Status
To estimate the overall number of PLHIV who know their status, we used data from the 2013 Namibia Demographic and Health Survey (2013 NDHS) [3], a comprehensive, national-level population and health survey in Namibia. Data collection for the 2013 NDHS was conducted among adults (15–64 years) between May and September 2013 [3]. The survey’s objective was to provide population-based demographic, socioeconomic, and health data including knowledge and prevalence of HIV. A total of 9849 households from all regions of Namibia were included in the survey, and a total of 14 499 individuals (10 018 females and 4481 males) were interviewed. Participants were asked to self-report their HIV status and were offered HIV testing services (HTS) as part of the survey.
To calculate the proportion of PLHIV who know their HIV status, we applied the percentage of 2013 NDHS participants who tested HIV-positive and who reported they were ever tested for HIV and received the results of their last test to the number of PLHIV from the 2016 SPECTRUM model [4]. The 2016 SPECTRUM model estimates are for the 2016 calendar year and are based on ART and prevention of mother-to-child transmission of HIV program data from previous years, as well as data from biennial surveys of pregnant women who attended antenatal clinics (1992–2016) and the 2013 NDHS population-based HIV prevalence estimate.
We calculated a confidence interval for the first 90 by multiplying the lower and upper 95 percent confidence limits for the 2013 NDHS estimate of PLHIV who knew their status by the lower and upper 95% uncertainty bounds for PLHIV from the 2016 SPECTRUM model [4].
The Second 90: Proportion of People With Diagnosed HIV Infection Receiving Sustained ART
At the time of NDHS implementation, the National HIV Treatment Guidelines recommended ART for patients with a CD4 count <350 cells/mm3, all HIV-infected tuberculosis patients, patients co-infected with HIV and hepatitis B virus, and patients with World Health Organization (WHO) clinical stages III and IV [5]. Currently in Namibia, all PLHIV are eligible to start ART upon diagnosis (test and treat) [5]. All individuals testing HIV positive are referred to an ART clinic for clinical assessment and to start ART. At public-run ART facilities, patients who are ready and willing to start treatment are assigned a unique ART number, and a file (patient care booklet) is opened for them. Standardized demographic and clinical data are collected and recorded in these patient booklets, which are updated after each clinical encounter with relevant clinical information. Data clerks routinely transcribe clinical and demographic information from the patient booklets into an electronic patient management system (ePMS). At each visit to the pharmacy for antiretroviral drug (ARV) refills, data on the number of pills dispensed and the patient’s next appointment are entered into the national electronic dispensing tool (EDT) system, which tracks patient adherence to ART and compliance with appointments. Aggregate data from EDT and ePMS are compiled on a monthly basis and reported to the national HIV program. Aggregate data on patients who access HIV services in the private sector are routinely submitted by providers to the Namibian Association for Medical Aid Funds (NAMAF), and then to the Ministry of Health and Social Services (MOHSS).
To assess progress toward the second 90, we used data from the ePMS and NAMAF to generate a list of patients active on ART at the end of 2016 at government facilities and in the private sector. We calculated the second 90 in the same manner as the UNAIDS target for the second 90 by using the number of PLHIV with known status as the denominator. We also calculated the second 90 using total PLHIV as the denominator.
The Third 90: Proportion of People Receiving ART who Have Viral Suppression
National HIV treatment guidelines [6] recommend HIV viral load (VL) testing 6 months after ART initiation and every 6 months thereafter for pediatric patients younger than 20 years of age or every 12 months thereafter for adults older than 19 years of age. Repeat VL testing is recommended for patients with virologic failure (defined as VL >1000 copies/mL) [6] after 3 months of intensive adherence counseling.
HIV VL testing for both public and private ART facilities is performed at 35 Namibia Institute of Pathology (NIP) laboratories. Coverage of VL testing in Namibia is quite high. At the end of 2016, MOHSS estimated that >90% of patients on ART had access to VL testing [7]. The rest of the patients were on ART at remote facilities that could not get plasma specimens transported to any of the NIP laboratories within a reasonable amount of time for VL testing.
With each VL testing request, the NIP collects basic patient demographic information, name of facility, name of the health care provider requesting the test, patient name, and patient date of birth. During the second half of 2016, the NIP also started recording the patient’s ART number on the VL request form. The NIP uses a separate electronic laboratory system (MEDITECH) to record and report VL test results.
To estimate Namibia’s progress toward achieving the third 90, VL testing data, disaggregated by sex and age, were collected from the NIP and MEDITECH from January to December 2016. To account for duplicates in the VL tests performed by the NIP in 2016, VL test results from the last quarter of 2016 were analyzed separately. This was done because HIV patients on ART are routinely seen once every 3 months and quarterly results should not have duplicates.
We calculated the third 90 in the same manner as the UNAIDS target for the third 90 by using the number of PLHIV on ART as the denominator. We also calculated the third 90 using total PLHIV as the denominator.
Impact of Progress Toward 90-90-90 on Reducing HIV Incidence
We used a simple model or risk equation [7] to evaluate the impact of progress made thus far toward achieving the 90-90-90 targets and reducing HIV incidence. This simple risk equation was derived from the HIV treatment cascade and formulates incidence as a function of the 90-90-90 targets. In the equation, each stage of the HIV cascade is represented by a parameter, and for linking HIV incidence to the HIV cascade, 2 additional parameters are required: the rate of HIV transmission and the factor by which this transmission rate is reduced on average among people who have suppressed virus. Using these parameters and the HIV cascade values that reflect current progress toward the 90-90-90 targets, we estimated HIV incidence and determined the relative contribution of each gap in the HIV cascade to the number of new HIV infections in 2016.
RESULTS
The First 90: Proportion of PLHIV who Know Their HIV Status
In the 2013 NDHS, 88.3% (95% confidence interval [CI], 86.3%–90.1%) of HIV-positive adults 15–64 years of age reported being tested for HIV in the past and knowing their HIV status. Among those ever tested for HIV, knowledge of HIV status was higher among HIV-positive women (91.8%; 95% CI, 89.4%–93.7%) than HIV-positive men 82.5% (95% CI, 78.1%–86.1%). Knowledge of HIV status among adults aged 15–64 years in the general population was 73.5% (95% CI, 72.5%–74.5%). Men 15–24 years of age reported the lowest rate (42.0%) of ever being tested and receiving the test results, whereas the highest reported rates of ever being tested were among women aged 30–39 years, at 94.9% (Table 1). Assuming the overall proportion of 88.3% of PLHIV who knew their status, we estimated that 191 003 PLHIV in Namibia knew their HIV status at the end of 2016.
Table 1.
Weighted Numbers and Percentages of HIV-Positive Adults Aged 15–64 Years Ever Tested for HIV and Received Results of the Last Test, by Age Category and Sex, 2013 NDHS
| HIV-Positive 2013 NDHS Participants Ever Tested and Received Results | ||||||
|---|---|---|---|---|---|---|
| Men | Women | Total | ||||
| Age, y | Percentage | Number | Percentage | Number | Percentage | Numbera |
| 15–24 | 42.0 | 43 | 83.9 | 73 | 68.5 | 116 |
| 25–29 | 84.6 | 58 | 94.0 | 106 | 90.7 | 164 |
| 30–39 | 84.9 | 174 | 94.9 | 317 | 91.3 | 491 |
| 40–49 | 87.8 | 127 | 92.1 | 188 | 90.3 | 314 |
| 50–64 | 89.6 | 70 | 85.9 | 115 | 87.3 | 185 |
| Totala | 82.5 | 471 | 91.8 | 798 | 88.3 | 1270 |
Abbreviation: NDHS, Namibia Demographic and Health Survey.
aTotals may not match the sum of cell numbers due to rounding.
The Second 90: Proportion of People With Diagnosed HIV Infection Receiving Sustained ART
A query of EDT and ePMS at the end of 2016 revealed that a total of 151 076 patients, including 9623 pediatric patients younger than 14 years of age, were active on ART at public facilities by the end of 2016. A total of 14 863 patients, including 439 children younger than 17 years of age, were reported to MOHSS be on ART in the private sector, indicating that a total of 165 939 or 76.7% of the estimated 216 311 PLHIV in Namibia were on ART by the end of 2016 (Table 2).
Table 2.
Number of Adults and Children on ART From ePMS and EDT (Public Sector) and NAMAF (Private Sector) Databases, by Age Category and Sex, 2016
| Number on ART by the End of 2016 | |||
|---|---|---|---|
| Age | Males | Females | Total |
| 0–14 | 5061 | 5001 | 10 062a |
| ≥15 | 57 993 | 97 884 | 155 877b |
| Total | 63 054 | 102 885 | 165 939 |
Abbreviations: ART, antiretroviral therapy; EDT, electronic dispensing tool; ePMS, electronic patient management system; NAMAF, Namibian Association for Medical Aid Funds.
aIncludes 439 children aged ≤16 years from the NAMAF database, which only provides desegregation of 0–16 and ≥17 years.
bIncludes 14 424 adults aged ≥17 years from the NAMAF database.
The Third 90: Proportion of People Receiving ART who Have Viral Suppression
A total of 183 674 VL tests were performed by NIP from January to December 2016, of which 160 635 or 87% were suppressed (VL <1000 copies/mL). The suppression rate was 89% among females and 85% among males. The highest suppression rate was among adults ≥20 years of age (90%), and the lowest was among adolescents 15–19 years of age (68%) (Table 3). To account for duplicates in the 183 674 VL tests performed by the NIP in 2016, VL test results from the last quarter of 2016 were analyzed separately. This was done because HIV patients on ART are routinely seen once every 3 months, and quarterly results should not have duplicates. The overall VL suppression rate for the last quarter of 2016 was 87%, with relatively similar rates of suppression among sexes and age groups when compared with results from all 4 quarters of 2016 (Table 3).
Table 3.
HIV Viral Load Testing and Suppression Data From NIP, by Age Category, Jan–Dec 2016
| Jan–December 2016 (n = 183 674) | Oct–December 2016 (n = 34 368) | |||||
|---|---|---|---|---|---|---|
| Age, y | VL Tests Performed, No. | VL >1000+ Copies/ mL, No. | VL <1000 Copies/mL, No. (%) | VL Tests Performed, No. | VL >1000+ Copies/ mL, No. | VL <1000 Copies/ mL, No. (%) |
| 0–4 | 2119 | 449 | 1670 (79) | 487 | 95 | 392 (80) |
| 5–9 | 5111 | 1001 | 4110 (80) | 962 | 205 | 757 (79) |
| 10–14 | 9109 | 2240 | 6869 (75) | 1740 | 457 | 1283 (74) |
| 15–19 | 7515 | 2401 | 5114 (68) | 1437 | 479 | 958 (67) |
| ≥20 | 155 523 | 16 237 | 139 286 (90) | 28 878 | 3071 | 25 807 (89) |
| Not reported | 4297 | 711 | 3586 (83) | 864 | 145 | 719 (83) |
| Total | 183 674 | 23 039 | 160 635 (87) | 34 368 | 4452 | 29 916 (87) |
Abbreviations: NIP, Namibia Institute of Pathology; VL, viral load.
Impact of Progress Toward 90-90-90 on Reducing HIV Incidence
The progress toward the 90-90-90 targets at the end of 2016 indicates that a 21% reduction in HIV incidence compared with the 2010 level has been achieved in Namibia (from 9238 new infections in 2010 to 7331 new infections in 2016) (Table 4). The breakdown of the 7331 new HIV infections in 2016 by their location along the HIV treatment cascade shows that the largest contributor to new infections is PLHIV who remain undiagnosed and unaware of their HIV infection (34%). This is followed by diagnosed PLHIV who are not yet on ART (33%) (Table 4). If we assume that the number of PLHIV is the upper 97.5% bound from the 2016 Spectrum model, then the largest contributor to new infections is diagnosed PLHIV not on ART (45%).
Table 4.
Impact of Progress Toward the 90-90-90 Targets on Reducing HIV Incidence in Namibia, 2016
| HIV Cascade | ||
|---|---|---|
| Sensitivity Analysis | ||
| No. of PLHIV in 2016a | 216 311 (median) | 236 933 (upper 97.5% bound) |
| No. (%) diagnosed among PLHIV (d) | 191 003 (88) | 209 212 (88) |
| No. (%) on ART among diagnosed (τ) | 165 939 (87) | 165 939 (79) |
| No. (%) with viral suppression among PLHIV on ART (σ) | 144 367 (87) | 144 367 (87) |
| HIV incidence | ||
| No. of new HIV infections in 2010 | 9238a (median) | 9238a (median) |
| No. of new HIV infections | 7331a (median) | 7331 (median) |
| In 2016 [Nβ (1–dτσϕ)]b | ||
| Percent reduction from 2010 level | 21 | 21 |
| HIV incidence attributable to each stage in HIV cascade | ||
| No. of new HIV infections in 2016 (%) | ||
| Total | 7331 (100) | 7331 (100) |
| Undiagnosed, virally not suppressed [N(1–d)β] | 2479 (34) | 2129 (29) |
| Diagnosed, not on ART, virally not suppressed [Nd(1- τ)β] | 2455 (33) | 3323 (45) |
| Diagnosed, on ART, virally not suppressed [Ndτ(1–σ)β] | 2113 (29) | 1657 (23) |
| Diagnosed, on ART, virally suppressed [Ndτσ(1–ϕ)β] | 283 (4) | 222 (3) |
Abbreviations: ART, antiretroviral therapy; PLHIV, people living with HIV; VL, viral load.
aThe number of PLHIV and the number of new HIV infections for 2010 and 2016 were estimated by the Namibia 2016 Spectrum model.
bThe formula for number of new HIV infections is a function of 2 additional HIV transmission parameters: (1) the β parameter (0.098) represents the average number of transmissions per person per year resulting from PLHIV without suppressed viral load and was consistently estimated from the other parameters in the incidence formula; (2) the ϕ parameter represents the percentage reduction in infectivity among people with suppressed virus and was estimated conservatively as 0.98, given that no linked infections were observed in index participants whose HIV infections were stably suppressed by ART in the HIV Prevention Trials Network 052 trial [28].
DISCUSSION
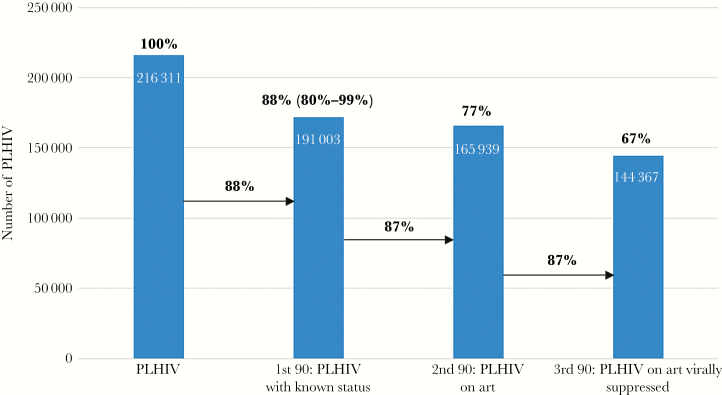
Namibia has made tremendous progress toward achieving the UNAIDS 90-90-90 HIV epidemic control targets. HIV program and population-based survey data indicate that of the estimated 216 311 PLHIV in Namibia in 2016, nearly 90% of them knew their HIV status. By the end of 2016, 87% of PLHIV diagnosed with HIV infection (77% of all PLHIV) in Namibia were on ART, and the VL suppression rate among those on ART was close to 90% (Figure 1). This indicates that once patients are diagnosed with HIV, the current strategies are effective in getting them linked to HIV treatment services, initiated on ART, and virally suppressed.
Figure 1.
Namibia’s progress toward UNAIDS 90-90-90 targets, December 2016. The 3 arrows represent estimates of Namibia’s three 90s. The last 3 histogram bars represent Namibia’s progress with respect to people living with HIV (PLHIV), represented by the first histogram bar.
What Has Led to Namibia’s Success?
Several factors have contributed to Namibia’s progress toward achieving the 90-90-90 targets and successfully reducing HIV incidence. These factors include:
Strong Political Will
Ending the HIV epidemic is a priority at all levels of the GRN, including the President. The government funds >60% of the national HIV response and rapidly adopts international guidelines. In fact, the high coverage of ART among PLHIV in Namibia can be explained by the progressive national HIV treatment guidelines in place since late 2013, which have allowed >85% of PLHIV to be eligible for ART [6]. Namibia recently adopted the latest WHO treatment guidelines, which recommend provision of ART to all HIV-infected patients irrespective of their immune status [5, 8]. The full implementation of these guidelines should allow Namibia to reach the second 90, or 81% of all PLHIV on ART, by 2020.
Implementation of Innovative HIV Testing Strategies to Identify PLHIV
The GRN and its implementing partners have implemented several strategies to improve HIV case finding, including index case testing, expansion of provider-initiated testing and counseling for inpatient and outpatient departments within health facilities, and targeted community testing aimed at supporting health facilities and communities experiencing human resource constraints and a high burden of undiagnosed HIV infection. In addition, subgroup analysis is being conducted to identify and target populations with a high burden of undiagnosed HIV infection for HTS.
Focus on Linking All Individuals Diagnosed as HIV Positive to HIV Treatment Services
Program data from the Namibian Ministry of Health and Social Services show that the total number of patients initiated on ART during 2016 was broadly similar to the total number of patients who tested positive for HIV in 2016 (data not reported). This suggests a strong linkage between HIV testing and treatment services. Within health facilities, the current practice in Namibia is to physically escort clients who test HIV positive to the ART clinic. Clients who test HIV-positive in the community are followed by community health care workers, who liaise with health facilities to ensure that all HIV-positive clients are linked to care. Although not evaluated in the Namibian context, similar approaches have been shown to improve linkages to care elsewhere [9, 10].
Strong Electronic Medical Systems to Improve Patient Care and Identify PLHIV in Need of Treatment Support
As previously mentioned, Namibia has several electronic medical systems, including ePMS, a national electronic dispensing tool, and a system to record and report VL test results. These systems strengthen patient care by allowing providers to identify patients who are nonadherent to their medications and/or in danger of treatment failure.
Implementation of Strategies to Address Geographic Constraints Associated With a Small Population Distributed Across a Wide Geographic Area
Namibia is a large country (824 292 square kilometers) with a small population (2.48 million people). This makes it difficult to ensure that all PLHIV have access to HIV testing and treatment services. To address these challenges, Namibia has been a leader in implementation of community-based ART services. Under this model, health care providers travel to remote villages on a quarterly basis, bringing patient medical files with them. They then dispense ARVs and other HIV medications and draw blood for VL testing. In addition, Namibia has implemented multimonth scripting and dispensing to reduce the number of trips stable patients need to make to a health facility from monthly to quarterly. Program data indicate that these strategies are leading to high 12-month retention rates, with a retention rate of 89% among adults on ART (Source: MOHSS, Directorate of Special Programmes, Unpublished, April 2015).
GRN, with support from PEPFAR, is engaged in rolling out Project Extension for Community Healthcare Outcomes (Project ECHO) [11] to all the main ART sites in Namibia. Project ECHO is a novel model of clinical mentorship and training that allows experts in HIV management to routinely share best practices for HIV care and treatment with health care workers throughout Namibia. To improve HIV VL testing capacity, NIP is in the process of decentralizing VL testing to a few additional regions and is also implementing the WHO-recommended collection of dried blood spot specimens for VL testing in remote areas [12].
What Are the Remaining Gaps?
Improving Coverage of HTS Among Adolescents and Men
The main gap preventing Namibia from reaching the 90-90-90 targets is identification of PLHIV through HTS. We estimated that this gap accounted for 34% of new HIV infections in 2016. Available data indicate that nearly half of men and women aged 15–24 years have never been tested for HIV and are unaware of their HIV status. Recent studies from Namibia and other countries indicates that HIV incidence is highest among females aged 15–24 years [13–15], which highlights the importance of expanding HTS among this population. Reasons for low testing among adolescents include poor knowledge of how HIV is transmitted and lack of awareness on how to access HIV services to prevent HIV transmission [16, 17]. Interventions that improve knowledge of HIV transmission risks and educate young people on how to access available HIV services and promote their use could help engage HIV-infected adolescents in HIV testing and treatment services. Additionally, adolescent-friendly HIV testing and treatment services, social network testing, and index partner testing are some of the other strategies that Namibia is planning to implement to improve HIV case detection among adolescents [16, 18–23].
NDHS data from 2013 also indicate low awareness of sero-status among men. This is similar to findings from other countries [24–26]. Some of the most common reasons for low coverage of HTS among men include stigma, poor health seeking behavior, socioeconomic and health facility barriers, and low perceived risk for HIV acquisition [24, 26, 27]. Several strategies are being implemented to improve men’s access to HTS, including index partner testing, work place testing, flexible clinic hours that allow men to come in for testing after hours or on weekends, and multidisease campaigns where men receive an HIV test in addition to other health screening tests (eg, hypertension, prostate cancer, diabetes) [27–30]. Plans are also underway to pilot HIV self-testing as a strategy for reaching adolescents and men with HTS.
Lower Viral Suppression Rates Among Pediatric and Adolescent Populations
The overall viral suppression rate among patients on ART is 87%. However, the suppression rates are about 10%−20% lower among pediatric and adolescent populations. This is similar to findings from other countries [31, 32]. Many factors account for low viral suppression rates among these groups, including poor adherence to ART, limited number of appropriate pediatric-friendly ARV formulations, nondisclosure of HIV status, lack of a support network, stigma, poor compliance with VL testing guidelines, and keeping patients on failing regimens [33–35]. Scaling up interventions to identify and address barriers to poor adherence among pediatric patients will help improve virologic suppression rates [36, 37]. This will also reduce the estimated 29% of new infections from patients who are not virally suppressed. To improve pediatric adherence, Namibia plans to roll out a standardized pediatric HIV disclosure program that has been demonstrated to improve HIV outcomes, including HIV VL suppression [38], and to expand teen clubs and other community-based HIV care and treatment programs for adolescents [24, 37].
There are several limitations to this report. The percentage of Namibians who know their HIV status is based on self-reports from the 2013 NDHS, along with SPECTRUM-modeled estimates of new HIV infections in subsequent years. Therefore, this estimate has a level of uncertainty, which we have tried to capture in our uncertainty bounds. In addition, we are not able to directly assess the percentage of patients who know their status and are linked to care and treatment services. Instead, we have relied on ART program data. Last, the overall VL suppression rates presented are based on the actual VL tests done, and repeat testers may be included. However, the fact that results from the last quarter of 2016 were identical to results from the entire year suggests that repeat testing had limited impact on the reported suppression rates. In addition, 2 papers presented at the 9th International AIDS Society Conference on HIV Science showed a VL suppression rate >90% in Namibia [39, 40]. During 2015, the most recent year with complete data, 91% of ART patients had at least 1 VL test [7]. It is possible that patients who did not have a VL test done were less compliant with their appointments. These patients could have lower virologic suppression rates. However, given that the VL suppression rate among patients who had the test done is about 90% and the tests were from patients from all the ART clinics in Namibia, it is unlikely that the overall VL suppression rate is significantly lower. A national household survey was carried out during June–December 2017 that should provide population-based estimates of HIV testing uptake, ART coverage, and VL suppression rates.
CONCLUSIONS
Namibia appears very close to reaching the UNAIDS 90-90-90 targets for HIV epidemic control. Some gaps remain in HTS and VL suppression, especially among children, adolescents, and men. Scaling up evidence-based interventions that target these groups will allow Namibia to reach the 90-90-90 targets by 2020 and ultimately help break the HIV transmission cycle and achieve HIV epidemic control. A national population-based survey in 2017 to estimate uptake of HTS, ART, and VL suppression should help validate the analyses presented here and identify geographic areas and population subgroups within Namibia that are still behind in meeting these targets.
Acknowledgments
The authors thank Honorable Dr. Bernard Haufiku, the Namibian Minister of Health and Social Services, and Ambassador Thomas Daughton, the US Ambassador to Namibia, for their leadership in the development and implementation of the Acceleration Plan.
Author contributions. S.A., M.K., A.B., S.S., N.M., N.P., N.S., A.W., N.T., G.M., A.J., A.M., E.D., D.P., and N.H. all contributed to the first or the final draft of the protocol. A.B. and M.K. did statistical analyses. S.A., S.S., N.K., N.P., N.S., A.W., N.T., G.M., D.P., and A.M. did the interpretation of results. S.A., S.S., N.T., and A.W. wrote the first draft of the report, which was revised by A.B., N.M., G.M., A.M., E.D., D.P., N.H., and A.M. All authors commented on earlier drafts of the report. All co-authors have seen and agree with the contents of the final manuscript.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of PEPFAR or the CDC.
Financial support. This work was supported in part by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC).
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Joint United Nations Programme on HIV/AIDS. 90-90-90: An Ambitious Treatment Target to Help End the AIDS Epidemic. Geneva: Joint United Nations Programme on HIV/AIDS; 2014. [Google Scholar]
- 2. Joint United Nations Programme on HIV/AIDS. Namibia/UNIADS. Geneva: Joint United Nations Programme on HIV/AIDS; 2017. http://www.unaids.org/en/regionscountries/countries/namibia. Accessed 22 August 2017. [Google Scholar]
- 3. Ministry of Health and Social Services. Namibia Demographic and Health Survey 2013. Windhoek, Namibia: Ministry of Health and Social Services; 2014. https://www.dhsprogram.com/pubs/pdf/FR298/FR298.pdf. Accessed 22 August 2017. [Google Scholar]
- 4. Stover J, Brown T, Puckett R, Peerapatanapokin W. Updates to the Spectrum/Estimations and Projections Package model for estimating trends and current values for key HIV indicators. AIDS 2017; 31:S5–11 [DOI] [PubMed] [Google Scholar]
- 5. Ministry of Health and Social Services. National Guidelines for Antiretroviral Therapy.Revised. 4th ed Windhoek, Namibia: Directorate of Special Programmes, Ministry of Health and Social Services; 2014. [Google Scholar]
- 6. Ministry of Health and Social Services. National Guidelines for Antiretroviral Therapy. Windhoek, Namibia: Directorate of Special Programs, Ministry of Health and Social Services; 2016. [Google Scholar]
- 7. Lecher S, Williams J, Fonjungo PN, et al. Progress with scale-up of HIV viral load monitoring – seven Sub-Saharan African countries, January 2015 – June 2016. MMWR Morb Mortal Wkly Rep 2016; 65:1332–5. [DOI] [PubMed] [Google Scholar]
- 8. Kelly SL, Wilson DP. HIV cascade monitoring and simple modeling reveal potential for reductions in HIV incidence. J Acquir Immune Defic Syndr 2015; 69:257–63. [DOI] [PubMed] [Google Scholar]
- 9. World Health Organization. Guideline on When to Start Antiretroviral Therapy and on Pre-Exposure Prophylaxisor HIV. Geneva: World Health Organization; 2015. [PubMed] [Google Scholar]
- 10. Brennan A, Browne JP, Horgan M. A systematic review of health service interventions to improve linkage with or retention in HIV care. AIDS Care 2014; 26:804–12. [DOI] [PubMed] [Google Scholar]
- 11. MacKellar DA, Williams D, Storer N, et al. Enrollment in HIV care two years after HIV diagnosis in the Kingdom of Swaziland: an evaluation of a National program of new linkage procedures. PLoS One 2016; 11:e0150086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. University of New Mexico. PROJECT ECHO. Albuquerque: University of New Mexico; 2017. https://echo.unm.edu/about-echo/. Accessed 22 August 2017. [Google Scholar]
- 13. World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. Geneva: World Health Organization; 2016. [PubMed] [Google Scholar]
- 14. World Health Organization. Health for the World’s Adolescents. A Second Chance in the Second Decade. Geneva: World Health Organization; 2014. http://apps.who.int/adolescent/seconddecade/files/1612_MNCAH_HWA_Executive_Summary.pdf. Accessed 22 August 2017. [Google Scholar]
- 15. UNICEF. UNICEF Data: Monitoring the Situation of Children and Women. Geneva: UNICEF; 2017. http://data.unicef.org/topic/hivaids/global-regional-trends/. Accessed 22 August 2017. [Google Scholar]
- 16. Tuli Nakanyala ADM, Mutenda N, Banda KM, et al. Namibia pilots sentinel population surveillance of HIV incidence and viral supression. Paper presented at: Conference on Retroviruses and Opportunistic Infections; July 13–16, 2017; Seattle, WA. [Google Scholar]
- 17. Chanda-Kapata P, Klinkenberg E, Maddox N, et al. The prevalence and socio-economic determinants of HIV among teenagers aged 15-18 years who were participating in a mobile testing population based survey in 2013-2014 in Zambia. BMC Public Health 2016; 16:789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Asaolu IO, Gunn JK, Center KE, et al. Predictors of HIV testing among youth in Sub-Saharan Africa: a cross-sectional study. PLoS One 2016; 11:e0164052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Balaji AB, Oraka E, Fasula AM, et al. Association between parent-adolescent communication about sex-related topics and HIV testing, United States. 2006-2013. AIDS Care 2017; 29:344–9. [DOI] [PubMed] [Google Scholar]
- 20. Reif LK, Rivera V, Louis B, et al. Community-based HIV and health testing for high-risk adolescents and youth. AIDS Patient Care STDS 2016; 30:371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sharma M, Ying R, Tarr G, Barnabas R. Systematic review and meta-analysis of community and facility-based HIV testing to address linkage to care gaps in sub-Saharan Africa. Nature 2015; 528:S77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Rooyen H, Essack Z, Rochat T, et al. Taking HIV testing to families: designing a family-based intervention to facilitate HIV testing, disclosure, and intergenerational communication. Front Public Health 2016; 4:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. World Health Organization. HIV and Adolescents: Guidance for HIV Testing and Counselling and Care for Adolescents Living With HIV: Recommendations for a Public Health Approach and Considerations for Policy-Makers and Managers. Geneva: World Health Organization; 2013. [PubMed] [Google Scholar]
- 24. Ayiga N, Nambooze H, Nalugo S, et al. The impact of HIV/AIDS stigma on HIV counseling and testing in a high HIV prevalence population in Uganda. Afr Health Sci 2013; 13:278–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. De Schacht C, Hoffman HJ, Mabunda N, et al. High rates of HIV seroconversion in pregnant women and low reported levels of HIV testing among male partners in Southern Mozambique: results from a mixed methods study. PLoS One 2014; 9:e115014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Johnson LF, Rehle TM, Jooste S, Bekker LG. Rates of HIV testing and diagnosis in South Africa: successes and challenges. AIDS 2015; 29:1401–9. [DOI] [PubMed] [Google Scholar]
- 27. Krakowiak D, Kinuthia J, Osoti AO, et al. Home-based HIV testing among pregnant couples increases partner testing and identification of serodiscordant partnerships. J Acquir Immune Defic Syndr 2016; 72(Suppl 2):S167–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Labhardt ND, Motlomelo M, Cerutti B, et al. Home-based versus mobile clinic HIV testing and counseling in rural Lesotho: a cluster-randomized trial. PLoS Med 2014; 11:e1001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Orne-Gliemann J, Balestre E, Tchendjou P, et al. ; Prenahtest ANRS 12127 Study Group Increasing HIV testing among male partners. AIDS 2013; 27:1167–77. [DOI] [PubMed] [Google Scholar]
- 30. Osoti AO, John-Stewart G, Kiarie JN, et al. Home-based HIV testing for men preferred over clinic-based testing by pregnant women and their male partners, a nested cross-sectional study. BMC Infect Dis 2015; 15:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Boerma RS, Boender TS, Bussink AP, et al. Suboptimal viral suppression rates among HIV-infected children in low-and middle-income countries: a meta-analysis. Clin Infect Dis 2016; 63:1645–54. [DOI] [PubMed] [Google Scholar]
- 32. Gaolathe T, Wirth KE, Holme MP, et al. ; Botswana Combination Prevention Project study team Botswana’s progress toward achieving the 2020 UNAIDS 90-90-90 antiretroviral therapy and virological suppression goals: a population-based survey. Lancet HIV 2016; 3:e221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bernheimer JM, Patten G, Makeleni T, et al. Paediatric HIV treatment failure: a silent epidemic. J Int AIDS Soc 2015; 18:20090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bienczak A, Denti P, Cook A, et al. Plasma efavirenz exposure, sex, and age predict virological response in HIV-infected African children. J Acquir Immune Defic Syndr 2016; 73:161–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schlatter AF, Deathe AR, Vreeman RC. The need for pediatric formulations to treat children with HIV. AIDS Res Treat 2016; 2016:1654938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dow DE, Shayo AM, Cunningham CK, Reddy EA. Durability of antiretroviral therapy and predictors of virologic failure among perinatally HIV-infected children in Tanzania: a four-year follow-up. BMC Infect Dis 2014; 14:567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fatti G, Shaikh N, Eley B, Grimwood A. Improved virological suppression in children on antiretroviral treatment receiving community-based adherence support: a multicentre cohort study from South Africa. AIDS Care 2014; 26:448–53. [DOI] [PubMed] [Google Scholar]
- 38. Beima-Sofie KM, Brandt L, Hamunime N, et al. Pediatric HIV disclosure intervention improves knowledge and clinical outcomes in HIV-infected children in Namibia. J Acquir Immune Defic Syndr 2017; 75:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kimelman F, Stopka T, Mutenda N, et al. Early warning indicators of HIV drug resistance among antiretroviral therapy sites in Namibia: a geospatial approach to population-based surveillance. Paper presented at: 9th International AIDS Conference; July 23–26, 2017; Paris, France. [Google Scholar]
- 40. Mutenda N, Taffa N, Baughman D, et al. Agolory outcomes of routine viral load monitoring in Namibia’s national antiretroviral treatment program. Paper presented at: 9th International AIDS Conference; July 23–26, 2017; Paris, France. [Google Scholar]