Abstract
Introduction: Gallic acid (GA) and curcumin (Cur) are natural phenolic compounds that their anti-tumor effects on many types of cancers have been proved. In the current study, the effect of the combination of these agents on MDA-MB-231 breast cancer cells was investigated.
Methods: Inhibition of cell proliferation (MTT assay), light microscopy, fluorescence microscopy, cell cycle analysis, nitrite detection, ROS levels, measurement of the mitochondrial membrane potential, GSH level, Annexin V assay, RT-PCR and Western blotting methods were applied.
Results: The results revealed the combination of GA and Cur strongly decreased MDA-MB-231 cell growth. Moreover, this combination increased ROS level and cytotoxic activity along with the glutathione depletion in MDA-MB-231 cells. Flow cytometry analysis showed the combination of GA and Cur increased sub-G1 cell population. Furthermore, fluorescent staining and Annexin V/PI assay showed that apoptotic cells were significantly increased in the presence of GA and Cur. At last, protein expression evaluation showed that the combination of GA and Cur significantly decreased Bcl-2 level while increased Bax, cleaved-caspase3 and PARP levels in MDA-MB-231 cells.
Conclusion: These results suggest that GA in combination with Cur could be a possible candidate for chemoprevention agent of triple negative breast cancer.
Keywords: Apoptosis, Breast cancer, Curcumin, Gallic acid, Mitochondria, ROS
Introduction
Breast cancer is the most prevalent type of cancer in women worldwide. Despite the impressive advances in therapeutic and diagnostic methods in oncology, breast cancer supposedly accounted for 29% of all new cancer cases among women in 2012. Resistance to drugs and metastasis in this type of cancer still seems to create main problems in spite of the immense progress that have been made in surgery, radio and targeted therapy. Therefore, the need for more effective therapies for breast cancer remains as a great demand.1 Nowadays many successful anti-cancer drugs are natural products or their analogs, and much more are under the clinical trials.2
Gallic acid (GA; 3, 4, 5- trihydroxy-benzoic acid) as a poly hydroxyl phenolic compound is broadly distributed in different kinds of plants, fruits and foods either in free form or more frequently, as an ingredient of tannins.3 Different biological activities of GA have been reported so far, including antibacterial, antiviral and anti-inflammatory effects, however, the main interest in GA is related to its anti-tumor activity and in a variety of cancer cells the anti-cancer activity of GA has been observed.4-6 Another natural product is named curcumin (Cur; diferuloylmethane), a biphenyl compound, which is extracted from the rhizome (Curcuma longa). This compound contains strong anti-proliferative, anti-inflammatory, anti-cancer and antioxidant effects and these features make it as a promising therapeutic agent.2,7 There are several publications concerning the Cur application in modern medicine.2,8 Both GA and Cur have been separately shown to exert anti-cancer effects through induction of apoptosis.2,4 However, previous studies have demonstrated that combination of Cur with the other anticancer compounds has a more effective anti-cancer activity compared with the pure Cur treatment.9 For instance, it was demonstrated that natural compounds such as resveratrol or diallyl disulfide can enhance the rate of Cur-induced apoptosis in the sarcoma cells.10 Moreover, another study has shown that in vitro and in vivo anti-tumoral effects of Cur in head and neck carcinomas could be improved by resveratrol.11 There is no report about the combined effects of these two natural agents together on cancer. Therefore, we tried to plan an effective protocol against cancer cells proliferation with a combination of Cur and GA.
One vital evolutionarily conserved mechanism for the development and maintenance of tissue homeostasis in multicellular organisms is apoptosis, or programmed cell death, which is disturbed in cancer cells.12 Apoptosis can lead to some changes in cell characteristics. These changes are activation of caspases, mitochondrial depolarization, cell volume loss, and DNA fragmentation. Proteins that function either to promote or inhibit apoptosis are encoded by the members of the Bcl-2 gene family. Anti-apoptotic members such as Bcl-2 and Bcl-xL inhibit apoptosis in response to a broad range of stimuli. On the other hand, pro-apoptotic proteins such as Bax can promote cell death and in some cases are sufficient to cause apoptosis independent of additional signals. A variety of biochemical changes has been suggested to be the main cascade events that result in the apoptosis process.13 Production of reactive oxygen species (ROS) and loss of mitochondrial membrane potential (MMP) are included in the events. The mitochondrial malfunction can lead to these biochemical fluctuations.14 Here, we attempted to explore the apoptotic effects of GA in combination with Cur in MDA-MB-231 breast cancer cells. Furthermore, we have investigated the mechanism of action of such a natural combination structure in this cellular system.
Materials and Methods
Materials
GA was purchased from Merck Co. (Germany). Cur was supplied from Serva Co. (USA). The following chemicals were bought from Sigma-chemical Co. (St. Louis, USA); 2,7- dichlorodihydrofluorescein diacetate (DCFD), rhodamin123, 5-5-dithiobis (2-nitrobenzoic acid) (DTNB), 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide) (MTT), Acridine Orange (AO), Ethidium Bromide (EB), sulfanilamide, N-1- naphthyl ethylenediamine dihydrochloride (NED) and RNase A.
The antibodies were obtained from Cell Signaling Co. (UK). RPMI 1640 media, Fetal Bovine Serum (FBS) and Trypsin/EDTA were purchased from Gibco Co. (Grand Island, USA). The Polyvinylidene difluoride (PVDF) membrane and ECL kit were from Amersham Bioscience (Arlington Heights, USA). Annexin V-Fluos staining kit was purchased from Roche Life Science (Penzberg, Germany). TRIzol reagent and PCR kit were provided from Invitrogen Corp. (Carlsbad USA).
Cell culture
The MDA-MB-231 cells and HUVECs were procured from National Cell Bank of Iran (NCBI). The cells were cultured in RPMI 1640 medium containing 10% FBS, in a 5 % CO2 humidified incubator at 37οC.15
Cell growth assay
Anti-proliferative effects of GA and Cur on the breast cancer cells and HUVECs were conducted with MTT assay. The cells grown in 96-well plates were treated with adequate concentrations of GA or Cur for 24 and 48 hours. These agents were dissolved in DMSO at 100mM as the stock solution and DMSO (solvent) was used as the control. After periods of 24 and 48h, the MTT (5 mg/mL in PBS) was added to each well and then incubated at 37°C for 3 hours. The insoluble formazan crystals were dissolved in DMSO and the absorbance values were measured at 490 nm with a Microplate Reader (BioTek ELX 800, UK). The results were expressed as the percentage of cell growth relative to the control.16 The cell morphology was also photographed under light microscopy (CETI, Belgium). In the combination mode, the effective doses of the drugs prepared and simultaneously treated on the cells.
Acridine orange and ethidium bromide double staining
The cells treated with the combination of GA (50 μM) and Cur (30 μM) were stained with a mixture of acridine orange/ethidium bromide (AO/EB,100 μg/mL) at room temperature for 5 minutes. The stained cells were observed by a fluorescence microscope (Zeiss, Germany) at 100X magnification. In each experiment, more than 300 cells/ samples were counted.17
Nitrite (NO2-) detection
For the assessment of the amount of NO production, the Griess reagent (sulfanilamide and NED) under acidic conditions was employed. Under this condition, the accumulated nitrite (NO2-), which is a stable breakdown product of NO can be recorded. The conditioned medium aliquots were mixed with equal volumes of the Griess reagent and incubated at room temperature for 15 minutes. The amounts of the diazo production were read with a spectrophotometer (BioTek ELX 800, UK) at 490 nm, and sodium nitrite was used as a standard.18
Detection of intracellular reactive oxygen species levels
Intracellular ROS was measured using an oxidation-sensitive fluorescent probe dye; 2, 7-dichlorodihydrofluorescein diacetate (DCFD). Concisely, the treated cells were washed in phosphate buffer saline (PBS) and incubated with 20 μM DCFD in PBS for 30 minutes at 37◦C. The samples were analyzed for forward- and side-scattering properties, as well as green and red fluorescence intensity using the FACScan flow cytometer (BD FACS Calibur, BD Biosciences, San Jose, USA).19
Cell cycle analysis
The cells were fixed in cold 70% ethanol and washed at least once with cold PBS. Finally, the cells resuspend in PI staining solution containing DNAse-free RNAse A in PBS and incubate at 37°C for 30 minutes. The resulting DNA distribution was detected by FlowJo software for the proportions of cells in the G1, S, and G2 phases of the cycle.20
Annexin V assay
The treated cells were stained with an annexin FITC/ PI staining kit according to the manufacturer. In brief, the cells were washed with PBS and resuspended in a mixture of binding buffer, PI, and Annexin V-FITC. The samples were incubated 15 min in the dark at room temperature (RT) and then were tested for Annexin V binding using flow cytometry.21
Measurement of mitochondrial membrane potential
MMP was estimated by applying a fluorescent dye, rhodamine 123 (Ex/Em = 485 nm/535 nm). The cells were detached and resuspended in PBS. Next, 1mg/ml rhodamine 123 was added and the samples were incubated for 10 minutes at 25οC. The cells were then washed twice with PBS and the stained cells were observed with a fluorescence microscope (Zeiss, Germany) at 200 X magnifications.
The amount of MMP loss was measured using the BD FACS Calibur flow cytometer. In brief, the cells were washed twice with PBS and incubated with rhodamine 123 (0.1 μg/mL) at 37οC for 30 min. The absence of rhodamine 123 from cells represented the loss of MMP in the cells which was analyzed with flow cytometer.22-23
Measurement of glutathione levels
The basis of glutathione assay is oxidation of GSH by the sulfhydryl reagent 5,5'-dithio-bis(2-nitrobenzoic acid) (DTNB), following the production of the yellow derivative 5'-thio-2-nitrobenzoic acid (TNB) which can be measured at 412 nm. At first, the cell lysate was prepared and the glutathione level was determined with DTNB reagent and a spectrophotometer set at 412 nm.24
Reverse transcription-PCR
RNA isolation was performed with the TRIzol reagent. RT-PCR preparation was done according to the kit manufacturer instructions and gene-specific forward and reverse primers (Table 1). The PCR product was electrophoresed on 1.7% agarose gel containing 1% ethidium bromide dye and the bands visualized under UV light. Normalization of PCR products was performed to β-actin expression and all PCR reactions were carried out at least three times.25
Table 1. Sequence of primers for RT- PCR .
| Gene Name | Forward | Reverse |
| Bax | 5′-TGG CAG CTG ACA TGT TTT CTG AC-3′ | 5′-TCA CCC AAC CAC CCT GGT CTT-3′ |
| Bcl-2 | 5′-CTG GTG AAC ATC GC-3′ | 5′-GGA GAA ATC AAA CAG AGG C-3′ |
| β-actin | 5′-ATC TGG CAC CAC ACC TTC TA-3′ | 5′-CGT CAT ACT CCT GCT TGC TG-3′ |
Western blot analysis
Following a period of 48 hours, the cells were lysed with the lysis buffer and the total protein concentration was measured by Bradford assay.27 The lysate was electrophoresed in 12.5% SDS-page gel and the separated proteins were transferred onto the PVDF membrane using a semi-dry transfer technique. Following the blocking stage with 3% skimmed milk in TBST buffer, the membrane was treated in the presence of first and secondary antibodies in 3% skimmed milk in TBST. Immunoreactive bands were observed using the ECL chemiluminescence detection kit and subsequent autoradiography. The quantification of the developed blot was done with densitometry using ImageJ software. β-actin was applied as a loading control in all western blotting analyses.27
Data analysis
All the experiments were done in triplicated and the quantitative data are represented as mean ± SEM. Comparison between the groups was made by one-way analysis of variance (ANOVA) followed by a specific post hoc test to analyze the difference. The statistical significances were achieved when P<0.05.
Results
Combination of GA and Cur decreased cell growth in MDA-MB-231 cells
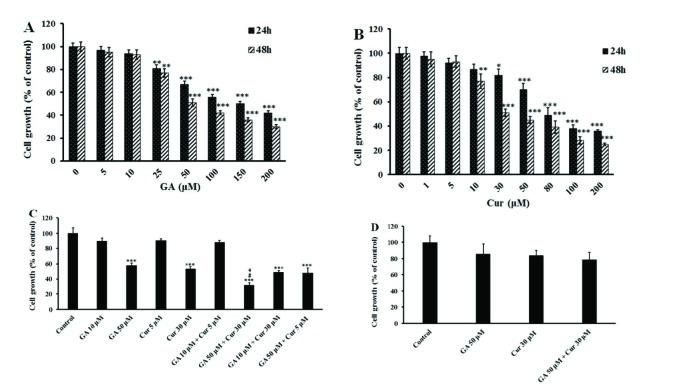
We applied the MTT colorimetric assay to investigate the effect of GA and Cur on the growth of the MDA-MB-231 breast cancer cell line. In order to assess the cell viability, the cells incubated with different concentrations of either GA or Cur alone for 24 and 48 hours. The results showed that GA and Cur alone inhibited cell growth dose-dependently (Figs. 1A and 1B). The IC50 of GA was approximately 150 μM in 24 hours and 50 μM in 48 hours, and that of Cur in 24 hours and 48 hours was approximately 80 μM and 30 μM, respectively. Therefore, for the next examinations, 48 hours of treatment was chosen. Consequently, the non-effective dose of GA (10 µM) and its effective dose (50 µM) were chosen. For Cur, the none-effective and effective doses were 5 μM and 30 μM, respectively. The results showed that only the combination of effective doses of GA (50 μM) and Cur (30 μM) could decrease cell growth significantly compared to either GA (50 μM) or Cur (30 μM) alone. Indeed, in this group cell growth decreased to 64% ± 3.2, 23% ± 2.25 and 21% ± 3.45 compared to the control (DMSO1% + media), GA (50 μM) and Cur (30 μM) groups, respectively (Fig. 1C). Interestingly, the combination of effective doses of GA and Cur had no significant effect on the growth of HUVEC normal cells (Fig. 1D). Therefore, we have employed the combination of GA (50 μM) and Cur (30 μM) for the rest of the study.
Fig. 1.
Effects of GA and Cur on the growth of MDA-MB-231 and HUVEC cell lines. Cell growth was assessed by MTT assay. The results are expressed as mean±SEM (n=3). (A) GA (1, 5, 10, 25, 50, 100 and 200 μM). B) Cur (1, 5, 10, 30, 50, 80, 100 and 200 μM) at 24 and 48 h. C) The combination of GA (10 and 50 μM) and Cur (5 and 30 μM) at 48 h. *** P<0.001, ** P<0.01 and * P<0.05 compared to the control, # P<0.05 compared to GA (5 0μM), + P<0.05 compared to Cur (30 μM). D) The combination of GA (50 μM) and Cur (30 μM) on the growth of HUVEC normal cells at 48 h.
AO/EB double staining cell analysis
In AO/EB double staining the viable cells with normal DNA show green nuclei, while the apoptotic and necrotic cells with fragmented DNA demonstrate orange and red nuclei. Our results showed that the combination of GA (50 μM) with Cur (30 μM) increased the number of apoptotic cells significantly in comparison with those treated with GA (50 μM) or Cur (30 μM) alone (Fig. 2B). It is worthy to note that the number of necrotic cells did not increase in the combination group (Fig. 2C).
Fig. 2.
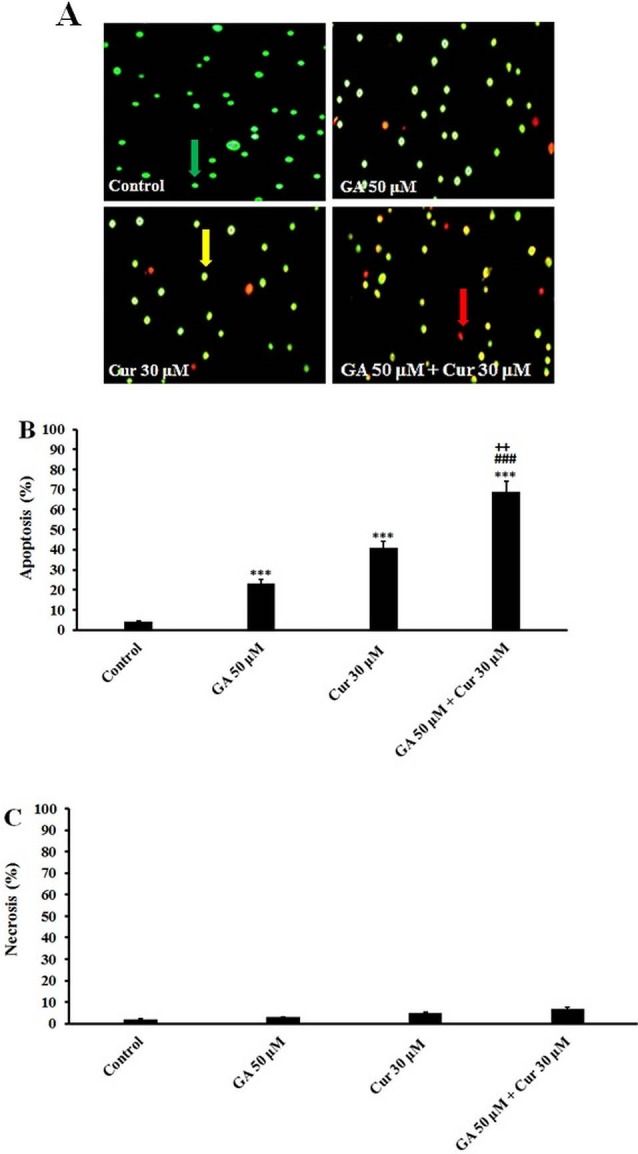
Acridine Orange/Ethidium Bromide Staining. A) Morphological changes in MDA-MB-231 cells after 48 h treatment with GA (50 μM) and Cur (30 μM). The cells were stained with Acridine orange and Ethidium bromide. Green cells indicate live cells; orange cells indicate apoptotic cells, and red cells indicate necrotic cells (×200). B) Apoptosis (%). C) Necrosis (%). The results are expressed as mean ± SEM (n=3), *** P<0.001 compared to control, ### P<0.001 compared to GA (50 μM), ++ P<0.01 compared to Cur (30 μM). The live, apoptotic, and necrotic cells were indicated by green, orange, and red arrows, respectively.
Combination of GA and Cur increased NO2- production in MDM-MB-231 cells
The MDA-MB-231 cells were treated with GA (50 μM), Cur (30 μM) and the combination of both for 48 hours. Our observation showed that in the presence of GA (50 μM) and Cur (30 μM), nitrite (NO2-) production increased. The combination of GA (50 μM) and Cur (30 μM) increased the nitrite (NO2-) production in a significant manner compared with the application of the aforementioned compounds alone (1.7±0.25, fold compared to the control, 1.40±0.2 fold compared to (GA 50 μM) and 1.3± 0.2 fold compared to Cur (30 μM)) (Fig. 3A).
Fig. 3.
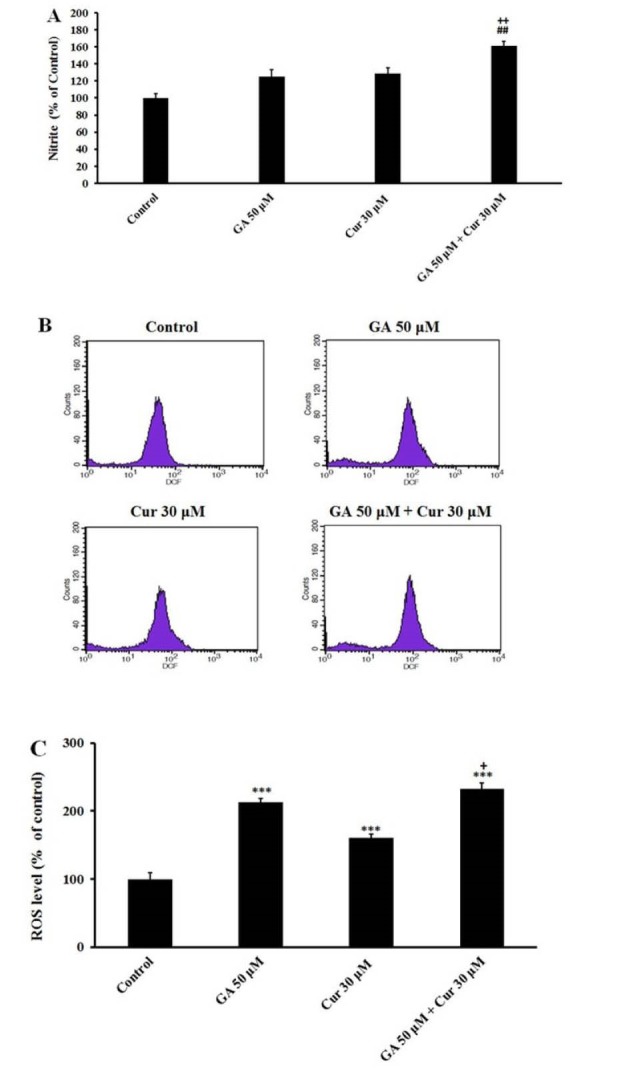
Effect of GA (50 μM) and Cur (30μM). A) On NO production in MDA-MB-231 cell line after 48 h treatment. B) ROS levels in breast cancer cells. Exponentially growing cells were treated with the indicated concentrations of GA and Cur for 48h incubation (The histogram was obtained from flow cytometry analysis). C) Graphs indicate ROS percentage in MDA-MB-231 cells. The results are expressed as mean±SEM (N=3), *** P<0.001, compared to the control. ## P<0.01 compared to GA (50 μM), + P<0.05 compared to Cur (30μM).
Combination of GA and Cur increased intracellular ROS level in MDA-MB-231 cells
Flow cytometry analysis showed that treatment of cells with GA and Cur caused an increase in the intracellular ROS levels (Fig. 3B). Also, it was demonstrated that the effect of GA on ROS level is more than that of Cur (Fig. 3C). The combination of GA and Cur enhanced ROS level in a significant way compared to the control group (2.4±0.3 fold change). Moreover, in the combination group, ROS level increased significantly relative to the Cur group (1.6±0.3 fold change) (Fig. 3C).
Combination of GA and Cur increased the population of sub-G1 phase in MDA-MB-231 cells
Flow cytometry analysis was carried out to demonstrate the distribution of the cell cycle. As shown in Fig. 4A in either GA or Cur groups a sub-G1 phase (apoptotic cells) appeared in the cell cycle distribution. However, the combination of GA (50 μM) and Cur (30 μM) caused a significant increase in the sub-G1 population compared to each compound used alone.
Fig. 4.
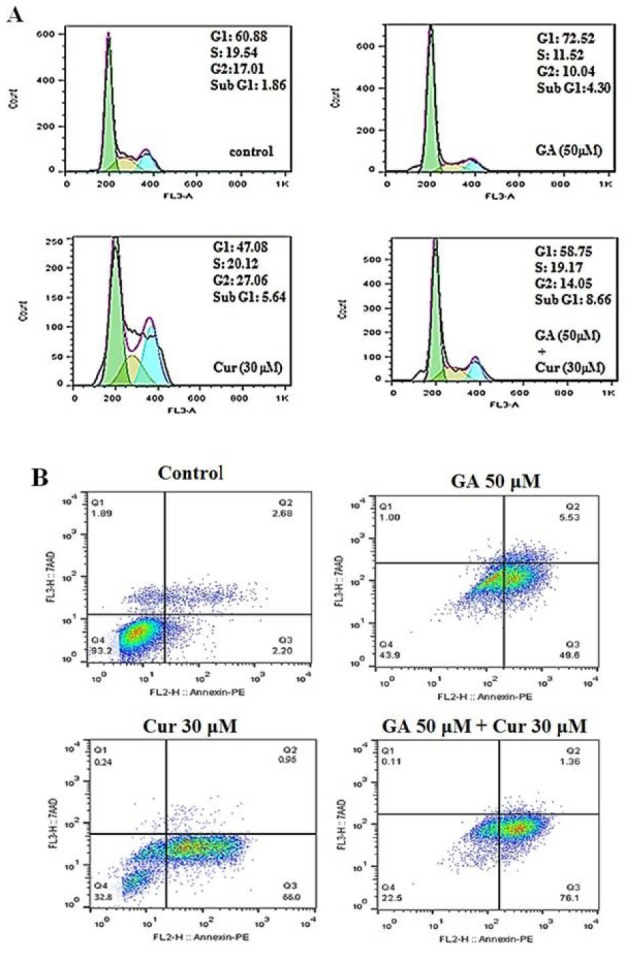
Effect of GA (50 μM) and Cur (30 μM) on cell cycle and apoptosis of MDA-MB-231 cells. A) The histograms show the effect of GA and Cur on the cell cycle with flow cytometry analysis. B) Flow cytometry analysis of Annexin-PI staining. Q1 shows necrotic cells, Q2 shows late apoptotic cells, Q3 shows early apoptotic cells, and Q4 shows the viable cells.
Combination of GA and Cur promoted apoptosis in MDA-MB-231cells
We employed the flow cytometric analysis of Annexin V-FITC and PI double staining was employed to study the effect of GA and Cur on the apoptosis of breast cancer cells. The results showed, when given as single agents, GA or Cur could induce apoptosis in a low percentage. However, as shown in Fig. 4B. Combination of GA (50 μM) and Cur (30 μM) increased the percentage of apoptotic cells compared with the other groups (16.4±0.6 fold compared to the control, 1.5±0.3 fold compared to GA [50 μM] and 1.3±0.25 fold compared to Cur [30 μM]).
Combination of GA and Cur decreased MMP in MDA-MB-231 cells
We assessed the effect of the combination of GA and Cur on MMP, as an important mitochondrial parameter that controls the cellular processes. Mitochondrial function induces quenching of rhodamine 123 fluorescence and the rate of fluorescence decay is proportional to the MMP and the absence of rhodamine 123 lights from the cells represents the loss of MMP in the mitochondria. The data demonstrated that in the combination mode of GA and Cur (GA/Cur) a severe decline in MMP in comparison with GA or Cur alone is seen (Fig. 5).
Fig. 5.
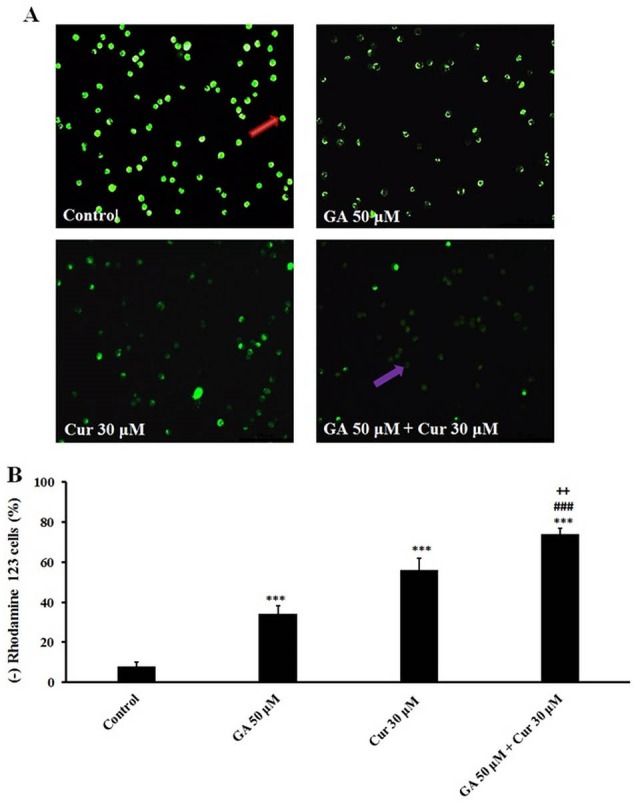
Effect of GA (50 μM) and Cur (30 μM) on MMP in MDA-MB-231 cells after 48 h. A) MMP loss in cells was observed with a fluorescent microscope. Bright cells indicate the cells with normal MMP, B) Graphs indicate the percent of MMP loss in the treated cells. The results are expressed as mean±SEM (N=3), *** P<0.001, compared to control, ### P<0.001compared to GA (50 μM), and ++ P<0.01 compared to Cur (30 μM). The cells with normal and dysfunctional mitochondria cells were distinguished by red and purple arrows, respectively.
GA/Cur decreased intracellular GSH level in breast cancer cells
The combination of GA (50 μM) and Cur (30 μM) resulted in a dramatic decrease of GSH level in comparison with GA or Cur alone. GSH levels in GA/Cur group decreased to 45%±2.5, 27%±3.5 and 25%±1.7 compared to control, GA (50 μM) and Cur (30 μM) groups, respectively (Table 2).
Table 2. The results of GSH levels in the treated MDA-MB-231 cells (mean±SEM) .
| Treatment | GSH levels (absorbance at 412 nm) |
| Control | 0.52 ± 0.14 |
| GA (50 μM) | 0.39 ± 0.15** |
| Cur (30 μM) | 0.38 ± 0.05** |
| GA (50 μM) + Cur (30 μM) | 0.29 ± 0.12***#+ |
*** P<0.001 and ** P<0.01 compared to control group, # P<0.05 compared to GA (50 μM), + P<0.05 compared to Cur (30 μM). (n= 3)
GA/Cur changed apoptotic gene expression
RT-PCR analysis showed that GA/Cur significantly augmented Bax/Bcl-2 gene expression ratio in comparison with either GA or Cur alone (3.1±0.3 fold compared to control, 1.9±.2 fold compared to GA (50 μM) and 1.3±0.4 fold compared to Cur (30 μM)) (Figs. 6A and 6B). It was also detected that caspase 3 gene expression did not change in the combination group in comparison with each drug alone, while it was enhanced significantly as compared to the control group (Figs. 6A and 6C).
Fig. 6.
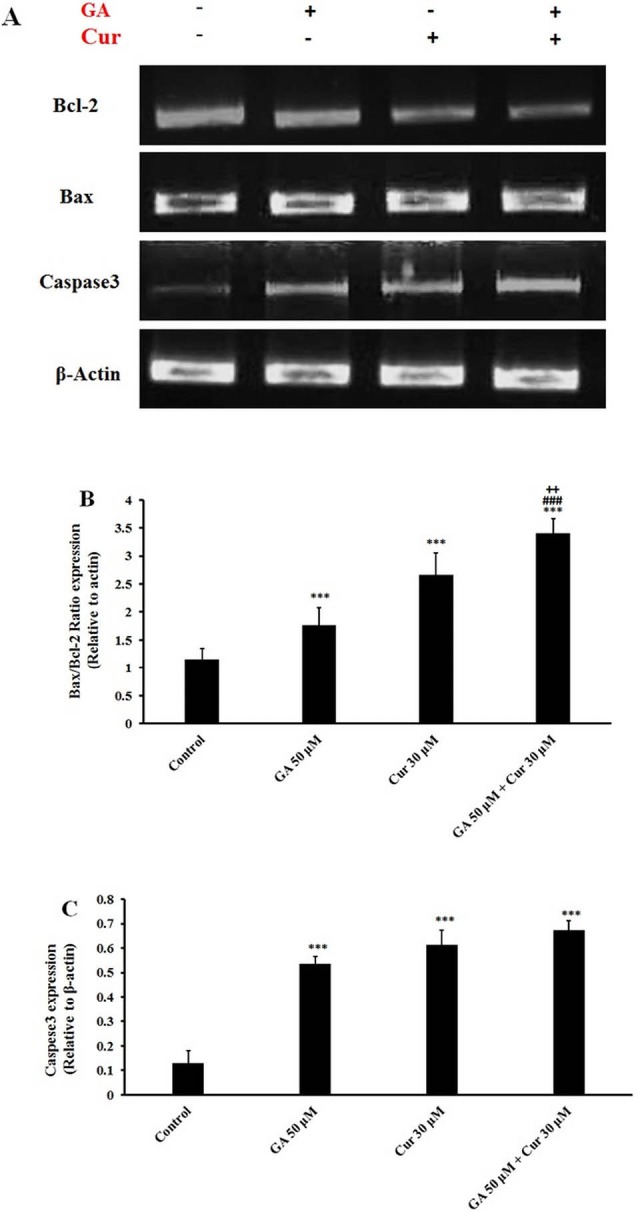
Gene expression analysis of apoptotic cells. A) RT-PCR gene expression of caspase-3, Bax, and Bcl-2. B) Bax/Bcl-2 ratio relative to β-actin. C) Caspese3 expression relative to β-actin. The results are expressed as mean±SEM (N=3), *** p<0.001 compared to control, ### p<0.001 compared to GA (50 μM) and + P<0.05 compared to Cur (30 μM) group.
GA/Cur increased expression of apoptotic proteins
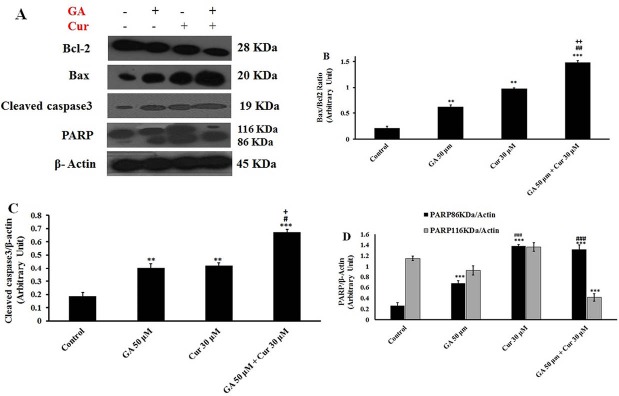
Western blotting analysis showed that GA or Cur alone could increase Bax/Bcl-2 ratio. The combination of these compounds significantly increased Bax/Bcl-2 ratio in comparison with either GA or Cur alone (7.1±0.18 fold compared to the control, 2.3±0.25 fold compared to GA (50 μM) and 1.6±0.15 fold compared to Cur (30 μM)) (Figs. 7A and 7B). Furthermore, GA/Cur led to an enhanced level of cleaved-caspase 3 (3.7±0.2 fold compared to the control, 1.6±0.15 fold compared to GA (50 μM) and 1.6±0.3 fold compared to Cur (30 μM) groups) (Figs. 7A and 7C). Moreover, the cleaved-PARP level in the combination group was increased in comparison with GA (50 μM) group. However, it showed no significant change compared to Cur group (Figs. 7A and 7D).
Fig. 7.
Effect of GA (50 μM) and Cur (30 μM) on cell cycle and apoptosis of MDA-MB-231 cells. A) The histograms show the effect of GA and Cur on the cell cycle with flow cytometry analysis. B) Flow cytometry analysis of Annexin-PI staining. Q1 shows necrotic cells, Q2 shows late apoptotic cells, Q3 shows early apoptotic cells, and Q4 shows the viable cells.
Discussion
The use of a combination of drugs may target multiple objectives in a disease simultaneously. The use of multiple drugs with different mechanisms or modes of action may also augment their effects and treat the disease more effectively. In the case of cancer, it is believed that combinations of different drugs may also overcome or delay the development of drug resistance. There are several natural products that known as anti‐cancer agents such as GA and Cur which are derived from the fruits and C. longa, respectively.28,29 Both of these phenolic compounds are vastly used in traditional medicine to treat various diseases.
The anti-cancer efficacy of natural polyphenols has been depended on their antioxidant potency and anti-inflammatory activities, which they can be associated with cell survival, proliferation, and differentiation.9 Previous studies have shown that GA and Cur separately possess antitumor effects on several types of human cancers.3,30 and various groups have worked to understand their mechanism of action.2,23 Numerous studies have been shown that a combination of polyphenols would lead to improving cancer treatment compared to the therapy with just a single polyphenol.9 As a case in point, it is demonstrated that combination of Cur and the green tea polyphenol named Epigallocatechin gallate (EGCG), strongly repress the growth of breast cancer cells both in vitro and in vivo. In fact, EGCG plus Cur caused a synergic cytotoxic effect on the human breast cancer cell line MDA-MB-231, which was associated with the arrest of G2/M-phase in the cell cycle. Interestingly, this combination allowed for the decreased concentration of Cur for tumor suppression purpose.31 In the other study on neuroblastoma cell lines, the combination of Resveratrol and Cur resulted in the depletion of cell proliferation, cell cycle arrest and apoptosis in vitro.32
In the present study, the combination of 2 natural agents, GA and Cur, was under the investigation. The present data showed that this phenolic combination significantly could inhibit the cell growth and induce apoptosis in the human breast cancer MDA-MB-231 cells as compared to the dose-effect each drug alone. The MDA-MB-231 cell is a triple-negative breast cancer (TNBC) cell line that does not have the ability to express estrogen receptor, progesterone receptor, and HER2 protein.33 Therefore, therapies for TNBC are scarce and the additional therapeutic approaches are required.33,34 Since the MDA-MB-231 cell is hardly treated with chemotherapy, we focused on exploring the anti-cancer effects of GA and Cur combination on these cells. Former studies have shown that treatment of cancer cells with GA or Cur can decrease their growth.28,29 Our results demonstrated that the combination of non-effective doses of GA and Cur and also the combination of effective and non-effective doses had no significant effects on the growth of MDA-MB-231 cells. However, the combination of effective doses of GA and Cur enhanced synergistically cell growth inhibition, as the CampuSyn analysis (data not shown) verified increasing the efficacy of the drug’s effects. On the other hand, the combination of effective doses of these compounds caused no significant effect on the growth of the HUVEC normal cells.
It is now proven that most cytotoxic anticancer agents induce apoptosis. Expressions of Bax and Bcl-2 can mediate apoptosis by controlling the mitochondrial permeability and the release of cytochrome C. Unlike the anti-apoptotic properties of Bcl-2, Bax has pro-apoptotic activities. The ratio of Bax/Bcl-2 appears to be a critical determinant of the cell threshold to undergo apoptosis.13 Gene expression analysis showed that combination of GA and Cur, up-regulated the expression of Bax and caspase 3 genes and down-regulated the expression of Bcl-2 gene. It seems that the GA/Cur combination upregulate pro-apoptotic protein Bax and downregulate the anti-apoptotic protein Bcl-2. This demonstrates that GA in combination with Cur promoted apoptosis of MDA-MB-231 cells. It has been shown previously that GA and Cur induce apoptosis in cancer cells via caspase 3 activation and PARP cleavage.7,23 In accordance with this, our data also demonstrated that the GA/Cur combination induces caspase 3 and PARP cleavage, and can increase the number of apoptotic cells much stronger than the drug was used alone.
The role of GSH content of cancer cells is prevalent in regulating of the mutagenic mechanisms, DNA synthesis, multidrug and radiation resistance. In malignant tumors, as compared with normal tissues, the resistance is connected in most cases with higher GSH levels within the cancer cells. Thus, GSH modulation-based methods for cancer treatment should control possible growth-associated changes in GSH content in these cells.35 A previous studies have revealed that GA acts as a pro-oxidant reagent in cancer cells, and also can deplete GSH content. GA caused a dose-dependent increase in the number of GSH-depleted cells in the lung cancer cells.23 The other studies reported that Cur induced apoptosis in the cancerous cells in a dose-dependent manner. This apoptosis was mediated through the generation of ROS and depletion of GSH.36,37 Our findings showed that the combination mode of GA and Cur decreased GSH levels than either when GA and Cur were used alone. Also, we found that the combination of these compounds increased ROS levels. It is reported that the increase of ROS (such as O2-, NO2- and H2O2) levels can lead to the mitochondrial dysfunction, and this process has a central role in the apoptosis initiation.35,37,38 As a matter of fact, the opening of the mitochondrial permeability transition pores induces changes of the membrane potential (Δψm) and release of apoptotic factors.39
Former studies indicated that GA or Cur can decrease MMP and increase ROS level in cancer cells.29,37 Consistent with this, our results demonstrated that the combination of GA and Cur decreased mitochondrial membrane potential via increased ROS level and apoptosis percentage. It seems that the loss of membrane potential resulted in the release of apoptotic factors from mitochondria and induction of apoptosis in MDA-MB-231 cells. Apoptosis, most of the times accompanies with augmentation in the sub-G1 population of the cell cycle. Previous studies confirmed that either GA or Cur separately could increase the population of sub-G1 phase in the cell cycle of different cancer cells.40,41 According to the previous study about successive G(1)/S and G(2)/M phase arrest followed by apoptosis in the presence of Cur and GA,42 our results also demonstrated that the combination of GA and Cur increased the number of cells in the sub-G1 phase of the cell cycle compared to each compound used separately. This phenolic combination may affect the expression of cyclins and cyclin dependent kinase (cdk) proteins and it seems that GA combined with Cur could induce apoptosis in breast cancer cells.
It is explicated the ATP-binding cassette (ABC) transporters are a family of transporter proteins responsible for drug resistance by pumping the drugs out of cells. The ABC transporters operate as an efflux pump for lipid, multiple drugs, natural products and peptides.42 Cancer cells express efflux pump due to the exposure to anti-cancer drugs which result in the resistance to the anti-cancer drugs.43 Previously it was explored that Cur reverses the drug resistance of cancer cells by modulating the activity of multi-drug efflux pumps.44 Finally, it seems that the combination of GA with Cur may potentially affect the function of ABC transporters in MDA-MB-231 breast cancer cell lines in comparison with Cur alone and the combination natural anti-cancer agents (as our model) might overcome multiple drug resistance in the cancer therapy.
Conclusion
The present study revealed that combining 2 phenolic natural agents GA and Cur enhanced their apoptotic properties synergistically. It was verified that GSH diminishing and ROS increasing followed by mitochondrial dysfunction are the main cause of apoptosis induction by the combination of GA and Cur. The ability of the combination of GA and Cur to stimulate apoptosis in TNBCs, which respond rarely to chemotherapeutic agents, points to the possibility of developing combination therapy as a potential universal cancer treatment.
Ethical approval
None to be declared.
Competing interests
There is no conflict of interests to be reported.
Acknowledgments
This study was supported by a research grant from the College of Sciences, University of Tehran (Iran). We acknowledge the contribution of Dr. Farnoosh Attari, Nazanin Namazi, Zahra Ghasemzadeh, and Kaveh Darabi.
Research Highlights
What is current knowledge?
√ Breast cancer is the most common cancer and the second cause of death in women.
√ Plants are reservoirs for novel chemical and provide a promising effect on cancer.
√ Gallic acid and curcumine, two naturally plant deriviteis contained anticancer effects in vitro.
What is new here?
√ Combination of curcumin and GA decreased the cell growth and promoted apoptosis more than the effective doses of each of these agents alone in breast cancer cells in vitro.
√ GA in combination with Cur could be a possible candidate for chemoprevention agent of triple negative breast cancer.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;62(1):10–29. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB. Curcumin and cancer: an “old-age” disease with an “age-old” solution. Cancer Lett. 2008;267(1):133–164. doi: 10.1016/j.canlet.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 3.Verma S, Singh A, Mishra A. Gallic acid: molecular rival of cancer. Environ Toxicol Pharmacol. 2013;35(3):473–485. doi: 10.1016/j.etap.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Tan S, Guan X, Grün C, Zhou Z, Schepers U, Nick Nick, P P. Gallic acid induces mitotic catastrophe and inhibits centrosomal clustering in HeLa cells. Toxicol In Vitro. 2015;30(1):506–513. doi: 10.1016/j.tiv.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Reddy TC, Reddy DB, Aparna A, Arunasree KM, Gupta G, Achari C. et al. Anti-leukemic effects of gallic acid on human leukemia K562 cells: Down regulation of COX-2, inhibition of BCR/ABL kinase and NF-κB inactivation. Toxicol In Vitro. 2012;26(3):396–405. doi: 10.1016/j.tiv.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 6.Yoshioka K, Kataoka T, Hayashi T, Hasegawa M, Ishi Y, Hibasami H. Induction of apoptosis by gallic acid in human stomach cancer KATO III and colon adenocarcinoma COLO 205 cell lines. Oncol Rep. 2000;7(6):1221–1224. doi: 10.3892/or.7.6.1221. [DOI] [PubMed] [Google Scholar]
- 7.Chen QY, Lu GH, Wu YQ, Zheng Y, Xu K, Wu LJ. et al. Curcumin induces mitochondria pathway mediated cell apoptosis in A549 lung adenocarcinoma cells. Oncol Rep. 2010;23(5):1285–12 92. doi: 10.3892/or_00000762. [DOI] [PubMed] [Google Scholar]
- 8.Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008;269(2):199–225. doi: 10.1016/j.canlet.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Fantini M, Benvenuto M, Masuelli L, Frajese GV, Tresoldi I, Modesti A. et al. In vitro and in vivo antitumoral effects of combinations of polyphenols, or polyphenols and anticancer drugs: perspectives on cancer treatment. Int J Mol Sci. 2015;16(5):9236–9282. doi: 10.3390/ijms16059236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masuelli L, Marzocchella L, Focaccetti C, Tresoldi I, Palumbo C, Izzi V. et al. Resveratrol and diallyl disulfide enhance curcumin-induced sarcoma cell apoptosis. Front Biosci. 2012;17:498–508. doi: 10.2741/3940. [DOI] [PubMed] [Google Scholar]
- 11.Masuelli L, Di Stefano E, Fantini M, Mattera R, Benvenuto M, Marzocchella L. et al. Resveratrol potentiates the in vitro and in vivo anti-tumoral effects of curcumin in head and neck carcinomas. Oncotarget. 2014;5(21):10745–10762. doi: 10.18632/oncotarget.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.George BP, Abrahamse H, Parimelazhagan T. Caspase dependent apoptotic activity of Rubus fairholmianus Gard on MCF-7 human breast cancer cell lines. J Appl Biomed. 2016;14(3):211–219. doi: 10.1016/j.jab.2016.02.001. [DOI] [Google Scholar]
- 13.Ouyang L, Shi Z, Zhao S, Wang F, Zhou T, Liu B. et al. Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012;45(6):487–498. doi: 10.1111/j.1365-2184.2012.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liou GY, Storz P. Reactive oxygen species in cancer. Free Radic Res. 2010;44(5):479–496. doi: 10.3109/10715761003667554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou DH, Wang X, Yang M, ShiX ShiX, Huang W, Feng Q. Combination of low concentration of (−)-epigallocatechin gallate (EGCG) and curcumin strongly suppresses the growth of non-small cell lung cancer in vitro and in vivo through causing cell cycle arrest. Int J Mol Sci. 2013;14(6):12023–12036. doi: 10.3390/ijms140612023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delphi L, Sepehri H. Apple pectin: A natural source for cancer suppression in 4T1 breast cancer cells in vitro and express p53 in mouse bearing 4T1 cancer tumors, in vivo. Biomed Pharmacother. 2016;84:637–644. doi: 10.1016/j.biopha.2016.09.080. [DOI] [PubMed] [Google Scholar]
- 17.Attari F, Sepehri H, Delphi L, Goliaei B. Apoptotic and necrotic effects of pectic acid on rat pituitary GH3/B6 tumor cells. Iran Biomed J. 2009;13(4):229–236. [PubMed] [Google Scholar]
- 18.Wang L, Wu B, Sun Y, Xu T, Zhang X, Zhou M. et al. Translocation of protein kinase C isoforms is involved in propofol-induced endothelial nitric oxide synthase activation. Br J Anaesth. 2010;104(5):606–612. doi: 10.1093/bja/aeq064. [DOI] [PubMed] [Google Scholar]
- 19.Moghtaderi H, Sepehri H, Attari F. Combination of arabinogalactan and curcumin induces apoptosis in breast cancer cells in vitro and inhibits tumor growth via overexpression of p53 level in vivo. Biomed Pharmacother. 2017;88:582–594. doi: 10.1016/j.biopha.2017.01.072. [DOI] [PubMed] [Google Scholar]
- 20.Berrak Ö, Akkoç Y, Arısan ED, Çoker-Gürkan A, Obakan- Yerlikaya P, Palavan-Ünsal N. The inhibition of PI3K and NFκB promoted curcumin-induced cell cycle arrest at G2/M via altering polyamine metabolism in Bcl-2 overexpressing MCF-7 breast cancer cells. Biomed Pharmacother. 2016;77:150–160. doi: 10.1016/j.biopha.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Niu Y, Wu H, Sun Y, Li Q, Kong X. et al. Modified apple polysaccharides could induce apoptosis in colorectal cancer cells. J Food Sci. 2010;75(8):H224–H229. doi: 10.1111/j.1750-3841.2010.01781.x. [DOI] [PubMed] [Google Scholar]
- 22.Liu E, Wu J, Cao W, Zhang J, Liu W, Jiang X. et al. Curcumin induces G2/M cell cycle arrest in a p53-dependent manner and upregulates ING4 expression in human glioma. J Neurooncol. 2007;85(3):263–270. doi: 10.1007/s11060-007-9421-4. [DOI] [PubMed] [Google Scholar]
- 23.You B R, Park WH. Gallic acid-induced lung cancer cell death is related to glutathione depletion as well as reactive oxygen species increase. Toxicol In Vitro. 2010;24(5):1356–1362. doi: 10.1016/j.tiv.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Sarvestani NN, Khodagholi F, Ansari N, Farimani MM. Involvement of p-CREB and phase II detoxifying enzyme system in neuroprotection mediated by the flavonoid calycopterin isolated from Dracocephalum kotschyi. Phytomedicine. 2013;20(10):939–946. doi: 10.1016/j.phymed.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 25.Attari F, Sepehri H, Ansari H, Hassani SN, Esfandiari F, Asgari B. et al. Efficient induction of pluripotency in primordial germ cells by dual inhibition of TGF-β and ERK signaling pathways. Stem Cells Dev. 2014;23(10):1050–1061. doi: 10.1089/scd.2013.0438. [DOI] [PubMed] [Google Scholar]
- 26.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1-2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 27.Altenburg JD, Bieberich AA, Terry C, Harvey KA, Vanhorn JF, Xu Z. et al. A synergistic antiproliferation effect of curcumin and docosahexaenoic acid in SK-BR-3 breast cancer cells: unique signaling not explained by the effects of either compound alone. BMC Cancer. 2011;11(1):1. doi: 10.1186/1471-2407-11-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo J, Li W, Shi H, Xie X, Li L, Tang H. et al. Synergistic effects of curcumin with emodin against the proliferation and invasion of breast cancer cells through upregulation of miR-34a. Mol Cell Biochem. 2013;382(1-2):103–111. doi: 10.1007/s11010-013-1723-6. [DOI] [PubMed] [Google Scholar]
- 29.You BR, Kim SZ, Kim SH, Park WH. Gallic acid-induced lung cancer cell death is accompanied by ROS increase and glutathione depletion. Mol Cell Biochem. 2011;357(1-2):295–303. doi: 10.1007/s11010-011-0900-8. [DOI] [PubMed] [Google Scholar]
- 30.Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB. Curcumin and cancer: an "old-age" disease with an "age-old" solution. Cancer Lett. 2008;267(1):133–64. doi: 10.1016/j.canlet.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 31.Somers‐Edgar TJ, Scandlyn MJ, Stuart EC, Le Nedelec MJ, Valentine SP, Rosengren RJ. The combination of epigallocatechin gallate and curcumin suppresses ERα‐breast cancer cell growth in vitro and in vivo. Int J Cancer. 2008;122(9):1966–1971. doi: 10.1002/ijc.23328. [DOI] [PubMed] [Google Scholar]
- 32.Liontas A, Yeger H. Curcumin and resveratrol induce apoptosis and nuclear translocation and activation of p53 in human neuroblastoma. Anticancer Res. 2004;24(2B):987–998. [PubMed] [Google Scholar]
- 33.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K. et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295(21):2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 34.Anders C K, Carey L A. Biology, metastatic patterns, and treatment of patients with triple-negative breast cancer. Clin Breast Cancer. 2009;9:S73–S81. doi: 10.3816/CBC.2009.s.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Estrela J M, Ortega A, Obrador E. Glutathione in cancer biology and therapy. Crit Rev Clin Lab Sci. 2006;43(2):143–181. doi: 10.1080/10408360500523878. [DOI] [PubMed] [Google Scholar]
- 36.Sun B, Ross SM, Trask OJ, Carmichael PL, Dent M, White A. et al. Assessing dose-dependent differences in DNA-damage, p53 response and genotoxicity for quercetin and curcumin. Toxicol In Vitro. 2013;27(6):1877–1887. doi: 10.1016/j.tiv.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 37.Syng-ai C, Kumari AL, Khar A. Effect of curcumin on normal and tumor cells: role of glutathione and bcl-2. Mol Cancer Ther. 2004;3(9):1101–1108. [PubMed] [Google Scholar]
- 38.Ly JD, Grubb DR, Lawen A. The mitochondrial membrane potential (deltapsi(m)) in apoptosis; an update. Apoptosis. 2003;8(2):115–28. doi: 10.1023/a:1022945107762. [DOI] [PubMed] [Google Scholar]
- 39.Gottlieb RA. Mitochondria and apoptosis. Neurosignals. 2001;10(3-4):147–161. doi: 10.1159/000046884. [DOI] [PubMed] [Google Scholar]
- 40.Marín YE, Wall BA, Wang S, Namkoong J, Martino JJ, Suh J. et al. Curcumin downregulates the constitutive activity of NF-κB and induces apoptosis in novel mouse melanoma cells. Melanoma Res. 2007;17(5):274–283. doi: 10.1097/CMR.0b013e3282ed3d0e. [DOI] [PubMed] [Google Scholar]
- 41.Subramanian AP, Jaganathan SK, Mandal M, Supriyanto E, Muhamad II. Gallic acid induced apoptotic events in HCT-15 colon cancer cells. World J Gastroenterol. 2016;22(15):3952–3961. doi: 10.3748/wjg.v22.i15.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheol-Hee CH. ABC transporters as multidrug resistance mechanisms and the development of chemosensitizers for their reversal. Cancer Cell Int. 2005;5:30. doi: 10.1186/1475-2867-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Housman G, Byler S, Heerboth S, Lapinska K, Longacre M, Snyder N, Sarkar S. Drug Resistance in Cancer: An Overview. Cancers (Basel) 2014;6(3):1769–1792. doi: 10.3390/cancers6031769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xue X, Xing-Jie L. Overcoming drug efflux-based multidrug resistance in cancer with nanotechnology. Chin J Cancer. 2012;31(2):100–109. doi: 10.5732/cjc.011.10326. [DOI] [PMC free article] [PubMed] [Google Scholar]