Abstract
Background
Osteosarcoma is the most common primary bone cancer and has a broad spectrum of histological subtypes. Stathmin 1 (STMN1) is a cytosolic phosphoprotein that is expressed in several types of cancer. The aim of this study was to evaluate the expression levels of STMN1 in osteosarcoma with clinicopathological characteristics and patient prognosis.
Material/Methods
The expression of STMN1 in tumor tissue from 94 patients with OS was detected and evaluated using an immunohistochemical score to divide the patients into low expression and high expression groups. Correlation between STMN1 expression and clinicopathological factors were analyzed with Fisher’s test, the prognostic value of expression levels of STMN1 in tumor tissue was evaluated by Kaplan-Meier univariate analysis, and independent prognostic factors were identified using the Cox regression model.
Results
Low expression of STMN1 was found in 43.62% of cases and high expression of STMN1 was found in 56.38% of cases of osteosarcoma. High tumor expression of STMN1 was significantly associated with the presence of metastases (P=0.028), Enneking surgical stage (P=0.030), tumor response to chemotherapy (P=0.011), and the site of tumor origin (P=0.023). High tumor expression of STMN1 was a prognostic marker in patients with osteosarcoma for poor prognosis (P=0.016), poor response to chemotherapy (P=0.004), the presence of metastases (P=0.003), advanced Enneking surgical stage (P=0.014), and the chondroblastic osteosarcoma subtype (P=0.004). The expression STMN1 was identified as an independent prognostic biomarker of osteosarcoma.
Conclusions
Increased expression of STMN1 in tumor tissue was an independent prognostic biomarker in patients with osteosarcoma.
MeSH Keywords: Biological Markers; Chemotherapy, Adjuvant; Osteosarcoma; Prognosis; Stathmin
Background
Osteosarcoma is one of the most common forms of malignancy of bone and is characterized by early metastasis [1]. Children and adolescents between the ages of 10–20 years have the highest morbidity and mortality from osteosarcoma [2]. Osteosarcoma has a broad spectrum of histological subtypes, which are considered to have different biological behavior [3]. According to the World Health Organization (WHO) classification, osteosarcoma includes three major subtypes, osteoblastic osteosarcoma, chondroblastic osteosarcoma, and fibroblastic osteosarcoma, based on the predominant type of matrix within the tumor; other histological variants include telangiectatic osteosarcoma and small cell osteosarcoma [4].
Osteosarcoma has a poor prognosis, as local invasion and metastatic disease can progress rapidly, and patients have a variable response to therapy. More than half of patients with primary osteosarcoma who do not receive postoperative adjuvant therapy suffer from metastases within a six-month period and >80% of patients undergo relapse within two years of diagnosis. Local invasion of the adjacent bone tissue occurs in between 15–30% of patients with osteosarcoma, and metastasis to the lungs occurs to approximately 95% of the patients [5]. These clinicopathological characteristics of osteosarcoma explain the poor prognosis. The 5-year overall survival rate is approximately 20% in patients with osteosarcoma who have metastases on initial diagnosis, and the 5-year survival rates for patients with non-metastatic osteosarcoma at initial presentation range from 65–70% when treated with adjuvant therapy in addition to surgery [6]. Given the aggressive clinical course and high rate of metastases of primary osteosarcoma of bone, there is an urgent need to find new biomarkers to patient prognosis and to assist in treatment decisions.
Stathmin 1 (STMN1) is a cytosolic phosphoprotein (18 kDa) that is involved in the regulation of the cell microtubule filament system by destabilizing microtubules [7]. STMN1 is widely expressed in the cell cytoplasm and is involved in multiple cellular functions, including cell proliferation, differentiation, motility, clonogenicity, and cell survival by preventing assembly and promoting disassembly of microtubules [8]. Because of its role in destabilizing the cell microtubules, STMN1 has a key role in carcinogenesis and cancer progression, including cell migration, invasion, and metastasis [9]. The overexpression of STMN1 has been reported in several types of human cancer, including breast cancer, prostate cancer, gastric cancer, and hepatocellular carcinoma [10–12]. Also, previous studies have shown that the progression of tumor cells was promoted by regulating STMN1 expression, indicating the potential of STMN1 as a biomarker for malignancy and the behavior of malignant cell [8].
Although studies on the role of STMN1 in human osteosarcoma are lacking, a recently published in vitro study of human osteosarcoma cell lines showed that knockdown of the STMN1 gene enhanced osteosarcoma cell chemosensitivity by inhibiting autophagy, indicating that STMN1 might be a potential target for the treatment of chemoresistance in osteosarcoma [13]. However, the clinical significance of STMN1 expression in human osteosarcoma in vivo remains to be elucidated, and the underlying molecular mechanisms for the effects of STMN1 are still unknown.
Therefore, the aim of this study was to evaluate the expression levels of STMN1 in osteosarcoma tissue, using immunohistochemistry (IHC), and to evaluate these expression levels with clinicopathological characteristics and patient prognosis.
Material and Methods
Patients studied
The study was approved by the Ethics Broad of Yidu Central Hospital and Shenzhen Second Peoples’ Hospital. All patients who provided osteosarcoma tissue for this study provided written informed consent. Between 2002–2016, a total of 223 patients underwent surgery for osteosarcoma in Yidu Central Hospital and Shenzhen Second Peoples’ Hospital, with the histological diagnosis of osteosarcoma confirmed by two senior pathologists. There were 94 patients selected in the study cohort who had no systemic therapy prior to surgery, but who underwent standard postoperative adjuvant therapy. Patients who had died within two months of surgery were excluded from the study as these patients were considered to have died from perioperative complications.
The overall survival rates for each patient were identified as being from the date of surgery to the date of death or the date of the last clinical follow-up. After discharge from the hospital, patients were followed-up by telephone, with follow-up ranging from between 4–107 months, with the average follow-up time being 27.2 months. The clinical stages of osteosarcoma were defined by the standard developed by Enneking et al. [14].
Immunohistochemistry (IHC) for stathmin 1 (STMN1)
Immunohistochemical staining was used for the detection of STMN1 using the streptavidin-peroxidase complex method, as previously described [15,16]. Osteosarcoma tissue obtained at surgery from each patient was routinely processed for diagnosis using formalin-fixed, paraffin wax-embedded tissue blocks, from which sections were cut onto glass slides. Xylene and graded alcohol were used for deparaffinization and rehydration of the tissue sections. Sections were incubated in citrate buffer for antigen retrieval, and in 3% hydrogen peroxide to block endogenous peroxidase. Nonspecific binding of antibodies was blocked by incubation of the tissue sections in 5% bovine serum albumin (BSA) and the sections were washed. The sections were incubated with the anti-STMN1 primary antibody (sc-48362) (Santa Cruz, CA, USA) at 4°C overnight. After rinsing with phosphate buffered saline (PBS), the sections were incubated with the secondary antibodies (Sangon, Shanghai, China) for 2 hours at room temperature. The streptavidin-peroxidase complex reagent (Sangon, Shanghai, China) and 3,3′-diaminobenzidine (DAB) (brown) solution (Sangon, Shanghai, China) were applied for visualization of the localization of the STMN1 immunostaining.
Evaluation of the IHC results
The results of IHC were semi-quantified with a visual scoring system, as previously described [17,18]. The evaluation of the IHC staining was performed by two pathologists who were unaware of the patients’ clinical data. This IHC scoring system consisted of two components: the score of staining intensity and the score of the percentage of positively-stained osteosarcoma cells. Staining intensity scores were defined as follows: 0, negative staining; 1, weak staining; 2, medium staining; and 3, strong staining. The scores for the percentage of positively-stained cells included: 1, between 0–25%; 2, between 25–50%; 3, as >50%. The final IHC staining score was defined as the product of these two scores and ranged from an IHC staining score of between 0–9.
Using the IHC staining scores, cut-off values were used to divide the patients into different groups. The value of the cut-off score was identified as the value that gave the highest summary of sensitivity plus specificity derived from the receiver-operating characteristic (ROC) curve, according to the method previously described [19,20].
Statistical analysis
Data were analyzed with SAS software (Cary, NC, USA) or SPSS software (IBM Co., Chicago, IL, USA). The correlation between STMN1 expression and clinicopathological factors were analyzed with Fisher’s test, the prognostic value of expression levels of STMN1 in tumor tissue was evaluated by Kaplan-Meier univariate analysis and the log-rank test, and independent prognostic factors were identified using the Cox regression model to identify the independent prognostic risk. A P-value of <0.05 was considered to be statistically significant.
Results
Expression and localization of stathmin 1 (STMN1) in osteosarcoma
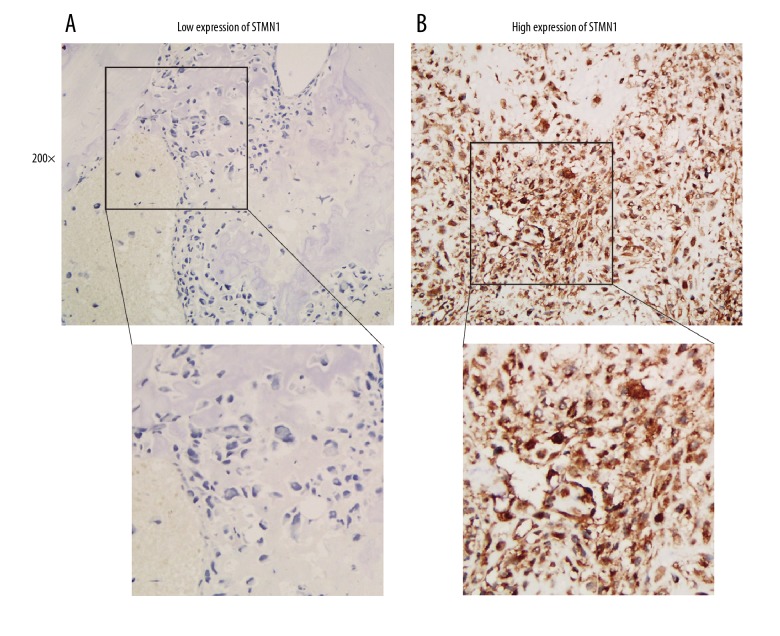
Immunohistochemistry (IHC) showed that stathmin 1 (STMN1) was mainly expressed in the cell cytoplasm of osteosarcoma cells, which was consistent with its function as a microtubules destabilizer. Representative images of the light microscopy of low STMN1 immunostaining and high STMN1 immunostaining are shown in the photomicrographs presented in Figure 1A and 1B. As shown in Table 1, the study cohort consisted of 19 female patients and 75 male patients with osteosarcoma. Patients who were <20 years-of-age accounted for 80.85% of all cases of osteosarcoma. The percentage of tumors showing low expression and high expression of STMN1 was 43.62% and 56.38%, respectively.
Figure 1.
Immunohistochemistry (IHC) shows the expression of stathmin 1 (STMN1) in osteosarcoma tissues. (A) Photomicrograph of the immunohistochemistry staining for stathmin 1 (STMN 1) in osteosarcoma tissue shows low expression levels. Magnification ×200. (B) Photomicrograph of the immunohistochemistry staining for STMN 1 in osteosarcoma tissue shows high expression levels. Magnification ×200.
Table 1.
Basic data of test cohort.
| Characters | Number | Percentage |
|---|---|---|
| Sex | ||
| Female | 19 | 20.21% |
| Male | 75 | 79.79% |
| Age | ||
| <20 | 76 | 80.85% |
| ≥20 | 18 | 19.15% |
| Tumor size (cm) | ||
| <8 | 61 | 64.89% |
| ≥8 | 33 | 35.11% |
| Enneking stage | ||
| I | 10 | 10.64% |
| II | 62 | 65.96% |
| III | 22 | 23.40% |
| Site | ||
| Femur | 44 | 46.81% |
| Tibia | 20 | 21.28% |
| Humerus | 15 | 15.96% |
| Fibula | 7 | 7.45% |
| Others | 8 | 8.51% |
| Histopathology | ||
| Osteoblastic | 33 | 35.11% |
| Fibroblastic | 21 | 22.34% |
| Chondroblastic | 16 | 17.02% |
| Telangiectatic | 11 | 11.70% |
| Others | 13 | 13.83% |
| Metastasis | ||
| No | 72 | 76.60% |
| Yes | 22 | 23.40% |
| Response to chemotherapy | ||
| Good | 40 | 42.55% |
| Poor | 54 | 57.45% |
| STMN1 | ||
| Low | 41 | 43.62% |
| High | 53 | 56.38% |
Correlation between STMN1 expression in osteosarcoma tissue and clinicopathological findings
The correlation between STMN1 expression in osteosarcoma tissue and patient clinicopathological factors was evaluated with Fisher’s test and are shown in Table 2. A high tissue expression level of STMN1 was significantly associated with the presence of metastases (P=0.028) and an advanced Enneking surgical stage (P=0.030), which were consistent findings, as metastatic status is an important parameter in the Enneking surgical stage for osteosarcoma. Also, STMN1 expression was significantly correlated with the site of origin of osteosarcoma (P=0.023), with an origin from the tibia or humerus being associated with an increased level of tissue STMN1 expression. Patients with high tissue expression levels of STMN1 had a poorer response to chemotherapy (P=0.011), indicating that STMN1 might be involved in the chemoresistance of osteosarcoma cells.
Table 2.
Correlation between STMN1 and clinicopathologic factors.
| Characters | STMN1 | P* | |
|---|---|---|---|
| Low | High | ||
| Sex | |||
| Female | 10 | 9 | 0.442 |
| Male | 31 | 44 | |
| Age | |||
| <20 | 37 | 39 | 0.063 |
| ≥20 | 4 | 14 | |
| Tumor size (cm) | |||
| <8 | 27 | 34 | 0.864 |
| ≥8 | 14 | 19 | |
| Enneking stage | |||
| I | 7 | 3 | 0.030 |
| II | 29 | 33 | |
| III | 5 | 17 | |
| Site | |||
| Femur | 24 | 20 | 0.023 |
| Tibia | 9 | 11 | |
| Humerus | 2 | 13 | |
| Fibula | 1 | 6 | |
| Others | 5 | 3 | |
| Histopathology | |||
| Osteoblastic | 13 | 20 | 0.727 |
| Fibroblastic | 11 | 10 | |
| Chondroblastic | 8 | 8 | |
| Telangiectatic | 5 | 6 | |
| Others | 4 | 9 | |
| Metastasis | |||
| No | 36 | 36 | 0.028 |
| Yes | 5 | 17 | |
| Response to chemotherapy | |||
| Poor | 11 | 29 | 0.011 |
| Good | 30 | 24 | |
Fisher test.
Survival rates and clinicopathological factors in patients with osteosarcoma
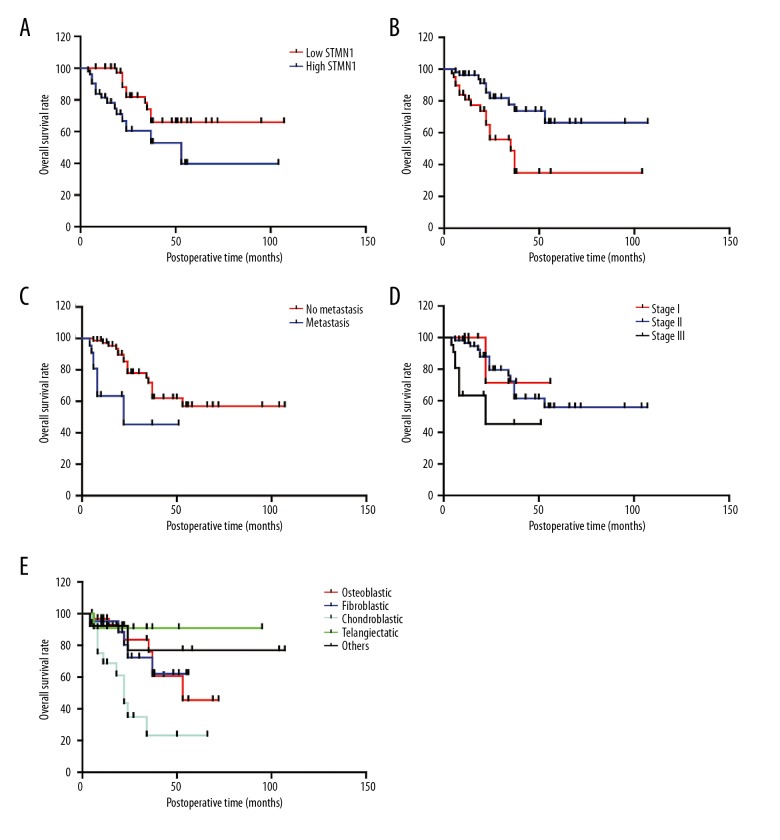
The correlation between the overall survival rates and the clinicopathological factors were evaluated using univariate analysis to identify the prognostic associations (Table 3). High expression levels of STMN1 was significantly associated with poor prognosis (P=0.016) (Figure 2A). Poor response to chemotherapy (P=0.004), the presence of metastases (P=0.003) and advanced Enneking surgical stage (P=0.014) were all associated with poor prognosis (Figure 2B–2D). Also, chondroblastic osteosarcoma had the lowest survival rates when compared with the other tumor types, including osteoblastic osteosarcoma, fibroblastic osteosarcoma, and telangiectatic osteosarcoma (Figure 2E). This finding might be explained by the fact that most cases of chondroblastic osteosarcoma were insensitive to chemotherapy, as previously reported [21].
Table 3.
Correlation between clinicopathologic factors and survival rates.
| Characters | 5-year survival rate | P* |
|---|---|---|
| Sex | ||
| Female | 58.4 | 0.274 |
| Male | 37.3 | |
| Age | ||
| <20 | 50.4 | 0.412 |
| ≥20 | 69.5 | |
| Tumor size (cm) | ||
| <8 | 63.0 | 0.902 |
| ≥8 | 43.7 | |
| Enneking stage | ||
| I | 71.4 | |
| II | 55.0 | 0.014 |
| III | 45.4 | |
| Site | ||
| Femur | 60.1 | |
| Tibia | 86.5 | 0.056 |
| Humerus | 35.5 | |
| Fibula | 40.0 | |
| Others | 0.0 | |
| Histopathology | ||
| Osteoblastic | 45.6 | |
| Fibroblastic | 62.0 | 0.004 |
| Chondroblastic | 23.3 | |
| Telangiectatic | 90.9 | |
| Others | 76.9 | |
| Metastasis | ||
| No | 56.9 | 0.003 |
| Yes | 45.4 | |
| Response to chemotherapy | ||
| Poor | 34.9 | 0.004 |
| Good | 66.3 | |
| STMN1 | ||
| Low | 65.9 | 0.016 |
| High | 39.7 | |
Log-rank test to analyzed the difference of 5-year survival rate.
Figure 2.
Survival curves for patients with osteosarcoma stratified by stathmin 1 (STMN1) expression, response to chemotherapy, metastasis, Enneking stage and histological type. (A) Patients with high levels of tumor tissue expression of stathmin 1 (STMN1) had lower survival rates compared with those with low levels of tumor tissue expression of STMN1. (B) Patients with a poor response to chemotherapy had lower survival rates compared with patients with a good response to chemotherapy. (C) Patients who were negative for tumor metastasis had lower survival rates compared with patients who had tumor metastases. (D) Patients with an advanced Enneking stage had lower survival rates compared with patients with an early Enneking stage. (E) Patients with chondroblastic osteosarcoma had the lowest survival rates; patients with telangiectatic osteosarcoma had the highest survival rates.
Independent prognostic risk factors
The independent prognostic factors of osteosarcoma were identified with multivariate analysis following univariate statistical analysis. The clinicopathological factors with P<0.10 in univariate analysis were enrolled into the multivariate analysis with the Cox-regression model for the identification of independent prognostic factors (Table 4). Enneking surgical stage was excluded from the analysis because this staging system includes the presence of metastasis. In this study, expression of STMN1 in osteosarcoma tissue using IHC was confirmed as an independent prognostic biomarker predicting the poor prognosis of osteosarcoma, confirmed by P-values, the hazard ratio (HR) and 95% confidence interval (CI) (P=0.012; HR=4.31; 95% CI, 1.48–13.4). Also, a poor response to chemotherapy (P=0.023) and positive distant metastases (P=0.005) were shown to be significant independent prognostic factors in the patients with osteosarcoma. The chondroblastic type of osteosarcoma was an independent risk factor of poor prognosis (P=0.009; HR=4.25), while the telangiectatic type of osteosarcoma was an independent predictor of favorable prognosis (P=0.034; HR=0.07). Osteosarcoma that originated from the tibia was a favorable prognostic factor when compared with osteosarcoma from the other sites (P=0.035; HR=0.16).
Table 4.
Multivariate analysis.
| Characters | HR | 95%CI | P* |
|---|---|---|---|
| Site | |||
| Femur | 1 | ||
| Tibia | 0.16 | 0.03–0.88 | 0.035 |
| Humerus | 1.47 | 0.47–4.58 | 0.512 |
| Fibula | 0.66 | 0.12–3.62 | 0.636 |
| Others | 0.98 | 0.27–3.63 | 0.980 |
| Histopathology | |||
| Osteoblastic | 1 | ||
| Fibroblastic | 1.05 | 0.33–3.35 | 0.940 |
| Chondroblastic | 4.25 | 1.44–12.5 | 0.009 |
| Telangiectatic | 0.07 | 0.01–0.82 | 0.034 |
| Others | 0.25 | 0.04–1.43 | 0.120 |
| Metastasis | |||
| No | 1 | ||
| Yes | 4.47 | 1.60–13.4 | 0.005 |
| Response to chemotherapy | |||
| Poor | 1 | ||
| Good | 0.35 | 0.14–0.87 | 0.023 |
| STMN1 | |||
| Low | 1 | ||
| High | 4.31 | 1.48–13.4 | 0.012 |
Means calculated with Cox-regression model.
Discussion
Stathmin 1 (STMN1) is a cytosolic phosphoprotein that is involved in the regulation of the cell microtubule filament system by destabilizing microtubules [7]. The aim of this study was to evaluate the expression levels of STMN1 in osteosarcoma tissue, using immunohistochemistry (IHC), and to evaluate these expression levels with clinicopathological characteristics and patient prognosis. The findings showed that increased expression of STMN1 in tumor tissue was an independent prognostic biomarker in patients with osteosarcoma.
In proliferating cells, including malignant cells, microtubules are essential components involved in cell division through the formation of the mitotic spindle. The regulation of microtubule dynamics mainly includes microtubule destabilization or stabilization [22]. The destabilization of microtubules can drive tubulin polymerization, resulting in activation of microtubule dynamics, which is essential for progression through mitosis [22]. Some agents, such as paclitaxel, function mainly by stabilizing microtubules and suppress tumor cells progression. Knockdown of the STMN1 gene has been shown to cause a delayed G2 phase and an increase in the rate of apoptosis in several cancer cell lines [23]. Studies undertaken in vitro and in vivo have previously shown that downregulation of STMN1 resulted in the inhibition of mesenchymal cell motility, while high levels of STMN1 was correlated with the metastatic phenotype of human sarcomas [24]. These previous study findings have suggested that STMN1 should be further investigated as a potential drug target in the treatment of the tumors, including sarcoma.
The oncogenic role of STMN1 in cancer cells proliferation and migration has been previously studied, and in sarcoma cells, the interaction of STMN1 with p27Kip1 was shown to regulate cell proliferation and invasion [24]. The oncogenic role of STMN1 has been previously demonstrated in several types of cancer or in cancer cell lines [25]. In osteosarcoma cells, knockdown of the STMN1 gene was previously shown to enhance chemosensitivity of osteosarcoma cells to paclitaxel through inhibition of autophagy [13].
In the present study, the prognostic role of STMN1 expression in osteosarcoma was demonstrated using IHC and showed, for the first time, that increased expression levels of STMN1 were correlated with several clinicopathologic factors including the presence of metastases and response to chemotherapy. These preliminary findings support the need for further controlled clinical studies on the role of STMN1 in the progression of human osteosarcoma and might trigger interest in further studies on the role of STMN1 as a prognostic biomarker in osteosarcoma. Importantly, the underlying molecular mechanisms on the role of STMN1 leading to tumor metastasis and chemoresistance in osteosarcoma should be explored further.
The establishment of new chemotherapeutic agents and targeted therapy in malignancy relies on the finding of effective diagnostic and prognostic biomarkers. However, as supported by the latest version of the National Comprehensive Cancer Network (NCCN) guidelines, there are still no satisfactory prognostic biomarkers for osteosarcoma [26]. The identification of new prognostic biomarkers for osteosarcoma may result in the development of new targeted drugs to prolong patient survival. Following several controlled clinical trials, sorafenib and everolimus are now used as second-line treatments for patients with advanced osteosarcoma.
In the present study, STMN1 was identified as an independent prognostic biomarker for osteosarcoma and was significantly correlated with the presence of metastases, which raises the possibility that future development of drugs that target STMN1 could be developed for osteosarcoma therapy. Currently, although there are no marketable specific inhibitors of STMN1, other drugs affecting microtubule dynamics may have a similar inhibitory function, such as eribulin, a drug targeting microtubule dynamics, which has been shown to inhibit mitosis and induce cell necrosis in osteosarcoma cells via suppressing microtubule dynamic stability in preclinical models of osteosarcoma [27]. It is hoped that the findings of the present study will encourage future studies on drug discovery and development in the area of microtubule dynamics and the treatment of osteosarcoma.
Conclusions
The findings of this study showed that increased expression of stathmin 1 (STMN1) in human osteosarcoma tissue was identified as an independent prognostic biomarker. In future, the detection of STMN1 expression could be used to stratify patients with osteosarcoma patients more precisely and to identify high-risk patients. Furthermore, future studies may be undertaken to determine whether treatment targeted to STMN1 might improve patient prognosis in osteosarcoma.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Ritter J, Bielack SS. Osteosarcoma. Ann Oncol. 2010;21(Suppl 7):vii320–25. doi: 10.1093/annonc/mdq276. [DOI] [PubMed] [Google Scholar]
- 2.Zandueta C, Ormazabal C, Perurena N, et al. Matrix-Gla protein promotes osteosarcoma lung metastasis and associates with poor prognosis. J Pathol. 2016;239(4):438–49. doi: 10.1002/path.4740. [DOI] [PubMed] [Google Scholar]
- 3.Moore DD, Luu HH. Osteosarcoma. Cancer Treat Res. 2014;162:65–92. doi: 10.1007/978-3-319-07323-1_4. [DOI] [PubMed] [Google Scholar]
- 4.Ballinger ML, Goode DL, Ray-Coquard I, et al. Monogenic and polygenic determinants of sarcoma risk: an international genetic study. Lancet Oncol. 2016;17(9):1261–71. doi: 10.1016/S1470-2045(16)30147-4. [DOI] [PubMed] [Google Scholar]
- 5.Kager L, Zoubek A, Kastner U, et al. Skip metastases in osteosarcoma: Experience of the Cooperative Osteosarcoma Study Group. J Clin Oncol. 2006;24(10):1535–41. doi: 10.1200/JCO.2005.04.2978. [DOI] [PubMed] [Google Scholar]
- 6.Aung L, Gorlick R, Healey JH, et al. Metachronous skeletal osteosarcoma in patients treated with adjuvant and neoadjuvant chemotherapy for nonmetastatic osteosarcoma. J Clin Oncol. 2003;21(2):342–48. doi: 10.1200/JCO.2003.06.177. [DOI] [PubMed] [Google Scholar]
- 7.Rubin CI, Atweh GF. The role of stathmin in the regulation of the cell cycle. J Cell Biochem. 2004;93(2):242–50. doi: 10.1002/jcb.20187. [DOI] [PubMed] [Google Scholar]
- 8.Nemunaitis J. Stathmin 1: Aprotein with many tasks. New biomarker and potential target in cancer. Expert Opin Ther Targets. 2012;16(7):631–34. doi: 10.1517/14728222.2012.696101. [DOI] [PubMed] [Google Scholar]
- 9.Singer S, Ehemann V, Brauckhoff A, et al. Protumorigenic overexpression of stathmin/Op18 by gain-of-function mutation in p53 in human hepatocarcinogenesis. Hepatology. 2007;46(3):759–68. doi: 10.1002/hep.21736. [DOI] [PubMed] [Google Scholar]
- 10.Alli E, Yang JM, Ford JM, Hait WN. Reversal of stathmin-mediated resistance to paclitaxel and vinblastine in human breast carcinoma cells. Mol Pharmacol. 2007;71(5):1233–40. doi: 10.1124/mol.106.029702. [DOI] [PubMed] [Google Scholar]
- 11.Golouh R, Cufer T, Sadikov A, et al. The prognostic value of Stathmin-1, S100A2, and SYK proteins in ER-positive primary breast cancer patients treated with adjuvant tamoxifen monotherapy: An immunohistochemical study. Breast Cancer Res Treat. 2008;110(2):317–26. doi: 10.1007/s10549-007-9724-3. [DOI] [PubMed] [Google Scholar]
- 12.Jeon TY, Han ME, Lee YW, et al. Overexpression of stathmin1 in the diffuse type of gastric cancer and its roles in proliferation and migration of gastric cancer cells. Br J Cancer. 2010;102(4):710–18. doi: 10.1038/sj.bjc.6605537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Z, He R, Xia H, et al. Knockdown of STMN1 enhances osteosarcoma cell chemosensitivity through inhibition of autophagy. Oncol Lett. 2017;13(5):3465–70. doi: 10.3892/ol.2017.5941. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Enneking WF. A system of staging musculoskeletal neoplasms. Clin Orthop Relat Res. 1986;(204):9–24. [PubMed] [Google Scholar]
- 15.Xu YF, Ge FJ, Han B, et al. High-mobility group box 1 expression and lymph node metastasis in intrahepatic cholangiocarcinoma. World J Gastroenterol. 2015;21(11):3256–65. doi: 10.3748/wjg.v21.i11.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang XQ, Xu YF, Guo S, et al. Clinical significance of nerve growth factor and tropomyosin-receptor-kinase signaling pathway in intrahepatic cholangiocarcinoma. World J Gastroenterol. 2014;20(14):4076–84. doi: 10.3748/wjg.v20.i14.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Z, Qiu S, Lu X. The expression and clinical significance of HDGF in osteosarcoma. Onco Targets Ther. 2015;8:2509–17. doi: 10.2147/OTT.S91708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu Y, Yang X, Li Z, et al. Sprouty2 correlates with favorable prognosis of gastric adenocarcinoma via suppressing FGFR2-induced ERK phosphorylation and cancer progression. Oncotarget. 2017;8(3):4888–900. doi: 10.18632/oncotarget.13982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu H, Xu Y, Zhang Q, et al. Prognostic significance of TBL1XR1 in predicting liver metastasis for early stage colorectal cancer. Surg Oncol. 2017;26(1):13–20. doi: 10.1016/j.suronc.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Liu H, Xu Y, Zhang Q, et al. Correlations between TBL1XR1 and recurrence of colorectal cancer. Sci Rep. 2017;7:44275. doi: 10.1038/srep44275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bacci G, Ferrari S, Delepine N, et al. Predictive factors of histologic response to primary chemotherapy in osteosarcoma of the extremity: Study of 272 patients preoperatively treated with high-dose methotrexate, doxorubicin, and cisplatin. J Clin Oncol. 1998;16(2):658–63. doi: 10.1200/JCO.1998.16.2.658. [DOI] [PubMed] [Google Scholar]
- 22.Chandrasekaran G, Tatrai P, Gergely F. Hitting the brakes: Targeting microtubule motors in cancer. Br J Cancer. 2015;113(5):693–98. doi: 10.1038/bjc.2015.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carney BK, Cassimeris L. Stathmin/oncoprotein 18, a microtubule regulatory protein, is required for survival of both normal and cancer cell lines lacking the tumor suppressor, p53. Cancer Biol Ther. 2010;9(9):699–709. doi: 10.4161/cbt.9.9.11430. [DOI] [PubMed] [Google Scholar]
- 24.Baldassarre G, Belletti B, Nicoloso MS, et al. p27(Kip1)-stathmin interaction influences sarcoma cell migration and invasion. Cancer Cell. 2005;7(1):51–63. doi: 10.1016/j.ccr.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 25.Wang J, Yao Y, Ming Y, et al. Downregulation of stathmin 1 in human gallbladder carcinoma inhibits tumor growth in vitro and in vivo. Sci Rep. 2016;6:28833. doi: 10.1038/srep28833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biermann JS, Chow W, Reed DR, et al. NCCN guidelines insights: Bone cancer, Version 2.2017. J Natl Compr Canc Netw. 2017;15(2):155–67. doi: 10.6004/jnccn.2017.0017. [DOI] [PubMed] [Google Scholar]
- 27.Sampson VB, Vetter NS, Zhang W, et al. Integrating mechanisms of response and resistance against the tubulin binding agent Eribulin in preclinical models of osteosarcoma. Oncotarget. 2016;7(52):86594–607. doi: 10.18632/oncotarget.13358. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]