Abstract
Objectives
This study aimed to identify the epidemiology, clinical characteristics, aetiology and seasonality of sporadic infectious diarrhoea in adults in Shanghai.
Setting
This study was based on a city-wide, active continuous hospital-based diarrhoea surveillance network established by Shanghai Municipal Center for Disease Control and Prevention. There were 22 sentinel hospitals in all 16 districts (9 primary-level hospitals, 6 secondary-level hospitals and 7 tertiary-level hospitals) which were selected using probability proportionate to size sampling method.
Participants
From 1 May 2012 to 31 May 2016, 90 713 patients were included in this study. Among 8797 patients whose stool samples were collected and detected, 4392 patients were male.
Results
The positive rate was 47.96%. Bacterial and viral infections accounted for 27.19% and 69.07% separately. Norovirus was the most common pathogen (43.10%), followed by rotavirus, Vibrio parahaemolyticus, diarrhoeagenic Escherichia coli (DEC) and Salmonella spp. Patients between 30–44 and 45–59 years were more likely to have infectious diarrhoea and viral diarrhoea. Those aged 30–44 years were the most likely to get infected with V. parahaemolyticus (adjusted OR, aOR vs 60+ years: 2.04, 95% CI 1.47 to 2.78) and norovirus (aOR vs 60+ years: 1.32, 95% CI 1.12 to 1.56). Bacterial (except V. parahaemolyticus) diarrhoea was characterised by fever, abdominal pain and loose stool; while viral diarrhoea was characterised by nausea, vomiting and watery stool. A seasonal distribution of infectious diarrhoea was observed with larger peaks in winter and smaller peaks in summer. Winter peaks were mainly due to norovirus and rotavirus, and summer peaks were due to bacterial infections. An emerging spring peak of norovirus around March was observed in the past 3 years.
Conclusion
Viral infections were predominant, and norovirus played a leading role. A seasonal distribution was observed and an emerging spring peak of norovirus was noted. Our findings highlight the necessity for conducting an active, comprehensive surveillance in adults, to monitor changing dynamics in the epidemiology and aetiology of infectious diarrhoea.
Keywords: infectious diseases, bacteriology, virology
Strengths and limitations of this study.
This is the first study in Shanghai identifying the aetiology and epidemiology of adult infectious diarrhoea in sporadic outpatients from a continuous active diarrhoea surveillance enhanced with comprehensive laboratory testing for common enteric bacteria and virus.
Seasonality of adult infectious diarrhoea and relevant contribution of different enteric pathogens in seasonal trend were demonstrated in detail.
Aetiology of adult infectious diarrhoea in Shanghai, including bacteria and virus, was detailed in this study.
Since information and detection results were collected from 22 hospitals and 16 laboratories, there was a chance of bias caused by different levels and conditions of hospitals and laboratories. Also, admission rate bias and recall bias were difficult to avoid.
As for seasonality, only descriptive data of every month or statistical tests of seasons were demonstrated. No statistical methods were used to analyse the successive time series.
Background
Diarrhoea is generally characterised by the frequent passage of loose or liquid stools. It is usually a symptom of gastrointestinal infections caused by bacterial, viral or parasitic pathogens, which spread through contaminated food or drinking water or from person to person.1 According to WHO, rotavirus and diarrhoeagenic Escherichia coli (DEC) are the two most common aetiological agents of diarrhoea in low/middle-income countries.1 However, norovirus was found the most prevalent pathogen of infectious diarrhoea in adults in Chinese Center For Disease Control And Prevention research,2 and Vibrio parahaemolyticus was the most common enteric pathogen in acute bacterial gastroenteritis.3 The aetiology of infectious diarrhoea differs among regions depending on economic development, local climate and geography.4 5
Nearly 1.7 billion cases and 1.3 million deaths due to diarrhoea occur worldwide every year.1 6Diarrhoea causes substantial medical and healthcare costs and thus has a high economic impact on society.7 Diarrhoea remains one of the major causes of disease burden worldwide, despite significant progress in sanitation status and public health awareness. Mortality due to diarrhoea fell 20% in recent 10 years, while it is still leading common cause of life loss (ranking fifth) globally.6 To react to this worldwide health issue, Shanghai CDC have established the Shanghai diarrhoea comprehensive surveillance system since 2012 which is an active continuous surveillance system this research is based on.
Most of current studies of diarrhoea have focused on children under 5 years old.8–12 Consequently, limited data about the epidemiology and aetiology of infectious diarrhoea in adults are available.13–15 Although diarrhoea accounts for only 2% deaths of adults,16 they may play a role in enteric infection transmission to other susceptible populations such as immunocompromised patients. Furthermore, there is rare research on the aetiology of infectious diarrhoea in adults in China,2 3 17 18 especially based on a continuous active surveillance with comprehensive laboratory detection of enteric bacteria and viruses. Better understanding of the epidemiology, aetiology and seasonality of infectious diarrhoea in adults would be valuable for planning and adopting targeted preventive measures and antimicrobial therapy.
The objectives of this study were to identify the epidemiology, clinical characteristics, aetiology and pathogen seasonality of infectious diarrhoea in adult sporadic outpatients through an active continuous hospital-based diarrhoea surveillance in Shanghai, and to explore to develop targeted policy of disease prevention and control in the future.
Methods
Shanghai diarrhoea comprehensive surveillance system
The Shanghai diarrhoea comprehensive surveillance system conducts active, population-based surveillance on diarrhoea outpatients. It consists of adult surveillance and children surveillance. The adult surveillance was established with 6 sentinel hospitals in May 2012, and incorporated 16 additional sentinel hospitals in August 2013. Municipal CDC, district CDCs and sentinel hospitals cooperate to maintain the surveillance, and share information and detection results through a dedicated online system. The 22 sentinel hospitals (9 primary-level hospitals, 6 secondary-level hospitals and 7 tertiary-level hospitals) were selected using probability proportionate to size (PPS) sampling method among all hospitals which had enteric disease clinics in all 16 districts of Shanghai. Different sampling intervals were allocated to different sentinel hospitals considering the hospitals’ location (district distribution), classification (hospital-level distribution) and annual number of diarrhoea patients (workload and operability) comprehensively, for use of collecting faecal specimens, ranging from 3:1 to 20:1.
Surveillance subjects were defined as patients who visited the enteric disease clinics of sentinel hospitals, with three or more loose or liquid stools per day, or more frequent than normal for the individual (WHO’s definition of diarrhoea).19 Demographic, epidemiological and medical information of all surveillance subjects was obtained using a standardised questionnaire, and recorded into the dedicated online system. Epidemiologically linked outbreak cases were excluded via inquiry.
Patient and public involvement
Patients involved were informed about the development and procedure of the surveillance, and interviewed by doctors in sentinel hospitals.
Laboratory tests
Faecal specimens were collected from surveillance subjects in accordance with sampling intervals by trained medical staff, as a part of standard medical care. If the sampling interval of a sentinel hospital is X, then faecal specimens are collected from the Xth, 2Xth, 3Xth,…nXth surveillance subjects in this sentinel hospital. Approximately, 8~10 g (mL) of stool was collected and then dispensed into two containers: (1) a tube with Cary-Blair (C-B) culture medium for bacteria testing and (2) a sterile plastic cup for virus testing. Nucleic acid was extracted from faecal specimens (20% w/v or v/v suspensions in PBS) using the QIAamp Viral RNA Mini Kit (Qiagen, Hilden, Germany).
All specimens were detected for eight bacterial pathogens (Vibrio cholera, Shigella spp, Salmonella spp, V. parahaemolyticus, Campylobacter jejuni, Yersinia enterocolitica, Campylobacter coli, DEC (including enteropathogenic Escherichia coli, enterotoxigenic Escherichia coli, enterohemorrhagic Escherichia coli, enteroaggregative Escherichia coli, enteroinvasive Eescherichia coli)) and five viral pathogens (norovirus, rotavirus, astrovirus, sapovirus and enteric adenovirus). Bacteria were isolated using different mediums at proper temperatures after preparation. The mediums included ChromID Vibrio and TCBS for V. cholera and V. parahaemolyticus, MAC for DEC, XLD for Shigella spp and Salmonella spp. Bacteria were identified using biochemical tests. An automatic biochemical identification system was used for DEC. Serum agglutination tests were employed to subtype Shigella spp, Salmonella spp, V. cholera and DEC. Astrovirus, norovirus, sapovirus and rotavirus were detected using real-time reverse transcription-PCR assays and enteric adenovirus was detected using rPCR. All molecular assays were performed using the appropriate respective commercial kits (Shanghai Zhejiang Biotechnology) according to the manufacturer’s instructions.
Samples were scored as positive if at least one of enteric pathogens was isolated or identified. A bacterial infection means enteric bacteria were isolated and no viruses were identified. A viral infection means enteric virus was identified and no bacteria were isolated. Samples were scored as simplex infection if 1 of the 13 enteric pathogens was isolated or identified; as a mixed infection if at least two of these pathogens were isolated or identified; as a bacterial–viral mixed infection if at least one bacterium was isolated and one virus was identified.
Statistical analysis
Data were analysed using SAS V.9.3. Numbers and percentages were computed for categorical variables. Cochren-Mantel-Haenszel test was used for comparison of categorical variables. Binary logistic model and general logit model were used for binary dependent variables and multicategory disordered dependent variables, respectively, to calculated adjusted OR (aOR) and to explore the association between aetiology and characteristics of infectious diarrhoea after adjusting for confounders. Variables of age group, suburb, gender, season and epidemiological histories were put into model and selected by stepwise methods. Age group, gender, suburb, season, consumption of suspicious food, medical history of enteric disease and whether to keep a pet were included in the final model. Two-tailed p values <0.05 were considered statistically significant.
This study focused on the adult diarrhoea patients with age ≥18 years. Age group was defined as 18–29, 30–44, 45–59 and 60+ years, according to the Global Burden of Disease 2000 and surveillance diarrhoea patients ‘age distribution’.20 Patients who visited hospitals in suburb areas were grouped in ‘suburb’. Patients who visited hospitals in rural areas were grouped in ‘rural’. Season was defined by the climatic characteristics of Shanghai, spring means March to May and summer means June to August, and autumn means September to November and winter means December to February. Suspicious food meant the suspicious food that patients self-reported and doctors thought that may cause diarrhoea, such as food which was contaminated by diarrhoea pathogen.
Results
From 1 May 2012 to 31 May 2016, a total of 95 284 patients were enrolled in Shanghai diarrhoea comprehensive surveillance system, of whom 4571 (4.80%) were not included in this study for the following reasons: 401 (0.42%) patients did not report clinical signs of diarrhoea, 379 (0.40%) patients visited the enteric disease clinics within 14 days and thus were considered as the same episodes, 11 (0.01%) patients sought clinical care >60 days after onset of diarrhoea, 212 (0.22%) patients were not infectious diarrhoea and have other explicit diagnosis, and 3568 (3.74%) patients were younger than 18 years. Among 90 713 adult diarrhoea patients, 8797 (9.70%) patients’ stool samples were collected and detected. These 8797 patients were included for further analysis.
Prevalence of enteric bacteria and viruses
A total of 4657 pathogens were identified or isolated from 4219 (positive rate 47.96%) stool samples of the 8797 samples. There are 1147 bacterial infections (27.19%), 2914 viral infections (69.07%) and 158 bacterial–viral mixed infections (infected with at least one bacterium and one virus, 3.74%). Excluding mixed-infection samples, V. parahaemolyticus infections, DEC infections and Salmonella spp infections were the most frequent bacterial infections, respectively, with positive rate 4.50%, 3.43% and 2.90%. Excluding mixed-infection samples, norovirus infections and rotavirus infections were the most frequent viral infections, with positive rates 19.82% and 8.12%, respectively. Positive rates of other enteric viral infections were as follows: sapovirus, 1.93%; astrovirus, 1.56% and enteric adenovirus, 0.35%. Positive rates of enteric bacterial infections were as follows: C. jejuni, 1.13%; Shigella spp, 0.22%; C. coli, 0.08%; Y. enterocolitica, 0.01% and Staphylococcus aureus, 0.01%. In addition, there were 343 (3.90%) mixed infections.
Isolated DEC consisted of 216 enterotoxigenic E. coli, 131 enteropathogenic E. coli, 84 enteroaggregative E. coli, 2 enteroinvasive E. coli and 1 enterohemorrhagic E. coli. Identified noroviruses consisted of 281 GI and 1726 GII. Identified rotaviruses consisted of 766 rotavirus group A, 6 rotavirus group B and 15 rotavirus group C.
Demographic and epidemiological characteristics
The median age was 46 (IQR 30–60) years. Of 8797 patients, 22.94% aged 18–29 years, 24.57% aged 30–44 years, 25.79% aged 45–59 years and 26.70% aged equal to or older than 60 years. A significant difference in positive rate within different age groups could be found among comparison of positive and negative diarrhoea patients (p=0.0150), comparison of bacterial and viral and bacterial–viral infections (p=0.0074), and comparison of different enteric pathogens infections (p<0.0001) (table 1). There were 4392 (49.93%) male patients, with a higher male proportion in positive diarrhoea patients (p=0.0472), DEC infections (aOR 1.29, 95% CI 1.02 to 1.64) and norovirus infections (aOR 1.22, 95% CI 1.08 to 1.36) (tables 1 and 2).
Table 1.
Demographic and epidemiological characteristics of diarrhoea outpatient adults by different infections
| Positive (n=4219) |
Negative (n=4578) |
P values | Bacterial infections (n=1147) | Viral infections (n=2914) | Bacterial–viral mixed infections (n=158) | P values |
Vibrio parahaemolyticus
* (n=396) |
DEC* (n=302) |
Salmonella spp* (n=255) | Norovirus* (n=1744) | Rotavirus* (n=714) | Other infections* (n=808) | P values | |
| Gender, N (%) | ||||||||||||||
| Male | 2153 (51.03) |
2239 (48.91) |
0.0472 | 577 (50.31) |
1497 (51.37) |
79 (50.00) |
0.8005 | 184 (46.46) |
164 (54.30) |
128 (50.20) |
946 (54.24) |
326 (45.66) |
405 (50.12) |
0.0011 |
| Age, positive rate (%) | ||||||||||||||
| 18–29 years | 941 (46.70) |
1074 | 0.0150 | 292 (14.49) |
611 (30.32) |
38 (1.89) |
0.0074 | 109 (5.41) |
74 (3.67) |
43 (2.13) |
384 (19.06) |
118 (5.86) |
213 (10.57) |
<0.0001 |
| 30–44 years | 1084 (53.80) |
1074 | 298 (14.79) |
748 (37.12) |
38 (1.89) |
119 (5.91) |
72 (3.57) |
57 (2.83) |
473 (23.47) |
158 (7.84) |
205 (10.17) |
|||
| 45–59 years | 1112 (55.19) |
1153 | 294 (14.59) |
768 (38.11) |
50 (2.48) |
105 (5.21) |
78 (3.87) |
76 (3.77) |
426 (21.14) |
231 (11.46) |
196 (9.73) |
|||
| 60+ years | 1079 (53.55) |
1266 | 262 (13.00) |
786 (39.01) |
31 (1.54) |
63 (3.13) |
78 (3.87) |
78 (3.87) |
460 (22.83) |
207 (10.27) |
193 (9.58) |
|||
| Living region, positive rate (%) | ||||||||||||||
| Suburb | 2401 (44.66) |
2975 | <0.0001 | 665 (12.37) |
1645 (30.60) |
91 (1.69) |
0.6661 | 257 (4.78) |
170 (3.16) |
149 (2.77) |
1019 (18.95) |
403 (7.50) |
403 (7.50) |
<0.0001 |
| Rural | 1818 (53.14) |
1603 | 482 (14.09) |
1269 (37.09) |
67 (1.96) |
139 (4.06) |
132 (3.86) |
255 (3.10) |
725 (21.19) |
311 (9.09) |
405 (11.84) |
|||
| Epidemiological history, N (%) | ||||||||||||||
| Had a medical history of enteric disease in the past 6 months | 17 (0.40) |
47 (1.03) |
0.0006 | 5 (0.44) |
12 (0.41) |
0 (0.00) |
0.7132 | 1 (0.25) |
1 (0.33) |
2 (0.78) |
8 (0.46) |
2 (0.28) |
3 (0.37) |
0.9001 |
| Had consumed suspicious food within 5 days before onset | 1914 (45.37) |
1865 (40.74) |
<0.0001 | 490 (42.72) |
1350 (46.33) |
74 (46.84) |
0.1073 | 179 (45.20) |
111 (36.75) |
117 (45.88) |
847 (48.57) |
282 (39.50) |
378 (46.78) |
<0.0001 |
| Had went out within 7 days before onset | 78 (1.85) |
46 (1.00) |
0.0010 | 29 (2.53) |
48 (1.65) |
1 (0.63) |
0.0881 | 7 (1.77) |
8 (2.65) |
5 (1.96) |
34 (1.95) |
6 (0.84) |
18 (2.23) |
0.3226 |
| Had kept or had contact with pets | 814 (19.29) |
604 (13.19) |
<0.0001 | 224 (19.53) |
556 (19.08) |
34 (21.52) |
0.7304 | 55 (13.89) |
65 (21.52) |
41 (16.08) |
323 (18.52) |
123 (17.23) |
207 (25.62) |
<0.0001 |
Bold face: p<0.05.
Cochren-Mantel-Haenszel test was used for comparison of categorical variables.
*Simplex infections.
DEC, diarrhoeagenic E coli.
Table 2.
Adjusted OR (aOR) of demographic and epidemiological characteristics comparing positive detection with negative detection in diarrhoea outpatients*
| Positive (n=4219) |
Bacterial infections (n=1147) | Viral infections (n=2914) | Bacterial-viral Mixed infections (n=158) |
Vibrio parahaemolyticus† (n=396) |
DEC† (n=302) |
Salmonella spp† (n=255) | Norovirus† (n=1744) | Rotavirus† (n=714) | |
| Male versus female | 1.09 (1.00 to 1.19) | 1.07 (0.94 to 1.22) | 1.1 (0.99 to 1.22) | 1.04 (0.75 to 1.43) | 0.89 (0.72 to 1.09) | 1.29 (1.02 to 1.64) | 1.11 (0.86 to 1.44) | 1.22 (1.08 to 1.36) | 0.88 (0.75 to 1.05) |
| Age (years) | |||||||||
| 18–29 | 1.10 (0.97 to 1.25) | 1.32 (1.09 to 1.59) | 0.99 (0.85 to 1.14) | 1.52 (0.93 to 2.44) | 1.92 (1.41 to 2.7) | 1.11 (0.79 to 1.54) | 0.64 (0.44 to 0.94) | 1.03 (0.88 to 1.22) | 0.75 (0.58 to 0.97) |
| 30–44 | 1.28 (1.14 to 1.45) | 1.28 (1.06 to 1.56) | 1.28 (1.11 to 1.47) | 1.54 (0.94 to 2.50) | 2.04 (1.47 to 2.78) | 1.02 (0.73 to 1.43) | 0.83 (0.58 to 1.19) | 1.32 (1.12 to 1.56) | 1.08 (0.84 to 1.35) |
| 45–59 | 1.19 (1.06 to 1.35) | 1.2 0 (1.00 to 1.47) | 1.16 (1.01 to 1.33) | 1.85 (1.18 to 2.94) | 1.72 (1.25 to 2.38) | 1.06 (0.77 to 1.47) | 1.06 (0.76 to 1.47) | 1.09 (0.93 to 1.28) | 1.33 (1.08 to 1.67) |
| 60+‡ | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Suburb | 0.80 (0.72 to 0.88) | 0.80 (0.68 to 0.92) | 0.80 (0.71 to 0.89) | 0.83 (0.58 to 1.19) | 0.96 (0.76 to 1.23) | 0.75 (0.57 to 0.98) | 0.82 (0.62 to 1.09) | 0.85 (0.75 to 0.97) | 0.79 (0.66 to 0.95) |
| Had a medical history of enteric disease in the past 6 months | 0.41 (0.23 to 0.73) | 0.42 (0.17 to 1.08) | 0.43 (0.22 to 0.85) | 0 | 0.24 (0.03 to 1.76) | 0.34 (0.05 to 2.46) | 0.71 (0.17 to 2.94) | 0.47 (0.21 to 1.01) | 0.32 (0.07 to 1.39) |
| Had consumed suspicious food within 5 days before onset | 1.18 (1.08 to 1.29) | 1.06 (0.93 to 1.22) | 1.24 (1.12 to 1.38) | 1.26 (0.82 to 1.75) | 1.22 (0.99 to 1.51) | 0.84 (0.66 to 1.08) | 0.24 (0.96 to 1.61) | 1.31 (1.17 to 1.48) | 0.99 (0.83 to 1.18) |
| Had kept or had contact with pets | 1.33 (1.17 to 1.5) | 1.57 (1.30 to 1.90) | 1.21 (1.04 to 1.40) | 1.62 (1.05 to 2.48) | 1.17 (0.85 to 1.63) | 1.79 (1.29 to 2.47) | 1.20 (0.83 to 1.75) | 1.26 (1.06 to 1.48) | 1.00 (0.78 to 1.27) |
Bold face: p<0.05.
*Data are aOR (95% CI) in binary logistic model or general logit model.
†Simplex infections.
‡Reference group in logistic regression model.
DEC, diarrhoeagenic E coli.
AORs of age were shown in table 2. Patients between 30–44 and 45–59 years were more likely to have infectious diarrhoea and viral diarrhoea. Those aged 30–44 years were the most likely to get infected with V. parahaemolyticus (aOR vs 60+ years group: 2.04, 95% CI 1.47 to 2.78) and norovirus (aOR vs 60+ years group: 1.32, 95% CI 1.12 to 1.56). In addition, patients in 18–29 years group had a significantly lower odds of experiencing infectious diarrhoea (aOR 0.85, 95% CI 0.76 to 0.97), viral infections (aOR 0.78, 95% CI 0.67 to 0.90), norovirus infections (aOR 0.78, 95% CI 0.66 to 0.92) and rotavirus infections (aOR 0.70, 95% CI 0.54 to 0.92) compared with 30–44 years group. Patients in 18–29 years group had a significantly lower odds of experiencing viral infections (aOR 0.85, 95% CI 0.74 to 0.98), Salmonella spp infections (aOR 0.61, 95% CI 0.41 to 0.89) and rotavirus infections (aOR 0.56, 95% CI 0.44 to 0.72) compared with 45–59 years group. Patients in 30–44 years group had a significantly higher odds experiencing norovirus infections (aOR 1.22, 95% CI 1.03 to 1.43) compared with 40–45 years group.
Among diarrhoea patients, 5376 (85.67%) visited the hospitals in suburb. The positive rates in suburb and rural groups were significantly different (p<0.0001, table 1). Comparing different enteric pathogen infections, the positive rates of patients in suburb and rural groups were significantly different (p<0.0001). More diarrhoea patients infected with V. parahaemolyticus (64.90%) lived in suburb areas. Patients living in suburb areas were less likely to get infected with enteric pathogens (aOR 0.75–0.85) except V. parahaemolyticus infections and Salmonella spp infections (table 2).
Sixty-four (0.73%) patients had a medical history of enteric disease in the past 6 months. Within 5 days before onset, 3779 (42.96%) patients had a history of consuming suspicious food. One hundred and twenty-four (1.41%) patients had a history of going out within 7 days before onset. And 1418 (16.12%) patients kept or had contact with pets. When compared with negative patients, a higher proportion of positive patients had a history of consuming suspicious food within 5 days before onset (p<0.0001), had a history of going out within 7 days before onset (p=0.0010) and kept or had contact with pets (p<0.0001), while a lower proportion had a medical history of enteric disease in the past 6 months (p=0.0006) (table 1). Epidemiological history, including consuming suspicious food and keeping or contacting with pets, was significantly associated with higher odds of infectious diarrhoea, viral infections and norovirus infections. A medical history of enteric disease was significantly associated with lower odds of infectious diarrhoea (table 2).
Clinical symptoms
Of positive diarrhoea patients, 13.11% reported fever, 41.91% reported nausea, 28.21% reported vomiting and 49.09% reported abdominal pain (table 3). Watery stool and loose stool were common, respectively, accounting for 76.27% and 20.93%. Compared with negative diarrhoea patients, positive patients reported more fever (p=0.0009), nausea (p<0.0001), vomiting (p<0.0001) and watery stool (p<0.0001), but fewer abdominal pain (p<0.0001).
Table 3.
Clinical symptoms in diarrhoea outpatients by different infections
| Positive (n=4219) |
Negative (n=4578) |
P values | Bacterial infections (n=1147) | Viral infections (n=2914) | Bacterial–viral mixed infections (n=158) | P values |
Vibrio parahaemolyticus
* (n=396) |
DEC* (n=302) |
Salmonella spp* (n=255) | Norovirus* (n=1744) | Rotavirus* (n=714) | Other infections* (n=808) | P values | |
| Fever, N (%) | 553 (13.11) |
495 (10.81) |
0.0009 | 219 (19.09) |
312 (10.71) |
22 (13.92) |
<0.0001 | 46 (11.62) |
43 (14.24) |
72 (28.24) |
169 (9.69) |
96 (13.45) |
127 (15.72) |
<0.0001 |
| Nausea, N (%) | 1768 (41.91) |
1561 (34.10) |
<0.0001 | 442 (38.54) |
1263 (43.34) |
63 (39.87) |
0.0175 | 224 (56.27) |
87 (28.81) |
71 (27.84) |
790 (45.30) |
309 (43.28) |
287 (35.52) |
<0.0001 |
| Vomiting, N (%) | 1190 (28.21) |
916 (20.01) |
<0.0001 | 269 (23.45) |
878 (30.13) |
43 (27.22) |
0.0001 | 164 (41.41) |
41 (13.58) |
37 (14.51) |
595 (34.12) |
195 (27.31) |
158 (19.55) |
<0.0001 |
| Abdominal pain, N (%) | 2071 (49.09) |
2446 (53.43) |
<0.0001 | 741 (64.60) |
1257 (43.14) |
73 (46.20) |
<0.0001 | 285 (71.97) |
170 (56.29) |
151 (59.22) |
777 (44.55) |
321 (44.96) |
367 (45.42) |
<0.0001 |
| Faecal property, N (%) | ||||||||||||||
| Watery | 3218 (76.27) |
3150 (68.81) |
<0.0001 | 814 (70.97) |
2283 (78.35) |
121 (76.58) |
<0.0001 | 323 (81.57) |
202 (66.89) |
179 (70.20) |
1344 (77.06) |
583 (81.65) |
587 (72.65) |
<0.0001 |
| Loose | 883 (20.93) |
1202 (26.26) |
267 (23.28) |
583 (20.01) |
33 (20.89) |
54 (13.64) |
85 (28.15) |
61 (23.92) |
372 (21.33) |
121 (16.95) |
190 (23.51) |
|||
| Mucous | 72 (1.71) |
143 (3.12) |
38 (3.31) |
31 (1.06) |
3 (1.90) |
8 (2.02) |
11 (3.64) |
11 (4.31) |
18 (1.03) |
6 (0.84) |
18 (2.23) |
|||
| Else | 46 (1.09) |
83 (1.81) |
28 (2.44) |
17 (0.58) |
1 (0.63) |
11 (2.78) |
4 (1.32) |
4 (1.57) |
10 (0.57) |
4 (0.56) |
13 (1.61) |
Bold face: p<0.05.
Cochren-Mantel-Haenszel test was used for comparison of categorical variables.
*Simplex infections.
DEC, diarrhoeagenic E. coli.
The distributions of clinical symptoms by different infections were significantly different (table 3). Diarrhoea patients infected with bacteria reported more fever (19.09%, p<0.0001), abdominal pain (64.60%, p<0.0001) and loose stool (23.28%, p<0.0001). Diarrhoea patients infected with virus reported more nausea (43.34%, p=0.0175), vomiting (30.13%, p=0.0001) and watery stool (78.35%, p<0.0001).
Diarrhoea patients infected with V. parahaemolyticus featured more nausea (56.27%), vomiting (41.41%), abdominal pain (71.9%) and watery stool (81.57%). Patients infected with DEC featured fewer nausea (28.81%) and vomiting (13.58%). Patients infected with Salmonella spp featured more fever (28.24%). Patients infected with norovirus featured fewer fever (9.69%) and abdominal pain (44.55%).
Pathogen spectrum and seasonality
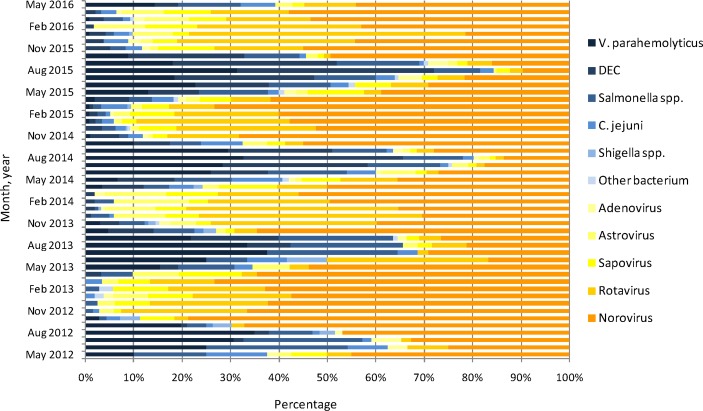
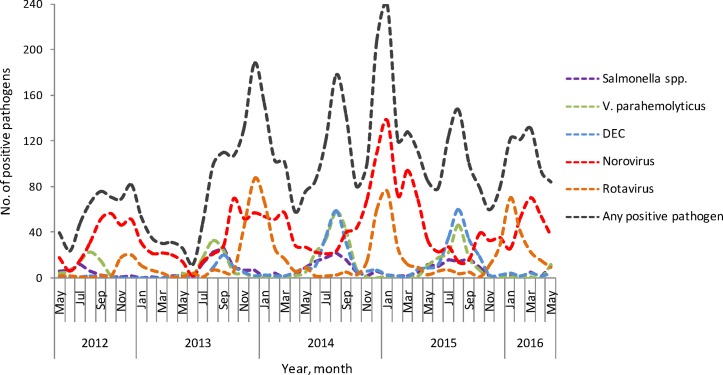
In term of descriptive data, the enteric pathogens spectrum of infectious diarrhoea patients displayed a yearly seasonal trend (figure 1). In general, viruses were predominant during November to March of every seasonal cycle, accounting for more than 80% in every month. Bacteria were predominant during June to August of almost every seasonal cycle, accounting for more than 60% in every month. September and October were the transition period from bacteria to viruses, and April and May were the transition period from viruses to bacteria. Norovirus and rotavirus both showed yearly seasonal trends. Rotavirus peaked in winter months, especially in December and January. Norovirus displayed a less distinct and broader seasonality. Norovirus clustered around autumn and winter, while a smaller peak appeared in March of 2014 and 2015. In the seasonal cycle from 2015 to 2016, norovirus peaked in March 2016. V. parahaemolyticus, DEC and Salmonella spp all showed yearly seasonal trends. These three enteric bacteria peaked in August, and Salmonella spp showed a smaller peak (figure 2).
Figure 1.
Pathogen spectrum of major enteric pathogens in adults with infectious diarrhoea by month in Shanghai, May 2012 to May 2016. (DEC, diarrhoeagenic E. coli.)
Figure 2.
Seasonality of major enteric pathogens in adult with infectious diarrhoea in Shanghai, May 2012 to May 2016. (DEC, diarrhoeagenic E. coli).
In term of statistical analysis, there were significantly different season distributions in comparison of positive and negative diarrhoea patients (p<0.0001), comparison of bacterial and viral and bacterial–viral infections (p<0.0001), and comparison of different enteric pathogens infections (p<0.0001). More bacterial infections appeared in summer (54.58%) and more viral infections appeared in winter (44.51%). The proportion of winter was lower among norovirus infections (34.86%) compared with among rotavirus infections (67.37%).
Patients in summer were 1.55–4.39 times more likely to have simplex bacterial diarrhoea and 0.16–0.20 times less likely to have simplex viral diarrhoea compared with in spring. Patients in autumn were 2.02–3.38 times more likely to have V. parahaemolyticus infections and DEC infections, and 0.69–0.77 times less likely to have simplex viral diarrhoea compared with in spring. Patients in winter were 1.60–5.61 times more likely to have simplex viral infections, and 0.14–0.56 times less likely to have simplex bacterial diarrhoea compared with in spring (table 4).
Table 4.
Seasonality of diarrhoea outpatients by different infections*
| Negative (n=4578) |
Positive (n=4219) |
Bacterial infections (n=1147) | Viral infections (n=2914) | Bacterial–viral mixed infections (n=158) |
Vibrio parahaemolyticus
† (n=396) |
DEC† (n=302) |
Salmonella spp† (n=255) | Norovirus† (n=1744) | Rotavirus† (n=714) | |
| Season (no (%)) | P<0.0001 | P<0.0001 | P<0.0001 | |||||||
| Spring | 867 (18.94) | 877 (20.79) | 149 (12.99) | 695 (23.85) | 33 (20.89) | 34 (8.59) | 21 (6.95) | 41 (16.08) | 462 (26.49) | 101 (14.15) |
| Summer | 1746 (38.14) | 927 (21.97) | 626 (54.58) | 260 (8.92) | 41 (25.95) | 252 (63.64) | 178 (58.94) | 123 (48.24) | 180 (10.32) | 32 (4.48) |
| Autumn | 1238 (27.04) | 1031 (24.44) | 322 (28.07) | 662 (22.72) | 47 (29.75) | 106 (26.77) | 96 (31.79) | 72 (28.24) | 494 (28.33) | 100 (14.01) |
| Winter | 727 (15.88) | 1384 (32.80) | 50 (4.36) | 1297 (44.51) | 37 (23.42) | 4 (1.01) | 7 (2.32) | 19 (7.45) | 608 (34.86) | 481 (67.37) |
| Season (aOR (95% CI)) | ||||||||||
| Spring‡ | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |
| Summer | 0.54 (0.48 to 0.61) | 2.16 (1.77 to 2.64) | 0.18 (0.16 to 0.23) | 0.62 (0.39 to 1.00) | 3.65 (2.53 to 5.29) | 4.39 (2.77 to 6.96) | 1.55 (1.07 to 2.23) | 0.20 (0.17 to 0.24) | 0.16 (0.10 to 0.24) | |
| Autumn | 0.85 (0.75 to 0.97) | 1.59 (0.28 to 1.97) | 0.69 (0.60 to 0.79) | 1.04 (0.66 to 1.65) | 2.2 (1.48 to 3.28) | 3.38 (2.09 to 5.47) | 1.26 (0.85 to 1.87) | 0.77 (0.66 to 0.9) | 0.69 (0.52 to 0.93) | |
| Winter | 1.91 (1.67 to 2.18) | 0.40 (0.29 to 0.56) | 2.26 (1.98 to 2.59) | 1.36 (0.84 to 2.20) | 0.14 (0.05 to 0.40) | 0.39 (0.16 to 0.92) | 0.56 (0.32 to 0.97) | 1.60 (1.37 to 1.88) | 5.61 (4.42 to 7.11) | |
Bold face: p<0.05.
Cochren-Mantel-Haenszel test was used for comparison of categorical variables.
*Data are aOR (95% CI) in binary logistic model or general logit model.
†Simplex infections.
‡Reference group in logistic regression model.
aOR, adjusted OR; DEC, diarrhoeagenic E. coli.
Discussion
This study is the first study in Shanghai to identify the aetiology and epidemiology of adult infectious diarrhoea in sporadic outpatients from a continuous active diarrhoea surveillance enhanced with comprehensive laboratory testing for common enteric bacteria and virus. It also adds to the limited number of studies investigating adult cases of infectious diarrhoea in China. The Shanghai diarrhoea comprehensive surveillance system used PPS sampling method and was conducted among 22 sentinel hospitals in all 16 districts of Shanghai continuously since May 2012, data from which are more representative and more feasible to be extrapolated to the city’s population by avoiding the influence of clusters and season-specific cases.
Aetiology of adult infectious diarrhoea in Shanghai was detailed in this study. At least one enteric pathogen was found in 47.96% of adult diarrhoea patients’ stools. Viral infections are predominant and bacteria were isolated from many cases. These findings were consistent with those of Wang’s research in Beijing.2 We found that norovirus was the most common enteric pathogen, accounting for over 40% of all cases, followed by rotavirus, V. parahaemolyticus, DEC and Salmonella spp. The proportion of norovirus was higher than the sum proportion of rotavirus, V. parahaemolyticus, DEC and Salmonella spp. This result confirmed norovirus’s leading role in adult infectious diarrhoea in China, and was similar to the research finding in sporadic gastroenteritis in both low/middle-income and developed countries.21–24 And it is observed that norovirus infections were more than two times that of rotavirus in adult patients in Shanghai. Rotavirus ranked second to norovirus. These results were consistent with studies in Russia24 and Shanghai, China,25 while inconsistent with study in France.26 Yet according to WHO, rotavirus is most common aetiological agents of diarrhoea in low/middle-income countries which may be due to rotavirus’s important role in children. As the leading cause of severe diarrhoea in children, the pathogenic role and disease burden of rotavirus in adults has been underestimated. Rotavirus needs more attention in routine clinical diagnosis and vaccination programme.
According to this study, V. parahaemolyticus, DEC and Salmonella spp were common bacteria in adult infectious diarrhoea. The prevalence of these three bacterial infections was between 2.90% and 4.50%, much lower than viral infections. In previous studies, V. parahaemolyticus, DEC and Salmonella spp were also among the most prevalent pathogen in adult infectious diarrhoea in different regions of China and worldwide.1 2 17 18 27 Although diarrhoea due to V. parahaemolyticus has decreased since 1998,28 29 V. parahaemolyticus was still the leading cause of adult bacterial infections in this study. However, Shigella spp was also among frequent bacteria in several studies before 2013.18 28 29 This study showed that positive rate of Shigella spp infections was only 0.22% during 2012–2016 in Shanghai, which may be due to the downward trend of Shigella spp infections over time.29
This study showed that there was association between adult infectious diarrhoea and patient age. In general, patients between 30 and 59 years were more likely to have infectious diarrhoea and viral diarrhoea than age groups of 18–29 and 60+ years. This was partly consistent with a study in France which found incidence of acute diarrhoea in youth group was higher than elderly group.26 Elderly people (≥60 years) were the least likely to get infected with V. parahaemolyticus, whereas people aged 30–44 years were the most likely among adult age groups. The similar findings were observed in a study in Shanghai.29 This may be related to more seafood consumption in young adults, which is an important risk factor in V. parahaemolyticus infections.30 In contrast to other studies which found elderly people more likely to get infected with norovirus,22 31 our study discovered that the highest proportion in norovirus infections was 30–44 years old. And considering the results of general logit model adjusting for other factors, patients aged 30–44 years were the most likely to get infected to norovirus. Patients aged 18–29 years had the lowest odds experiencing rotavirus diarrhoea.
People living in rural areas were more susceptible to DEC, norovirus and rotavirus, which may be because city environment provided more chances for pathogens to transmit.
In regard to clinical symptoms in general, bacterial diarrhoea was characterised by fever, abdominal pain and loose stool, while viral diarrhoea was characterised by nausea, vomiting and watery stool. However the symptoms of V. parahaemolyticus infections showed more like viral infections. In addition, abdominal pain was common in V. parahaemolyticus infections. These findings of V. parahaemolyticus were in accordance with a research in Shanghai during 1998–2013.28 The symptoms of DEC and Salmonella spp were similar except fever. The proportion of fever was the highest in Salmonella spp (28.24%) while lowest in norovirus (9.69%). The proportion of fever in norovirus infections was much lower in comparison with some studies,26 31 while the proportion in Salmonella spp infections was close to another research.28 The proportion of abdominal pain was the highest in V. parahaemolyticus (71.97%) while much lower in norovirus (44.55%) and rotavirus (44.96%).
This study also demonstrated the seasonality of adult infectious diarrhoea and relevant contribution of different enteric pathogens in seasonal trend. A seasonal distribution of adult infectious diarrhoea was observed with a large peak in winter and a small peak in summer. Winter peak was mainly due to norovirus and rotavirus, which was in line with previous study.32 33 Summer peak was smaller, due to low proportion of bacterial infections. What should be noted was that there was a peak around March due to norovirus in 2014–2016, even higher than the summer peak in 2015–2016 season cycle. This emerging spring peak was possibly because of the increased activity of a novel norovirus GII.17.34 Rotavirus showed a distinct peak in December and January (significantly winter vs summer aOR 35.67), which was consistent with researches in Shanghai and Iran,25 35 while different from a study in London (peak from January to May)36 and a study in Russia (peak from December to May).24 However, norovirus displayed a broader seasonality peaking around autumn and winter (significantly winter vs summer OR 8.00) in this study and a study in Netherlands.9 Bacterial infections, included V. parahaemolyticus, DEC and Salmonella spp, showed a yearly seasonality peaking in summer (often in August), with significantly summer vs winter OR 25.00, 11.11 and 2.78, respectively. This was similar in Enserink’s study,9 whereas autumn peak of bacterial infections was observed in some studies.25 37 The seasonality of infectious diarrhoea may be due to the climate, biological characteristics of pathogens and people’s diet habit of Shanghai.
There are several limitations that need to be acknowledged. First, information and detection results were collected from 22 hospitals and 16 laboratories. Though detection methods and materials were unified and regular trainings were held, there was still a chance of bias caused by the different levels and conditions of hospitals and laboratories. Admission rate bias should also be taken into consideration as patients may have a preference when visiting hospitals of different levels or in different regions. Second, the recall bias of epidemiological information was difficult to avoid. And the data of exposure history were important for infectious diarrhoea. Third, only diarrhoea patients who visited the enteric disease clinics were included in surveillance, severe diarrhoea patients or asymptomatic patients were possibly not studied in our research. Fourth, as for seasonality, only descriptive data of every month or statistical tests of seasons were demonstrated. No statistical methods were used to analyse the successive time series, because of the limit seasonal cycles of existing data. In the future, after accumulating enough data for several years, time series analysis could be taken to explore the inherent natural order and to forecast prospective trend.
Conclusions
In conclusion, this study provides a detailed picture about the epidemiology, aetiology and seasonal pathogen spectrum of adult infectious diarrhoea in Shanghai. Viral infections are predominant, and norovirus is the most common enteric pathogen detected in our surveillance. Other common pathogens include rotavirus, V. parahaemolyticus, DEC and Salmonella spp. Patients between 30 and 59 years were more likely to have infectious diarrhoea and viral diarrhoea. A seasonal distribution was observed with larger peaks in winter and smaller peaks in summer. Winter peak was mainly due to norovirus and rotavirus, and summer peak was due to bacterial infections. An emerging spring peak of norovirus around March was observed in recent 3 years. Our findings highlight the necessity for conducting an active, comprehensive surveillance for both bacterial and viral enteric pathogens in adults, to monitor the changing dynamics in the epidemiology and aetiology of infectious diarrhoea. These findings help us to understand adult infectious diarrhoea better and to develop targeted prevention strategies.
Supplementary Material
Acknowledgments
The authors of this study thank all of the patients, the public health workers in the CDCs and healthcare workers in the hospitals involved in the surveillance. We are grateful to Fan Wu and Yi He for their intellectual contributions to the study’s design. We are grateful to Martin C W Chan and She-Lan Liu for their valuable revision advice on the manuscript.
Footnotes
X-HG and H-YW contributed equally.
Contributors: X-HG performed the statistical analysis and drafted the manuscript. HP and H-YW put the surveillance system into effect. JL designed the study of the surveillance system. W-JX participated in the management of the system. XZ, MC and ZT carried out the management and quality control of the laboratory tests. Z-AY conceived of the study. All authors read and approved of the final manuscript.
Funding: This work was supported by the fourth Round of Three-year Action Plan on Public of Health of Shanghai: Key Discipline-Epidemiology and Hygiene Microbiology (No. 15GWZK0101), Shanghai Public Health Professional Overseas Training Grant (No. GWTD2015S02), from the Shanghai Municipal Commission of Health and Family Planning.
Competing interests: None declared.
Patient consent: Not required.
Ethics approval: The study protocol was approved by the Institutional Ethics Review Committee of the Shanghai Municipal Centers for Disease Control and Prevention.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional unpublished data are available.
References
- 1. The World Health Organization. Diarrhoeal disease, 2013. [Google Scholar]
- 2. Wang X, Wang J, Sun H, et al. Etiology of childhood infectious diarrhea in a developed region of China: compared to childhood diarrhea in a developing region and adult diarrhea in a developed region. PLoS One 2015;10:e0142136 10.1371/journal.pone.0142136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chan SS, Ng KC, Lyon DJ, et al. Acute bacterial gastroenteritis: a study of adult patients with positive stool cultures treated in the emergency department. Emerg Med J 2003;20:335–8. 10.1136/emj.20.4.335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thapar N, Sanderson IR. Diarrhoea in children: an interface between developing and developed countries. Lancet 2004;363:641–53. 10.1016/S0140-6736(04)15599-2 [DOI] [PubMed] [Google Scholar]
- 5. Podewils LJ, Mintz ED, Nataro JP, et al. Acute, infectious diarrhea among children in developing countries. Semin Pediatr Infect Dis 2004;15:155–68. 10.1053/j.spid.2004.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1459–544. 10.1016/S0140-6736(16)31012-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jones TF. A population-based estimate of the substantial burden of diarrhoeal disease in the United States; FoodNet, 1996 – 2003, 2007:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Benhafid M, Youbi M, Klena JD, et al. Epidemiology of rotavirus gastroenteritis among children <5 years of age in Morocco during 1 year of sentinel hospital surveillance, June 2006-May 2007. J Infect Dis 2009;200(Suppl 1):S70–S75. 10.1086/605048 [DOI] [PubMed] [Google Scholar]
- 9. Enserink R, van den Wijngaard C, Bruijning-Verhagen P, et al. Gastroenteritis attributable to 16 enteropathogens in children attending day care. Pediatr Infect Dis J 2015;34:5–10. 10.1097/INF.0000000000000472 [DOI] [PubMed] [Google Scholar]
- 10. Chhabra P, Payne DC, Szilagyi PG, et al. Etiology of viral gastroenteritis in children <5 years of age in the United States, 2008-2009. J Infect Dis 2013;208:790–800. 10.1093/infdis/jit254 [DOI] [PubMed] [Google Scholar]
- 11. Grant L, Vinjé J, Parashar U, et al. Epidemiologic and clinical features of other enteric viruses associated with acute gastroenteritis in American Indian infants. J Pediatr 2012;161:110–5. 10.1016/j.jpeds.2011.12.046 [DOI] [PubMed] [Google Scholar]
- 12. Fletcher S, Van Hal S, Andresen D, et al. Gastrointestinal pathogen distribution in symptomatic children in Sydney, Australia. J Epidemiol Glob Health 2013;3:11–21. 10.1016/j.jegh.2012.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sambe-Ba B, Espié E, Faye ME, et al. Community-acquired diarrhea among children and adults in urban settings in Senegal: clinical, epidemiological and microbiological aspects. BMC Infect Dis 2013;13:580 10.1186/1471-2334-13-580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Franck KT. Norovirus epidemiology in community and health care settings and association with patient age, Denmark, 2014:1123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Parry CM, Thomas S, Aspinall EJ, et al. A retrospective study of secondary bacteraemia in hospitalised adults with community acquired non-typhoidal Salmonella gastroenteritis. BMC Infect Dis 2013;13:107 10.1186/1471-2334-13-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095–128. 10.1016/S0140-6736(12)61728-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dong B, Liang D, Lin M, et al. Bacterial etiologies of five core syndromes: laboratory-based syndromic surveillance conducted in Guangxi, China. PLoS One 2014;9:e110876 10.1371/journal.pone.0110876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Qu M, Deng Y, Zhang X, et al. Etiology of acute diarrhea due to enteropathogenic bacteria in Beijing, China. J Infect 2012;65:214–22. 10.1016/j.jinf.2012.04.010 [DOI] [PubMed] [Google Scholar]
- 19. WHO. Diarrhoea. 2016. http://www.who.int/topics/diarrhoea/en/
- 20. Mathers CD. Global burden of disease 2000: version 2 methods and results: WHO, 2002. [Google Scholar]
- 21. Tam CC, Rodrigues LC, Viviani L, et al. Longitudinal study of infectious intestinal disease in the UK (IID2 study): incidence in the community and presenting to general practice. Gut 2012;61:69–77. 10.1136/gut.2011.238386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Patel MM, Widdowson MA, Glass RI, et al. Systematic literature review of role of noroviruses in sporadic gastroenteritis. Emerg Infect Dis 2008;14:1224–31. 10.3201/eid1408.071114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morillo SG, Timenetsky MC. Norovirus: an overview. Rev Assoc Med Bras 2011;57:453–8. 10.1016/S0104-4230(11)70094-X [DOI] [PubMed] [Google Scholar]
- 24. Podkolzin AT, Fenske EB, Abramycheva NY, et al. Hospital-based surveillance of rotavirus and other viral agents of diarrhea in children and adults in Russia, 2005-2007. J Infect Dis 2009;200(Suppl 1):S228–S233. 10.1086/605054 [DOI] [PubMed] [Google Scholar]
- 25. Wang Y, Zhang J, Liu P. Clinical and molecular epidemiologic trends reveal the important role of rotavirus in adult infectious gastroenteritis, in Shanghai, China. Infect Genet Evol 2017;47 10.1016/j.meegid.2016.11.018 [DOI] [PubMed] [Google Scholar]
- 26. Arena C, Amoros JP, Vaillant V, et al. Acute diarrhea in adults consulting a general practitioner in France during winter: incidence, clinical characteristics, management and risk factors. BMC Infect Dis 2014;14:1–7. 10.1186/s12879-014-0574-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu HX, Zhang J. [Analysis of reported infectious diarrhea (other than cholera, dysentery, typhoid and paratyphoid) in China in 2011]. Zhonghua Yu Fang Yi Xue Za Zhi 2013;47:328–32. [PubMed] [Google Scholar]
- 28. Qi XL, Wang HX, Bu SR, et al. Incidence rates and clinical Symptoms of Salmonella, Vibrio parahaemolyticus, and Shigella infections in China, 1998-2013. J Infect Dev Ctries 2016;10:127–33. 10.3855/jidc.6835 [DOI] [PubMed] [Google Scholar]
- 29. Zhang Y, Zhao Y, Ding K, et al. Analysis of bacterial pathogens causing acute diarrhea on the basis of sentinel surveillance in Shanghai, China, 2006-2011. Jpn J Infect Dis 2014;67:264–8. 10.7883/yoken.67.264 [DOI] [PubMed] [Google Scholar]
- 30. Su YC, Liu C. Vibrio parahaemolyticus: a concern of seafood safety. Food Microbiol 2007;24:549–58. 10.1016/j.fm.2007.01.005 [DOI] [PubMed] [Google Scholar]
- 31. Tang MB, Chen CH, Chen SC, et al. Epidemiological and molecular analysis of human norovirus infections in Taiwan during 2011 and 2012. BMC Infect Dis 2013;13:338 10.1186/1471-2334-13-338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Karsten C, Baumgarte S, Friedrich AW, et al. Incidence and risk factors for community-acquired acute gastroenteritis in north-west Germany in 2004. Eur J Clin Microbiol Infect Dis 2009;28:935–43. 10.1007/s10096-009-0729-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. de Wit MA, Koopmans MP, Kortbeek LM, et al. Gastroenteritis in sentinel general practices, The Netherlands. Emerg Infect Dis 2001;7:82–91. 10.3201/eid0701.010113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gao Z, Liu B, Huo D, et al. Increased norovirus activity was associated with a novel norovirus GII.17 variant in Beijing, China during winter 2014–2015. BMC Infect Dis 2015;15 10.1186/s12879-015-1315-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Eesteghamati A, Gouya M, Keshtkar A, et al. Sentinel hospital-based surveillance of rotavirus diarrhea in iran. J Infect Dis 2009;200(Suppl 1):S244–S247. 10.1086/605050 [DOI] [PubMed] [Google Scholar]
- 36. Iturriza-Gómara M, Dallman T, Bányai K, et al. Rotavirus surveillance in europe, 2005-2008: web-enabled reporting and real-time analysis of genotyping and epidemiological data. J Infect Dis 2009;200(Suppl 1):S215–S221. 10.1086/605049 [DOI] [PubMed] [Google Scholar]
- 37. Liang Z, Ke B, Deng X, et al. Serotypes, seasonal trends, and antibiotic resistance of non-typhoidal Salmonella from human patients in Guangdong Province, China, 2009-2012. BMC Infect Dis 2015;15:53 10.1186/s12879-015-0784-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.