Abstract
Objective
To determine which service models and organisational structures are effective and cost-effective for delivering tuberculosis (TB) services to hard-to-reach populations.
Design
Embase and MEDLINE (1990–2017) were searched in order to update and extend the 2011 systematic review commissioned by National Institute for Health and Care Excellence (NICE), discussing interventions targeting service models and organisational structures for the identification and management of TB in hard-to-reach populations. The NICE and Cochrane Collaboration standards were followed.
Setting
European Union, European Economic Area, European Union candidate countries and Organisation for Economic Co-operation and Development countries.
Participants
Hard-to-reach populations, including migrants, homeless people, drug users, prisoners, sex workers, people living with HIV and children within vulnerable and hard-to-reach populations.
Primary and secondary outcome measures
Effectiveness and cost-effectiveness of the interventions.
Results
From the 19 720 citations found, five new studies were identified, in addition to the six discussed in the NICE review. Community health workers from the same migrant community, street teams and peers improved TB screening uptake by providing health education, promoting TB screening and organising contact tracing. Mobile TB clinics, specialised TB clinics and improved cooperation between healthcare services can be effective at identifying and treating active TB cases and are likely to be cost-effective. No difference in treatment outcome was detected when directly observed therapy was delivered at a health clinic or at a convenient location in the community.
Conclusions
Although evidence is limited due to the lack of high-quality studies, interventions using peers and community health workers, mobile TB services, specialised TB clinics and improved cooperation between health services can be effective to control TB in hard-to-reach populations. Future studies should evaluate the (cost-)effectiveness of interventions on TB identification and management in hard-to-reach populations and countries should be urged to publish the outcomes of their TB control systems.
PROSPERO registration number
CRD42015017865.
Keywords: tuberculosis, public health
Strengths and limitations of this study.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses and Cochrane Collaboration reporting guidelines for systematic reviews were followed.
The search was highly sensitive, but we might have missed important information as many European countries do not publish their tuberculosis identification and management data in journals; our search focused on Embase and MEDLINE.
We identified five studies and discuss the results together with the six studies identified by the National Institute for Health and Care Excellence review to give the complete body of evidence.
None of the included studies was of high quality, and there was high heterogeneity across the studies prohibiting a meta-analysis.
Introduction
Prevention and control of tuberculosis (TB) is based on early detection and diagnosis of TB followed by effective treatment. In 2015, there were an estimated 10.4 million incident TB cases worldwide, an estimated 4.3 million cases were either not diagnosed or diagnosed but not reported to national TB programmes.1 Trends for TB treatment are encouraging, with most notified TB cases completing their treatment successfully, although treatment success rates in some regions, such as the European region, were considerably below the WHO World Health Assembly target of 85%.1
In many countries with a low TB incidence (less than 10 TB cases per 100 000 population),2 TB prevails in the big cities where vulnerable and hard-to-reach (underserved) populations are concentrated.3 These populations, such as people who are homeless (or have insecure accommodation), misuse drugs or are migrants, are at higher risk of contracting TB and are more likely unable or unwilling to seek medical care and comply with the long-term TB treatment. Managing TB in those populations is therefore challenging, due to barriers caused by stigma, cultural barriers, poor access to healthcare services and low levels of accurate TB knowledge.4–7 This therefore requires special efforts. Healthcare services need to be organised effectively to identify and diagnose TB cases and to provide adequate treatment and support. This can be organised in different ways, for example, mainly as hospital based8 or health centre based,9 including the public sector, private sector,10 or civil society and other partners.11 Sometimes, organisation of the services has proven ineffective in managing TB.12
The review question of this systematic review with a scoping component was: ‘Which service models and organisational structures, including different types of healthcare workers and settings, are effective and cost-effective for delivering TB services to hard-to-reach populations in low- and medium-incidence countries?’.
Findings of this review and the previously published review series4 13 formed the base for the guidance document by the European Centre for Disease Prevention and Control (ECDC) on controlling TB in hard-to-reach and vulnerable populations.14
Methods
In 2011, the Matrix Knowledge Group published a review, commissioned by the National Institute for Health and Clinical Excellence (NICE), on effectiveness and cost-effectiveness of service models or structures, focusing on the type of healthcare worker and setting, to identify and manage TB in hard-to-reach populations. We updated and extended the NICE review15 using the same methodology but adjusting the focus by excluding latent TB infection and including additional hard-to-reach populations. The review was conducted following standards described by the Cochrane Collaboration16 and NICE methods guidelines.17 Results are reported according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.18 The review protocol was registered in advance in the database of prospectively registered systematic reviews in health and social care, PROSPERO (CRD42015017865).
Selection of studies and data management
The same search strategy as for the previous NICE review15 and the previous published review by Heuvelings et al 13 was used, searching Embase and MEDLINE through the Ovid platform. The search was expanded by including all European Union (EU)/European Economic Area and EU candidate countries to the Organisation for Economic Co-operation and Development countries (see box 1).15 Two hard-to-reach populations (people living with HIV and children within vulnerable and hard-to-reach populations) were added in addition to the hard-to-reach populations included by the NICE review (migrants including refugees, asylum seekers and the Roma population, homeless people including rough sleepers and shelter users, drug users, prisoners and sex workers).15 The update of the search conducted for the NICE review15 covered the period 1 January 2010 (overlapping the end of the search period of the NICE review15 with a few months) to 24 February 2017. The search for the expanded geographical area and newly included hard-to-reach populations covered a time period from 1 January 1990 (beginning of the search period used in the NICE review15) to 24 February 2017.
Box 1. Inclusion/exclusion criteria for this review.
Inclusion criteria
Discussing service models and organisational structures, different types of healthcare workers and settings for delivering TB services to hard-to-reach populations.
Having been conducted in any of the EU/EEA countries (only updated review), the candidate countries* (only updated review) and the other OECD countries.†
Having been published in 2010 or later for the OECD countries.†
Having been published in 1990 or later for the EU/EEA countries and the EU candidate countries* not being one of the OECD countries (only updated review).
-
Including data from any hard-to-reach population:
Homeless people.
People who abuse drugs or alcohol.
Sex workers.
Prisoners or people with a history of imprisonment.
Migrants, including vulnerable migrant populations such as asylum seekers, refugees and the Roma population.
Children within vulnerable and hard-to-reach populations (only updated review).
People living with HIV (only updated review).
Present quantitative empirical data.
Being a (cost)-effectiveness study or any other type of quantitative primary research, discussing (cost-)effectiveness.
Exclusion criteria
Latent TB infection (only updated review).
Systematic review (only used for reference searching).
*EU candidate countries: Albania, Montenegro, Serbia, the former Yugoslav Republic of Macedonia and Turkey.
†OECD countries: Australia, Austria, Belgium, Canada, Chile, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Israel, Italy, Japan, Korea, Luxembourg, Mexico, the Netherlands, New Zealand, Norway, Poland, Portugal, Slovak Republic, Slovenia, Spain, Sweden, Switzerland, Turkey, UK and USA.
EU, European Union; EEA, European Economic Area; OECD, Organisation for Economic Co-operation and Development; TB, tuberculosis.
Reference lists of relevant systematic reviews were scanned. No language restrictions were applied.
Studies focusing on the effectiveness and/or cost-effectiveness of interventions for service models and organisational structures supporting TB identification and management of hard-to-reach populations (see box 1) were included.
Predefined interventions were using more convenient locations (like specialised TB centres, shelters for homeless people or drug users, needle exchange/methadone programme locations, port of arrival, schools or mobile clinics) and peers or healthcare workers with the same ethnic or cultural background; however, other interventions could also be included if they supported TB identification or management in hard-to-reach populations. TB identification tools, TB diagnostics, incentives, social support, directly observed therapy and treatment of comorbidities are discussed in another review.13 In this review, we aim to identify the effectiveness of the type of health worker and setting to identify and manage TB in hard-to-reach and vulnerable populations.
The comparator was defined during the review process; interventions were compared with a relevant comparator, for example, usual care or no intervention, another intervention or historical comparison.
Outcomes were defined as any measure of TB identification and management (eg, number of people screened, screening coverage, proportion receiving treatment and treatment completion rate). Effectiveness was defined as an improvement in any measure of TB identification and/or management. Randomised and non-randomised studies were eligible for inclusion.
See online supplementary material I for the PROSPERO study protocol, online supplementary material II for Population-Intervention-Comparator-Outcome-Study design) questions and online supplementary material III for the complete search strategy and search results.
bmjopen-2017-019642supp001.pdf (128.2KB, pdf)
bmjopen-2017-019642supp002.pdf (298.9KB, pdf)
bmjopen-2017-019642supp003.pdf (246.2KB, pdf)
Data extraction, data items and synthesis
Identified citations were entered into an EndNote database, and duplicates were removed (EndNote X7.1, Thomson Reuters 2014). The inclusion criteria were piloted and refined using the first 25 citations. Double screening was conducted by one reviewer screening 100% of the citations (CCH), while another two reviewers screened 50% of the citations each (PFG and SGdV) for inclusion on title and abstract. Disagreement was resolved by discussion. Full-text files of included citations were retrieved; irretrievable articles (not available after attempts online, from the university library or through contacting authors) were excluded. Two reviewers assessed full-text records for inclusion (CCH and PFG). Disagreement was resolved by discussion. Agreement after screening on title and abstract was 99.6% with an inter-rater reliability (Cohen’s kappa) of κ=0.985.
Data extraction forms from the NICE review15 were used to extract information on participant characteristics, settings, types of services/organisational structures, types of healthcare workers delivering the service, outcome measures, methods of analysis and results. For one study, data extraction was conducted by two reviewers (CCH and PFG) independently. For the remaining studies, data extraction was conducted by one reviewer (CCH) and checked by a second (PFG); disagreement was resolved by discussion. In one case, the study author was contacted to verify data and obtain additional data.19
To facilitate comparability, data synthesis was structured in a similar way to that of the NICE review.15 Studies were divided into those examining service models and organisational structures for TB identification (screening) and those examining service models and organisational structures for TB management (treatment and support) in hard-to-reach populations. Data were analysed narratively, and appropriateness of meta-analysis was considered. Findings were reported as stated by the study authors.
Risk of bias in individual studies and overall strength of evidence
The modified NICE Quality Assessment Tools17 (based on the Graphical Appraisal Tool for Epidemiological studies) were used to assess quality and risk of bias of included studies. This included an assessment of selection of study sample, minimisation of selection bias and contamination, controlling confounding, outcome measurements, analytical methods and risk of bias. Two reviewers (CCH and PFG) assessed one study independently; the remaining studies were assessed by one reviewer (CCH) and checked by a second reviewer (PFG). Any disagreement was resolved by discussion. Studies were given a quality rating based on the quality assessment: high quality [++], medium quality [+] or low quality [−]. The strength of the evidence was assessed and reported as described in the previous NICE review15 (online supplementary material IV).
bmjopen-2017-019642supp004.pdf (233.2KB, pdf)
Patient and public involvement statement
Patient and public were not involved in the design of this systematic review.
Results
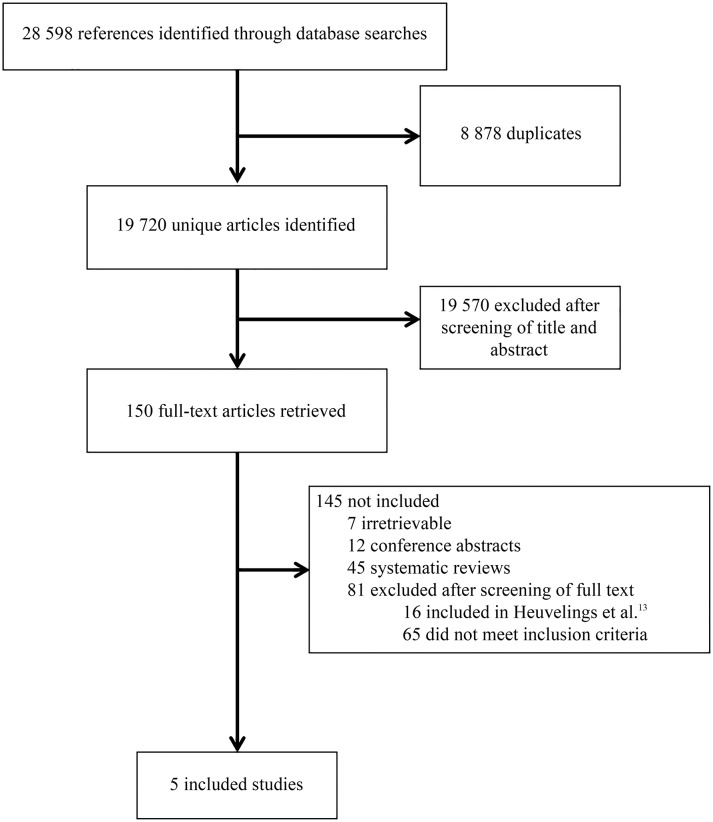
Of the 19 720 citations identified by the literature search five studies were included in this review (figure 1).11 19–22 These five studies are in addition to the six studies23–28 included in the NICE review.15 The results section in this paper focuses on the evidence of the five studies identified in our updated review. The evidence statements (presented in online supplementary material IV) summarise evidence identified in terms of consistency, quality and applicability, combining evidence from the NICE review15 and this update.
Figure 1.
Study selection process.
All five studies were conducted in the EU; two in the UK,19 22 one in Germany,20 one in Portugal11 and one in Spain.21 Two studies focused on homeless people,19 20 one on homeless people and drug users,22 one on drug users alone11 and one on migrants.21 Four studies5 19–21 addressed the influence of the type of healthcare worker on TB identification and TB management and one study focused on the influence of different settings on TB identification.22 A variety of study designs were included: one study was a prospective cluster randomised controlled trial (RCT),19 one was an economic evaluation using a compartmental model of treated and untreated active TB cases22 and three studies were retrospective comparison studies.11 20 21 Study characteristics of included studies are described in table 1. The data extraction forms by study are presented in online supplementary material V.
Table 1.
Characteristics of studies applying different service models and organisational structures to improve TB identification and TB management
| First author (year), country | Population | Aims | Intervention | Comparator | Study design | Outcome measure | Quality score |
| TB identification (studies identified by this review) | |||||||
| Jit22 (2011), UK |
Homeless people and drug users. | To assess the effectiveness and cost-effectiveness of the Find and Treat service for diagnosing and managing hard-to-reach individuals with active TB in London. | Period 2007–2010: Find and Treat service:
|
Passive case detection and standard treatment at a London TB clinic. | Observational and cost-effectiveness study. | Identified TB cases, treatment completion, lost to follow-up and incremental costs from healthcare taxpayer perspective. | + |
| Duarte11 (2011), Portugal |
Drug users. | To evaluate the effect of an intervention with key partners (TB clinic, drug users support centres, shelters, street teams, public health department and hospital) delivering promotion of health-seeking behaviour, eliminating potential barriers for TB screening at a chest clinic and DOT on identifying TB cases and treatment compliance. | Improved cooperation of key partners (2005–2007):
|
Period before the intervention (2001–2003):
|
Before–after study. | Identified TB cases and treatment compliance. | − |
| Goetsch20 (2012), Germany |
Homeless people and drug users. | To estimate the coverage of a low-threshold CXR screening programme for pulmonary TB among illicit drug users and homeless persons. | CHWs providing TB education and promoting voluntary CXR screening 1–2×/year. | Comparing the beginning of the 5-year intervention period with the end (2002–2007). | Retrospective effectiveness study. | Screening coverage. | − |
| Ospina21 (2012), Spain |
Migrants. | To evaluate the effectiveness of an intervention with CHWs to improve contact tracing among migrants. | CHWs active follow-up of cases and contacts, including visits of the cases at home, accompanying at outpatient appointments, providing counselling and information on treatments (2003–2005). | Preintervention period (2000–2002). | Before–after study. | Number of migrants who were included in contact tracing. | + |
| Aldridge19 (2015), UK |
Homeless people. | To compare TB screening uptake between current practice of encouraging homeless people by shelter staff and encouragement by shelter staff plus volunteer peer educators. | Encouragement of TB screening by peers in addition to shelter staff. | Encouragement of TB screening by shelter staff only. | Cluster RCT. | Screening uptake. | + |
| TB identification (studies identified by the previous NICE review15) | |||||||
| El-Hamad24 (2001), Italy |
Migrants | To compare the completion rates of screening procedures for TB infection among undocumented migrants at specialised TB units and non-specialised health clinics. | TB screening at specialised TB clinic. | TB screening at a general health service for migrants. | Prospective cohort. | Screening completion. | + |
| Bothamley25 (2002), UK |
Migrants and homeless people. | To compare the yield and costs of TB screening in three settings: a new entrants’ clinic within the POA scheme; a large general practice; and centres for the homeless. | TB screening at a GP. | TB screening at POA and at homeless centres. | Cost analysis. | Cost per person screened per case of TB prevented. | − |
| Deruaz28 (2004), Switzerland |
Migrants, alcohol or drug users, homeless people and prisoners. | Evaluation of first experience of the DOT programme for TB introduced in the Canton of Vaud in 1997. |
|
|
Before–after study. | Adherence to treatment and outcome. | − |
| Miller26 (2006), USA |
Homeless people and prisoners. | To evaluate and compare the efficiency of a non-state-law-mandated TB screening programme for homeless persons with a state-law-mandated TB screening programme for prisoners. | Non-state-law-mandated TB screening programme for homeless persons. | State-law-mandated TB screening programme for prisoners. | Retrospective comparison of the cost and health impacts. | TB cases averted and cost. | + |
| Ricks23 (2008), USA |
Drug users. | To compare the effectiveness of using peers versus ‘standard’ public health workers to coordinate TB treatment. | Enhanced case management by peers. | Limited case management by healthcare professionals. | RCT. | Adherence to treatment. | ++ |
| Mor27 (2008), Israel |
Migrants. | To examine the effectiveness and cost-effectiveness of premigration screening and postmigration screening at POA. | Premigration screening. | Postmigration screening. | Retrospective cohort analysis. | Active TB cases, time between migration and diagnosis, and cost-savings. | − |
Study quality: high quality [++], medium quality [+] or low quality [−].
CHWs, community health workers; CXR, chest X-ray; DOT, direct observed treatment; GP, general practice; MXU, mobile X-ray unit; n, number of participants; POA, port of arrival; RCT, randomised controlled trial; TB, tuberculosis.
bmjopen-2017-019642supp005.pdf (311.7KB, pdf)
None of the included studies in this review had a low risk of bias, three studies19 21 22 had a medium risk of bias and the other two studies5 20 were assessed as having a high risk of bias (online supplementary material VI).
bmjopen-2017-019642supp006.pdf (149.6KB, pdf)
We did not perform a meta-analysis due to study heterogeneity. Results were synthesised narratively.29
Main outcomes for services structures and organisational models for TB identification among hard-to-reach populations, combined with the findings of the NICE review,15 are summarised in table 2. For full evidence statements, see online supplementary material IV.
Table 2.
Effectiveness of service models and organisational structures interventions to improve TB identification and TB management
| Population | Intervention (I) | Comparator (C) | Studies (first author, year, country) | No. of participants | Comparison | Outcome | Risk of bias | |
| I | C | |||||||
| Homeless people | Health/TB education and promotion of screening by street teams, drug users support centres, shelters and CHWs. | Beginning of the intervention when CHWs were just introduced. | Goetsch,20 2012, Germany. | 465 | 125 | Retrospective comparison over intervention period. | Improved annual TB screening uptake among homeless people and drug users (from 10.0% to 15.0% at the peak).20 The percentage of all drug users with active TB identified by screening increased from 13.4% to 61.0% (OR 10.1 (95%CI 4.44 to 23.0)).11 | High* |
| Drug users | No active screening policy. | Duarte,11 2011, Portugal. | Retrospective before–after comparison. | High† | ||||
| Homeless people | TB education and promotion of screening by peers and shelter staff. | TB education and promotion of screening by shelter staff only. | Aldridge,19 2015, UK. | 1150 | 1192 | Comparing randomised intervention cluster with comparator cluster. | No difference in screening uptake (I=40% (IQR 25–61) versus C=45% (IQR 33–55), aRR=0.98 (95% CI 0.80 to 1.20)). | Medium‡ |
| Migrants | Premigration screening | Postmigration screening at POA. | Mor,27 2008, cited in the NICE review, Israel. | 162 | 105 | Retrospective Intervention versus comparator comparison. | Reduced the risk of developing TB in the new country and was cost-effective (0.28% of the premigration versus 0.32% of the postmigration screening migrants developed TB; RR 0.82, p<0.01). The detection period was shorter as well (193 days vs 487 days between entry and diagnosis; OR=0.72 (95% CI 0.59 to 0.89) p=0.002). | High§ |
| Prisoners and homeless people | TB screening in a prison. | TB screening at a homeless centre. | Miller,26 2006, cited in the NICE review, USA. | 22 920 | 822 | Retrospective comparison of two cohorts. | No difference in screening uptake (94.7% in prison vs 95% in homeless centre p=0.179) but higher proportion of active TB cases were identified at the homeless centre (1.2% vs 0.03% at a prison setting, p<0.001). | Medium¶ |
| Homeless people and migrants | Active case finding by symptom-based questionnaire at homeless centres. | Active case finding by symptom-based questionnaire at POA. | Bothamley,25 2002, cited in the NICE review, UK. | 262 | 199 | Cost analysis. | Active case finding at POA was most cost-effective (costs per person screened for every case prevented at POA £10.00, at homeless centre £23.00). | High** |
| Migrants | Active case finding at a specialised TB clinic using two visits. | Active case finding at a general primary care clinic, with referral for CXR, using three visits. | El-Hamad,24 2001, cited in the NICE review, Italy. | 749 | 483 | Prospective intervention versus comparator comparison. | Improved screening completion among migrants (85.6% in TB clinic vs 71.4% at primary care clinic, p=not reported; OR=2.57 (95% CI 1.92 to 3.42)). | Medium†† |
| Drug users | Contact tracing by peers or CHWs from the same migrant community. | Peers versus other healthcare workers. | Ricks,23 2008, cited in the NICE review, USA.48 | 46 | RCT | Improved contact tracing among drug users (75% by peers vs 47% by healthcare workers, p=0.03)23 and migrants (from 55.4% without CHWs to 66.2% with CHWs; aOR 1.8 (95% CI 1.3 to 2.5) p<0.001).21 | Low | |
| Migrants | Normal practice before introducing CHWs. | Ospina, 21 2012, Spain. |
388 | 572 | Before–after comparison. | Medium‡‡ | ||
| Drug users and homeless people | Mobile TB screening and treatment service at convenient location in the community. | Passive case detection and management at a TB clinic. | Jit,22 2011, UK. | 48 | 252 | Prospective intervention versus comparator comparison plus economic evaluation. | Improved TB identification among homeless people and drug users; particularly in asymptomatic patients (35.4% extra identified) and those who delay seeking healthcare (22.2% extra identified). Higher treatment completion rate (67.1% vs 56.8%) and lower lost to follow-up rate (2.1% vs 17.2%). Both parts of the service are cost-effective (screening= £18 000/QALY gained, treatment is £4100/QALY gained). | Medium§§ |
| Drug users | Enhanced case management by peers. | Limited case management by regular healthcare workers. | Ricks,23 2008, cited in the NICE review, USA. | 48 | 46 | RCT | Improved treatment completion in drug users (85% by peers vs 61% by healthcare workers, RR=2.68 (95% CI 1.24 to 5.82) p=0.01). | Low |
| Drug users | DOT and active follow-up of non-compliant patients by ‘key partners’. | Non-compulsory TB treatment and education about TB disease and treatment to improve compliance. | Duarte,11 2011, Portugal. | 465 | 125 | Retrospective before–after comparison. | Reduced treatment default rates (from 35.4% to 10.2%; OR 0.21 (95% CI 0.08 to 0.54)). | High** |
| Migrants, drug users, homeless people and prisoners | DOT at a convenient location in the community. | DOT at a health clinic. | Dèruaz,28 2004, cited in the NICE review, Switzerland. | 36 | 18 | Retrospective before–after comparison. | No significant difference in successful treatment outcome, treatment completion and cure rate (85.2% at convenient location vs 92.6% at health clinic, p=0.67). | High¶¶ |
Footnotes risk of bias:
*Not adjusted for important confounding factors (intervention and comparator group were recruited over different time periods). Denominator not given therefore unable to calculate screening coverage.
†Risk of selection bias as participation was voluntary. Not adjusted for important confounding factors (intervention and comparator group were recruited over different time periods). No statistical test used to show statistical significance of the findings; an estimated number was used for the denominator.
‡Most comparator sites were not naïve for peer intervention, no individual information of the participants was collected and the characteristics between the two groups might have been significantly different.
§Not adjusted for important confounding factors (intervention and comparator group were recruited over different time periods), premigration group had a shorter follow-up period than postmigration group what may have influenced the detection of number of TB cases in the premigration group.
¶Unclear if the differences in outcome was caused by the setting or by the different methods or to differences in TB prevalence in the different populations.
**TB prevalence might be different in the different populations as the costs are calculated per active case detected this is a major issue, there were only three active TB cases detected, all in the POA group. The economic perspective used was not reported, and the costs of identification were not discounted.
††Not adjusted for difference in baseline characteristics.
‡‡Not adjusted for important confounding factors (intervention and comparator group were recruited over different time periods). Contact tracing of only one contact was enough to be called contact tracing, and the ultimate aim of contact tracing (increase cased detection and reduce transmission) was not analysed in this study.
§§Study was designed to evaluate the cost-effectiveness, no statistical test used to evaluate statistical significant findings. The ‘Find and Treat’ service identifies extremely hard-to-reach populations that would never self-present, and the findings would underestimate the benefit of the service. The economical evaluation is based on a compartmental model that does not take secondary transmission and drug resistance into account.
¶¶Risk of bias due to difference in collecting treatment adherence outcome at the health clinic a nurse recorded treatment adherence at time of visit, in the social outreach group a healthcare worker was interviewed up to 6 months after treatment completion and was asked about the treatment adherence, risk of recall bias. Not recorded how many people per setting received 6 months of DOT (full DOT) and how many received 2 months of DOT and 4 months of self-treatment (partial DOT), what was another intervention in this study. Allocation to setting was based on needs of participants what might have caused bias.
aOR, adjusted ORs; aRR, adjusted risk ratio; CHWs, community health workers; CXR, chest X-ray; DOT, directly observed treatment; POA, port of arrival; QALYs, quality-adjusted life years; RCT, randomised controlled trial; TB, tuberculosis.
Three studies19–21 compared the effect of the type of healthcare worker on TB identification.
In the UK, a cluster randomised trial found that peer educators working together with shelter staff to encourage homeless people to participate in a TB screening programme using mobile X-ray units did not improve screening uptake compared with encouragement by shelter staff only (respectively 40%, IQR 25–61 vs 45%, IQR 33–55; adjusted risk ratio 0.98, 95% CI 0.80 to 1.20).19 Control sites were not ‘naïve’ for peer intervention, which could have caused contamination of the control sites and contributed to the negative finding.
In Germany, introduction of TB education and promotion of voluntary chest X-ray screening at least once every 2 years by community health workers (CHWs) improved screening uptake in homeless people and drug users. Annual screening coverage increased from 10.0% at the beginning of the study period (2002–2004) to 15.0% during the middle part of the study period (2004–2006); the last part of the study period had a 13.4% annual screening coverage (2005–2007). Screening once every 2 years increased screening coverage from 18.0% (2002–2004) to 26.4% (2004–2006). Coverage was 23.4% at the third and final study period (spanning 2005–2007).20 The authors did not test for statistical significance, and denominator data (the number of homeless people and drug users in the study area) were estimated.
In Barcelona, Spain, contact tracing organised by CHWs coming from the same migrant community as the person diagnosed with TB improved contact tracing among migrants to 66.2% (2003–2005) compared with 55.4% (2000–2002) in the period before the implementation of the intervention using CHWs (adjusted OR of an index case having their contacts screened before and after the intervention was 1.8, 95% CI 1.3 to 2.5, p<0.001).21 Identification and tracing of at least one contact was taken as appropriate contact tracing, where all contacts at risk should be traced to detect and treat TB transmission early. The population characteristics varied, and the age and country of origin were different between both periods. The importance of contact tracing is to identify cases early to reduce transmission; the authors did not report if any of the contacts traced had active TB.
Two studies11 22 evaluated the effect of the type of healthcare worker and the setting on TB identification and TB management.
In Portugal, improved cooperation of ‘key partners’ (street teams, TB clinics, drug user support centres, local public health department and local hospital) for TB identification and management in drug users was evaluated in a before-and-after study. Representatives of all ‘key partners’ (authors’ term) worked on improving policies, clinic screening procedures and cooperation. Key partners were trained in identifying drug users in their population, and offering health promotion, notification cards, free transport to the TB clinic, free medical and substance abuse care, directly observed therapy (DOT) for active TB cases, identification of non-compliant patients and the cause of non-compliance and tailor-made strategies to improve compliance. This resulted in an increase of TB screening uptake, from 52 drug users being screened before the intervention (2001–2003 when there was no active screening policy) to 465 drug users screened thereafter (2005–2007). Of all people misusing drugs taking up screening, the proportion without TB symptoms increased from 41.6% to 93.5% (OR=21.76; 95% CI 13.03 to 36.33) indicating improved TB awareness and access to screening facilities for drug users. Of all drug users with active TB, the proportion identified by screening increased from 13.4% to 61.0% (OR 10·1; 95% CI 4.44 to 23.0). Treatment default rates decreased from 35.4% to 10.2% (OR 0.21, 95% CI 0.08 to 0.54), compared with the period before the intervention (2001–2003) when TB treatment was not compulsory and compliance was stimulated by TB education and providing information on the importance of treatment completion.11 Although the absolute number of drug users screened increased, information on the screening coverage was not available as denominator data were not provided. Another limitation is that the results were not adjusted for confounding factors, baseline characteristics might have been different as the two cohorts were recruited over different time periods and participation was voluntary which may have led to selection bias.
In the UK, the effectiveness and cost-effectiveness of the ‘Find and Treat’ service (raising awareness of TB screening and providing a mobile TB screening and treatment service) for homeless people and drug users was evaluated and compared with people (with a history of homelessness, imprisonment, drug abuse or mental health problems) self-presenting to a London TB clinic receiving standard TB care at the clinic.22 The authors estimated that 22.9% of the patients detected by the ‘Find and Treat’ service with the longest first symptom-to-detection time would not have self-presented plus 35.4% were asymptomatic at time of detection and would not have self-presented, only part of the asymptomatic patients would self-present to a TB clinic at a later stage when symptoms would have developed. The ‘Find and Treat’ service had a higher treatment completion rate (67.1% vs 56.8%) and a lower lost to follow-up rate (2.1% vs 17.2%) compared with the control group receiving standard TB care at a TB clinic. The authors concluded that the ‘Find and Treat’ service was cost-effective when using the threshold used by NICE of £20 000 to £30 000/QALY gained, with an incremental cost ratio of £18 000 per QALY gained for the TB screening service and £4100 per QALY gained for the TB management service. This study has a few limitations: first, it is a non-randomised study, second, the ‘Find and Treat’ service identifies extremely hard-to-reach populations of which some would never self-present, therefore the findings could be even better in less hard-to-reach populations, and third, the economical evaluation is based on a compartmental model that does not take secondary transmission and drug resistance into account.
Discussion
To tackle TB and disrupt transmission in high-income, low TB incidence settings, improvement of TB care in hard-to-reach populations is of vital importance. In this updated review, five studies,11 19–22 published between 1 January 2010 and 24 February 2017, evaluating effectiveness of services models and organisational structures supporting TB identification and management of hard-to-reach populations, were identified in addition to the six studies considering active TB23–28 identified by the NICE review.15 Only one study22 evaluated cost-effectiveness. Although the evidence from two reviews is limited, it highlights those interventions that are likely to be effective and those that have no clear evidence of being effective (table 2). For development of the ECDC guidance document,14 a scientific panel compiled by ECDC carefully considered these findings. Their main suggestions for action were to involve CHWs or peers to improve TB screening uptake and TB treatment completion among homeless people20 and drug users5 20 23; to use outreach teams to improve TB screening uptake and TB treatment completion among vulnerable populations22; and to strengthen relationships and good collaboration between healthcare workers, peers, communities and patients to improve treatment outcome among vulnerable populations.5 20 22 23 The updated systematic review provided evidence for all suggestions except for using peers to improve screening uptake. This is in contrast to an American study23 included in the original NICE review,15 which showed that peers improved contact tracing and treatment adherence among drug users.
Strengths and limitations
PRISMA and Cochrane Collaboration reporting guidelines for systematic reviews were followed. Established screening protocols were used, including double screening, and the search was highly sensitive. The methodology from the previous NICE review15 was followed, in order to connect this update and, so, describe the full body of relevant evidence. High-quality evidence is lacking. Only one23 study from the NICE review15 was considered to be of high quality; all other studies had some risk of bias (five medium risk19 21 22 24 26 and five high risk11 20 25 27 28). Therefore, only limited conclusions can be drawn. Most studies lacked identification and adjustment for confounding factors and the use of appropriate analytical methods. In addition, many studies were biased, particularly with regard to potential selection bias. A meta-analysis could not be performed because of heterogeneity across the studies. Gaps in evidence exist; no studies focusing on children within vulnerable and hard-to-reach populations or on people living with HIV or sex workers were identified. Only three studies provided economic data; one study identified by this review22 and two studies25 27 by the NICE review.15
Our search focused on publications in databases Embase and MEDLINE. Many European countries have strong organisational structures for TB identification and management, but these countries did not publish their data on these organisational structures in journals, which may have caused a publication bias. Comparing findings of the NICE review15 with this review comes with some limitations. For the NICE review, only 10% of the citations were double screened,15compared with 100% for this updated review; therefore, studies conducted between 1990 and 2010 might have been missed. The NICE review focused their recommendations on the population in the UK,15 and this review focused on populations in high-income, low TB incidence countries. Further methodology was identical.
The evidence identified by this review and the previous NICE review15 along with evidence presented in a review series covering the barriers and facilitators of seeking TB care,6 and the effectiveness of interventions for TB identification and management in hard-to-reach populations,13 was used to develop the ECDC guidance on improving TB identification and management among hard-to-reach and vulnerable populations in Europe.14 ECDC recommended that implementation of the interventions is context specific; it depends on the setting, target population, resources available and healthcare systems in place. Interventions focusing on one specific hard-to-reach population might not work in another hard-to-reach population; therefore, the interventions have to be adapted and reassessed per target population.14 Given the scope of this review, considering settings across Europe, findings presented here are potentially relevant to any low incidence region and are relevant to other institutions/governmental organisations seeking to improve service structures for TB identification and management among hard-to-reach populations.
Characteristics of different hard-to-reach populations and their TB epidemiology vary per country and setting. Challenges in identification and management of TB should be identified and targeted, tailored to the specific setting and hard-to-reach population. These TB interventions could be integrated within broader programmes targeting specific populations. A follow-up systematic review should include information from national public health services about their organisational structures for TB identification and management. National public health services are urged to regularly analyse their organisational structures for TB identification and management and publish these data.
Efforts to improve quality of research on service models and organisational structures should be made, even though it is often challenging to perform ‘clean’, unbiased and unconfounded trials in hard-to-reach populations, as attrition rates are often high, and confounding factors are plentiful. This includes conducting (cluster) RCTs and before-and-after studies where appropriate, recruiting an adequate number of participants, using relevant control groups and minimising selection bias. Standardised case definitions for hard-to-reach populations should be created. Feasibility, effectiveness, cost-effectiveness and impact of interventions should be evaluated. Mathematical economic models can be used to evaluate costs.14
Conclusions
Identification and management of TB in hard-to-reach populations is suboptimal.2 Therefore, service models and organisational structures to identify and manage TB in hard-to-reach populations should be improved and evaluated regularly.
Our systematic review, in conjunction with the original NICE review,15 provides limited evidence, due to the lack of high-quality studies, that interventions such as using peers and CHWs, mobile TB services, specialised TB clinics, screening or active case finding in non-healthcare settings, as well as improved cooperation between key services can help to improve TB identification and management.
Further research should be undertaken to evaluate other effective and cost-effective ways to identify and manage TB in hard-to-reach populations, and countries with good TB control systems are urged to evaluate their system and publish the data.
Supplementary Material
Footnotes
Contributors: CCH: created the protocol, performed study selection, collected the data, performed quality/risk of bias assessment, synthesised the data, interpreted the data and created the manuscript and supplementary files. PFG: performed the study selection, collected the data, performed quality/risk of bias assessment, synthesised the data, involved in interpretation of the data and contributed to and endorsed the final version of the manuscript. SGdV: created the protocol, performed study selection, involved in interpretation of the data and contributed to and endorsed the final version of the manuscript. BJV: created the protocol, involved in interpretation of the data and contributed to and endorsed the final version of the manuscript. SB, SJ, ALC and AS: involved in interpretation of the data and contributed to and endorsed the final version of the manuscript. RS: conducted the literature search, involved in interpretation of the data and contributed to and endorsed the final version of the manuscript. ES and RAH: expert input especially on the interpretation of the NICE findings, involved in interpretation of the data and contributed to and endorsed the final version of the manuscript. AZ: expert input, involved in interpretation of the data and contributed to and endorsed the final version of the manuscript. MJvdW: involved in the study design, data interpretation and contributed to and endorsed the final version of the manuscript. MPG: is the guarantor of this review, supervised every step in the process, commented and provided input at every stage of the review process, was involved in creating the protocol, interpretation of the data and contributed to and endorsed the final version of the manuscript.
Funding: This study was funded by the European Centre for Disease Prevention and Control (contract reference OJ/02/05/2014-PROC/2014/014). None of the authors have received payment from a pharmaceutical company or other agency to write this article.
Disclaimer: The funder of the study was involved in study design, data interpretation, and reporting. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
Competing interests: MPG reports grants from ECDC, for the conduct of part of the study. ES reports that NICE—her employing organisation—has published guidance in this area.
Patient consent: Not required.
Ethics approval: Ethics approval was not required for this systematic review.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Data extraction forms and quality assessment forms are available from supplementary files V and VI.
References
- 1. World Health Organization (WHO). Global Tuberculosis report 2016. Geneva: WHO, 2016. WHO/HTM/TB/2016.13. [Google Scholar]
- 2. World Health Organization (WHO) and European Respiratory Society. Towards TB elimination: an action framework for low-incidence countries. Geneva: WHO, 2014. WHO/HTM/TB/2014.13. [Google Scholar]
- 3. de Vries G, Aldridge RW, Cayla JA, et al. . Epidemiology of tuberculosis in big cities of the European Union and European Economic Area countries. Euro Surveill 2014;19 10.2807/1560-7917.ES2014.19.9.20726 [DOI] [PubMed] [Google Scholar]
- 4. de Vries SG, Cremers AL, Heuvelings CC, et al. . Barriers and facilitators to the uptake of tuberculosis diagnostic and treatment services by hard-to-reach populations in countries of low and medium tuberculosis incidence: a systematic review of qualitative literature. Lancet Infect Dis 2017;17:e128–43. 10.1016/S1473-3099(16)30531-X [DOI] [PubMed] [Google Scholar]
- 5. Kamholz SL. Resurgence of tuberculosis: the perspective a dozen years later. J Assoc Acad Minor Phys 1996;7:83–6. [PubMed] [Google Scholar]
- 6. de Vries G, van Hest RA, Richardus JH. Impact of mobile radiographic screening on tuberculosis among drug users and homeless persons. Am J Respir Crit Care Med 2007;176:201–7. 10.1164/rccm.200612-1877OC [DOI] [PubMed] [Google Scholar]
- 7. van Hest NA, Aldridge RW, de Vries G, et al. . Tuberculosis control in big cities and urban risk groups in the European Union: a consensus statement. Euro Surveill 2014;19 10.2807/1560-7917.ES2014.19.9.20728 [DOI] [PubMed] [Google Scholar]
- 8. Belling R, McLaren S, Boudioni M, et al. . Pan-London tuberculosis services: a service evaluation. BMC Health Serv Res 2012;12:203 10.1186/1472-6963-12-203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ohkado A, Williams G, Ishikawa N, et al. . The management for tuberculosis control in Greater London in comparison with that in Osaka City: lessons for improvement of TB control management in Osaka City urban setting. Health Policy 2005;73:104–23. 10.1016/j.healthpol.2004.11.006 [DOI] [PubMed] [Google Scholar]
- 10. Lei X, Liu Q, Escobar E, et al. . Public-private mix for tuberculosis care and control: a systematic review. Int J Infect Dis 2015;34:20–32. 10.1016/j.ijid.2015.02.015 [DOI] [PubMed] [Google Scholar]
- 11. Duarte R, Santos A, Mota M, et al. . Involving community partners in the management of tuberculosis among drug users. Public Health 2011;125:60–2. 10.1016/j.puhe.2010.09.002 [DOI] [PubMed] [Google Scholar]
- 12. Frieden TR, Fujiwara PI, Washko RM, et al. . Tuberculosis in New York City--turning the tide. N Engl J Med 1995;333:229–33. 10.1056/NEJM199507273330406 [DOI] [PubMed] [Google Scholar]
- 13. Heuvelings CC, de Vries SG, Greve PF, et al. . Effectiveness of interventions for diagnosis and treatment of tuberculosis in hard-to-reach populations in countries of low and medium tuberculosis incidence: a systematic review. Lancet Infect Dis 2017;17:e144–58. 10.1016/S1473-3099(16)30532-1 [DOI] [PubMed] [Google Scholar]
- 14. European Centre for Disease Prevention and Control (ECDC). Guidance on tuberculosis control in vulnerable and hard-to-reach populations. Stockholm: ECDC, 2016. [Google Scholar]
- 15. Rizzo M, Martin A, Jamal F, et al. . Evidence review on the effectiveness and cost-effectiveness of service models or structures to manage tuberculosis in hard-to-reach groups. London: Matrix evidence: NICE – National Institute for Health and Clinical Excellence, 2011. [Google Scholar]
- 16. Higgins J, Green S, Cochrane handbook for systematic reviews of interventions version 5.1.0. London: The Cochrane Collaboration, 2011. [Google Scholar]
- 17. National Institute for Health and Clinical Excellence. Methods for the development of NICE public health guidance. 2nd edn London: National Institute for Health and Clinical Excellence, 2009. [PubMed] [Google Scholar]
- 18. Moher D, Liberati A, Tetzlaff J, et al. . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aldridge RW, Hayward AC, Hemming S, et al. . Effectiveness of peer educators on the uptake of mobile X-ray tuberculosis screening at homeless hostels: a cluster randomised controlled trial. BMJ Open 2015;5:e008050 10.1136/bmjopen-2015-008050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goetsch U, Bellinger OK, Buettel KL, et al. . Tuberculosis among drug users and homeless persons: impact of voluntary X-ray investigation on active case finding. Infection 2012;40:389–95. 10.1007/s15010-011-0238-x [DOI] [PubMed] [Google Scholar]
- 21. Ospina JE, Orcau A, Millet JP, et al. . Community health workers improve contact tracing among immigrants with tuberculosis in Barcelona. BMC Public Health 2012;12:158 10.1186/1471-2458-12-158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jit M, Stagg HR, Aldridge RW, et al. . Dedicated outreach service for hard to reach patients with tuberculosis in London: observational study and economic evaluation. BMJ 2011;343:d5376 10.1136/bmj.d5376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ricks PM. Tuberculosis control among substance users: the indigenous leadership outreach model vs. standard care. Chicago, IL: University of Illinois, 2008. [Google Scholar]
- 24. El-Hamad I, Casalini C, Matteelli A, et al. . Screening for tuberculosis and latent tuberculosis infection among undocumented immigrants at an unspecialised health service unit. Int J Tuberc Lung Dis 2001;5:712–6. [PubMed] [Google Scholar]
- 25. Bothamley GH, Rowan JP, Griffiths CJ, et al. . Screening for tuberculosis: the port of arrival scheme compared with screening in general practice and the homeless. Thorax 2002;57:45–9. 10.1136/thorax.57.1.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miller TL, Hilsenrath P, Lykens K, et al. . Using cost and health impacts to prioritize the targeted testing of tuberculosis in the United States. Ann Epidemiol 2006;16:305–12. 10.1016/j.annepidem.2005.07.053 [DOI] [PubMed] [Google Scholar]
- 27. Mor Z, Lerman Y, Leventhal A. Pre-immigration screening process and pulmonary tuberculosis among Ethiopian migrants in Israel. Eur Respir J 2008;32:413–8. 10.1183/09031936.00145907 [DOI] [PubMed] [Google Scholar]
- 28. Dèruaz J, Zellweger JP. Directly observed therapy for tuberculosis in a low prevalence region: first experience at the Tuberculosis Dispensary in Lausanne. Swiss Med Wkly 2004;134:552–8. doi:2004/37/smw-10643 [DOI] [PubMed] [Google Scholar]
- 29. Popay J, Roberts H, Sowden A, et al. . Guidance on the conduct of narrative synthesis in systematic reviews. A product from the ESRC methods programme. Lancaster, 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2017-019642supp001.pdf (128.2KB, pdf)
bmjopen-2017-019642supp002.pdf (298.9KB, pdf)
bmjopen-2017-019642supp003.pdf (246.2KB, pdf)
bmjopen-2017-019642supp004.pdf (233.2KB, pdf)
bmjopen-2017-019642supp005.pdf (311.7KB, pdf)
bmjopen-2017-019642supp006.pdf (149.6KB, pdf)