Abstract
We report a case of a 70-year-old farmer admitted for viper bite who presented with bilateral hyphema and angle closure attack. He was managed conservatively with topical steroids and cycloplegics. He responded well and was discharged after 2 weeks.
Keywords: anterior chamber, pupil, ophthalmology
Background
Snakebite is an occupational health problem in the tropical countries. The highest incidence and mortality was reported in South and Southeast Asia. The accurate incidence and prevalence of snakebite are unknown because many cases are not reported. Myanmar (Burma) is one of the countries in the ‘Global Burden Region’ with estimated incidence of 17.68–20.67 per 100 000 population with high mortality of 0.69–1.33 per 100 000 population reported in 2008.1 According to public health statistics report (2014–2016) from Myanmar Ministry of Health, the incidence of poisonous snakebite was 17.5 with mortality rate 1.2/100 000 population in the year 2016.2 It is an important health problem in the rice growing areas of Myanmar and 3 years hospital-based study across 87 hospitals in Myanmar between 1998 and 2000 reported that majority of snakebite is accounted by Russell’s vipers (Daboia russelii and Daboia siamensis) 60%, followed by cobra (Naja kaouthia) 6%, green pit viper (Trimeresurus sp) 5%, sea snake 0.4% and unknown 29%. Highest fatality is associated with Russell’s viper and cobra which accounts for 8.2% and 8%, respectively.3
Snake venoms are complex mixture of natural venoms and toxins which has local and systemic envenoming effects.4 Local features include fang marks, pain and burning sensation, bleeding from the wound, localised swelling and tissue necrosis. Systemic envenoming effects are mainly caused by haematotoxins and neurotoxins. Cobra and kraits typically produce neurotoxicity causing flaccid paralysis of the muscles of eyes, tongue, throat and chest leading to respiratory failure. Viper envenoming is characterised by vasculotoxicity, haematotoxicity and coaguloapthies.
The ocular complications are uncommon in snakebite but they may lead to significant sight-threatening conditions. Studies on ocular involvement in venomous snakebite are still under reported.5 Reported ocular features include subconjunctival haemorrhage, hyphema, vitreous, retinal and orbital haemorrhages due to haematotoxicity; acute angle closure attack associated with capillary leak syndrome secondary to haematotoxic envenomation, ophthalmoplegia due to neurotoxicity; panuveitis due to serum sickness secondary to antisnake venoms (ASVs) and direct injury to the eyes causing chemical and mechanical injuries such as globe lacerations.5–8 In this report, we describe a rare ocular presentation of bilateral and simultaneous manifestation of hyphema and angle closure attack following snakebite.
Case presentation
A 70-year-old farmer with no relevant medical and ocular history from Magway region in Central Myanmar was referred to the ophthalmology department, Magway General Hospital with visual loss in both eyes (BE) after unknown snakebite a week prior. He was bitten to the dorsum of his right foot around 10:00 hours while working in a farm and was brought to the emergency department.
On examination, the 20 min whole blood clotting test (20WBCT) was non-clotting (positive), that led to a conclusion as a viper bite. Physical examination revealed a pulse rate (PR) of 70/min, blood pressure (BP) of 140/70 mm Hg, respiratory rate of 20/min and SpO2 of 97%. There was localised swelling at the wound but no active bleeding or tissue necrosis was noted. He was treated with intravenous ASV, 6 hourly intravenous crystalline penicillin 1 million units and 6 hourly intravenous hydrocortisone 100 mg.
The first dose of ASV (viper antivenom eight phials—batch number DD17018, expiry date 11 August 2022 Myanmar Pharmaceutical Factory, Ministry of Industry) was administered immediately at emergency department, approximately 90 min after he was bitten.
The 20WBCT was monitored hourly which revealed non-clotted for next 3 hours so that additional dose of ASV (eight phials) was administered 4 hours after the first dose. Three hours after the second dose of ASV, 20WBCT revealed clotted. The patient received total 16 phials of ASV. On admission, haemoglobin 10.9 g/dL, white cell count 12.3×109/L, platelet 190×109/L, urea 46.6 mg/dL, creatinine 1.06 mg/dL and urine output was 200 mL for first 24 hours. The other tests for clotting profile (PT, INR and APTT) were not performed due to limited facilities. The treatment continued.
On day 3, he could not open his eyes associated with bilateral periorbital swelling. By the same time, his general condition became critical as he developed breathlessness, mild pleural effusion and ascites. PR 56/min, BP 120/70 mm Hg, SpO2 93% and the blood creatinine dramatically increased to 3.44 mg/dL and urine output was 200 mL/24 hours. He was treated as acute renal failure and 8 hourly intravenous frusemide 120 mg was given but urine output became 50 mL on day 4. He had first dialysis on day 4, and subsequently on days 6, 8 and 10. Platelet counts fell to 120×109/L on day 5 for which PRP 3 units were administered on days 5, 6 and 8. The PR ranged from 56 to 70/min, BP 120–140/70 mm Hg and SpO2 from 93% to 97% throughout the illness. Details of laboratory results, fluid intake and urine output are shown in tables 1 and 2.
Table 1.
Blood tests results
| Investigation | Day 1 | Day 3 | Day 5 | Week 1 | Week 2 |
|---|---|---|---|---|---|
| Hb (g/dL) | 10.9 | 8.7 | 8.2 | 7.2 | 8.8 |
| WCC (×109/L) | 12.32 | 26.71 | 16.83 | 11.20 | 6.03 |
| Platelet (×109/L) | 190 | 160 | 120 | 380 | 440 |
| Urea (mg/dL) | 46.6 | ||||
| Creatinine (mg/dL) | 1.06 | 3.44 | 8.72 | 8.67 | 1.43 |
| K+ (mEq/L) | 3.06 | 3.69 | 3.38 | 3.33 |
Hb, haemoglobin; WCC, white cell count.
Table 2.
Urine output and albuminuria
| Day | Fluid intake mL/24 hours | Urine output mL/24 hours | Urine albumin | Remark |
|---|---|---|---|---|
| 1 | 200 | 200 | ||
| 2 | 500 | 200 | 3+ | |
| 3 | 300 | 200 | 4+ | |
| 4 | 800 | 50 | 4+ | First dialysis on day 4 |
| 5 | 700 | 10 | 4+ | |
| 6 | 400 | 30 | 4+ | Second dialysis on day 6, ophthalmic consultation was made |
| 7 | 400 | 50 | ||
| 8 | 500 | 100 | 1+ | Third dialysis on day 8 |
| 9 | 1000 | 100 | 1+ | |
| 10 | 600 | 200 | 1+ | Fourth dialysis on day 10 |
| 11 | 500 | 200 | ||
| 12 | 500 | 400 | Trace | |
| 13 | 1000 | 800 | ||
| 14 | 1000 | 950 |
When the periorbital swelling relieved 3 days later, he was able to open the eyes and noticed the visual loss and ophthalmic consultation was made. By the time he was first seen by ophthalmologist, it was day 6 after viper bite and he had renal dialysis for two times.
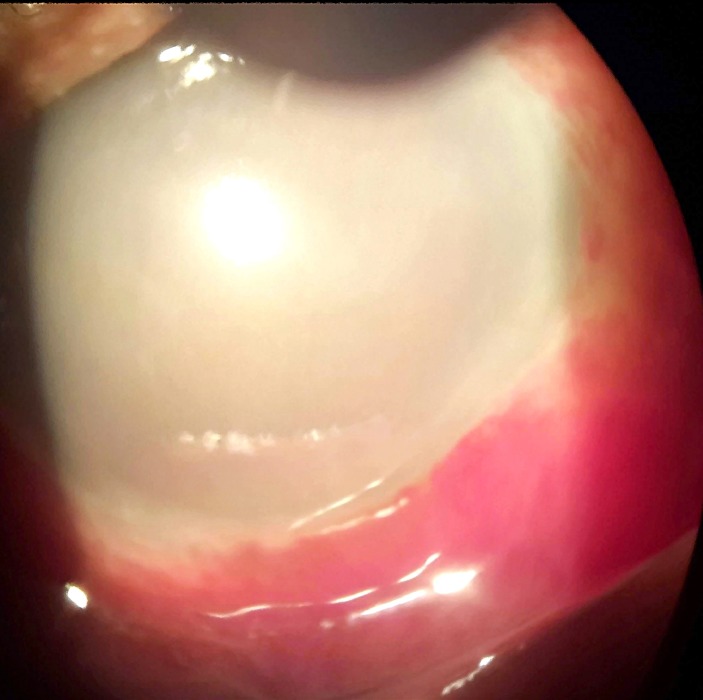
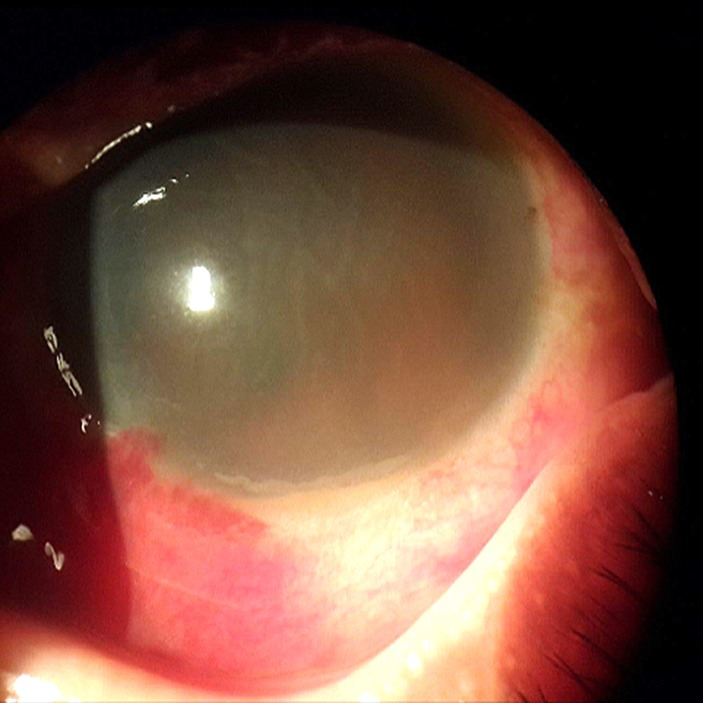
Initial visual acuity (VA) was only light perception in BE. The eyelids were normal with the absence of swelling or discolouration. Slit lamp biomicroscopy at that time revealed subconjunctival haemorrhage, very hazy cornea with 2 mm of hypopyon without any details of anterior chamber (AC) visible in right eye (RE) (figure 1). Left eye (LE) showed subconjunctival haemorrhage, very marked cornea striae with 1 mm of hyphema, fixed and dilated pupil with posterior synechiae at 4 and 5 o’clock position (figure 2).
Figure 1.
Day 6, right eye: marked congestion, very hazy cornea, no details of anterior chamber with 2 mm of pseudohypopyon.
Figure 2.
Day 6, left eye: hazy cornea with striae, fixed pupil and 1 mm of hyphema.
Intraocular pressure (IOP) was 10 mm Hg in BE. Topical dexamethasone 2 hourly and topical homatropine three times a day to BE were given.
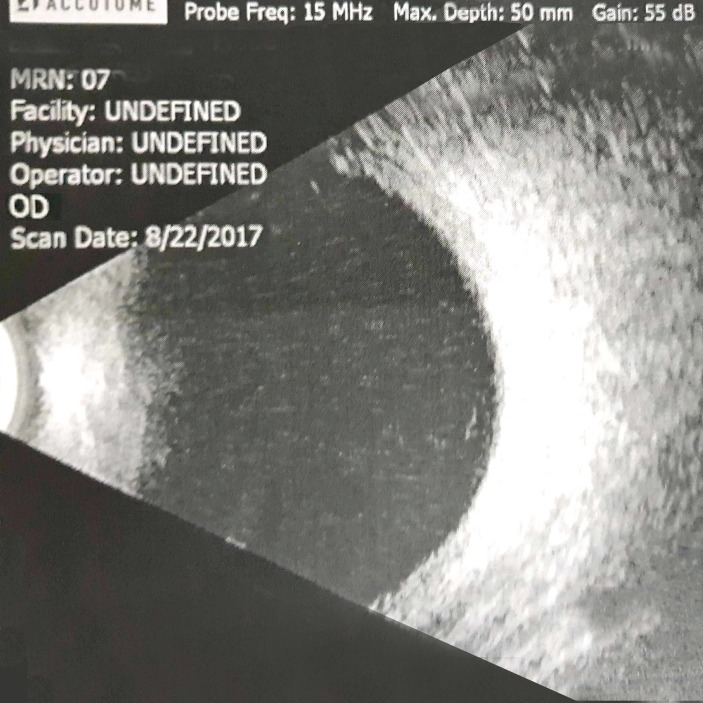
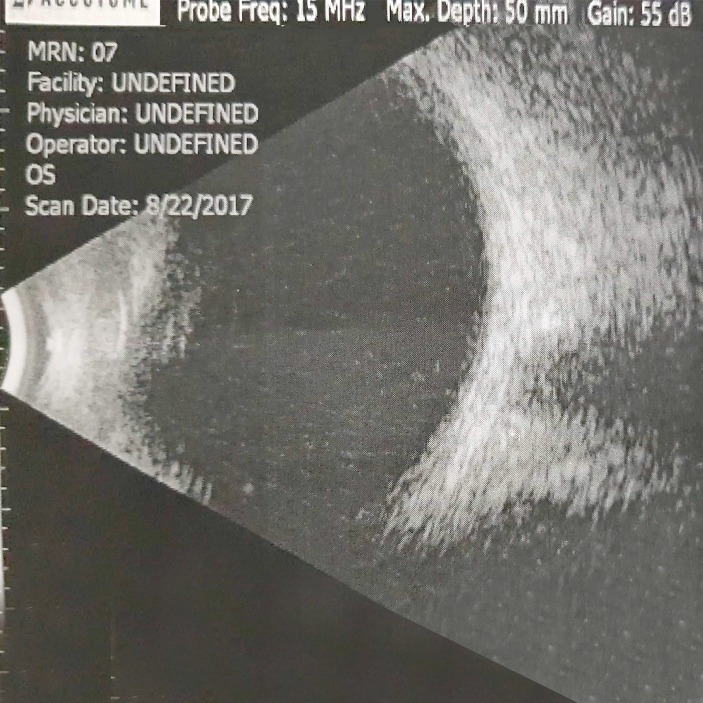
On the next day, cornea of BE became clearer, hypopyon in RE was reduced with iris details more visible and hyphema in LE became dehaemoglobinised. Both corneas showed Descemet folds and both AC had moderate depth. IOP was 10 and 12 mm Hg in RE and LE, respectively. With relatively clearer cornea, it was noticed that altered blood was mistaken as hypopyon on the earlier day in the RE. The treatment continued. Three days later, VA in RE improved to HM and LE to CF 2 feet, with clearing of hyphema. There was no fundal view bilaterally, but ultrasound B scan revealed no vitreous haemorrhage in BE (figures 3 and 4).
Figure 3.
Day 8, right eye: ultrasound B scan.
Figure 4.
Day 8, left eye: ultrasound B scan.
One week after, hyphema resolved from BE. VA continued to improve to CF 1 foot in RE and CF 2 feet in LE. Descemet folds reduced particularly in LE. AC depth was moderate in BE.
360° of posterior synechiae was noted in LE. IOP was within normal range throughout.
All vital signs were good. Urine output was adequate after total four sessions of dialysis. CT scan of head and brain disclosed absence of any intracranial bleeds.
Outcome and follow-up
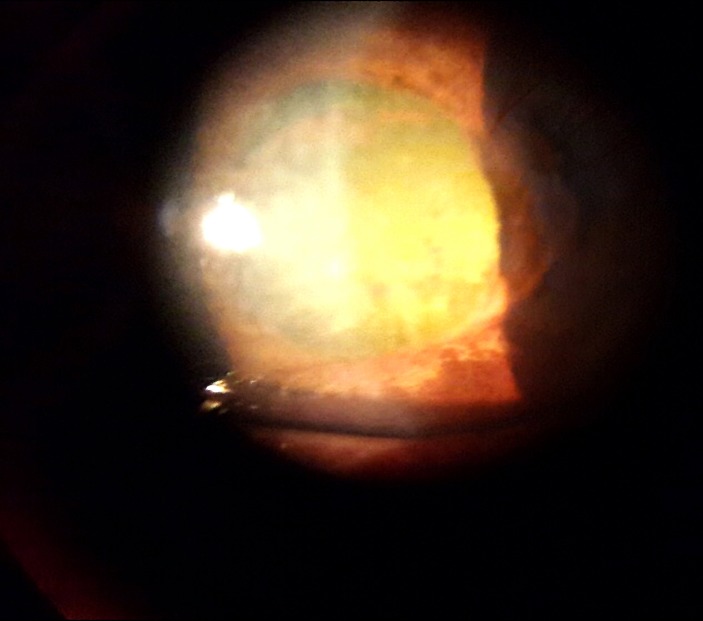
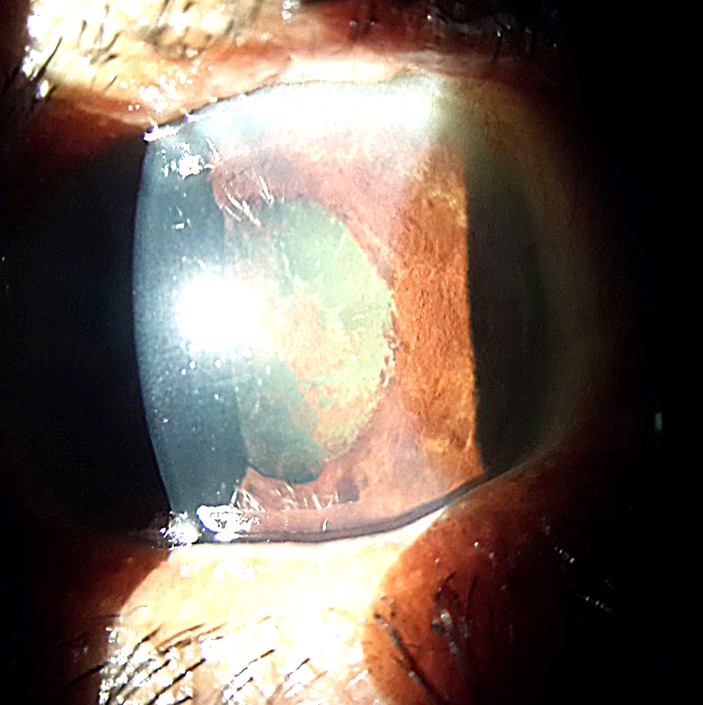
At 2 weeks follow-up, VA had improved to RE CF 5 feet and LE 6/24. Right cornea showed moderate striae but the LE cornea cleared up. In BE, inflammation resolved with moderate AC depth, fixed and mid dilated pupils, cataract with glaucomflecken detected (figure 5 and 6). No fundus detail was visible in the RE due to the presence of corneal striae and cataract; LE fundus showed a small haemorrhage over the disc but we were not able to take photograph since there was no fundus camera available. The patient was discharged from medical ward 2 weeks after admission.
Figure 5.
Day 14, right eye: cornea clearer, hyphema resolved, moderate anterior chamber, mid dilated pupil and significant cataract with glaucomflecken.
Figure 6.
Day 14, left eye: cornea clearer, hyphema resolved, moderate anterior chamber, mid dilated pupil and mild cataract with glaucomflecken.
Discussion
Myanmar (formerly known as Burma) is an agricultural country in tropical South East Asia region. The highest prevalence and fatality due to snakebite were reported in Magway division among 14 of its state and divisions. Russell’s viper bite accounted for 60% with 8.2% fatality rate in Myanmar.3
Snake venoms are complex poisons which display multisystem damage. Generally, ocular manifestations are rare but they may lead to sight-threatening conditions. Lists of ocular manifestations were documented in the literature particularly over the last decades.5–8 Although our patient presented with bilateral hyphema, uveitis and angle closure attack, he had mainly haematotoxic manifestations evident by the presence of subconjunctival haemorrhage with hyphema in BE and retinal haemorrhage in LE. RE was more severely affected than the left.
At initial presentation, it was thought as severe uveitis which is a known ocular manifestation in snakebite. Two mechanisms postulated for uveitis; the first being acute uveitis due to direct toxic effect of venoms which usually presents early within 2–3 days.5 The second is related with the adverse effect of ASV and presents later around 10–15 days after ASV.9 At first, altered blood in the RE was mistaken as hypopyon. On the next day, the hyphema change to white in colour in the LE making us realised that the patient had bilateral hyphema. The blood in the AC changed its colour within 24 hours and it would have been missed if it was not monitored closely. Although the AC depth was moderate, the presence of Descemet folds, fixed and mid dilated pupils (even before the start of mydriatics) and glaucomflecken in BE indicated postcongestive angle closure attack. It has been reported that angle closure attack after snakebite is associated with capillary leak syndrome secondary to haematotoxic envenomation.5 6 10 Kulkarni et al reported that the onset of acute attack is on the day 2 and at recovery all patients with bilateral angle closure attack had normal AC depth.10 Our patient noticed blurred vision on day 3. This might be the onset of acute angle closure but he was referred to ophthalmology department 3 days later due to the fact that he was very ill at the time of onset of blurring. One credible explanation for low IOP recorded throughout the course is that the patient received intravenous frusemide 120 mg three times a day as part of treatment for renal failure. However, to our best knowledge, our case is the first case that presented with bilateral uveitis, hyphema, angle closure attack and retinal haemorrhage. Capillary leak syndrome is considered to be associated with haematotoxic envenomation and is characterised by hypotension, hypoalbuminaemia, haemoconcentration, generalised oedema, ascites and renal failure. Our patient had periorbital oedema, minimal pleural effusion, ascites and renal failure suggesting that he suffered from capillary leak syndrome. It was also reported that renal failure is associated with high risk of angle closure.10
Learning points.
Snakebite is associated with sight-threatening conditions.
Early identification and recognition of ocular manifestations in patients with snakebite.
Close monitoring of the ocular manifestations in the early course of disease (particularly in the first week).
Prompt referral and appropriate treatment are associated with favourable outcome.
Footnotes
Contributors: Patient was under MTHA’s care. MTHA contributed for conception, acquisition of data or analysis and interpretation of data and revising it critically for intellectual content. KTM contributed for conception, design, analysis and interpretation of data, drafting the article and revising it critically for intellectual content. TN contributed for analysis and interpretation of data and revising it critically for important intellectual content.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Kasturiratne A, Wickremasinghe AR, de Silva N, et al. The global burden of snakebite: a literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med 2008;5:e218 10.1371/journal.pmed.0050218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ministry of Health and Sports. Indicators for morbidity and mortality of diseases under national surveillance. Nay Pyi Taw, Myanmar: Public Health Statistics (2014-2016), 2017:79. [Google Scholar]
- 3.Myint AA, Pe T, Maw TZ. An epidemiological study of snakebite and venomous snake survey in Myanmar. Paper presented. Yangon, Myanmar: Management of Snakebite and Research Seminar, 2001. [Google Scholar]
- 4.Warrell DA. Snake bite. The Lancet 2010;375:77–88. 10.1016/S0140-6736(09)61754-2 [DOI] [PubMed] [Google Scholar]
- 5.George TA, A. V. A, Ravindran R, et al. Ocular manifestations of snake bites in a tertiary care hospital in rural Northern Kerala, India. Int J Res Med Sci 2017;5:829–34. doi:10.18203/2320-6012.ijrms20170456 [Google Scholar]
- 6.Praveen Kumar KV, Praveen Kumar S, Kasturi N, et al. Ocular manifestations of venomous snake bite over a one-year period in a tertiary care hospital. Korean J Ophthalmol 2015;29:256–62. 10.3341/kjo.2015.29.4.256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Das S, Samaddar S, Jana S. Ocular manifestation and long standing visual impairment following venomous snake bite. J Evol Med Dent Sci 2015;4:7919–23. doi:10.14260/jemds/2015/1153 [Google Scholar]
- 8.Sithole HL. The ocular complications of an envenomous snakebite. South African Family Practice 2013;55:161–3. 10.1080/20786204.2013.10874325 [DOI] [Google Scholar]
- 9.Nayak SG, Satish R, Nityanandam S, et al. Uveitis following anti-snake venom therapy. J Venom Anim Toxins Incl Trop Dis 2007;13:130–4. 10.1590/S1678-91992007000100010 [DOI] [Google Scholar]
- 10.Kulkarni C, George TA, Av A, et al. Acute angle closure glaucoma with capilllary leak syndrome following snake bite. J Clin Diagn Res 2014;8:vc01–vc03. 10.7860/JCDR/2014/10716.4924 [DOI] [PMC free article] [PubMed] [Google Scholar]