Abstract
A previously healthy 22-year-old woman presented with acute, unilateral facial and neck swelling, associated with fever and malaise. She was initially treated with intravenous antibiotics; however, CT imaging showed unilateral, synchronous swelling and inflammation of the parotid and submandibular glands, and a PCR swab from the parotid duct was positive for mumps. She was fully immunised and had no contact in the preceding period with anyone diagnosed with mumps. She responded to supportive management and her symptoms resolved over the course of her admission. Unilateral, synchronous swelling and severe inflammation of both the parotid and submandibular glands in mumps is a very unusual presentation, and not one previously reported in the literature.
Keywords: otolaryngology / ENT; ear, nose and throat/otolaryngology
Background
Mumps is a common viral infection, caused by the mumps virus (MuV), part of the Paramyxoviridae family of RNA viruses.1 There is an incubation period of 12–24 days,2 and the virus most commonly affects the parotid glands.3 Those affected can present with salivary gland swelling, fever and fatigue,4 and later go on to develop serious complications like acute pancreatitis, chronic encephalitis and orchitis.5
The introduction of vaccination against the mumps virus in the UK in 1988 saw a decline in the incidence of the condition6; however, heavily publicised (and ultimately unfounded) controversy regarding the measles, mumps, rubella (MMR) vaccine7 led to a decline in vaccination uptake, and the incidence of mumps has subsequently risen. There were 6239 cases in the UK in 2016, compared with only 3907 in the UK between 1998 and 2002.8
Previous data suggest that Scotland has a high MMR vaccine uptake with 93% of children receiving two doses of vaccine by the age of 5.9 However, in recent years, there have been a number of outbreaks among university students.10–12 During the latest reported outbreak in 2014, two distinct genotype G clusters of the mumps virus were identified. One strain was detected circulating before the outbreak and the other thereafter, which suggests that the virus responsible for the outbreak was genetically different from the previously circulating one. Poor vaccine effectiveness during this outbreak was thought to be caused not only due waning immunity over time but also possibly because the current mumps vaccine is less effective against some genotypes.13
We present a case report of a young woman presenting with unusual unilateral, synchronous swelling of both the parotid and submandibular gland, initially suspected of having a deep neck space abscess, but who was subsequently discovered to have mumps.
Case presentation
A 22-year-old, previously healthy woman presented to the Ear, Nose and Throat (ENT) Unit for review, with a 2-day history of swelling and worsening pain on the left side of the face and neck. She worked in a local bank, was not a student and did not have (and had not recently had) a partner. There was no recent travel history. She had attended her general practitioner (GP) the previous day, who had commenced antibiotics for suspected parotitis (amoxicillin–clavulanic acid, 625 mg orally, 8 hourly). However, she continued to deteriorate in the community and was referred to ENT services.
She felt systemically unwell, with malaise and anorexia. On systemic examination, her temperature was 39.3°C, with a sinus tachycardia of 119bpm and normal blood pressure (121/84 mm Hg). Her oxygen saturations were 98% on room air, and there was no suggestion of stridor or impending airway compromise.
She had severe pain on the left side of the face and neck, and there was diffuse swelling noted. She had a degree of trismus, and neck movement was limited both laterally and in flexion/extension, worse on the left than the right. She did not report any specific symptoms of otalgia or pharyngotonsillitis.
She had no clinical signs or symptoms of respiratory, intra-abdominal, neurological or urinary infection. The only medical history of note was recurrent urinary tract infections as a child, for which she was followed up and subsequently discharged by urology. She had previously had multidrug-resistant Escherichia coli cultured from the urine but reported no urinary symptoms within the previous 12 months. She was not on regular medication. She was fully up to date with her immunisations and confirmed that she received the complete MMR vaccination in her youth.
Investigations
Investigations on admission revealed a peripheral white cell count (WCC) of 3.2×109/L (normal range 4–11×109/L), neutrophil count of 1.61 (normal range 2–7.5×109/L), lymphocyte count of 1.14 (normal range of 1.5–4×109/L) and C reactive protein of 33 mg/L (normal range 0–5 mg/L). She had a platelet count of 129×109/L (normal range 140–200×109/L) and her adjusted serum calcium was 2.11 mmol/L (normal range 2.2–2.6 mmol/L). The rest of her blood results were within normal ranges.
She had flexible nasendoscopy performed on admission, which did not show any obvious asymmetry in the laryngeal soft tissue or in the pharyngeal region.
The following morning she appeared to have clinically deteriorated; she remained feverish, with a temperature of 39.1°C, and the left-sided facial and neck swelling had increased. Repeat blood tests showed a worsening leucopaenia (with a WCC of 2.5×109/L), thrombocytopaenia (platelet count of 114) and mild hypocalcaemia (adjusted serum calcium of 2.11 mmol/L). The repeat C reactive protein count was 24 mg/L.
A range of blood tests for serology were sent, on the advice of Microbiology, to investigate any other underlying cause. These included cytomegalovirus, Epstein-Barr virus (EBV), HIV and toxoplasma. All of these were negative. Blood cultures taken on admission also showed no growth of organisms. Her vitamin B12 and folate levels were also within the normal range, as were her thyroid function tests.
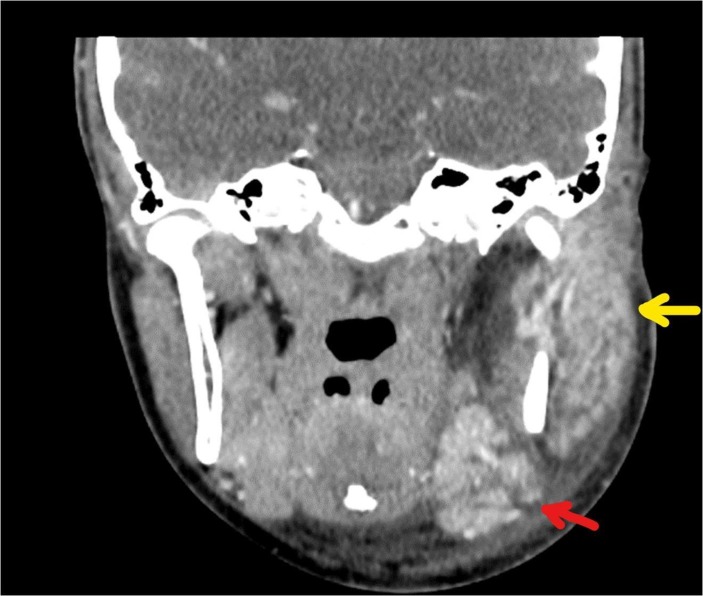
She had a CT scan of the neck on day 2 of her admission (with intravenous contrast), which showed marked asymmetry of the parotid glands, with enlargement and marked enhancement of the left parotid, extending into the deep gland posterior to the angle of the mandible. There was no evidence of a discrete abscess. The left submandibular gland was similarly enlarged and hyperenhancing. There were multiple enlarged adjacent submandibular and level II/III neck nodes, individually up to 17 mm, consistent with reactive changes. The left oropharynx was displaced secondary to soft tissue swelling (figures 1–3).
Figure 1.
Coronal CT neck with contrast showing enlarged left parotid (yellow arrow) and submandibular (red arrow) glands.
Figure 2.
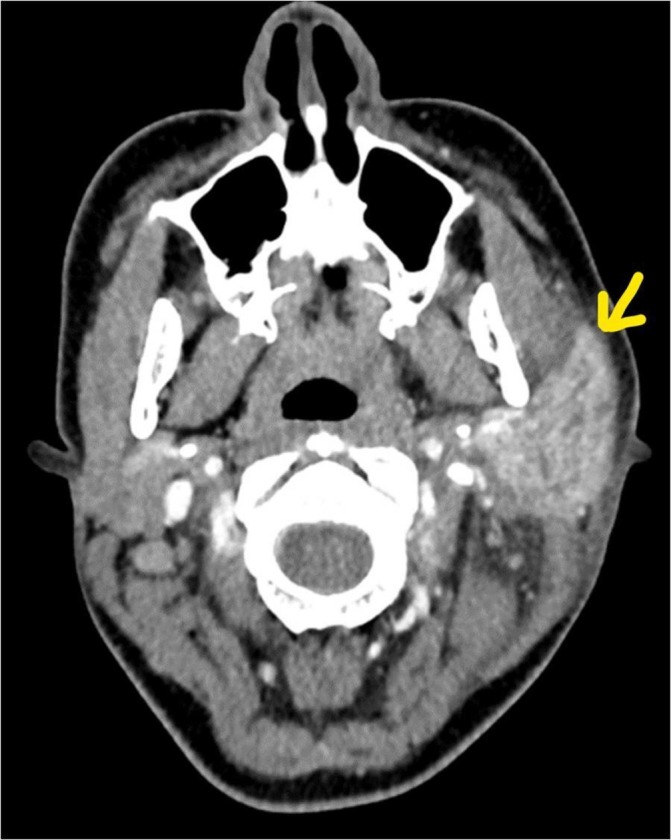
Axial CT neck with contrast showing marked left parotid enlargement (yellow arrow).
Figure 3.
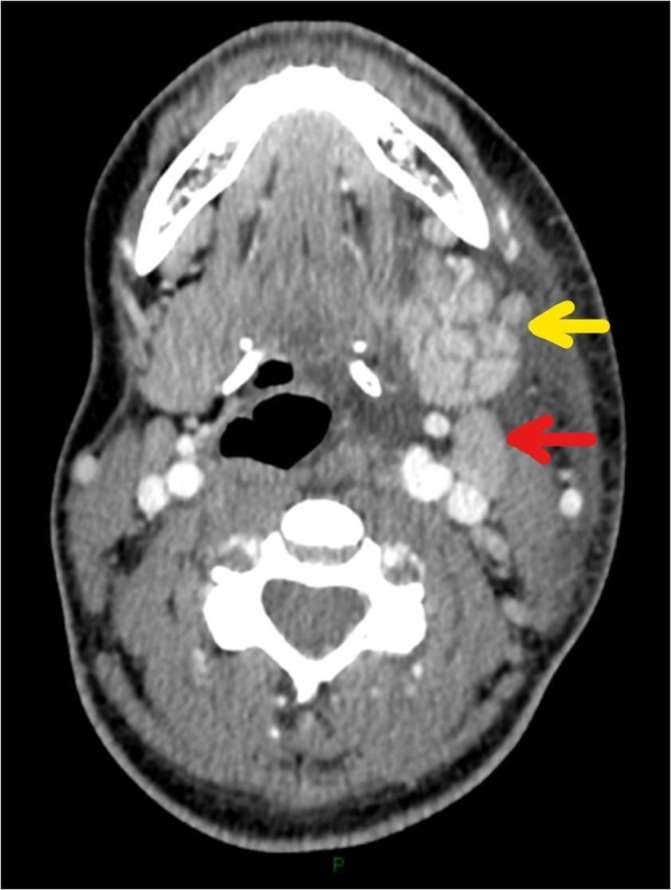
Axial CT neck with contrast showing enlargement of the left submandibular gland (yellow arrow). Adjacent reactive left submandibular lymph node (red arrow).
Differential diagnosis
The working diagnosis on admission was parotitis; however, due to her ongoing clinical deterioration and presenting symptoms, concern arose that she had an infection or abscess in the deep neck space, and she was treated with intravenous antibiotics that would cover both. The diffuse nature of the neck swelling meant that mumps was also a differential diagnosis.
Infectious mononucleosis is quite common in this age group and can present with neck swelling. The patient’s EBV serological test was however negative and she lacked any other signs such as the characteristic exudative appearances of the palatine tonsils. As with any unilateral swelling in the head and neck region, it was important to consider a neoplastic cause for the presentation. Lymphoma, specifically Hodgkin’s lymphoma, would be the most common neoplastic cause in someone of this age. Another possible cause would be a malignant salivary gland mass, although the speed of onset of the patient’s symptoms, the nature of her symptoms and her age make this less likely.14
Treatment
Her observations and initial blood results on admission met the criteria for a patient with sepsis, and she was commenced on intravenous fluid replacement and intravenous antibiotics (amoxicillin–clavulanic acid 1.2 g, 8 hourly and metronidazole, 500 mg, 8 hourly) to cover pathogens for a suspected deep neck space abscess. She continued to display clinical signs of sepsis and increasing swelling on the second day of admission. Her antimicrobial treatment was therefore broadened to include vancomycin (dose calculated as 1000 mg intravenously, 12 hourly, after an initial 1500 mg intravenously loading dose) and clindamycin (450 mg intravenously, 6 hourly) while awaiting results of cultures and imaging.
She remained on the above four intravenous antibiotics for the next 24 hours, and there was gradually a clinical improvement in her condition, with a reduction in trismus and slightly more movement of the neck achievable. She was nursed in isolation to prevent spread of infection to other patients and remained in a side room on the ward for the duration of her admission.
The thrombocytopaenia was discussed with haematology, who thought it likely a reactive response to severe illness, with no acute action necessary. In addition to the above blood tests, a viral throat swab was taken after the CT scan result, and a swab was taken from the opening of the parotid gland (Stensen’s duct) on the left side of the mouth, to test for mumps. The viral throat swab (tested for influenza, parainfluenza, respiratory synctical virus, adenovirus and rhinovirus) was negative, but mumps PCR on the parotid duct swab was positive.
Outcome and follow-up
After the positive mumps result, we discussed the case with virology, microbiology and infectious diseases, all of whom advised continuing supportive management (with intravenous fluids and analgesia). The four intravenous antibiotics were not stopped simultaneously, in case of a super-added infection; they were stopped gradually over the following 24 hours, with the patient remaining apyrexial and haemodynamically stable throughout.
Four days after admission, the neck swelling had significantly reduced, her trismus had resolved and she was clinically much improved. The discussion with Virology had established that routine follow-up in secondary care for patients with mumps was not required if the patient was clinically improving. Detailed worsening advice and warning about the symptoms of complications was given. We advised having repeat blood tests taken in the community after discharge to ensure that the neutropaenia and thrombocytopaenia resolved, and these tests (taken 10 days postdischarge) showed both the WCC and platelets to be within the normal range.
Discussion
Mumps is a viral infection, which primarily causes fever and salivary gland swelling. It can affect multiple organs in the body via haematogenous spread, with the subsequent spectrum of complications ranging from subclinical infection to orchitis, pancreatitis, meningoencephalitis and cardiomyositis.
The parotid glands are most commonly affected, with the submandibular glands less so. Parotid gland swelling appears in approximately 85% of patients; infection of both the parotid and submandibular glands appears in approximately 11% and submandibular swelling alone in 1.3%.3 The incidence of bilateral salivary gland swelling is quoted at 70%–80%.15
There have been reports of isolated submandibular gland swelling due to mumps,3 16 but to our knowledge, this is the first reported case in the literature of mumps presenting with both unilateral and synchronous parotid and submandibular gland swelling.
The treatment for mumps is symptomatic, with oral fluid intake and analgesia being encouraged.17 However, despite the fact that there is no specific treatment, the importance of correctly diagnosing the condition cannot be overstated, both in terms of ruling out a deep neck space infection and to allow quicker identification of developing complications secondary to mumps. In other atypical presentations of mumps, patients have been known to develop meningitis and orchitis, before any salivary gland swelling is even evident,3 which can have subsequent serious long-term sequelae.
One such serious complication is profound sensorineural hearing loss. The incidence of hearing loss in children diagnosed with mumps has been estimated at 1 in 1000,18 and a study by Schreiber et al estimated that mumps was the cause of approximately 7% of cases of sudden sensorineural hearing loss (SSHL) in adults.19 The clinical concern with this would be evidence that shows over 90% of patient with mumps-associated SSHL do not achieve a complete recovery.20
As the treatment for mumps is symptomatic, rather than curative, the most effective strategy for preventing complications is through vaccination programmes against the disease. In a group of male patients with mumps, those who had been previously vaccinated were shown to have a nearly 70% reduced risk of developing orchitis,21 and overall patients with mumps, who have had at least 1 dose of the vaccine, are 50% less likely to be admitted to hospital with the disease or with complications. Patients who have had two doses of the vaccine are 63% less likely to require a hospital admission with the disease.22 The patient in this report stated that she had previously been vaccinated, and as mentioned above did not suffer any further complications during her admission.
Learning points.
Early recognition of mumps, even with an unusual presentation, is important to allow appropriate management and to prevent the development of serious complications, such as pancreatitis, encephalitis, hearing loss and orchitis.
There should be continued public health awareness regarding mumps vaccination programmes, as this is the most effective strategy to prevent complications.
Significant unilateral facial and neck swelling, with associated fever, trismus and restriction of neck movement, should still be urgently investigated for an underlying deep neck space abscess.
Footnotes
Contributors: CB, PA, EK and GV: involved in the clinical management of the patient. PA: identified the case as worth sharing. CB and PA: involved in the conception of the article and literature search. CB: was responsible for the drafting of the article, with all authors responsible for the subsequent revision of the article and approval of the submitted draft.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Rubin S, Eckhaus M, Rennick LJ, et al. Molecular biology, pathogenesis and pathology of mumps virus. J Pathol 2015;235:242–52. 10.1002/path.4445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henle G, Henle W, Wendell K, Rosenberg P. Isolation of mumps virus from human beings with induced apparent or inapparent infections. J Exp Med 1948;88:223–32. 10.1084/jem.88.2.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Myung NS, Kim YJ, Kim YJ, et al. Complicated mumps viral infection: an unusual presentation affecting only submandibular gland. Am J Otolaryngol 2013;34:600–2. 10.1016/j.amjoto.2013.02.004 [DOI] [PubMed] [Google Scholar]
- 4.Cole AS, Trotter MI, O’Connell J. Follicular lymphoma presenting as mumps, with persistent cervical lymphadenopathy. J Laryngol Otol 2007;121:501–2. 10.1017/S0022215106003549 [DOI] [PubMed] [Google Scholar]
- 5.Yung CF, Andrews N, Bukasa A, et al. Mumps complications and effects of mumps vaccination, England and Wales, 2002-2006. Emerg Infect Dis 2011;17:661–7. 10.3201/eid1704.101461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta RK, Best J, MacMahon E. Mumps and the UK epidemic 2005. BMJ 2005;330:1132–5. 10.1136/bmj.330.7500.1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Godlee F, Smith J, Marcovitch H. Wakefield’s article linking MMR vaccine and autism was fraudulent. BMJ 2011;342:c7452. [DOI] [PubMed] [Google Scholar]
- 8.Public Health England. Mumps: confirmed cases. https://www.gov.uk/government/publications/mumps-confirmed-cases.
- 9.NHS National Services Scotland, Information Services Division. Childhood Immunisation Statistics. http://www.isdscotland.org/Health-Topics/Child-Health/Immunisation/ (accessed 25 May 2018).
- 10.Health Protection Scotland. http://www.hps.scot.nhs.uk/ewr/index.aspx (accessed 25 May 2018).
- 11.MacKenzie DG, et al. Mumps in a boarding school: description of an outbreak and control measures. British Journal of General Practice 2006;56:526–9. [PMC free article] [PubMed] [Google Scholar]
- 12.Donaghy M, Cameron JC, Friederichs V. Increasing incidence of mumps in Scotland: options for reducing transmission. Journal of Clinical Virology 2006;35:121–9. 10.1016/j.jcv.2005.09.009 [DOI] [PubMed] [Google Scholar]
- 13.Willocks LJ, Guerendiain D, Austin HI, et al. An outbreak of mumps with genetic strain variation in a highly vaccinated student population in Scotland. Epidemiol Infect 2017;145:3219–25. 10.1017/S0950268817002102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horn-Ross PL, Ljung B-M, Morrow M. Environmental factors and the risk of salivary gland cancer. Epidemiology 1997;8:414–9. 10.1097/00001648-199707000-00011 [DOI] [PubMed] [Google Scholar]
- 15.Nussinovitch M, Volovitz B, Varsano I. Complications of mumps requiring hospitalization in children. Eur J Pediatr 1995;154:732–4. 10.1007/BF02276717 [DOI] [PubMed] [Google Scholar]
- 16.Cheung L, Henderson AH, Banfield G, et al. Bilateral isolated submandibular gland mumps. BMJ Case Rep 2017:bcr-2017-220103 10.1136/bcr-2017-220103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knipe D, Howley P. Virology. 4th edn Birmingham: Lippincott, Williams & Wilkins, 2001;5:1381–400. [Google Scholar]
- 18.Hashimoto H, Fujioka M, Kinumaki H. An office-based prospective study of deafness in mumps. Pediatr Infect Dis J 2009;28:173–5. 10.1097/INF.0b013e31818a8ca8 [DOI] [PubMed] [Google Scholar]
- 19.Schreiber BE, Agrup C, Haskard DO, et al. Sudden sensorineural hearing loss. The Lancet 2010;375:1203–11. 10.1016/S0140-6736(09)62071-7 [DOI] [PubMed] [Google Scholar]
- 20.Morita S, Fujiwara K, Fukuda A, et al. The clinical features and prognosis of mumps-associated hearing loss: a retrospective, multi-institutional investigation in Japan. Acta Otolaryngol 2017;137:S44–S47. 10.1080/00016489.2017.1290826 [DOI] [PubMed] [Google Scholar]
- 21.Hahné S, Whelan J, van Binnendijk R, et al. Mumps vaccine effectiveness against orchitis. Emerg Infect Dis 2012;18:191–3. 10.3201/eid1801.111178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yung C-F, Andrews N, Bukasa A, et al. Mumps complications and effects of mumps vaccination, England and Wales, 2002–2006. Emerg Infect Dis 2011;17:661–7. 10.3201/eid1704.101461 [DOI] [PMC free article] [PubMed] [Google Scholar]