Abstract
Objectives
To investigate the characteristics and healthcare utilisation of high-cost patients and to compare high-cost patients across payers and countries.
Design
Systematic review.
Data sources
PubMed and Embase databases were searched until 30 October 2017.
Eligibility criteria and outcomes
Our final search was built on three themes: ‘high-cost’, ‘patients’, and ‘cost’ and ‘cost analysis’. We included articles that reported characteristics and utilisation of the top-X% (eg, top-5% and top-10%) patients of costs of a given population. Analyses were limited to studies that covered a broad range of services, across the continuum of care. Andersen’s behavioural model was used to categorise characteristics and determinants into predisposing, enabling and need characteristics.
Results
The studies pointed to a high prevalence of multiple (chronic) conditions to explain high-cost patients’ utilisation. Besides, we found a high prevalence of mental illness across all studies and a prevalence higher than 30% in US Medicaid and total population studies. Furthermore, we found that high costs were associated with increasing age but that still more than halve of high-cost patients were younger than 65 years. High costs were associated with higher incomes in the USA but with lower incomes elsewhere. Preventable spending was estimated at maximally 10% of spending. The top-10%, top-5% and top-1% high-cost patients accounted for respectively 68%, 55% and 24% of costs within a given year. Spending persistency varied between 24% and 48%. Finally, we found that no more than 30% of high-cost patients are in their last year of life.
Conclusions
High-cost patients make up the sickest and most complex populations, and their high utilisation is primarily explained by high levels of chronic and mental illness. High-cost patients are diverse populations and vary across payer types and countries. Tailored interventions are needed to meet the needs of high-cost patients and to avoid waste of scarce resources.
Keywords: high-need high-cost, integrated delivery of health care, health care utilization, health care costs
Strengths and limitations of this study.
Based on an extensive literature search, this review included 55 studies of high-cost patients’ characteristics and healthcare utilisation.
Andersen’s behavioural model was used to categorise the characteristics of high-cost patients into predisposing, enabling and need characteristics.
Grey literature was not included in our systematic review. However, we identified 55 studies and compared high-cost patients’ characteristics and healthcare utilisation across payers and countries.
We did not assess the quality of the studies because of the methodological diversity of the studies.
Background
It is widely known that healthcare costs are concentrated among a small group of ‘high-cost’ patients.1 Although they receive substantial care from multiple sources, critical healthcare needs are unmet and many receive unnecessary and ineffective care.2–5 This suggests that high-cost patients are a logical group to seek for quality improvement and cost reduction.
Especially in the USA, many providers or insurance plans have pursued this logic and developed programmes for ‘high-need, high-cost patients’. So far, such programmes, including, for example, care coordination and disease management, have had favourable results in quality of care and health outcomes and mixed results in their ability to reduce hospital use and costs.6 Research has shown that the effectiveness and efficiency of the programmes increase when interventions are targeted to the patients that most likely benefit.2 7 8 Little is known, however, about variations in clinical characteristics and care-utilisation patterns across payer-defined groups or countries.9 Such insight in the health requirements of high-cost patients is prerequisite for designing effective policy or programme responses.
We conducted this systematic review to synthesise the literature on high-cost patients’ characteristics and healthcare utilisation. Andersen’s behavioural model (see Methods section) was used to organise the findings. Our analysis was aimed at identifying drivers of costs that matter across payer types and countries. We aimed to inform the development of new interventions and policy, as well as future research in high-cost patients.
Methods
Our methodology was based on established guidance for conducting systematic reviews.10 11 Our main research questions was ‘Who are the most expensive patients, what health care services do they use, what drives these high costs, and what drivers matter across payers and countries?’.
Study selection
A preliminary search in PubMed was conducted to identify key articles and keywords. On the basis of these findings, we developed a search strategy covering the most important terms. We then reshaped the search strategy by consulting an information specialist of our university. The final search was built on three themes: ‘high-cost’, ‘patients’, and ‘cost’ and ‘cost analysis’. The sensitivity of the search was verified with the key articles we found earlier. We searched PubMed and Embase on 30 October 2017. Full details of our search strategy are attached in online supplementary appendix 1.
bmjopen-2018-023113supp001.pdf (7.3KB, pdf)
Inclusion and exclusion criteria
Articles were reviewed by author A using title and abstract to identify potentially eligible studies. Author B verified a random sample of articles to guarantee specificity and sensitivity of the selection process. Only studies from high-income countries—as defined by the World Bank12—and studies published in 2000 and later were included. Studies not written in English and conference abstracts were excluded. In the second step, titles and abstracts were reviewed by author A to assess whether articles fit within our definition of high-cost patients: the article reported characteristics and utilisation of the top-X% (eg, top-5% and top-10%) patients of costs of a given population. Author B verified a random sample of articles at this selection step. In the third step, full-text articles were retrieved and independently screened by author A and author B for our inclusion criteria. At this step, we aimed for studies covering a broad range of services across the continuum of care at health system level and excluded all studies with a narrow scope of costs (eg, hospital costs and pharmaceutical costs) and all studies with a narrow population base (primarily disease oriented studies, or studies in children). At each step of this selection process, (in-)consistencies were discussed until consensus was reached. On basis of the discussions, the criteria were refined, and the prior selection process was repeated.
Data extraction
A data extraction form was developed by the research team to ensure the approach was consistent with the research question. Author A extracted all data. To guarantee specificity and sensitivity of data extraction, author B and author C both independently extracted the data of five random articles. A meeting was held to discuss (in-)consistencies in extraction results. On basis of this discussion, the data extraction form was refined, and the prior data extraction was repeated. Per article, the following key elements were extracted: author, year, country, definition of high-cost patients, inclusion and exclusion criteria of the study population, cost data used to determine total costs, characteristics of the high-cost patients such as diagnoses, age, gender, ethnicity, determinants for high costs including associated supply side factors (concerning the supply of health services), subpopulations and healthcare use and costs (per subpopulation). We also made a narrative summary of the findings per article (provided in online supplementary appendix 2). To identify the most important medical characteristics, only those diseases with a high prevalence (≥10%) among high-cost patient populations or medical characteristics overrepresented in high-cost populations were extracted. Medical characteristics (prevalent diseases) were categorised and presented at the level of International Statistical Classification of Diseases, 10th Revision (ICD-10) chapters.
bmjopen-2018-023113supp002.pdf (97.9KB, pdf)
Data synthesis
Andersen’s behavioural model was used to categorise characteristics and determinants for high costs into predisposing, enabling and need characteristics. Andersen’s model assumes that healthcare use is a function of (1) characteristics that predispose people to use or not to use services, although such characteristics are not directly responsible for use (eg, age, gender, education, ethnicity and beliefs); (2) enabling characteristics that facilitate or impede use of services (income/wealth/insurance as ability to pay for services, organisation of service provision and health policy); and (3) needs or conditions that laypeople or healthcare providers recognise as requiring medical treatment. The model also distinguishes between individual and contextual (measured at aggregate level, such as measures of community characteristics) determinants of service use. Andersen hypothesised that the variables would have differential ability to explain care use, depending on the type of service. For example, dental care (and other discretionary services) would be explained by predisposing and enabling characteristics, whereas hospital care would primarily be explained by needs and demographic characteristics.13 14
We presented all data according to five general categories, including study characteristics, predisposing characteristics, enabling characteristics, need characteristics, and expenditure categories and healthcare utilisation. We presented summary tables of results, extracted central themes and topics from the studies and summarised them narratively. All studies were analysed according to payer and country to identify the most important drivers across settings.
Patient and public involvement
Patients and or public were not involved in the conduct of this study.
Results
General information
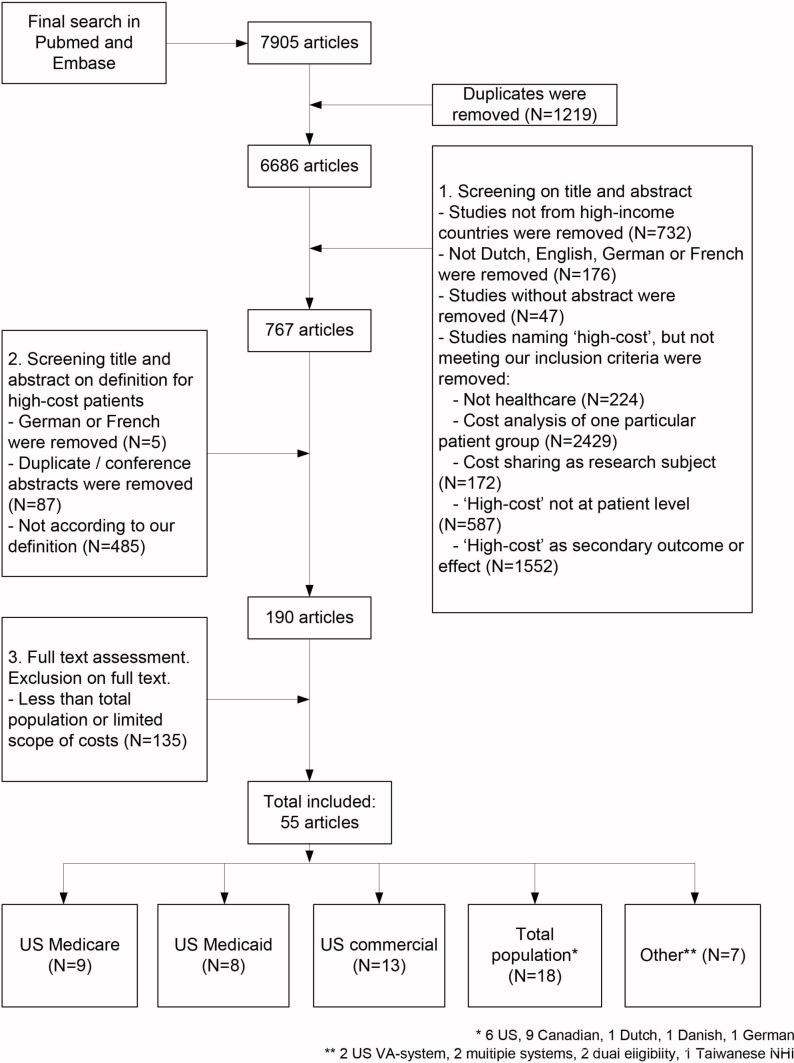
Our search strategy resulted in 7905 articles. After first broad eligibility assessment, 767 articles remained. After screening of titles and abstracts, 190 articles remained for full-text screening, from which 55 were ultimately included (figure 1).
Figure 1.
Flow diagram of article selection.
A description of the studies is given in table 1. The majority of the studies were conducted in the USA (n=42). The remaining studies were conducted in Canada (n=9), Germany (n=1), Denmark (n=1), the Netherlands (n=1) and Taiwan (n=1). All were retrospective cohort studies, and descriptive and logistic regression analysis were the main analytic approaches used. The study period ranged from 6 months to 30 years. The most frequent observation period was 1 year.
Table 1.
Description of the included studies
| Author(s), country | Methodological approach | Study period | Definition high-cost | Study population: inclusion and exclusion criteria | Cost data |
| Aldridge and Kelly,57 USA | Descriptive | 2011 | Top-5% | US population | Total spending was identified from a combination of data from Medical Expenditure Panel Survey, the Health and Retirement Study, peer-reviewed literature, published reports, 2011 MEPS and 2011 National Health Expenditure Accounts. |
| Ash et al,58 USA | Descriptive, logistic regression | 1997–1998 | Top-0.5% with highest predicted costs, top-0.5% prior cost. | Individuals eligible for at least 1 month in each of the two study years. | MEDSTAT MarketScan Research Database, consisting of inpatient and outpatient care from individuals covered by employee-sponsored plans. Outpatient pharmacy costs were excluded. |
| Bayliss et al,59 USA | Predictive modelling, cluster analysis | 2014 | Top-25% | Members with new Kaiser Permanente Colorado benefits and who completed the Brief Health Questionnaire. | Per-member per-month costs from Kaiser Permanente Colorado health system. |
| Beaulieu et al,28 USA | Descriptive, logistic regression | 2011–2012 | Top-10% | Fee-For-Service Medicare population. Excluding patients <65 years, enrolled in Medicare advantage and those not continuously enrolled in parts A and B. | Standardised Medicare costs, excluding prescription drug charges. |
| Boscardin et al,60 USA | Descriptive, logistic regression | 2009 | Top-10% | Employees enrolled in the Safeway health insurance programme in 2009, with biometric and self-reported health status data (Health Risk Questionnaire). Excluding dependents covered through a family member. |
Safeway’s health plan. |
| Buck et al,61 USA | Descriptive | 1995 | Top-10% | Medicaid population in 10 states. Excluding dually eligible, ≥65 years, enrolled in capitated plans, missing sex or birthdate. |
Total Medicaid expenditures. |
| Bynum et al,16 USA | Descriptive, multinominal logistic regression | 2010–2011 | Top-10% in each state Persistently HC, died in 2011, or converted |
Dually eligible adults with full Medicaid eligibility; in the 36 states that had usable and complete Medicaid data. | Medicare and Medicaid. |
| Chang et al,62 USA | Descriptive, logistic regression | 2007–2009 | Consistent high-user: top-20% in four consecutive half year periods (≡ 6.14% of the population) Point high-user: top-6.14% in 1 year |
Enrollees from four health plans who were (1) continuously enrolled, (2) incurred ≥$100 each year, (3) from the 4 largest plans; (4) aged between 18 and 62 years in 2007. Excluding those who died. |
Commercial health plans. |
| Charlson et al,63 USA | Quantile regression | 2007 (6 months) | Top-5%, top-10% | All enrollees of the MMC Plan who had an assigned primary care provider at Lincoln Medical and Mental Health Center. | Metroplus Medicaid Managed Care costs, including inpatient, outpatient, emergency room, laboratory tests and prescription drugs. |
| Charlson et al,64 USA | Quantile regression | 2009–2010 | Top-5%, top-10% | Union of health and hospital workers in the Northeast, those who were consistently eligible for benefits over at least 22 months in 2009 and 2010 (self-insured trust fund), who also received DCG codes. | Inpatient, outpatient, emergency room, laboratory tests, behavioural health and prescription drugs. |
| Chechulin et al,22 Canada | Logistic regression | 2007/2008–2010/2011 | Top-5% | All Ontario residents serviced by the Ontario healthcare system during the fiscal year 2009/2010. Patients under 5 years or who died during this year were excluded. | Total health system costs (including Long Term Care), excluding outpatient oncology, outpatient dialysis, and outpatient clinic. |
| Cohen et al,65 USA | Logistic regression | 1996–2002 | Top-10%, | Nationally representative sample of the Medical Expenditure Panel Survey. | All direct payments to providers by individuals, private insurance, Medicare, Medicaid and other payment sources for: inpatient and outpatient care, emergency room services, office-based medical provider services, home healthcare, prescription medicines and other medical services and equipment. |
| Coughlin et al,66 USA | Descriptive | 2006–2007 (1 year) |
Top-10% | Medicare beneficiaries and dual eligibles. | Spending paid for by the public programmes. |
| Coughlin and Long,67 USA | Descriptive | 2002–2004 | Various. Top-1%, Top-5%, Top-10%, Top-25%, Top-50% |
2002 national Medicaid population (living in institutions and community). Excluding who received only State Children’s Health Insurance Program (SCHIP) coverage or never full benefits. Top-0.1% of spenders. |
Medicaid. |
| Crawford et al,68 USA | Neural network modelling | 1999–2001 | Top-15% | Members of a health plan, where American Healthways, Inc. provided disease management services. Only members with 24 months continuous enrolment were included. | Health plan costs. |
| DeLia,20 USA | Descriptive, multinomial regression | 2011–2014 | Top-1%, top-2%–10%, Persistently extreme: 4 years top-1% Persistently high: 4 years in top-10% |
Medicaid/Children’s Health Insurance Program (CHIP) beneficiaries in New Jersey, newly covered individuals under the Affordable Care Act (ACA) (2014) were excluded; Medicaid/Medicare dual eligibles were excluded. | Medicaid FFS claims and managed care encounters and CHIP. |
| de Oliveira et al,18 Canada | Descriptive | 2012 | Top-10%, top-5%, top-1%. Mental health HC patients: mental health>50% of total costs. | All adult patients (18 years and older) who had at least one encounter with the Ontario healthcare system in 2012. Excluding all individuals who did not have a valid Ontario Health Insurance Plan number. |
Most publicly funded healthcare services. |
| Figueroa et al,30 USA | Descriptive, χ2 | 2012 | Top-10% | Adults 18–64 year without FFS Medicare coverage or Medicare Advantage coverage. | Massachusetts All-Payer Claims database; nearly a universal account of all healthcare delivered in the state with the exception of Medicare FFS. |
| Figueroa et al,39 USA | Descriptive | 2012 | Top-10% | All Medicare patients, excluding those with Medicare Advantage coverage, who were not continually enrolled in parts A and B. | Standardised Medicare costs. |
| Fitzpatrick et al,21 Canada | Descriptive, logistic regression | 2003/2005 and 5-year follow-up | Top-5% | Participants from two cycles of Canadian Community Health Survey (CCHS) surveys, representative of the population ≥12 years and living in private dwellings. ≥18 years. Excluding baseline high cost. | Ontario health insurance plan. |
| Fleishmann and Cohen,69 USA | Logistic regression | 1996–2003 | Top-10%, top-5% | Nationally representative sample of the Medical Expenditure Panel Survey. | All direct payments to providers by individuals, private insurance, Medicare, Medicaid and other payment sources for: inpatient and outpatient care, emergency room services, office-based medical provider services, home healthcare, prescription medicines and other medical services and equipment. |
| Ganguli et al,23 USA | Descriptive, retrospective chart review, interview analysis | 2005–2011 | Five archetypal patients among the 50 costliest/1500 highest cost patients | Patients selected by costs and a prospective risk score to participate in a Centers for Medicare and Medicaid care management project, >18 years and had sufficient cognitive capacity to participate in an interview, or if deceased had family members who were able to give sufficient information. | Total Medicare payments. |
| Graven et al,29 USA | Descriptive | 2011–2013 | Top-10%, Episodically high-cost, persistently high-cost |
Adults ages 19 and over, enrolled in Oregon Medicaid, commercial or Medicare Advantage programmes. Only those with continuous enrolment in 2011 and 2012 were included. Excluding dual eligibles and individuals who had ‘coordination of benefit’- laims or with negative total spending in any of the quarters. | Total Medicaid, commercial or Medicare Advantage payments (acute care expenditures), excluding spending on prescription drugs. |
| Guilcher et al,19 Canada | Descriptive | 1 April 2010–31 March 2011 | Top-5% | All persons eligible for provincial health insurance residing in the community, who had at least one interaction with the system in the last 5 years. | All publicly funded healthcare in a universal public healthcare system. |
| Guo et al,36 USA | Descriptive, logistic regression | 1999–2000 | Top-10% of average monthly expenses | Medicaid, FFS recipients younger than 65 years. Excluding nursing home recipients. |
Medicaid costs. |
| Hartmann et al,70 Germany | Logistic regression | 2010–2011 | Top-10% | Enrollees 18 years and older of AOK Lower Saxony, Germany’s 10th largest statutory health insurer. | Inpatient and outpatient care, sickness benefits, rehabilitation, home nursing, ambulatory drug supply, prescribed therapeutic appliances and remedies. |
| Hensel et al,71 Canada | Descriptive, logistic regression | 1 April 2011–31 March 2012 | Top-1%, top-2%–5%, top-6%–50%, bottom-50%, and zero-cost referent group | All Ontario residents, with a valid Ontario healthcare, 18 years of age or older and medical care costs greater than zero. | Ontario health insurance plan, for all hospital and home care services, including physician care, costs related to outpatient physician services were not included |
| Hirth et al,72 USA | Descriptive, logistic regression | 2003–2008 | High: top-10% Moderate: top-10%–30% Low: bottom-70% Usually low Low/moderate Sometimes high Often high Usually high |
Under-65 population (Truven Health MarketScan database); enrollees and dependents of more than 100, mainly self-insured, medium and large employers. Only people enrolled continuously are included. Attrition (a minority was enrolled each year) due to several reasons: death, retirement, children ageing out of dependent status and so on. |
Data from all carve-outs (eg, prescription drug and mental health), including claims for which the deductible is imposed. All spending was adjusted to 2008 dollars using the medical cost Consumer Price Index. Excluding out-of-plan spending (eg, OTC drugs and travel costs). |
| Hunter et al,73 USA | Descriptive, linear regression | Fiscal year 2010 | Top-5% | Cohort from Veterans Affairs (VA) administrative records, who were eligible for and received care in study period. Excluding individuals with schizophrenia, bipolar depression, other psychosis, alcohol dependence and abuse, drug dependence and abuse, post-traumatic stress disorder and/or depression. | Inpatient, outpatient, pharmacy and non-VA contract care. |
| Hwang et al,37 USA | Descriptive, logistic regression | 2008–2011 | Top-10% | Employees from a large employer in Pennsylvania and the employees’ dependents. Only those continuously enrolled. | Amount paid by the insurer and the amount of cost sharing paid by individuals. |
| Izad Shenas et al,74 USA | Data mining techniques/predictive modelling | 2006–2008 | Top-5%, top-10%, top-20% | Nationally representative sample of the Medical Expenditure Panel Survey, household individuals ≥17 years (redundant records, or with zero personal-level weights were removed). | All direct payments to providers by individuals, private insurance, Medicare, Medicaid and other payment sources for: inpatient and outpatient care, emergency room services, office-based medical provider services, home healthcare, prescription medicines and other medical services and equipment. |
| Joynt et al,75 USA | Descriptive | 2011 and 2012 | Top-10% | All Medicare patients, excluding those with Medicare Advantage coverage, who were not continually enrolled in parts A and B, or who died during the study period. | Standardised Medicare costs. |
| Joynt et al,26 USA | Descriptive, linear regression | 2009–2010 | Top-10% | Medicare >65 years population. Excluding decedents, any Medicare advantage enrolment, not continuously enrolled. |
Inpatient and outpatient services. |
| Krause et al,76 USA | Logistic regression | 2009–2011 | Top-5%, top-1%, >$1 00 000 | Enrollees of Blue Cross Blue Shield of Texas, only members 18–63 years, with a zip code in Texas and continuous enrolment in 2009 were included. | Total claims expense, including expenditures for hospital care, outpatient facility services and professional services. |
| Ku et al,34 Taiwan | Descriptive, generalised estimating equations | 2005–2009 | Top-10%, top-11%–25% | Survey respondents 65 years of age and older. | National health insurance. |
| Lauffenburger et al,77 USA | Descriptive, group-based trajectory modelling | 2009–2011 | Top-5% | Patients ≥18 years, with continuous eligibility for the entire calendar year, with ≥1 calendar year before their entry year and with ≥1 medical and pharmacy claim in both the baseline and entry year. | Medical and prescription data of Aetna, a large US nationwide insurer. |
| Lee et al,78 USA | Descriptive, cluster analysis | 2012 | Top-10% | Medicare patients hospitalised exclusively at Cleveland Clinic Health System and received at least 90% of their primary care services at a CCHS facility. | CCHS facility costs, postacute care services were only included for those patients who were admitted to a CCHS postacute care facility. |
| Leininger et al,79 USA | Descriptive, logistic regression | 2009–2010 (1 year) | Top-10% | New enrollees for Medicaid who completed a self-reported health needs assessment. | Medicaid costs. |
| Lieberman et al,33 USA | Descriptive | 1995–1999 | Top-5% | Medicare FFS beneficiaries. | Medicare spending. |
| Meenan et al,80 USA | Risk modelling. | 1995–1996 | Top-0.5%, top-1% | Enrollees of six Health Maintenance Organizations (HMOs), eligible for some period in 1995 and 1996 and who had an outpatient pharmacy benefit. Medicare Cost enrollees were excluded. | Total claims, including inpatient, outpatient, radiology, pharmacy, durable medical equipment, long-term care, laboratory. |
| Monheit,31 USA | Descriptive, logistic regression | 1996–1997 | Various. Top-1%, Top-2%, Top-5%, Top-10%, Top-20%, Top-30%, Top-50%. |
Representation of non-institutionalised civilian US population (survey respondents). | Total payments (including Out-Of-Pocket, uncovered services and third-party payments). |
| Powers and Chaguturu,9 USA | Descriptive | 2014 | Top-1% | Patients of Partners HealthCare integrated delivery system. | Medicare, Medicaid, commercial insured populations are compared. |
| Pritchard et al,35 USA | Descriptive | 2011 | Top-5% | Managed care population, of all ages, with at least 180 days continuous enrolment prior 1 January 2011, patients with gaps in enrolment greater than 30 days were excluded (so no uninsured or patients enrolled in traditional FFS Medicare or Medicaid programmes). | Medical and pharmaceutical claims for more than 80 US health plans, the total amount reimbursed by the insurer plus the plan member’s out-of-pocket share. |
| Rais et al,38 Canada | Descriptive | 2009–2010 (1 year) |
Top-5% | Cost consuming users of hospital and home care services at the provincial level. | Hospital and home care services. Excluding: primary care and long-term care use. |
| Reid et al,81 Canada | Descriptive | 1996–1997 (1 year) |
Top-5% | ≥18 years and older enrolled in the province’s universal healthcare plan. | Medical services costs in a universal healthcare plan (physician and hospital services). |
| Reschovsky et al,27 USA | Descriptive, logistic regression | 2006 or 12 months before death | Top-25% | Medicare FFS beneficiaries, ≥1 Community Tracking Survey survey, with usual source of care physician. Excluding end-stage renal disease beneficiaries. |
Standardised total costs of Medicare parts A and B. |
| Riley,82 USA | Descriptive | 1975–2004 | Top-1% Top-5% |
Medicare, beneficiaries entitled to parts A and B. | Medicare costs. |
| Robst,83 USA | Descriptive, logistic regression | 2005–2010 | Top-1% in some years, or in 6 years | Medicaid beneficiaries with fee-for-service coverage for at least 6 months in all 6 years. | Medicaid. |
| Rosella et al,24 Canada | Descriptive, multinomial logistic regression | 2003–2008 | Top-5% Top-1%, top-2%–5%, top-6%–50% |
Ontario residents. Participants of the CCH Survey. Excluding: institutionalised. Full-time members of the Canadian forces. Persons living in remote areas/aboriginal reserves. Ages 12–18 years. |
Those covered by Ontario’s Universal Health Insurance Plan. Excluding some prescription drug costs, allied health services, dental care, eye care and assistive devices. |
| Snider et al,25 USA | Logistic regression | 2004–2009 | Top-20% | Employees from large US employers, from the Thomson Reuters Marketscan Commercial Claims and Encounters database with both body mass index and claims in any given year. Pregnant women and underweight employees were excluded. | All inpatient, outpatient and prescription claims. |
| Tamang et al,32 Denmark | Descriptive, prediction modelling | 2004–2011 | Top-10% | Entire population of Western Denmark, with a full year of active residency in year 1. | Danish National Health Service. |
| Wammes et al,17 the Netherlands | Descriptive | 2013 | Top-1%, top-2%–5%, bottom-95% | Beneficiaries of one Dutch health insurer. | Dutch curative health system, basic benefit package including voluntary complementary insurance benefits. |
| Wodchis et al,15 Canada | Descriptive | 1 April 2009–31 March 2012 | Top-1% Top-5% Top-10% Top-50% |
People with a recorded age of less than 105 years who were alive on 1 April in any of the three study years and who had a valid Ontario healthcare at any time between 1 April 2009 and 3 March 2012. | Costs refer to healthcare expenditures that have been allocated to patient encounters for healthcare. All medically necessary care, both acute and long term, as covered by public health insurance. Excluding public health, community service agencies and many other programmes, as well as for administrative (government) staff. Private home care, privately insured medication costs. |
| Zhao et al,84 USA | Descriptive, linear regression | 1997–1999 | Top-0.5% | Private insured, whose claims were covered in the Medstat MarketScan Research Database; a multisource private sector healthcare database. All cases with a pharmacy benefit and at least 1 month of eligibility in each of the first two study years, or the last two study years. | Total medical costs, including inpatient plus ambulatory plus pharmacy costs, and deductibles, coinsurance and coordination-of-benefit payments. |
| Zulman et al,85 USA | Descriptive, regression analyses | Fiscal year 2010 | Top-5% | Veterans served by the VA System, who received inpatient or outpatient VA care. | Outpatient and inpatient, pharmacy, VA-sponsored contract care. |
A range of definitions for high-cost patients were used, and some studies used more than one definition to distinguish between age groups, between high-cost and very high-cost patients or to study persistently high-cost patients (>1 year high costs). In general, patients belonging to the top-1%, top-5%, top-10% or top-20% of spending were considered high-cost patients.
The study population differed between the studies. We categorised eighteen studies as ‘total population’ studies, including studies in universal insurance schemes (of all ages; nine Canadian studies, one Dutch, one German and one Danish study), studies that combined data of different payers or survey studies. Respectively 9, 7 and 14 studies were among US Medicare, US Medicaid or US commercial populations. The remaining studies compared high-cost patients in multiple US payers or were among US dual eligibles (eligible for both Medicare and Medicaid), US Veterans Affairs (VA) beneficiaries or among elderly in the Taiwanese insurance system. Some studies used additional criteria to determine the population. Age, healthcare use or insurance were most frequently used as secondary condition to determine the population.
In 50 studies, total costs per patient were based on the insurance plan or public programme. In the remaining studies, total costs were based on a survey or identified from a variety of sources.
Predisposing characteristics
Table 2 presents predisposing, enabling and need characteristics associated with high-cost patients. Age was related to high-cost patients in several ways. First, high-cost patients were generally older, and higher age was associated with high costs. This held for each payer type. Second, persistently high-cost patients were generally older than episodic high-cost patients, and higher ages were associated with persistently high costs. Third, the magnitude of cost concentration and the threshold for high costs differed between age groups.15 As younger groups are generally healthier, costs are concentrated among fewer individuals. Fourth, clinical diagnoses and utilisation patterns varied across age groups,15–17 and some subgroups were related to particular ages, including mental health high-cost patients among younger ages.18 Finally, although age was related to high costs, total population studies showed that approximately half of the high-cost populations were younger than 65 years.17 19
Table 2.
Predisposing, enabling and need factors for high-cost patients
| Variables | Number of studies |
| Predisposing factors | |
| Age | 3217 20–22 24–31 34–36 57 59–62 65 67–72 76 77 79 81 82 |
| Gender=male | 917 18 20 22 26 27 36 78 83 |
| Gender=female | 1617 19 20 24 25 29–31 59–61 65 67 72 75 81 |
| Ethnicity=black/African–American | 426–28 82 |
| Ethnicity=white | 521 24 61 67 83 |
| Ethnicity=less likely black or Hispanic | 331 67 83 |
| Ethnicity=less likely immigrant | 121 |
| Ethnicity=less likely whites | 2 75 76 |
| Region | 426 67 72 74 |
| Urban residence | 619 26 28 34 36 75 |
| Rural residence | 2 22 72 |
| Living institutionalised | 320 27 66 |
| Employment status: early retiree | 172 |
| Job satisfaction | 160 |
| Marital status: divorced/widow/separated/living alone | 2 34 65 |
| Dependents less likely to incur high costs | 170 |
| Receive care in many census divisions | 127 |
| Harmful habits | 324 60 79 |
| Union membership | 172 |
| Education: less than a high-school degree (neighbourhod level) | 176 |
| Enabling factors | |
| Health insurance | |
| Medicare: more likely dual eligible | 626–28 39 75 82 |
| Medicaid: specific eligibility status | 436 61 67 83 |
| Commercial: increased insurance | 2 59 72 |
| Total population: insurance status had no effect | 131 |
| Type of insurance | 170 |
| Income | |
| Positive relation with high costs | 331 65 72 |
| Negative relation | 518 21 22 71 81 |
| No relation | 324 27 34 |
| Organisational enabling factors | |
| Primary care physician supply | 126 |
| Specialist physician supply | 126 |
| Hospital bed supply | 126 |
| Medical specialist as usual source of care | 127 |
| Proportion of physicians who are medical specialists | 2 28 27 |
| Inadequate time during office visits | 127 |
| Proportion of providers operating for profit | 2 28 27 |
| Teaching hospitals | 128 |
| Low nurse-to-staffing ratios | 128 |
| Low supply of long-term care beds | 128 |
| Regular medical doctor or hospital | 179 |
| Regular medical doctor (negative relation) | 124 |
| Need factors | |
| A00–B99 Certain infectious and parasitic diseases | 915 17 20 21 25 62 65 72 83 |
| C00–D48 Neoplasms | 2115 17 21 22 25 26 28 29 34 35 37 38 72 73 75–78 81 82 85 |
| D50–D89 Diseases of the blood and blood-forming organs and certain disorders involving the immune mechanism | 416 20 35 81 |
| E00–E90 Endocrine, nutritional and metabolic diseases | 3216 17 20–22 25 26 28–30 32–34 36 37 58–60 62 66–68 70 73 75 77–79 81 82 84 85 |
| F00–F99 Mental and behavioural disorders | 349 15–18 20–22 24 26 28 29 33 36 38 39 60–63 66 67 70–73 75 77–79 81–83 85 |
| G00–G99 Diseases of the nervous system | 1017 20 25 32 37 38 62 75 81 83 |
| H00–H59 Diseases of the eye and adnexa | 517 21 36 38 81 |
| I00–I99 Diseases of the circulatory system | 369 15–18 20–22 26 28 29 32–35 37–39 58–60 62 66 68 70 72 73 75–79 81 82 84 85 |
| J00–J99 Diseases of the respiratory system | 309 15–17 20–22 26 28–30 32 34 36–38 58 59 62 65 67 70 73 75 77–79 81 82 84 |
| K00–K93 Diseases of the digestive system | 917 18 20 21 38 72 73 81 83 |
| L00–L99 Diseases of the skin and subcutaneous tissue | 517 20 21 36 81 |
| M00–M99 Diseases of the musculoskeletal system and connective tissue | 159 17 20 21 28 35 60 62 72 73 75 77 78 81 85 |
| N00–N99 Diseases of the genitourinary system | 229 16 17 20–22 26 28–30 32 34 35 37 38 70 72 73 75 78 81 82 |
| O00–O99 Pregnancy, childbirth and the puerperium | 515 36 39 63 81 |
| Q00–Q99 Congenital malformations, deformations and chromosomal abnormalities | 132 |
| R00–R99 Symptoms, signs and abnormal clinical and laboratory findings, not elsewhere classified | 617 21 36 60 78 81 |
| S00–T98 Injury, poisoning and certain other consequences of external causes | 915 17 21 36 38 72 75 76 78 |
| Z00–Z99 Factors influencing health status and contact with health services | 317 21 38 |
| Chronic illness | 2215 17 20 24 28–30 32–34 36 39 57 59 69 70 73 75 77 81 82 85 |
| Multimorbidity/burden of comorbid illness | 319 17 19 20 24–27 29 35 36 39 57 58 60 63 64 68–70 72–75 77 81–85 |
| Decedents/survival | 1415–17 19 20 27 31–33 36 57 75 81 82 |
| Activities daily living | 731 34 59 65 66 69 74 |
| Health status | 924 31 33 34 37 59 65 69 74 |
Studies showed inconsistent results for gender. Respectively 9 and 16 studies noted males and females were overrepresented in high-cost patients. Besides, gender was associated with different segments of the high-cost population, including males in top-1% or persistently extreme-cost patients, and females in top-2%–5% or persistently high-cost patients,17 20 or males in mental health high-cost patients.18
Eleven studies reported the association between ethnicity and high costs. In two Canadian total population studies and three US Medicaid studies, whites were over-represented among high-cost populations, whereas in four US Medicare studies blacks were over-represented.
Socioeconomic status is regarded as both a predisposing characteristic and an enabling characteristic in Andersen’s model, and we found evidence for both relationships. One Canadian study found that high costs were most strongly associated with food insecurity, lower personal income, non-homeownership and living in highly deprived or low ethnic concentration neighbourhoods.21 Other studies found that social deprivation seemed to increase risk for high costs more than material deprivation.22
Ganguli et al studied health beliefs among high-cost US Medicare patients: socioeconomic status, social network, patient activation and relationships with and trust in the clinician and the health system all increased or decreased costs, depending on the context. Trust was particularly important and modified the interaction between patient activation and costs: when patients trusted their physicians, patient activation was associated with lower costs. When trust was lacking, patient activation was associated with higher costs.23
Health behaviours, including underweight, obesity, physical inactivity and former smoking were significantly related to high costs.24 25
Enabling characteristics
The studies’ abilities to assess the effect of insurance were limited because most study populations were determined by insurance. Nevertheless, the studies indicated that increased insurance may have indicated specific or additional care needs. For example, six US Medicare studies reported that high-cost patients were more likely dually eligible, and four US Medicaid studies reported that certain eligibility statuses were associated with high costs. In addition, increased insurance was associated with high costs because it lowers costs. Two US commercial studies mentioned that high-cost patients were more likely to have a health maintenance organisation plan, a preferred provider organisation plan or comprehensive insurance compared with high-deductible health plans, and insured status was associated with less consideration of costs in decision making.23
Twelve studies addressed the relationship between income and high costs. In three US studies, higher incomes were associated with high costs, whereas five Canadian studies found that lower incomes were associated with (mental health) high costs. However, one US, one Taiwanese and one Canadian study reported that income was not significantly related to high costs. Finally, among high-cost US Medicare patients, personal resources and education were associated with increased use of resources (higher socioeconomic status (SES) was linked to higher priced care) and also with lower resources use.23
Organisational enabling factors
The number of primary care physicians, specialists and hospital beds were associated with higher per capita preventable costs among high-cost US Medicare patients.26 Reschovsky et al 27 found several weak or insignificant relationships between organisational factors and high costs within the high-cost population but found that high-cost US Medicare patients more likely had a medical specialist as usual source of care than a primary care physician or surgeon. Finally, high-cost US Medicare patients were only modestly concentrated in hospitals and markets (they were widely distributed through the system). High concentration hospitals (with relatively many high-cost patients) had a 15% higher median cost per claim, were more likely for-profit and teaching hospitals, had lower nurse-to-patient ratios, were more likely to care for the poor and had higher 30-day readmission rates and lower 30-day mortality rates. High concentration hospital referral regions had higher annual median costs per beneficiary, a larger supply of specialists but equal supply of total physicians, a lower supply of long-term care beds, higher hospital care intensity and higher end-of-life spending.28
Need characteristics
Medical characteristics of high-cost patients are presented in table 2. We categorised medical characteristics to ICD-10 chapters. Circulatory diseases, mental and behavioural disorders, endocrine, nutritional and metabolic, diseases of the respiratory system, diseases of the genitourinary system, neoplasms and diseases of the musculoskeletal system and connective tissue were most frequently reported among high-cost patients. The prevalence of chronic disease(s) and multimorbidity were also dominant among high-cost patients. For example, Bynum et al 16 showed that over 26.4% of high-cost US dual eligibles suffered from five or more chronic conditions.
Two studies presented medical characteristics across US payers. Both studies showed that high-cost commercial patients had the lowest numbers of comorbidities and that high-cost Medicaid patients had the highest prevalence of mental illness.9 29 We further compared the prevalence of diabetes, congestive heart failure, lung disease and mental disorders across the studies. The prevalence of diabetes, congestive heart failure and lung disease was relatively low (≈5%–25%) in US commercial and total population studies. In US Medicaid, the prevalence of congestive heart failure and lung disease were relatively high (≈15%–40%; one study reported a prevalence of diabetes and lung disease >60%30), and the prevalence of mental illness was particularly high (≈30%–75%). In US Medicare, the prevalence of diabetes, congestive heart failure and lung disease were highest (≈20%–55%) and the prevalence of mental illness more modest (≈10%–25%). In total populations, approximately 30%–40% of high-cost patients were treated for mental illness. Besides, the prevalence of each of the chronic diseases in the Dutch study was comparable with the prevalence in other total population studies. Finally, persistent high-cost patients had a higher number of comorbidities and a higher prevalence of each of the diseases compared with episodic high-cost patients.
High-cost patients were more likely to die, and those in the process of dying were more likely to incur high costs. The mortality differed between payers, much less between countries. The mortality among Danish and Dutch high-cost patients was comparable with the mortality in other total population studies. In US Medicare studies, the mortality ranged from 14.2% to 27.4%, compared with 11.7% in one US Medicaid study and 5%–13% in total populations. In addition, top-1% patients were more likely to die compared with top-5% patients,17 31 and persistent high-cost patients were more likely to die than episodic high-cost patients.32 Finally, among US dual eligibles, mortality varied much across age and residence groups; nearly half of dual eligibles aged 65 years and older died.16
Expenditure patterns and healthcare utilisation
In each study, costs were heavily concentrated. The top-10% patients roughly accounted for about 68% of costs (range: 55%–77%), the top-5% patients accounted for about 55% of costs (range: 29%–65%) and top-1% patients for approximately 24% (range: 14%–33%) within a given year. Costs were generally less concentrated in US Medicare and more concentrated in total populations.
A wide range of parameters were used to describe high-cost patients’ healthcare utilisation (table 3). Inpatient acute hospital care was most often reported as a primary expenditure category for high-cost patients. In line with this, 17 studies reported hospitalisations, admissions or inpatient days as important cost drivers. Lieberman found that total spending per beneficiary correlated strongly with the use of inpatient services,33 likewise several studies found that increasing levels of use (ie, top-1% compared with top-5%) were associated with increasing proportions of spending on (inpatient) hospital care.15 17 23 24 34 35 Guo et al 36 reported that high-cost users consumed more units of each of the service category analysed, with the exception of laboratory tests; these findings were confirmed elsewhere.35 37 In addition, it was found that 91% of high-cost patients received care in multiple care types.38 Mental care services were listed as expenditure category only in studies of total populations, US Medicaid and US VA. Finally, one study determined the frequency use of expensive services among high-cost patients: expensive treatments (expensive drugs, intensive care unit treatment, dialysis, transplant care, and Diagnosis Related Groups >€30 000) contributed to high cost in approximately one-third of top-1% patients and in less than 10% of top-2%–5% patients.17
Table 3.
Expenditure patterns and utilisation of high-cost patients
| Spending category | Number of studies |
| (Inpatient) hospital care | 3115–19 22–25 27–30 32–39 60 66–68 73 75 78 79 82 85 |
| Subacute care/postacute care services rehabilitation | 119 15 22 27 30 35 38 39 66 67 75 |
| Hospitalisations/admission/ patient days/length of stay | 1717–19 23 26 35 36 39 60 68 73 74 77–79 81 85 |
| Emergency department | 1219 26 29 35–38 60 73 77 78 85 |
| Outpatient (physician) visits | 1319 27 34–37 39 65 73 77 82 83 85 |
| Long-term care | 1115 16 22 30 39 66 67 70 73 78 83 |
| Mental health | 1017 18 22 36 38 61 67 73 83 85 |
| Physician services | 1315 18 27 35–37 68 73 74 81–83 85 |
| Intensive care unit | 2 78 17 |
| Prescription drugs | 1617 19 23 30 35–37 62 65 67 68 75 77–79 85 |
| Persistency | |
| Subsequent use | 1316 20 21 23 29 31–33 62 67 72 82 83 |
| Prior use | 521 32 58 60 65 |
| Persistent users | 2115 16 20–23 26 29 31–33 37 57 58 60 62 65 67 72 82 83 |
| Prediction of high-cost patients* | 1622 25 58–60 63–65 68–70 77 79 80 83 84 |
*An in-depth discussion of prediction models for high costs is beyond the scope of the article (though individual predictors are used throughout the paper). Generally, diagnosis-based models outperform prior cost models, and combinations accurately predict high-cost patients. Besides, comorbidity indices also accurately predict high-cost patients, and self-reported health data meaningfully improved existing models.
Four studies quantified the amount of ‘preventable’ spending (based on preventable emergency department visits and preventable (re-)admissions) among high-cost patients. As shown above, various supply side characteristics were associated with higher preventable costs among high-cost US Medicare patients, and approximately 10% of total costs were preventable.26 Another study found that 4.8% of US Medicare spending was preventable and that high-cost patients accounted for 73.8% of preventable spending. Moreover, 43.8% of preventable spending was accounted for by frail elderly, and preventable spending was particularly high for heart failure, pneumonia, chronic obstructive pulmonary disease/asthma and urinary tract infections.39 Figueroa et al 30 found that preventable spending differed by insurance type among US non-elderly: 3.5%, 2.8%, and 1.4% of spending were preventable among US Medicaid, US Medicaid managed care and privately insured high-cost patients, respectively. Similarly, Graven et al 29 found that proportions of preventable spending differed between payers and that persistent high-cost patients had higher proportions of preventable spending.
Twenty-one studies reported on the persistency of high costs. We found three approaches for studying persistency. First, studies reported prior healthcare use and/or reported posterior healthcare use for patients with high costs in a given index year. In other studies, persistent high-cost patients were compared with episodic high-cost patients. Spending persistency varied between 24% and 48% for top-5% patients, and between 28% and 45% for top-10% patients. Spending persistence was relatively high in US Medicaid and relatively low in US Medicare. Increasing persistence was associated with increasing expenditures on all service types.37
Discussion
We reviewed 55 studies on high-cost patients’ characteristics and healthcare utilisation and made comparisons across payers and countries. The studies consistently point to a high prevalence of multiple (chronic) conditions to explain high-cost patients’ utilisation. Besides, we found a high prevalence of mental illness across all the studies, most notably in US Medicaid and total population studies. We found that various health system characteristics may contribute to high costs. Preventable spending was estimated at maximally 10% of spending. Furthermore, we found that high costs are associated with increasing age and that clinical diagnoses and utilisation patterns varied across age groups. However, still more than half of high-cost patients are younger than 65 years. High costs were associated with higher incomes in the USA, but with lower incomes elsewhere. Finally, we confirmed that high-cost patients are more likely to die, and decedents are more likely to incur high-costs. However, no more than 30% of high-cost patients were in their last year of life.
Strengths and weaknesses
This is the first systematic review of scientific literature on high-cost patients’ characteristics and healthcare utilisation. Future studies might consider inclusion of grey literature. We included studies of various payer types and countries, allowing comparisons across settings. However, most studies were conducted in the USA and Canada, which limits the generalisability of the findings. Although our comparison across countries did not reveal large differences in mortality or prevalence of common chronic diseases, these analyses were based on a limited number of variables, studies and countries. It is likely that the specific characteristics and utilisation of high-cost patients vary across localisations due to a wide range of epidemiological and health system factors. One limitation is that we, because of methodological diversity, did not assess the quality of the included studies, and some studies by design did not control for confounding. To our knowledge, no agreed on framework exists for risk of bias assessment of the kind of studies included in our review. One limitation in current frameworks for observation/cross-sectional studies is that these are primarily designed for studies that aim to assess intervention effects in comparative studies. The internal validity of the findings in our included studies is mainly contingent on its ability to control for relevant confounders. However, no consensus exists about what factors should reasonably be controlled for. The external validity of the findings of each of the studies depend on the breadth of the population studied and the scope of the costs considered for establishing total costs. Our study selection process was aimed at identifying studies with a broad population studies and a wide range of costs considered. Finally, the studies used various approaches for defining the needs and measuring multimorbidity among their populations, which limits the comparability across studies.
Reflections on our findings
Current research in high-cost patients has focused on care redesign of the treatment of patients with multiple chronic morbidities.7 40 One contribution of our review is our identification of notable differences in characteristics and utilisation across payers and countries. This (clinical) diversity of high-cost patients may even be larger at a local level. Segmentation analysis has been suggested as a method to identify homogenous and meaningful segments of patients with similar characteristics, needs and behaviour, which allows for tailored policy.41 Such segmentation analysis may powerfully inform population health management initiatives. Given the multiple needs and cross-sectoral utilisation of high-cost patients, we suggest such analyses should capture both characteristics and utilisation as broadly as possible, to fully apprehend high-cost patients care needs and utilisation. In the context of high-cost patients, multimorbidity complicates segmentation, and the usefulness of segmentation may depend on the way multimorbidity is dealt with. To illustrate a potent example, Hayes et al 42 defined high-need, high-cost patients as ‘people having three or more chronic conditions and a functional limitation that makes it hard for them to perform basic daily tasks’.
Our findings also reveal several supply-side factors that contribute to high costs. However, no firm conclusions can be drawn about the strength of these effects. The apparent limited impact of organisational factors on spending is in line with Andersen’s model predictions, where multimorbidity and health status are prime determinants of healthcare costs.43 However, such findings are surprising given the abundance of evidence for supplier induced demand and medical practice variation.44 High-cost populations may be too diverse for studying the impact of organisational factors; for such studies, more homogenous populations may be prerequisite.
Four of our included studies estimated the amount of ‘preventable’ spending among high-cost patients. Preventable spending was estimated at maximally 10% of spending, which is relatively low compared with the amounts of savings that have been reported elsewhere.8 Preventable spending was mainly defined as preventable emergency department visits or preventable (re-)admissions, as such echoing the two primary targets of most high-need high-cost programmes, including care coordination and disease management. The algorithms used were said to be relatively narrow and could have included other diagnostic categories.29 Besides, future studies might consider more broad measures of preventable or wasteful spending and develop algorithms to identify duplicate services, contraindicated care, unnecessary laboratory testing, unnecessary prolonged hospitalisations or any other kinds of lower value services.
It was striking that three US studies reported that higher incomes were associated with high costs, whereas other studies found that lower incomes were associated with high costs. These findings may point to disparities in health, the price that some Americans pay for their care and the reduced accessibility to care of low-income patients. This may particularly hold for the uninsured. Besides, these findings suggest tailored interventions for lower income patients may be worthwhile.
Policy and research implications
Based on our findings, we deduced four major segments of high-cost patients for which separate policy may be warranted, including patients in their last year of life, patients experiencing a significant health event who return to stable health (episodically high-cost patients), patients with mental illness and patients with persistently high costs characterised by chronic conditions, functional limitations and elder age.
Many interventions have been taken to increase value of end-of-life care. Advance care planning has shown to increase the quality of end-of-life care and decrease costs.45–47 In addition, health systems might consider strengthening their palliative care systems.48 Increasing value for episodically high-cost patients requires appropriate pricing of procedures and drugs, for example, through selective contracting of providers, reference pricing or competitive bidding.49 In addition, bundled payments for procedures and associated care may improve care coordination and reduce the use of duplicative or unnecessary services.50 Multidisciplinary needs assessment and shared decision making may reduce unwarranted variation in expensive procedures. Mental health high-cost patients are known for their medical comorbidities, which suggests these patients might benefit from multidisciplinary cross-sectoral healthcare delivery, for example, through collaborative care.51 52 Finally, persistent high-cost patients might benefit from a variety of models, including disease management, care coordination or ambulatory intensive care units, depending on the needs of the population and local circumstances.8 53–55 Especially population health management approaches may be beneficial for these populations. Sherry et al recently examined five community-oriented programmes that successfully improved care for high-need, high-cost patients. The five programmes shared common attributes, including a ‘whole person’ orientation, shared leadership, flexible financing and shared cross-system governance structures.56
One study addressed health beliefs and patient networks among high-cost patients.23 More of such research is needed as health beliefs may be more amenable to change than other drivers of high costs. One study analysed the use of expensive treatments by high-cost patients.17 Better insight in such healthcare utilisation patterns is needed to inform interventions and policy aimed at high-cost populations. There is a need for segmentation variables and logic that is informative at either microlevel, mesolevel and macrolevel. More research is needed to identify determinants of preventable and wasteful spending.
In conclusion, high-cost patients make up the sickest and most complex populations, and their high utilisation is primarily explained by high levels of chronic and mental illness. High-cost patients are diverse populations and vary across payer types and countries. Tailored interventions are needed to meet the needs of high-cost patients and to avoid waste of scarce resources.
Supplementary Material
Footnotes
Contributors: JJGW drafted the first manuscript and conducted the analyses. JJGW and PJvdW selected eligible studies. JJGW, PJvdW and MACT conceptualised the study and interpreted the data. GPW and PPTJ made a substantial contribution to the development of the research question and interpretation and presentation of the findings. All authors provided feedback to and approved the final manuscript.
Funding: The study was conducted as part of a research program funded through the Dutch Ministry of Health.
Disclaimer: The funding source had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the manuscript for publication.
Competing interests: None declared.
Patient consent: None required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Detailed forms with extracted data are available from the authors upon request.
References
- 1. Zook CJ, Moore FD. High-cost users of medical care. N Engl J Med 1980;302:996–1002. 10.1056/NEJM198005013021804 [DOI] [PubMed] [Google Scholar]
- 2. Blumenthal D, Chernof B, Fulmer T, et al. Caring for High-Need, High-Cost Patients - An Urgent Priority. N Engl J Med 2016;375:909–11. 10.1056/NEJMp1608511 [DOI] [PubMed] [Google Scholar]
- 3. Bodenheimer T, Fernandez A. High and rising health care costs. Part 4: can costs be controlled while preserving quality? Ann Intern Med 2005;143:26–31. 10.7326/0003-4819-143-1-200507050-00007 [DOI] [PubMed] [Google Scholar]
- 4. Colla CH, Lewis VA, Kao LS, et al. Association Between Medicare Accountable Care Organization Implementation and Spending Among Clinically Vulnerable Beneficiaries. JAMA Intern Med 2016;176:1167–75. 10.1001/jamainternmed.2016.2827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wennberg JE, Bronner K, Skinner JS, et al. Inpatient care intensity and patients' ratings of their hospital experiences. Health Aff 2009;28:103–12. 10.1377/hlthaff.28.1.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blumenthal D, et al. Tailoring Complex-Care Management, Coordination, and Integration for High-Need, High-Cost Patients, 2016., in Vital Directions for Health and Health Care Series . Discussion paper. National Academy of Medicine. Washington DC., Editor 2016. [Google Scholar]
- 7. Anderson GF, Ballreich J, Bleich S, et al. Attributes common to programs that successfully treat high-need, high-cost individuals. Am J Manag Care 2015;21:e597–600. [PubMed] [Google Scholar]
- 8. Brown RS, Peikes D, Peterson G, et al. Six features of Medicare coordinated care demonstration programs that cut hospital admissions of high-risk patients. Health Aff 2012;31:1156–66. 10.1377/hlthaff.2012.0393 [DOI] [PubMed] [Google Scholar]
- 9. Powers BW, Chaguturu SK. ACOs and High-Cost Patients. N Engl J Med 2016;374:203–5. 10.1056/NEJMp1511131 [DOI] [PubMed] [Google Scholar]
- 10. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. World bank. cited 2017; http://en.wikipedia.org/wiki/World_Bank_high-income_economy
- 13. Andersen RM. Revisiting the behavioral model and access to medical care: does it matter? J Health Soc Behav 1995;36:1–10. 10.2307/2137284 [DOI] [PubMed] [Google Scholar]
- 14. Kominski GF. Changing the U.S. Health Care System: Key Issues in Health Services Policy and Management: Wiley, 2013. [Google Scholar]
- 15. Wodchis WP, Austin PC, Henry DA. A 3-year study of high-cost users of health care. CMAJ 2016;188:182–8. 10.1503/cmaj.150064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bynum JPW, Austin A, Carmichael D, et al. High-Cost Dual Eligibles' Service Use Demonstrates The Need For Supportive And Palliative Models Of Care. Health Aff 2017;36:1309–17. 10.1377/hlthaff.2017.0157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wammes JJG, Tanke M, Jonkers W, et al. Characteristics and healthcare utilisation patterns of high-cost beneficiaries in the Netherlands: a cross-sectional claims database study. BMJ Open 2017;7:e017775 10.1136/bmjopen-2017-017775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Oliveira C, Cheng J, Vigod S, et al. Patients With High Mental Health Costs Incur Over 30 Percent More Costs Than Other High-Cost Patients. Health Aff 2016;35:36–43. 10.1377/hlthaff.2015.0278 [DOI] [PubMed] [Google Scholar]
- 19. Guilcher SJ, Bronskill SE, Guan J, et al. Who Are the High-Cost Users? A Method for Person-Centred Attribution of Health Care Spending. PLoS One 2016;11:e0149179 10.1371/journal.pone.0149179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. DeLia D. Mortality, Disenrollment, and Spending Persistence in Medicaid and CHIP. Med Care 2017;55:220–8. 10.1097/MLR.0000000000000648 [DOI] [PubMed] [Google Scholar]
- 21. Fitzpatrick T, Rosella LC, Calzavara A, et al. Looking Beyond Income and Education: Socioeconomic Status Gradients Among Future High-Cost Users of Health Care. Am J Prev Med 2015;49:161–71. 10.1016/j.amepre.2015.02.018 [DOI] [PubMed] [Google Scholar]
- 22. Chechulin Y, Nazerian A, Rais S, et al. Predicting patients with high risk of becoming high-cost healthcare users in Ontario (Canada). Healthc Policy 2014;9:68–79. 10.12927/hcpol.2014.23710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ganguli I, Thompson R, Ferris TG. What can five high cost patients teach us about health care spending? Journal of General Internal Medicine 2016;1:S469. [DOI] [PubMed] [Google Scholar]
- 24. Rosella LC, Fitzpatrick T, Wodchis WP, et al. High-cost health care users in Ontario, Canada: demographic, socio-economic, and health status characteristics. BMC Health Serv Res 2014;14:532 10.1186/s12913-014-0532-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Snider JT, Bognar K, Globe D, et al. Identifying patients at risk for high medical costs and good candidates for obesity intervention. Am J Health Promot 2014;28:218–27. 10.4278/ajhp.121116-QUAN-561 [DOI] [PubMed] [Google Scholar]
- 26. Joynt KE, Gawande AA, Orav EJ, et al. Contribution of preventable acute care spending to total spending for high-cost Medicare patients. JAMA 2013;309:2572–8. 10.1001/jama.2013.7103 [DOI] [PubMed] [Google Scholar]
- 27. Reschovsky JD, Hadley J, Saiontz-Martinez CB, et al. Following the money: factors associated with the cost of treating high-cost Medicare beneficiaries. Health Serv Res 2011;46:997–1021. 10.1111/j.1475-6773.2011.01242.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Beaulieu ND, Joynt KE, Wild R, et al. Concentration of high-cost patients in hospitals and markets. Am J Manag Care 2017;23:233–8. [PubMed] [Google Scholar]
- 29. Graven PF, et al. Preventable acute care spending for high-cost patients across payer types. Journal of Health Care Finance 2016;42. [Google Scholar]
- 30. Figueroa JF, Frakt AB, Lyon ZM, et al. Characteristics and spending patterns of high cost, non-elderly adults in Massachusetts. Healthc 2017;5:165–70. 10.1016/j.hjdsi.2017.05.001 [DOI] [PubMed] [Google Scholar]
- 31. Monheit AC. Persistence in health expenditures in the short run: prevalence and consequences. Med Care 2003;41 III–53. 10.1097/00005650-200307007-00007 [DOI] [PubMed] [Google Scholar]
- 32. Tamang S, Milstein A, Sørensen HT, et al. Predicting patient ’cost blooms' in Denmark: a longitudinal population-based study. BMJ Open 2017;7:e011580 10.1136/bmjopen-2016-011580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lieberman SM, Lee J, Anderson T, et al. Reducing the growth of Medicare spending: geographic versus patient-based strategies. Health Aff 2003;Suppl Web Exclusives:W3-603–13. [DOI] [PubMed] [Google Scholar]
- 34. Ku LJ, Chiou MJ, Liu LF. Variations in the persistence of health expenditures and the implications for the design of capitation payments in Taiwan. J Health Serv Res Policy 2015;20:146–53. 10.1177/1355819615577711 [DOI] [PubMed] [Google Scholar]
- 35. Pritchard D, Petrilla A, Hallinan S, et al. What Contributes Most to High Health Care Costs? Health Care Spending in High Resource Patients. J Manag Care Spec Pharm 2016;22:102–9. 10.18553/jmcp.2016.22.2.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Guo JJ, Ludke RL, Heaton PC, et al. Characteristics and risk factors associated with high-cost Medicaid recipients. Manag Care Interface 2004;17:20–7. [PubMed] [Google Scholar]
- 37. Hwang W, LaClair M, Camacho F, et al. Persistent high utilization in a privately insured population. Am J Manag Care 2015;21:309–16. [PubMed] [Google Scholar]
- 38. Rais S, Nazerian A, Ardal S, et al. High-cost users of Ontario’s healthcare services. Healthc Policy 2013;9:44–51. [PMC free article] [PubMed] [Google Scholar]
- 39. Figueroa JF, Joynt Maddox KE, Beaulieu N, et al. Concentration of Potentially Preventable Spending Among High-Cost Medicare Subpopulations: An Observational Study. Ann Intern Med 2017;167:706 10.7326/M17-0767 [DOI] [PubMed] [Google Scholar]
- 40. Bleich SN, Sherrod C, Chiang A, et al. Systematic Review of Programs Treating High-Need and High-Cost People With Multiple Chronic Diseases or Disabilities in the United States, 2008-2014. Prev Chronic Dis 2015;12:E197 10.5888/pcd12.150275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vuik SI, Mayer EK, Darzi A. Patient Segmentation Analysis Offers Significant Benefits For Integrated Care And Support. Health Aff 2016;35:769–75. 10.1377/hlthaff.2015.1311 [DOI] [PubMed] [Google Scholar]
- 42. Hayes SL, Salzberg CA, McCarthy D, et al. High-Need, High-Cost Patients: Who Are They and How Do They Use Health Care? A Population-Based Comparison of Demographics, Health Care Use, and Expenditures. Issue Brief 2016;26:1–14. [PubMed] [Google Scholar]
- 43. Heider D, Matschinger H, Müller H, et al. Health care costs in the elderly in Germany: an analysis applying Andersen’s behavioral model of health care utilization. BMC Health Serv Res 2014;14:71 10.1186/1472-6963-14-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wennberg JE. Tracking Medicine:A Researcher’s Quest to Understand Health Care: A Researcher’s Quest to Understand Health Care. USA: Oxford University Press, 2010. [Google Scholar]
- 45. Brinkman-Stoppelenburg A, Rietjens JA, van der Heide A. The effects of advance care planning on end-of-life care: a systematic review. Palliat Med 2014;28:1000–25. 10.1177/0269216314526272 [DOI] [PubMed] [Google Scholar]
- 46. Dixon J, Matosevic T, Knapp M. The economic evidence for advance care planning: Systematic review of evidence. Palliat Med 2015;29:869–84. 10.1177/0269216315586659 [DOI] [PubMed] [Google Scholar]
- 47. Klingler C, in der Schmitten J, Marckmann G. Does facilitated Advance Care Planning reduce the costs of care near the end of life? Systematic review and ethical considerations. Palliat Med 2016;30:423–33. 10.1177/0269216315601346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. World Health Organization. Strengthening of palliative care as a component of integrated treatment throughout the life course. J Pain Palliat Care Pharmacother 2014;28:130–4. 10.3109/15360288.2014.911801 [DOI] [PubMed] [Google Scholar]
- 49. Stadhouders N, Koolman X, Tanke M, et al. Policy options to contain healthcare costs: a review and classification. Health Policy 2016;120:486–94. 10.1016/j.healthpol.2016.03.007 [DOI] [PubMed] [Google Scholar]
- 50. Miller DC, Gust C, Dimick JB, et al. Large variations in Medicare payments for surgery highlight savings potential from bundled payment programs. Health Aff 2011;30:2107–15. 10.1377/hlthaff.2011.0783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Camacho EM, Ntais D, Coventry P, et al. Long-term cost-effectiveness of collaborative care (vs usual care) for people with depression and comorbid diabetes or cardiovascular disease: a Markov model informed by the COINCIDE randomised controlled trial. BMJ Open 2016;6:e012514 10.1136/bmjopen-2016-012514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Druss BG, Walker ER. Mental disorders and medical comorbidity, in Research Synthesis Report 21. NJ: Robert Wood Johnson Foundation: Princeton, 2011. [PubMed] [Google Scholar]
- 53. Bodenheimer T. Strategies to Reduce Costs and Improve Care for High-Utilizing Medicaid Patients: Reflections on Pioneering Programs: Centre for Health Care Strategies, 2013. [Google Scholar]
- 54. Tricco AC, Antony J, Ivers NM, et al. Effectiveness of quality improvement strategies for coordination of care to reduce use of health care services: a systematic review and meta-analysis. CMAJ 2014;186:E568–E578. 10.1503/cmaj.140289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vrijhoef B, Thorlby R. Developing care for a changing population: supporting patients with costly, complex needs: Nuffield Trust, 2016. [Google Scholar]
- 56. Sherry M, Wolff JL, Ballreich J, et al. Bridging the Silos of Service Delivery for High-Need, High-Cost Individuals . Popul Health Manag 2016;19:421–8. 10.1089/pop.2015.0147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Aldridge MD, Kelley AS. The Myth Regarding the High Cost of End-of-Life Care. Am J Public Health 2015;105:2411–5. 10.2105/AJPH.2015.302889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ash AS, Zhao Y, Ellis RP, et al. Finding future high-cost cases: comparing prior cost versus diagnosis-based methods. Health Serv Res 2001;36(6 Pt 2):194–206. [PMC free article] [PubMed] [Google Scholar]
- 59. Bayliss EA, Powers JD, Ellis JL, et al. Applying Sequential Analytic Methods to Self-Reported Information to Anticipate Care Needs. EGEMS 2016;4:14 10.13063/2327-9214.1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Boscardin CK, Gonzales R, Bradley KL, et al. Predicting cost of care using self-reported health status data. BMC Health Serv Res 2015;15:406 10.1186/s12913-015-1063-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Buck JA, Teich JL, Miller K. Use of mental health and substance abuse services among high-cost Medicaid enrollees. Adm Policy Ment Health 2003;31:3–14. 10.1023/A:1026089422101 [DOI] [PubMed] [Google Scholar]
- 62. Chang HY, Boyd CM, Leff B, et al. Identifying Consistent High-cost Users in a Health Plan: Comparison of Alternative Prediction Models. Med Care 2016;54:852–9. 10.1097/MLR.0000000000000566 [DOI] [PubMed] [Google Scholar]
- 63. Charlson ME, Wells MT, Kanna B, et al. Medicaid managed care: how to target efforts to reduce costs. BMC Health Serv Res 2014;14:461 10.1186/1472-6963-14-461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Charlson M, Wells MT, Ullman R, et al. The Charlson comorbidity index can be used prospectively to identify patients who will incur high future costs. PLoS One 2014;9:e112479 10.1371/journal.pone.0112479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cohen SB, Ezzati-Rice T, Yu W. The utility of extended longitudinal profiles in predicting future health care expenditures. Med Care 2006;44(5 Suppl):I–45. 10.1097/01.mlr.0000208200.31206.38 [DOI] [PubMed] [Google Scholar]
- 66. Coughlin TA, Waidmann TA, Phadera L. Among dual eligibles, identifying the highest-cost individuals could help in crafting more targeted and effective responses. Health Aff 2012;31:1083–91. 10.1377/hlthaff.2011.0729 [DOI] [PubMed] [Google Scholar]
- 67. Coughlin TA, Long SK. Health care spending and service use among high-cost Medicaid beneficiaries, 2002-2004. Inquiry 2010;46:405–17. 10.5034/inquiryjrnl_46.4.405 [DOI] [PubMed] [Google Scholar]
- 68. Crawford AG, Fuhr JP, Clarke J, et al. Comparative effectiveness of total population versus disease-specific neural network models in predicting medical costs. Dis Manag 2005;8:277–87. 10.1089/dis.2005.8.277 [DOI] [PubMed] [Google Scholar]
- 69. Fleishman JA, Cohen JW. Using information on clinical conditions to predict high-cost patients. Health Serv Res 2010;45:532–52. 10.1111/j.1475-6773.2009.01080.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hartmann J, Jacobs S, Eberhard S, et al. Analysing predictors for future high-cost patients using German SHI data to identify starting points for prevention. Eur J Public Health 2016;26:549–55. 10.1093/eurpub/ckv248 [DOI] [PubMed] [Google Scholar]
- 71. Hensel JM, Taylor VH, Fung K, et al. Rates of Mental Illness and Addiction among High-Cost Users of Medical Services in Ontario. Can J Psychiatry 2016;61:358–66. 10.1177/0706743716644764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hirth RA, Gibson TB, Levy HG, et al. New evidence on the persistence of health spending. Med Care Res Rev 2015;72:277–97. 10.1177/1077558715572387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hunter G, Yoon J, Blonigen DM, et al. Health Care Utilization Patterns Among High-Cost VA Patients With Mental Health Conditions. Psychiatr Serv 2015;66:952–8. 10.1176/appi.ps.201400286 [DOI] [PubMed] [Google Scholar]
- 74. Izad Shenas SA, Raahemi B, Hossein Tekieh M, et al. Identifying high-cost patients using data mining techniques and a small set of non-trivial attributes. Comput Biol Med 2014;53:9–18. 10.1016/j.compbiomed.2014.07.005 [DOI] [PubMed] [Google Scholar]
- 75. Joynt KE, Figueroa JF, Beaulieu N, et al. Segmenting high-cost Medicare patients into potentially actionable cohorts. Healthc 2017;5(1-2):62–7. 10.1016/j.hjdsi.2016.11.002 [DOI] [PubMed] [Google Scholar]
- 76. Krause TM, Yay Donderici E, Ganduglia Cazaban C, et al. Future expenditure risk of silent members: a statistical analysis. BMC Health Serv Res 2016;16:319 10.1186/s12913-016-1552-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lauffenburger JC, Franklin JM, Krumme AA, et al. Longitudinal Patterns of Spending Enhance the Ability to Predict Costly Patients: A Novel Approach to Identify Patients for Cost Containment. Med Care 2017;55:64–73. 10.1097/MLR.0000000000000623 [DOI] [PubMed] [Google Scholar]
- 78. Lee NS, Whitman N, Vakharia N, et al. High-Cost Patients: Hot-Spotters Don’t Explain the Half of It. J Gen Intern Med 2017;32:28–34. 10.1007/s11606-016-3790-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Leininger LJ, Friedsam D, Voskuil K, et al. Predicting high-need cases among new Medicaid enrollees. Am J Manag Care 2014;20:e399–e407. [PubMed] [Google Scholar]
- 80. Meenan RT, Goodman MJ, Fishman PA, et al. Using risk-adjustment models to identify high-cost risks. Med Care 2003;41:1301–12. 10.1097/01.MLR.0000094480.13057.75 [DOI] [PubMed] [Google Scholar]
- 81. Reid R, Evans R, Barer M, et al. Conspicuous consumption: characterizing high users of physician services in one Canadian province. J Health Serv Res Policy 2003;8:215–24. 10.1258/135581903322403281 [DOI] [PubMed] [Google Scholar]
- 82. Riley GF. Long-term trends in the concentration of Medicare spending. Health Aff 2007;26:808–16. 10.1377/hlthaff.26.3.808 [DOI] [PubMed] [Google Scholar]
- 83. Robst J. Developing Models to Predict Persistent High-Cost Cases in Florida Medicaid. Popul Health Manag 2015;18:467–76. 10.1089/pop.2014.0174 [DOI] [PubMed] [Google Scholar]
- 84. Zhao Y, Ash AS, Haughton J, et al. Identifying Future High-Cost Cases Through Predictive Modeling. Disease Management & Health Outcomes 2003;11:389–97. 10.2165/00115677-200311060-00005 [DOI] [Google Scholar]
- 85. Zulman DM, Pal Chee C, Wagner TH, et al. Multimorbidity and healthcare utilisation among high-cost patients in the US Veterans Affairs Health Care System. BMJ Open 2015;5:e007771 10.1136/bmjopen-2015-007771 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-023113supp001.pdf (7.3KB, pdf)
bmjopen-2018-023113supp002.pdf (97.9KB, pdf)