Malaria induces increase in circulating regulatory T cells and triggers their expression of inhibitory molecules such as PD-1. This phenomenon is associated with changes in the Treg phenotype, suggesting both plasticity and altered suppressive functions during Plasmodium vivax infection.
Keywords: regulatory T cells, inhibitory molecules, PD-1, Plasmodium vivax, malaria
Abstract
The balance between pro- and antiinflammatory mechanisms is essential to limit immune-mediated pathology, and CD4+ forkhead box P3 (Foxp3+) regulatory T cells (Treg) play an important role in this process. The expression of inhibitory receptors regulates cytokine production by Plasmodium vivax-specific T cells. Our goal was to assess the induction of programmed death-1 (PD-1) and cytotoxic T-lymphocyte antigen (CTLA-4) on Treg during malaria and to evaluate their function. We found that P. vivax infection triggered an increase in circulating Treg and their expression of CTLA-4 and PD-1. Functional analysis demonstrated that Treg from malaria patients had impaired suppressive ability and PD-1+Treg displayed lower levels of Foxp3 and Helios, but had higher frequencies of T-box transcription factor+ and interferon-gamma+ cells than PD-1−Treg. Thus malaria infection alters the function of circulating Treg by triggering increased expression of PD-1 on Treg that is associated with decreased regulatory function and increased proinflammatory characteristics.
Malaria remains a major health problem in tropical countries. It is estimated that 212 million people were clinically affected by malaria and almost half a million deaths occurred in 2015 [1]. Plasmodium vivax is the most widespread parasite causing malaria and mechanisms of immune evasion are important for virulence [2]. Nevertheless, pathogen-associated molecular patterns expressed by Plasmodium are recognized and activate cells of the innate immune system, such as neutrophils and monocytes [3–7]. This can lead to massive cytokine production, which can cause immunopathology and therefore needs to be regulated [8]. Several mechanisms can contribute to the modulation of inflammatory responses, including antiinflammatory mediators and forkhead box P3 (Foxp3)-expressing CD4+ regulatory T cells (Treg) [9–11].
Foxp3 has been defined as a key regulatory transcription factor for the development and function of Treg [11] and its expression is commonly associated with high levels of CD25 and low levels of CD127 [12, 13]. However, Treg can also express other transcription factors depending on their activation status and differentiation. For example, Helios, a member of the Ikaros transcription factor family, is expressed by Treg and it is associated with T-cell activation and cellular division [14]. Despite T-box transcription factor (Tbet) being considered a master transcriptional regulator of T-helper (TH1) differentiation, its expression is also found in different populations of innate and adaptive immune cells, including Treg under some circumstances [15–17].
The inflammatory scenario triggered during malaria also induces the expression of inhibitory molecules by activated T cells, and the coexpression of these molecules often indicates T-cell exhaustion [18–20]. The impact of the inhibitory molecules on different outcomes of malaria was demonstrated both in animal models and in human disease. The blockade of cytotoxic T-lymphocyte antigen (CTLA-4) and programmed cell death protein 1 (PD-1) during mild malaria, in humans and mice, was able to restore the production of inflammatory cytokines, contributing to the immune response against Plasmodium. On the other hand, during severe murine malaria, the blockade of inhibitory molecules was associated with deleterious inflammation and reduced survival [20–24]. These inhibitory molecules can be also expressed by Treg [25, 26]. CTLA-4 is constitutively expressed by Treg and is responsible for the regulation of T-cell effector functions by transendocytosis [27, 28]. The expression of PD-1 on Treg, on the other hand, is induced during viral infections, such as human immunodeficiency virus (HIV), lymphocytic choriomeningitis virus (LCMV), hepatitis C virus (HCV), and the Friend virus murine model [26, 29–31]. These reports suggest that the expression of PD-1 by Treg represents a more complex and controversial mechanism of regulation of the immune responses.
We previously demonstrated that P. vivax infection triggers the expression of inhibitory molecules by T lymphocytes, modulating cytokine production by antigen-specific effector T cells [20]. Here, we address the hypothesis that inhibitory molecules are also induced on Treg during malaria and that this may affect their regulatory functions. Indeed, we found a higher proportion of PD-1 and CTLA-4 expressing Treg in P. vivax-infected patients, and these Treg were less capable of suppressing proliferation of other CD4+ T cells. We further show that upregulation of PD-1 was associated with decreased expression of Foxp3 and Helios and increased expression of Tbet in Treg during P. vivax infection, suggesting that malaria promotes a more proinflammatory population of Treg cells.
MATERIALS AND METHODS
Patients and Healthy Donors
P. vivax-infected patients were enrolled in this study during acute infection (before treatment [BT]) and 30–45 days after treatment (AT) at Centro de Pesquisa de Medicina Tropical de Rondônia in Porto Velho, a malaria endemic area in the Amazon, Brazil. The group of malaria patients consisted of 31 individuals (5 females, 26 males) with ages raging from 23 to 62 years (39.56 ± 10.68) (Supplementary Table 1). A total of 100 mL of peripheral blood was collected from each patient, after confirmation of P. vivax infection by thick blood smear film. Patients were treated according to the Brazilian Ministry of Health recommendations. The second sample from each patient was collected 30–45 days AT, after confirmation of negative parasitemia. Healthy donors (HD) consisted of 12 individuals with ages ranging from 25 to 65 years (39.17 ± 15.18) from the same endemic area, who did not have any malaria episode on the dates the blood samples were collected, did not present with any other symptoms and were not on medication for chronic diseases.
Ethics
This study was performed under protocols approved by the Ethical Committees on Human Experimentation from Instituto René Rachou-Fiocruz (CEP-CPqRR24/2011). Patients were enrolled in the study after providing written informed consent.
Reticulocyte Purification
Red blood cells (1 mL) were thawed at 37°C and transferred to a 50-mL tube where 200 µL of a saline solution (12% NaCl) was slowly added. The cell suspension was incubated for 5 minutes at room temperature and then 10 mL of 1.6% NaCl solution was added. After homogenization, the cell suspension was centrifuged at 600g for 10 minutes. Cells were then suspended in 10 mL of 0.9% NaCl solution with 0.2% dextrose. Different concentrations of saline solutions were used to avoid hemolysis. Cell suspension was centrifuged and red blood cells were incubated in 10 mL McCoy’s 5A-Medium (Gibco) 0.5% of glucose (Sigma), and 25% of human AB serum (Cellgro) for 20 hours at 37°C, 5% CO2/5% O2 + 90% N2 to allow parasite maturation. After incubation, the cell suspension was centrifuged and suspended in 5 mL Roswell Park Memorial Institute 1640 (RPMI) (Sigma Aldrich), overlayed on 5 mL of 45% Percoll (Sigma Aldrich) solution and centrifuged at 1600g for 10 minutes. Enriched P. vivax-infected reticulocytes (iRet) were washed and diluted to be incubated with peripheral blood mononuclear cells (PBMC) or leukocyte subpopulations [33–35].
T-Cell Immunophenotyping and Intracellular Cytokine Measurement
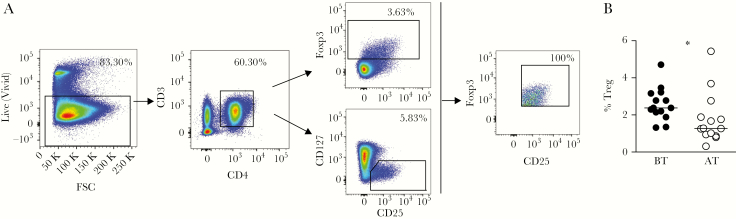
PBMC were prepared from heparinized blood samples with Ficoll-Hypaque gradient (GE Healthcare Life Sciences) and frozen in fetal bovine serum (FBS; GIBCO, Life Technologies) containing 20% dimethyl sulphoxide (Sigma). PBMC were thawed in RPMI supplemented with 10% fetal calf serum and benzonase nuclease (20 U/mL; Novagen), washed, incubated with Live/Dead Fixable cell stain (ThermoFisher) and monoclonal antibodies (mAb) (Supplementary Table 2). Cells were then washed, fixed, and permeabilized according to the manufacture’s instructions (Foxp3 staining buffer set, eBioscience). Stained cell suspensions were acquired on a BD LSR-FORTESSA totaling at least 500000 events per sample for phenotypic characterization and at least 3000 events per sample for functional characterization. A forward scatter area (FSC-A) versus forward scatter height (FSC-H) gate was used to remove doublets. Size scatter area (SSC-A) versus time and combinations of fluorochromes gates were used to exclude debris and flux interruptions. Viable T cells were gated using a Live/Dead gate versus FSC-A gate and Treg were defined as Foxp3+CD127lowCD25+CD4+CD3+ cells (Figure 1) [12, 13]. To do so, the Treg population was gated using a tool available in the software Flowjo called AND-Boolean gate, which defines a population of an area of overlap between cell populations. In this case, we select FoxP3+ cells (among CD4+CD3+ live cells) AND CD25+CD127low cells (among CD4+CD3+ live cells). GraphPad Prism-V5.0 (GraphPad software) and FlowJo-V10 (TreeStar) were used for data analyses and graphical representation.
Figure 1.
Plasmodium vivax infection induces increased proportion of regulatory T cells (Treg). A, Representative density plots showing the gating strategy. Treg were analyzed after gating live cells and based on the expression of CD3, CD4, CD25, forkhead box P3 (Foxp3), and excluding those bearing CD127. B, Peripheral blood mononuclear cells from malaria patients were analyzed ex vivo during acute infection with P. vivax before treatment (BT; ●; n = 14) and 30–45 days after treatment (AT; ○; n = 14) and frequencies are represented as dispersion symbols. Lines represent median values in each group. * P < .05, using the Wilcoxon test when comparing BT versus AT. Abbreviation: FSC, forward scatter.
Cell Purification and Culture
After staining, PBMC from HD and P. vivax-acutely infected patients were sorted into monocytes, Treg subsets, and the remaining PBMC based on the expression of CD14, CD4, CD25, CD127, and PD-1, using FACSAria II and III (BD Biosciences). Gates were set to exclude doublets and then to select T-cell populations using FCS-A versus SSC-A. The first round of sorting was based on expression of CD4 and CD14 (monocytes). CD4+ Treg subsets were obtained in a second round of sorting, based on expression of CD25, CD127, and PD-1: Treg (CD25+CD127loCD4+), PD-1+ Treg (CD4+CD25+CD127lowPD-1+), and PD-1− Treg (CD4+CD25+CD127lowPD-1−). The remaining cell subpopulations, comprising CD4− T cells and CD4+CD25−CD127high cells from the first and second sorts, were pooled and considered as PBMC−. CD4+CD25−CD127high cells were named effector CD4+ T cells.
PBMC− were cocultivated without or with Treg, or their subpopulations (PD-1+ or PD-1− Treg), at a ratio of 4:1 (105 PBMC−:2.5 × 104 Treg) [36]. 2.5 × 104 CD14+ monocytes were added to each well. Cells were cultured with anti-CD3 (1 µg/mL, clone HIT3a) and anti-CD28 (0.5 µg/mL, clone CD28.2) mAb. 105 iRet were added to each well where antigen-specific responses were assessed. Cultures were prepared in the presence of RPMI 10% plasma from infected patients, maintained for 5 days in 5% CO2 at 37°C, before supernatants were removed for cytokine measurement. The frequencies of interferon-gamma (IFN-γ) producing cells were analyzed by flow cytometry. To confirm whether malaria impairs specifically Treg function, Treg from P. vivax-infected patients were isolated from frozen PBMC (both BT and AT) and cocultured with autologous effector CD4+ T cells (AT).
CFSE Proliferation Assay
Sorted effector CD4+ T cells were incubated with 1.25 μM carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes) for 8 minutes and subsequently washed 3 times with RPMI 10% FBS. Labeled CD4+ T cells were cultured with Treg in the presence of anti-CD3 and anti-CD28 mAb, as described above. Cells were harvested after 5 days, incubated with Live/Dead Fixable cell stain to exclude dead cells, then washed and incubated with fluorochrome-labeled anti-CD3 and anti-CD4 mAb. Cells were acquired on LSRII Fortessa and the frequency of CFSELowCD4+ T cells was evaluated to characterize proliferation.
Statistical Analysis
Statistical analysis was performed using GraphPad PrismV5.0. Differences were considered significant when P ≤ .05. Results were analyzed using a 2-tailed paired or unpaired t test. Wilcoxon and Mann-Whitney were used when data did not fit a Gaussian distribution and paired t test was used when data exhibited a Gaussian distribution. Correlations were evaluated by Spearman test and a third order polynomial cubic to fit the line was used due to the distribution of the data.
RESULTS
P. vivax Infection Triggers an Increase in the Frequency of Treg
We first investigated the frequencies of Treg in P. vivax-infected patients during acute infection (BT) and 30–45 days AT. Representative density plots show the gating strategy used for Treg, as described in Methods. Treg were characterized as Foxp3+CD127lowCD25+CD4+CD3+ cells (Figure 1A).
We observed significantly higher frequencies of Treg in patients during acute P. vivax infection when compared with the same patients AT (Figure 1B).
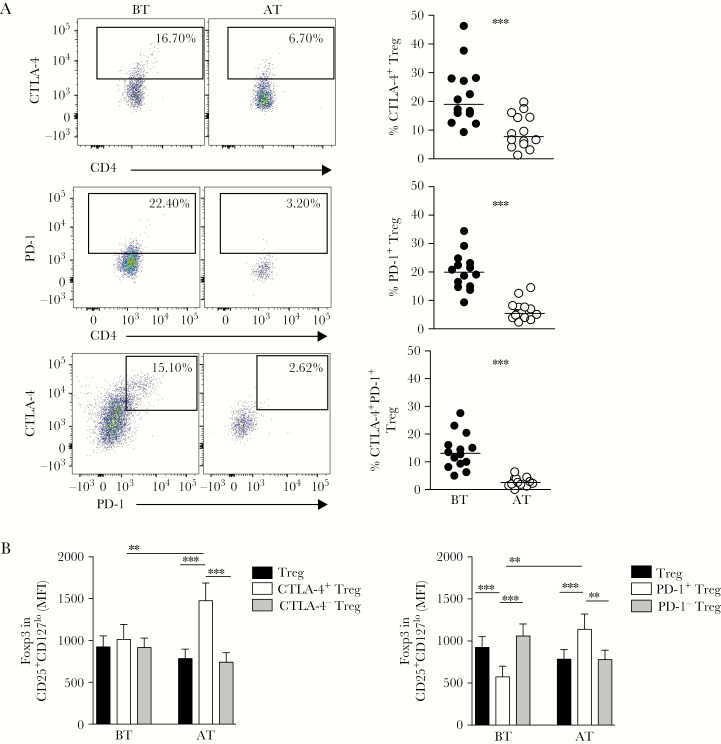
Malaria Induces Expression of CTLA-4 and PD-1 on Treg and Decreases the Expression of Foxp3 in PD-1+ Treg
We previously described that P. vivax triggers the expression of inhibitory molecules on T cells and that blockade of their signaling pathways restores cytokine production by antigen-specific T cells [20]. We therefore evaluated the expression of two of these molecules, CTLA-4 and PD-1, on Treg from malaria patients. The analyses used the gate strategy displayed in Figure 1A and Figure 2A (left panels). We found significantly higher frequencies of both CTLA-4 and PD-1 expression on Treg from patients BT, when compared with Treg assessed in the same individuals AT (Figure 2A, upper and middle panels). We also observed that the coexpression of CTLA-4 and PD-1 on Treg from patients BT was higher during P. vivax infection compared to Treg analyzed AT (Figure 2A, lower panels). The coexpression of inhibitory molecules is known to lead to T-cell exhaustion [37], which suggested that the increase in inhibitory molecules expression by Treg during malaria might have altered their effector functions.
Figure 2.
Plasmodium vivax infection triggers the expression of cytotoxic T-lymphocyte antigen (CTLA-4) and programmed cell death protein 1 (PD-1), and decreases forkhead box P3 (Foxp3) expression in PD-1+ regulatory T cells (Treg). A, Representative density plots showing the proportion of Treg expressing CTLA-4, PD-1, and coexpressing CTLA-4 and PD-1 (left panels) from a single P. vivax-infected patient before (BT; left plots) and after treatment (AT; right plots). Percentage of Treg expressing CTLA-4, PD-1, and co-expressing CTLA-4 and PD-1 from P. vivax-infected patients BT (●, n = 14) and AT (○, n = 14) (right panels). Lines represent the median values of a given measurement in each group. B, Mean fluorescence intensity (MFI) of Foxp3 expression analyzed ex vivo in CD127lowCD25+CD4+ T cells (Treg), and their subpopulations expressing or not CTLA-4 (left graph) and PD-1 (right graph) in P. vivax-infected patients BT (n = 14) and AT (n = 14). ** .001 < P < .05 and *** P < .001 using the Wilcoxon Test.
To examine Treg function during P. vivax infection and its relationship with inhibitory molecule expression, we first assessed the expression of Foxp3, the transcription factor that serves as a lineage signature of Treg [11]. We evaluated Foxp3 expression among CTLA-4 and PD-1–expressing Treg subsets from patients during P. vivax infection (BT) and AT (Figure 2B). We found no differences in Foxp3 levels when total Treg, CTLA-4+ Treg, and CTLA-4− Treg were compared before treatment (Figure 2B, left panel). In contrast, PD-1+ Treg had significantly decreased Foxp3 levels during P. vivax infection (Figure 2B, right panel). However, AT, the levels of Foxp3 were significantly higher among CTLA-4+ Treg and PD-1+ Treg, when compared to total Treg and to the CTLA-4− Treg or PD-1− Treg subsets, respectively (Figure 2B).
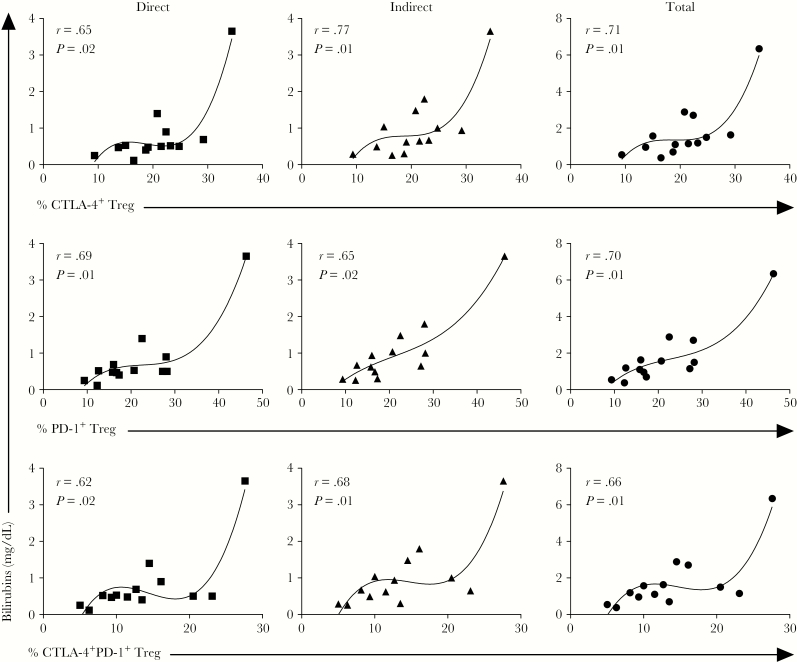
Expression of Inhibitory Molecules by Treg Is Associated With the Levels of Bilirubins
In order to investigate the association between the expression of inhibitory molecule and clinical manifestations during malaria, correlation analyses were performed with markers associated with clinical disease. We found that there was a positive correlation between the frequencies of CTLA-4+, PD-1+, and CTLA-4+PD-1+ Treg with serum levels of direct, indirect, and total bilirubins during P. vivax infection (Figure 3). A third-order polynomial equation was used to fit the line due the distribution of the data and no data were excluded from the analysis.
Figure 3.
Higher expression of cytotoxic T-lymphocyte antigen (CTLA-4)+, programmed cell death protein 1 (PD-1)+ , and CTLA-4+PD-1+ regulatory T cells (Treg) correlate with higher levels of bilirubin during Plasmodium vivax infection. Levels of direct, indirect, and total bilirubin were assessed in serum and correlated with proportions of CTLA-4+, PD-1+ and CTLA+PD-1+ Treg analyzed ex vivo in peripheral blood mononuclear cells from P. vivax-infected patients. P value and r, using Spearman test, are depicted in each graph.
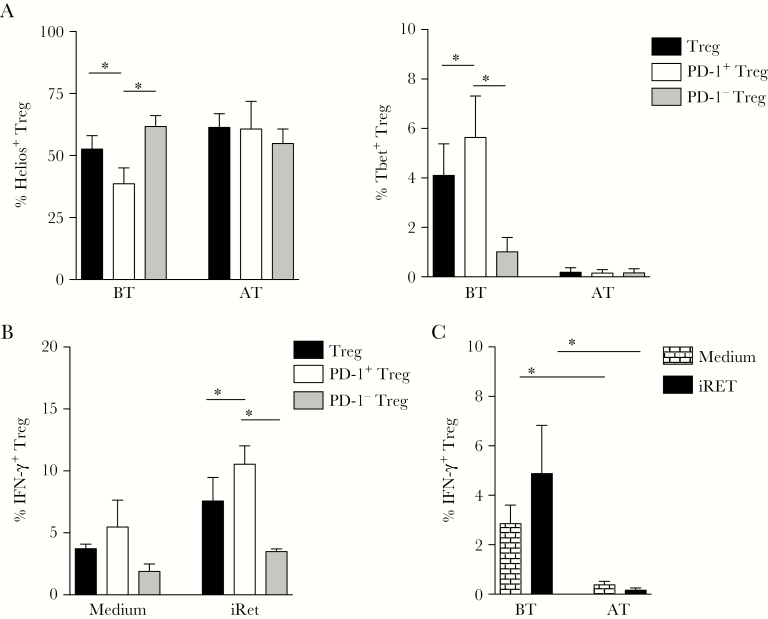
PD-1 Expressing Treg Display Decreased Levels of Helios and Increased Expression of Tbet and IFN-γ During P. vivax Infection
Through the phenotypic characterization of PD-1 and CTLA-4–expressing Treg, we observed that most of the PD-1+ Treg also expressed CTLA-4, whereas a minority of Treg exclusively expressed CTLA-4. Indeed, it was shown that simultaneous expression of inhibitory receptors leads to impaired CD4+ T-cell effector functions [23]. Thus, we choose to further analyze the PD-1–expressing Treg population for expression of transcription factors and secretion of IFN-γ.
Because the levels of Foxp3 among PD-1–expressing Treg were affected by P. vivax infection (Figure 2), we also analyzed the expression of two other transcription factors, Helios and Tbet, respectively associated with the differentiation of Treg and TH1 cells (Figure 4A). Consistent with their reduced expression of Foxp3, lower frequencies of Helios+ cells were found among PD-1+ Treg from acute malaria patients when compared to total Treg or PD-1− Treg (Figure 4A, left panel). In contrast, the frequency of Tbet+ cells was higher among PD-1+ Treg than among the other Treg subsets, when analyzed during acute infection (Figure 4A, right panel). However, AT, no differences were observed in the frequencies of Helios+ or Tbet+ cells among the PD-1+ Treg and other Treg subsets (Figure 4A). To further assess whether the changes in the PD-1+ Treg phenotype correlated with alterations in their function, we assessed the ability of PD-1+ Treg to produce IFN-γ during P. vivax infection. We found significantly higher frequencies of IFN-γ+ cells among PD-1+ Treg in comparison to other Treg subsets, in response to restimulation with iRet (Figure 4B). In addition, when we compared the IFN-γ production between whole Treg from patients BT and AT, we observed significantly higher frequency of IFN-γ–secreting Treg in acutely P. vivax-infected patients (Figure 4C). Although these differences were observed when comparing PD-1+ and PD-1-Treg, as far as we could investigate, PD-1 expression was not directly associated with the ability of Treg to modulate cytokine production during malaria (Supplementary Figure 1).
Figure 4.
The decreased levels of Helios in programmed cell death protein 1 (PD-1)+ regulatory T cells (Treg) are accompanied by increased expression of T-box transcription factor (Tbet) and higher production of interferon-gamma (IFN-γ). A, The expression of Helios (left panel) and Tbet (right panel) were analyzed among Treg (forkhead box P3 [Foxp3]+CD127lowCD25+CD4+ T cells), and their subpopulations expressing or not PD-1 in the same patients before (BT, n = 5) and after treatment (AT, n = 5). B, Treg, effector cells and monocytes from Plasmodium vivax-infected patients were cultured in medium alone or with P. vivax-infected reticulocytes (iRet) and the frequencies of IFN-γ–producing cells were assessed among total Treg (n = 5), PD-1+ Treg (n = 6), and PD-1-Treg (n = 6). C, Cells from P. vivax-infected patients (n = 5) and from patients after treatment (n = 4) were cultured in medium alone or with iRet and the frequencies of IFN-γ–producing cells were assessed among total Treg. Bars indicate mean values and lines represent standard error. * P < .05, using the Wilcoxon Test.
Regulatory Function of Treg Is Impaired During P. vivax Infection
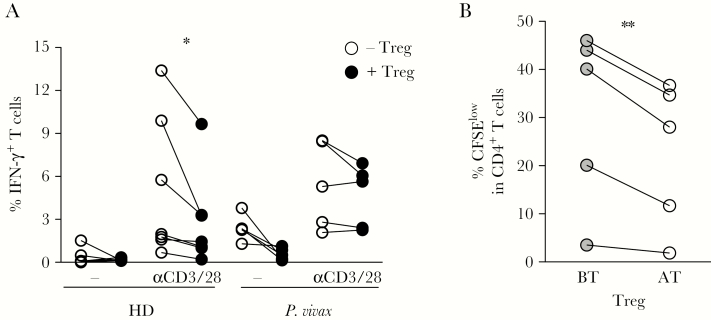
To evaluate the functionality of Treg during malaria, PBMC (depleted of Treg) from P. vivax-infected patients or HD were cocultivated in the presence or absence of autologous Treg, in medium alone or stimulated with anti-CD3 and CD28 mAb (Figure 5A). The ability of Treg to modulate T-cell effector function was assessed by analyzing the production of IFN-γ. Anti-CD3 and CD28 mAb induced IFN-γ production by T cells from P. vivax-infected patients and HD (Figure 5A). Addition of autologous Treg decreased IFN-γ production by CD4+ T cells from HD, whereas Treg from P. vivax-infected patients were not able to modulate IFN-γ production by CD4+ T cells (Figure 5A).
Figure 5.
Regulatory T cells (Treg) from acutely infected patients display impaired ability to modulate cytokine production and proliferation of CD4+ T cells during malaria. A, Peripheral blood mononuclear cells (depleted of Treg) from healthy donors (HD; n = 7) or Plasmodium vivax-infected patients (n = 5) were cultured for 24 hours in medium alone and with anti-CD3 and anti-CD28 monoclonal antibodies (mAb), in the absence or presence of Treg. Each pair of symbol (with connecting line) shows the frequencies of interferon-gamma (IFN-γ)–producing T cells from an individual. B, CD4+ T cells (depleted of Treg) were purified from individuals after treatment, labeled with carboxyfluorescein succinimidyl ester, and cocultured with autologous Treg collected either during P. vivax infection (BT) or after treatment (AT), for 5 days in the presence of anti-CD3 and anti-CD28 mAb. * P < .05; ** .001 < P < .05, using the paired t test.
Additionally, CD4+ T cells (depleted of Treg) were purified from individuals AT and CFSE labeled, then cocultured with autologous Treg from the same patients collected either during P. vivax infection or AT, in the presence of anti-CD3 and CD28 mAb for 5 days. We observed significantly lower frequencies of CFSElow CD4+ T cells after coculture with autologous Treg isolated from patients AT, compared to those cultured with autologous Treg isolated from patients BT (Figure 5B). These results show that Treg isolated from patients with acute malaria display impaired ability to modulate the proliferation of autologous CD4+ T cells compared to Treg isolated from the same patients after malaria cure.
DISCUSSION
Treg have been shown to play important roles in the control of exacerbated immune responses during several parasite infections through pathways involving cell-cell contact and cytokine production [38–41]. Studies have shown that P. vivax triggers an increase in circulating Treg; however, whether and how these cells control deleterious effector functions during malaria infection are still unclear [25, 42, 43]. Our findings demonstrate that malaria induces increased frequencies of circulating Treg and also increases their expression of CTLA-4 and PD-1. Moreover, increased expression of these inhibitory molecules was associated with changes in the Treg phenotype, suggesting both plasticity and altered suppressive functions during P. vivax infection.
The strong systemic proinflammatory response observed during malaria can trigger the expression of inhibitory molecules. It is broadly accepted that expression of these inhibitory molecules is involved in the process of T-cell exhaustion [37, 44]. Indeed, our previous study reported that the expression of a combination of inhibitory molecules in effector T cells was triggered by P. vivax infection, leading to attenuated cytokine production [20]. Our data show that the expression of CTLA-4 and PD-1 is also increased on Treg upon P. vivax infection. Recently, studies reported that both P. vivax and P. falciparum infection leads to an increase in the expression of CTLA-4 in Treg [25, 45]. Although the induction of PD-1 on Treg has not been previously described in malaria, its roles in the development and function of Treg are well recognized [46].
During acute P. vivax infection, we found that the expression of Foxp3 was selectively decreased among PD-1+ Treg. As P. vivax infection led to a reduction in expression of FoxP3, this analysis gated on CD127loCD25+CD4+ T cells as Treg, because cells expressing lower levels of Foxp3 would probably be missed if we gated on Foxp3+ cells to analyze the further levels of this transcription factor. Similar to our findings, lower levels of Foxp3 were observed among PD-1highTreg than among PD-1lowTreg in another inflammatory condition, a mouse model of lupus [47]. Consistent with reduced Foxp3 expression, the transcription factor Helios was also downregulated in PD-1+ Treg during P. vivax infection. Helios is known to bind the Foxp3 promoter, leading to increased Foxp3 expression and reinforcement of regulatory function [48, 49]. Along with the decreased Foxp3 and Helios expression, a higher frequency of Tbet+ cells was observed within PD-1+ Treg during acute malaria. Moreover, PD-1+ Treg displayed a higher frequency of IFN-γ+ cells upon antigen-specific stimulation with iRet. A previous study also reported that PD-1+ Treg may acquire the ability to produce IFN-γ and this was associated with Treg dysfunction [50]. It is known that plasticity is a characteristic of Treg and in specific scenarios they can express Tbet and other signature molecules of the TH1 profile, such as CXCR3 and IFN-γ [16].
In addition to the altered phenotype displayed by Treg during acute malaria, higher expression of CTLA-4 and PD-1 on Treg correlated with higher levels of bilirubins. As PD-1 expression is frequently associated with intense inflammatory response and high pathogen load, its expression by Treg could regulate suppressive effects and favor parasite control. On the other hand, the lack of this suppression may lead to tissue damage. Although our data suggest that PD-1 expression is involved with impaired suppressive function of Treg, a direct association remains to be demonstrated. However, even if the expression of inhibitory molecules is not directly responsible for the impaired suppressive function of Treg, it at least may be considered a biomarker of morbidity during malaria. Alterations of bilirubin levels are indicative of hemolysis with the involvement of hepatic tissue, a compartment known to be affected during malaria and contributing to immunopathology [51].
Indeed, the impact of PD-1 on therole of Treg in regulating suppressive effects suppressive function is still controversial. The expression of PD-1 on Treg induced by LCMV infection was reported to boost their regulatory functions [30]. On the other hand, the expression of PD-1 leading to impaired Treg function has been demonstrated in patients chronically infected with HCV [26]. Similarly, PD-1 expression negatively regulated the suppressive capacity of Treg in a murine model of lupus [47]. Despite malaria patients having increased frequencies of PD-1+ Treg, this phenotype was not differently associated with the ability to suppress cytokine production by effector T cells. In fact, although PD-1 is also increased on Treg during HIV infection, PD-L1 blockade had no effect on their suppressive function [31].
A previous study attempted to characterize the effector functions of Treg during P. vivax infection by assessing their ability to suppress proliferation of PBMC [43]. Although they claimed that Treg were able to modulate the proliferation of PBMC from P. vivax-infected patients, this conclusion was limited because only 2 patients were assessed and there were no HD included as controls. Here, we found that, in contrast to Treg obtained from HD, Treg isolated from P. vivax-infected patients were unable to regulate the production of IFN-γ by CD4+ T cells upon polyclonal stimulation. Moreover, coculture of purified CD4+ T cells from patients with autologous Treg, isolated either during or after the resolution of P. vivax infection, confirmed that Treg had impaired suppressive capacity during malaria infection. The decrease in regulatory ability of Treg during acute malaria could be an attempt to favor the control of P. vivax infection. However, in the absence of regulation, the impairment of Treg suppressive function may lead to tissue damage.
Taken together, our results demonstrate that Treg display impaired function during P. vivax infection and this phenomenon is not directly associated with the expression of PD-1 on Treg. Nevertheless, the expression of PD-1 is associated with altered Treg phenotype and is accompanied by increased levels of bilirrubins in P. vivax-infected patients, suggesting that PD-1 can be employed as a biomarker of immunopathology. Despite our efforts, the cause of the impaired suppressive function of Treg during malaria requires further investigation, alongside the potential role of other inhibitory molecules.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Dragana Jankovic for reviewing the manuscript and Cristina Toscano Fonseca and Rosiane Aparecida Pereira da Silva for scientific discussions. We are also grateful to the nurses and to Ana Beatriz R. Queiroz, Clécia O. Vieira, and Cristiane P. Gomes for excellent technical assistance. We acknowledge the Program for Technological Development in Tools for Health, Fundação Oswaldo Cruz for the use of its facilities.
Financial support. This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (grant number PQ-307408/2016-7); Fundação de Amparo à Pesquisa do Estado de Minas Gerais (grant number APQ-00616-16); CNPq/FAPEMIG-National Institute of Science and Technology for Vaccines (grant number CNPq-573547/2008-4/FAPEMIG/MS-CBB-APQ00077-09); FAPEMIG, PRONEX-Malaria (grant number CBB-APQ-01355-14); National Institute of Allergy and Infectious Diseases (grant number NIH/NIAID/ICEMR/U19 AI089681); CNPq/Ciencias sem fronteiras (grant number 402553/2012–8); and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Reference
- 1. World Health Organization (WHO). World Malaria Report 2015. Geneva, Switzerland: WHO, 2015. [Google Scholar]
- 2. Guerra CA, Howes RE, Patil AP, et al. The international limits and population at risk of Plasmodium vivax transmission in 2009. PLoS Negl Trop Dis 2010; 4:e774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Antonelli LR, Leoratti FM, Costa PA, et al. The CD14+CD16+ inflammatory monocyte subset displays increased mitochondrial activity and effector function during acute Plasmodium vivax malaria. PLoS Pathog 2014; 10:e1004393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gazzinelli RT, Kalantari P, Fitzgerald KA, Golenbock DT. Innate sensing of malaria parasites. Nat Rev Immunol 2014; 14:744–57. [DOI] [PubMed] [Google Scholar]
- 5. Leoratti FM, Trevelin SC, Cunha FQ, et al. Neutrophil paralysis in Plasmodium vivax malaria. PLoS Negl Trop Dis 2012; 6:e1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Franklin BS, Ishizaka ST, Lamphier M, et al. Therapeutical targeting of nucleic acid-sensing Toll-like receptors prevents experimental cerebral malaria. Proc Natl Acad Sci U S A 2011; 108:3689–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Parroche P, Lauw FN, Goutagny N, et al. Malaria hemozoin is immunologically inert but radically enhances innate responses by presenting malaria DNA to Toll-like receptor 9. Proc Natl Acad Sci U S A 2007; 104:1919–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Artavanis-Tsakonas K, Tongren JE, Riley EM. The war between the malaria parasite and the immune system: immunity, immunoregulation and immunopathology. Clin Exp Immunol 2003; 133:145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Borsellino G, Kleinewietfeld M, Di Mitri D, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood 2007; 110:1225–32. [DOI] [PubMed] [Google Scholar]
- 10. de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med 1991; 174:1209–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 2003; 4:330–6. [DOI] [PubMed] [Google Scholar]
- 12. Liu W, Putnam AL, Xu-Yu Z, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med 2006; 203:1701–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 1995; 155:1151–64. [PubMed] [Google Scholar]
- 14. Akimova T, Beier UH, Wang L, Levine MH, Hancock WW. Helios expression is a marker of T cell activation and proliferation. PLoS One 2011; 6:e24226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Townsend MJ, Weinmann AS, Matsuda JL, et al. T-bet regulates the terminal maturation and homeostasis of NK and Valpha14i NKT cells. Immunity 2004; 20:477–94. [DOI] [PubMed] [Google Scholar]
- 16. Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol 2009; 10:595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Duhen T, Duhen R, Lanzavecchia A, Sallusto F, Campbell DJ. Functionally distinct subsets of human FOXP3+ Treg cells that phenotypically mirror effector Th cells. Blood 2012; 119:4430–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Illingworth J, Butler NS, Roetynck S, et al. Chronic exposure to Plasmodium falciparum is associated with phenotypic evidence of B and T cell exhaustion. J Immunol 2013; 190:1038–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Butler NS, Moebius J, Pewe LL, et al. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol 2012; 13:188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Costa PA, Leoratti FM, Figueiredo MM, et al. Induction of inhibitory receptors on T cells during Plasmodium vivax malaria impairs cytokine production. J Infect Dis 2015; 212:1999–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hafalla JC, Claser C, Couper KN, et al. The CTLA-4 and PD-1/PD-L1 inhibitory pathways independently regulate host resistance to Plasmodium-induced acute immune pathology. PLoS Pathog 2012; 8:e1002504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lepenies B, Gaworski I, Tartz S, Langhorne J, Fleischer B, Jacobs T. CTLA-4 blockade differentially influences the outcome of non-lethal and lethal Plasmodium yoelii infections. Microbes Infect 2007; 9:687–94. [DOI] [PubMed] [Google Scholar]
- 23. Mackroth MS, Abel A, Steeg C, Schulze Zur Wiesch J, Jacobs T. Acute malaria induces PD1+CTLA4+ effector T cells with cell-extrinsic suppressor function. PLoS Pathog 2016; 12:e1005909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Horne-Debets JM, Faleiro R, Karunarathne DS, et al. PD-1 dependent exhaustion of CD8+ T cells drives chronic malaria. Cell Rep 2013; 5:1204–13. [DOI] [PubMed] [Google Scholar]
- 25. Gonçalves-Lopes RM, Lima NF, Carvalho KI, Scopel KK, Kallás EG, Ferreira MU. Surface expression of inhibitory (CTLA-4) and stimulatory (OX40) receptors by CD4+ regulatory T cell subsets circulating in human malaria. Microbes Infect 2016; 18:639–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Franceschini D, Paroli M, Francavilla V, et al. PD-L1 negatively regulates CD4+CD25+Foxp3+ Tregs by limiting STAT-5 phosphorylation in patients chronically infected with HCV. J Clin Invest 2009; 119:551–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Qureshi OS, Zheng Y, Nakamura K, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science 2011; 332:600–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wing K, Onishi Y, Prieto-Martin P, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science 2008; 322:271–5. [DOI] [PubMed] [Google Scholar]
- 29. Joedicke JJ, Dietze KK, Zelinskyy G, Dittmer U. The phenotype and activation status of regulatory T cells during Friend retrovirus infection. Virol Sin 2014; 29:48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Park HJ, Park JS, Jeong YH, et al. PD-1 upregulated on regulatory T cells during chronic virus infection enhances the suppression of CD8+ T cell immune response via the interaction with PD-L1 expressed on CD8+ T cells. J Immunol 2015; 194:5801–11. [DOI] [PubMed] [Google Scholar]
- 31. Peligero C, Argilaguet J, Güerri-Fernandez R, et al. PD-L1 blockade differentially impacts regulatory T cells from HIV-infected individuals depending on plasma viremia. PLoS Pathog 2015; 11:e1005270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Carvalho BO, Lopes SC, Nogueira PA, et al. On the cytoadhesion of Plasmodium vivax-infected erythrocytes. J Infect Dis 2010; 202:638–47. [DOI] [PubMed] [Google Scholar]
- 33. Ihalamulla RL, Mendis KN. Plasmodium vivax: isolation of mature asexual stages and gametocytes from infected human blood by colloidal silica (Percoll) gradient centrifugation. Trans R Soc Trop Med Hyg 1987; 81:25–8. [DOI] [PubMed] [Google Scholar]
- 34. Noulin F, Borlon C, van den Eede P, et al. Cryopreserved reticulocytes derived from hematopoietic stem cells can be invaded by cryopreserved Plasmodium vivax isolates. PLoS One 2012; 7:e40798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. de Araújo FF, Corrêa-Oliveira R, Rocha MO, et al. Foxp3+CD25(high) CD4+ regulatory T cells from indeterminate patients with Chagas disease can suppress the effector cells and cytokines and reveal altered correlations with disease severity. Immunobiology 2012; 217:768–77. [DOI] [PubMed] [Google Scholar]
- 36. Blackburn SD, Shin H, Haining WN, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol 2009; 10:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mills KH. Regulatory T cells: friend or foe in immunity to infection?Nat Rev Immunol 2004; 4:841–55. [DOI] [PubMed] [Google Scholar]
- 38. Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 2002; 420:502–7. [DOI] [PubMed] [Google Scholar]
- 39. Hesse M, Piccirillo CA, Belkaid Y, et al. The pathogenesis of schistosomiasis is controlled by cooperating IL-10-producing innate effector and regulatory T cells. J Immunol 2004; 172:3157–66. [DOI] [PubMed] [Google Scholar]
- 40. Hisaeda H, Maekawa Y, Iwakawa D, et al. Escape of malaria parasites from host immunity requires CD4+ CD25+ regulatory T cells. Nat Med 2004; 10:29–30. [DOI] [PubMed] [Google Scholar]
- 41. Kho S, Marfurt J, Noviyanti R, et al. Preserved dendritic cell HLA-DR expression and reduced regulatory T cell activation in asymptomatic Plasmodium falciparum and P. vivax infection. Infect Immun 2015; 83:3224–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bueno LL, Morais CG, Araújo FF, et al. Plasmodium vivax: induction of CD4+CD25+FoxP3+ regulatory T cells during infection are directly associated with level of circulating parasites. PLoS One 2010; 5:e9623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006; 439:682–7. [DOI] [PubMed] [Google Scholar]
- 44. Kurup SP, Obeng-Adjei N, Anthony SM, et al. Regulatory T cells impede acute and long-term immunity to blood-stage malaria through CTLA-4. Nat Med 2017; 23:1220–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Francisco LM, Salinas VH, Brown KE, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med 2009; 206:3015–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wong M, La Cava A, Hahn BH. Blockade of programmed death-1 in young (New Zealand Black x New Zealand White)F1 mice promotes the suppressive capacity of CD4+ regulatory T cells protecting from lupus-like disease. J Immunol 2013; 190:5402–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Getnet D, Grosso JF, Goldberg MV, et al. A role for the transcription factor Helios in human CD4(+)CD25(+) regulatory T cells. Mol Immunol 2010; 47:1595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Khaitan A, Kravietz A, Mwamzuka M, et al. FOXP3+Helios+ regulatory T cells, immune activation, and advancing disease in HIV-infected children. J Acquir Immune Defic Syndr 2016; 72:474–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lowther DE, Goods BA, Lucca LE, et al. PD-1 marks dysfunctional regulatory T cells in malignant gliomas. JCI Insight 2016; 1:pii:e85935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mitra S, Abhilash K, Arora S, Miraclin A. A prospective study from south India to compare the severity of malaria caused by Plasmodium vivax, P. falciparum and dual infection. J Vector Borne Dis 2015; 52:281–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.