Abstract
Background
Sepsis remains a major problem in intensive care medicine. It is often accompanied by coagulopathies, leading to thrombotic occlusion of small vessels with subsequent organ damage and even fatal multi-organ failure. Prediction of the clinical course and outcome—especially in the heterogeneous group of pediatric patients—is difficult. Antithrombin, as an endogenous anticoagulant enzyme with anti-inflammatory properties, plays a central role in controling coagulation and infections. We investigated the relationship between antithrombin levels and organ failure as well as mortality in pediatric patients with sepsis.
Methods
Data from 164 patients under the age of 18, diagnosed with sepsis, were retrospectively reviewed. Antithrombin levels were recorded three days before to three days after peak C-reactive protein to correlate antithrombin levels with inflammatory activity. Using the concept of developmental haemostasis, patients were divided into groups <1 yr and ≥1 yr of age.
Results
In both age groups, survivors had significantly higher levels of antithrombin than did deceased patients. An optimal threshold level for antithrombin was calculated by ROC analysis for survival: 41.5% (<1 yr) and 67.5% (≥1 yr). The mortality rate above this level was 3.3% (<1 yr) and 9.5% (≥1 yr), and below this level 41.7% (<1 yr) and 32.2% (≥1 yr); OR 18.8 (1.74 to 1005.02), p = 0.0047, and OR 4.46 (1.54 to 14.89), p = 0.003. In children <1 yr with antithrombin levels <41.5% the rate of respiratory failure (66.7%) was significantly higher than in patients with antithrombin levels above this threshold level (23.3%), OR 6.23 (1.23 to 37.81), p = 0.0132. In children ≥1 yr, both liver failure (20.3% vs 1.6%, OR 15.55 (2.16 to 685.01), p = 0.0008) and a dysfunctional intestinal tract (16.9% vs 4.8%, OR 4.04 (0.97 to 24.08), p = 0.0395) occurred more frequently above the antithrombin threshold level of 67.5%.
Conclusion
In pediatric septic patients, significantly increased mortality and levels of organ failure were found below an age-dependent antithrombin threshold level. Antithrombin could be useful as a prognostic marker for survival and occurrence of organ failure in pediatric sepsis.
Keywords: Children, Antithrombin, Mortality, Sepsis, Organ failure, C-reactive protein, Threshold level
Introduction
Despite considerable progress in treatment, sepsis is still associated with a high mortality rate and is the leading cause of death in patients with infectious diseases (Vincent et al., 2006; Angus et al., 2001). In children, it is one of the main causes of mortality and morbidity (Schlapbach et al., 2015; Boeddha et al., 2018; Hazelzet et al., 1996; Despond et al., 2001) and the most common cause of death in children under 5 years of age (Rudan et al., 2008).
Sepsis initiates diffuse activation of the coagulation system and at the same time inactivates anticoagulation as well as fibrinolysis. In its maximal variant, coagulation activity develops up to an uncontrolled coagulation process of disseminated intravascular coagulation (DIC). It leads to the formation of small intravascular blood clots that clog vessels and prevent the organs from being sufficiently supplied with blood, thus causing lasting damage to the organs. This condition is referred to as Multiple Organ Dysfunction Syndrome (MODS) (Schouten et al., 2008; Nimah & Brilli, 2003).
Biomarkers are needed to predict mortality and organ failure with high sensitivity and specificity in terms of diagnosis and prognosis. Due to the close correlation between coagulation and inflammation, coagulation markers are suitable for this purpose.
Septic patients usually have very low levels of antithrombin as antithrombin is increasingly consumed as a result of uncontrolled effluent coagulation in accordance with progress of sepsis (Mihajlovic et al., 2017). In many cases, synthesis is also impaired because of decreased liver performance in sepsis, and elastase released from activated neutrophils also inactivates antithrombin, a process promoted by heparin (Jordan, Kilpatrick & Nelson, 1987). Beside neutrophil elastase, syndecan shedding is another reason for the decrease in antithrombin (Chung et al., 2008).
An acquired antithrombin deficiency causes a dangerous imbalance in the coagulation system (Hayakawa et al., 2018). Antithrombin is not only an indispensable physiological anticoagulant, but also has anti-inflammatory properties—independent of its anticoagulant activity.
Although studies in adult patients report on the association between antithrombin and outcome during sepsis (Mihajlovic et al., 2017; Hayakawa et al., 2018), there is little literature available on children broken down into age groups (Ersoy et al., 2007; Hazelzet et al., 1996).
In particular, the relationship between antithrombin levels and the failure of various organs in the context of sepsis in children is poorly understood. Neither in adults nor in infants has the correlation between antithrombin and an organ failure of the gastrointestinal tract really been studied so far. No biomarkers have yet been confirmed for the diagnosis of Acute Respiratory Distress Syndrome (ARDS) or prediction of its prognosis (Garcia-Laorden et al., 2017; Cartin-Ceba et al., 2015). There may be an association with decreased antithrombin levels and acute liver failure at least in adult patients with end-stage heart failure (Hoefer et al., 2017).
In general, younger patients have physiologically lower levels of procoagulant factors as well as lower levels of fibrinolytic proteins (Attard et al., 2013; Jaffray & Young, 2013; Andrew et al., 1992). Coagulase inhibitors such as protein C, protein S and antithrombin are also reduced (Appel et al., 2012). While protein C and protein S remain reduced by 10%–20% of adult levels in childhood (Attard et al., 2013; Monagle et al., 2006; Andrew et al., 1988), antithrombin reaches adult levels only after 7–12 months (Appel et al., 2012).
Because antithrombin plays such an important role in sepsis, it may also be used as a predictive parameter for clinical outcome. In view of the much-discussed antithrombin administration with still unclear clinical benefit (Allingstrup et al., 2016), the question of threshold levels arises for a possible substitution trigger. Similar studies have found a threshold for antithrombin, but mainly for the adult (Pettila et al., 2002; Iba et al., 2015) or neonatal (Ersoy et al., 2007) patient population. The knowledge concerning our targeted pediatric patient population is poor, with especially a lack of recent studies. The aim of this study was to examine whether children with sepsis have a threshold level for antithrombin activity, at which antithrombin deficiency increases the probability of a negative outcome in terms of mortality and organ failure.
Methods
Patients aged 0–18 years treated at the Pediatric Intensive Care Unit (PICU) of Innsbruck Medical University Hospital between January 2000 and December 2014 were screened for suspicious or proven infections. A total of 250 patients met the sepsis criteria of the international definitions for pediatric sepsis and organ dysfunction of 2005 (Goldstein, Giroir & Randolph, 2005). Furthermore, the children had to meet the inclusion criterion of an available antithrombin measurement at the peak level of C-reactive protein during sepsis. Finally, 164 pediatric patients were included in this retrospective analysis. Clinical data as well as the routine laboratory parameters C-reactive protein (CRP) and antithrombin (AT) levels were recorded for these patients. The study was permitted by the institutional review board of the Medical University of Innsbruck (AN2013-0044).
We collected the demographic variables age, sex, and the diagnosed underlying disease of the children. Characteristics of patients are listed in Table 1. We screened the children’s hospital stay for the day with the most severe C-reactive protein rash to observe the most severe stage of sepsis in every child, regardless of the underlying disease or any received sepsis treatement. The peak level of C-reactive protein was defined as day 0 and was used to objectify sepsis progression (Povoa et al., 2005; Schmit & Vincent, 2008). Due to a possible temporal displacement of the C-reactive protein peaks and the sepsis maximum, the available antithrombin levels were observed from three days before until three days after day 0 (C-reactive protein peak).
Table 1. Comparison of patient characteristics in children younger than 1 year (<1 year) and older than 1 year (≥1 year).
| Characteristicsa | Total (n = 164) | <1 year (n = 42) | ≥1 year (n = 122) | Estimate with 95% CIb | p valuec |
|---|---|---|---|---|---|
| Female gender | 72/164 (43.9%) | 19/42 (45.2%) | 53/122 (43.4%) | 1.07 (0.5 to 2.31) | 0.8586 |
| Age (months) | 41.95 (9.72–134.6) | 1.87 (0.78–4.32) | 77.98 (34.22–163.19) | 75.33 (49.5 to 105.03) | <0.0001 |
| PIM2d predicted mortality (%) | 3.9 (1.1–8.3) | 3.8 (1.1–15.1) | 3.9 (1.1–7.2) | −0.2 (−2.3 to 0.9) | 0.6229 |
| Diagnosed underlying disease | |||||
| Central nervous system | 35/164 (21.3%) | 8/42 (19%) | 27/122 (22.1%) | 0.83 (0.3 to 2.11) | 0.8278 |
| Cardiovascular system | 30/164 (18.3%) | 16/42 (38.1%) | 14/122 (11.5%) | 4.69 (1.89 to 11.89) | 0.0003 |
| Digestive tract | 29/164 (17.7%) | 17/42 (40.5%) | 12/122 (9.8%) | 6.14 (2.43 to 16.11) | <0.0001 |
| Respiratory system | 40/164 (23.8%) | 15/42 (35.7%) | 25/122 (20.5%) | 2.14 (0.92 to 4.94) | 0.0608 |
| Oncologic | 25/164 (15.2%) | 2/42 (4.8%) | 23/122 (18.9%) | 0.22 (0.02 to 0.95) | 0.0269 |
| Kidney | 21/164 (12.8%) | 5/42 (11.9%) | 16/122 (13.1%) | 0.9 (0.24 to 2.8) | 1 |
| Liver | 15/164 (9.1%) | 5/42 (11.9%) | 10/122 (8.2%) | 1.51 (0.38 to 5.23) | 0.5362 |
| Skin | 6/164 (3.7%) | 0/42 (0%) | 6/122 (4.9%) | 0 (0 to 2.46) | 0.3396 |
Notes.
Binary data are presented as no./total no. (%), continuous data as medians (25th–75th percentile).
Odds ratio for binary variables and estimated median difference for continuous variables.
Differences between groups assessed with Fisher’s exact test for binary variables and Wilcoxon rank sum test for continuous variables.
Pediatric Index of Mortality Score 2 (not known for nine patients younger than 1 year and six patients older than 1 year).
The level of C-reactive protein was measured with an immunological turbidity test (Roche cobas® system) and for the antithrombin level a functional chromogenic assay (Siemens Berichrom® Antithrombin III (A) on Siemens BCS XP) was used (Price et al., 1987; Eda et al., 1998; Friberger et al., 1982).
Based on the fact that antithrombin activity reaches the adult level at the age of 7–12 months (Andrew et al., 1987; Andrew et al., 1992), patients were classified in age groups of <1 yr (younger than one year) and ≥1 yr (one year or older). Organ failure and in-hospital mortality were chosen as outcome parameters.
Statistical analysis
A mathematician not involved in the study procedures or patient assessment was responsible for the statistical analyses using R version 3.4.1 (R Core Team, 2017). All statistical assessments were two-sided and a significance level of 5% was used. The hypothesis of a normal distribution was not reasonable for most of the continuous variables (Shapiro–Wilk normality test). To rule out possible confounding due to the protracted study period (2000–2014), we grouped patients into four time-cohorts: 54 patients had their C-reactive protein peak in 2000–2004, 35 in 2005–2007, 47 in 2008–2010 and 28 in 2011–2014. The Kruskal–Wallis test was used to look for differences between the time cohorts and also between the antithrombin levels stratified by underlying disease. The Wilcoxon rank sum test and Fisher’s exact test were applied to assess differences between the two age groups and between patients with antithrombin levels above and below the computed threshold.
We present continuous data as medians (25th–75th percentile) and binary variables as no./total no. (%). We show effect size or precision with estimated median differences between groups for continuous data and odds ratios (OR) for binary variables, with 95% CIs.
We perform a ROC curve analysis for survival predicted by antithrombin levels at the peak level of C-reactive protein to compute optimal thresholds for antithrombin. For children older than one year and stratified according to survival, the evolution antithrombin levels from three days prior to three days after the peak level of C-reactive protein are illustrated by the sequence of medians with corresponding 95% CIs in a purely descriptive manner.
Results
A total of 164 critically ill children with an available antithrombin measurement at the time of peak level of C-reactive protein were included in the final analysis. For patient characteristics see Table 1. The children older than one year had a median age of 6.5 years, whereas those younger than 1 year were median 1.9 months old. Predicted mortality in both groups was comparable.
In children <1 yr significantly more organs were affected than in children ≥1 yr. Children aged less than 1 year most commonly showed an underlying disease in the form of disorders of the digestive tract (40.5%) followed by cardiovascular complications (38.1%) and problems of the respiratory system (35.7%). Children older than 1 year most often presented with disorders of the central nervous system (22.1%), followed by complications of the respiratory system (19.7%) and oncologic diseases (18.9%).
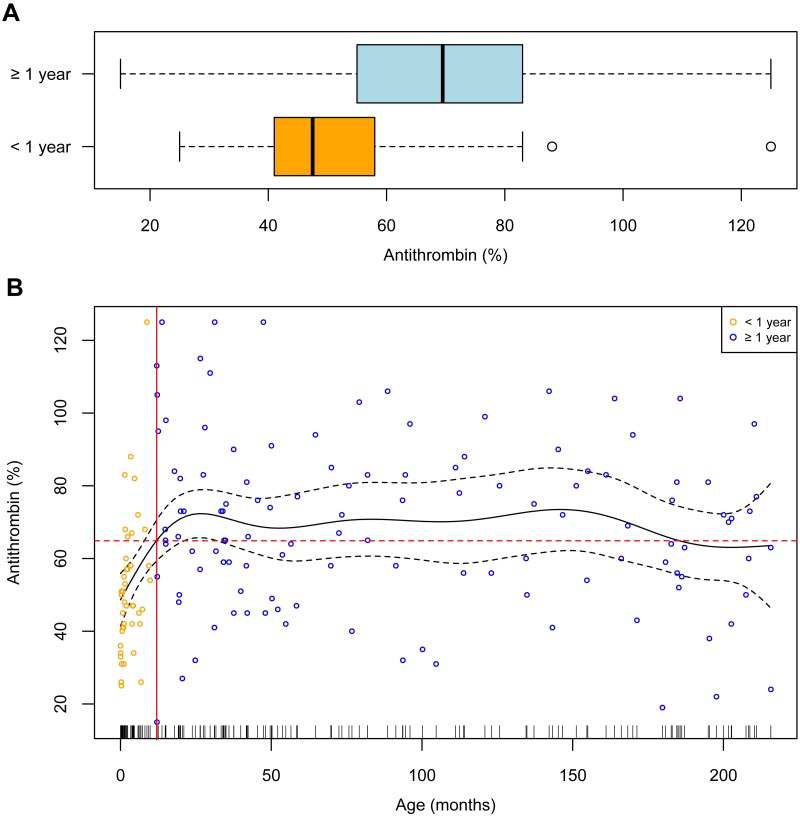
As depicted in Fig. 1A, antithrombin levels at the peak level of C-reactive protein were significantly lower in children <1 yr, namely 47.5 (41–58), than in children ≥1 yr, 69.5 (55.25–83); estimated median difference 19 (12 to 26), p < 0.0001. In children ≥1 yr the antithrombin levels were systematically higher, as seen in Fig. 1B. For progression analysis in children <1 yr, too few antithrombin measurements were available. Therefore, the analysis is restricted to day 0 in this patient group.
Figure 1. Relationship between antithrombin levels and age.
(A) Boxplots of antithrombin levels for patients younger (<1 year) and older than one year (≥1 year). (B) Smoothing spline (black solid line) with 95% CI (dashed black lines). The solid red line marks 12 months of age and the dashed red line corresponds to the mean antithrombin level of 64.87%.
To evaluate the protracted study period (2000–2014), we grouped patients into four time-cohorts: 54 patients had their C-reactive protein peak in 2000–2004, 35 in 2005–2007, 47 in 2008–2010 and 28 in 2011–2014. The mortality rate was 13.0%, 31.4%, 14.9% and 21.4%, respectively, and was not significantly associated with the time periods (Fisher’s Exact test: p = 0.1527). Median Paediatric Index of Mortality, Version 2 (PIM2), scores were accordingly higher in time periods with higher mortality: 3.3 (1.1–5.9) in 2000–2004, 4.35 (1.3–14.95) in 2005–2007, 2.05 (0.92–6.6) in 2008–2010 and 5.9 (3.9–13.9) in 2011–2014 (Kruskal–Wallis test: p = 0.0324). Moreover, C-reactive protein levels and antithrombin levels did not significantly differ between time cohorts (Kruskal–Wallis test: p = 0.1642 and p = 0.2437, respectivley).
Antithrombin threshold levels for survival
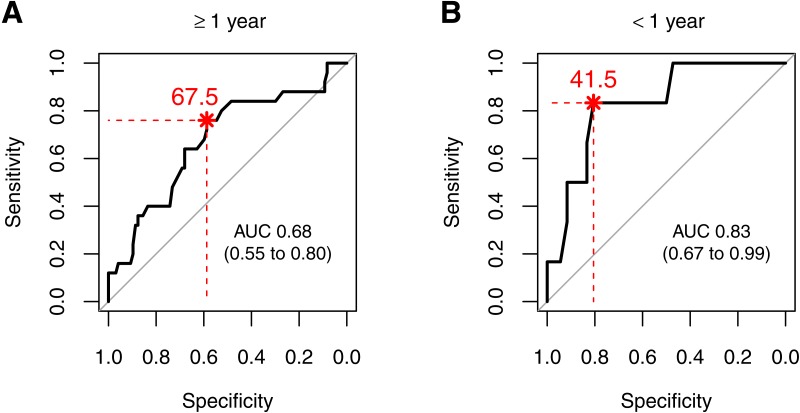
Statistical significance for survival depending on antithrombin was found in both age groups when calculating a threshold level. An antithrombin level above 67.5% was associated with a better outcome in children ≥1 yr, whereas in younger children an antithrombin threshold level of 41.5% was associated with an increased survival rate (Fig. 2). Due to the heterogeneity of patients the antithrombin levels were tested for differences between the groups of underlying diseases. This resulted in significantly not different antithrombin levels in both age classes, children younger than one year (p = 0.7614) and older than one year (p = 0.1309).
Figure 2. ROC curves for survival predicted by antithrombin levels for patients (A) older than one year (≥1 year) and (B) younger than one year (<1 year).
The optimal threshold for antithrombin levels (%) are written in red text and corresponds to the point closest to the asterisk.
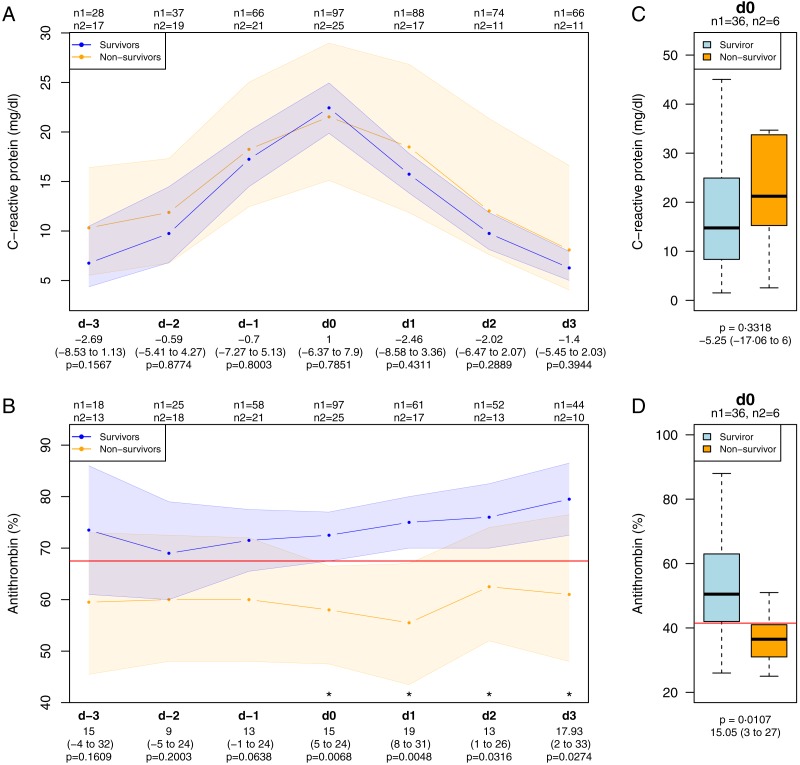
In children ≥1 yr, progression of C-reactive protein during the observation period did not discriminate between survivors and non-survivors, whereas the antithrombin levels were significantly different in survivors and non-survivors from day 0, as depicted in Figs. 3A and 3B). Similarly, in children <1 yr the C-reactive protein levels did not differ between survivors and non-survivors at day 0, but antithrombin did (Figs. 3C and 3D).
Figure 3. C-reactive protein and antithrombin levels in children with sepsis.
Progression of C-reactive protein levels (A) and antithrombin levels (B) in children older than one year stratified by survival. Depicted are medians with 95% CIs. Boxplots of C-reactive protein levels (C) and antithrombin levels (D) for patients younger than one year at the peak levels of C-reactive protein. The red lines refer to the computed threshold levels for antithrombin. n1, children who survived; n2, children who deceased. d-3 to d3 describe the days of the observation period whereas d0 is the day of C-reactive protein peak level, starting with d-3 three days before and ending with d3 three days after d0.
Of the children <1 yr with respiratory failure 66.7% showed antithrombin levels below the threshold of 41.5%. Of the older children with liver failure 20.3% and of those with complications of the intestinal tract 16.9% revealed antithrombin levels lower than the calculated threshold of 67.5%. Other single organ failures and the multiple organ dysfunction syndrome were evenly distributed in dependence on antithrombin threshold levels (Table 2).
Table 2. Children’s morbidity stratified by age and antithrombin (AT) levels (%).
| Morbiditya | Children <1 year | Children≥1 year | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (n = 42) | AT ≥41.5 (n = 30) | AT<41.5 (n = 12) | OR with 95% CIb | p valuec | Total (n = 122) | AT ≥67.5 (n = 63) | AT<67.5 (n = 59) | OR with 95% CIb | p valuec | |
| Survivor | 36/42 (85.7%) | 29/30 (96.7%) | 7/12 (58.3%) | 18.8 (1.74 to 1005.02) | 0.0047 | 97/122 (79.5%) | 57/63 (90.5%) | 40/59 (67.8%) | 4.46 (1.54 to 14.89) | 0.003 |
| Diagnosed organ failure | ||||||||||
| Cardiovascular system | 16/42 (38.1%) | 10/30 (33.3%) | 6/12 (50%) | 1.97 (0.41 to 9.63) | 0.483 | 21/122 (17.2%) | 9/63 (14.3%) | 12/59 (20.3%) | 1.53 (0.54 to 4.5) | 0.4734 |
| Central nervous system | 2/42 (4.8%) | 1/30 (3.3%) | 1/12 (8.3%) | 2.57 (0.03 to 213.54) | 0.4948 | 10/122 (8.2%) | 3/63 (4.8%) | 7/59 (11.9%) | 2.67 (0.57 to 16.82) | 0.1951 |
| Intestinal tract | 9/42 (21.4%) | 6/30 (20%) | 3/12 (25%) | 1.32 (0.18 to 7.98) | 0.6987 | 13/122 (10.7%) | 3/63 (4.8%) | 10/59 (16.9%) | 4.04 (0.97 to 24.08) | 0.0395 |
| Kidney | 6/42 (14.3%) | 4/30 (13.3%) | 2/12 (16.7%) | 1.29 (0.1 to 10.78) | 1 | 28/122 (23%) | 14/63 (22.2%) | 14/59 (23.7%) | 1.09 (0.43 to 2.77) | 1 |
| Liver | 3/42 (7.1%) | 3/30 (10%) | 0/12 (0%) | 0 (0 to 6.15) | 0.5453 | 13/122 (10.7%) | 1/63 (1.6%) | 12/59 (20.3%) | 15.55 (2.16 to 685.01) | 0.0008 |
| Respiratory | 15/42 (35.7%) | 7/30 (23.3%) | 8/12 (66.7%) | 6.23 (1.23 to 37.81) | 0.0132 | 28/122 (23%) | 13/63 (20.6%) | 15/59 (25.4%) | 1.31 (0.52 to 3.35) | 0.6671 |
| Multiple organ dysfunction syndrome | 22/42 (52.4%) | 15/30 (50%) | 7/12 (58.3%) | 1.39 (0.3 to 6.92) | 0.7385 | 46/122 (37.7%) | 22/63 (34.9%) | 24/59 (40.7%) | 1.28 (0.58 to 2.84) | 0.5768 |
| Thromboembolic event | 5/42 (11.9%) | 2/30 (6.7%) | 3/12 (25%) | 4.46 (0.44 to 61.51) | 0.1309 | 4/122 (3.3%) | 2/63 (3.2%) | 2/59 (3.4%) | 1.07 (0.08 to 15.21) | 1 |
| Bleeding event | 2/42 (4.8%) | 1/30 (3.3%) | 1/12 (8.3%) | 2.57 (0.03 to 213.54) | 0.4948 | 5/122 (4.1%) | 1/63 (1.6%) | 4/59 (6.8%) | 4.46 (0.42 to 225.31) | 0.1964 |
Notes.
Data are presented as no./total no. (%).
Odds ratio for binary variables.
Differences between groups assessed with Fisher’s exact test.
Discussion
Sepsis with concomitant disruption of the coagulation system up to DIC and the associated consumption of coagulation factors lead to a condition of acquired antithrombin deficiency (Amaral, Opal & Vincent, 2004). It can be deduced that the level of antithrombin is related to the severity of the coagulation disorder and consequently affects the extent of organ damage and survival (Fourrier et al., 1992). The aim of this study was to investigate the relationship between antithrombin activity and the likelihood of survival and organ damage in pediatric patients with sepsis. The significantly lower antithrombin levels in the age group <1 yr than in the age group ≥1 yr confirm the concept of developmental hemostasis introduced by Andrew et al. (1987); Andrew et al. (1992). In studies conducted in septic adult patients, an association between increased mortality and low antithrombin levels of around 63% has been demonstated (LaRosa et al., 2006; Taylor Jr et al., 1988). The Kypercept trial revealed that in patients with antithrombin levels <60% the mortality rate was elevated to up to 47.5% in comparison to patients with a higher antithrombin level, where the mortality rate was increased to up to 29.1% (Warren et al., 2001). In our study a threshold level of 67.5% was calculated, which discriminates between survivors and non-survivors on the day of C-reactive protein peak in children older than one year. The lower threshold value, namely an antithrombin level of 41.5% in children <1 yr, is in line with other studies: comparable levels of antithrombin (52.0%) were found in neonates with suspected sepsis (Bartolovic et al., 2011), and also in this patient group the survivors had higher antithrombin levels (Ersoy et al., 2007; Lauterbach et al., 2006).
C-reactive protein levels did not differ between survivors and non-survivors in either age group, although C-reactive protein was shown to be of diagnostic value in sepsis in other studies (Pasternak et al., 2016; Lautz et al., 2016; Carcillo et al., 2017).
In our study, it was not only possible to statistically differentiate between survival and non-survival (p = 0.003) based on the antithrombin level in the age group ≥1 yr, but also to determine whether a failure of certain organs occurs or not. In the context of sepsis, microangiopathy caused by disruption of clotting activity can lead to impairment of organ functions including organ failure (Amaral, Opal & Vincent, 2004). The highest statistical relevance was observed in the prediction of liver failure (p = 0.0008) in the patient group ≥1 yr, which in children with antithrombin deficiency occurred at a significantly higher frequency of more than 20%.
The association between impaired liver function and low antithrombin levels, which was clearly demonstrated in this study, can be explained by a pre-existing liver failure and the resulting reduced antithrombin production (Sheikh Sajjadieh & Vasilovna Viunytska, 2009). On the other hand, this can also be attributed to DIC-induced hypoperfusion of the liver in the context of sepsis. Antithrombin not only optimizes coagulation, but is also a potential regulator of inflammatory processes and subsequent tissue damage. Injection of antithrombin directly into the portal vein after LPS-induced acute liver failure resulted in a significant reduction in inflammatory cytokines, reduced intrahepatic fibrin deposition and improvement of the histological findings (Miyazaki et al., 2012). In a rat model, systemic administration of antithrombin was seen to improve liver function in liver failure and attenuate damage of the liver tissue in a dose-dependent manner (Fujiwara et al., 1988). As an underlying mechanism increased anithrombin-intitated prostacyclin distribution was suspected (Harada et al., 1999), which might have been the reason for inhibition of platelet aggregation and reduction of thrombocytopenia (Fujiwara et al., 1988). In adult cancer patients, antithrombin levels of <50%–61.5% predicted postoperative liver dysfunction (Pereyra et al., 2017; Hoffmann et al., 2006), and administration of antithrombin was able to reduce this rate (Kuroda et al., 2015).
In the age group ≥1 yr, a significant correlation (p = 0.0395) was found between lower antithrombin and increased rate of organ damage of the gastrointestinal tract (16.9%). So far, there are hardly any studies that describe this effect between antithrombin and organ failure of the gastrointestinal tract in humans. In rat models antithrombin had a positive effect on reperfusion after intestinal injury due to a reduction in fibrin deposition and micro-vascular thrombotic obstruction as well as anti-inflammatory action (Schoots et al., 2004). Furthermore, decreased leukocyte migration and adhesion along mesenterial venoles after endotoxin treatment was proven (Neviere et al., 2001). The reason was the inhibition of thrombin action on the endothelium, resulting in the expression of adhesion molecules (Ostrovsky et al., 1997). Consequntly, antithrombin is able to reduce organ tissue damage and subsequent failure.
In the patient group <1 yr, low antithrombin levels were associated with an increased rate of respiratory failure (p = 0.0132). Like other organs, the lung is affected by DIC and inflammatory reactions in sepsis. Several studies suggested that low levels of antithrombin are combined with poor outcomes in lung disease such as acute lung injury (ALI) and idiopathic respiratory distress syndrome (IRDS) in neonates (Peters et al., 1984; Van den Berg et al., 1989) and adults (Owings & Gosselin, 1997). Again, in rat models less tissue destruction of the pulmonary vessels was detected after antithrombin administration following LPS-induced sepsis, which was attributed to the ability of antithrombin to induce prostacyclin release and thereby reduce leukocyte activation (Uchiba & Okajima, 1997). In another rat model treatment of pneumonia, triggered by S. pneumoniae, with antithrombin resulted in a marked reduction in neutrophil cells in the lung, decreased levels of pro-inflammatory cytokines, and a reduction in NET (neutrophil extracellular trap) formation (Ishikawa et al., 2017; Choi et al., 2008). Here, too, a reduction in lung tissue destruction as well as a significant reduction in colony formation of S. pneumoniae was demonstrated (Choi et al., 2008). This leads us to conclude that by interacting with the complexity of an inflammatory reaction antithrombin exerts a certain protective effect on the lung in the context of sepsis.
Surprisingly, we found no association between low levels of antithrombin and kidney failure or underlying kidney disease. Other studies in adults have clearly shown that antithrombin may help limit acute kidney injury (Yin et al., 2017; Kong et al., 2017; Wang et al., 2015; Rameshkumar et al., 2017).
Although not significantly different, more bleeding complications as well as thromboembolic events are observed in children with antithrombin levels below the calculated threshold levels, especially in children younger than 1 year.
Although the results suggest that higher levels of antithrombin are associated with a better outcome, the data do not suggest that antithrombin as a drug also improves the outcome of sepsis patients. Early studies in human populations showed controversial effects; the benefit of antithrombin in the treatment of critically ill patients remained unclear (Fourrier et al., 1993; Baudo et al., 1998; Inthorn et al., 1998; Eisele et al., 1998; Gando et al., 2013; Hoffmann et al., 2004). The Kypersept trial was not able to prove a survival benefit, but even caused higher bleeding rates in septic adults receiving antithrombin substitution (Warren et al., 2001). Nevertheless, critical analysis of this study showed a clear tendency (p = 0.058) to a lower rate of new organ failure after treatment in the antithrombin group as compared to the placebo group (Eid, Wiedermann & Kinasewitz, 2008). Moreover, the mortality rate significantly decreased in patients with antithrombin administration, namely from 44.9% in the placebo group to 52.5% when not concomitantly receiving heparin (Hoffmann et al., 2006).
Our study design does not allow a conclusion about a trigger limit with respect to administration. However, our levels of safekeeping certainly indicate a critical threshold of antithrombin activity, which may possibly be used as a therapy-critical basis.
Compared to adults, only few studies of antithrombin substitution have been conducted in children. These also report conflicting results regarding efficacy and safety (Kreuz, Schneider & Nowak-Gottl, 1999; St Peter et al., 2007; Wong et al., 2016), but the administration of antithrombin in children is increasing (Wong et al., 2013). Especially in infants, antithrombin administration showed an improvement in multiple-organ dysfunction including an increase in platelet count with a good safety profile (Novakova et al., 2000).
Patients in this study did not receive antithrombin rountinely, but we were not able to discriminate patients who had been administered antithrombin from those who had not. Even if both production and consumption are certainly involved in the pathophysiologic pathways of coagulation, especially the aspects of liver synthesis on the one hand and coagulation on the other hand, as prognostic factors should be examined in future studies focusing on differences between substituted and non-substituted antithrombin levels.
In this study, patients were included over a 14-year period. The increased mortality in the children in the period from 2005–2007 is probably attributed to the admission of initially more severely ill patients and not to to a change in practice during this time.
Our study demonstrates that a low antithrombin level in septic children is a good prognostic marker for certain organ failures depending on the age group and is associated with higher mortality throughout childhood. If antithrombin supplementation reaches levels above the calculated thresholds, the improving organ function and mortality rate need to be evaluated in further studies.
Limitations
An important limitation of our study is the heterogeneity of the patients regarding the different underlying diseases. Unfortunately, the sample size was too small to calculate threshold levels for each affected system, especially in oncologic patients, which is difficult to achieve since the overall number of critically ill children is low. Nevertheless, there was no difference in antithrombin levels regarding the underlying diseases in our patient population.
Another confounding factor is that treatments, e.g., antithrombin supplementation were not taken into account in this analysis and so could have influenced the levels of both C-reactive protein and antithrombin. C-reactive protein was used only as a surrogate marker in order to identify the most severe period of the sepsis in each child independently of any treatment; the absolute levels of C-reactive protein are secondary for the objective of this study. Furthermore, we assumed in this study that administered antithrombin has the same efficacy as endogenous antithrombin and therefore no discrimination was necessary.
Due to the reaction time and the limited duration of the elevated serum level, the C-reactive protein is also unsuitable for immediate diagnosis and prognosis (Haupt et al., 1996; Martin et al., 1997). Thus, the C-reactive protein peak does not coincide exactly with the maximum temporal manifestation of sepsis but is close to it.
Nonetheless, the antithrombin threshhold levels for survival and organ failure should be analysed in a larger patient cohort stratified according to underlying disease since the levels could be influenced by these illnesses.
Unfortunately, the study design does not allow the evolution of sepsis to be observed from the beginning since the study center is a high-level PICU and many children were transferred to our hospital when sepsis had already progressed. This also explains the high mortality since our PICU treats the most severe cases of sepsis.
Conclusion
Children with sepsis revealed age-dependent antithrombin threshold levels, below which dysfunction of particular organs and mortality significantly increased. At an antithrombin level of 41.5% the threshold level for significantly higher morbidity and mortality was lower in children <1 yr than it was in children ≥1 yr, with a threshold value of 67.5%. Lower antithrombin levels may be seen as a prognostic tool for increased morbidity and mortality in pediatric sepsis patients.
Supplemental Information
Boxplots of antithrombin levels (%) by underlying diseases of (A) children older (≥1 year) and (B) younger than one year (<1 year). CNS refers to underlying diseases affecting the central nervous system.
Data set of antithrombin in septic children
Acknowledgments
We thank Dr. Katharina Auer, Dr. Christina Schoner, Dr. Daniela Hainz, Dr. Ahmed Fideh, who helped with data acquisition, and Dr. Dietmar Fries for his assistance in conducting this project.
Abbreviations
- <1 yr
younger than one year
- ≥1 yr
one year or older
- PIM2
Pediatric Mortality Index, Version 2
- AT
antithrombin
- CRP
C-reactive protein
Funding Statement
The authors received no funding for this work.
Additional Information and Declarations
Competing Interests
The authors state that they have no competing interests in regard to this study. Mirjam Bachler has received personal fees from LFB Biomedicaments, Baxter GmbH, CSL Behring GmbH, Mitsubishi Tanabe and non-financial support from TEM International outside the submitted work.
Author Contributions
Christian Niederwanger and Mirjam Bachler conceived and designed the experiments, analyzed the data, authored or reviewed drafts of the paper, approved the final draft.
Tobias Hell conceived and designed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Sophie Hofer, Christina Salvador, Bettina Schenk and Benedikt Treml authored or reviewed drafts of the paper, approved the final draft.
Human Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
The study was permitted by the institutional review board of the Medical University of Innsbruck (AN2013-0044).
Data Availability
The following information was supplied regarding data availability:
The raw dataset is provided as a Supplemental File.
References
- Allingstrup et al. (2016).Allingstrup M, Wetterslev J, Ravn FB, Moller AM, Afshari A. Antithrombin III for critically ill patients: a systematic review with meta-analysis and trial sequential analysis. Intensive Care Medicine. 2016;42:505–520. doi: 10.1007/s00134-016-4225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral, Opal & Vincent (2004).Amaral A, Opal SM, Vincent JL. Coagulation in sepsis. Intensive Care Medicine. 2004;30:1032–1040. doi: 10.1007/s00134-004-2291-8. [DOI] [PubMed] [Google Scholar]
- Andrew et al. (1988).Andrew M, Paes B, Milner R, Johnston M, Mitchell L, Tollefsen DM, Castle V, Powers P. Development of the human coagulation system in the healthy premature infant. Blood. 1988;72:1651–1657. [PubMed] [Google Scholar]
- Andrew et al. (1987).Andrew M, Paes B, Milner R, Johnston M, Mitchell L, Tollefsen DM, Powers P. Development of the human coagulation system in the full-term infant. Blood. 1987;70:165–172. [PubMed] [Google Scholar]
- Andrew et al. (1992).Andrew M, Vegh P, Johnston M, Bowker J, Ofosu F, Mitchell L. Maturation of the hemostatic system during childhood. Blood. 1992;80:1998–2005. [PubMed] [Google Scholar]
- Angus et al. (2001).Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Critical Care Medicine. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- Appel et al. (2012).Appel IM, Grimminck B, Geerts J, Stigter R, Cnossen MH, Beishuizen A. Age dependency of coagulation parameters during childhood and puberty. Journal of Thrombosis and Haemostasis. 2012;10:2254–2263. doi: 10.1111/j.1538-7836.2012.04905.x. [DOI] [PubMed] [Google Scholar]
- Attard et al. (2013).Attard C, Van der Straaten T, Karlaftis V, Monagle P, Ignjatovic V. Developmental hemostasis: age-specific differences in the levels of hemostatic proteins. Journal of Thrombosis and Haemostasis. 2013;11:1850–1854. doi: 10.1111/jth.12372. [DOI] [PubMed] [Google Scholar]
- Bartolovic et al. (2011).Bartolovic D, Ignjatovic S, Stankovic S, Nada Majkic S. Procalcitonin and other biomarkers of sepsis in newborns in the intensive care unit. Ejifcc. 2011;22:24–30. [PMC free article] [PubMed] [Google Scholar]
- Baudo et al. (1998).Baudo F, Caimi TM, de Cataldo F, Ravizza A, Arlati S, Casella G, Carugo D, Palareti G, Legnani C, Ridolfi L, Rossi R, D’Angelo A, Crippa L, Giudici D, Gallioli G, Wolfler A, Calori G. Antithrombin III (ATIII) replacement therapy in patients with sepsis and/or postsurgical complications: a controlled double-blind, randomized, multicenter study. Intensive Care Medicine. 1998;24:336–342. doi: 10.1007/s001340050576. [DOI] [PubMed] [Google Scholar]
- Boeddha et al. (2018).Boeddha NP, Schlapbach LJ, Driessen GJ, Herberg JA, Rivero-Calle I, Cebey-Lopez M, Klobassa DS, Philipsen R, De Groot R, Inwald DP, Nadel S, Paulus S, Pinnock E, Secka F, Anderson ST, Agbeko RS, Berger C, Fink CG, Carrol ED, Zenz W, Levin M, Van der Flier M, Martinon-Torres F, Hazelzet JA, Emonts M. Mortality and morbidity in community-acquired sepsis in European pediatric intensive care units: a prospective cohort study from the European Childhood Life-threatening Infectious Disease Study (EUCLIDS) Critical Care. 2018;22 doi: 10.1186/s13054-018-2052-7. Article 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carcillo et al. (2017).Carcillo JA, Sward K, Halstead ES, Telford R, Jimenez-Bacardi A, Shakoory B, Simon D, Hall M. A systemic inflammation mortality risk assessment contingency table for severe sepsis. Pediatric Critical Care Medicine. 2017;18:143–150. doi: 10.1097/PCC.0000000000001029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartin-Ceba et al. (2015).Cartin-Ceba R, Hubmayr RD, Qin R, Peters S, Determann RM, Schultz MJ, Gajic O. Predictive value of plasma biomarkers for mortality and organ failure development in patients with acute respiratory distress syndrome. Journal of Critical Care. 2015;30:219.e1–219.e7. doi: 10.1016/j.jcrc.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Choi et al. (2008).Choi G, Hofstra JJ, Roelofs JJ, Rijneveld AW, Bresser P, Van der Zee JS, Florquin S, Van der Poll T, Levi M, Schultz MJ. Antithrombin inhibits bronchoalveolar activation of coagulation and limits lung injury during Streptococcus pneumoniae pneumonia in rats. Critical Care Medicine. 2008;36:204–210. doi: 10.1097/01.CCM.0000292012.87482.F4. [DOI] [PubMed] [Google Scholar]
- Chung et al. (2008).Chung MC, Jorgensen SC, Popova TG, Bailey CL, Popov SG. Neutrophil elastase and syndecan shedding contribute to antithrombin depletion in murine anthrax. FEMS Immunology and Medical Microbiology. 2008;54:309–318. doi: 10.1111/j.1574-695X.2008.00480.x. [DOI] [PubMed] [Google Scholar]
- Despond et al. (2001).Despond O, Proulx F, Carcillo JA, Lacroix J. Pediatric sepsis and multiple organ dysfunction syndrome. Current Opinion in Pediatrics. 2001;13:247–253. doi: 10.1097/00008480-200106000-00006. [DOI] [PubMed] [Google Scholar]
- Eda et al. (1998).Eda S, Kaufmann J, Roos W, Pohl S. Development of a new microparticle-enhanced turbidimetric assay for C-reactive protein with superior features in analytical sensitivity and dynamic range. Journal of Clinical Laboratory Analysis. 1998;12:137–144. doi: 10.1002/(SICI)1098-2825(1998)12:3<137::AID-JCLA2>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid, Wiedermann & Kinasewitz (2008).Eid A, Wiedermann CJ, Kinasewitz GT. Early administration of high-dose antithrombin in severe sepsis: single center results from the KyberSept-trial. Anesthesia and Analgesia. 2008;107:1633–1638. doi: 10.1213/ane.0b013e318184621d. [DOI] [PubMed] [Google Scholar]
- Eisele et al. (1998).Eisele B, Lamy M, Thijs LG, Keinecke HO, Schuster HP, Matthias FR, Fourrier F, Heinrichs H, Delvos U. Antithrombin III in patients with severe sepsis. A randomized, placebo-controlled, double-blind multicenter trial plus a meta-analysis on all randomized, placebo-controlled, double-blind trials with antithrombin III in severe sepsis. Intensive Care Medicine. 1998;24:663–672. doi: 10.1007/s001340050642. [DOI] [PubMed] [Google Scholar]
- Ersoy et al. (2007).Ersoy B, Nehir H, Altinoz S, Yilmaz O, Dundar PE, Aydogan A. Prognostic value of initial antithrombin levels in neonatal sepsis. Indian Pediatrics. 2007;44:581–584. [PubMed] [Google Scholar]
- Fourrier et al. (1992).Fourrier F, Chopin C, Goudemand J, Hendrycx S, Caron C, Rime A, Marey A, Lestavel P. Septic shock, multiple organ failure, and disseminated intravascular coagulation. Compared patterns of antithrombin III, protein C, and protein S deficiencies. Chest. 1992;101:816–823. doi: 10.1378/chest.101.3.816. [DOI] [PubMed] [Google Scholar]
- Fourrier et al. (1993).Fourrier F, Chopin C, Huart JJ, Runge I, Caron C, Goudemand J. Double-blind, placebo-controlled trial of antithrombin III concentrates in septic shock with disseminated intravascular coagulation. Chest. 1993;104:882–888. doi: 10.1378/chest.104.3.882. [DOI] [PubMed] [Google Scholar]
- Friberger et al. (1982).Friberger P, Egberg N, Holmer E, Hellgren M, Blomback M. Antithrombin assay—the use of human or bovine thrombin and the observation of a second heparin cofactor. Thrombosis Research. 1982;25:433–436. doi: 10.1016/0049-3848(82)90133-5. [DOI] [PubMed] [Google Scholar]
- Fujiwara et al. (1988).Fujiwara K, Ogata I, Ohta Y, Hirata K, Oka Y, Yamada S, Sato Y, Masaki N, Oka H. Intravascular coagulation in acute liver failure in rats and its treatment with antithrombin III. Gut. 1988;29:1103–1108. doi: 10.1136/gut.29.8.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gando et al. (2013).Gando S, Saitoh D, Ishikura H, Ueyama M, Otomo Y, Oda S, Kushimoto S, Tanjoh K, Mayumi T, Ikeda T, Iba T, Eguchi Y, Okamoto K, Ogura H, Koseki K, Sakamoto Y, Takayama Y, Shirai K, Takasu O, Inoue Y, Mashiko K, Tsubota T, Endo S. A randomized, controlled, multicenter trial of the effects of antithrombin on disseminated intravascular coagulation in patients with sepsis. Critical Care. 2013;17 doi: 10.1186/cc13163. Article R297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Laorden et al. (2017).Garcia-Laorden MI, Lorente JA, Flores C, Slutsky AS, Villar J. Biomarkers for the acute respiratory distress syndrome: how to make the diagnosis more precise. Annals of Translational Medicine. 2017;5 doi: 10.21037/atm.2017.06.49. Article 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein, Giroir & Randolph (2005).Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatric Critical Care Medicine. 2005;6:2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- Harada et al. (1999).Harada N, Okajima K, Kushimoto S, Isobe H, Tanaka K. Antithrombin reduces ischemia/reperfusion injury of rat liver by increasing the hepatic level of prostacyclin. Blood. 1999;93:157–164. [PubMed] [Google Scholar]
- Haupt et al. (1996).Haupt W, Fritzsche H, Hohenberger W, Zirngibl H. Selective cytokine release induced by serum and separated plasma from septic patients. European Journal of Surgery. 1996;162:769–776. [PubMed] [Google Scholar]
- Hayakawa et al. (2018).Hayakawa M, Yamakawa K, Kudo D, Ono K. Optimal antithrombin activity threshold for initiating antithrombin supplementation in patients with sepsis-induced disseminated intravascular coagulation: a multicenter retrospective observational study. Clinical and Applied Thrombosis/Hemostasis. 2018;24(6):874–883. doi: 10.1177/1076029618757346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelzet et al. (1996).Hazelzet JA, Risseeuw-Appel IM, Kornelisse RF, Hop WC, Dekker I, Joosten KF, De Groot R, Hack CE. Age-related differences in outcome and severity of DIC in children with septic shock and purpura. Thrombosis and Haemostasis. 1996;76:932–938. doi: 10.1055/s-0038-1650688. [DOI] [PubMed] [Google Scholar]
- Hoefer et al. (2017).Hoefer J, Ulmer H, Kilo J, Margreiter R, Grimm M, Mair P, Ruttmann E. Antithrombin III is associated with acute liver failure in patients with end-stage heart failure undergoing mechanical circulatory support. Journal of Thoracic and Cardiovascular Surgery. 2017;153:1374–1382. doi: 10.1016/j.jtcvs.2017.01.053. [DOI] [PubMed] [Google Scholar]
- Hoffmann et al. (2004).Hoffmann JN, Muhlbayer D, Jochum M, Inthorn D. Effect of long-term and high-dose antithrombin supplementation on coagulation and fibrinolysis in patients with severe sepsis. Critical Care Medicine. 2004;32:1851–1859. doi: 10.1097/01.CCM.0000139691.54108.1F. [DOI] [PubMed] [Google Scholar]
- Hoffmann et al. (2006).Hoffmann JN, Wiedermann CJ, Juers M, Ostermann H, Kienast J, Briegel J, Strauss R, Warren BL, Opal SM. Benefit/risk profile of high-dose antithrombin in patients with severe sepsis treated with and without concomitant heparin. Thrombosis and Haemostasis. 2006;95:850–856. doi: 10.1160/TH05-07-0530. [DOI] [PubMed] [Google Scholar]
- Iba et al. (2015).Iba T, Saitoh D, Gando S, Thachil J. The usefulness of antithrombin activity monitoring during antithrombin supplementation in patients with sepsis-associated disseminated intravascular coagulation. Thrombosis Research. 2015;135:897–901. doi: 10.1016/j.thromres.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Inthorn et al. (1998).Inthorn D, Hoffmann JN, Hartl WH, Muhlbayer D, Jochum M. Effect of antithrombin III supplementation on inflammatory response in patients with severe sepsis. Shock. 1998;10:90–96. doi: 10.1097/00024382-199808000-00002. [DOI] [PubMed] [Google Scholar]
- Ishikawa et al. (2017).Ishikawa M, Yamashita H, Oka N, Ueda T, Kohama K, Nakao A, Kotani J. Antithrombin III improved neutrophil extracellular traps in lung after the onset of endotoxemia. Journal of Surgical Research. 2017;208:140–150. doi: 10.1016/j.jss.2016.09.041. [DOI] [PubMed] [Google Scholar]
- Jaffray & Young (2013).Jaffray J, Young G. Developmental hemostasis: clinical implications from the fetus to the adolescent. Pediatric Clinics of North America. 2013;60:1407–1417. doi: 10.1016/j.pcl.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Jordan, Kilpatrick & Nelson (1987).Jordan RE, Kilpatrick J, Nelson RM. Heparin promotes the inactivation of antithrombin by neutrophil elastase. Science. 1987;237:777–779. doi: 10.1126/science.3649921. [DOI] [PubMed] [Google Scholar]
- Kong et al. (2017).Kong Y, Yin J, Cheng D, Lu Z, Wang N, Wang F, Liang M. Antithrombin III Attenuates AKI Following Acute Severe Pancreatitis. Shock. 2017;49(5):572–579. doi: 10.1097/SHK.0000000000000946. [DOI] [PubMed] [Google Scholar]
- Kreuz, Schneider & Nowak-Gottl (1999).Kreuz WD, Schneider W, Nowak-Gottl U. Treatment of consumption coagulopathy with antithrombin concentrate in children with acquired antithrombin deficiency—a feasibility pilot study. European Journal of Pediatrics. 1999;158(Suppl 3):S187–S191. doi: 10.1007/PL00014353. [DOI] [PubMed] [Google Scholar]
- Kuroda et al. (2015).Kuroda S, Tashiro H, Kobayashi T, Hashimoto M, Mikuriya Y, Ohdan H. Administration of antithrombin III attenuates posthepatectomy liver failure in hepatocellular carcinoma. Digestive Surgery. 2015;32:173–180. doi: 10.1159/000379759. [DOI] [PubMed] [Google Scholar]
- LaRosa et al. (2006).LaRosa SP, Opal SM, Utterback B, Yan SC, Helterbrand J, Simpson AJ, Chaowagul W, White NJ, Fisher Jr CJ. Decreased protein C, protein S, and antithrombin levels are predictive of poor outcome in Gram-negative sepsis caused by Burkholderia pseudomallei. International Journal of Infectious Diseases. 2006;10:25–31. doi: 10.1016/j.ijid.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Lauterbach et al. (2006).Lauterbach R, Pawlik D, Radziszewska R, Wozniak J, Rytlewski K. Plasma antithrombin III and protein C levels in early recognition of late-onset sepsis in newborns. European Journal of Pediatrics. 2006;165:585–589. doi: 10.1007/s00431-006-0139-7. [DOI] [PubMed] [Google Scholar]
- Lautz et al. (2016).Lautz AJ, Dziorny AC, Denson AR, O’Connor KA, Chilutti MR, Ross RK, Gerber JS, Weiss SL. Value of procalcitonin measurement for early evidence of severe bacterial infections in the pediatric intensive care unit. Jornal de Pediatria. 2016;179:74–81. doi: 10.1016/j.jpeds.2016.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin et al. (1997).Martin C, Boisson C, Haccoun M, Thomachot L, Mege JL. Patterns of cytokine evolution (tumor necrosis factor-alpha and interleukin-6) after septic shock, hemorrhagic shock, and severe trauma. Critical Care Medicine. 1997;25:1813–1819. doi: 10.1097/00003246-199711000-00018. [DOI] [PubMed] [Google Scholar]
- Mihajlovic et al. (2017).Mihajlovic D, Brkic S, Lendak D, Mikic AN, Draskovic B, Mitic G. Endogenous thrombin potential as marker of procoagulant response that can be useful in early stage of sepsis. Blood Coagulation & Fibrinolysis. 2017;28:460–467. doi: 10.1097/MBC.0000000000000622. [DOI] [PubMed] [Google Scholar]
- Miyazaki et al. (2012).Miyazaki M, Kato M, Tanaka M, Tanaka K, Takao S, Kohjima M, Ito T, Enjoji M, Nakamuta M, Kotoh K, Takayanagi R. Antithrombin III injection via the portal vein suppresses liver damage. World Journal of Gastroenterology. 2012;18:1884–1891. doi: 10.3748/wjg.v18.i16.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monagle et al. (2006).Monagle P, Barnes C, Ignjatovic V, Furmedge J, Newall F, Chan A, De Rosa L, Hamilton S, Ragg P, Robinson S, Auldist A, Crock C, Roy N, Rowlands S. Developmental haemostasis. Impact for clinical haemostasis laboratories. Thrombosis and Haemostasis. 2006;95:362–372. doi: 10.1160/TH05-01-0047. [DOI] [PubMed] [Google Scholar]
- Neviere et al. (2001).Neviere R, Tournoys A, Mordon S, Marechal X, Song FL, Jourdain M, Fourrier F. Antithrombin reduces mesenteric venular leukocyte interactions and small intestine injury in endotoxemic rats. Shock. 2001;15:220–225. doi: 10.1097/00024382-200115030-00010. [DOI] [PubMed] [Google Scholar]
- Nimah & Brilli (2003).Nimah M, Brilli RJ. Coagulation dysfunction in sepsis and multiple organ system failure. Critical Care Clinics. 2003;19:441–458. doi: 10.1016/S0749-0704(03)00008-3. [DOI] [PubMed] [Google Scholar]
- Novakova et al. (2000).Novakova D, Simak J, Janota J, Stranak Z. Development of multiple organ failure in critically ill newborns under AT III substitution. Sbornik Lekarsky. 2000;101:143–148. [PubMed] [Google Scholar]
- Ostrovsky et al. (1997).Ostrovsky L, Woodman RC, Payne D, Teoh D, Kubes P. Antithrombin III prevents and rapidly reverses leukocyte recruitment in ischemia/reperfusion. Circulation. 1997;96:2302–2310. doi: 10.1161/01.CIR.96.7.2302. [DOI] [PubMed] [Google Scholar]
- Owings & Gosselin (1997).Owings JT, Gosselin R. Acquired antithrombin deficiency following severe traumatic injury: rationale for study of antithrombin supplementation. Seminars in Thrombosis and Hemostasis. 1997;23(1):17–24. doi: 10.1055/s-2007-996065. [DOI] [PubMed] [Google Scholar]
- Pasternak et al. (2016).Pasternak Y, Livni G, Ashkenazi S, Lowenthal A, Yarden-Bilavsky H. Extremely elevated C-reactive protein levels are associated with unfavourable outcomes, including death, in paediatric patients. Acta Paediatrica. 2016;105:e17–e21. doi: 10.1111/apa.13226. [DOI] [PubMed] [Google Scholar]
- Pereyra et al. (2017).Pereyra D, Offensperger F, Klinglmueller F, Haegele S, Oehlberger L, Gruenberger T, Brostjan C, Starlinger P. Early prediction of postoperative liver dysfunction and clinical outcome using antithrombin III-activity. PLOS ONE. 2017;12:e0175359. doi: 10.1371/journal.pone.0175359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters et al. (1984).Peters M, Ten Cate JW, Breederveld C, De Leeuw R, Emeis J, Koppe J. Low antithrombin III levels in neonates with idiopathic respiratory distress syndrome: poor prognosis. Pediatric Research. 1984;18:273–276. doi: 10.1203/00006450-198403000-00012. [DOI] [PubMed] [Google Scholar]
- Pettila et al. (2002).Pettila V, Pentti J, Pettila M, Takkunen O, Jousela I. Predictive value of antithrombin III and serum C-reactive protein concentration in critically ill patients with suspected sepsis. Critical Care Medicine. 2002;30:271–275. doi: 10.1097/00003246-200202000-00001. [DOI] [PubMed] [Google Scholar]
- Povoa et al. (2005).Povoa P, Coelho L, Almeida E, Fernandes A, Mealha R, Moreira P, Sabino H. C-reactive protein as a marker of infection in critically ill patients. Clinical Microbiology and Infection. 2005;11:101–108. doi: 10.1111/j.1469-0691.2004.01044.x. [DOI] [PubMed] [Google Scholar]
- Price et al. (1987).Price CP, Trull AK, Berry D, Gorman EG. Development and validation of a particle-enhanced turbidimetric immunoassay for C-reactive protein. Journal of Immunological Methods. 1987;99:205–211. doi: 10.1016/0022-1759(87)90129-3. [DOI] [PubMed] [Google Scholar]
- Rameshkumar et al. (2017).Rameshkumar R, Krishnamurthy S, Ganesh RN, Mahadevan S, Narayanan P, Satheesh P, Jain P. Histopathological changes in septic acute kidney injury in critically ill children: a cohort of post-mortem renal biopsies. Clinical and Experimental Nephrology. 2017;21:1075–1082. doi: 10.1007/s10157-016-1343-z. [DOI] [PubMed] [Google Scholar]
- R Core Team (2017).R Core Team . Vienna: R Foundation for Statistical Computing; 2017. [Google Scholar]
- Rudan et al. (2008).Rudan I, Boschi-Pinto C, Biloglav Z, Mulholland K, Campbell H. Epidemiology and etiology of childhood pneumonia. Bulletin of the World Health Organization. 2008;86:408–416. doi: 10.2471/BLT.07.048769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlapbach et al. (2015).Schlapbach LJ, Straney L, Alexander J, MacLaren G, Festa M, Schibler A, Slater A. Mortality related to invasive infections, sepsis, and septic shock in critically ill children in Australia and New Zealand, 2002-13: a multicentre retrospective cohort study. The Lancet Infectious Diseases. 2015;15:46–54. doi: 10.1016/S1473-3099(14)71003-5. [DOI] [PubMed] [Google Scholar]
- Schmit & Vincent (2008).Schmit X, Vincent JL. The time course of blood C-reactive protein concentrations in relation to the response to initial antimicrobial therapy in patients with sepsis. Infection. 2008;36:213–219. doi: 10.1007/s15010-007-7077-9. [DOI] [PubMed] [Google Scholar]
- Schoots et al. (2004).Schoots IG, Levi M, Van Vliet AK, Maas AM, Roossink EH, Van Gulik TM. Inhibition of coagulation and inflammation by activated protein C or antithrombin reduces intestinal ischemia/reperfusion injury in rats. Critical Care Medicine. 2004;32:1375–1383. doi: 10.1097/01.CCM.0000128567.57761.E9. [DOI] [PubMed] [Google Scholar]
- Schouten et al. (2008).Schouten M, Wiersinga WJ, Levi M, Van der Poll T. Inflammation, endothelium, and coagulation in sepsis. Journal of Leukocyte Biology. 2008;83:536–545. doi: 10.1189/jlb.0607373. [DOI] [PubMed] [Google Scholar]
- Sheikh Sajjadieh & Vasilovna Viunytska (2009).Sheikh Sajjadieh MR, Vasilovna Viunytska L. Antithrombin-III as a non-invasive marker of chronic liver disease. Hepatitis Monthly. 2009;9(2):128–132. [Google Scholar]
- St Peter et al. (2007).St Peter SD, Little DC, Calkins CM, Holcomb 3rd GW, Snyder CL, Ostlie DJ. The initial experience of antithrombin III in the management of neonates with necrotizing enterocolitis. Journal of Pediatric Surgery. 2007;42:704–708. doi: 10.1016/j.jpedsurg.2006.12.018. [DOI] [PubMed] [Google Scholar]
- Taylor Jr et al. (1988).Taylor Jr FB, Emerson Jr TE, Jordan R, Chang AK, Blick KE. Antithrombin-III prevents the lethal effects of Escherichia coli infusion in baboons. Circulatory Shock. 1988;26:227–235. [PubMed] [Google Scholar]
- Uchiba & Okajima (1997).Uchiba M, Okajima K. Antithrombin III (AT III) prevents LPS-induced pulmonary vascular injury: novel biological activity of AT III. Seminars in Thrombosis and Hemostasis. 1997;23:583–590. doi: 10.1055/s-2007-996140. [DOI] [PubMed] [Google Scholar]
- Van den Berg et al. (1989).Van den Berg W, Breederveld C, ten Cate JW, Peters M, Borm JJ. Low antithrombin III: accurate predictor of idiopathic respiratory distress syndrome in premature neonates. European Journal of Pediatrics. 1989;148:455–458. doi: 10.1007/BF00595913. [DOI] [PubMed] [Google Scholar]
- Vincent et al. (2006).Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, Moreno R, Carlet J, Le Gall JR, Payen D. Sepsis in European intensive care units: results of the SOAP study. Critical Care Medicine. 2006;34:344–353. doi: 10.1097/01.CCM.0000194725.48928.3A. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2015).Wang F, Zhang G, Lu Z, Geurts AM, Usa K, Jacob HJ, Cowley AW, Wang N, Liang M. Antithrombin III/SerpinC1 insufficiency exacerbates renal ischemia/reperfusion injury. Kidney International. 2015;88:796–803. doi: 10.1038/ki.2015.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren et al. (2001).Warren BL, Eid A, Singer P, Pillay SS, Carl P, Novak I, Chalupa P, Atherstone A, Penzes I, Kubler A, Knaub S, Keinecke HO, Heinrichs H, Schindel F, Juers M, Bone RC, Opal SM. Caring for the critically ill patient. High-dose antithrombin III in severe sepsis: a randomized controlled trial. JAMA. 2001;286:1869–1878. doi: 10.1001/jama.286.15.1869. [DOI] [PubMed] [Google Scholar]
- Wong et al. (2013).Wong TE, Huang YS, Weiser J, Brogan TV, Shah SS, Witmer CM. Antithrombin concentrate use in children: a multicenter cohort study. Jornal de Pediatria. 2013;163:1329–1334. doi: 10.1016/j.jpeds.2013.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong et al. (2016).Wong TE, Nguyen T, Shah SS, Brogan TV, Witmer CM. Antithrombin concentrate use in pediatric extracorporeal membrane oxygenation: a multicenter cohort study. Pediatric Critical Care Medicine. 2016;17:1170–1178. doi: 10.1097/PCC.0000000000000955. [DOI] [PubMed] [Google Scholar]
- Yin et al. (2017).Yin J, Wang F, Kong Y, Wu R, Zhang G, Wang N, Wang L, Lu Z, Liang M. Antithrombin III prevents progression of chronic kidney disease following experimental ischaemic-reperfusion injury. Journal of Cellular and Molecular Medicine. 2017;21:3506–3514. doi: 10.1111/jcmm.13261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Boxplots of antithrombin levels (%) by underlying diseases of (A) children older (≥1 year) and (B) younger than one year (<1 year). CNS refers to underlying diseases affecting the central nervous system.
Data set of antithrombin in septic children
Data Availability Statement
The following information was supplied regarding data availability:
The raw dataset is provided as a Supplemental File.