Abstract
We have recently described a novel therapeutic antibody product (IL2–F8–TNFmut), featuring the simultaneous fusion of murine IL2 and of a TNF mutant with scFv(F8), an antibody specific to the alternatively-spliced extra domain A of fibronectin (EDA). Here, we report on the in vivo characterization of the anti-cancer activity of IL2–F8–TNFmut in four immunocompetent murine models of cancer, CT26, WEHI-164, F9 teratocarcinoma and Lewis lung carcinoma (LLC), using the product alone or in combination with a monoclonal antibody specific to murine PD-L1. All four models exhibited a strong expression of EDA-fibronectin, which was confined to vascular structures for F9 tumors, while the other three malignancies exhibited a more stromal pattern of staining. A complete and long-lasting tumor eradication of CT26 and WEHI-164 tumors was observed in BALB/c mice when IL2–F8–TNFmut was used in combination with PD-L1 blockade. The combination treatment led to improved tumor growth inhibition in 129/SvEv mice bearing murine teratocarcinoma or in C57BL/6 mice bearing murine LLC, but those cancer cures were difficult to achieve in those models. A microscopic analysis of tumor sections, obtained 24 h after pharmacological treatment, revealed that the PD-L1 antibody had homogenously reached tumor cells in vivo and that the combination of PD-L1 blockade with IL2–F8–TNFmut stimulated an influx of NK cells and of T cells into the neoplastic mass. These data indicate that potency-matched dual-cytokine fusion proteins may be ideally suited to potentiate the therapeutic activity of immune check-point inhibitors.
Electronic supplementary material
The online version of this article (10.1007/s00262-018-2194-0) contains supplementary material, which is available to authorized users.
Keywords: Immunotherapy, PD-L1 blockade, Immunocytokines, IL2, TNF, EDA domain of fibronectin
Introduction
Immune check-point inhibitors are rapidly changing the clinical management of patients with cancer [1, 2]. Ipilimumab (blocking CTLA-4), Nivolumab or Pembrolizumab (blocking PD-1) and Avelumab (Blocking PD-L1) [3–6] have gained marketing authorization for the treatment of different types of malignancies, on the basis of an impressive clinical benefit offered to a subset of patients. Unfortunately, not all cancer types and not all patients respond equally well to immune oncology drugs and many combination strategies are currently being investigated, with the aim to improve therapeutic activity with acceptable toxicity [3–7].
The therapeutic activity of immune check-point inhibitors often correlates with the quantity and quality of lymphocyte infiltrate into the solid tumor mass [2]. On one hand, the nature of tumor rejection antigens presented by the tumor influences the anti-cancer activity of specific cytotoxic T cells [8, 9]. A growing body of experimental evidence indicates that both mutational load and HLA class I genotype potently influence response to immunotherapy in patients [10]. Moreover, various experimental strategies are under development, with the aim to turn “cold” tumors “hot”, by increasing the density of lymphocytes in the neoplastic lesions and by tilting the cytokine balance towards a more inflammatory phenotype [11].
Recombinant cytokines (e.g., IL2, TNF, IFN) have been used for many years, with the aim to boost the patient’s anti-cancer activity, with some encouraging results. Treatment with recombinant IL2 mediates a long-term survival for a relatively small proportion of patients with metastatic melanoma and renal cell carcinoma [12]. TNF has received marketing authorization in Europe for the treatment of soft-tissue sarcoma with isolated limb perfusion procedures [13], while recombinant IFN has been used for decades to treat various types of cancer [14]. However, the clinical use of anti-cancer cytokines is often limited to substantial toxicity (sometimes even at sub-milligram dose levels), preventing escalation to therapeutically active regimens [12–15].
To improve the therapeutic index of pro-inflammatory cytokines for oncological applications, the fusion of these immunomodulatory payloads with tumor-targeting monoclonal antibodies has been proposed [16–18]. Both intact immunoglobulins and antibody fragments have been used to generate fusion proteins with cytokines (“immunocytokines”). Some of these products have moved to clinical trials [19], on the basis of promising preclinical results.
Our group has previously described two antibody–cytokine fusion proteins (L19–IL2 and L19–TNF), which are currently being investigated in Phase III clinical trials [EudraCT number 2015-002549-72], after having shown encouraging activity in Phase II clinical studies [20–22]. These products recognize the alternatively-spliced extra domain B of fibronectin (EDB), a marker of tumor angiogenesis [23].
We have recently observed that the simultaneous delivery of two cytokine payloads to the tumor environment may exhibit a synergistic anti-cancer effect. For example, the combination of IL2- and TNF-based immunocytokine products was able to eradicate lesions in immunocompetent mouse models [24] and to induce complete responses in patients with stage IIIB/C melanoma [22]. In an attempt to combine the therapeutic activity of IL2 and of TNF into a single molecular entity, we have recently described a novel class of biopharmaceuticals, termed “potency-matched dual cytokine fusions” [25]. The possibility of having both IL2 and TNF moieties incorporated into a single polypeptide would be attractive from a pharmaceutical perspective, as the two synergistic payloads would require the development of only one antibody product. However, we needed to solve a technical challenge, considering the fact that the recommended dose of TNF is approximately ten times lower compared to the one of IL2 [20, 26, 27]. To solve this problem, we have generated a novel fusion protein (termed IL2–F8–TNFmut), featuring the scFv(F8) antibody fragment (specific to the alternatively-spliced EDA domain of fibronectin) fused to murine interleukin-2 (IL2) and to a single-aminoacid TNF mutant [25]. The R111W mutation in the TNF moiety was introduced to match the potency of the IL2 and TNF payloads [25]. The EDA domain of fibronectin is expressed in the majority of tumors both in mouse and in man, while being virtually undetectable in normal adult tissues [28]. The F8 antibody recognizes murine and human EDA with identical affinity [29].
In this article, we describe the anti-cancer properties of IL2–F8–TNFmut in combination with an anti-mouse PD-L1 antibody, previously reported to display some level of therapeutic activity in immunocompetent models of cancer [11]. Combination treatment induced cancer cures in BALB/c mice bearing CT26 and WEHI-164 tumors. The same therapeutic modality potentiated immune check-point inhibition and anti-cancer activity in 129/SvEv mice bearing murine F9 teratocarcinoma or in C57BL/6 mice bearing murine LLC, but complete tumor eradications were difficult to achieve in those models. A microscopic investigation of the nature of the immune cell infiltrate following combination treatment provided insights on the mechanism of action.
Materials and methods
Tumor cell lines and reagents
WEHI-164 fibrosarcoma cells, F9 teratocarcinoma cells, CT26 colon carcinoma cells and LLC Lewis lung carcinoma cells were obtained from the American Type Culture Collection (ATTC) between 2015 and 2017, expanded and stored as cryopreserved aliquots in liquid nitrogen. Cells were grown according to the supplier’s protocol and kept in culture for no longer than 14 passages. The production and purification of IL2–F8–TNFmut was performed as described before [25]. The commercial anti-PD-L1 was purchased at BioXCell (clone 10F.9G2; BE0101).
Tumor models and therapy studies
Tumor cells were implanted subcutaneously in the flank of BALB/c mice using 4 × 106 cells (CT26), 5 × 106 cells (WEHI-164). 15 × 106 cells (F9) in 129/ScEv mice and 2 × 106 cells (LLC) in C57BL/6 mice.
Mice were monitored daily and tumor volume was measured with a calliper (volume = length × width2 × 0.5). When tumors reached a suitable volume (approx. 70–80 mm3), mice were injected three times into the lateral tail vein with the pharmacological agents. IL2–F8–TNFmut and the commercial anti-PD-L1 antibody (clone 10F.9G2, BioXCell) were dissolved in PBS, also used as negative control, and administered every 48 h.
IL2–F8–TNFmut was administered at 50, 20, 40 and 40 µg for the therapy in CT26, WEHI-164, F9 and LLC, respectively. The commercial anti-PD-L1 antibody was administered at 200 µg. In a combination group, IL2–F8–TNFmut was administered 6 h before anti-PD-L1, while in a second combination group anti-PD-L1 was administered 6 h before IL2–F8–TNFmut.
For the tumor re-challenge study, mice with complete responses were injected subcutaneously with 4 × 106 CT26 cells in the flank.
Immunofluorescence studies
EDA expression was assessed on ice-cold acetone-fixed 8-µm cryostat sections of WEHI-164, CT26, F9 and LLC stained with IL2–F8–TNFmut (final concentration 5 µg/mL), as negative control IL2–KSF–TNFmut (specific for an irrelevant antigen) was used. Both antibodies were detected with rat anti-IL2 (eBioscience 14-7022-85) and anti-rat AlexaFluor488 (Invitrogen A21208). For vascular staining goat anti-CD31 (R&D AF3628) and anti-goat AlexaFluor594 (Invitrogen A11058) antibodies were used.
For ex-vivo immunofluorescence analysis, mice bearing CT26 or LLC lesions were injected once with IL2–F8–TNFmut + anti-PD-L1 or saline according to the therapy schedule and sacrificed 24 h after injection. Tumors were excised and embedded in cryoembedding medium (Thermo Scientific) and cryostat sections (8 µm) were stained using the following antibodies: rabbit anti-CD4 (SinoBiological 50134-R001), rabbit anti-CD8 (SinoBiological 50389-R208), rabbit anti-FoxP3 (Invitrogen 700914), rabbit anti-natural cytotoxicity receptor 1 (NCR1) (Abcam ab214468), goat anti-CD31 (R&D AF3628) and detected with anti-rat AlexaFluor488 (Invitrogen A21208), anti-rabbit AlexaFluor488 (Invitrogen A11008), and anti-goat AlexaFluor594 (Invitrogen A11058). Slides were mounted with fluorescent mounting medium and analyzed with Axioskop2 mot plus microscope (Zeiss).
MHC binding prediction of AH1 peptide
AH1 peptide was subjected to MHC class I-binding prediction analysis using NetMHCpan 4.0 net [30]. The peptide was assigned the minimal rank for the three BALB/C H-2 alleles Dd, Kd, and Ld, and for the two C57BL/6H-2 alleles Db and Kb. Weak binding was annotated if the rank calculated by NetMHCpan was below 2% and strong binding was annotated if the rank calculated by NetMHCpan was below 0.5%.
Identification of AH1 from MHC class I complexes of CT26 cells
The purification of MHC class I molecules, mass spectrometric and bioinformatics analysis were essentially performed as described [31]. MHC class I complexes were purified from 108 CT26 cells. Eluted peptides were analyzed on a Q Exactive mass spectrometer and data were processed with MaxQuant. After MaxQuant analysis, peptide-to-spectrum-matches of AH1 peptide within 1% false discovery rate were displayed with the MaxQuant Viewer using standard parameters and exported as pdf.
Results
Therapy experiments in immunocompetent mouse models of cancer
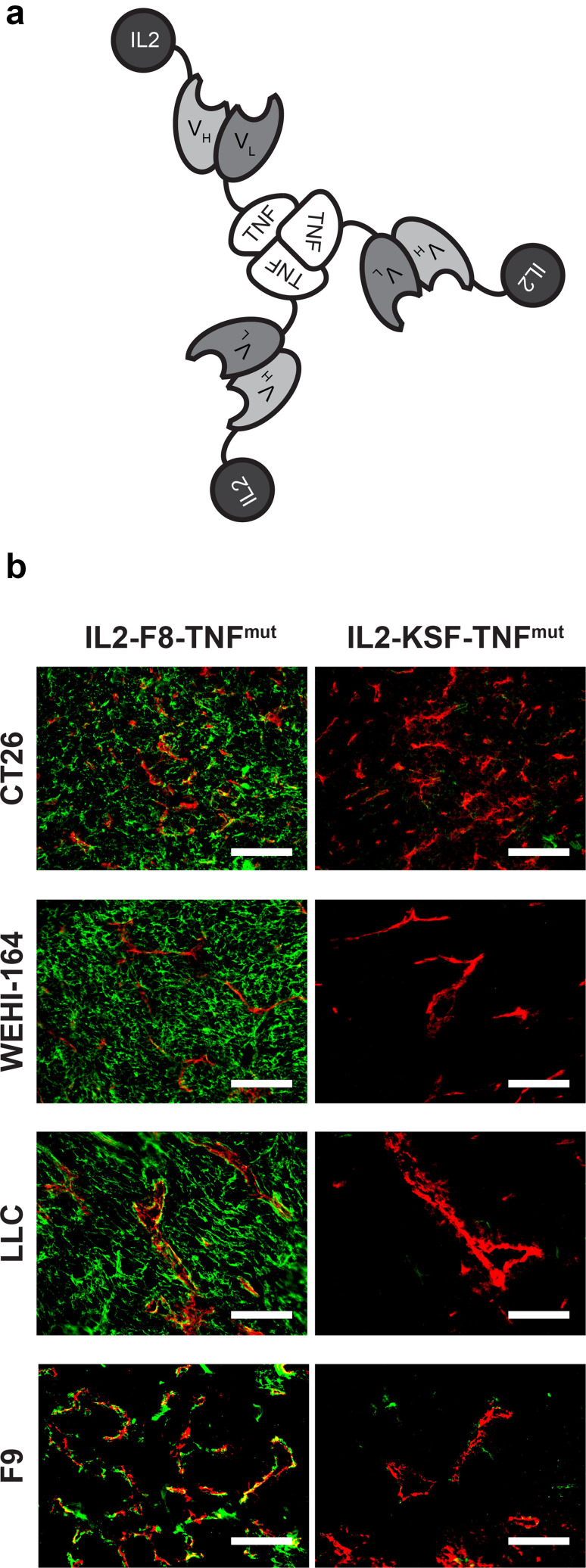
Figure 1 describes the reagents used for therapy experiments and provides information about the murine tumor models used in the study. IL2–F8–TNFmut, assembled into a stable non-covalent homotrimeric structure (Fig. 1a), was produced and purified as previously described [25], while a commercial antibody specific to murine PD-L1 was used as a surrogate for clinically-approved anti-PD-L1 products [11]. The IL2–F8–TNFmut product strongly stained the neo-vasculature of F9 teratocarcinomas (Fig. 1b), while the three other models used in our study (CT26, WEHI-164 and LLC) displayed a more diffuse stromal staining pattern. When IL2–KSF–TNFmut (a fusion protein with identical format but specific to hen egg lysozyme) was used as negative control in immunofluorescence procedures, no detectable staining was observed in addition to the CD31 signal.
Fig. 1.
Reagents and tumor models characterization. a Schematic representation of the domain assembly of IL2–F8–TNFmut. b Microscopic fluorescence analysis of EDA expression on CT26, WEHI-164, LLC and F9 tumor sections detected with IL2–F8–TNFmut or IL2–KSF–TNFmut (green for anti-murine IL2, Alexa Fluor 488) and anti-CD31 (red, Alexa Fluor 594), × 20 magnification, scale bars = 100 µm
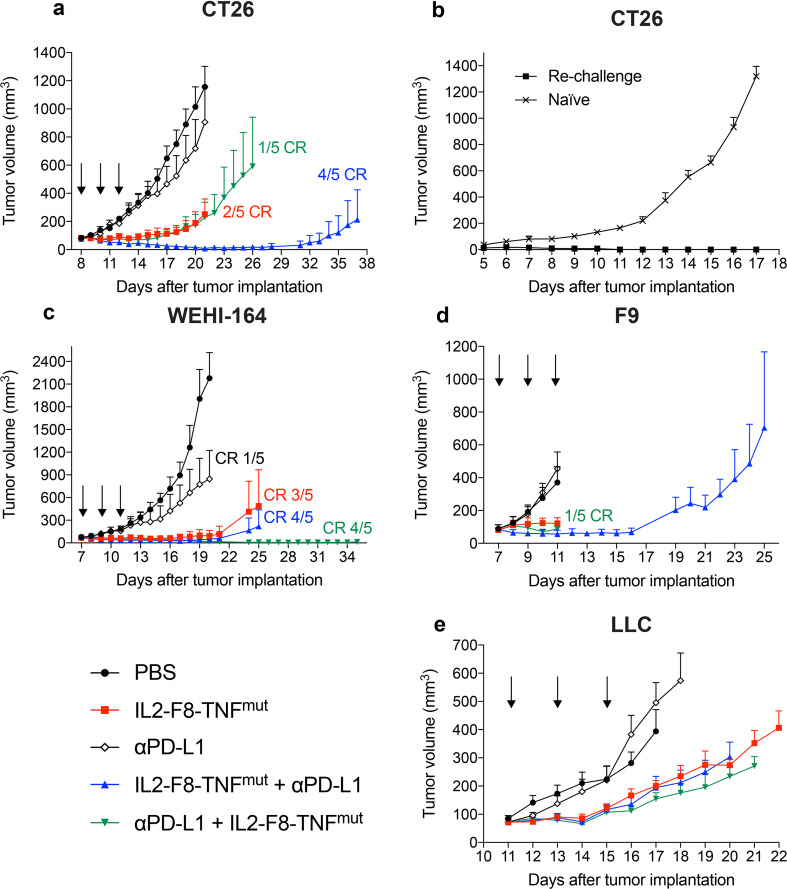
The therapeutic activity of IL2–F8–TNFmut (administered at a dose of 50 µg) was compared with the one of an anti-mouse PD-L1 antibody (used at 200 µg) in immunocompetent BALB/c mice bearing CT26 carcinomas (Fig. 2a). Treatment with the anti-PD-L1 antibody resulted in a tumor growth profile similar to the one obtained in the saline control group (PBS). A tumor growth inhibition was observed in the IL2–F8–TNFmut group, as well as in the combination group where the anti-PD-L1 antibody had been administered 6 h before IL2–F8–TNFmut. By contrast, the combination IL2–F8–TNFmut followed by anti-PD-L1 6 h later induced complete responses in 80% of the treated mice. Animals which had been cured by the combined administration of IL2–F8–TNFmut + anti-PD-L1 were re-challenged with CT26 cells and were found to have acquired a protective immunity against a second tumor implantation (Fig. 2b).
Fig. 2.
Therapeutic performance of IL2–F8–TNFmut in combination with anti-PD-L1 treatment. Data represent mean tumor volume ± SEM. For all therapies, mice were injected three times intravenously (black arrows) every 48 h with either PBS, IL2–F8–TNFmut, 200 µg anti-mouse PD-L1 or a combination of the two (IL2–F8–TNFmut 6 h before anti-PD-L1 or the opposite). n = 5 mice per group (unless stated elsewhere), CR = complete response. a Therapy in Balb/c mice bearing CT26 colon carcinoma lesions. Treatment started when tumors reached a volume of 80 mm3, IL2–F8–TNFmut was dosed at 50 µg. n = 5 mice per group, CR complete response. b Tumor re-challenge study. After 37 days, mice with complete responses were injected subcutaneously with 4 × 106 CT26 cells in the flank. n = 5 for PBS group (n = 4 from day 12), n = 4 for re-challenge group. c Therapy in Balb/c mice bearing WEHI-164 tumors. Treatment started when tumors reached a volume of 70 mm3, IL2–F8–TNFmut was dosed at 20 µg. d Therapy in 129/SvEv mice bearing F9 teratocarcinomas. Treatment started when tumors reached a volume of 80 mm3, IL2–F8–TNFmut was dosed at 40 µg. e Therapy in C57BL/6 mice bearing LLC tumors. Treatment started when tumors reached a volume of 70 mm3, IL2–F8–TNFmut was dosed at 40 µg. n = 4 from day 15 for groups IL2–F8–TNFmut + anti-PD-L1 and PBS, from day 16 for group anti-PD-L1 and from day 20 for groups anti-PD-L1 + IL2–F8–TNFmut and IL2–F8–TNFmut
In a second therapy experiment, immunocompetent mice bearing WEHI-164 sarcomas were treated (Fig. 2c). In this model, which is very sensitive to the action of TNF, the dose of IL2–F8–TNFmut was reduced to 20 µg, to assess whether a synergistic effect with anti-PD-L1 treatment could be observed [25]. An extremely potent anti-tumor activity was observed for IL2–F8–TNFmut used as single agent and its combination with PD-L1 blockade led to cancer cures in 4/5 mice, irrespective of the order of administration of the two biopharmaceuticals.
A potent inhibition of tumor cell growth was observed in mice bearing F9 and LLC lesions (Fig. 2d, e), but complete cancer eradication was difficult to achieve in these models. All regimens were tolerated as evidenced by the comparison of body weight profiles (Supplementary Fig. 1).
Microscopic analysis of immune cell infiltrate
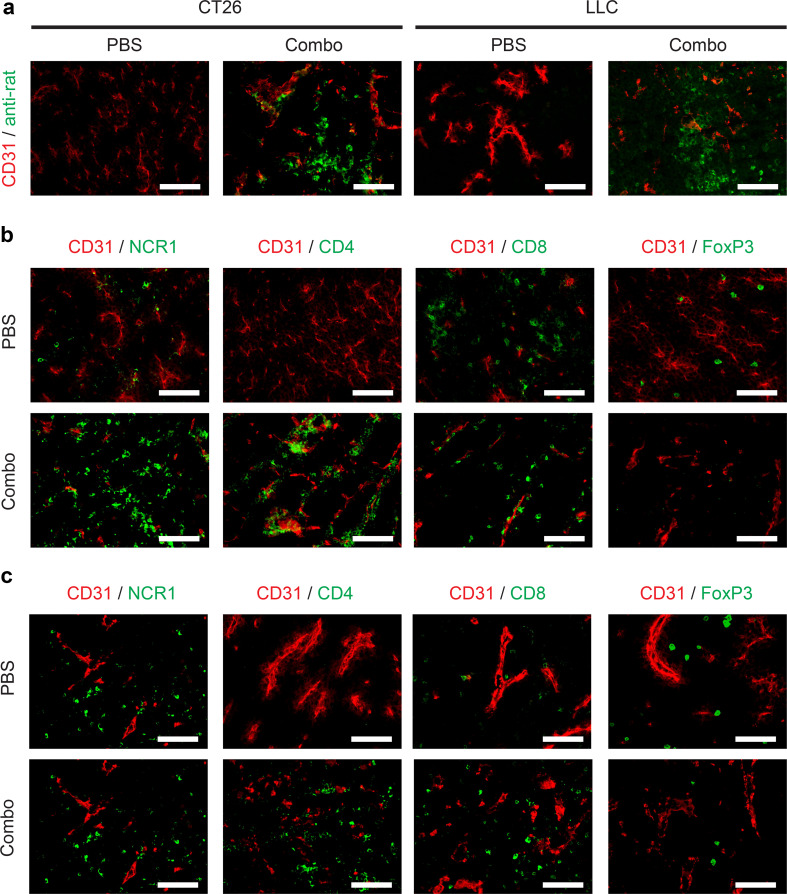
The tumor-targeting properties of the commercial anti-PD-L1 antibody were investigated by analyzing sections of CT26 (a tumor that could be cured) or LLC (a tumor that could not be cured in our experimental setting) 24 h after intravenous administration of 200 µg of this product. An ex vivo immunofluorescence staining, performed using an anti-rat secondary antibody reagent, revealed a patchy uptake of the anti-PD-L1 antibody at the tumor site in both models (Fig. 3a).
Fig. 3.
Microscopic analysis of therapeutic performance of IL2–F8–TNFmut in combination with anti-PD-L1 treatment. a Immunofluorescence analysis of tumor-targeting properties of an anti-PD-L1 antibody 24 h after IL2–F8–TNFmut + anti-PD-L1 treatment in mice bearing CT26 or LLC lesions, cryosections were stained with anti-rat IgG (green, Alexa Fluor 488) and anti-CD31 (red, Alexa Fluor 594), × 20 magnification, scale bars = 100 µm. Immunofluorescence analysis of tumor-infiltrating cells on CT26 (b) or LLC (c) tumor sections 24 h after treatment with PBS or IL2–F8–TNFmut + anti-PD-L1, marker specific for NK cells (NCR1), CD4+ T cells (CD4), CD8+ T cells (CD8) and T regs (FoxP3) were stained in green (Alexa Fluor 488), anti-CD31 (red, Alexa Fluor 594), ×20 magnification, scale bars = 100 µm
A microscopic analysis of leukocyte infiltrate in sections of CT26 tumors (Fig. 3b), obtained 24 h after a single injection of IL2–F8–TNFmut + anti-PD-L1 or saline, revealed a substantial increase in the intratumoral density of NK cells and CD4+ T cells after combination therapy. Tumor-resident CD8+ T cells were detectable also prior to treatment. By contrast, the density of FoxP3-positive lymphocytes decreased, as a result of pharmacological intervention, indicating product activity against regulatory T cells.
A similar analysis, performed in sections of LLC tumors (Fig. 3c), revealed a distinct increase in the intratumoral density of NK cells, CD4+ T cells and CD8+ T cells after combination therapy. Only a small reduction in the density of FoxP3-positive lymphocytes was observed.
Discussion
The targeted delivery of cytokines to the tumor environment increases the therapeutic index of those immune modulators [19, 32–40] and various antibody–cytokine fusions are currently being investigated in clinical trials for oncological applications. As the combination of cytokines is often required to control immunological processes [41], it is attractive to use mixtures of antibody–cytokine fusions in therapy experiments [24, 42–45] or to develop antibody fusions, featuring multiple cytokine payloads [25, 44, 46]. Here, we studied the therapeutic activity of a novel antibody–cytokine fusion protein (IL2–F8–TNFmut) in four immunocompetent mouse models of cancer (CT26, WEHI-164, F9 and LLC). The product was used alone or in combination with an anti-PD-L1 antibody (a surrogate for the human-specific PD-L1 blockers Avelumab, Durvalumab and Atezolizumab, which have gained marketing authorization for cancer therapy) [6, 47, 48].
A potent tumor growth inhibition was observed for IL2–F8–TNFmut used as single agents in CT26 tumors, while PD-L1 blockade had a minimal anti-cancer activity in all experimental models, in keeping with previous reports [11]. The combination of the two products led to long-lasting complete responses, when the immune check-point inhibitor was given after immunocytokine treatment. Pre-administration of IL2–F8–TNFmut may increase vascular permeability of tumor blood vessels, promoting a higher uptake of anti-PD-L1 in the neoplastic lesion. Similar effects in biodistribution studies following pre-administration of TNF fusion proteins have previously been reported by our group [46], the group of Jean-Pierre Mach [49] and the group of Alan Epstein [50]. Analogous findings have also been reported for IL2-based therapeutics [51]. High concentrations of TNF may promote hemorrhagic necrosis [25] and, in certain cases, intravascular blood coagulation [52]. This observation and studies of the immune infiltrate into the tumor mass suggests that the targeted delivery of pro-inflammatory cytokines (IL2 and TNF) increase the density and activity of lymphocytes in the neoplastic mass, whose activity can be further modulated by PD-L1 blockade.
A potentiation of the therapeutic activity of IL2–F8–TNFmut in combination with the anti-PD-L1 antibody was observed in WEHI-164, leading to cures in most treated animals. Sarcomas are extremely sensitive to the targeted delivery of TNF, which rapidly kills the majority of tumor cells. The anti-cancer role of lymphocytes is mainly confined to the eradication of the few tumor cells, which survive the induction of hemorrhagic necrosis into the neoplastic mass [25, 31].
A potent tumor growth inhibition was observed when F9 tumors were treated with IL2–F8–TNFmut, alone or in combination with anti-PD-L1. However, only one mouse could be cured in the combination group. For LLC tumors, no cures were observed and the potency-matched immunocytokine product could mediate only a partial tumor growth inhibition. No apparent benefit was observed in the combination therapy group. No obvious correlation between target antigen expression and therapeutic outcome was observed in these therapy settings, as all models were strongly positive for EDA.
The acquired protective immunity in BALB/c-derived tumors indicates a possible role for the AH1 peptide (a peptide derived from the gp70 envelope protein of the murine leukemia virus, endogenous in the BALB/c genome) as a dominant tumor-rejection antigen [31]. The anti-cancer activity of IL2–F8–TNFmut is mainly driven by CD4+ and CD8+ T cells [25], supporting the notion that the presentation of a suitable tumor-associated antigen on MHC molecules contributes to therapeutic outcome.
At this moment in time, we do not know whether the lower anti-cancer activity observed in the LLC model, strongly positive for EDA(+)-fibronectin, is due to the higher concentration of immunosuppressive mediators or to the absence of suitable immunogenic peptides to support immune surveillance. It has previously been reported that LLC tumors display an anti-inflammatory environment and that they do not respond well to immune check-point inhibition [11]. The observation that the dense infiltration of leukocytes at the tumor site, promoted by pharmacological intervention, does not result in a complete response, and correlates with the persistence of regulatory T cells within the tumor mass (Fig. 3c).
A series of publications has shown that LLC and CT26 express MHC-I on the cell surface and that specific T cells (directed against the tumor) recognize putative rejection antigens [31, 53–58]. Moreover, the different mouse strain used for LLC tumors (C57BL/6) and for WEHI-164 and CT26 tumors (BALB/c) correlates with a difference in MHC class I alleles [59].
Interestingly, the immunodominant AH1 peptide can be efficiently displayed on H-2Ld of BALB/c tumors, while the same sequence cannot be displayed on H-2Db or H-2Kb of C57BL/6 tumors. Efficient presentation of AH1 on MHC class I molecules in CT26 cancer cells could be predicted using software tools and was experimentally confirmed by mass-spectrometry-based MHC peptidome analysis (Supplementary table 1 and Supplementary Fig. 2). This observation also correlates with the finding that gp70 is strongly expressed in many tumors of BALB/c origin and also in B16F10 melanoma of C57BL/6 origin, while a very weak expression was seen for LLC, using quantitative PCR analysis [31]. Rejection of LLC tumors and induction of protective immunity has been claimed in the literature [44, 60–64]. It might be possible that LLC cells present a different rejection antigen, perhaps derived from gp70, but with a different sequence from the AH1 peptide. The seminal work of Thierry Boon and collaborators [61] featured the treatment of LLC with mutagenic agents. This procedure is likely to increase the immunogenicity of the tumor, as highlighted by Goodenow and collaborators [60]. Virus-mediated transfection of LLC with cytokine genes (e.g., IL2, IL6, IL12) has led to the complete eradication of tumor cells and to the induction of protective immunity [62–64]. These findings can be mimicked by the intratumoral injection of a cocktail of antibody–cytokine fusions [65]. Overall, LLC remains one of the most difficult experimental tumors to treat and cure, using systemic pharmacological approaches.
It is becoming increasingly clear that the ability of tumor cells to efficiently present tumor rejection antigen may represent a dominant feature, that discriminates between tumors that respond (or do not respond) to immunotherapy [10].
IL2–F8–TNFmut is a biopharmaceutical with excellent biodistribution properties in tumor-bearing mice [25]. The product recognizes the alternatively-spliced EDA domain of fibronectin, a marker of tumor angiogenesis. The F8 antibody binds to EDA with identical affinity in mouse and man, thus facilitating clinical translation. EDA is strongly expressed in a large variety of human malignancies, including several types of lymphomas [66], renal cell carcinomas [67], acute leukemias [68], melanoma [69], head and neck tumors [70], as well as high-grade astrocytomas, lung and liver metastases, colon carcinomas, rhabdomyosarcomas and other tumor types [28]. The product may be used alone or, preferably, in combination with immune check-point inhibitors, which are rapidly gaining acceptance as broad-spectrum agents for many cancer indications [1, 2].
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Patrizia Murer, Marco Stringhini and Gianluca Buttinoni for their help with experimental procedures, as well as Dr. Tim Fugmann and Philipp Probst for the MHC class I analysis.
Abbreviations
- EDA
Alternatively-spliced extra domain A of fibronectin
- EDB
Alternatively-spliced extra domain B of fibronectin
- gp70
Envelope protein of the murine leukemia virus
- LLC
Lewis lung carcinoma
- NCR1
Natural Cytotoxicity receptor 1
Author Contributions
RD and DN: conception and design; development of methodology; acquisition, analysis and interpretation of data; writing, review and revision of the manuscript. DN: study supervision.
Funding
We gratefully acknowledge funding from ETH Zürich, the Swiss National Science Foundation, the European Research Council (ERC Advanced Grant “Zauberkugel”), the Swiss Federal Commission for Technology and Innovation (CTI Project “DUAL CYTOKINE-ANTIBODY FUSIONS”) and the “Stiftung zur Krebsbekämpfung”.
Compliance with ethical standards
Conflict of interest
Dario Neri is co-founder, shareholder and member of the board of Philogen, a company working on antibody therapeutics. The authors declare no additional conflict of interest.
Animal source
Eight-week-old female BALB/c mice, 129/SvEv mice and C57BL/6 were obtained from Janvier Labs.
Animal research
Experiments were performed under a project license (license number 27/2015) granted by the Veterinäramt des Kantons Zürich, Switzerland, in compliance with the Swiss Animal Protection Act (TSchG) and the Swiss Animal Protection Ordinance (TSchV).
Cell line authentication
Authentication of the cell lines also including check of post-freeze viability, growth properties and morphology, test for mycoplasma contamination, isoenzyme assay and sterility test was performed by the cell bank before shipment.
References
- 1.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161(2):205–214. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei SC, Levine JH, Cogdill AP, Zhao Y, Anang NAS, Andrews MC, Sharma P, Wang J, Wargo JA, Pe’er D, Allison JP. Distinct cellular mechanisms underlie anti-CTLA-4 and anti-PD-1 checkpoint blockade. Cell. 2017;170(6):1120–1133 e1117. doi: 10.1016/j.cell.2017.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, Davidson N, Richards J, Maio M, Hauschild A, Miller WH, Jr, Gascon P, Lotem M, Harmankaya K, Ibrahim R, Francis S, Chen TT, Humphrey R, Hoos A, Wolchok JD. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 4.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, Savage KJ, Hernberg MM, Lebbe C, Charles J, Mihalcioiu C, Chiarion-Sileni V, Mauch C, Cognetti F, Arance A, Schmidt H, Schadendorf D, Gogas H, Lundgren-Eriksson L, Horak C, Sharkey B, Waxman IM, Atkinson V, Ascierto PA. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 5.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns B, Blank CU, Hamid O, Mateus C, Shapira-Frommer R, Kosh M, Zhou H, Ibrahim N, Ebbinghaus S, Ribas A, investigators K- Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 6.Kaufman HL, Russell J, Hamid O, Bhatia S, Terheyden P, D’Angelo SP, Shih KC, Lebbe C, Linette GP, Milella M, Brownell I, Lewis KD, Lorch JH, Chin K, Mahnke L, von Heydebreck A, Cuillerot JM, Nghiem P. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17(10):1374–1385. doi: 10.1016/S1470-2045(16)30364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, Wolchok JD. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balachandran VP, Luksza M, Zhao JN, Makarov V, Moral JA, Remark R, Herbst B, Askan G, Bhanot U, Senbabaoglu Y, Wells DK, Cary CIO, Grbovic-Huezo O, Attiyeh M, Medina B, Zhang J, Loo J, Saglimbeni J, Abu-Akeel M, Zappasodi R, Riaz N, Smoragiewicz M, Kelley ZL, Basturk O, Australian Pancreatic Cancer Genome I, Garvan Institute of Medical R, Prince of Wales H, Royal North Shore H, University of G, St Vincent’s H, Institute QBMR, University of Melbourne CfCR, University of Queensland IfMB, Bankstown H, Liverpool H, Royal Prince Alfred Hospital COBL, Westmead H, Fremantle H, St John of God H, Royal Adelaide H, Flinders Medical C, Envoi P, Princess Alexandria H, Austin H, Johns Hopkins Medical I, Cancer AR-NCfARo, Gonen M, Levine AJ, Allen PJ, Fearon DT, Merad M, Gnjatic S, Iacobuzio-Donahue CA, Wolchok JD, DeMatteo RP, Chan TA, Greenbaum BD, Merghoub T, Leach SD (2017) Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer. Nature 551(7681):512–516. 10.1038/nature24462 [DOI] [PMC free article] [PubMed]
- 9.Luksza M, Riaz N, Makarov V, Balachandran VP, Hellmann MD, Solovyov A, Rizvi NA, Merghoub T, Levine AJ, Chan TA, Wolchok JD, Greenbaum BD. A neoantigen fitness model predicts tumour response to checkpoint blockade immunotherapy. Nature. 2017;551(7681):517–520. doi: 10.1038/nature24473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chowell D, Morris LGT, Grigg CM, Weber JK, Samstein RM, Makarov V, Kuo F, Kendall SM, Requena D, Riaz N, Greenbaum B, Carroll J, Garon E, Hyman DM, Zehir A, Solit D, Berger M, Zhou R, Rizvi NA, Chan TA. Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science. 2018;359(6375):582–587. doi: 10.1126/science.aao4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mosely SI, Prime JE, Sainson RC, Koopmann JO, Wang DY, Greenawalt DM, Ahdesmaki MJ, Leyland R, Mullins S, Pacelli L, Marcus D, Anderton J, Watkins A, Coates Ulrichsen J, Brohawn P, Higgs BW, McCourt M, Jones H, Harper JA, Morrow M, Valge-Archer V, Stewart R, Dovedi SJ, Wilkinson RW. Rational selection of syngeneic preclinical tumor models for immunotherapeutic drug discovery. Cancer Immunol Res. 2017;5(1):29–41. doi: 10.1158/2326-6066.CIR-16-0114. [DOI] [PubMed] [Google Scholar]
- 12.Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol. 2014;192(12):5451–5458. doi: 10.4049/jimmunol.1490019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jakob J, Hohenberger P. Role of isolated limb perfusion with recombinant human tumor necrosis factor alpha and melphalan in locally advanced extremity soft tissue sarcoma. Cancer. 2016;122(17):2624–2632. doi: 10.1002/cncr.29991. [DOI] [PubMed] [Google Scholar]
- 14.Mellstedt H, Björkholm M, Johansson B, Ahre A, Holm G, Strander H. Interferon therapy in myelomatosis. Lancet. 1979;313(8110):245–247. doi: 10.1016/S0140-6736(79)90770-0. [DOI] [PubMed] [Google Scholar]
- 15.Gollob JA, Mier JW, Veenstra K, McDermott DF, Clancy D, Clancy M, Atkins MB. Phase I trial of twice-weekly intravenous interleukin 12 in patients with metastatic renal cell cancer or malignant melanoma: ability to maintain IFN-γ induction Is associated with clinical response. Clin Cancer Res. 2000;6(5):1678. [PubMed] [Google Scholar]
- 16.Pasche N, Neri D. Immunocytokines: a novel class of potent armed antibodies. Drug Discov Today. 2012;17(11–12):583–590. doi: 10.1016/j.drudis.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Bootz F, Neri D. Immunocytokines: a novel class of products for the treatment of chronic inflammation and autoimmune conditions. Drug Discov Today. 2016;21(1):180–189. doi: 10.1016/j.drudis.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hess C, Venetz D, Neri D. Emerging classes of armed antibody therapeutics against cancer. Med Chem Commun. 2014;5(4):408. doi: 10.1039/c3md00360d. [DOI] [Google Scholar]
- 19.Neri D, Sondel PM. Immunocytokines for cancer treatment: past, present and future. Curr Opin Immunol. 2016;40:96–102. doi: 10.1016/j.coi.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spitaleri G, Berardi R, Pierantoni C, De Pas T, Noberasco C, Libbra C, Gonzalez-Iglesias R, Giovannoni L, Tasciotti A, Neri D, Menssen HD, de Braud F. Phase I/II study of the tumour-targeting human monoclonal antibody-cytokine fusion protein L19-TNF in patients with advanced solid tumours. J Cancer Res Clin Oncol. 2013;139(3):447–455. doi: 10.1007/s00432-012-1327-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papadia F, Basso V, Patuzzo R, Maurichi A, Di Florio A, Zardi L, Ventura E, Gonzalez-Iglesias R, Lovato V, Giovannoni L, Tasciotti A, Neri D, Santinami M, Menssen HD, De Cian F. Isolated limb perfusion with the tumor-targeting human monoclonal antibody-cytokine fusion protein L19-TNF plus melphalan and mild hyperthermia in patients with locally advanced extremity melanoma. J Surg Oncol. 2013;107(2):173–179. doi: 10.1002/jso.23168. [DOI] [PubMed] [Google Scholar]
- 22.Danielli R, Patuzzo R, Di Giacomo AM, Gallino G, Maurichi A, Di Florio A, Cutaia O, Lazzeri A, Fazio C, Miracco C, Giovannoni L, Elia G, Neri D, Maio M, Santinami M. Intralesional administration of L19-IL2/L19-TNF in stage III or stage IVM1a melanoma patients: results of a phase II study. Cancer Immunol Immunother. 2015;64(8):999–1009. doi: 10.1007/s00262-015-1704-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pini A, Viti F, Santucci A, Carnemolla B, Zardi L, Neri P, Neri D. Design and use of a phage display library: human antibodies with subnanomolar affinity against a marker of angiogenesis eluted from a two-dimensional gel. J Biol Chem. 1998;273(34):21769–21776. doi: 10.1074/jbc.273.34.21769. [DOI] [PubMed] [Google Scholar]
- 24.Schwager K, Hemmerle T, Aebischer D, Neri D. The immunocytokine L19-IL2 eradicates cancer when used in combination with CTLA-4 blockade or with L19-TNF. J Invest Dermatol. 2013;133(3):751–758. doi: 10.1038/jid.2012.376. [DOI] [PubMed] [Google Scholar]
- 25.De Luca R, Soltermann A, Pretto F, Pemberton-Ross C, Pellegrini G, Wulhfard S, Neri D. Potency-matched dual cytokine-antibody fusion proteins for cancer therapy. Mol Cancer Ther. 2017;16(11):2442–2451. doi: 10.1158/1535-7163.MCT-17-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johannsen M, Spitaleri G, Curigliano G, Roigas J, Weikert S, Kempkensteffen C, Roemer A, Kloeters C, Rogalla P, Pecher G, Miller K, Berndt A, Kosmehl H, Trachsel E, Kaspar M, Lovato V, Gonzalez-Iglesias R, Giovannoni L, Menssen HD, Neri D, de Braud F. The tumour-targeting human L19-IL2 immunocytokine: preclinical safety studies, phase I clinical trial in patients with solid tumours and expansion into patients with advanced renal cell carcinoma. Eur J Cancer. 2010;46(16):2926–2935. doi: 10.1016/j.ejca.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 27.Eigentler TK, Weide B, de Braud F, Spitaleri G, Romanini A, Pflugfelder A, Gonzalez-Iglesias R, Tasciotti A, Giovannoni L, Schwager K, Lovato V, Kaspar M, Trachsel E, Menssen HD, Neri D, Garbe C. A dose-escalation and signal-generating study of the immunocytokine L19-IL2 in combination with dacarbazine for the therapy of patients with metastatic melanoma. Clin Cancer Res. 2011;17(24):7732–7742. doi: 10.1158/1078-0432.CCR-11-1203. [DOI] [PubMed] [Google Scholar]
- 28.Rybak JN, Roesli C, Kaspar M, Villa A, Neri D. The extra-domain A of fibronectin is a vascular marker of solid tumors and metastases. Cancer Res. 2007;67(22):10948–10957. doi: 10.1158/0008-5472.CAN-07-1436. [DOI] [PubMed] [Google Scholar]
- 29.Villa A, Trachsel E, Kaspar M, Schliemann C, Sommavilla R, Rybak JN, Rosli C, Borsi L, Neri D. A high-affinity human monoclonal antibody specific to the alternatively spliced EDA domain of fibronectin efficiently targets tumor neo-vasculature in vivo. Int J Cancer. 2008;122(11):2405–2413. doi: 10.1002/ijc.23408. [DOI] [PubMed] [Google Scholar]
- 30.Jurtz V, Paul S, Andreatta M, Marcatili P, Peters B, Nielsen M. NetMHCpan-4.0: improved peptide-MHC class I interaction predictions integrating eluted ligand and peptide binding affinity data. J Immunol. 2017;199(9):3360–3368. doi: 10.4049/jimmunol.1700893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Probst P, Kopp J, Oxenius A, Colombo MP, Ritz D, Fugmann T, Neri D. Sarcoma eradication by doxorubicin and targeted TNF relies upon CD8(+) T-cell recognition of a retroviral antigen. Cancer Res. 2017;77(13):3644–3654. doi: 10.1158/0008-5472.CAN-16-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halin C, Rondini S, Nilsson F, Berndt A, Kosmehl H, Zardi L, Neri D. Enhancement of the antitumor activity of interleukin-12 by targeted delivery to neovasculature. Nat Biotechnol. 2002;20(3):264–269. doi: 10.1038/nbt0302-264. [DOI] [PubMed] [Google Scholar]
- 33.Yang RK, Kalogriopoulos NA, Rakhmilevich AL, Ranheim EA, Seo S, Kim K, Alderson KL, Gan J, Reisfeld RA, Gillies SD, Hank JA, Sondel PM. Intratumoral hu14.18-IL-2 (IC) induces local and systemic antitumor effects that involve both activated T and NK cells as well as enhanced IC retention. J Immunol. 2012;189(5):2656–2664. doi: 10.4049/jimmunol.1200934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muller D. Antibody fusions with immunomodulatory proteins for cancer therapy. Pharmacol Ther. 2015;154:57–66. doi: 10.1016/j.pharmthera.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Penichet ML, Morrison SL. Antibody-cytokine fusion proteins for the therapy of cancer. J Immunol Methods. 2001;248(1–2):91–101. doi: 10.1016/S0022-1759(00)00345-8. [DOI] [PubMed] [Google Scholar]
- 36.Kontermann RE. Antibody-cytokine fusion proteins. Arch Biochem Biophys. 2012;526(2):194–205. doi: 10.1016/j.abb.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 37.Lode HN, Xiang R, Becker JC, Gillies SD, Reisfeld RA. Immunocytokines: a promising approach to cancer immunotherapy. Pharmacol Ther. 1998;80(3):277–292. doi: 10.1016/S0163-7258(98)00033-3. [DOI] [PubMed] [Google Scholar]
- 38.Gillies SD. A new platform for constructing antibody-cytokine fusion proteins (immunocytokines) with improved biological properties and adaptable cytokine activity. Protein Eng Des Sel. 2013;26(10):561–569. doi: 10.1093/protein/gzt045. [DOI] [PubMed] [Google Scholar]
- 39.Fellermeier S, Beha N, Meyer JE, Ring S, Bader S, Kontermann RE, Muller D. Advancing targeted co-stimulation with antibody-fusion proteins by introducing TNF superfamily members in a single-chain format. Oncoimmunology. 2016;5(11):e1238540. doi: 10.1080/2162402X.2016.1238540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klein C, Waldhauer I, Nicolini VG, Freimoser-Grundschober A, Nayak T, Vugts DJ, Dunn C, Bolijn M, Benz J, Stihle M, Lang S, Roemmele M, Hofer T, van Puijenbroek E, Wittig D, Moser S, Ast O, Brunker P, Gorr IH, Neumann S, de Vera Mudry MC, Hinton H, Crameri F, Saro J, Evers S, Gerdes C, Bacac M, van Dongen G, Moessner E, Umana P. Cergutuzumab amunaleukin (CEA-IL2v), a CEA-targeted IL-2 variant-based immunocytokine for combination cancer immunotherapy: overcoming limitations of aldesleukin and conventional IL-2-based immunocytokines. Oncoimmunology. 2017;6(3):e1277306. doi: 10.1080/2162402X.2016.1277306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murphy K, Weaver C (2017) Janeway’s Immunobiology, 9th Edition. Garland Science
- 42.Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, Smith M, Anderson B, Villablanca JG, Matthay KK, Shimada H, Grupp SA, Seeger R, Reynolds CP, Buxton A, Reisfeld RA, Gillies SD, Cohn SL, Maris JM, Sondel PM, ’s ChildrenOncology G. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363(14):1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wigginton JM, Wiltrout RH. IL-12/IL-2 combination cytokine therapy for solid tumours: translation from bench to bedside. Expert Opin Biol Ther. 2002;2(5):513–524. doi: 10.1517/14712598.2.5.513. [DOI] [PubMed] [Google Scholar]
- 44.Gillies SD, Lan Y, Brunkhorst B, Wong WK, Li Y, Lo KM. Bi-functional cytokine fusion proteins for gene therapy and antibody-targeted treatment of cancer. Cancer Immunol Immunother. 2002;51(8):449–460. doi: 10.1007/s00262-002-0302-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hemmerle T, Neri D. The antibody-based targeted delivery of interleukin-4 and 12 to the tumor neovasculature eradicates tumors in three mouse models of cancer. Int J Cancer. 2014;134(2):467–477. doi: 10.1002/ijc.28359. [DOI] [PubMed] [Google Scholar]
- 46.Halin C, Gafner V, Villani ME, Borsi L, Berndt A, Kosmehl H, Zardi L, Neri D. Synergistic therapeutic effects of a tumor targeting antibody fragment, fused to interleukin 12 and to tumor necrosis factor alpha. Cancer Res. 2003;63(12):3202–3210. [PubMed] [Google Scholar]
- 47.Planchard D, Yokoi T, McCleod MJ, Fischer JR, Kim YC, Ballas M, Shi K, Soria JC. A phase III study of Durvalumab (MEDI4736) with or without tremelimumab for previously treated patients with advanced NSCLC: rationale and protocol design of the ARCTIC Study. Clin Lung Cancer. 2016;17(3):232–236 e231. doi: 10.1016/j.cllc.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 48.Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D, Artal-Cortes A, Lewanski C, Braiteh F, Waterkamp D, He P, Zou W, Chen DS, Yi J, Sandler A, Rittmeyer A, Group PS. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837–1846. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 49.Folli S, Épèlegrin A, Chalandon Y, Yao X, Buchegger F, Lienard D, Lejeune F, Mach J-P. Tumor-necrosis factor can enhance radio-antibody uptake in human colon carcinoma xenografts by increasing vascular permeability. Int J Cancer. 1993;53(5):829–836. doi: 10.1002/ijc.2910530521. [DOI] [PubMed] [Google Scholar]
- 50.Khawli LA, Miller GK, Epstein AL. Effect of seven new vasoactive immunoconjugates on the enhancement of monoclonal antibody uptake in tumors. Cancer. 1994;73:824–831. doi: 10.1002/1097-0142(19940201)73:3+<824::AID-CNCR2820731312>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 51.Carnemolla B, Borsi L, Balza E, Castellani P, Meazza R, Berndt A, Ferrini S, Kosmehl H, Neri D, Zardi L. Enhancement of the antitumor properties of interleukin-2 by its targeted delivery to the tumor blood vessel extracellular matrix. Blood. 2002;99(5):1659–1665. doi: 10.1182/blood.V99.5.1659. [DOI] [PubMed] [Google Scholar]
- 52.Lejeune FJ, Lienard D, Matter M, Ruegg C. Efficiency of recombinant human TNF in human cancer therapy. Cancer Immun. 2006;6(1):6. [PubMed] [Google Scholar]
- 53.Juul-Madsen HR, Olsson L. Discrepancy between transcriptional products and cell surface expression of MHC class I antigens in metastatic and non-metastatic Lewis Lung tumor cells. APMIS. 1990;98(7-12):624–636. doi: 10.1111/j.1699-0463.1990.tb04980.x. [DOI] [PubMed] [Google Scholar]
- 54.Garrido G, Rabasa A, Garrido C, Chao L, Garrido F, Garcia-Lora AM, Sanchez-Ramirez B. Upregulation of HLA class I expression on tumor cells by the anti-EGFR antibody nimotuzumab. Front Pharmacol. 2017;8:595. doi: 10.3389/fphar.2017.00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Imboden M, Murphy KR, Rakhmilevich AL, Neal ZC, Xiang R, Reisfeld RA, Gillies SD, Sondel PM. The level of MHC class I expression on murine adenocarcinoma can change the antitumor effector mechanism of immunocytokine therapy. Can Res. 2001;61(4):1500. [PubMed] [Google Scholar]
- 56.Ohno Y, Toyoshima Y, Yurino H, Monma N, Xiang H, Sumida K, Kaneumi S, Terada S, Hashimoto S, Ikeo K, Homma S, Kawamura H, Takahashi N, Taketomi A, Kitamura H. Lack of interleukin-6 in the tumor microenvironment augments type-1 immunity and increases the efficacy of cancer immunotherapy. Cancer Sci. 2017;108(10):1959–1966. doi: 10.1111/cas.13330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Storey BT, Christian JF. Characterization of Lewis lung clonal variants in a model of syngeneic pulmonary murine metastases. Clin Exp Metastasis. 2004;21(3):265. doi: 10.1023/B:CLIN.0000037728.44457.13. [DOI] [PubMed] [Google Scholar]
- 58.Eisenbach L, Segal S, Feldman M. MHC imbalance and metastatic spread in Lewis lung carcinoma clones. Int J Cancer. 1983;32(1):113–120. doi: 10.1002/ijc.2910320118. [DOI] [PubMed] [Google Scholar]
- 59.Scrimieri F, Askew D, Corn DJ, Eid S, Bobanga ID, Bjelac JA, Tsao ML, Allen F, Othman YS, Wang SC, Huang AY. Murine leukemia virus envelope gp70 is a shared biomarker for the high-sensitivity quantification of murine tumor burden. Oncoimmunology. 2013;2(11):e26889. doi: 10.4161/onci.26889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gelber C, Eisenbach L, Feldman M, Goodenow RS. T-cell subset analysis of lewis lung carcinoma tumor rejection: heterogeneity of effectors and evidence for negative regulatory lymphocytes correlating with metastasis. Can Res. 1992;52(23):6507. [PubMed] [Google Scholar]
- 61.Van Pel A, Georlette M, Boon T. Tumor cell variants obtained by mutagenesis of a Lewis lung carcinoma cell line: immune rejection by syngeneic mice. Proc Natl Acad Sci USA. 1979;76(10):5282–5285. doi: 10.1073/pnas.76.10.5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tanaka M, Saijo Y, Sato G, Suzuki T, Tazawa R, Satoh K, Nukiwa T. Induction of antitumor immunity by combined immunogene therapy using IL-2 and IL-12 in low antigenic Lewis lung carcinoma. Cancer Gene Ther. 2000;7:1481. doi: 10.1038/sj.cgt.7700251. [DOI] [PubMed] [Google Scholar]
- 63.Ohe YP, Podack ER, Shirahige KO, Miyahara Y, Tamura Y, Miura T, Kubo K, Morikage S, Saijo T. Effect of IL-2, IL-6, and IL-8 transfection on the rejection of Lewis lung carcinoma. Lung Cancer. 1991;7:26. doi: 10.1016/0169-5002(91)91444-G. [DOI] [Google Scholar]
- 64.Ohe Y, Podack Eckhard R, Olsen Kristin J, Miyahara Y, Ohira T, Miura K, Nishio K, Saijo N. Combination effect of vaccination with IL2 and IL4 cdna transfected cells on the induction of a therapeutic immune response against lewis lung carcinoma cells. Int J Cancer. 1993;53(3):432–437. doi: 10.1002/ijc.2910530314. [DOI] [PubMed] [Google Scholar]
- 65.Ziffels B, Pretto F, Neri D. Intratumoral administration of IL2- and TNF-based fusion proteins cures cancer without establishing protective immunity. Immunotherapy. 2018;10(3):177–188. doi: 10.2217/imt-2017-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schliemann C, Wiedmer A, Pedretti M, Szczepanowski M, Klapper W, Neri D. Three clinical-stage tumor targeting antibodies reveal differential expression of oncofetal fibronectin and tenascin-C isoforms in human lymphoma. Leuk Res. 2009;33(12):1718–1722. doi: 10.1016/j.leukres.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 67.Galler K, Junker K, Franz M, Hentschel J, Richter P, Gajda M, Gohlert A, von Eggeling F, Heller R, Giavazzi R, Neri D, Kosmehl H, Wunderlich H, Berndt A. Differential vascular expression and regulation of oncofetal tenascin-C and fibronectin variants in renal cell carcinoma (RCC): implications for an individualized angiogenesis-related targeted drug delivery. Histochem Cell Biol. 2012;137(2):195–204. doi: 10.1007/s00418-011-0886-z. [DOI] [PubMed] [Google Scholar]
- 68.Gutbrodt KL, Schliemann C, Giovannoni L, Frey K, Pabst T, Klapper W, Berdel WE, Neri D. Antibody-based delivery of interleukin-2 to neovasculature has potent activity against acute myeloid leukemia. Sci Transl Med. 2013;5(201):201ra118. doi: 10.1126/scitranslmed.3006221. [DOI] [PubMed] [Google Scholar]
- 69.Frey K, Fiechter M, Schwager K, Belloni B, Barysch MJ, Neri D, Dummer R. Different patterns of fibronectin and tenascin-C splice variants expression in primary and metastatic melanoma lesions. Exp Dermatol. 2011;20(8):685–688. doi: 10.1111/j.1600-0625.2011.01314.x. [DOI] [PubMed] [Google Scholar]
- 70.Schwager K, Villa A, Rosli C, Neri D, Rosli-Khabas M, Moser G. A comparative immunofluorescence analysis of three clinical-stage antibodies in head and neck cancer. Head Neck Oncol. 2011;3(1):25. doi: 10.1186/1758-3284-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.