Abstract
Background
Children with primary hypertension have been reported to have diminished scores in measures of cognition. However, little is known about the relative correlation between office and ambulatory blood pressure (BP) and neurocognitive test performance, and whether short-term BP variability is associated with decreased neurocognitive function. We sought to determine whether ambulatory BP monitoring (ABPM) was more strongly associated with neurocognitive test performance compared with office BP, and whether increased short-term BP variability was associated with lower neurocognitive scores.
Methods
Seventy-five subjects ages 10 – 18 y, with untreated primary hypertension and 75 matched normotensive controls completed neurocognitive testing. All subjects had office BP and ABPM prior to neurocognitive testing.
Results
On multivariate analyses, there was no significant association between office BP and neurocognitive tests. However, several ABPM parameters were significantly associated with neurocognitive test scores in the lower quartile, in particular 24hr SBP load and wake SBP index [Rey Auditory Verbal learning Test (RAVLT) List A Trial 1, 24hr SBP load, odds ratio (OR) = 1.02, wake SBP index, OR = 1.06; List A Total, 24hr SBP load, OR = 1.02, wake SBP index, OR = 1.06; Short delay recall, wake SBP index, OR = 1.06; CogState Maze delayed recall, 24hr SBP load, OR = 1.03, wake SBP index, OR = 1.08; Grooved Pegboard, 24hr SBP load, OR = 1.02; all p<0.05]. In contrast, short-term BP variability measures were not associated with neurocognitive test performance.
Conclusion
ABPM is superior to office BP in distinguishing hypertensive youth with lower neurocognitive test performance.
Keywords: neuropsychological testing, hypertension, obesity, pediatric, adolescence
Introduction
Children and adolescents with primary hypertension (HTN) can develop subclinical target organ damage (TOD), including left ventricular hypertrophy (LVH), increased carotid intima-media thickness (cIMT), and increased arterial stiffness, similar to adults.[1-4] In addition, recent studies also suggest that hypertensive children have diminished scores on measures of cognition, a potential early manifestation of hypertensive TOD to the brain.[5-7]
As in adults, ambulatory blood pressure (BP) measurements are more accurate than office BP for the diagnosis of HTN in children and adolescents,[8, 9] and correlate more strongly with cardiovascular hypertensive TOD.[1, 9-12] Furthermore, increases in short-term and long-term BP variability have been associated with TOD, including decreased cognitive function, in hypertensive adults.[13-16] However, little is known about the relative correlation between ambulatory and office BP and cognitive test performance, and about the relation between short-term BP variability and cognitive function in children with primary HTN.
With funding from the National Institutes of Health, we have established a prospective, multicenter study of neurocognition in children with primary HTN.[17] The specific aims of this multicenter study were to compare the performance on neurocognitive testing of newly diagnosed subjects with untreated HTN with that of the performance of matched normotensive controls at baseline, and to evaluate the effect of 1-year of antihypertensive therapy on neurocognitive test performance. We recently reported results of the baseline comparison, showing that children with HTN had worse performance on neurocognitive testing compared with that of the normotensive control subjects, particularly in the domains of attention, learning, and memory.
The objectives of the current analysis were: (1) to determine the strength of the relationship of ambulatory BP parameters and office BP with neurocognitive test performance, and (2) to determine the relation between ambulatory BP variability and neurocognitive test performance in both groups. We hypothesized that ambulatory BP would be more strongly associated with neurocognitive test performance compared with office BP, and that increased short-term BP variability on 24-hr BP monitoring would be associated with lower neurocognitive test performance.
METHODS
The details of the overall study design and methods have been previously reported.[7, 17] We enrolled 75 newly diagnosed untreated children with primary HTN and 75 frequency matched normotensive control subjects aged 10 - 18 years through the Pediatric Hypertension Clinics at each site. Participating recruitment sites included the University of Rochester, Emory University, Maimonides Medical Center, and the McGovern Medical School at UTHealth. All hypertensive subjects were required to have office BP ≥95th percentile and sustained HTN confirmed by 24-hr ambulatory BP monitoring (ABPM). Control subjects were required to have office BP < 95th percentile and normotension confirmed by 24-hr ABPM. The control group was frequency matched to the HTN group for maternal education, sex, and proportion with obesity (body mass index [BMI] ≥95th percentile). Exclusion criteria were stringent, particularly with respect to conditions that could influence the neurocognitive testing, and included: medication for Attention-Deficit/Hyperactivity Disorder, the presence of a pre-existing learning problem/disability, any disorder of cognitive impairment, history of chelation treatment for elevated lead level, history of chronic disease, pregnancy or breast feeding, previous sleep study diagnosis of obstructive sleep apnea, a diagnosis of secondary HTN, and previous or current treatment with antihypertensive medication.
At the baseline neurocognitive-testing visit, each subject’s office BP was measured by automated oscillometric device. Mean office BP for this analysis was the average of 3 readings taken 5 minutes apart, at that study visit. In addition, all subjects underwent 24-hr ABPM at baseline according to the American Heart Association guidelines utilizing the Spacelabs 90217 oscillometric monitor.[18] BP measurements were recorded every 20 minutes for the entire 24-hr period, and wake and sleep periods were determined by patient diary. BP load was defined as the percentage of readings above the 95th percentile for ambulatory norms in the 24-hr period. Subjects with HTN were required to have sustained ambulatory HTN, defined as mean wake or sleep systolic BP or diastolic BP ≥95th percentile for ambulatory norms. A subject with HTN could also be included if the mean ambulatory BP was < 95th percentile, but the subject had both BP load >25% (ambulatory prehypertension) and LVH on echocardiogram. Control subjects were required to have mean wake and sleep systolic BP and diastolic BP <95th percentile and 24hr BP load <25% to be included. BP index was defined as the obtained BP reading divided by the 95th percentile for the specific BP parameter. Short-term BP variability was assessed as the weighted standard deviation (wSD) of the mean systolic and diastolic BP for the wake and sleep periods of the 24-hr ambulatory BP study. [19] Both groups underwent the same neurocognitive test battery at baseline as described previously, and parents completed the Sleep-Related Breathing Disorder Scale of the Pediatric Sleep Questionnaire (PSQ-SRBD).
We previously reported that hypertensive subjects were best distinguished from normotensive control subjects by the subset of 6 neurocognitive tests shown in Table 1. For the current analysis, we examined the association of baseline office BP and ambulatory BP with performance on these 6 neurocognitive measures. For these analyses, the normotension and hypertension subject BP data were combined in order to examine the relation of BP to neurocognitive test performance across a broad range of BP values.
Table 1.
Neurocognitive Measures
| Cognitive Subtest | Domain |
|---|---|
| Rey Auditory Verbal Learning Test (RAVLT) List A Trial 1[32] | Attention |
| Rey Auditory Verbal Learning Test (RAVLT) List A Total | Learning, Verbal Memory |
| Rey Auditory Verbal Learning Test (RAVLT) Short delay recall | Learning, Verbal Memory |
| CogState Maze Delayed Recall[33] | Visual Memory |
| Wechsler Abbreviated Scale of Intelligence (WASI) Vocabulary[34] | Vocabulary |
| Grooved Pegboard, dominant hand[32] | Manual Speed and Dexterity |
Statistical Analyses
Office BP and ambulatory BP measures were summarized using mean ± standard deviation (SD), or median and interquartile range where appropriate. Two sample t test was used to compare baseline BP measures between HTN and control patients. Pearson correlation coefficients were calculated to assess the linear relationship between BP measure and neurocognitive test performance. To address the two research questions (to determine the strength of the relationship of ambulatory BP parameters and office BP with neurocognitive test performance; to determine the relation between ambulatory BP variability and neurocognitive test performance in both groups), multiple logistic regression analyses were carried out to evaluate the independent association between ambulatory BP parameters or office BP and poor cognitive test performance (lower quartile or worse), after adjustment for age, sex, maternal education, race, ethnicity, PSQ score, glucose and triglycerides. To maximize the unique information provided by each neurocognitive subtest, separate models were examined for each test as a dependent measure. Similarly, to maximize the unique information provided by each BP parameter, separate models were examined for each BP parameter as an independent variable. P<0.05 was considered statistically significant. All analyses were performed with SAS (version 9.4, SAS Institute Inc; Cary, North Carolina).
RESULTS
Sample Description
Complete blood pressure and neurocognitive testing data were available for 150 subjects (75 HTN and 75 control subjects). Only 2 subjects with prehypertension were included with the alternate criteria of mean ambulatory BP < 95th percentile but BP load > 25% and LVH on echocardiogram. Because the number of subjects with hypertension included under the alternate criteria was so small, all 75 hypertension subjects were combined for all analyses. As previously described, the HTN and control groups were similar in age, sex, race, ethnicity, percent with obesity, BMI Z-score, maternal education, household income, total, LDL and HDL cholesterol, insulin level, homeostatic model assessment for insulin resistance, glucose and CRP.[7] Groups differed in Pediatric Sleep Questionnaire scores and triglyceride levels. The range of BP across groups was similar for both office and ambulatory BP (Control vs HTN; office SBP index, 0.85 ± 0.12 vs 1.0 ± 0.08, P < 0.001; mean wake SBP index, 0.87 ± 0.05 vs 1.02 ± 0.05, p < 0.001).
Relationship of Ambulatory BP and Office BP with Neurocognitive Performance
Table 2 shows the results of individual multiple logistic regression analyses for association between increased office BP and poor performance on the six individual neurocognitive subtests (lower quartile or worse), after adjusting for patient demographics, socioeconomic status (SES), obesity, disordered sleep, and other potential confounders. Office BP was not independently associated with low performance on any of the neurocognitive measures. By contrast, several ABPM parameters were significantly associated with worse cognitive test performance, with the strongest associations for day systolic BP index and 24hr systolic BP load. Figure 1 shows a representative example of the relation between mean BP and neurocognitive test scores for both office and ambulatory mean SBP index, using performance on the RAVLT short delay recall, a measure of verbal memory, in the example. Table 3 reports the adjusted Odds Ratio estimates with 95% confidence intervals for predicting poor performance (lower quartile or worse) on the six individual neurocognitive subtests when ambulatory measures increase.
Table 2.
Odds ratios with 95% confidence intervals for the association between office blood pressure (BP) index and neurocognitive test performance in the worst quartile
| Office BP Parameter (N = 150) | RAVLT List A Trial 1 | RAVLT List A Total | RAVLT Short Delay Recall | WASI Vocabulary | CogState Maze Delayed Recall error | Grooved Pegboard, dominant hand |
|---|---|---|---|---|---|---|
| Office SBP index | 1.04 (0.99, 1.08) |
1.02 (0.98, 1.06) |
0.99 (0.95, 1.02) |
1.03 (0.97, 1.08) |
1.03 (0.99, 1.07) |
1.02 (0.97, 1.07) |
| Office DBP index | 1.03 (1, 1.06) |
1.02 (0.99, 1.05) |
0.98 (0.95, 1.01) |
0.99 (0.95, 1.03) |
1.01 (0.98, 1.04) |
1.02 (0.981, 1.0) |
All data shown are adjusted for age, sex, maternal education, race, ethnicity, PSQ score, glucose and triglycerides; *p < 0.05 SBP systolic blood pressure, DBP diastolic blood pressure
Figure 1.
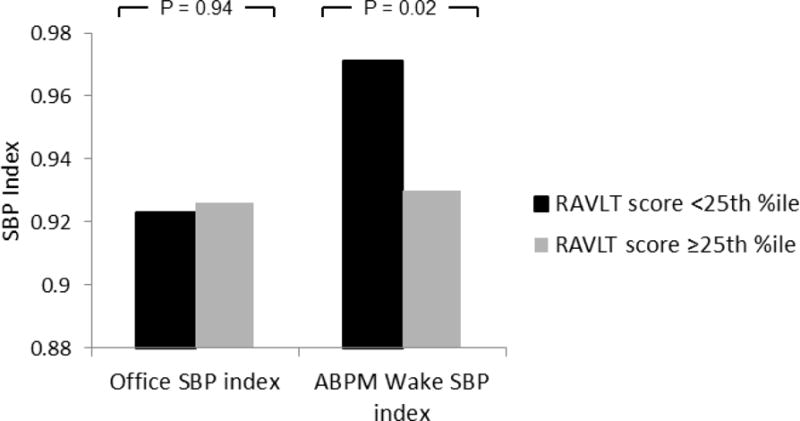
The relation between scores on the Rey Auditory Verbal learning Test (RAVLT) short delay recall subtest mean systolic blood pressure (SBP) index on office and ambulatory blood pressure monitoring (ABPM) measures. Subjects who scored in the lower quartile on the short delay recall test are shown in black and subjects who scored higher are shown in grey. There was no difference in office SBP index between subjects who scored in the lower quartile and those who scored better. By contrast, subjects with worse performance on the short delay recall subtest had significantly higher ambulatory mean wake SBP index compared with that of subjects who scored better.
Table 3.
Odds ratios with 95% confidence intervals for the association between ambulatory blood pressure (BP) parameters and neurocognitive test performance in the worst quartile
| ABPM Parameter (N = 150) | RAVLT List A Trial 1 | RAVLT List A Total | RAVLT Short Delay Recall | WASI Vocabulary | CogState Maze Delayed Recall error | Grooved Pegboard, dominant hand |
|---|---|---|---|---|---|---|
| 24hr SBP load | 1.02* (1.00, 1.03) |
1.02* (1.00, 1.03) |
1.01 (0.99, 1.02) |
1.01 (0.99, 1.03) |
1.03** (1.01, 1.04) |
1.02* (1.00, 1.03) |
| 24hr DBP load | 1.02 (0.99, 1.04) |
1.03* (1.00, 1.06) |
1.01 (0.98, 1.04) |
0.99 (0.96, 1.02) |
1.03* (1.00, 1.06) |
1.02 (0.99, 1.04) |
| Wake SBP Index | 1.06* (1.00, 1.11) |
1.07** (1.02, 1.13) |
1.06* (1.01, 1.11) |
1.02 (0.96, 1.08) |
1.08** (1.03, 1.14) |
1.04 (0.99, 1.10) |
| Wake DBP Index | 1.04 (0.99, 1.09) |
1.06* (1.01, 1.11) |
1.04 (0.99, 1.09) |
1.03 (0.97, 1.09) |
1.04 (0.99, 1.10) |
1.04 (0.99, 1.1) |
| Sleep SBP Index | 1.03* (0.99, 1.07) |
1.07** (1.02, 1.12) |
0.99 (0.97, 1.03) |
1.01 (0.96, 1.06) |
1.01 (0.97, 1.04) |
1.03 (0.99, 1.07) |
| Sleep DBP Index | 1.06* (1.01, 1.11) |
1.06* (1.01, 1.11) |
0.98 (0.95, 1.01) |
0.98 (0.94, 1.03) |
1.00 (0.97, 1.03) |
1.03 (0.99, 1.07) |
All data shown are adjusted for age, sex, maternal education, race, ethnicity, PSQ score, glucose and triglycerides;
p < 0.05,
p < 0.01
RAVLT Rey Auditory Verbal Learning Test, ABPM ambulatory blood pressure monitoring, SBP systolic blood pressure, DBP diastolic blood pressure, WASI Wechsler Abbreviated Scale of Intelligence, RAVLT Rey Auditory Verbal Learning Test
Relationship of Ambulatory BP Variability and Neurocognitive Performance
The HTN group had greater systolic and diastolic BP variability as measured by wSD (HTN vs control; systolic wSD, 11.8 ± 2.4 vs 10.3 ± 1.7 mmHg, p < 0.001; diastolic wSD, 10.2 ±.2.0 vs 9.2 ± 1.6 mmHg, p = 0.002). Table 4 shows the results of individual multiple logistic regression analyses for the association between increased wSD and neurocognitive test performance in the lower quartile for the six neurocognitive subtests. In contrast to studies on BP variability in adults,[15] increased BP variability on the 24-hr ABPM study in our pediatric subjects was not independently associated with lower neurocognitive test scores.
Table 4.
Odds ratios with 95% confidence intervals for the association between wSD and neurocognitive test performance in the worst quartile
| wSD Parameter (N = 150) | RAVLT List A Trial 1 | RAVLT List A Total | RAVLT Short Delay Recall | WASI Vocabulary | CogState Maze Delayed Recall error | Grooved Pegboard, dominant hand |
|---|---|---|---|---|---|---|
| SBP wSD | 1.05 (0.84, 1.31) | 0.99 (0.80, 1.22) | 1.04 (0.84, 1.29) | 0.94 (0.73, 1.20) | 1.04 (0.84, 1.29) | 1.20 (0.96, 1.50) |
| DBP wSD | 1.07 (0.84, 1.37) | 0.95 (0.74, 1.21) | 1.02 (0.80, 1.30) | 0.99 (0.96, 1.03) | 0.85 (0.63, 1.16) | 1.24 (0.96, 1.59) |
All data shown are adjusted for age, sex, maternal education, race, ethnicity, PSQ score, glucose and triglycerides; *p < 0.05 wSD weighted standard deviation, SBP systolic blood pressure, DBP diastolic blood pressure
DISCUSSION
Our group has recently reported that children with newly diagnosed primary HTN had decreased performance on neurocognitive testing compared with that of the normotensive control subjects, particularly in the domains of attention, learning, and memory, even adjusting for multiple variables such as age and socioeconomic status. To our knowledge this is the first report to compare the strength of the association of ambulatory and office BP with neurocognitive function. Similar to findings with cardiovascular markers of target organ damage, we found that ABPM is superior to office BP in distinguishing youth with decreased neurocognitive test performance. Ambulatory mean BP level and BP load were significantly associated with cognitive test scores; more specifically, ambulatory BP was independently associated with decreased performance on tests of memory and learning (Rey Auditory Verbal Learning Test, Cogstate Maze Delayed Recall Error Test), and fine-motor speed and dexterity (Grooved Pegboard, Dominant Hand). In contrast, office BP was not associated with low performance on any of the neurocognitive measures.
In this study, we also assessed the effects of short-term BP variability on 24-hr ABPM in relation to neurocognitive performance in both the HTN and control groups. Although the HTN group showed greater short-term BP variability compared with the control group, both during daytime and nighttime, none of the ambulatory BP variability measures were significantly associated with cognitive test scores.
Ambulatory BP measurements are more accurate than office BP for the diagnosis of HTN [8, 9] and correlate more strongly with cardiovascular hypertensive TOD in both children and adults.[1, 9-12] Similar to the subclinical effects of HTN on the heart (i.e., LVH) and vessels (i.e., increased cIMT, arterial stiffness), subclinical TOD may occur in the brain and affect cognition.[20-23] In adults, nocturnal BP abnormalities have been associated with cognitive dysfunction.[20, 21] In a sub-study of the Coronary Artery Risk Development in Young Adults (CARDIA) Study, an abnormal nocturnal BP pattern (less systolic BP dipping and higher diastolic BP) was associated with lower executive function in midlife, independent of multiple measures of office BP during long-term follow up.[20] Similarly, in a large cohort of children with mild-to-moderate chronic kidney disease (CKD), increased diastolic load and decreased diastolic nocturnal dipping were independently associated with lower neurocognitive test performance when compared to matched controls.[23]
Increased short and long-term BP variability has been associated with TOD and lower cognitive function in hypertensive adults.[13, 15, 24-28] Kanemaru et al. showed that increased short-term variability of daytime BP and high nighttime systolic BP were associated with cognitive impairment as assessed by the Raven’s Colored Progressive Matrices Test, a test that assesses nonverbal problem solving.[24] Similarly, using data from the CARDIA Study, Yano et al. demonstrated that long-term BP variability (for over 25 years) was associated with worse psychomotor speed and verbal memory tests in midlife, independent of cumulative exposure to BP during follow-up.[15] Similarly, in children with mild-to-moderate CKD, higher long-term BP variability, specifically higher systolic visit-to-visit BP variability, was independently associated with decreased D-KEFS Category switching scores.[22] However, in our study of hypertensive children without CKD, increased BP short-term variability was not independently associated with worse neurocognitive test scores. These results suggest heightened BP variability may not impact cognition until later in the lifespan. It is known that BP variability increases with age and is most prominent in the elderly.[29] It is possible that the magnitude of BP variability is not severe enough or that increased BP variability has not been present for long enough in youth with primary hypertension to effect neurocognitive test performance.
The current study has several limitations. The sample size was relatively small which may have limited our ability to detect more modest associations with office BP and BP variability with neurocognitive testing results. We did not standardize the office automated oscillometric device across participating study centers. In addition, BP was obtained with an oscillometric monitor on the day of the neurocognitive testing. It is possible that, as oscillometric measurements overestimate BP,[30] the differences between office and ambulatory BP and cognition may have been even greater had we obtained manual, auscultatory BP at that visit. Also, we did not evaluate longer term, visit-to-visit BP variability. Lastly, we did not have information on low birth weight or prematurity, factors that can influence BP level.[31]
In conclusion, we showed that 24-hr ABPM (mean BP and BP load, but not BP variability) is more likely than office BP to identify youth with diminished neurocognitive test performance. These findings need to be confirmed with studies that include a larger sample size and a greater range of hypertension severity. Our findings underscore the importance of 24-hr ABPM in the assessment of children with elevated BP for assessment of TOD. Future studies directed at the amelioration of the potential cognitive effects of hypertension in youth should include 24-hr ABPM for assessment of severity of BP elevation and evaluation of hypertension control.
Acknowledgments
This work was funded by a grant from the National Heart, Lung, and Blood Institute (R01HL098332, MBL).
We thank the study coordinators at each participating center for all their work in keeping this study going, and the study participants and their parents.
Footnotes
Compliance with ethical standards
The Institutional Review Board of each site approved the study. Parental permission was obtained, as well as subject assent.
Conflicts of interest: none. Payments to write the manuscript: none
References
- 1.McNiece KL, Gupta-Malhotra M, Samuels J, Bell C, Garcia K, Poffenbarger T, Sorof JM, Portman RJ, National High Blood Pressure Education Program Working G Left ventricular hypertrophy in hypertensive adolescents: analysis of risk by 2004 National High Blood Pressure Education Program Working Group staging criteria. Hypertension. 2007;50:392–395. doi: 10.1161/HYPERTENSIONAHA.107.092197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kupferman JC, Paterno K, Mahgerefteh J, Pagala M, Golden M, Lytrivi ID, Ramaswamy P. Improvement of left ventricular mass with antihypertensive therapy in children with hypertension. Pediatr Nephrol. 2010;25:1513–1518. doi: 10.1007/s00467-010-1511-4. [DOI] [PubMed] [Google Scholar]
- 3.Lande MB, Carson NL, Roy J, Meagher CC. Effects of childhood primary hypertension on carotid intima media thickness: a matched controlled study. Hypertension. 2006;48:40–44. doi: 10.1161/01.HYP.0000227029.10536.e8. [DOI] [PubMed] [Google Scholar]
- 4.Stabouli S, Papakatsika S, Kotronis G, Papadopoulou-Legbelou K, Rizos Z, Kotsis V. Arterial stiffness and SBP variability in children and adolescents. J Hypertens. 2015;33:88–95. doi: 10.1097/HJH.0000000000000369. [DOI] [PubMed] [Google Scholar]
- 5.Lande MB, Kaczorowski JM, Auinger P, Schwartz GJ, Weitzman M. Elevated blood pressure and decreased cognitive function among school-age children and adolescents in the United States. J Pediatr. 2003;143:720–724. doi: 10.1067/S0022-3476(03)00412-8. [DOI] [PubMed] [Google Scholar]
- 6.Kupferman JC, Lande MB, Adams HR, Pavlakis SG. Primary hypertension and neurocognitive and executive functioning in school-age children. Pediatr Nephrol. 2013;28:401–408. doi: 10.1007/s00467-012-2215-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lande MB, Batisky DL, Kupferman JC, Samuels J, Hooper SR, Falkner B, Waldstein SR, Szilagyi PG, Wang H, Staskiewicz J, Adams HR. Neurocognitive Function in Children with Primary Hypertension. J Pediatr. 2017;180:148–155. e141. doi: 10.1016/j.jpeds.2016.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stergiou GS, Alamara CV, Salgami EV, Vaindirlis IN, Dacou-Voutetakis C, Mountokalakis TD. Reproducibility of home and ambulatory blood pressure in children and adolescents. Blood Press Monit. 2005;10:143–147. doi: 10.1097/00126097-200506000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Macumber I. Ambulatory Blood Pressure Monitoring in Children and Adolescents: a Review of Recent Literature and New Guidelines. Curr Hypertens Rep. 2017;19:96. doi: 10.1007/s11906-017-0791-5. [DOI] [PubMed] [Google Scholar]
- 10.Richey PA, Disessa TG, Hastings MC, Somes GW, Alpert BS, Jones DP. Ambulatory blood pressure and increased left ventricular mass in children at risk for hypertension. J Pediatr. 2008;152:343–348. doi: 10.1016/j.jpeds.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kollias A, Dafni M, Poulidakis E, Ntineri A, Stergiou GS. Out-of-office blood pressure and target organ damage in children and adolescents: a systematic review and meta-analysis. J Hypertens. 2014;32:2315–2331. doi: 10.1097/HJH.0000000000000384. discussion 2331. [DOI] [PubMed] [Google Scholar]
- 12.Mancia G, Parati G. Ambulatory blood pressure monitoring and organ damage. Hypertension. 2000;36:894–900. doi: 10.1161/01.hyp.36.5.894. [DOI] [PubMed] [Google Scholar]
- 13.Parati G, Ochoa JE, Bilo G. Blood pressure variability, cardiovascular risk, and risk for renal disease progression. Curr Hypertens Rep. 2012;14:421–431. doi: 10.1007/s11906-012-0290-7. [DOI] [PubMed] [Google Scholar]
- 14.Parati G, Schumacher H. Blood pressure variability over 24 h: prognostic implications and treatment perspectives. An assessment using the smoothness index with telmisartan-amlodipine monotherapy and combination. Hypertens Res. 2014;37:187–193. doi: 10.1038/hr.2013.145. [DOI] [PubMed] [Google Scholar]
- 15.Yano Y, Ning H, Allen N, Reis JP, Launer LJ, Liu K, Yaffe K, Greenland P, Lloyd-Jones DM. Long-term blood pressure variability throughout young adulthood and cognitive function in midlife: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Hypertension. 2014;64:983–988. doi: 10.1161/HYPERTENSIONAHA.114.03978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dore GA, Elias MF, Crichton GE, Robbins MA. Age modifies the relation between intraindividual measurement-to-measurement variation in blood pressure and cognitive function: the Maine-Syracuse Study. J Hypertens. 2017 doi: 10.1097/HJH.0000000000001510. [DOI] [PubMed] [Google Scholar]
- 17.Lande MB, Adams HR, Kupferman JC, Hooper SR, Szilagyi PG, Batisky DL. A multicenter study of neurocognition in children with hypertension: methods, challenges, and solutions. J Am Soc Hypertens. 2013;7:353–362. doi: 10.1016/j.jash.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flynn JT, Daniels SR, Hayman LL, Maahs DM, McCrindle BW, Mitsnefes M, Zachariah JP, Urbina EM, American Heart Association Atherosclerosis H, Obesity in Youth Committee of the Council on Cardiovascular Disease in the Y Update: ambulatory blood pressure monitoring in children and adolescents: a scientific statement from the American Heart Association. Hypertension. 2014;63:1116–1135. doi: 10.1161/HYP.0000000000000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bilo G, Giglio A, Styczkiewicz K, Caldara G, Maronati A, Kawecka-Jaszcz K, Mancia G, Parati G. A new method for assessing 24-h blood pressure variability after excluding the contribution of nocturnal blood pressure fall. J Hypertens. 2007;25:2058–2066. doi: 10.1097/HJH.0b013e32829c6a60. [DOI] [PubMed] [Google Scholar]
- 20.Yano Y, Ning H, Muntner P, Reis JP, Calhoun DA, Viera AJ, Levine DA, Jacobs DR, Jr, Shimbo D, Liu K, Greenland P, Lloyd-Jones D. Nocturnal Blood Pressure in Young Adults and Cognitive Function in Midlife: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Hypertens. 2015;28:1240–1247. doi: 10.1093/ajh/hpv028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riba-Llena I, Nafria C, Filomena J, Tovar JL, Vinyoles E, Mundet X, Jarca CI, Vilar-Bergua A, Montaner J, Delgado P. High daytime and nighttime ambulatory pulse pressure predict poor cognitive function and mild cognitive impairment in hypertensive individuals. J Cereb Blood Flow Metab. 2016;36:253–263. doi: 10.1038/jcbfm.2015.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lande MB, Mendley SR, Matheson MB, Shinnar S, Gerson AC, Samuels JA, Warady BA, Furth SL, Hooper SR. Association of blood pressure variability and neurocognition in children with chronic kidney disease. Pediatr Nephrol. 2016;31:2137–2144. doi: 10.1007/s00467-016-3425-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruebner RL, Laney N, Kim JY, Hartung EA, Hooper SR, Radcliffe J, Furth SL. Neurocognitive Dysfunction in Children, Adolescents, and Young Adults With CKD. Am J Kidney Dis. 2016;67:567–575. doi: 10.1053/j.ajkd.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 24.Kanemaru A, Kanemaru K, Kuwajima I. The effects of short-term blood pressure variability and nighttime blood pressure levels on cognitive function. Hypertens Res. 2001;24:19–24. doi: 10.1291/hypres.24.19. [DOI] [PubMed] [Google Scholar]
- 25.Yamaguchi Y, Wada M, Sato H, Nagasawa H, Koyama S, Takahashi Y, Kawanami T, Kato T. Impact of ambulatory blood pressure variability on cerebral small vessel disease progression and cognitive decline in community-based elderly Japanese. Am J Hypertens. 2014;27:1257–1267. doi: 10.1093/ajh/hpu045. [DOI] [PubMed] [Google Scholar]
- 26.Stevens SL, Wood S, Koshiaris C, Law K, Glasziou P, Stevens RJ, McManus RJ. Blood pressure variability and cardiovascular disease: systematic review and meta-analysis. BMJ. 2016;354:i4098. doi: 10.1136/bmj.i4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu D, Li C, Chen Y, Xiong H, Tian X, Wu W, Huang W, Zhang YT, Zhang H. Influence of blood pressure variability on early carotid atherosclerosis in hypertension with and without diabetes. Medicine (Baltimore) 2016;95:e3864. doi: 10.1097/MD.0000000000003864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDonald C, Pearce MS, Kerr SR, Newton JL. Blood pressure variability and cognitive decline in older people: a 5-year longitudinal study. J Hypertens. 2017;35:140–147. doi: 10.1097/HJH.0000000000001120. [DOI] [PubMed] [Google Scholar]
- 29.Imai Y, Aihara A, Ohkubo T, Nagai K, Tsuji I, Minami N, Satoh H, Hisamichi S. Factors that affect blood pressure variability. A community-based study in Ohasama, Japan. Am J Hypertens. 1997;10:1281–1289. doi: 10.1016/s0895-7061(97)00277-x. [DOI] [PubMed] [Google Scholar]
- 30.Flynn JT, Pierce CB, Miller ER, 3rd, Charleston J, Samuels JA, Kupferman J, Furth SL, Warady BA, Chronic Kidney Disease in Children Study G Reliability of resting blood pressure measurement and classification using an oscillometric device in children with chronic kidney disease. J Pediatr. 2012;160:434–440. e431. doi: 10.1016/j.jpeds.2011.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hovi P, Vohr B, Ment LR, Doyle LW, McGarvey L, Morrison KM, Evensen KA, van der Pal S, Grunau RE, Collaboration AABPI. Brubakk AM, Andersson S, Saigal S, Kajantie E. Blood Pressure in Young Adults Born at Very Low Birth Weight: Adults Born Preterm International Collaboration. Hypertension. 2016;68:880–887. doi: 10.1161/HYPERTENSIONAHA.116.08167. [DOI] [PubMed] [Google Scholar]
- 32.Strauss E, Sherman E, Spreen O. A compendium of neuropsychological tests: administration, norms, and commentary. Oxford University Press; New York: 2006. [Google Scholar]
- 33.Pietrzak RHMP, Mayes LC, Roman SA, Sosa JA, Snyder PJ. An examination of the construct validity and factor structure of the groton maze learning test, a new measure of spatial working memory, learning efficiency, and error monitoring. Arch Clin Neuropsychol. 2008;23:433–445. doi: 10.1016/j.acn.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Wechsler D. Wechsler intelligence scale for children. The Psychological Corporation; San Antonio, TX: 2004. [Google Scholar]


