Abstract
Objective
To determine whether family history of coronary heart disease (FH) definitions differ in their association with atherosclerotic cardiovascular disease (ASCVD) events.
Patients/Methods
Participants who provided FH data between July 17, 2000 to February 24, 2004 were identified. FH definitions were: any, premature, and a familial-risk-assessment (FRA) tool. Outcomes included coronary heart disease (CHD), stroke, peripheral artery disease, angina, and congestive heart failure. Multivariable-adjusted Cox models examined the association of FH definitions with events. C-statistics and net reclassification improvement (NRI) examined the incremental prognostic contribution of each definition.
Results
In 6,200 participants, the proportion of any-FH and premature-FH were 36% and 16%, respectively. Using the FRA, the proportions for weak, moderate, and strong-familial-risk were 20%, 16%, and 20%, respectively. Over 10.1-years median follow-up (range 0.02–11.5 years), 741 participants suffered a composite event. Compared to no-FH, any-FH was associated with incident CHD, angina, and composite ASCVD [HR (95% CI)]: 1.4 (1.1–1.8), 1.6 (1.2–2.1), and 1.3 (1.1–1.5), respectively]. Similar results were obtained for premature-FH compared to no-FH, and for strong compared to weak-FRA for these three outcomes. There was no association between the FH definitions and non-coronary cardiovascular events. Compared to traditional risk-factors (C-statistic 0.740), any-FH, premature-FH and FRA all improved discrimination of composite ASCVD (all P<.01); however, the differences in C-statistic between any-FH (0.743), premature-FH (0.742), and FRA (0.744) were numerically small, as were differences in NRI.
Conclusions
A single question of the presence of FH in any first-degree relative performs just as well as more complicated assessments in predicting CHD.
Clinical Trials Registration Number
Keywords: prevention, ethnic, genetic, atherosclerosis, inflammation
INTRODUCTION
Primary prevention of atherosclerotic cardiovascular disease (ASCVD) is based on the accurate identification of adults who will benefit from lifestyle and pharmacologic interventions that are aimed at risk reduction. In this context, the collection of information regarding family history (FH) of coronary heart disease (CHD) may represent an inexpensive and evidence-based tool to improve the assessment of ASCVD risk and guide preventive therapies.1 Current guidelines recommend considering the presence of premature CHD when the decision to initiate pharmacological therapy remains uncertain after considering other risk factors.2 However, the collection and assessment of familial risk is often not performed in routine clinical practice and, when it is, providers differ in how much detail they obtain.3
While FH is an established risk factor for developing future ASCVD events, prior studies have differed widely in the applied definition of FH, have been racially homogenous, have not considered gender differences, or have exclusively evaluated CHD and/or stroke as the ASCVD endpoint.4–13 Possibly resulting in part from these limitations, minimal prognostic discrimination is typically seen with the addition of FH to models containing traditional risk factors.5–7,14 For this reason, information on FH is not included in standard ASCVD risk equations, such as Framingham risk score (FRS) or the Pooled Cohorts Equation (PCE).2,15
Given these considerations, further characterization of the association between various definitions FH and ASCVD among an ethnically diverse population could provide stronger evidence for the routine incorporation of FH assessment into primary prevention efforts. We also hypothesized that FH definitions may differ in their association with various ASCVD outcomes over extended follow-up out to 10-years.
METHODS
Study Population and Data Collection
The Multi-Ethnic Study of Atherosclerosis (MESA) recruited 6,814 participants between 2000 and 2002 across 6 field centers, with full details previously published.16 Participants were between 45 and 84 years of age, identified themselves as white, black, Hispanic, or Chinese American, and were free of clinical ASCVD at baseline. Data pertaining to FH was obtained at the baseline visit (July 17, 2000 to September 5, 2002) and visit 2 (September 9, 2002 to February 24, 2004). As such, only persons who attended both visits (n=6201) were included. Overall, the final analysis consisted of 6,200 participants, with one person excluded due to unavailable FH data. Institutional Review Boards at each site approved the study and all participants gave written informed consent.
At the baseline visit, demographic information, medical history, anthropometric measurements, and laboratory data were collected. Body mass index was calculated as weight in kilograms divided by height in meters squared. High-density lipoprotein-cholesterol (HDL-C) was measured using the cholesterol oxidase method. Low-density lipoprotein-cholesterol (LDL-C) was calculated using the Friedewald equation.17 Diabetes mellitus (DM) was defined as use of insulin or oral hypoglycemic medications, or a fasting glucose ≥126 mg/dL.18 Hypertension was defined as either a history of physician-diagnosed hypertension, taking a medication for hypertension, a systolic blood pressure (SBP) ≥140 mmHg, or a diastolic blood pressure (DBP) ≥90 mmHg.19 Study participants also self-reported personal habits, such as alcohol and current tobacco use (defined as having smoked a cigarette in the past 30 days and >100 cigarettes in a lifetime).
Family History Assessment
At baseline (visit 1), participants reported on the presence or absence of a FH of heart attack or stroke in any first-degree relative: mother, father, siblings, or child. Response options were “yes”, “no”, and “do not know”. At visit 2, participants were asked if any relative had CHD, stroke or cerebral hemorrhage, or DM; with response options the same as those at visit 1. If a participant reported a disease in a relative, the age at diagnosis was ascertained. For the purposes of this analysis, “do not know” responses were counted as “no” responses.
We defined “any-FH” as CHD occurring in a first-degree relative, irrespective of age, and “premature-FH” as having at least one relative with CHD occurring before the age of 55 years in males and 65 years in females, respectively. We also used the validated Familial Risk Assessment (FRA) tool to categorize FH risk as strong, moderate, or weak. The FRA is based on the number, sex, lineage, and age at onset of relatives with CHD, stroke, and DM (Table 1).20,21
Table 1.
Familial risk stratification rules considered the presence of coronary heart disease, stroke, and diabetes in first-degree relativesa
| Strong Familial Risk | Moderate Familial Risk | Weak Familial Risk |
|---|---|---|
|
|
|
Familial risk stratification rules considered the presence of coronary heart disease (CHD), stroke, and diabetes in first-degree relatives. Information regarding second-degree relatives was not collected, although this information may have contributed further to the risk stratification. Early-onset CHD was defined as occurring before the age of 65 years in women and before the age of 55 years in men. Early-onset stroke was defined as occurring before the age of 50 years in both women and men. CHD and stroke occurring before the age of 25 years were not considered, because we suspected that these were most likely cases of congenital or hereditary forms of CVD. CHD or stroke after the age of 85 years was also not considered. From the same lineage refers to maternal (mother and siblings or children), paternal (father and siblings or children), or nuclear (siblings or children) lineage.20
CHD = Coronary Heart Disease
Ascertainment of Incident ASCVD
A detailed description of the event adjudication process in MESA has been previously published.22 We analyzed 5 separate clinical end-points. First, hard CHD: defined as myocardial infarction, resuscitated cardiac arrest, or CHD death. Second, angina: defined as definite, probable, or absent. For definite or probable angina, participants required physician diagnosed typical or atypical symptoms of chest pain. Definite angina further required history of a CABG or revascularization procedure, ≥70% obstruction on angiography, or evidence of ischemia by stress testing or resting ECG.23,24 Third, stroke: defined as fatal or nonfatal due to hemorrhage or infarct. Fourth, peripheral artery disease (PAD): defined as an ABI value of <0.9 and did not require symptoms.25 Fifth, congestive heart failure (CHF): defined as having shortness of breath or peripheral edema, plus pulmonary edema by chest X-ray, and/or dilated ventricle or poor left ventricular (LV) function by echocardiography or ventriculography, or evidence of LV diastolic dysfunction.26
Statistical Analysis
Baseline characteristics of the study participants in each of the family history status groups (none, any, and premature) and for each of the familial risk assessment categories (weak, moderate, strong) were compared using ANOVA for continuous variables and chi square test for categorical variables, respectfully.
We used Cox proportional hazard regression models to study the association of FH status and outcomes. The proportionality assumption was tested and satisfied using graphical methods (log-log plots). Models were adjusted for age, sex, race, education, MESA site, body mass index (BMI), cigarette smoking, SBP, LDL-C, HDL-C, anti-hypertensive medication use, and lipid-lowering therapy. We also tested for multiplicative interaction between FH status and either sex or race/ethnicity in the association with ASCVD risk.
Area under the receiver operating characteristic curves (AUC) was used to assess discrimination of ASCVD events. The likelihood ratio test examined the improvement in discrimination when FH information was added to variables included in the 2013 PCE for ASCVD risk estimate (age, sex, race, SBP, treatment for hypertension, HDL-C, total cholesterol, diabetes mellitus, and smoking). Net reclassification improvement (NRI) was used to assess the incremental contribution of FH information for reclassification of ASCVD events when added to categories of the FRS (calibrated for individual CVD outcomes), plus race/ethnicity.27
In a sensitivity analysis, we assessed the incremental contribution of traditional risk factor elements of the FRS for ASCVD reclassification when added to baseline models containing the various FH definitions, plus race/ethnicity. Additionally, we calculated the same NRI analyses among only those categorized as intermediate risk by the FRS. Lastly, we used Cox proportional hazard regression models to study the association of the number of relatives with any-FH and premature-FH, and composite ASCVD events, respectively. All statistical analysis was performed using Stata version 13 (StataCorp). A 2-tailed P<.05 was considered statistically significant.
RESULTS
Our study population consisted of 6,200 individuals (mean age 62±10 years, 48% men, 40% White). Overall, 36% (n=2211) and 16% (n=983) MESA participants reported any-FH or premature-FH, respectively. The corresponding proportions for weak, moderate, and strong FHA were 20%, 16%, and 20%, respectively (Supplementary Table 1). Compared to no FH, those with any-FH had a higher percentage of individuals who were white, overweight, current smokers, and on blood pressure and lipid lowering therapy (Table 2). In the premature-FH group, a higher percentage of individuals were younger, female, black or Hispanic, overweight, current smokers, and on therapy for blood pressure and lipid management. When stratified by FRA, individuals with a strong FH had a higher percentage of individuals who were female, black, diabetic, and on therapy for blood pressure and lipid management (Supplementary Table 1).
Table 2.
| Total population (N= 6200) |
No FH (N= 3989) |
Any FH (N= 2211) |
Premature FH N= (983) |
p-valuec | p-valued | ||
|---|---|---|---|---|---|---|---|
| Age, years | 62 (10) | 62 (10) | 62(10) | 61(10) | .64 | .003e | |
| Males, % | 2954 (48) | 1984 (50) | 970 (44) | 406 (41) | <.001e | <.001e | |
| Ethnicity, % | <.001e | <.001e | |||||
| White | 2453 (40) | 1378 (35) | 1075 (49) | 433(44) | |||
| African American | 1673 (27) | 1092 (27) | 581 (26) | 305 (31) | |||
| Hispanic | 1347 (22) | 890 (22) | 457 (21) | 216 (22) | |||
| Chinese-American | 727 (12) | 629 (16) | 98 (4) | 29 (3) | |||
| BMI, kg/m2 | 28.2 (5.4) | 28.0 (5.3) | 28.9 (5.6) | 29.4 (5.7) | <.001e | <.001e | |
| LDL, mg/dL | 117 (31) | 116 (31) | 119 (31) | 118 (31) | .002e | .19 | |
| HDL, mg/dL | 51 (15) | 51 (15) | 51 (15) | 51 (15) | .10 | .57 | |
| SBP, mmHg | 126 (21) | 126 (21) | 127 (21) | 126(20) | .12 | .73 | |
| Diabetes mellitus, % | 741 (12) | 478 (12) | 263 (12) | 130 (13) | .93 | .19 | |
| Cigarette smoking, % | <.001e | <.001e | |||||
| Never | 3124 (51) | 2077 (52) | 1047 (47) | 459(47) | |||
| Former | 2283 (37) | 1417 (36) | 866 (39) | 367 (37) | |||
| Current | 776 (13) | 482 (12) | 294 (13) | 155(16) | |||
| Lipid-lowering therapy, % | 1008 (16) | 585 (15) | 423 (19) | 191 (19) | <.001e | .003e | |
| Antihypertensive therapy, % | 2277 (37) | 1397 (35) | 880 (40) | 395 (40) | <.001e | .02e | |
BMI = body mass index, FH = family history, HDL = high density lipoprotein cholesterol, LDL = low density lipoprotein cholesterol, SBP = systolic blood pressure.
Baseline characteristics expressed as means (standard deviation) for continuous variables and numbers, percentages for categorical variables. P-value for difference calculated using ANOVA test for continuous variables and chi-square test for categorical variables.
P-value comparing presence of any FH to no FH.
P-value comparing presence of premature FH to no FH.
Indicates statistically significant results.
After a median of 10.1 years follow up (range 0.02–11.5 years), a total of 741 composite ASCVD events occurred overall (not accounting for repeat events occurring after the first occurrence of any of the individual components of the composite in a given participant) (Supplementary Table 2). For individual outcomes, there were 250 CHD, 258 angina pectoris, 150 stroke, 75 PAD, and 206 CHF events, respectively. Among those with any-FH and premature-FH, a total of 317 and 142 composite events occurred, representing a cumulative incidence of 14% and 14%, respectively (Supplementary Table 2 for individual outcomes). In unadjusted analyses, point estimates for incident event rates (IR) (per 1000 person-years) for composite and individual outcomes were qualitatively similar between any-FH and premature-FH, respectively (Supplementary Table 2).”
Family History Status and ASCVD Risk
A) Adjusted HR based on Any Family History
Relative to persons without a FH, any-FH was significantly associated with composite ASCVD, hard CHD, and angina in all models, respectively (Table 3). No significant associations were found for stroke, PAD, or CHF (Supplementary Table 3). Associations between any-FH and ASCVD events were also calculated within each race/ethnicity category (HR [95% CI]): 1.27 (1.02–1.58) in Whites, 1.34 (0.99–1.81) in Blacks, 1.21 (0.88–1.68) in Hispanic, and 1.39 (0.61–3.14) in Chinese Americans). There was no significant interaction between any-FH and either ethnicity or sex in the association of composite ASCVD, CHD, and angina (data not shown).
Table 3.
Hazard ratios (95% confidence interval) for the association of family history definitions and cardiovascular eventsa
| CHD | Angina | Composite ASCVD | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Model 1b | Model 2c | Unadjusted | Model 1b | Model 2c | Unadjusted | Model 1b | Model 2c | |
| Family History Status | |||||||||
| No FH (ref) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Any | 1.37 (1.07,1.76)d | 1.39 (1.07,1.79)d | 1.37 (1.06,1.77)d | 1.71 (1.34,2.18)d | 1.76 (1.37,2.26)d | 1.60 (1.24,2.06)d | 1.37 (1.19,1.59)d | 1.34 (1.16,1.56)d | 1.28 (1.10,1.49)d |
| Premature | 1.26 (0.92,1.73) | 1.33 (0.97,1.83) | 1.33 (0.96,1.83) | 1.52 (1.13,2.04)d | 1.66 (1.23,2.22)d | 1.58 (1.17,2.14)d | 1.28 (1.06,1.53)d | 1.34 (1.10,1.61)d | 1.29 (1.07,1.55)d |
| Familial Risk | |||||||||
| Weak (ref) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Moderate | 1.38 (0.99,1.92) | 1.41 (1.01,1.97)d | 1.40 (1.00,1.96)d | 1.47 (1.06,2.04)d | 1.48 (1.06,2.06)d | 1.33 (0.94,1.87) | 1.27 (1.05,1.55)d | 1.24 (1.01,1.51)d | 1.20 (0.98,1.47) |
| Strong | 1.39 (1.03,1.88)d | 1.40 (1.03,1.90)d | 1.37 (1.00,1.87)d | 1.88 (1.42,2.49)d | 1.98 (1.49,2.64)d | 1.80 (1.35,2.40)d | 1.45 (1.22,1.72)d | 1.43 (1.20,1.70)d | 1.35 (1.13,1.61)d |
ASCVD = atherosclerotic cardiovascular disease, CHD = coronary heart disease, FH = family history
Model 1: Adjusted for age, sex, ethnicity, education, and MESA study site
Model 2: Adjusted for Model 1 covariates + body mass index, diabetes mellitus, cigarette smoking status, systolic blood pressure, LDL-C, HDL-C, lipid-lowering therapy, and anti-hypertensive medication use.
Indicates statistically significant results.
B) Adjusted HR based on Premature Family History
Among those who reported a premature-FH (compared to no FH), significant associations were seen with composite ASCVD and angina in all models, respectively (Table 3). No significant association was found between premature-FH and incident CHD. Similar to any-FH, no significant association was found with any of the non-coronary cardiovascular events (Supplementary Table 3). According to each ethnicity, associations between premature FH and total ASCVD events were as follows (HR [95% CI]): 1.31 (0.99–1.72) in Whites, 1.75 (1.26–2.45) in Blacks, 0.79 (0.51–1.23) in Hispanic, and 0.59 (0.08–4.36) in Chinese Americans. There was no significant interaction between premature-FH and either ethnicity or sex in the association of composite ASCVD, CHD, and angina (data not shown).
C) Adjusted HR based on Number of Relatives with a Family History
Among those who reported any-FH, similar significant associations were seen with composite ASCVD when one of or multiple relatives were affected (Supplementary Table 4). Among those who reported a premature-FH, the presence of multiple affected relatives predicted composite ASCVD events.
Familial Risk Assessment and ASCVD Risk
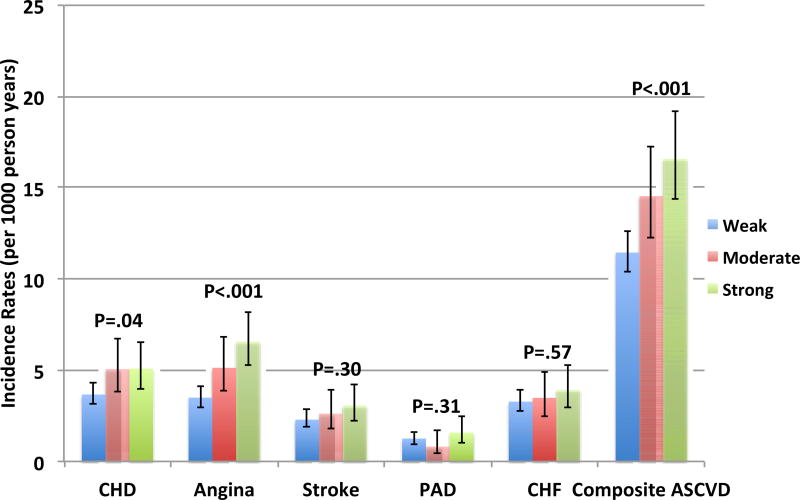
When participants were stratified by the FRA, a significant graded relationship was seen for crude IR of the composite ASCVD outcome, hard CHD, and angina (Figure 1). For example, the highest IR was in those with a strong FH (16.6 per 1000 person-years). Additionally, the highest IRs were found in outcomes for CHD and angina in those with a strong FH: 5.1 and 6.6 (per 1000 person-years), respectively.
Figure 1.
Crude incidence rates of cardiovascular outcomes by familial risk stratification*†
* ASCVD = atherosclerotic cardiovascular disease, CHD = Coronary Heart Disease, CHF = Congestive Heart Failure, PAD – Peripheral Artery Disease
† P-value refers to the comparison of incidence rates using non-parametric testing
Relative to a weak FH, a strong FH by FRA was associated with composite ASCVD, CHD, and angina in all models, respectively (Table 3). For moderate FRA, associations were seen in demographic adjusted models for composite ASCVD, CHD, and angina; however, this relationship persisted only for CHD after risk factor adjustment. Similar to FH status, none of the FRA categories were significantly associated with non-coronary cardiovascular events in all models. Furthermore, there was no significant interaction for any event between FRA and either ethnicity or sex (data not shown).
In a sensitivity analysis, we repeated the models reported above for each of the three FH definitions after stratification by whether the source of the FH was a sibling, parent, child, or spouse (the latter was only used in the FRA analyses). These demonstrated qualitatively similar findings, though having a child or parent with a FH (any and premature) appeared to indicate a higher-risk for composite ASCVD and angina (Supplementary Table 5).
Addition of FH Status and Familial Risk Assessment for the Discrimination of CHD and composite ASCVD Events
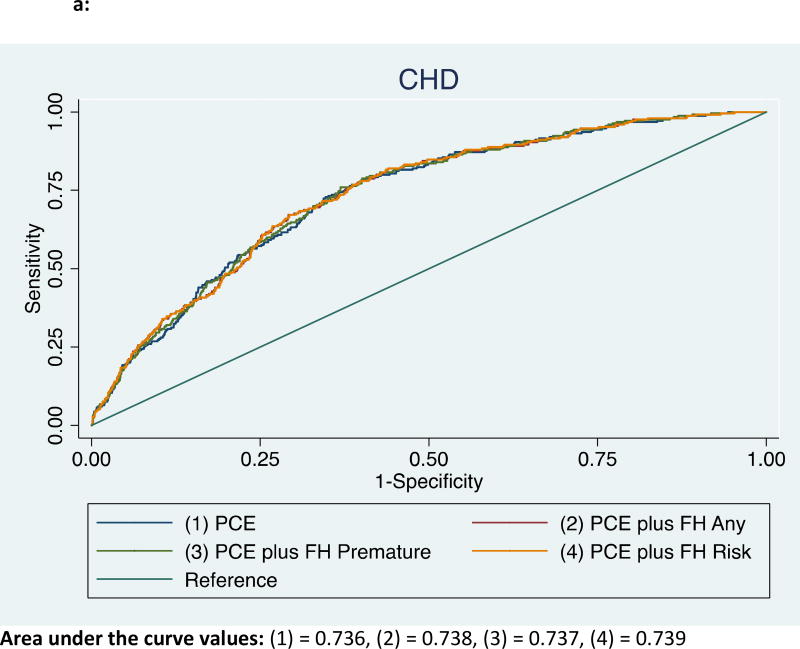
For CHD events, the addition of FH status to the base model comprising traditional risk factors led to an increase in the C-statistic from 0.736 to 0.738 (P=.02) for any-FH, and from 0.736 to 0.737 for premature-FH (P=.09) (Figure 2a). The FRA also improved the c-statistic from 0.736 to 0.739 (P=.05) when added to the base model.
Figure 2.
a: Receiver operator curves showing area under the curve for incident CHD among those with any-FH, premature-FH, and FRA*
*ASCVD – atherosclerotic cardiovascular disease, FH = family history of coronary heart disease, FRA – familial risk assessment, PCE – pooled cohorts equation
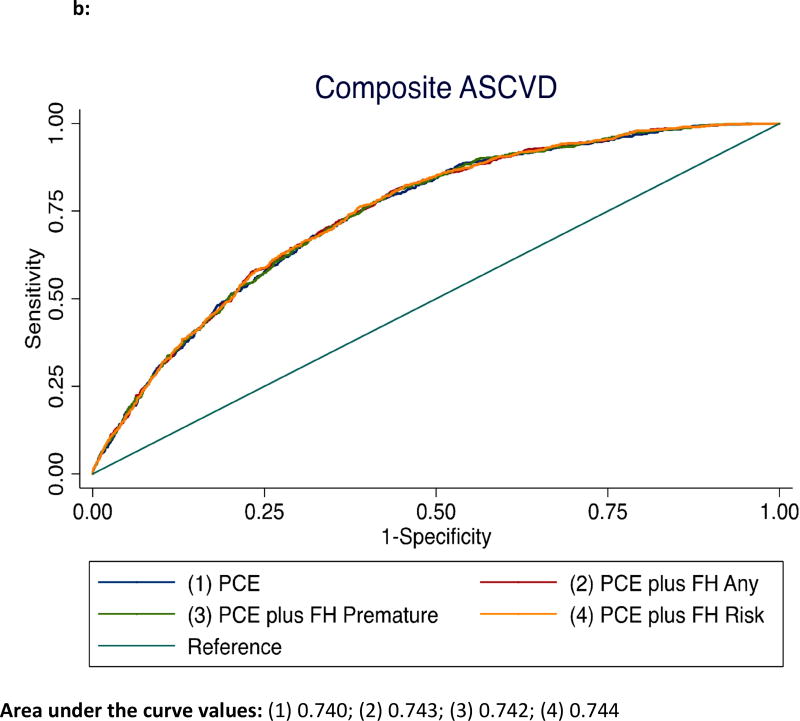
b: Receiver operator curves showing area under the curve for incident ASCVD among those with any-FH, premature-FH, and FRA*
*ASCVD – atherosclerotic cardiovascular disease, FH = family history of coronary heart disease, FRA – familial risk assessment, PCE – pooled cohorts equation.
For composite ASCVD events, the addition of FH status to the base model comprising traditional risk factors led to an increase in the C-statistic from 0.740 to 0.743 (P<.001) for any-FH, and from 0.740 to 0.742 for premature-FH (P<.05) (Figure 2b). The FRA also improved the c-statistic from 0.740 to 0.744 (P=.001) when added to the base model and provided further discrimination over and above premature FH status for composite ASCVD (c-statistic from 0.742 to 0.744, P=.05).
Reclassification Based on FH Status and Familial Risk Assessment
Table 4 displays the net reclassification for the various FH definitions. The addition of any-FH and FRA to traditional Framingham risk factors reclassified incident CHD. For the composite ASCVD outcome, similar results were found for any-FH and FRA, however premature-FH also reclassified this outcome. Among intermediate risk participants, any-FH reclassified risk for the composite outcome. In a sensitivity analysis (Supplementary Table 6), the addition of Framingham risk factors to a base model including each FH definition, resulted in a significant and larger reclassification improvement for incident CHD and ASCVD events, respectfully, in the total population and among those at intermediate risk by the FRS.
Table 4.
Net reclassification improvement analysis for incident cardiovascular events with addition of FH definitions to models adjusted for FRS in the entire study population and those at intermediate risk, respectivelya
| FH Variable | CHD NRI (95%CI) |
Composite ASCVD NRI (95%CI) |
|---|---|---|
| Total Population | ||
| FRS+ FH Any | 0.162 (0.061,0.264)b | 0.166 (0.094,0.243)b |
| FRS+ FH Premature | 0.069 (−0.106,0.179) | 0.076 (0.014,0.135)b |
| FRS+ FH Risk Strata | 0.164 (0.067,0.260)b | 0.165 (0.090,0.237)b |
| Population at Intermediate Risk by FRS | ||
| FRS+ FH Any | 0.160 (−0.200, 0.323) | 0.143 (0.041, 0.244)b |
| FRS+ FH Premature | 0.064 (−0.190, 0.206) | 0.036 (−0.108, 0.111) |
| FRS+ FH Risk Strata | 0.159 (−0.201, 0.318) | −0.209 (−0.155, 0.205) |
ASCVD= atherosclerotic cardiovascular disease, CHD = coronary heart disease, FH = family history, FRS = Framingham Risk Score, NRI = net reclassification index.
Indicated statistically significant results.
DISCUSSION
In this contemporary, multi-ethnic cohort, FH definitions of differing complexity were all demonstrated to be independent risk factors for ASCVD events. Interestingly, the magnitude of these associations was similar for each FH definition. While statistically significant, the incremental prognostic contribution to the C-statistic for ASCVD was qualitatively similar for each FH definition. The association of FH and events was limited to CHD and angina, while other non-coronary cardiovascular outcomes were not significantly associated. As such, our main finding is that all of the approaches to defining FH considered in this analysis appeared to perform similarly in improving CHD risk prediction, supporting the use of a simple and practical approach to defining FH in routine clinical practice towards CHD prevention.
The comparison between simple and other more comprehensive assessments of FH has been described in other populations.28–31 However, comparisons between studies can be challenging given the different definitions of FH used, variable study designs, and duration of follow-up. For example, ORs have been reported from cross-sectional and retrospective studies for the association between premature-FH (applying various definitions) and CHD ranging from 1.4 to 5.9.5,10,11,13,30,31 This study has a lower (and perhaps more valid) risk ratio for any-FH or premature-FH (both RRs were 1.3) given its prospective nature.32 Consistent with this, the association of FH with CHD in our study is similar in magnitude to other prospective studies, which reported HRs ranging from 1.3 to 1.7.8,9
Adoption of FH into risk prediction models has been limited as: 1) FH risk is often not independent of other established risk factors, 2) FH is non-modifiable, and 3) because of the varied temporal association between FH and ASCVD events (i.e. greater influence of shared, genetic component for premature events, and a more balanced contribution of environmental and acquired CVD risk factors for later onset events).4,8,33–35 Indeed, traditional risk factors account for the majority of attributable CHD risk, with only 1% contributed by FH, which provides a plausible explanation for the limited improvement in discrimination and reclassification with FH seen in the current study.35 Despite these limitations, current guidelines recommend that clinicians assess premature-FH status, particularly among intermediate risk individuals to potentially reclassify risk.2,36 However, some providers may be dissuaded from querying FH status given time-constraints and the perceived complexity and unreliability of premature-FH assessment.3 Given our results and the historical challenges implementing FH information into risk assessment tools, it may be reasonable to consider using a simple definition of “any-FH” to identify individuals at increased risk for CHD, both in clinical practice and for research purposes.
Notably, the application of our results is most valid in the routine care of patients drawn from the general community when traditional risk factors are already known and CHD is the outcome of primary interest. We are unable to speculate whether detailed FH assessments may have added value at the individual treatment level in high risk subsamples of the population.37 For example, by incorporating the FRA, the Family Healthware™ tool accounts for the number, sex, onset, and lineage relatives with CHD, with classification into three risk categories, and has been developed by the Center for Disease Control.38 This MESA study cannot rule out that more detailed FH tools like the FRA have value in some high risk pedigrees. For example, we found that the number of relatives with premature FH (a form of high risk pedigree) was incrementally associated with outcomes in this analysis, a situation where these more detailed tools may have value. Additionally, depending on the definition used, a vastly different number of patients could fall into different categories of risk. This has implications when considering how the FH assessment may change the care being offered a given patient. For example, recent Society of Cardiovascular Computed Tomography guidelines recommend CAC imaging if ASCVD risk by the PCE is <5% in patients with a premature-FH.39 However, many more people would have been eligible using the any FH definition. Thus, for certain applications that have cost or safety implications, an assessment of premature FH may be preferred.
LIMITATIONS
The results of our study should be interpreted in the context of several limitations. Self-reports of CHD in a first-degree relative are subject to a high specificity and comparatively lower sensitivity, limiting accurate FH assessment by reporting errors.40 Selection bias may have been introduced, as detailed information on FH was collected at visit 2. As a result, participants who died from an ASCVD related event before visit 2 were excluded from the analysis. However, of those who did die (n=159), the proportion of those with any-FH was statistically similar to the proportion of those with any–FH included in our analysis and the time between visits was short (median time, 1.6 years).41 Given the number of ASCVD outcomes compared, multiple comparisons would have to be considered. Data collection may have been affected by recall bias (if participants were unable to accurately recollect the number family members who suffered a CHD event) and by ascertainment bias related to the participant’s age. Another limitation is the inclusion of persons responding ‘do not know’ in the no FH group. However, we conducted a sensitivity analysis excluding such ‘do not know’ individuals from the analysis and the results were all quantitatively similar (data not shown). Our definition of PAD may not have allowed for an accurate assessment with various FH-phenotypes, as the ABI measurement is limited by non-compressible arteries, rendering it a sensitive but not specific test.42 Given the age cutoff used for this study, our results may not be generalizable to those less than age 45 who may have a strong premature family history.
CONCLUSIONS
Various definitions for a family history of CHD can add significant prognostic information to traditional risk factors in the prediction of CHD events. Current prevention guidelines endorse the assessment of premature-FH to guide ASCVD risk prevention, particularly when therapeutic uncertainty exists. Our MESA data would suggest that, in aggregate and when other traditional risk factors are already known, a simple and single-question assessment of family history in a first-degree relative of any age may be more efficient and as effective as more complex FH definitions for identifying individuals with heightened CHD risk; a finding that should motivate more routine assessment of FH in ASCVD prevention.
Supplementary Material
Acknowledgments
MESA was supported by contracts HHSN268201500003I, N01-HC-95159/60/61/62/63/64/65/66/67/69 from the NHLBI, and by grants UL1-TR-000040/001079/001420 from NCATS. The content is solely the responsibility of the authors and does not necessarily represent the views of the NHLBI or the NIH. We would like to thank the staff and participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa.nhlbi.org.
Dr. Scheuner hold two patents for familial risk assessment algorithms: 1) US 7,951,078 B2 - Method and apparatus for determining familial risk of disease; and 2) US 8,719,045 - Personal assessment including familial risk analysis for personalized disease prevention plan. Dr. Nasir has reported consulting for Regeneron; and is on the advisory board of Quest Diagnostics.
ABBREVIATIONS
- ABI
ankle brachial index
- ASCVD
atherosclerotic cardiovascular heart disease
- CHD
coronary heart disease
- CHF
congestive heart failure
- DM
diabetes mellitus
- FH
family history of coronary heart disease
- FRA
familial risk assessment
- FRS
Framingham risk score
- MESA
Multiethnic Study of Atherosclerosis
- NRI
net reclassification index
- PAD
peripheral artery disease
- PCE
pooled cohorts equation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COMPETING INTERESTS
Drs. Patel, Al Rifai, Shea, Blumenthal, Blaha, and McEvoy do not have any disclosures to report.
References
- 1.Safarova MS, Bailey KR, Kullo IJ. Association of a Family History of Coronary Heart Disease with Initiation of Statin Therapy in Individuals at Intermediate Risk: Post Hoc Analysis of a Randomized Clinical Trial. JAMA Cardiol. 2016;1(3):364–366. doi: 10.1001/jamacardio.2016.0227. [DOI] [PubMed] [Google Scholar]
- 2.Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2935–2959. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dhiman P, Kai J, Horsfall L, et al. Availability and quality of coronary heart disease family history in primary care medical records: implications for cardiovascular risk assessment. PLoS One. 2015;9(1):e81998. doi: 10.1371/journal.pone.0081998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sesso HD, Lee IM, Gaziano JM, et al. Maternal and paternal history of myocardial infarction and risk of cardiovascular disease in men and women. Circulation. 2001;104(4):393–398. doi: 10.1161/hc2901.093115. [DOI] [PubMed] [Google Scholar]
- 5.Lloyd-Jones DM, Nam BH, D'Agostino RB, Sr, et al. Parental cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults: a prospective study of parents and offspring. JAMA. 2004;291(18):2204–2211. doi: 10.1001/jama.291.18.2204. [DOI] [PubMed] [Google Scholar]
- 6.Chow CK, Islam S, Bautista L, et al. Parental history and myocardial infarction risk across the world: the INTERHEART Study. J Am Coll Cardiol. 2011;57(5):619–627. doi: 10.1016/j.jacc.2010.07.054. [DOI] [PubMed] [Google Scholar]
- 7.Sivapalaratnam S, Boekholdt SM, Trip MD, et al. Family history of premature coronary heart disease and risk prediction in the EPIC-Norfolk prospective population study. Heart. 2010;96(24):1985–1989. doi: 10.1136/hrt.2010.210740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bachmann JM, Willis BL, Ayers CR, et al. Association between family history and coronary heart disease death across long-term follow-up in men: the Cooper Center Longitudinal Study. Circulation. 2012;125(25):3092–3098. doi: 10.1161/CIRCULATIONAHA.111.065490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li R, Bensen JT, Hutchinson RG, et al. Family risk score of coronary heart disease (CHD) as a predictor of CHD: the Atherosclerosis Risk in Communities (ARIC) study and the NHLBI family heart study. Genet Epidemiol. 2000;18(3):236–250. doi: 10.1002/(SICI)1098-2272(200003)18:3<236::AID-GEPI4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 10.Murabito JM, Pencina MJ, Nam BH, et al. Sibling cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults. JAMA. 2005;294(24):3117–3123. doi: 10.1001/jama.294.24.3117. [DOI] [PubMed] [Google Scholar]
- 11.Leander K, Hallqvist J, Reuterwall C, Ahlbom A, de Faire U. Family history of coronary heart disease, a strong risk factor for myocardial infarction interacting with other cardiovascular risk factors: results from the Stockholm Heart Epidemiology Program (SHEEP) Epidemiology. 2001;12(2):215–221. doi: 10.1097/00001648-200103000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Jousilahti P, Puska P, Vartiainen E, Pekkanen J, Tuomilehto J. Parental history of premature coronary heart disease: an independent risk factor of myocardial infarction. J Clin Epidemiol. 1996;49(5):497–503. doi: 10.1016/0895-4356(95)00581-1. [DOI] [PubMed] [Google Scholar]
- 13.Friedlander Y, Kark JD, Stein Y. Family history of myocardial infarction as an independent risk factor for coronary heart disease. Br Heart J. 1985;53(4):382–387. doi: 10.1136/hrt.53.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeboah J, Young R, McClelland RL, et al. Utility of Nontraditional Risk Markers in Atherosclerotic Cardiovascular Disease Risk Assessment. J Am Coll Cardiol. 2016;67(2):139–147. doi: 10.1016/j.jacc.2015.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D'Agostino RB, Sr, Grundy S, Sullivan LM, Wilson P CHD Risk Prediction Group. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286(2):180–187. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 16.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(5):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 17.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 18.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2006;29(Suppl 1):S43–S48. [PubMed] [Google Scholar]
- 19.Joint National Committee. The sixth report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Arch Intern Med. 1997;157(21):2413–2446. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]
- 20.Scheuner MT, Whitworth WC, McGruder H, Yoon PW, Khoury MJ. Familial risk assessment for early-onset coronary heart disease. Genet Med. 2006;8(8):525–531. doi: 10.1097/01.gim.0000232480.00293.00. [DOI] [PubMed] [Google Scholar]
- 21.Scheuner MT, Setodji CM, Pankow JS, Blumenthal RS, Keeler E. General Cardiovascular Risk Profile identifies advanced coronary artery calcium and is improved by family history: the multiethnic study of atherosclerosis. Circ Cardiovasc Genet. 2010;3(1):97–105. doi: 10.1161/CIRCGENETICS.109.894527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeboah J, Folsom AR, Burke GL, et al. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation. 2009;120(6):502–509. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Budoff MJ, McClelland RL, Nasir K, et al. Cardiovascular events with absent or minimal coronary calcification: the Multi- Ethnic Study of Atherosclerosis (MESA) Am Heart J. 2009;158(4):554–561. doi: 10.1016/j.ahj.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coylewright M, Rice K, Budoff MJ, et al. Differentiation of severe coronary artery calcification in the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2011;219(2):616–622. doi: 10.1016/j.atherosclerosis.2011.08.038. [DOI] [PubMed] [Google Scholar]
- 24.Criqui MH, McClelland RL, McDermott MM, et al. The ankle-brachial index and incident cardiovascular events in the MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2010;56(18):1506–1512. doi: 10.1016/j.jacc.2010.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bahrami H, Kronmal R, Bluemke DA, et al. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med. 2008;168(19):2138–2145. doi: 10.1001/archinte.168.19.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeboah J, McClelland RL, Polonsky TS, et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308(8):788–795. doi: 10.1001/jama.2012.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunt SC, Williams RR, Barlow GK. A comparison of positive family history definitions for defining risk of future disease. J Chronic Dis. 1986;39(10):809–821. doi: 10.1016/0021-9681(86)90083-4. [DOI] [PubMed] [Google Scholar]
- 29.Silberberg JS, Wlodarczyk J, Fryer J, Robertson R, Hensley MJ. Risk associated with various definitions of family history of coronary heart disease. The Newcastle Family History Study II. Am J Epidemiol. 1998;147(12):1133–1139. doi: 10.1093/oxfordjournals.aje.a009411. [DOI] [PubMed] [Google Scholar]
- 30.Scheuner MT, Whitworth WC, McGruder H, Yoon PW, Khoury MJ. Expanding the definition of a positive family history for early-onset coronary heart disease. Genet Med. 2006;8(8):491–501. doi: 10.1097/01.gim.0000232582.91028.03. [DOI] [PubMed] [Google Scholar]
- 31.Eaton CB, Bostom AG, Yanek L, et al. Family history and premature coronary heart disease. J Am Board Fam Pract. 1996;9(5):312–318. [PubMed] [Google Scholar]
- 32.Song JW, Chung KC. Observational studies: cohort and case-control studies. Plast Reconstr Surg. 2010;126(6):2234–2242. doi: 10.1097/PRS.0b013e3181f44abc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Donnell CJ. Family history, subclinical atherosclerosis, and coronary heart disease risk: barriers and opportunities for the use of family history information in risk prediction and prevention. Circulation. 2004;110(15):2074–2076. doi: 10.1161/01.CIR.0000145539.77021.AC. [DOI] [PubMed] [Google Scholar]
- 34.Hippe M, Vestbo J, Bjerg AM, et al. Cardiovascular risk factor profile in subjects with familial predisposition to myocardial infarction in Denmark. J Epidemiol Community Health. 1997;51(3):266–271. doi: 10.1136/jech.51.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yusuf S, Hawken S, Ounpuu S, et al. INTERHEART Study Investigators. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 36.Piepoli MF, Hoes AW, Agewall S, et al. Authors/Task Force Members. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Eur Heart J. 2016;37(29):2315–2381. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rose G. Sick individuals and sick populations. Int J Epidemiol. 2001;30(3):427–432. doi: 10.1093/ije/30.3.427. [DOI] [PubMed] [Google Scholar]
- 38.Yoon PW, Scheuner MT, Jorgensen C, Khoury MJ. Developing Family Healthware, a family history screening tool to prevent common chronic diseases. Prev Chronic Dis. 2009;6(1):A33. [PMC free article] [PubMed] [Google Scholar]
- 39.Hecht H, Blaha MJ, Berman DS, et al. Clinical indications for coronary artery calcium scoring in asymptomatic patients: Expert consensus statement from the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr. 2017;1(2):157–168. doi: 10.1016/j.jcct.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 40.Khoury MJ, Flanders WD. Bias in using family history as a risk factor in case-control studies of disease. Epidemiology. 1995;6(5):511–519. doi: 10.1097/00001648-199509000-00009. [DOI] [PubMed] [Google Scholar]
- 41.Patel J, Al Rifai M, Blaha MJ, et al. Coronary Artery Calcium Improves Risk Assessment in Adults With a Family History of Premature Coronary Heart Disease: Results From Multiethnic Study of Atherosclerosis. Circ Cardiovasc Imaging. 2015;8(6):e003186. doi: 10.1161/CIRCIMAGING.115.003186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mohler ER., 3rd Peripheral arterial disease: identification and implications. Arch Intern Med. 2003;163(19):2306–2314. doi: 10.1001/archinte.163.19.2306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.