Abstract
Study Design
Cross-sectional design
Objectives
This study examined the relationships between circulating adiponectin levels, body composition, metabolic profile, and measures of skeletal muscle mitochondrial enzyme activity and biogenesis.
Settings
Clinical Research in a Medical Center
Methods
Plasma adiponectin was quantified in nineteen individuals with chronic spinal cord injury (SCI). Body composition was evaluated by dual x-ray absorptiometry and magnetic resonance imaging. Metabolic profile was assessed by basal metabolic rate (BMR), oxygen uptake (VO2), and intravenous glucose tolerance testing. Mitochondrial enzyme activity of skeletal muscle was obtained by spectrophotometric assays and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) and 5′ AMP-activated protein kinase (AMPK) protein expression was assessed by Western blots.
Results
Adiponectin was negatively related to both total and regional fat mass and positively related to lean mass and muscle mass. Furthermore, there were positive relationships between adiponectin and BMR (r=0.52, P=0.02) and VO2 (r=0.73, P=0.01). Furthermore, adiponectin was positively related to citrate synthase (r=0.68, P=0.002) and complex III activity (r=0.57, P=0.02). The relationships between adiponectin and body composition remained significant after accounting for age. The relationships between adiponectin, metabolic profile and markers of mitochondria mass and activity were influenced by age.
Conclusions
The study demonstrated that adiponectin is closely related to body composition and metabolic profile in persons with SCI and further supports mechanistic studies suggesting that adiponectin may stimulate mitochondrial biogenesis.
Keywords: Adiponectin, mitochondrial biogenesis, PGC-1alpha, BMR, spinal cord injury
Introduction
Adiponectin is a circulating hormone released predominately from adipocytes [1–4]. Paradoxically, levels have shown to be decreased in obese individuals and are negatively related to percent body fat mass [2,5–7]. Adiponectin levels are also decreased in individuals with type II diabetes and cardiovascular disease [8,9]. Previous studies in animal and cell culture models have shown that adiponectin has anti-inflammatory properties and insulin-sensitizing effects on muscle and liver [10,11]. Increasing adiponectin has been shown reverse insulin resistance and reduce atherosclerosis in animal models [12,13].
One mechanism by which adiponectin exerts beneficial effects may be by increasing mitochondrial bioenergetics [9,14]. Mitochondria undergo a cycle of biogenesis, remodeling, and degradation based on the energy needs of the cell. Upstream regulators of mitochondrial biogenesis include 5′ AMP-activated protein kinase (AMPK) and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α). Moreover, AMPK is an important sensor of energy balance and phosphorylation of AMPK increases production of PGC-1α [15]. Treatment of primary human myotubes with adiponectin resulted in increased PGC-1α, fatty acid oxidation, mitochondrial DNA content and citrate synthase, a marker of mitochondrial mass [9]. Inhibition of AMPK blunted fatty acid oxidation and citrate synthase [9,14]. Similarly, adiponectin signaling has been shown to activate AMPK in vivo and increase skeletal muscle glucose uptake [16]. Lack of skeletal muscle adiponectin signaling resulted in decreased oxidative muscle fibers and exercise intolerance [17]. In humans, mitochondrial DNA copy number was positively related to skeletal muscle adiponectin receptor expression [9].
Persons with spinal cord injury (SCI) are at high risks of developing obesity, type II diabetes and cardiovascular disease [18,19]. The extensive loss of lean mass and increased fat mass that occurs after injury negatively impacts basal metabolic rate (BMR) and peripheral glucose utilization [20,21]. Furthermore, the negative changes in body composition and metabolic profile are likely to be impacted by decreased level of physical activity after SCI [22,23]. Previous work indicated that serum adiponectin was negatively correlated with body mass index (BMI) and visceral fat in individuals with SCI [24,25]. One study showed that persons with SCI have a higher level of circulating adiponectin compared to able-bodied controls [24]. The difference in circulating adiponectin was explained by loss in inhibitory effects of sympathetic nervous system. It was also recommended that adipose tissue may secrete different inactive forms of adiponectin that may serve as a protective mechanism of decreased BMR after SCI [24].
Recent work indicated that increase in both lean mass and thigh muscle cross-sectional area is associated with increased citrate synthase (CS), a marker of mitochondrial density, that was positively related to BMR [26, 27]. The increase in CS was associated with decreased ectopic adiposity and improvement in lipid profile in persons with SCI [26,27]. However, it is unclear whether circulating adiponectin may trigger increase in citrate synthase and subsequent increase in BMR after SCI [25]. The current study investigated the relationships between adiponectin, body composition, metabolic profile and mitochondrial enzyme activity and biogenesis in individuals with chronic SCI. We hypothesized that circulating adiponectin would be negatively related to measures of body fat and positively related to lean mass and metabolic profile. Furthermore, we hypothesized that mitochondrial enzyme activity and proteins involved in mitochondrial biogenesis would be positively related to adiponectin.
Methods
Ethical Approval
This study was approved by the McGuire VA Medical Center institutional review board. Participants provided written informed consent as part of a clinical trial, registered at clincialtrials.gov (NCT01652040). Data presented are prior to any intervention.
Participants
Twenty-two men with motor complete SCI were recruited to participate in the current study. Participants were recruited for a clinical trial investigating the effects of evoked resistance training and testosterone replacement therapy in men with chronic SCI [28]. Persons with motor complete SCI were only studied to ensure a homogenous sample and to decrease the inadvertent effects on body composition and metabolic profile. Individuals were between the ages of 18 and 50 and were at least one-year post injury. The study protocol has been recently published [28] and participants are the same cohort as in previously published studies [26, 27]. Participant characteristics are shown in Table 1.
Table 1.
Physical characteristics of persons with motor complete SCI
| Total participants | |
|---|---|
|
| |
| Demographics | |
| Age (years) | 36.0±10.0 |
| BMI (kg/m2) | 25± 4 |
| TSI (years) | 8. ± 8 |
| White, n (%) | 14 (64%) |
| Injury Level | |
| Paraplegia, n (%), AIS | 14 (T4-T11 ;64%), AIS A (11) & AIS B (3) |
| Tetraplegia, n (%), AIS | 8 (C5-C7 ; 36%), AIS A (5) & AIS B (3) |
Values are mean ± SD; AIS, American Spinal Injury Association Impairment Scale; BMI, body mass index; TSI, time since injury; n=22
Metabolic profile assessment
Basal metabolic rate (BMR) was measured using a COSMED K4b2 (Cosmed USA Inc, Chicago, IL) with canopy after overnight fast for 10-12 hours followed by an intravenous glucose tolerance test as previously described [27,29, 30]. After calibration, participants were gently awakened in the morning and a see-through canopy was placed over the participant’s head while lying flat with a clear instruction to lay still during the 20-minute test. The first 5 minutes of the test was disregarded and the last 15 minutes were analyzed after ensuring a steady state has been reached for VO2, VCO2 and respiratory exchange ratio. The Weir equation was used to calculate BMR [29]. Blood was collected and fasting lipid profile, plasma glucose and glycated hemoglobin (HbA1c) were sent out to a clinical diagnostic lab. Insulin sensitivity (Si) and glucose effectiveness (Sg) were determined using the MinMod software (MinMod Inc., Pasadena, CA) [31]. Three Si values were excluded because they were outside the physiological range. Fasting plasma glucose and HbA1c values from one individual were unavailable due to lab error.
Oxygen uptake (VO2) was measured in a subset of participants (n=15) during functional electrical stimulation cycling on a RTI-300 bike (Restorative Therapies) using a COSMED K4b2 [32]. Electrical stimulation was applied bilaterally to the quadriceps, hamstrings, and gluteal muscles to achieve exercise in a cyclical pattern [28, 32]. Stimulation parameters were set at 30 Hz, pulse duration at 450 μs and current amplitude ranged from 100-140 mA [32]. After a three-minute resting period, participant cycled for a three-minute warm-up period with the motor on. The testing period involved cycling at speed of 40-45 revolutions per minute (RPM) and manually increasing the resistance by 2 Nm every two minutes with the motor off until fatigue (i.e., speed below 18 RPM). This was followed by a cool down period of one minute and a 5-minute period of rest as previously described [32]. Blood pressure and heart rate were monitored every 2-3 minutes to protect against episodes of autonomic dysreflexia that may occur especially in those with high level of injury. Data are then normalized to body mass (ml/min/kg).
Body Composition
Total body and regional dual-energy x-ray absorptiometry (DXA) scans were performed with a Lunar Prodigy Advance scanner (Lunar Inc., Madison, WI) by a trained operator to measure lean mass and fat mass. Once participant was transferred to the DXA table, both legs were strapped proximal to the knee joints and around both feet to avoid any movement artifact due to muscle spasms during scanning. Participants were allowed 20 minutes in a supine position before conducting the scan to minimize the effects of fluid shift on body composition assessment. The RMS-CV% for legs and total body for same day repeated scans was 2.7% and 2.3%, respectively [33]. Weight and height were determined as previously described [26–30].
Transaxial magnetic resonance imaging was captured to measure whole thigh skeletal muscle and knee extensor cross-sectional area [slice thickness was 8 mm and inter-slice space was 16 mm apart] from the hip joint to the knee joint as previously described [23,34]. Analysis was performed using Win-vessel software (Ronald Meyer, MSU) by an experimenter blinded to the experimental conditions [23,34]. Data presented are the average of 12-15 slices from the right leg. MRI data were not available from one participant with a previous gunshot wound. The magnetic field may possibly cause movement of the sharpeners in the spinal canal and may lead to further damage.
Adiponectin
Blood samples were obtained between 6:00 and 7:00 AM after an overnight fast and plasma samples were stored at −70°C until batch analysis. Total adiponectin was quantified in duplicate by colorimetric enzyme-linked immunosorbent assay (ELISA; Alpco Diagnostics, Salem, NH, USA). This assay has a detection limit of 0.034 ng/ml. Blood samples from three individuals were unavailable for analysis, because of shortage in the blood volumes drawn from these participants Adiponectin was expressed in absolute form (ng/ml) and relative to body weight, lean mass and fat mass (ng/ml/kg) to control for variability relative to body size and body composition compartments.
Enzyme Activities
Muscle biopsies were collected from the vastus lateralis of the right leg using a 14-gauge tru-cut™ needle under local anesthesia (2% lidocaine). Samples were immediately frozen in liquid nitrogen and stored at −70°C until analysis. A portion of the sample (~10-25 mg) was removed of visible connective and adipose tissue and homogenized in ice cold buffer containing 220 mM mannitol, 70 mM sucrose, 5 mM MOPS, 2 mM EDTA, with cOmplete™ protease inhibitor cocktail (Sigma-Aldrich), pH 7.4. The homogenate was centrifuged at 371 g (5 min, 4°C) and the supernatant was used for analysis. Protein concentration was determined and samples were solubilized in 1% potassium cholate. Assays were completed on the same day as homogenization. CS and complex III (CIII) activity were measured spectrophotometrically in duplicate or triplicate as previously described [26,27,35]. Insufficient muscle tissues precluded analysis of four samples for CS analysis and seven samples for CIII analysis.
Western Blot
Muscle tissue was homogenized in RIPA buffer with containing protease and phosphatase inhibitors (Halt Protease and Phosphatase, ThermoScientific) using an electric tissue homogenizer [30]. The homogenate was briefly sonicated and centrifuged for 10 minutes at 4°C at 10,000 g. A microBCA kit (Thermo Scientific) was used to measure the protein content of the supernatant using bovine serum albumin as the standard. Samples were mixed 1:1 in 2× Laemlli sample buffer with 2-mercaptoethanol and boiled for 3 minutes. 50 μg of protein was loaded onto a 10% polyacrylamide gel and run for 60 minutes at 185 V. Protein was electrophoretically transferred to a PVDF membrane and stained with Ponceau S to visualize protein loading. The membrane was destained with a 10-15 min wash in Tris-buffered saline with 0.05% Tween 20 (TBS-T) and blocked in a solution of 5% milk and TBS-T for one hour followed by overnight incubation at 4°C with primary antibody diluted 1:1000 in 1% milk and TBS-T. Primary antibodies included PGC1α (sc-13067; Santa Cruz Biotechnology), total AMPK (#2532; Cell Signaling) and AMPKT172 (#50081; Cell Signaling). Membranes were washed in TBS-T with 3 10 min washes then incubated for an hour with a horseradish peroxidase-conjugated secondary antibody (1:2000, Cell Signaling). The membranes were washed as mentioned above then incubated for five minutes with a horseradish peroxidase chemiluminescence detection reagent (ECL Prime: Amersham) and digitaly imaged (A600; Amersham) [30]. Densitometry was analyzed with iQuant software (Amersham). AMPK data are shown as the ratio of phosphorylated AMPK/total AMPK.
Statistical Analyses
Data are expressed as mean ± standard deviation. Data are checked for normal distribution using Shapiro-Wilk test and then log-transformed if needed (P<0.05) prior to any statistical analyses. Independent two-tailed t-tests were used to evaluate differences in adiponectin based on SCI characteristics including level of injury, age (≥ 40 or <40 years), time since injury (≥6 or < 6 years), BMI (>25 or <25) and ethnicity (African American vs Caucasian) Bivariate Pearson correlations were used to assess the relationship between lipid and metabolic variables and plasma adiponectin. Partial correlations were conducted to consider age as a confounding variable. Statistical significance was set at P<0.05. All statistical analyses were performed using SPSS version 23 (Armonk, NY).
Results
Participant characteristics
Participant demographics are shown in Table 1. Participants ranged in age from 18 to 50 with a mean age of 36±10 years and were an average of 8±8 years post injury (range from 1 to 28). All participants were motor complete (no motor function below the level of injury). Table 2 shows values for body composition, metabolic profile, skeletal muscle mitochondrial enzyme activities, and absolute adiponectin as well as adiponectin adjusted to body weight, lean mass and fat mass. Values of PGC-1 α, phosphorylated AMPK and phosphorylated AMPK to total AMPK ratio are also listed.
Table 2.
Body composition and metabolic outcomes of persons with motor complete SCI.
| Outcome Variables | Mean ± SD |
|---|---|
|
| |
| Body Composition | |
| Total %Fat | 32.6 ± 9.7, n=22 |
| Total LM (kg) | 49.5 ± 7.5, n=22 |
| Leg %Fat | 34.1 ± 9.6, n=22 |
| Leg LM (kg) | 14.2 ± 3.6, n=22 |
| %IMF (thigh) | 14.1 ± 8.8, n=20 |
| Thigh muscle CSA (cm2) | 82.2 ± 21.2, n=20 |
| knee extensor CSA (cm2) | 37.1 ± 10.4, n=20 |
| Metabolic Profile | |
| Fasting glucose (mg/dl) | 107.1 ±13.0, n=21 |
| HbA1c (%) | 5.35 ± 0.45, n=21 |
| Si ((mU/L)−1 min−1 | 8.6 ± 6.3, n=19 |
| Sg (10−2 × min−1) | 2.01 ± 0.88, n=22 |
| BMR (kcal/day) | 1547 ± 177, n=22 |
| VO2 (ml/min/kg) | 7.04 ± 3.18, n=15 |
| Mitochondrial Enzyme Activity | |
| CS activity (nmol/min/mg) | 52.7 ± 28.5, n=18 |
| CIII activity (nmol/min/mg) | 34.2 ± 18.0, n=15 |
| Adiponectin (ng/ml) | 3892.6 ± 1493.9; n=19 |
| Adiponectin/body weight (ng/ml/kg) | 52.2 ± 23.3; n=19 |
| Adiponectin/lean mass (ng/ml/kg) | 78.6 ± 26; n=19 |
| Adiponectin/fat mass (ng/ml/kg) | 207 ± 175; n=19 |
| Protein expression (arbitrary units) | |
| PGC-1α | (744.2 ± 1269) × 10−4; n=18 |
| Phospho-AMPK | (360.2 ± 284) × 10−4; n=18 |
| Phospho AMPK/total AMPK | 1.23 ± 1.30; n=18 |
Values are means ± SD; n, number of subjects; LM, lean mass; IMF, intramuscular fat; CSA, cross sectional area; KE, knee extensor; HbA1c, glycated hemoglobin; Si, insulin sensitivity; Sg, glucose effectiveness; BMR, basal metabolic rate, VO2, oxygen uptake; CS, citrate synthase; CIII, complex III
Adiponectin and SCI Characteristics
There was a trend (P = 0.06) of greater adiponectin between individuals age < 40 years (n=13; 4318±1474.5 ng/dl) and age ≥ 40 years (n=6; 2972±1153 ng/dl). Caucasians (n=12; 4478±1407 ng/dl) have greater (P =0.007) adiponectin compared to African-Americans (n=7; 2889±1097 ng/dl) men with SCI. There were no effects of level of injury (tetraplegia vs. paraplegia), time since injury (≥ 6 or < 6 years), AIS classification (A vs. B) and BMI (≥25 or <25 kg/m2) on adiponectin level. Adiponectin was negatively related to age (r=−0.62, P=0.005) and adiponectin adjusted to fat mass was negatively related to age (r=−0.62, P=0.004) and body weight (r=−0.48, P=0.035). Time since injury was not related to adiponectin (r=−0.2, P =0.2).
Relationship between adiponectin and body composition
Total %fat (r=−0.71, P=0.001), leg %fat (r=−0.65, P=0.003) and trunk % fat (r=−0.75, P < 0.0001) were negatively related to adiponectin levels (Fig. 1A). Adjusted adiponectin demonstrated similar relationships with total %fat (r=−0.63 to −0.89; P ≤ 0.001-0.004), leg% fat (r=−0.53 to −0.83; P < 0.0001-0.018) and trunk% fat (r=−0.70 to −0.92; P ≤ 0.0001-0.001). There was a positive relationship between adiponectin and total lean mass (r=0.62, P=0.005) and as well as leg lean mass (r=0.61, P=0.006; Fig. 1B). Similarly, there was a positive relationship between adiponectin and thigh muscle CSA (r=0.58, P=0.011) as well as knee extensor CSA (r=0.64, P=0.005; Fig. 2A). there was a negative relationship between percent intramuscular fat (%IMF) and adiponectin (r=−0.66, P=0.003; Fig. 2B).
Figure 1.
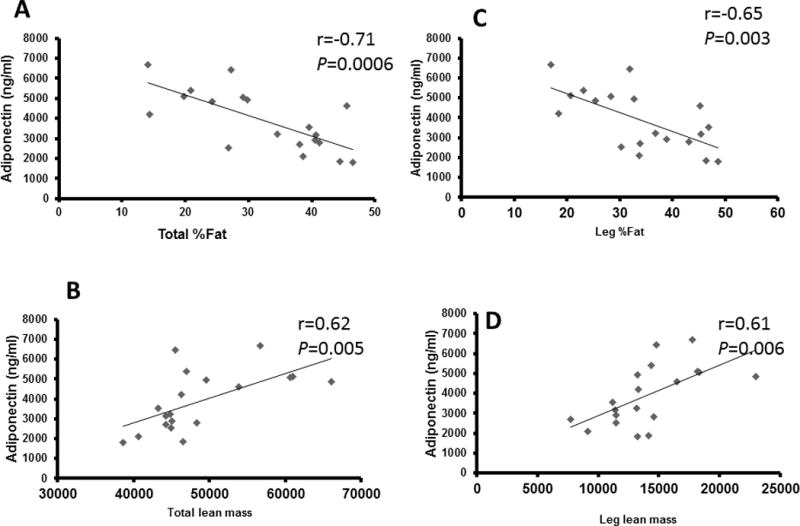
Total and leg %fat (A) and LM (B) related to adiponectin in 19 men with SCI
Figure 2.
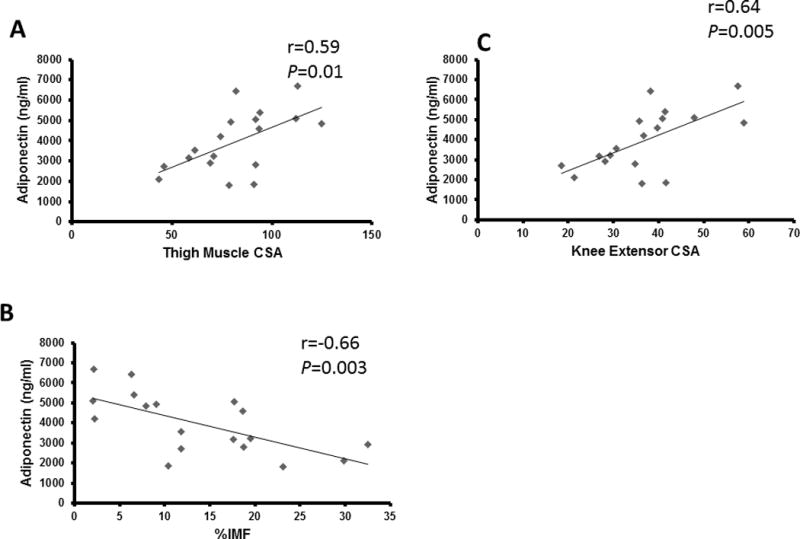
Adiponectin related to A) whole thigh muscle cross sectional area (CSA), knee extensor (KE) CSA (n=18) and B) %intramuscular fat (%IMF)
The relationships between adiponectin and total %fat, total LM, leg %fat, and leg LM remained significant when partial correlations were run to account for age as a confounding variable (Table 3). Similarly, there was a trend for a relationship between adiponectin and thigh muscle CSA (r=0.43, P=0.09) and adiponectin and knee extensor CSA (r= 0.45, P=0.07) when age was accounted for. However, the relationships between adiponectin and %IMF were no longer significant after accounting for age.
Table 3.
Correlation coefficients for Adiponectin, body composition and metabolic Profile in persons with motor complete SCI
| Pearson (r) | Partial (age-adjusted) | |
|---|---|---|
|
| ||
| Total %Fat | −0.71** | −0.56* |
| Total lean mass | 0.62** | 0.59* |
| Trunk %Fat | −0.75** | −0.57* |
| Leg %Fat | −0.65** | −0.59* |
| Leg lean mass | 0.61** | 0.52* |
| %IMF | −0.66** | −0.34 |
| Thigh Muscle CSA | 0.59* | 0.43ˆ |
| Knee extensor CSA | 0.64** | 0.45ˆ |
| BMR | 0.52* | 0.46ˆ |
| VO2 | 0.63* | 0.29 |
| CS | 0.68** | 0.48ˆ |
| CIII | 0.57* | 0.38 |
IMF, intramuscular fat; CSA, cross sectional area; BMR, basal metabolic rate, VO2 peak, peak oxygen uptake; CS, citrate synthase; CIII, complex III.
P < 0.0001;
P < 0.02;
P > 0.05-0.07
Relationship between adiponectin and metabolic profile
As shown in Fig. 3, BMR (r=0.52, P=0.02; Fig. 3A) and VO2 (r=0.73, P=0.01; Fig. 3B) were positively related to adiponectin. No significant relationships were observed between adiponectin and fasting glucose, HbA1c, Si, Sg, or lipid profile. A trend toward significance remained between BMR and adiponectin when age was taken into consideration (r=0.46, P=0.06; Table 3). However, the relationship with VO2 was no longer significant after accounting for age.
Figure 3.
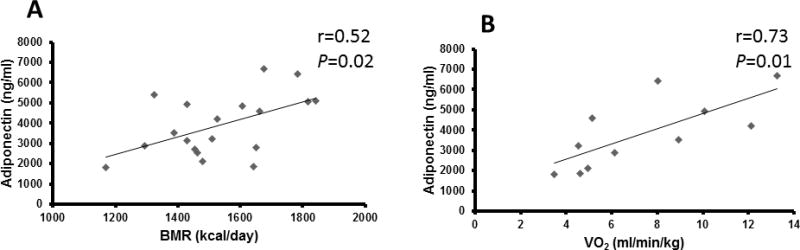
Basal metabolic rate (BMR; n=19) and oxygen uptake (VO2; n=11) related to adiponectin.
Relationship between adiponectin and markers of mitochondrial mass and biogenesis
Adiponectin was positively related to CS (r=0.68, P =0.002) and CIII activity (r=0.57, P =0.02) (Fig. 4). Similarly, there was a trend for a positive relationship between adiponectin and protein levels of PGC-1α (r=0.46, P=0.06); this relationship is no longer valid after accounting for the non-normal distribution pattern of PGC-1 α. There was also no significant relationship between adiponectin and CIII normalized to CS. After accounting for age, trend toward significance remained between CS and adiponectin (r=0.48, P=0.05) but not PGC-1α. There was also a trend of adiponectin adjusted to lean mass to PGC-1 α (r=0.47, P =0.07) and negative relationship between adiponectin and phosphorylated AMPK (r=−0.59, P =0.019).
Figure 4.
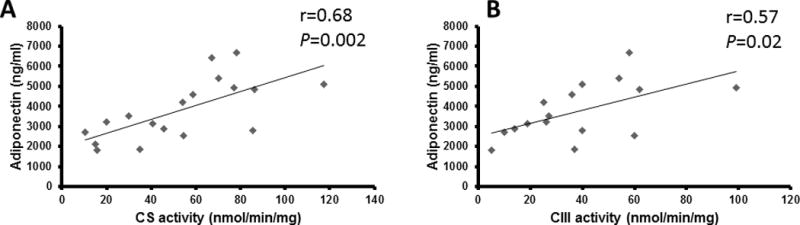
Skeletal muscle citrate synthase (A: CS; n=18) and complex III (B; CIII; n=16) activity related to adiponectin in individuals with SCI.
Discussion
The relationships between serum adiponectin levels, glucose levels and parameters of muscle size, exercise capacity, BMR, and muscle mitochondrial density and activity were examined in persons with SCI. Serum adiponectin levels were inversely related to fat with good concordance for this relationship between whole body fat mass, regional fat mass and IMF. Similarly, adiponectin levels were positively related to total and leg lean mass, thigh muscle CSA and knee extensor CSA, again demonstrating good concordance between multiple measures of skeletal muscle and adiponectin. A relationship between adiponectin and BMR, which is greatly impacted by muscle mass and reduced after SCI. An intriguing additional finding was the relationships between adiponectin, VO2 and levels of citrate synthase and CIII which may reflect an effect of adiponectin on mitochondrial biogenesis or health. Relationships between adiponectin and body composition remained significant after accounting for age. However, relationships between adiponectin and metabolism as well as mitochondrial parameters were influenced by age.
Adiponectin and body composition
Persons with SCI at heightened risk for obesity, insulin resistance and diabetes. A common and vexing secondary complication of SCI is the constellation of changes affecting metabolism that include a dramatic increase in body fat mass, visceral adiposity, fatty liver and elevation of a variety of cytokines [36–38]. Given that muscle is responsible for at least 60% of insulin-mediated glucose uptake, skeletal muscle atrophy after SCI accompanied with decreased mitochondrial density and activity may alter intracellular signaling [26,27,30]. Our understanding of the cellular and molecular mechanisms responsible for these alterations is incomplete. No prior work examined the relationships between adiponectin levels and muscle mass or mitochondria after SCI. Thus, our findings support a new role for adiponectin in regulating properties of skeletal muscle below the level of injury. The relationship between adiponectin and body composition are consistent with previous reports in able bodied individuals [6] and those with SCI [23,24]. Compared to previous work that used BMI [23], we have performed body composition assessment using both DXA and MRI to accurately determine the relationships between adiponectin and total as well as sublesional fat mass or lean mass. BMI underestimates the percentage of fat mass in persons with SCI. Adiponectin was related to regional measurements of leg fat and lean mass measured by both DXA and MRI. We sought to investigate the relationships with body composition, because of the extreme level of muscle atrophy accompanied with infiltration of IMF below the level of injury. The inverse relationship between adiponectin and IMF is consistent with previous studies showing that adiponectin decreased triglyceride content in muscle [12,39]. Decreased IMF may be due to increased muscle fatty acid oxidation induced by adiponectin [15]. This is in line with our recent findings that mitochondrial mass and activity was negatively related to IMF [26,27].
Adiponectin and metabolic profile
Consistent with the observations in body composition, a positive relationship was seen between adiponectin, BMR, and VO2. Contrasting to previous studies that used arm-cycling ergometer [30,40], we have used FES-cycling to determine VO2 [32]. The limitation of this approach is that attaining peak VO2 may be limited by peripheral neuromuscular fatigue occurred during electrical stimulation [39]. However, this approach is beneficial in grouping both individuals with tetraplegia and paraplegia without considering the discrepancy in upper extremity strength. The findings are in contrast to a previous study that found a negative relationship between circulating adiponectin and metabolic rate [42]. However, the difference between the current study and that of Ruige et al. [42] with regard to the methods for measuring body composition (bio-electrical impedance vs. DXA) and adiponectin, as well as participant demographics (able-bodied vs. SCI), makes it difficult to compare the two studies. Moreover, Rugie et al.[42] adjusted BMR to fat-free mass before determining the relationship with adiponectin compared to the current work. Similar to previous studies, steady state was reached within 5 minutes from starting the test and the respiratory exchange ratio was within 0.7-0.9 with coefficient of variations of less than 10% for both VO2 and VCO2 across the last 15 minutes [29, 43]. Another study showed a positive relationship between mean VO2 and adiponectin in able-bodied individuals [44]. The significance of these findings may rely on the fact that persons with SCI experience diminished BMR that is likely to expose them to increased obesity [36, 37].
Adiponectin and protein expression
Important determinants of mitochondrial biology include levels and activation of PGC-1alpha and activity of AMPK. Levels of PGC-1alpha were very low in the majority of subjects, consistent with prior data in rodents following complete spinal cord transection [45]. In subjects for whom higher PGC-1alpha levels were observed these appeared to correlate positively with adiponectin raising intriguing questions about the underlying mechanisms. Skeletal muscle expresses adiponectin receptors and adiponectin is well known to increase insulin action and to activate AMPK [12, 16]. AMPK is turn is a potent regulator of cellular metabolism which activates PGC-1alpha, regulates fatty acid metabolism and insulin action, and drives mitochondrial biogenesis in tissues including skeletal muscle [15]. The relationship between adiponectin and phosphorylated AMPK may suggest that adiponectin regulates the interplay between AMPK and PGC-1alpha. An increase in mitochondrial function may underlie the positive relationship between adiponectin and BMR. Activation of AMPK by adiponectin is a transient event, making it difficult to study in vivo. Peak AMPK activation was observed 20-60 minutes after adiponectin treatment of C2C12 myoblasts [46] and isolated human skeletal muscle preparations [47] and 5 minutes in vivo [16]. A trend for non- significant relationship was observed between adiponectin and another upstream regulator of mitochondrial biogenesis, PGC-1α, in the current study. However, there was considerable variability in PGC-1α protein expression between individuals. The current study only measured total PGC-1α; however, there are a number of PGC-1α isoforms that have different functions in skeletal muscle [48]. Specifically, PGC-1α 4 is associated with skeletal muscle hypertrophy but not mitochondrial biogenesis [49]. Furthermore, PGC-1α activity is modulated by acetylation, ubiquitylation and phosphorylation and the current study did not investigate these post translational modifications. Even so, the current results suggest a moderate relationship between PGC-1α and adiponectin which may inform future studies. Further research will be required to unravel how adiponectin levels may contribute to insulin resistance or diabetes in persons with SCI.
Significance of the work & future implications
The current findings have significant clinical relevance to persons with SCI. Prevalence of obesity, impaired glucose tolerance, insulin resistance and are at a heightened and alarming rate after SCI [37]. Along with decreased level of physical activity, persons with SCI experienced reduced BMR that is likely to be a predisposing factor for several comorbidities. Previous work demonstrated that the frequency and the intensity of the exercise paradigms may not be sufficient to counterbalance several of the health-related comorbidities after SCI. Exercise induced muscle hypertrophy may be a potent stimulus that is associated with increase of circulating plasma adiponectin. The circulating adiponectin will be associated with downstream stimulation of PGC-1 alpha that will lead to increase in body mitochondrial biogenesis and subsequent increase in BMR. The findings may recommend potential pathways that are likely to be used as a countermeasure to resolve this expensive and time-consuming problems. Therefore, recommending pathways that are likely to be target in future pharmaceutical or rehabilitation intervention are of paramount significance to this clinical population.
Limitations
A major limitation of the current study is that only total adiponectin was measured. Adiponectin exists in multiple forms, only some of which are biologically active [50]. Similarly, only total PGC-1α was measured. Furthermore, the small sample size and inclusion of only men may limit the generalization of the current findings. Future studies may investigate various forms of adiponectin as well as PGC-1α isoforms in a larger sample size of both men and women with SCI. The current findings indeed do not support causality but it can be simply used to drive future mechanistic research hypothesis that investigate the role of adiponectin on mitochondrial health and BMR after SCI. In the current study, we did not have a matched control group. Previous studies comparing adiponectin levels in individuals with spinal cord compared to able bodied individuals suggests a trend for higher adiponectin levels after SCI [24]; however, these results were not statistically significant. The primary research hypothesis was aimed to investigate the relationships between adiponectin and possible mechanistic associations with BMR.
Conclusions
This study provides preliminary evidence for a link between adiponectin and metabolic health as demonstrated by increase in BMR and mitochondrial enzyme activity in individuals with SCI. The preliminary evidence presented above supports a negative association between adiponectin and body fat mass, and a positive association with muscle size and mitochondrial parameters after. That is accompanied with concomitant increase in BMR and oxygen uptake. The links between adiponectin levels and the size of paralyzed muscle suggest previously unknown effects of this hormone on muscle homeostasis. These results are consistent with the hypothesis that adiponectin stimulates mitochondrial biogenesis through PGC-1α. Given the critical role of muscle in insulin action, the marked atrophy of muscle below the level of injury, the numerous positive effects adiponectin on metabolism, there is a strong impetus to more fully understand the role of adiponectin on paralyzed muscles after SCI.
Acknowledgments
We would like to thank the Hunter Holmes McGuire Medical Center for allowing us the opportunity to conduct clinical research and to all of our participants. We would like also to thank Refka Khalil for research coordination and to Jeremy Thompson and Ying Hu for technical assistance.
Grants
This work was supported by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs grant # B7867-W and grant # B-2020-C.
Abbreviations
- BMR
basal metabolic rate
- CIII
complex III
- CS
citrate synthase
- CSA
cross sectional area
- DXA
dual energy x-ray absorptiometry
- FFA
free fatty acids
- HbA1c
glycated hemoglobin
- IMF
intramuscular fat
- LOI
level of injury
- PGC-1α
peroxisome-proliferator-activated receptor-gamma co-activator 1alpha
- SCI
spinal cord injury
- Si
insulin sensitivity
- Sg
glucose effectiveness
- TSI
time since injury
- VO2
oxygen uptake
Footnotes
Disclosures
The authors declare no conflict of interest.
References
- 1.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to c1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 2.Hu E, Liang P, Spiegelman BM. Adipo Q is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 3.Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apm1 (adiposemost abundant gene transcript Biochem. Biophys Res Commun. 1996;221:286–289. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- 4.Nakano Y, Tobe T, Choi-Miura NH, Mazda T, Tomita M. Isolation and characterization of gbp28, a novel gelatin-binding protein purified from human plasma. J Biochem. 1996;120:803–812. doi: 10.1093/oxfordjournals.jbchem.a021483. [DOI] [PubMed] [Google Scholar]
- 5.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;425:560–564. doi: 10.1016/j.bbrc.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 6.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clinic Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 7.Rasmussen MS, Lihn A, Pedersen SB, Bruun J, Rasmussen M, Richelsen B. Adiponectin receptors in human adipose tissue: effects of obesity, weight loss, and fat depots. Obes Res. 2006;14:28–35. doi: 10.1038/oby.2006.5. [DOI] [PubMed] [Google Scholar]
- 8.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 9.Civitarese AE, Jenkinson CP, Richardson D, Bajaj M, Cusi K, Kashyap S, et al. Adiponectin receptors gene expression and insulin sensitivity in non-diabetic Mexican Americans with or without a family history of Type 2 diabetes. Diabetologia. 2004;47:816–820. doi: 10.1007/s00125-004-1359-x. [DOI] [PubMed] [Google Scholar]
- 10.Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, et al. Targeted disruption of adipor1 and adipor2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332–339. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 11.Nawrocki AR, Rajala MW, Tomas E, Pajvani UB, Saha AK, Trumbauer ME, et al. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. Biol Chem. 2006;281:2654–2660. doi: 10.1074/jbc.M505311200. [DOI] [PubMed] [Google Scholar]
- 12.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 13.Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, et al. Adiponectin reduces atherosclerosis in apolipoprotein e-deficient mice. Circulation. 2002;106:2767–2770. doi: 10.1161/01.cir.0000042707.50032.19. [DOI] [PubMed] [Google Scholar]
- 14.Yoon MJ, Lee GY, Chung JJ, Ahn YH, Hong SH, Kim JB. Adiponectin increases fatty acid oxidation in skeletal muscle cells by sequential activation of AMP-activated protein kinase, p38 mitogen-activated protein kinase, and peroxisome proliferator-activated receptor alpha. Diabetes. 2006;55:2562–2570. doi: 10.2337/db05-1322. [DOI] [PubMed] [Google Scholar]
- 15.Jäger S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci USA. 2007;104(29):12017–22. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 17.Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M, et al. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+)and AMPK/SIRT1. Nature. 2010;464:1313–1319. doi: 10.1038/nature08991. [DOI] [PubMed] [Google Scholar]
- 18.Nash MS, Mendez AJ. A guideline-driven assessment of need for cardiovascular disease risk intervention in persons with chronic paraplegia. Arch Phys Med Rehabil. 2007;88:751–757. doi: 10.1016/j.apmr.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 19.Bauman WA, Spungen AM. Disorders of carbohydrate and lipid metabolism in veterans with paraplegia or quadriplegia: a model of premature aging. Metabolism. 1994;43:749–756. doi: 10.1016/0026-0495(94)90126-0. [DOI] [PubMed] [Google Scholar]
- 20.Buchholz AC, McGillivray CF, Pencharz PB. Differences in resting metabolic rate between paraplegic and able-bodied subjects are explained by differences in body composition. Am J Clin Nutr. 2003;77:371–8. doi: 10.1093/ajcn/77.2.371. [DOI] [PubMed] [Google Scholar]
- 21.Gorgey AS, Chiodo AE, Zemper ED, Hornyak JE, Rodriguez GM, Gater DR. Relationship of spasticity to soft tissue body composition and the metabolic profile in persons with chronic motor complete spinal cord injury. J Spinal Cord Med. 2010;33:6–15. doi: 10.1080/10790268.2010.11689669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castro MJ, Apple DF, Jr, Staron RS, Campos GE, Dudley GA. Influence of complete spinal cord injury on skeletal muscle within 6 mo of injury. J Appl Physiol. 1999;86:350–358. doi: 10.1152/jappl.1999.86.1.350. [DOI] [PubMed] [Google Scholar]
- 23.Gorgey AS, Dudley GA. Skeletal muscle atrophy and increased intramuscular fat after incomplete spinal cord injury. Spinal Cord. 2007;45:304–309. doi: 10.1038/sj.sc.3101968. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Huang T, Liang H, Su T, Chen S, Wang T. Fasting serum levels of adiponectin, ghrelin, and leptin in men with spinal cord injury. Arch Phys Med Rehabil. 2005;86:1964–8. doi: 10.1016/j.apmr.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 25.Maruyama Y, Mizuguchi M, Yaginuma T, Kusaka M, Yoshida H, Yokoyama K, et al. Serum leptin, abdominal obesity and the metabolic syndrome in individuals with chronic spinal cord injury. Spinal Cord. 2008;46:494–499. doi: 10.1038/sj.sc.3102171. [DOI] [PubMed] [Google Scholar]
- 26.O’Brien LC, Wade RC, Segal L, Chen Q, Savas J, Lesnefsky EJ, et al. Mitochondrial mass and activity as a function of body composition in individuals with spinal cord injury. Physiol Rep. 2017a;5:e13080. doi: 10.14814/phy2.13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Brien LC, Chen Q, Savas J, Lesnefsky EJ, Gorgey AS. Skeletal muscle mitochondrial mass is linked to lipid and metabolic profile in individuals with spinal cord injury. Eur J Appl Physiol. 2017b;117:2137–2147. doi: 10.1007/s00421-017-3687-9. [DOI] [PubMed] [Google Scholar]
- 28.Gorgey AS, Khalil RE, Gill R, O’Brien LC, Lavis T, Castillo T, et al. Effects of Testosterone and Evoked Resistance Exercise after Spinal Cord Injury (TEREX-SCI): study protocol for a randomised controlled trial. BMJ Open. 2017;7:e014125. doi: 10.1136/bmjopen-2016-014125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nightingale TE, Gorgey AS. Predicting Basal Metabolic Rate in Men with Motor Complete Spinal Cord Injury. Med Sci Sports Exerc. doi: 10.1249/MSS.0000000000001548. In Press. [DOI] [PubMed] [Google Scholar]
- 30.Gorgey AS, Graham ZA, Bauman WA, Cardozo C, Gater DR. Abundance in proteins expressed after functional electrical stimulation cycling or arm cycling ergometry training in persons with chronic spinal cord injury. J Spinal Cord Med. 2017;40(4):439–448. doi: 10.1080/10790268.2016.1229397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boston RC, Stefanovski D, Moate PJ, Sumner AE, Watanabe RM, Bergman RN. MINMOD Millennium: a computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes Technol Ther. 2003;5:1003–15. doi: 10.1089/152091503322641060. [DOI] [PubMed] [Google Scholar]
- 32.Gorgey AS, Lawrence J. Acute Responses of Functional Electrical Stimulation Cycling on the Ventilation-to-CO2 Production Ratio and Substrate Utilization After Spinal Cord Injury. PMR. 2016;8:225–34. doi: 10.1016/j.pmrj.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 33.Gorgey AS, Cirnigliaro CM, Bauman W, Adler RA. Estimates of the precision of regional and whole-body composition by dual-energy x-ray absorptiometry in persons with chronic spinal cord injury. Spinal Cord. doi: 10.1038/s41393-018-0079-x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gorgey AS, Mather KJ, Cupp HR, Gater DR. Effects of resistance training on adiposity and metabolism after spinal cord injury. Med Sci Sports Exerc. 2012;44(1):165–74. doi: 10.1249/MSS.0b013e31822672aa. [DOI] [PubMed] [Google Scholar]
- 35.Brass EP, Hiatt WR, Gardner AW, Hoppel CL. Decreased NADH dehydrogenase and ubiquinol-cytochrome c oxidoreductase in peripheral arterial disease. Am J Physiol Heart Circ Physiol. 2001;280(2):H603–9. doi: 10.1152/ajpheart.2001.280.2.H603. [DOI] [PubMed] [Google Scholar]
- 36.Gorgey AS, Gater DR. Demographic of obesity after spinal cord injury. Top Spinal Cord Inj Rehabil. 2007;12:1–7. doi: 10.1310/sci1204-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gorgey AS, Dolbow DR, Dolbow JD, Khalil RK, Castillo C, Gater DR. Effects of spinal cord injury on body composition and metabolic profile - part I. J Spinal Cord Med. 2014;37:693–702. doi: 10.1179/2045772314Y.0000000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rankin KC, O’Brien LC, Segal L, Khan MR, Gorgey AS. Liver Adiposity and Metabolic Profile in Individuals with Chronic Spinal Cord Injury. Biomed Res Int. 2017;2017:1364818. doi: 10.1155/2017/1364818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mullen K, Pritchard J, Ritchie I, Snook L, Chabowski A, Bonen A, et al. Adiponectin resistance precedes the accumulation of skeletal muscle lipids and insulin resistance in high-fat-fed rats. Am J Physiol Regul Integr Comp Physiol. 2009;296:R243–51. doi: 10.1152/ajpregu.90774.2008. [DOI] [PubMed] [Google Scholar]
- 40.Simmons OL, Kressler J, Nash MS. Reference fitness values in the untrained spinal cord injury population. Arch Phys Med Rehabil. 2014;95(12):2272–8. doi: 10.1016/j.apmr.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 41.Gorgey AS, Poarch HJ, Dolbow DD, Castillo T, Gater DR. Effect of adjusting pulse durations of functional electrical stimulation cycling on energy expenditure and fatigue after spinal cord injury. J Rehabil Res Dev. 2014;51(9):1455–68. doi: 10.1682/JRRD.2014.02.0054. [DOI] [PubMed] [Google Scholar]
- 42.Ruige JB, Ballaux DP, Funahashi T, Mertens IL, Matsuzawa Y, Van Gaal LF. Resting metabolic rate is an important predictor of serum adiponectin concentrations: potential implications for obesity-related disorders. Am J Clin Nutr. 2005;82:21–5. doi: 10.1093/ajcn.82.1.21. [DOI] [PubMed] [Google Scholar]
- 43.Nevin A, Mayr H, Atresh S, Kemp I, Simmons J, Vivanti A, et al. Feasibility and Acceptability of Implementing Indirect Calorimetry into Routine Clinical Care of Patients with Spinal Cord Injury. Top Spinal Cord Inj Rehabil. 2016;22(4):269–276. doi: 10.1310/sci2016-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jurimae J, Vaiksaar S, Purge P, Jurimae T. Adiponectin and osteocalcin responses to rowing exercise, and the relationship to substrate oxidation in female rowers. Phys Int. 2016;103:220–230. doi: 10.1556/036.103.2016.2.9. [DOI] [PubMed] [Google Scholar]
- 45.Wu Y, Zhao J, Zhao W, Guo S, Bauman WA, Cardozo CP. Nandrolone normalizes determinants of muscle mass and fiber type after spinal cord injury. J Neurotrauma. 2012;29(8):1663–75. doi: 10.1089/neu.2011.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou L, Deepa SS, Etzler JC, Ryu J, Mao X, Fang Q, et al. Adiponectin activates AMP-activated protein kinase in muscle cells via APPL1/LKB1-dependent and phospholipase C/Ca2+/Ca2+/calmodulin-dependent protein kinase kinase-dependent pathways. Journal of Biological Chemistry. 2009;284:22426–35. doi: 10.1074/jbc.M109.028357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bruce CR, Mertz VA, Heigenhause GJ, Dyck DJ. The stimulatory effect of globular adiponectin on insulin-stimulated glucose uptake and fatty acid oxidation is impaired in skeletal muscle from obese subjects. Diabetes. 2005;54:3154–3160. doi: 10.2337/diabetes.54.11.3154. [DOI] [PubMed] [Google Scholar]
- 48.Martínez-Redondo V, Pettersson AT, Ruas JL. The hitchhiker’s guide to PGC-1α isoform structure and biological functions. Diabetologia. 2015;58(9):1969–77. doi: 10.1007/s00125-015-3671-z. [DOI] [PubMed] [Google Scholar]
- 49.Ruas JL, White JP, Rao RR, Kleiner S, Brannan KT, Harrison BC, et al. A PGC-1α isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell. 2012;151:1319–1331. doi: 10.1016/j.cell.2012.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Achari AE, Jain SK. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int J Mol Sci. 2017;18:1321. doi: 10.3390/ijms18061321. [DOI] [PMC free article] [PubMed] [Google Scholar]


