Abstract
Objective:
To examine the bidirectional relationship between weight change and obstructive sleep apnea (OSA) in the context of a behavioral weight loss intervention.
Patients and Methods:
Adults who were overweight or obese (N=114) participated in a 12-month behavioral weight loss intervention from April 17, 2012, through February 9, 2015. The apnea-hypopnea index (AHI), our marker of the presence and severity of OSA, was assessed at baseline, 6 months, and 12 months. Linear mixed models evaluated the effect of weight change on AHI and the effect of OSA (AHI≥5) on subsequent weight loss. Secondary analyses evaluated the effect of OSA on intervention attendance, meeting daily calorie goals, and accelerometer-measured physical activity.
Results:
At baseline, 52% of the sample had OSA. Adults who achieved ≥5% weight loss had an AHI reduction that was 2.1±0.9 (adjusted mean±standard error) events/h greater than those with <5% weight loss (P<.05). Adults with OSA lost 2.2±0.9% less weight during the subsequent 6-month interval compared to those without OSA (P=.02). Those with OSA were less adherent to daily calorie goals (25.2±3.3 vs. 34.8±3.4% of days; P=.006) and had a smaller increase in daily activity (378.3±353.7 vs. 1060.1±377.8 steps/day; P<.05) over 12 months than those without OSA.
Conclusion:
Behaviorally induced weight loss in overweight/obese adults was associated with significant AHI reduction. However, the presence of OSA was associated with blunted weight loss, potentially via reduced adherence to behaviors supporting weight loss. These results suggest that OSA screening prior to attempting weight loss may be helpful to identify who may benefit from additional behavioral counseling.
INTRODUCTION
Obstructive sleep apnea (OSA) is a prevalent sleep disorder whose underlying pathogenesis is often driven by excess weight.1-3 Multiple studies have found that weight loss is effective at reducing OSA severity.4,5 In particular, behavioral weight loss interventions, which typically incorporate dietary modification and physical activity to induce weight loss, reduce the apnea-hypopnea index (AHI) by 20-50%.6 Accordingly, weight loss is a common first-line recommendation for overweight adults with OSA.7
In contrast, less attention has been placed on the potential impact of underlying OSA on attempted weight loss. Evidence suggests that OSA can impede weight loss,8-10 but these studies are limited by small sample sizes8,10 and many did not consider adherence to behaviors supporting weight loss.9,10 Moreover, these samples included few females, who are more likely to engage in weight loss efforts than men,11 yet among whom OSA is less likely to be recognized.12
The purpose of this study was to examine the bidirectional association between weight change and OSA in the context of a 12-month behavioral weight loss intervention. We examined the association between behaviorally induced weight loss and OSA severity, and whether underlying OSA was associated with subsequent weight loss. Based on prior evidence, we hypothesized that OSA severity would be significantly reduced as a result of the lifestyle intervention. We also hypothesized that the presence of OSA would be associated with blunted weight loss, and that adults with OSA would be less adherent at modifying caloric intake and physical activity compared to adults without OSA.
METHODS
Study Design and Sample
The current study was an ancillary component of the EMPOWER study. The primary aim of EMPOWER was to determine, using ecological momentary assessment methods, the triggers for lapses during intentional weight loss.13 As a longitudinal descriptive study, all participants received a group-delivered behavioral weight loss intervention over 12 months. Additional details regarding participants and the intervention are described below and in Burke et al.13 The University of Pittsburgh Institutional Review Board approved the study protocol and participants provided written informed consent prior to participating. The study was conducted from April 17, 2012, through February 9, 2015.
Adults ≥18 years old with a body mass index (BMI) between 27 and 44 kg/m2 who had not participated in a weight loss program in the prior 3 months were eligible. Exclusion criteria included the presence of medical conditions that could confound study findings (e.g., diabetes), pregnancy, or other conditions that would prevent completion of the intervention.13 All participants who were not being treated for OSA (e.g., continuous positive airway pressure [CPAP]) were eligible to participate in the ancillary study; see Supplemental Figure for data loss through the study. Thirty-seven of the 151 participants enrolled in EMPOWER were excluded from the present analyses, leaving 114 participants; primary reasons for exclusion included study participation prior to the addition of OSA assessments (n=18) and current OSA treatment (n=9). In addition, 12 of the 114 participants withdrew prior to study completion. Compared to those who completed the study, participants who withdrew were younger and less likely to be of white race (44.1±13.2 years vs. 51.2±9.9 years [P=.03], 58.3% vs. 85.3% white [P=.02]); however, they did not differ on other factors, including baseline BMI or AHI.
All participants received a standard behavioral weight loss intervention delivered in groups.14 Key intervention components included provision of behavior change strategies such as daily dietary and weekly exercise goals along with self-monitoring of dietary intake, physical activity, and weight.13 Daily caloric intake goals were based on initial body weight and gender. For those weighing <90.9 kg (i.e., 200 pounds), 1200 kcal/day and 1500 kcal/day were prescribed for women and men, respectively; for participants weighing ≥90.9 kg, 1500 kcal/day and 1800 kcal/day were prescribed for women and men, respectively. Participants were instructed to gradually increase their physical activity over the initial six weeks until they reached a goal of 150 min/wk. Sleep was not addressed as part of the intervention. Daily self-monitoring of dietary intake, physical activity, and weight were performed using a smartphone application (Lose It!; FitNow, Inc., Boston, MA). Twenty-four group sessions were offered: meetings occurred weekly for the initial 3 months, biweekly during months 4 through 6, then monthly over the final 6 months.
Measures
Sociodemographics.
Sociodemographic data (age, sex, marital status, race/ethnicity, smoking status) were collected using a self-administered questionnaire.
Weight.
Body weight was measured at baseline (BL), 6 months (6M), and 12 months (12M) by a digital scale (Tanita; Arlington Heights, IL). Weight data at 6M and 12M were expressed as the percentage change from BL. Weight loss ≥5% was considered clinically significant.15
Obstructive sleep apnea.
OSA was assessed with a limited-channel home sleep testing device (ApneaLink [firmware version 05.02]; ResMed, San Diego, CA) over single nights at BL, 6M, and 12M. This device records nasal pressure and pulse oximetry signals to derive summary measures of OSA.16 Records were scored automatically using manufacturer-supplied software (version 9.30); an apnea was defined as a ≥80% decline in airflow for ≥10 sec and a hypopnea was defined as a ≥30% decline in airflow for ≥10 sec. The AHI was calculated as the sum of apneas and hypopneas divided by recording time in hours. Presence of OSA was defined as an AHI≥5. Two hours of valid recording time was required to include an OSA assessment in analyses.
Intervention behaviors.
Three behaviors related to the intervention were assessed: group session attendance, adherence to daily calorie goals, and objectively monitored physical activity.
Group session attendance.
We calculated the percentage of group sessions attended over the first 6 months (18 possible) and the final 6 months (6 possible).
Caloric intake.
Participants reported their caloric intake each day using a self-monitoring application (Lose It!; FitNow, Inc., Boston, MA). Daily adherence to the prescribed caloric intake goal was calculated by dividing the calories consumed on a specific day by the daily caloric goal and then multiplying by 100. Participants were categorized as being adherent (reported consuming 85-115% of the calorie goal) or non-adherent (reported consuming <85% or >115% of the calorie goal) each day; days without reported caloric intake were categorized as non-adherent.17 We calculated the percentage of days the participant met their individual daily calorie goal as a summary measure of adherence, calculated separately for the first and final 6 months.
Physical activity.
Participants wore an accelerometer (GT3x; ActiGraph Corp., Pensacola, FL) on their waist during waking hours for 7 days at BL, 6M, and 12M. Mean data from participants with ≥4 days of wear time ≥10 h/day were included in analyses. Daily durations of sedentary behavior and moderate-vigorous physical activity (MVPA) were established using standard cutpoints.18 Steps/day and daily time spent sedentary and in MVPA were retained for analysis, using change scores at 6M and 12M by subtracting them from BL values.
Statistical Analysis
All participants with valid baseline data were included in analyses. Data were first screened for any anomalies, which revealed several extreme AHI values at each time point. Although multiple strategies were considered to handle the extreme values (i.e., winsorization, natural logarithm transformation), similar results were obtained regardless of approach; therefore, for ease of interpretation, untransformed data are reported. Change scores for AHI were normally distributed and were not transformed. Analyses evaluated the effects of the intervention on weight and AHI, the association between changes in weight and AHI, and whether OSA predicted subsequent weight change and intervention behaviors. All analyses included covariates known to be associated with sleep and/or OSA: age (years), sex (male, female), race (white, black/other), marital status (never married, married/living with partner, widowed/divorced/separated), and smoking status (current/former, never). Unless otherwise noted, sample descriptive statistics are summarized using mean±standard deviation or n (%), while outcomes are summarized using adjusted means±standard error. All tests were two-tailed, with statistical significance set at P<.05, using SAS version 9.4 (SAS Institute, Inc.; Cary, NC).
Change in weight and AHI over time.
Using the marginal distribution of the observed data, linear mixed models were used to evaluate the trajectories of weight and AHI over time (BL, 6M, 12M). Models assumed a normal (Gaussian) error and identity link. Time point and covariates were included as fixed effects, with random effects for subjects; a random intercept model was fit and compound symmetry was assumed across repeated assessments. Pairwise comparisons across time points were made using adjusted means. We used a generalized linear mixed model to evaluate whether OSA prevalence (AHI ≥5) differed across time points assuming a binomial error structure and logit link to the linear predictors.
Association of weight change with change in OSA severity.
We used a linear mixed model, incorporating the BL-6M and BL-12M intervals and using the marginal distribution of available data, to assess whether ≥5% weight loss during a given interval was associated with AHI change. An interaction term evaluated whether the association differed according to the interval (BL-6M, BL-12M), and baseline AHI was included as a covariate.
Association of OSA prevalence and severity with weight change and intervention behaviors.
We evaluated whether the presence of at least mild OSA at the beginning of an interval (i.e., AHI≥5 at BL or 6M) was associated with weight change over the subsequent interval (BL-6M, 6M-12M) using a linear mixed model using the marginal distribution of available data. An interaction term evaluated whether the association differed according to the interval (BL-6M, 6M-12M), and BMI at the start of each interval was included as a covariate. We also repeated these analyses using continuous AHI values to examine whether OSA severity (regardless of ‘mild OSA’ or ‘no OSA’ classification) was related to weight change.
We used linear mixed models to evaluate whether the presence of OSA at the beginning of an interval was associated with intervention-related behaviors (session attendance, adherence to daily calorie goals, physical activity [change in steps/day, change in sedentary time/day, change in MVPA/day]). An interaction term was included in the models to investigate whether the associations differed according to the interval (BL-6M, 6M-12M). For session attendance and adherence to daily calorie goals, BMI at the start of each interval was included as a covariate in each model; for activity outcomes, change in wear time and the respective activity parameter at the start of each interval were included as covariates.
RESULTS
Participant Characteristics
Baseline characteristics of the study sample (N=114) are summarized in Table 1. On average, the sample was middle-aged (50.4±10.5 years) and obese (BMI=34.0±4.6 kg/m2), with most being female (90.4%) and white (82.5%). Mean BL AHI was 7.0±8.2, with a range of 0 to 53 events/hour. Approximately one-half of the sample (51.8%; n=59) had at least mild-severity OSA (AHI≥5) at BL; however, only 10.5% (n=12) had at least moderate-severity OSA (AHI≥15). Aside from having significantly higher AHI (P<.001), adults with at least mild OSA were older (P=.008), had a greater BMI (P=.02), and accumulated fewer steps/day at baseline (P<.05) than those without OSA.
Table 1.
| Characteristicsc | Total (N=114) |
BL AHI < 5 (n=55) |
BL AHI ≥ 5 (n=59) |
|---|---|---|---|
| Age (years) * | 50.4 (10.5) | 47.7 (10.7) | 52.9 (9.8) |
| Female sex | 103 (90.4) | 51 (92.7) | 52 (88.1) |
| White race | 94 (82.5) | 43 (78.2) | 51 (86.4) |
| Body mass index (kg/m2) * | 34.0 (4.6) | 33.0 (4.5) | 34.9 (4.5) |
| Marital status | |||
| Married/living with partner | 68 (59.6) | 30 (54.5) | 38 (64.4) |
| Never married | 22 (19.3) | 12 (21.8) | 10 (16.9) |
| Widowed/separated/divorced | 24 (21.1) | 13 (23.6) | 11 (18.6) |
| Never smoker | 79 (69.3) | 36 (65.5) | 43 (72.9) |
| AHI * | 7.0 (8.2) | 1.9 (1.3) | 11.8 (9.0) |
| Steps, no./day * | 6091.3 (1765.6) | 6433.0 (1603.1) | 5772.8 (1862.0) |
| SED, min/day | 634.7 (89.7) | 637.5 (95.7) | 632.1 (84.6) |
| MVPA, min/day | 10.9 (10.1) | 12.5 (11.0) | 9.5 (9.1) |
AHI=apnea-hypopnea index; BL=baseline; MVPA=moderate-vigorous physical activity; SD=standard deviation; SED=sedentary.
Data are presented as mean (standard deviation) or No. (percentage).
Between-group comparisons based upon analysis of variance or χ2, as appropriate:
P <.05.
Changes in Weight and AHI over Time
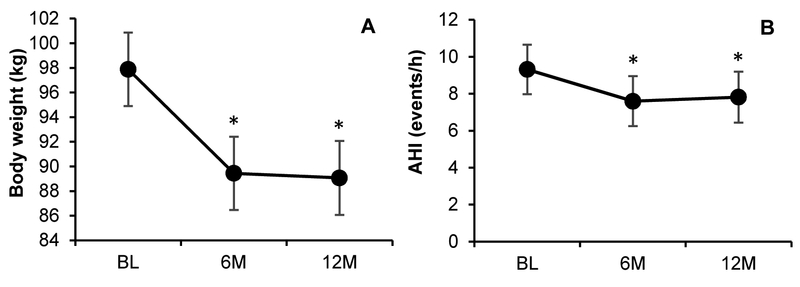
The changes in body weight and AHI over 12 months are depicted in Figure 1. There was a significant reduction in body weight over time (P<.001). Relative to BL, weight was lower at 6M and 12M (−8.4±0.6 kg [P<.001], −8.8±0.7 kg [P<.001], respectively); however, weight did not significantly change between 6M and 12M (−0.4±0.7 kg; P=.60). We also observed a significant AHI reduction over time (P=.009). AHI at 6M and 12M were significantly lower than AHI at BL (−1.7±0.6 events/h [P=.005], −1.5±0.6 events/h [P=.02], respectively); however, AHI did not significantly change between 6M and 12M (0.2±0.7 events/h; P=.75). In addition, the prevalence of OSA was reduced over time (P=.02). Relative to BL, the odds of having OSA were not significantly lower at 6M (odds ratio [OR]=0.69 [0.36,1.34]; P=.28) but were significantly lower at 12M (OR=0.36 [0.17,0.73]; P=.005).
Figure 1. Weight and AHI change according to intervention time point.
Data shown are adjusted means±standard error. Abbreviations: 6M=6-month; 12M=12-month; AHI=apnea-hypopnea index; BL=baseline. * indicates significant difference from baseline (P<.05).
Association Between Weight Change and OSA
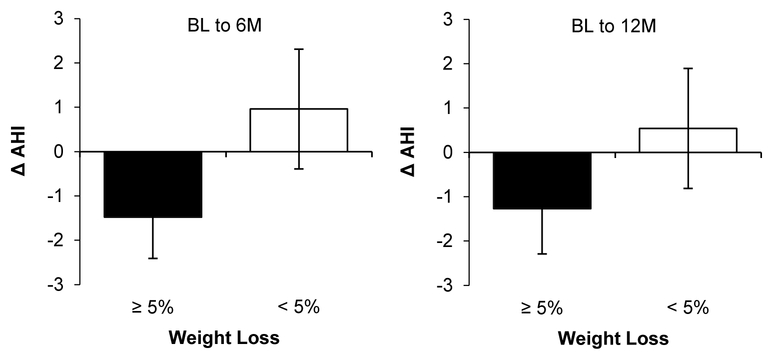
The relationship between weight loss and AHI change is depicted in Figure 2. Achievement of ≥5% weight loss was associated with 2.1±0.9 events/h greater AHI reduction relative to <5% weight loss (P<.05). The association between weight change and AHI change was consistent across time intervals (BL-6M, BL-12M; interaction P=.72).
Figure 2. Relationship between weight loss (≥5%, <5%) and AHI change.
Abbreviations: 6M=6-month; 12M=12-month; AHI=apnea-hypopnea index; BL=baseline.
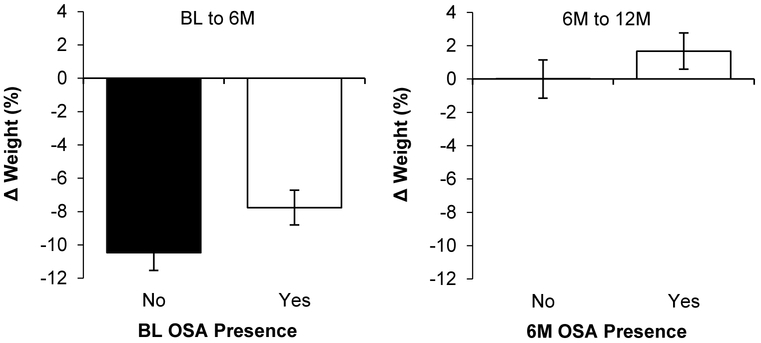
Association Between OSA Status and Severity with Weight Change
The association between OSA status and subsequent weight change is depicted in Figure 3. Participants with AHI≥5 lost 2.2±0.9% less weight than those with AHI<5 (P=.02), with the association between OSA status and weight change not differing over time (BL-6M, 6M-12M; interaction P=.51). When examined as a continuous variable, greater AHI was associated with lower weight loss over the subsequent 6 months (B=0.19 [0.11], P=.04), with the association being consistent across time intervals (interaction P=.40).
Figure 3. Relationship between OSA status and subsequent weight change.
Abbreviations: 6M=6-month; 12M=12-month; AHI=apnea-hypopnea index; BL=baseline; OSA=obstructive sleep apnea.
Association Between OSA Status and Intervention Behaviors
The association between OSA status and intervention behaviors is summarized in Table 2. Attendance to intervention sessions did not differ according to the presence of OSA (74.5±3.5% [OSA], 75.7±3.7% [no OSA] of sessions attended; P=.72), and the relationship was similar over time (interaction P=.32). Across 12 months, participants with OSA were adherent to their daily calorie goals on 25.2±3.3% of days, while those without OSA were adherent on 34.8±3.4% of days (P=.006); the association was similar over time (interaction P=.53). Across 12 months, participants with OSA increased their physical activity by 378.3±353.7 steps/day and 2.1±2.3 min/day of MVPA, while those without OSA increased their activity by 1060.1±377.8 steps/day (P<.05) and 6.4±2.5 min/day of MVPA (P=.06); both of these associations were similar over time (each interaction P≥.39). Sedentary time was reduced similarly among those with and without OSA (−7.1±9.3 min/day [OSA], −9.1±9.8 min/day [no OSA]; P=.81), with the relationship being similar over time (P=. 96).
Table 2.
| BL to 6M values |
6M to 12M values |
P values |
|||||
|---|---|---|---|---|---|---|---|
| BL OSA: |
6M OSA: |
BL- | 6M- | ||||
| Measure | No | Yes | No | Yes | Overall | 6M | 12M |
| Attendance, % | 84.7 (4.2) | 80.4 (4.2) | 66.8 (4.4) | 68.6 (4.2) | .72 | .33 | .71 |
| Caloric goal, % | 43.7 (3.9) | 35.9 (3.9) | 25.9 (4.1) | 14.5 (3.9) | .006 | .06 | .01 |
| Steps, no./day c | 1970.2 (416.9) | 1026.3 (397.9) | 150.1 (454.3) | −269.8 (439.0) | <.05 | .03 | .37 |
| SED, min/day c | −13.9 (11.2) | −12.3 (10.7) | −4.3 (11.4) | −2.0 (11.0) | .81 | .88 | .85 |
| MVPA, min/day c | 12.3 (2.7) | 6.9 (2.6) | 0.4 (3.00) | −2.6 (2.9) | .06 | <.05 | .31 |
6M=6-month; 12M=12-month; AHI=apnea-hypopnea index; BL=baseline; MVPA=moderate-vigorous physical activity; OSA=obstructive sleep apnea; SED=sedentary.
Data are presented as adjusted means (standard error).
Data represent changes from beginning to end of interval (e.g., change from baseline to 6 months).
DISCUSSION
This study evaluated the bidirectional association between weight change and OSA in the context of a 12-month behavioral weight loss intervention. Overall, we found that weight loss reduced OSA severity, but also that underlying OSA led to worse weight loss outcomes. In addition, those with OSA were less likely to adhere to daily calorie goals or increase physical activity, despite similar attendance at intervention sessions.
The behavioral weight loss intervention was associated with significant AHI reduction, which aligns with findings from most other behavioral weight loss interventions.6,19 While the 12-month reduction in AHI was relatively small given the weight loss observed—approximately 16% AHI reduction with 9% weight loss—this may be at least partially due to the mild OSA severity at baseline. Despite this small magnitude of improvement, the odds of having OSA were 64% lower following the 12-month intervention. Thus, our results support the existing recommendation of weight loss for overweight adults with OSA, especially those with milder severity OSA.20
While OSA is often implicated as a significant factor contributing to weight gain,21 less inquiry has been made on the influence of OSA on weight loss. Whited and colleagues found that adults who were designated ‘high risk’ for OSA based upon self-report lost 3% less weight following a 12-month dietary weight loss intervention than those screened as low risk.22 In a sample of 34 adults, Eraslan and colleagues observed that those with AHI≥30 were significantly less likely to achieve ≥3% weight loss following a 12-week lifestyle intervention compared to those with milder-severity OSA.10 Borel and colleagues reported that those with OSA at baseline (n=28) had significantly smaller reductions in body weight, waist circumference, and fat mass following a 12-month lifestyle intervention compared to those without OSA (n=49).8 More recently, in an analysis of ≥80,000 veterans enrolled in a 12-month weight loss program, Janney and colleagues found that those who were diagnosed with sleep-disordered breathing lost ~0.4 kg less weight than those without this diagnosis.9 Finally, in a sample of 816 bariatric surgery patients, de Raaff and colleagues found that those with OSA at preoperative screening experienced less weight loss at 6 and 12 months following surgery compared to those without OSA, though the strength of the relationship weakened considerably once confounders such as age and baseline BMI were considered.23 As we found an approximately 2% difference in weight loss between those with and without OSA in a predominantly female sample with mild OSA, our results are in agreement with prior behavioral weight loss interventions and indicate that even mild OSA may impair behavioral weight loss outcomes.
Compared to adults without OSA, those with OSA were less adherent to their daily calorie goals and had smaller improvements in daily physical activity despite similar attendance at intervention sessions. These results contrast with those of two prior studies that found no differences in self-reported caloric intake8,22 and self-reported22 or pedometer-assessed activity8 between those with and without OSA. Although limited research is available,21 our finding of lower adherence to caloric intake goals is in agreement with research indicating that OSA is associated with greater fat consumption and greater overall energy intake.24 Moreover, other studies have reported an inverse relationship between physical activity levels and OSA severity.25,26 The reduced adherence to behaviors supporting weight loss could be driven, at least in part, by the sleep disturbance brought upon by underlying OSA. Among adults without OSA, sleep restriction leads to increased energy intake and decreased physical activity.27,28 Our findings suggest that reduced adherence to these behaviors contributed to the lower weight loss observed among those with OSA in the current study. These results also raise the possibility that adults with OSA may need more extensive behavioral counseling to optimize adherence to behaviors supporting weight loss.
Although not measured in this study, the lower weight loss observed in those with OSA could also be due to metabolic dysregulation induced by OSA. Adults with OSA have altered glucose metabolism and impaired appetite regulation either as a direct result of OSA or its associated sleep disturbance.29,30 Borel and colleagues found an inverse relationship between baseline OSA severity and improvement in glucose tolerance following the 12-month lifestyle intervention, alongside a smaller increase in plasma adiponectin.8 Future research should evaluate how these parameters respond to weight loss in adults with OSA.
Our results, as well as those of others, raise the question as to whether standard treatment of OSA (e.g., CPAP) leads to greater weight loss in a behavioral weight loss intervention. However, the available evidence does not support this possibility.31,32 Most notably, in a sample of 181 adults with severe OSA, Chirinos and colleagues found that adding CPAP to a 6-month behavioral weight loss intervention did not result in greater weight loss compared to those who only received the weight loss intervention.31 In addition, outside of behavioral weight loss interventions, CPAP has been shown to lead to mild weight gain.33 Despite these findings, it remains possible that OSA treatment may be a necessary precursor to minimize the barrier of poor sleep on behavior modification.34,35
Study limitations should be noted. The main limitation was the assessment of OSA. Although common in both clinical and research settings, use of a limited channel device to measure OSA parameters is less accurate than laboratory-based polysomnography.36 Because respiratory events are expressed relative to recording duration rather than sleep duration, OSA parameters are often underestimated.16 In addition, the lack of a control group with no lifestyle restrictions alongside the behavioral intervention was a study limitation for this analysis. There were also multiple strengths to this study. The sample, the largest to date to examine the effect of directly measured OSA on weight loss, was a significant strength. In particular, the high prevalence of females and mild-severity OSA are significant strengths, as females are more likely than males to attempt weight loss yet are less likely to be considered for OSA,11,12 while weight loss is often recommended for mild OSA.37 Furthermore, we were able to assess multiple behaviors related to the weight loss intervention at multiple time points over the 12-month period, which allowed us to address potential behavioral factors that were related to the lower weight loss observed among those with OSA. Finally, although data loss occurred for a variety of other reasons (e.g., device recording failure), low participant attrition was a study strength, as 89.5% of the 114 participants included in these analyses completed the 12-month study.
CONCLUSION
Overall, we observed a bidirectional association between weight change and OSA in the context of a behavioral weight loss intervention. The blunted weight loss observed in those with OSA may be due to reduced adherence to behaviors supporting weight loss. This introduces a problematic cycle, wherein weight loss is recommended for mild and moderate OSA, but those with at least mild OSA are less likely to lose weight. Our results suggest that OSA screening prior to initiation of weight loss efforts may be helpful to identify who may benefit from additional behavioral counseling. Future research should examine whether screening and treatment of OSA prior to initiating a behavioral weight loss intervention leads to better weight loss outcomes.
Supplementary Material
ACKNOWLEDGMENTS
Home sleep testing devices used in this study were donated by ResMed (San Diego, CA). ResMed had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support: Funding for this study was provided by National Institutes of Health (NIH) grant R01HL107370 (PI: Burke). Additional investigator support for DDM, ERC, and CEK was provided by NIH grants R01HL107370-S1 (PI: Mendez), K24NR016685 (PI: Chasens), and K23HL118318 (PI: Kline), respectively. Investigator support for BR-W was provided by Agency for Healthcare Research and Quality grant K12HS022989 (PI: Kapoor).
ABBREVIATIONS
- 12M
12 months
- 6M
6 months
- AHI
apnea-hypopnea index
- BL
baseline
- BMI
body mass index
- CPAP
continuous positive airway pressure
- MVPA
moderate-vigorous physical activity
- OSA
obstructive sleep apnea
Footnotes
Conflicts of interest: PJS reports personal fees from Resmed, Inspire Medical Systems, and Philips Respironics; has served as a consultant for Jazz Pharmaceuticals and Itamar Medical; has been a member of the NFL General Medical Committee; and has given expert testimony for Harris v Emory. CEK, LEB, SMS, CCI, BR-W, DDM, YZ, SLR, and ERC have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartz AR, Patil SP, Laffan AM, Polotsky V, Schneider H, Smith PL. Obesity and obstructive sleep apnea: pathogenic mechanisms and therapeutic approaches. Proc Am Thorac Soc. 2008;5(2):185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol. 2005;99(4):1592–1599. [DOI] [PubMed] [Google Scholar]
- 4.Tuomilehto H, Seppa J, Uusitupa M. Obesity and obstructive sleep apnea--clinical significance of weight loss. Sleep Med Rev. 2013;17(5):321–329. [DOI] [PubMed] [Google Scholar]
- 5.Sarkhosh K, Switzer NJ, El-Hadi M, Birch DW, Shi X, Karmali S. The impact of bariatric surgery on obstructive sleep apnea: a systematic review. Obes Surg. 2013;23(3):414–423. [DOI] [PubMed] [Google Scholar]
- 6.Araghi MH, Chen YF, Jagielski A, et al. Effectiveness of lifestyle interventions on obstructive sleep apnea (OSA): systematic review and meta-analysis. Sleep. 2013;36(10):1553–1562E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Epstein LJ, Kristo D, Strollo PJ, Jr., et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263–276. [PMC free article] [PubMed] [Google Scholar]
- 8.Borel AL, Leblanc X, Almeras N, et al. Sleep apnoea attenuates the effects of a lifestyle intervention programme in men with visceral obesity. Thorax. 2012;67(8):735–741. [DOI] [PubMed] [Google Scholar]
- 9.Janney CA, Kilbourne AM, Germain A, et al. The influence of sleep disordered breathing on weight loss in a national weight management program. Sleep. 2016;39(1):59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eraslan A, Selcuk OT, Eyigor H, Erkal Z, Osma U. Success rate of outpatient weight management in obese patients with obstructive sleep apnea. Acta Medica Mediterr. 2016;32(5):1705–1712. [Google Scholar]
- 11.Kruger J, Galuska DA, Serdula MK, Jones DA. Attempting to lose weight: specific practices among U.S. adults. Am J Prev Med. 2004;26(5):402–406. [DOI] [PubMed] [Google Scholar]
- 12.Lindberg E, Benediktsdottir B, Franklin KA, et al. Women with symptoms of sleep-disordered breathing are less likely to be diagnosed and treated for sleep apnea than men. Sleep Med. 2017;35(17-22. [DOI] [PubMed] [Google Scholar]
- 13.Burke LE, Shiffman S, Music E, et al. Ecological momentary assessment in behavioral research: addressing technological and human participant challenges. J Med Internet Res. 2017;19(3):e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wing RR. Behavioral approaches to the treatment of obesity. In: Bray GA, Bouchard C, James WPT, eds. Handbook of Obesity: Clinical Applications. New York: Marcel Dekker; 2004:147–167. [Google Scholar]
- 15.Jensen MD, Ryan DH, Donato KA, et al. Guidelines (2013) for the management of overweight and obesity in adults. Obesity. 2014;22(S2):S5–S39.24961825 [Google Scholar]
- 16.Oktay B, Rice TB, Atwood CW Jr, et al. Evaluation of a single-channel portable monitor for the diagnosis of obstructive sleep apnea. J Clin Sleep Med. 2011;7(4):384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng Y, Burke LE, Danford CA, Ewing LJ, Terry MA, Sereika SM. Patterns of self-weighing behavior and weight change in a weight loss trial. Int J Obesity. 2016;40(9):1392–1396. [DOI] [PubMed] [Google Scholar]
- 18.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30(5):777–781. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell LJ, Davidson ZE, Bonham M, O'Driscoll DM, Hamilton GS, Truby H. Weight loss from lifestyle interventions and severity of sleep apnea: a systematic review and meta-analysis. Sleep Med. 2014;15(10):1173–1183. [DOI] [PubMed] [Google Scholar]
- 20.Tuomilehto H, Seppa J, Uusitupa M, et al. The impact of weight reduction in the prevention of the progression of obstructive sleep apnea: an explanatory analysis of a 5-year observational follow-up trial. Sleep Med. 2014;15(3):329–335. [DOI] [PubMed] [Google Scholar]
- 21.Shechter A Obstructive sleep apnea and energy balance regulation: a systematic review. Sleep Med Rev. 2017;34(59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whited MC, Olendzki E, Ma Y, et al. Obstructive sleep apnea and weight loss treatment outcome among adults with metabolic syndrome. Health Psychol. 2016;35(12):1316–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Raaff CA, Coblijn UK, de Vries N, et al. Predictive factors for insufficient weight loss after bariatric surgery: does obstructive sleep apnea influence weight loss? Obes Surg. 2016;26(5): 1048–1056. [DOI] [PubMed] [Google Scholar]
- 24.Cao Y, Wittert G, Taylor A, Adams R, Shi Z. Associations between macronutrient intake and obstructive sleep apnoea as well as self-reported sleep symptoms: results from a cohort of community dwelling Australian men. Nutrients. 2016;8(4):207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chasens ER, Sereika SM, Houze MP, Strollo PJ. Subjective and objective appraisal of activity in adults with obstructive sleep apnea. J Aging Res. 2011;2011(751819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verwimp J, Ameye L, Bruyneel M. Correlation between sleep parameters, physical activity and quality of life in somnolent moderate to severe obstructive sleep apnea adult patients. Sleep Breath. 2013;17(3):1039–1046. [DOI] [PubMed] [Google Scholar]
- 27.St-Onge MP, Roberts AL, Chen J, et al. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am J Clin Nutr. 2011;94(2):410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bromley LE, Booth JN III, Kilkus JM, Imperial JG, Penev PD Sleep restriction decreases the physical activity of adults at risk for type 2 diabetes. Sleep. 2012;35(7):977–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aronsohn RS, Whitmore H, Van Cauter E, Tasali E. Impact of untreated obstructive sleep apnea on glucose control in type 2 diabetes. Am J Respir Crit Care Med. 2010;181(5):507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harsch IA, Konturek PC, Koebnick C, et al. Leptin and ghrelin levels in patients with obstructive sleep apnoea: effect of CPAP treatment. Eur Respir J. 2003;22(2):251–257. [DOI] [PubMed] [Google Scholar]
- 31.Chirinos JA, Gurubhagavatula I, Teff K, et al. CPAP, weight loss, or both for obstructive sleep apnea. N Engl J Med. 2014;370(24):2265–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kajaste S, Brander PE, Telakivi T, Partinen M, Mustajoki P. A cognitive-behavioral weight reduction program in the treatment of obstructive sleep apnea syndrome with or without initial nasal CPAP: a randomized study. Sleep Med. 2004;5(2): 125–131. [DOI] [PubMed] [Google Scholar]
- 33.Drager LF, Brunoni AR, Jenner R, Lorenzi-Filho G, Bensenor IM, Lotufo PA. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials. Thorax. 2015;70(3):258–264. [DOI] [PubMed] [Google Scholar]
- 34.Igelstrom H, Martin C, Emtner M, Lindberg E, Asenlof P. Physical activity in sleep apnea and obesity—personal incentives, challenges, and facilitators for success. Behav Sleep Med. 2012;10(2):122–137. [DOI] [PubMed] [Google Scholar]
- 35.Sporndly-Nees S, Igelstrom H, Lindberg E, Martin C, Asenlof P. Facilitators and barriers for eating behaviour changes in obstructive sleep apnoea and obesity: a qualitative content analysis. Disabil Rehabil. 2014;36(1):74–81. [DOI] [PubMed] [Google Scholar]
- 36.Qaseem A, Dallas P, Owens DK, Starkey M, Holty JE, Shekelle P. Diagnosis of obstructive sleep apnea in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2014;161(3):210–220. [DOI] [PubMed] [Google Scholar]
- 37.Tuomilehto HP, Seppa JM, Partinen MM, et al. Lifestyle intervention with weight reduction: first-line treatment in mild obstructive sleep apnea. Am J Respir Crit Care Med. 2009;179(4):320–327. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.