Abstract
Hoarding disorder (HD) has been hypothesized to arise from deficits in error monitoring and abnormalities in emotional processing, but the relationship between error monitoring and emotional processing has not been examined. We examined measures of self-report, as well as behavioral, physiological, and facial responses to errors during a Stop-Change task. 25 participants with HD and 32 healthy controls (HC) were recruited. Participants reported on number of errors committed and pre/post emotional response to errors. Skin conductance response (SCR) during correct and error commission trials was examined. Facial expression during task performance was coded for self-conscious and negative emotions. HD and HC participants had significantly different error rates but comparable error correction and post-error slowing. SCR was significantly lower for HD during error commission than for HC. During error trials, HD participants showed a significant deficit in displays of self-conscious emotions compared to HC. Self-reported emotions were increased in HD, with more negative and self-conscious emotion reported than was reported for HC participants. These findings suggest that hypoactive emotional responding at a physiological level may play a role in how errors are processed in individuals with HD.
Keywords: Hoarding Disorder, Error Response, Emotions, Facial Emotion, Stop-Change Task
1. INTRODUCTION
Hoarding disorder (HD) is a highly impairing and distressing psychiatric disorder that poses a significant public health burden (Tolin et al. 2008). The prevalence of HD, estimated at 2–4% (Samuels et al. 2008), is higher than those of many other psychiatric disorders of adulthood (e.g., schizophrenia and bipolar disorder (McGrath et al. 2008) (Kessler et al. 2005), and increases with age, beginning around age 35, reaching >6% among adults over age 55 (Cath et al. 2017) (Samuels et al. 2008). It can be expected that the public health burden of this disorder will continue to grow due to increased global life expectancy(Collaborators 2016). However, in part because HD was not formally recognized as a separate psychiatric illness until 2013, research into its causes, prognosis, and treatment still lags behind that of other psychiatric disorders.
Although the pathophysiology of HD is not yet well understood, multiple theories about the fundamental causes of HD have been developed over the past two decades. Perhaps the best known of these is the cognitive-behavioral model of HD, developed by Frost and colleagues (Frost and Hartl 1996) (Steketee et. al., 2003) which posits that HD arises from four core deficits: 1) information processing deficits (specifically decision-making, categorization/organization, and memory functions), 2) problematic emotional attachments (or more specifically, highly emotional [abnormal] attachments to items), 3) behavioral avoidance, and 4) erroneous beliefs about the nature (value or replaceability) of possessions (Frost and Hartl 1996). Frost et al. postulated that these deficits are not mutually exclusive, but rather overlap and interact to create hoarding behaviors. For example, difficulty discarding may represent an avoidance behavior arising from indecisiveness and fear of making an error that serves to prevent or delay negative consequences, including negative emotions, that may arise from discarding an object that may be wanted later.
Subsequent studies have supported the hypothesis that hoarding behaviors are associated with strong negative and positive emotions. For example, positive emotions, such as happiness and pride, have been associated with excessive acquiring (Wheaton et al. 2011) (Timpano et al. 2014) (Steketee and Tolin 2011), while negative emotions such as anger, fear and sadness have been associated with the inability to discard (Fernández de la Cruz et al. 2013). Increased emotional reactivity and intense emotional reactions have been reported in a study of individuals who self-identified as having problematic hoarding when imagining both acquiring and discarding (Shaw et al. 2015). A study using a sample of college students also found that higher levels of hoarding symptoms were associated with a greater tendency towards impulsivity during negative mood states (Timpano and Schmidt 2013). However, to date, all the work on emotional reactivity in HD has relied on self-report measures, and to our knowledge, emotional reactivity in individuals with HD has not been quantified using multi-modal approaches. Despite some initial work suggesting that individuals with HD may avoid unpleasant emotions (Ayers et al. 2014) (Wheaton et al. 2011) and report having a lower tolerance for distressing situations in general (Timpano et al. 2014), it is still unclear if the reported increase in emotional reactivity occurs beyond hoarding-related scenarios.
More work has been done on the hypothesis that hoarding behaviors are related to indecision and a fear of making errors. Studies of decision-making as a neuropsychological construct (assessed by tools such as the Iowa Gambling Task) have been equivocal (Steketee et. al., 2003) (Frost and Gross 1993) (Mackin et al. 2011) (Mackin et al. 2016). In previous studies examining error commission on continuous performance tasks (such as the Sustained Attention to Response Task, the Stop Signal Reaction Time Task and Go/No-Go) hoarding participant’s mean number of errors were slightly higher and reaction time slower than control groups, although these differences were not statistically significant (Blom et al. 2011; Grisham et al. 2010) To date, current studies examining neuropsychological functioning in respect to decision-making, error commission and inhibitory control have yet to find clear indications of abnormalities in performance in HD samples.
Although the neuropsychological studies are equivocal, neuroimaging and neurophysiological studies suggest that HD may be characterized by more specific deficits in error processing. For example, recent work by Mathews et. al (2015) using electrophysiological approaches demonstrated that the error-related negativity (ERN), a pre-conscious event related potential that is thought to represent a mismatch between intended and actual responses (e.g. errors) on response conflict tasks, was hypoactive in individuals with HD compared to age-matched healthy controls and to individuals with OCD. Similarly, two neuroimaging studies using functional MRI (fMRI) found different patterns of neural activity in HD compared with healthy controls and participants with OCD during error processing, despite similar rates of error commission. One study found hypoactivity in the middle frontal gyrus and hyperactivity in the right precentral gyrus during error commission (Tolin et. al., 2014) and the other showed hyperactivity in orbitofrontal cortex (OFC), insula and striatum compared to healthy controls, and hyperactivity in the striatum and ventrolateral prefrontal cortex compared to individuals with OCD (Hough et al.). It is notable that in these studies, there were no significant differences in the number of errors made by individuals with HD compared to those with OCD or healthy controls; rather, the pre-conscious processing of errors was abnormal.
These studies suggest that abnormalities in error processing are a core feature of HD, although further research is needed to explore which aspects of error processing (e.g., conscious vs. unconscious processing of errors) are disrupted and how these deficits are related to experiential avoidance, emotional reactivity and ultimately, to hoarding behaviors. As noted above, much of the work on emotional reactivity in HD has been done in the context of imagined or real discarding tasks, and not in the context of emotionally neutral behavioral tasks that are designed to elicit errors but not emotional responses. The present study sought to objectively explore emotional responses to errors on a simple behavioral task in HD by examining physiological responses and emotional facial expressions during error commission, and to compare these emotional responses to behavioral performance (number of errors committed, reaction time) as well as to self-reports of performance and emotional reactions to errors. We hypothesized that, in comparison to healthy controls, individuals with HD would: 1) have similar responses in the number of errors committed and reaction times during a simple behavioral response task, but would over-report the number of errors committed and endorse more negative emotional responses to errors on self-report, and 2) have normal autonomic functioning at baseline but would show enhanced physiological response and more negative facial expression during error commission.
2. METHODS AND MATERIALS
2.1. Participants
Fifty-seven participants were recruited for this study, including 25 individuals with HD and 32 age-matched healthy controls (HC). HC were recruited from two ongoing research studies of neuropsychiatric disorders at UCSF: HC under age 60 were recruited from the control sample for research studies of HD, OCD, and depression at the OCD and Anxiety Clinic at UCSF, and HC ages 50 and older were recruited from the control sample for studies of dementia at the Memory and Aging Center (MAC) at UCSF. HD participants were recruited from the OCD and Anxiety Clinic at UCSF. Participants were compensated for participation. The UCSF Institutional Review Board approved the study and all participants reviewed and signed written informed consent materials prior to participation.
2.2. Clinical Assessments
HD participants and HC participants recruited through the OCD and Anxiety Clinic were assessed for HD, OCD, and history of other lifetime psychiatric disorders using the Structured Clinical Interview for Diagnosis of DSM-IV Axis I Disorders (SCID-I (Spitzer et al. 1992)), the Saving Inventory-Revised (SI-R (Fros et. al., 2004)), and the Yale-Brown Obsessive Compulsive Scale (YBOCS (Goodman et al. 1989)). The Beck Anxiety Inventory (BAI (Beck and Steer 1993)) and the Beck Depression Inventory (BDI (Beck et al. 1961)) were used to assess current symptoms of anxiety and depression. All participants completed neuropsychological testing. HC participants recruited through the MAC underwent a comprehensive evaluation that included a clinical history assessing current and past symptoms of psychiatric, neurological and medical disorders, with a focus on symptoms of dementia, depression and anxiety, a neurological examination, and neuropsychological assessment (Kramer et al. 2003). The Geriatric Depression Scale (GDS) (Yesavage et al. 1982) was used to assess current depressive symptoms. To correct for the use of two different depression symptom scales, z-scores of the total depression score for each participant from either the BDI or the GDS were calculated and reported as overall depression z-score in this study.
2.3. Inclusion/exclusion
Psychiatric diagnoses were assessed by a psychiatrist with experience in HD and OCD (C.A.M. or K.N.) who was blinded to group using DSM-5 criteria. HD participants were eligible if they meet DSM-5 criteria for HD but did not have OCD symptoms as defined by a YBOCs score of < 5. HC participants were age-matched to the HD group and were eligible if they did not meet DSM-5 criteria for HD or OCD, and did not meet criteria for a current mood or anxiety disorder. Individuals with a lifetime history of mood or anxiety disorders that were in remission at the time of assessment were not excluded. HC participants with a GDS score above 4 were excluded from the study. For both the HD and HC groups, individuals with active substance use, psychosis, dementia, and intellectual disabilities were excluded. Participants were also excluded if they were taking neuroleptic medications. No other medication use was excluded but participants were required to be on stable doses without change for ≥3 months prior to assessment, and to hold benzodiazepines for 12 hours prior to testing.
2.4. General Procedures
Study procedures required two hours of participation over one session and were explained and performed in the following order: 1) placement of physiological sensors, 2) initial self-report questionnaires, 3) startle response testing, 4) behavioral task, 5) behavioral task self-report measures, 6) removal of physiological sensors, 7) Neurological Assessment Battery (NAB) sub-tests, 8) final self-report measures. The experimental room was wired for remote-operated video and sound recording and participants were told when the recording of the session was started. After the initial self-report questionnaires were completed and recording quality of measures confirmed, headphones were positioned over the participant’s ears and sound levels were confirmed clear and at a comfortable volume.
2.5. Physiological Recording
Physiological responses were measured using skin conductance response (SCR), and were collected during the startle and behavioral tasks. SCR was collected using a James Long Company (Caroga Lake, New York, USA) bioamplifier. Two Ag-AgCl electrodes prepared with Biogel electrode gel (UFI Inc., Morro Bay, CA) were placed on the first and third index fingers of the non-dominant hand with isotonic paste. The sensors were connected to an SAI bioamplifier (0.5V constant voltage with a sensitivity of 600 pS, SAI Inc., Hauppauge, NY), which was in turn connected to a Biopac UMI100 to be digitized. The digitized signal was recorded using AcqKnowledge data acquisition software version 4.2 (Biopac systems Inc., Goleta, CA, USA). Maximum SCR was defined as the maximum or peak SCR measured within the first 5000 milliseconds (ms) after the presentation of the stimulus of interest (see section 2.8). For X trials, the stimulus of interest was the presentation of the X. For beep trials, the stimulus was the beginning of the beep tone. Raw SCR data was log-transformed to minimize skew (Boucsein et al. 2012).
2.6. Self-Report Measures
2.6.1. Prediction/Recall of Emotions:
Participants were asked to predict the strength of 10 specific emotions (embarrassment, guilt, shame, anger, sadness, surprise, fear, pride, disgust and happiness) on an 8 point Likert scale from 0 (no emotion at all) to 8 (strongest emotion ever felt) should they make a mistake on testing. These questions were repeated at the end of the session with regard to how they remembered actually feeling when making an error during the session.
2.6.2. Performance:
After completing the task instructions and practice trials, participants were asked to estimate how many errors they would make and how many they would correct if given 100 beep trials (see task explanation below). After task completion, participants were asked to recall how many errors they made and how many they corrected of the 40 beep trials administered.
2.7. Startle Response
Participants were asked to relax for two minutes. At thirty-four seconds, a 500ms, 105 decibel white noise burst was played through headphones followed by eighty-eight seconds of silence. SCR to the startle probe was measured at the onset of the probe, extending for five seconds. A baseline of one second prior to startle onset was subtracted from the peak SCR response post probe.
2.8. Behavioral (Stop-Change) Task
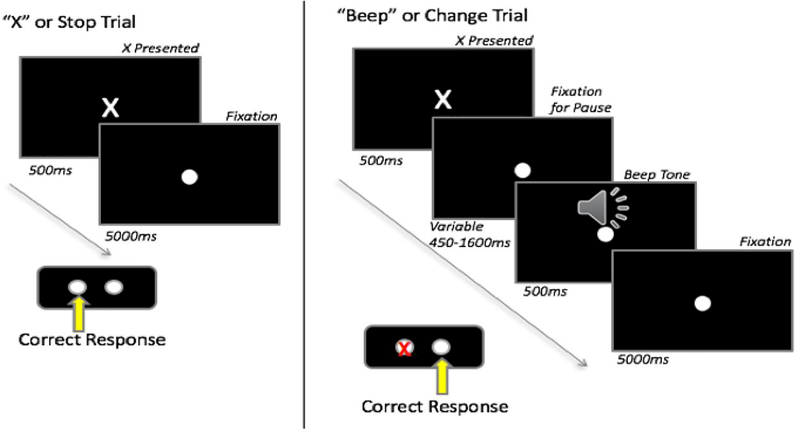
A modified stop-change task (Lappin and Eriksen 1966) (Logan 1994; Boecker et. al., 2013) was used to elicit and assess errors. This task was selected because it has a correction response as a behavioral measure of error awareness and because it allows for the ability to manipulate task difficulty through a variable pause duration before the second stimulus is presented. A video monitor was positioned in front of the participant and a two-button box was placed on a table in from of them. The task was synchronized with the physiological data collection and behavioral and reaction time data were recorded. Participants were given one block of six practice trials which were repeated until task understanding was confirmed. As illustrated in Figure 1, two types of stimuli were presented; an X displayed for 500ms and a beep tone lasting 500ms. Participants were instructed to press the left button if an X appeared; if the X was followed by a beep tone, they were to press the right button. If they pressed the wrong button (e.g., made an error), they were instructed to correct it by subsequently pressing the appropriate button. A total of 120 trials were presented; 80 trials were X-only (X trials), and 40 were X paired with a beep tone (beep trials). X trials consisted of a 500ms presentation of the X followed by 5000ms of the fixation dot. Beep trials consisted of an X presented for 500ms followed by a fixation dot that was present on the screen for the trial duration. The beep tone was presented between 400–1600ms after the X and was followed by 5000ms of remaining trial time. The pause duration prior to the beep tone was based on the previous beep trial response with a starting duration of 500ms. For correct responses, the next pause would increase by 50ms; if the response was incorrect, the next pause duration would decrease by 50ms. This change in pause duration was based on the initial response, and was not affected by any subsequent attempts to correct errors. Trials were given in two blocks of 60, with beep trials randomly dispersed throughout the block and separated by at least one X trial.
Figure 1:
Stop-Change Task Design. ms=milliseconds.
Reaction time was recorded in milliseconds from the first X presentation to the first button press response. Post-error slowing was measured as change scores created by subtracting the reaction time on every beep-trial from the reaction time on the subsequent trial, allowing for comparisons both within and across groups.
2.9. Emotional Facial Recording and Coding
Participants’ facial expressions were recorded throughout the behavioral task and video recordings were synchronized with the participant’s physiological responses using time stamping. Eight emotional behaviors were coded throughout the task on a second-by-second basis, (anger, disgust, happiness, contempt, sadness, embarrassment, fear, and surprise) on an intensity scale of 0 to 3 using a modified version of the Emotional Expressive Behavioral Coding System (Gross and Levenson 1993) (Sturm et al. 2008). Intensity was scored with 0 indicating no emotion, 1 indicating mild, 2 indicating moderate and 3 severe (strong) emotion. Coders were blind to participant group and trial type. Emotional codes were extracted by trial, and intensity scores were summed by emotion for the duration of trial to create an intensity X duration (IxD) score. Composite scores were generated to reduce the number of tests as well as to replicate previous research (Scherling et al. 2017; Sturm et al. 2006). Negative emotion composite scores were created for each trial by summing anger, sadness, fear, disgust, and contempt emotion IxD scores. Self-conscious emotion IxD scores were created following Scherling et al and Sturm et al. by combining happiness and embarrassment IxD scores, as these emotions cannot be reliably differentiated in the context of an emotionally neutral stimulus and a short trial time such as are present in our study (Scherling et al. 2017; Sturm et al. 2006).
2.10. Neuropsychological Assessment Battery (NAB)
The NAB, which is an assessment of real-world daily living skills designed to assess attention, language, memory, spatial and executive function, was completed by all participants and used to assess baseline cognitive functioning in real-life situations (Stern 2003). Raw scores for each subtest were summed to obtain a global performance score.
2.11. Statistical Analysis
All single observation per participant variables (age, education, etc) were compared using independent sample t-tests and chi-square analyses as appropriate using SPSS version 23. Post-test accuracy was normally distributed and independent sample t-test was conducted to examine the variability of responses for HD compared to HC. All other self-report measures were normalized using Blom transformations. Univariate general linear models using age and education as covariates were conducted for self-report, startle and NAB measures. Data consisting of trial-by-trial measures such as reaction time, SCR and emotional facial reactions were analyzed using a linear mixed effects (lme) model in R (R Core Team, 2015) (Pinheiro and Team 2015). Age and sex were used as covariates in each analysis. Two types of trials were used for the lme model, correct beep-trials and incorrect beep-trials. Trial type and diagnosis were included as fixed effects in each model. For facial emotion reactivity, IxD score was used as the outcome variable. Emotion type was included as a fixed variable and the interaction between diagnosis and emotion type was also examined. The model included random intercepts, nesting by emotion type. Similar analyses were performed for post-error slowing and SCR as outcome variables. For the lme analyses, we also report the loglikelihood ratio (X2(1)), which provides a measure of the fit of the observed model compared to the null. Effect sizes were calculated for all outcome variables of interest and are reported as Cohen’s d (for t-tests) or partial eta (for general linear models). Because this work is exploratory in nature, significance values are not corrected for multiple testing. For the linear mixed effects analyses, secondary sensitivity analyses including error rates as an additional covariate were conducted to assess the influence of possible differences in behavioral performance between groups on the outcomes of interest. As these analyses did not differ from the primary analyses, they are not reported here.
3. Results
3.1. Demographics
There were no differences in gender, age, medication rates or executive function as measured by the NAB between participant groups. HD participants were significantly less educated and more depressed than HC participants (Table 1). Of the nine individuals of our sample on medication (15.8% of the total sample), the majority were taking prescribed stimulants (36%) or anti-depressants (36%) with one person on mood stabilizers and two on combinations of anti-depressants and mood stabilizers.
Table 1:
Participant Demographics. HD = hoarding disorder. HC = healthy controls. NAB = Neurological Assessment Battery. SD = standard deviation. NS = not significant. Because different depression assessment instruments were used for some control participants, the respective depression scale total scores were z-scored to allow for group comparisons.
| Gender, N males (%) | 9 (36%) | 10 (31%) | X2 =0.004, NS |
| Mean age, years (SD) | 59.3 (10.1) | 55.3 (17.2) | t(55)=−1.05, NS |
| Mean education, years (SD) | 16.3 (2.6) | 17.6 (2.2) | t(55)=2.06, p=.04 |
| Medication use N (%) | 6 (24%) | 3 (9.4%) | X2 =2.39, NS |
| Depression z-scores | 0.35 | −0.33 | t(249)=−2.57, p=.013 |
| NAB overall raw scores (SD) | 25.5 (2.4) | 26.6 (1.8) | F(1, 50)=1.78, NS |
3.2. Behavioral data
There were significant differences in the number of errors made but not in the number of errors corrected between groups (Table 2). There was a significant difference for the main effect of reaction time on the trial following a beep-trial (F(1,2185)=41.4 p < .001, X2(1) = 110.6), with post-error slowing observed for all participant groups. However, there were no significant differences in reaction time by participant group (F(2,53)=0.002, p=0.959) or between participant groups by trial type (F(1,2185)=1.33, p=0.249).
Table 2:
Response Rates in Stop-Change Task by Group. HD = hoarding disorder. HC = healthy controls. SE = standard error. RT= reaction time. ms=milliseconds.
| Mean | SE | Mean | SE | ||
| Error rate | 7.7 | 0.9 | 5.3 | 0.6 | t(55)=−2.30, p=.025, d=0.604 |
| Correction rate (%) | 98.9% | 0.8 | 99.3% | 0.7 | t(52)=0.415, p=. 680, d=0.109 |
3.3. Perceived Task Accuracy
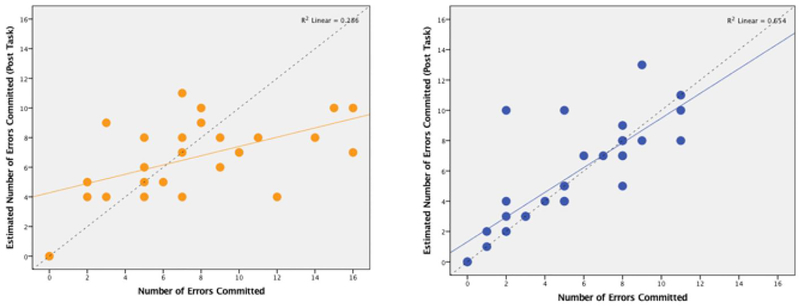
Pre- and post-task error estimates for each participant were assessed both as raw means and as accuracy rates. Accuracy rates were calculated by subtracting how many errors the participant estimated that they made from the number of errors they actually committed. HD participants were less accurate in their error commission estimates than HC participants, with a moderate effect size (t(1,55)=1.942, p=0.057, d=0.52), (Figure 2). In addition, we calculated the number percentage of non-overlapping responses by determining the 95% confidence interval (CI) and found that the proportion of HC which do not fall within the within the CI overlap is 50%, however, the number of HD which do not fall within the CI is 88%.
Figure 2:
Post-task error task accuracy scatterplots by group. X axis= number of errors actually committed, Y axis= number of errors committed as estimated by the participant immediately following the task. These scatterplots demonstrate that HD participants (left) have a more diffuse pattern of response than HC (right). The line in the corresponding color indicates linear R2, the dashed line indicates accurate (perfect) recall of errors.
3.4. Perceived emotional responses
There were significant between-group differences in pre and post-testing prediction and recall of participants’ emotional states during error commission, with a small to medium effect size (pre-test F(1,54)=5.58, p=0.022, η2=0.10; post F(1,54)=3.978, p=0.050, η2=0.07). HD participants reported that they would (and did) feel significantly more emotions during error commission than HC participants. To reduce the number of variables for analysis and to better match emotional facial response categories, emotions were split into three mutually exclusive categories; basic negative emotions (anger, sadness, fear, disgust, contempt), basic positive emotions (happiness) and self-conscious emotions (embarrassment, guilt, shame and pride)(Tangney 1999). Pre-test negative (F(1,53)=8.941, p=0.004, η2=0.14), and self-conscious emotions (F(1,53)=6.624, p=0.013, η2=0.11) were significantly different between groups with medium to large effect sizes; however, positive emotions were not (F(1,53)=0.75, p=0.392, η2=0.01). Similarly, post-test negative (F(1,53)=8.59, p=0.005, η2=0.14), and self-conscious emotions (F(1, 53)=8.05, p=0.006, η2=0.13) were significantly different between groups with a medium to large effect size; however, positive emotions were not (F(1, 53)=0.305, p=0.305, η2=0.02). HD participants reported higher levels of both pre- and post-test negative and self-conscious emotions compared with HC participants.
3.5. Startle Response:
Both groups showed a similar physiological (SCR) response to the startle probe with there was no significant difference in level of startle response between groups (F(1, 53)=0.766, p=0.385, η2=0.01).
3.6. Physiological Reactivity
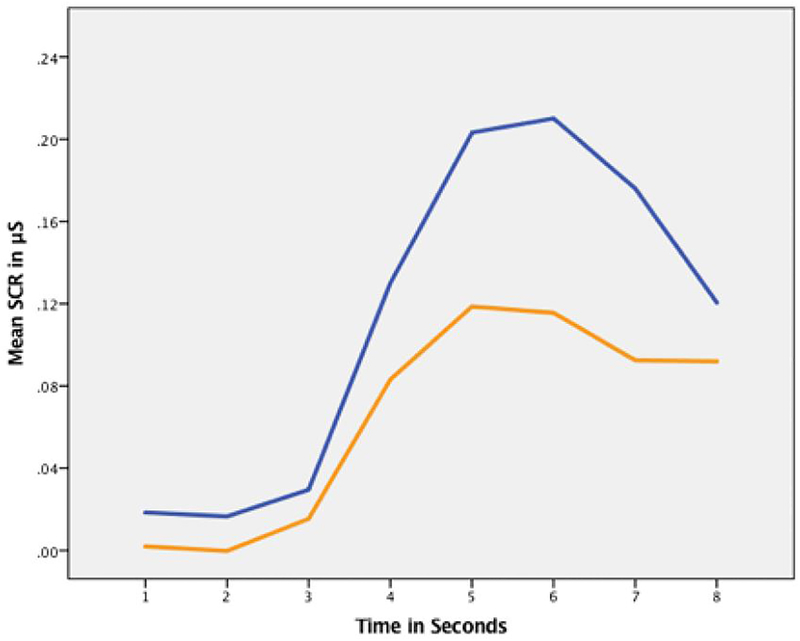
Both HD and HC groups showed a greater skin conductance response on error trials than correct trials (within-group t-tests: HD t=−7.5, SE=0.010, p<.001; HC t=−14.7, SE=0.010, p<.001). There were also significant differences in trial type by group (controlling for age and gender) in SCR (F(1,2065)=43.66, p<.001, X2(1) = 36.4). While there were no significant differences between groups during correct beep trials, HD participants had a significantly lower SCR than HC participants during error trials, demonstrating a large effect size as well (HC > HD t=3.58, SE=0.0265, p<.001, d=0.96) (Figure 3). Again, we also calculated the non-overlap of the 95% CI proportion for each group and found of the HC wo did not fall within the CI overlap were 72% whereas for HD there were 85.9%.
Figure 3:
SCR during stop-change error trials over time with hoarding disorder (HD) participants shown in orange, and healthy control (HC) participants in blue. HD participants had a significantly lower SCR than HC participants during error trials (HC > HD t=3.58, SE=0.0265, p<.001, d=0.956) as can be seen in the difference between response curves over time in uS=microseconds.
3.7. Emotional Facial Response
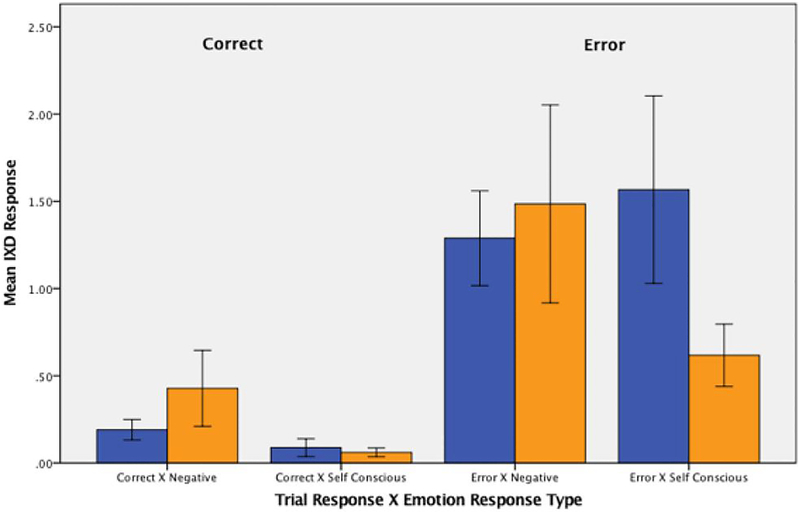
Video-recorded facial response data were available for 53 participants: 29 HC and 24 HD. Facial data from 3 HC participants and 1 HD participant could not be analyzed due to poor recording quality. A mixed model analysis examining facial response to errors indicated a significant three-way interaction between emotion type, trial type, and group (F(1,4130) = 8.77, p=0.003, X2(1) = 7.61). There were no significant differences in facial responses during correct beep-trials across either emotion or group. For error trials, HD participants showed significantly fewer self-conscious emotions than HC participants, with a medium effect size (−1.00 IxD units, SE=0.226, p=0.001, d=0.46). In calculating the non-overlap of the 95% CI proportion for each group for self-conscious emotions on error trials, we found of the HC wo did not fall within the CI overlap were 67% whereas for HD there were 78%. However, also on error trials, HD participants did not show a difference for negative emotional responses, however demonstrating a moderate effect as well (−0.07 IxD units, SE=0.226, p=0.728, d=0.46). (Figure 4). In calculating the non-overlap of the 95% CI proportion for each group for negative emotions on error trials, we found of the HC wo did not fall within the CI overlap were 70% whereas for HD there were 61%.
Figure 4:
Negative and self-conscious emotional facial response during the stop-change task in both correct and incorrect trials for hoarding disorder (HD) participants (orange) and healthy control (HC) participants (blue). Error bars = 1 standard errors. Y-axis = intensity of emotional response as measured by intensity of response × duration of response (IxD).
4. Discussion
In this study, we examined error processing and emotional reactivity in the context of error commission on a simple, non-emotional behavioral task in individuals with HD compared with healthy controls. As expected, we found that individuals with HD had similar behavioral responses when compared to HC, including correction of errors, reaction times, and post-error slowing, although HD participants made on average 2 more errors out of 40 trials than did healthy controls. All participants also performed similarly on the NAB daily living skill subtests, including the memory subtest, suggesting that there were no functional deficits in executive functioning. However, contrary to our hypotheses, individuals with HD had hypoactive rather than hyperactive physiological and facial emotional responses to errors compared to HC, although both groups had similar negative facial emotional responses to errors and similar facial responses for all emotions on correct trials. Interestingly, HD participants’ self-reported emotional reactions to errors were in direct opposition to their physiological and facial responses to errors. HD participants anticipated that they would feel significantly more emotions than HC participants anticipated they would feel; post task, participants with HD still reported feeling significantly more emotion overall and more self-conscious emotions in response to errors than did HC participants, despite physiological indications to the contrary. In line with this finding, HD participants’ self-report for the number of errors they committed during the stop-change task was much more variable (both over- and under-estimating the number of errors committed) than estimates from HC participants, although they had no difficulty in correcting errors during the task, suggesting intact in-the-moment recognition of error commission. Taken together, our data suggest that individuals with HD exhibit a dissociation between perception of performance and emotional reactions to errors and actual performance and emotional reaction to errors. These deficits appear to be specific to error commission, as there were no differences in baseline physiology or in physiological or facial expression of emotion on correct trials. Similarly, impairment in executive function and/or motivation cannot explain these deficits, as all groups performed comparably on the NAB daily living skill subtests, and all groups ranked the importance of the testing and their motivation to perform well at moderate to high levels both pre and post testing.
Although this study is in line with previous work showing that individuals with HD report intense emotional reactions, both in relation to discarding behaviors (Shaw et al. 2015) (Timpano et al. 2013) and in response to stimuli designed to evoke emotional responses (Timpano et al. 2014), it also suggests that self-report of emotion does not accurately reflect the underlying autonomic state in these individuals. Previous neuroscience research has shown that both the cognitive and emotional systems are engaged during error processing through the dorsal anterior cingulate cortex (dACC) and the amygdala (Pourtois et al. 2010). The hypoactive response to errors as measured by autonomic response and emotional facial reactivity for self-conscious emotions seen in this study corresponds to the decrease in electrophysiological responding (error related negativity or ERN) to errors among individuals with HD previously reported by our group, and thought to be mediated by the ACC (Mathews et al. 2016) (Gemba et. al., 1986) (Niki and Watanabe 1979).
It is also notable that individuals with HD evidenced deficits specifically in self-conscious emotional response. Self-conscious emotions of guilt, shame, pride and embarrassment are cognitively complex emotions that require an appreciation of self in a social context (Tangney 1999). Contextually, embarrassment to being recorded while making errors during a simple behavioral task can be expected and was demonstrated at similar intensities in our HC participants, but to a lesser extent with HD participants. The role of embarrassment is to indicate that a social transgression has occurred and to motivate attempts to correct or change the situation (Keltner and Anderson 2000) (Keltner and Buswell 1997) (Miller and Leary 1992). Brain regions associated with self-conscious emotions include the medial prefrontal cortex (mPFC), the dorsal ACC and the dorsolateral prefrontal cortex (DLPFC) (Gilead et al. 2016) (Sturm et al. 2013), areas also associated with executive function (Alvarez and Emory 2006). Currently published neuroimaging studies of HD, although hampered by methodological difficulties and small sample sizes, have implicated frontal and temporal regions involved in emotion regulation, decision making, response inhibition, error processing, and decision making (Tolin et al. 2012), most consistently, the ACC, orbitofrontal cortex (OFC), and dorsolateral and medial prefrontal regions (Tolin et al. 2012) (Tolin, Witt, and Stevens 2014) (Hough et al. 2016). Thus, although preliminary and requiring replication, our findings of hypoactive emotional responses to errors in participants with HD, despite normal correction of errors, adds to the growing literature suggesting that HD arises from an interaction between abnormal emotional responding and deficits in information processing (in particular, in error processing) as originally hypothesized by Frost and Hartl in 1996. In addition, when combined with the growing literature examining error processing and response inhibition using neuroimaging and electrophysiological approaches, the finding that individuals with HD have deficits specifically in self-conscious emotional responding further suggests that frontal dysfunction, in particular, dysfunction in the cingulate, medial and prefrontal cortical regions, may be implicated in the development of hoarding behaviors.
There are several limitations to this study. First, the sample sizes were relatively small, and there were significant differences in education between the HC and HD participants. However, we controlled for education in our analyses. Similarly, medication status varied among participants, with approximately 20% of HD (and none of the HC participants) using psychotropic medications. Due to small sample sizes, we did not covary for medication status. However, all participants were on stable doses of psychotropic medications, individuals on neuroleptic medications were excluded from participation, and all individuals were asked to hold benzodiazepines for 12 hours prior to testing, to minimize possible effects on physiological responding. Nevertheless, replication studies should include larger sample sizes, and more carefully control for education and medication status among all participant groups.
The results of this study lend further support to the hypothesis that a core deficit in HD is hypoactive response to errors and expands the currently available literature on error processing in HD to include emotional response to errors. The results also highlight the role of self-conscious emotional response and its relationship to error monitoring in HD. Further research is needed to understand whether this emotional hypoactivation generalizes across multiple contexts or if it is specific to online error monitoring. The results of this work also underscore the potential pitfalls of relying on self-report in this population, and the need for additional, objective multi-modal investigation of error processing, response inhibition, and emotional reactivity in HD.
Highlights.
Individuals with HD had hypoactive responses to errors.
Individuals with HD self-reports were opposite of their facial responses to errors.
Individuals with HD exhibit dissociation between perception and emotional reactions.
Acknowledgements & Disclosures
This work was supported by NIH Grant R21 MH087748, NIH/NIA Grant R01 AG030688, NIH/NIA Grant P50 AG023501, NIH/NIA Grant P01 AG019724, and The Hillbloom Network Program.
Authors Jessica J. Zakrzewski, MRes; Samir Datta, BA; Carole Scherling, PhD; Krystal Nizar, MD, PhD; Ofilio Vigil, MS; Howard Rosen, MD; Carol A. Mathews, MD all have no financial disclosures to declare.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez JA, and Emory E. 2006. ‘Executive function and the frontal lobes: a meta-analytic review’, Neuropsychol Rev, 16: 17–42. [DOI] [PubMed] [Google Scholar]
- Ayers Catherine R., Castriotta Natalie, Dozier Mary E., Espejo Emmanuel P., and Porter Ben. 2014. ‘Behavioral and experiential avoidance in patients with hoarding disorder’, Journal of Behavior Therapy and Experimental Psychiatry, 45: 408–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson MM, Mock JJ, and Erbaugh JJ. 1961. ‘An inventory for measuring depression’, Archives of General Psychiatry, 4: 561–71. [DOI] [PubMed] [Google Scholar]
- Beck AT, and Steer RA. 1993. Beck Anxiety Inventory (Harcourt Assessment Inc: San Antonio: ). [Google Scholar]
- Blom RM, Samuels JF, Grados MA, Chen Y, Bienvenu OJ, Riddle MA, Liang KY, Brandt J, and Nestadt G. 2011. ‘Cognitive functioning in compulsive hoarding’, J Anxiety Disord, 25: 1139–44. [DOI] [PubMed] [Google Scholar]
- Boecker M, Gauggel S, and Drueke B. 2013. ‘Stop or stop-change--does it make any difference for the inhibition process?’, Int J Psychophysiol, 87: 234–43. [DOI] [PubMed] [Google Scholar]
- Boucsein W, Fowles DC, Grimnes S, Ben-Shakhar G, roth WT, Dawson ME, Filion DL, and Measures Society for Psychophysiological Research Ad Hoc Committee on Electrodermal. 2012. ‘Publication recommendations for electrodermal measurements’, Psychophysiology, 49: 1017–34. [DOI] [PubMed] [Google Scholar]
- Cath Danielle C., Nizar Krystal, Boomsma Dorret, and Mathews Carol A.. 2017. ‘Age-Specific Prevalence of Hoarding and Obsessive Compulsive Disorder: A Population-Based Study’, The American Journal of Geriatric Psychiatry, 25: 245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborators, GBD 2015 Mortality and Causes of Death. 2016. ‘Global, regional, and national life expectancy, all-cause mortality, and cause-specifi c mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015’, Lancet, 388: 1459–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández de la Cruz Lorena, Landau Danielle, Iervolino Alessandra C., Santo Susan, Pertusa Alberto, Singh Satwant, and Mataix-Cols David. 2013. ‘Experiential avoidance and emotion regulation difficulties in hoarding disorder’, Journal of Anxiety Disorders, 27: 204–09. [DOI] [PubMed] [Google Scholar]
- Frost RO, and Gross RC. 1993. ‘The hoarding of possessions’, Behaviour Research and Therapy, 31: 367–81. [DOI] [PubMed] [Google Scholar]
- Frost Randy O., Steketee Gail, and Grisham Jessica. 2004. ‘Measurement of compulsive hoarding: saving inventory-revised’, Behaviour Research and Therapy, 42: 1163–82. [DOI] [PubMed] [Google Scholar]
- Frost RO, and Hartl TL. 1996. ‘A cognitive-behavioral model of compulsive hoarding’, Behav Res Ther, 34: 341–50. [DOI] [PubMed] [Google Scholar]
- Gemba H, Sasaki K, and Brooks VB. 1986. ‘‘Error’ potentials in limbic cortex (anterior cingulate area 24) of monkeys during motor learning’, Neurosci Lett, 70: 223–7. [DOI] [PubMed] [Google Scholar]
- Gilead M, Katzir M, Eyal T, and Liberman N. 2016. ‘Neural correlates of processing “self-conscious” vs. “basic” emotions’, Neuropsychologia, 81: 207–18. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, and et al. 1989. ‘The yale-brown obsessive compulsive scale: I. development, use, and reliability’, Archives of General Psychiatry, 46: 1006–11. [DOI] [PubMed] [Google Scholar]
- Grisham JR, Norberg MM, Williams AD, Certoma SP, and Kadib R. 2010. ‘Categorization and cognitive deficits in compulsive hoarding’, Behav Res Ther, 48: 866–72. [DOI] [PubMed] [Google Scholar]
- Gross JJ, and Levenson RW. 1993. ‘Emotional suppression: physiology, self-report, and expressive behavior’, J Pers Soc Psychol, 64: 970–86. [DOI] [PubMed] [Google Scholar]
- Hough CM, Luks TL, Lai K, Vigil O, Guillory S, Nongpiur A, Fekri SM, Kupferman E, Mathalon DH, and Mathews CA. 2016. ‘Comparison of brain activation patterns during executive function tasks in hoarding disorder and non-hoarding OCD’, Psychiatry Res, 255: 50–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keltner Dacher, and Anderson Cameron. 2000. ‘Saving face for Darwin: The functions and uses of embarrassment’, Current Directions in Psychological Science, 9: 187–92. [Google Scholar]
- Keltner Dacher, and Buswell Brenda N.. 1997. ‘Embarrassment: Its distinct form and appeasement functions’, Psychological Bulletin, 122: 250–70. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu W, Demler O, and Walters EE. 2005. ‘Prevalence, severity, and comorbidity of 12-month dsm-iv disorders in the national comorbidity survey replication’, Archives of General Psychiatry, 62: 617–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer JH, Jurik J, Sha SJ, Rankin KP, Rosen HJ, Johnson JK, and Miller BL. 2003. ‘Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease’, Cogn Behav Neurol, 16: 211–8. [DOI] [PubMed] [Google Scholar]
- Lappin JS, and Eriksen CW. 1966. ‘Use of a delayed signal to stop a visual reaction-time response’, Journal of Experimental Psychology, 72: 805–11. [Google Scholar]
- Logan GD. 1994. ‘On the ability to inhibit thought and action. A user’s guide to the stop-signal paradigm’ in Dagenbach D and Carr TH (eds.), Inhibitory Process in Attention, Memory, and Language (Academic Press: San Degio: ). [Google Scholar]
- Mackin RS, Arean PA, Delucchi KL, and Mathews CA. 2011. ‘Cognitive functioning in individuals with severe compulsive hoarding behaviors and late life depression’, Int J Geriatr Psychiatry, 26: 314–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackin RS, Vigil O, Insel P, Kivowitz A, Kupferman E, Hough CM, Fekri S, Crothers R, Bickford D, Delucchi KL, and Mathews CA. 2016. ‘Patterns of Clinically Significant Cognitive Impairment in Hoarding Disorder’, Depress Anxiety, 33: 211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews CA, Perez VB, Roach BJ, Fekri S, Vigil O, Kupferman E, and Mathalon DH. 2016. ‘Error-related brain activity dissociates hoarding disorder from obsessive-compulsive disorder’, Psychol Med, 46: 367–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath John, Saha Sukanta, Chant David, and Welham Joy. 2008. ‘Schizophrenia: A Concise Overview of Incidence, Prevalence, and Mortality’, Epidemiologic Reviews, 30: 67–76. [DOI] [PubMed] [Google Scholar]
- Miller Rowland S., and Leary Mark R.. 1992. ‘Social sources and interactive functions of emotion: The case of embarrassment’ in, Emotion and social behavior (Sage Publications, Inc: Thousand Oaks, CA, US: ). [Google Scholar]
- Niki H, and Watanabe M. 1979. ‘Prefrontal and cingulate unit activity during timing behavior in the monkey’, Brain Res, 171: 213–24. [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, & R Core, and Team. 2015. “nlme: Linear and nonlinear mixed effects models.” In, Retrieved from http://cran.r-project.org/web/packages/nlme.
- Pourtois G, Vocat R, N’Diaye K, Spinelli L, Seeck M, and Vuilleumier P. 2010. ‘Errors recruit both cognitive and emotional monitoring systems: simultaneous intracranial recordings in the dorsal anterior cingulate gyrus and amygdala combined with fMRI’, Neuropsychologia, 48: 1144–59. [DOI] [PubMed] [Google Scholar]
- Samuels JF, Bienvenu OJ, Grados MA, Cullen B, Riddle MA, Liang KY, Eaton WW, and Nestadt G. 2008. ‘Prevalence and correlates of hoarding behavior in a community-based sample’, Behav Res Ther, 46: 836–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherling Carole S., Zakrzewski Jessica, Datta Samir, Levenson Robert W., Shimamura Arthur P., Sturm Virginia E., Miller Bruce L., and Rosen Howard J.. 2017. ‘Mistakes, Too Few to Mention? Impaired Self-conscious Emotional Processing of Errors in the Behavioral Variant of Frontotemporal Dementia’, Frontiers in Behavioral Neuroscience, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw AM, Timpano KR, Steketee G, Tolin DF, and Frost RO. 2015. ‘Hoarding and emotional reactivity: The link between negative emotional reactions and hoarding symptomatology’, Journal of psychiatric research, 63: 84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, and First MB. 1992. ‘The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description’, Arch Gen Psychiatry, 49: 624–9. [DOI] [PubMed] [Google Scholar]
- Steketee G, Frost RO, and Kyrios M. 2003. ‘Cognitive aspects of compulsive hoarding’, Cognitive Therapy and Research, 27: 463–79. [Google Scholar]
- Steketee Gail, and Tolin David F.. 2011. ‘Cognitive-behavioral therapy for hoarding in the context of contamination fears’, Journal of Clinical Psychology, 67: 485–96. [DOI] [PubMed] [Google Scholar]
- Stern RA. 2003. “Neuropsychological Assessment Battery (NAB).” In. Lutz, Florida: Psychological Assessment Resources. [Google Scholar]
- Sturm VE, Ascher EA, Miller BL, and Levenson RW. 2008. ‘Diminished self-conscious emotional responding in frontotemporal lobar degeneration patients’, Emotion, 8: 861–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm VE, Rosen HJ, Allison S, Miller BL, and Levenson RW. 2006. ‘Self-conscious emotion deficits in frontotemporal lobar degeneration’, Brain, 129: 2508–16. [DOI] [PubMed] [Google Scholar]
- Sturm VE, Sollberger M, Seeley WW, Rankin KP, Ascher EA, Rosen HJ, Miller BL, and Levenson RW. 2013. ‘Role of right pregenual anterior cingulate cortex in self-conscious emotional reactivity’, Soc Cogn Affect Neurosci, 8: 468–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangney June Price. 1999. ‘The self-conscious emotions: Shame, guilt, embarrassment and pride’ in Dalgleish T Power MJ (ed.), Handbook of cognition and emotion (John Wiley & Sons Ltd: New York, NY, US: ). [Google Scholar]
- Timpano KR, Rasmussen J, Exner C, Rief W, Schmidt NB, and Wilhelm S. 2013. ‘Hoarding and the multi-faceted construct of impulsivity: a cross-cultural investigation’, J Psychiatr Res, 47: 363–70. [DOI] [PubMed] [Google Scholar]
- Timpano KR, and Schmidt NB. 2013. ‘The relationship between self-control deficits and hoarding: a multimethod investigation across three samples’, J Abnorm Psychol, 122: 13–25. [DOI] [PubMed] [Google Scholar]
- Timpano KR, Shaw AM, Cougle JR, and Fitch KE. 2014. ‘A multifaceted assessment of emotional tolerance and intensity in hoarding’, Behav Ther, 45: 690–9. [DOI] [PubMed] [Google Scholar]
- Tolin DF, Frost RO, Steketee G, Gray KD, and Fitch KE. 2008. ‘The economic and social burden of compulsive hoarding’, Psychiatry Res, 160: 200–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolin DF, Stevens MC, Villavicencio AL, Norberg MM, Calhoun VD, Frost RO, Steketee G, Rauch SL, and Pearlson GD. 2012. ‘Neural mechanisms of decision making in hoarding disorder’, Arch Gen Psychiatry, 69: 832–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolin DF, Witt ST, and Stevens MC. 2014. ‘Hoarding disorder and obsessive-compulsive disorder show different patterns of neural activity during response inhibition’, Psychiatry Res, 221: 142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheaton Michael G., Abramowitz Jonathan S., Franklin Joseph C., Berman Noah C., and Fabricant Laura E.. 2011. ‘Experiential Avoidance and Saving Cognitions in the Prediction of Hoarding Symptoms’, Cognitive Therapy and Research, 35: 511–16. [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, and Leirer VO. 1982. ‘Development and validation of a geriatric depression screening scale: a preliminary report’, J Psychiatr Res, 17: 37–49. [DOI] [PubMed] [Google Scholar]