Abstract
Objective
To determine the effect of feline congenital glaucoma (FCG) on corneal sensitivity, and relationships between corneal sensitivity, central corneal thickness (CT) and corneal diameter (CD).
Animals and Procedures
Corneal sensitivity (estimated by corneal touch threshold (CTT) using Cochet-Bonnet esthesiometry); CT using ultrasonic pachymetry; intraocular pressure (IOP) using rebound tonometry; and maximal horizontal CD were measured in 16 normal and 14 FCG cats, both males and females, aged 7mths–3.5yrs. All procedures complied with an Institutional Animal Care and Use Committee-approved protocol. Data were analyzed by linear regression; paired student t-tests for between eye comparisons, and unpaired student t-tests for comparisons between groups. Relationships between parameters were evaluated by Pearson correlation coefficients and linear mixed effects modeling. For statistical tests, with the exception of values that were Benjamini-Hochberg adjusted for multiple comparisons, p values <0.05 were considered significant.
Results
Mean CTT and CT values were lower in FCG eyes relative to normal eyes but differences were not statistically significant. Mean CD was significantly larger in FCG eyes relative to normal eyes and there was a significant negative correlation between CD and CTT in FCG (r=−0.8564, corrected p=0.005). These associations were confirmed in linear mixed effects models.
Conclusions
Eyes with FCG have significantly larger CDs when compared with normal eyes, and larger CDs correlated with decreased corneal sensitivity in this group. Further studies are warranted to explore the effect of buphthalmos and corneal enlargement on corneal sensitivity and innervation in feline subjects with chronic glaucoma.
Keywords: glaucoma, cat, feline, corneal sensitivity, corneal diameter, esthesiometry
Introduction
Glaucoma appears to be less prevalent in cats than in dogs, but this difference may reflect under-diagnosis of feline glaucoma, due to insidious onset and relatively subtle clinical signs in cats that include mydriasis and globe enlargement. [1] A form of inherited primary feline congenital glaucoma (FCG) was identified in Siamese cats [2] and a viable mixed-breed colony established that provides a valuable model for the study of glaucoma pathophysiology in cats as well as humans. [3] Corneal enlargement, often associated with more generalized globe enlargement, or buphthalmos, is a common secondary feature of chronic glaucoma in adult dogs and cats, and in both FCG and primary congenital glaucoma (PCG) in humans. [4–7] In particular, corneal enlargement can be very pronounced in patients that are affected by glaucoma at a very young age.[4–7] Chronic glaucoma has been associated with reduced corneal sensitivity in human PCG patients. [8, 9] In their study involving patients aged 8 to 16 years old with PCG, Gatzioufas et al. identified significant reduction in mean corneal sub-basal nerve density and total number of nerve fibers in the central cornea. Although number and density of corneal sub-basal nerve fibers were positively correlated with corneal sensitivity, corneal sensitivity was not demonstrably reduced in PCG eyes in that study. [10]
Corneal sensitivity provides an indirect estimate of corneal innervation, the latter being essential for corneal health, and can be affected by several factors including age, [11, 12] species, [11–14] ocular abnormalities, shape of the skull, [14, 15] and corneal region[14]. Corneal nerves provide neural signaling for the production of tears, help maintain structure and function of the cornea, and provide protective mechanisms to prevent physical corneal damage.[16] Thus, any loss or dysfunction of these nerves may lead to significant corneal health issues including neurotrophic keratitis, impaired wound healing, increased risk of infection, and enhanced inflammation. [13, 16, 17] A report documenting reduced corneal sensitivity in dogs with chronic glaucoma, [13] indicates that this parameter warrants evaluation in veterinary patients due to its potential to impact corneal health.
To the authors’ knowledge, there have been no prior published reports of corneal sensitivity in cats with glaucoma. The current study was designed to determine the effect of FCG on corneal sensitivity and examine relationships between corneal sensitivity and corneal biometric parameters, including central corneal thickness and corneal diameter.
Materials and Methods
Animals and inclusion criteria
All animal studies were performed in accordance with a protocol approved by the University of Wisconsin-Madison Institutional Animal Care and Use Committee and in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Forty adult domestic cats (Felis catus) that were housed in a laboratory animal facility at the University of Wisconsin-Madison for the purposes of other long-term, non-invasive studies (electrophysiology and ocular imaging), were used opportunistically for this study, between other testing events. Nineteen cats (13 males and 6 females) with FCG, that were homozygous for an LTBP2 gene mutation, [2] were evaluated in the glaucoma group and 21 normal cats (11 males and 10 females) were included in the age-matched control group. All subjects were domestic short-hair cats and were sexually intact. Cats in the control group did not have the LTBP2 gene mutation as determined by parentage or genotyping and were determined to have a normal ophthalmic examination as outlined below.
Five to seven days prior to conducting esthesiometry, all animals were examined by a board certified veterinary ophthalmologist (GJM). Examinations included slit-lamp biomicroscopy (SL-15 Kowa Company, Ltd, Tokyo, Japan), fluorescein staining (Ful-Glo, Akorn, Inc., Buffalo Grove, IL, USA), and indirect ophthalmoscopy (Keeler Vantage Plus, Keeler Instruments Inc., Broom, PA, USA). Intraocular pressures (IOPs) had been measured in all cats at least weekly from 8 weeks of age using a rebound tonometer (TonoVet®; iCare Finland Oy, Helsinki, Finland), to further confirm phenotype. Cats with potentially confounding ocular abnormalities in one or both eyes (corneal edema, anterior lens luxation, corneal scars), and fractious animals in which tests could not be conducted with only gentle manual restraint, were excluded from the study. From the population initially screened, a total of 14 normal and 16 FCG cats were ultimately included in the esthesiometry study, and ranged in age from 7 months to 3.5 years. All clinical examination and measurement procedures outlined below were conducted in awake, gently restrained cats by a single observer (MRT).
Intraocular pressures
Rebound tonometry (TonoVet®; Icare Finland Oy, Helsinki, Finland) was used to measure IOP in each eye of each subject. [18] Tonometry was performed before measuring other parameters in the study, using the tonometer’s “d” (dog) setting, until three readings with a standard deviation of ≤ 2.5 mmHg (no bar or a low bar on the instrument display) were obtained, and these triplicate values averaged for each eye.
Corneal touch threshold
A Cochet-Bonnet esthesiometer (Luneau Ophtalmologie, Chartres, France) with a 0.12 mm diameter monofilament nylon fiber was used to test corneal touch threshold (CTT) for the right and left eyes of each cat. The esthesiometer was held perpendicular to the cornea while the filament tip was advanced toward the axial corneal surface until there was a slight bend noted in the fiber. Starting with the longest filament length of 60 mm, the length was shortened in increments of 5 mm, with 3 repetitions performed at each filament length until a complete blink was consistently seen in response to appropriate corneal contact. In order to ensure the blink was not spontaneous or associated with inadvertent contact between the filament and adnexal structures, the filament length was shortened by another 5 mm once a complete blink was elicited and the procedure was repeated. If the cat also had a complete blink at that filament length, it was assumed that the previous complete blinks were real and representative of true CTT. If the blink response could not be verified, testing was continued by shortening the filament in 5 mm increments until a consistent full blink was obtained. The CTT value (in millimeters), defined as the longest filament length at which a blink response was consistently elicited, was considered proportional to corneal sensitivity (with shorter filament length values indicating lower corneal sensitivity). Esthesiometry was not performed in other regions of the cornea because the axial cornea is considered to be the most sensitive. [14, 19]
Corneal thickness
An Accutome AccuPach V Ultrasound pachymeter (Accutome, Inc., Malvern, PA, USA), calibrated in accordance with manufacturer’s instructions, was used to measure central corneal thickness (CT) in both eyes of each subject. Proparacaine hydrochloride ophthalmic solution, USP 0.5% (Akorn, Inc., Lake Forest, IL, USA) topical anesthestic was administered in each eye prior to pachymetry. The average value of nine readings, as calculated by the instrument software was recorded. This procedure was repeated to obtain three instrument-derived average values that were then averaged for each eye.
Corneal diameter
Maximum horizontal corneal diameter (CD) was measured using digital calipers (FisherScience Education Traceable Digital Carbon Fiber Calipers, Fisher Scientific, Waltham, MA, USA) from the temporal to nasal limbus. Triplicate measurements were obtained by the same observer (MRT) for each eye and averaged.
Statistical analyses
Data were assessed by Kolmogorov-Smirnov testing to confirm normal distribution. As data for two eyes of an individual animal are generally considered highly correlated and not truly independent, and mean values for the measured parameters were not statistically significantly different between eyes in either group, values were averaged for both eyes. A paired Student t-test was used for between-eye comparisons within groups; an unpaired Student t-test was used for comparisons of clinical parameters between groups. Relationships between different parameters were first evaluated by linear regression (Microsoft Excel) and subsequently assessed by Pearson correlation coefficients. Except where otherwise specified, statistical analyses were conducted using Instat (Graphpad Software Inc., San Diego, CA, USA) and data were presented as mean ± standard deviation. For all statistical tests, p values <0.05 were considered significant (Benjamini-Hochberg adjusted to account for multiple comparisons where appropriate).
However, as disease was asymmetric between eyes in some FCG cats in this study group, the convention of averaging of data to yield a single value for both eyes was not considered to fully reflect between-eye variability within subjects. Linear mixed effects models were therefore utilized to enable inclusion of data from both eyes in statistical analyses, while not neglecting the correlation between eyes within individual cats. This was done using the lme4 package[20] in R (version 3.4.1). For each pair of variables, one was fitted as the response on the other, including a random effect of cat ID, and results expressed as estimated regression coefficients, with 95% or 99% confidence intervals.
Results
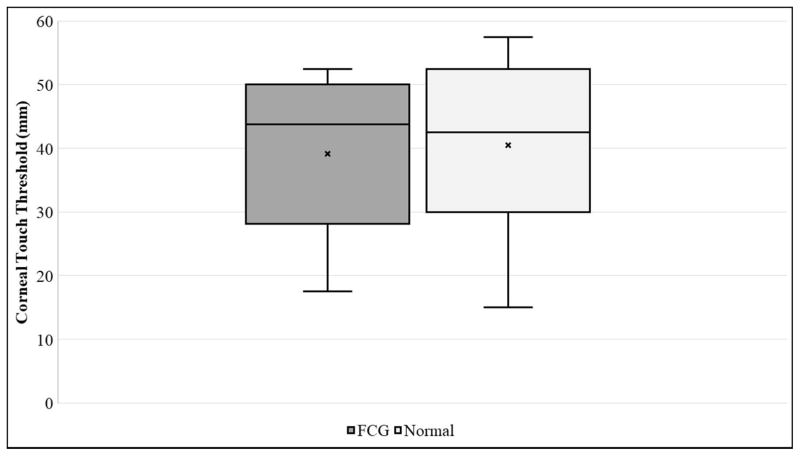
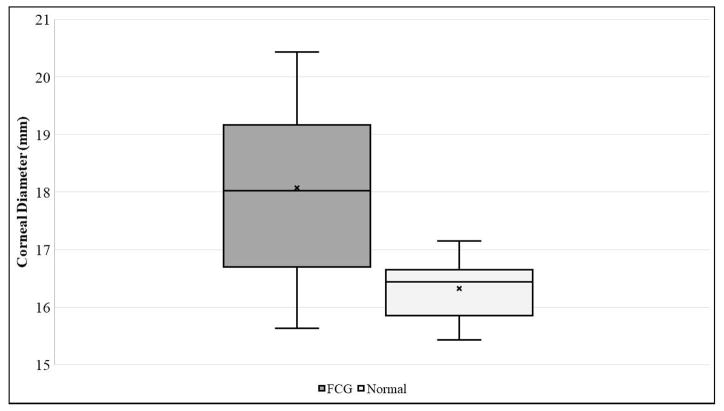
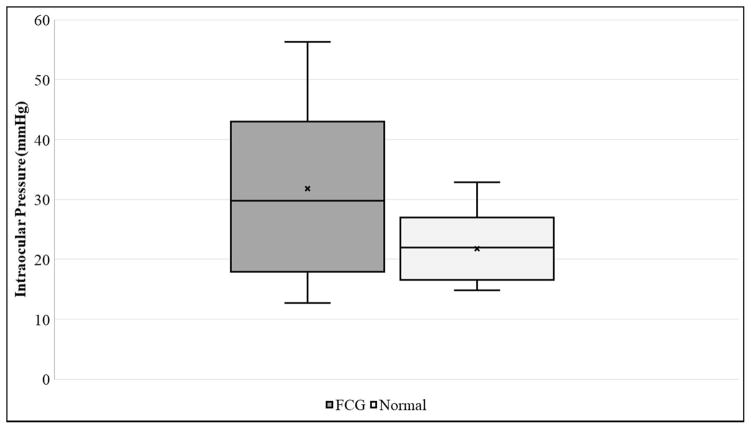
Corneal sensitivity, as determined by mean central CTT, was slightly lower in FCG cats (39.1 ± 12.2 mm) than in normal cats (40.2 ± 13.0 mm), but the difference between groups was not statistically significant (Table 1, Figure 1A). Although the cornea was slightly thinner in FCG cats (CT=564.4 ± 36.5 μm) than in normal cats (CT=583.1 ± 54.28 μm), the difference between groups was not statistically significant (Table 1). Mean CD was significantly larger in FCG cats (18.1 ± 1.5 mm) than in normal cats (16.3 ± 0.5 mm) (Table 1, Figure 1B; p=0.0004). As expected, IOP was significantly higher in glaucomatous cats (31.7 ± 14.9 mmHg) than in normal cats (21.8 ± 5.6 mmHg; p=0.0409) (Table 1, Fig. 1C).
Table 1.
Mean values (± standard deviation) for intraocular pressure (IOP), corneal touch threshold (CTT), central corneal thickness (CT), and corneal diameter (CD) for cats with feline congenital glaucoma compared to normal cats.[* indicates p value <0.05 (unpaired t test)]
| Parameter | Glaucoma (n=16) | Normal (n=14) |
|---|---|---|
| *IOP (mmHg) p=0.0409 |
31.7 ± 14.9 | 21.8 ± 5.6 |
| CTT (mm) p=0.7013 |
39.1 ± 12.2 | 40.15 ± 13.0 |
| CT (μm) p=0.5532 |
564.4 ± 36.5 | 583.1 ± 54.3 |
| *CD (mm) p=0.0004 |
18.1 ± 1.5 | 16.3 ± 0.5 |
Figure 1A–C.
Box and whisker plots depicting [A] Corneal Touch Threshold (CTT), [B] Corneal Diameter (CD), and [C] Intraocular Pressure (IOP) for FCG and normal cats. In each plot “x” represents the mean, the median is depicted by a horizontal line dividing the box, which represents the interquartile range, and the whiskers show the outlier range outside of the upper and lower quartiles (includes lowest and highest data points).
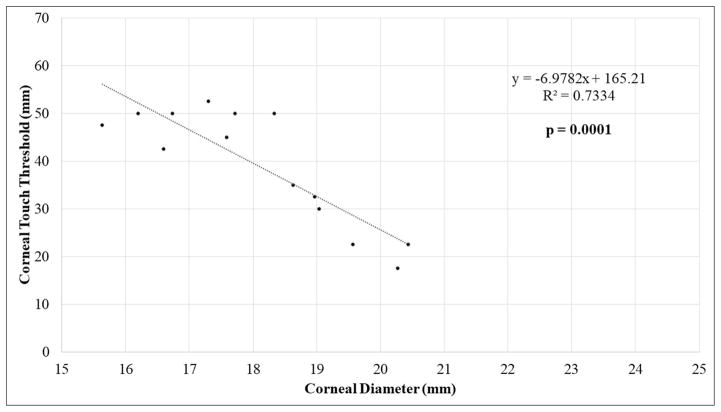
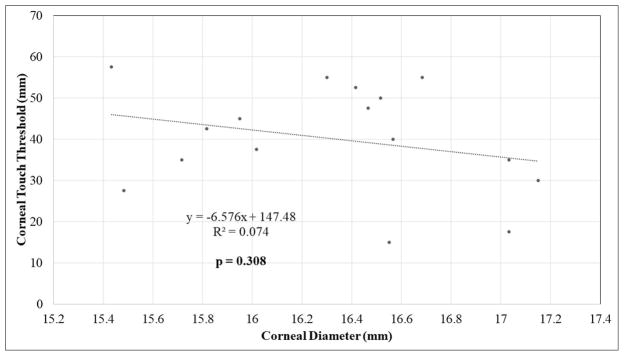
Additional statistical analyses were performed to examine the relationships between clinical parameters including age, CTT, CT, IOP, and CD for each study population (Tables 2 and 3). A statistically significant, strong negative correlation was identified between CD and corneal sensitivity (CTT) in FCG cats (Pearson, r=-0.8564; corrected p=0.0001) (Fig. 2A). A statistically significant negative correlation was also noted between CTT and age in FCG cats (r=−0.7421; corrected p=0.015). Corneal diameter was significantly positively correlated with age in cats with FCG (r=0.777; corrected p=0.01). There was no significant relationship between CTT and CD in normal cats (Fig. 2B). There was a significant trend for CT to increase with age in both normal and FCG cats, but this relationship was more robust in normal cats (r=0.6849; corrected p=0.0034). Although some were statistically significant, correlations between other the parameters presented in table 2 were determined to be relatively weak, based on linear regression and correlation coefficients.
Table 2.
Correlation between age, corneal touch threshold (CTT), corneal thickness (CT), intraocular pressure (IOP), and corneal diameter (CD) in normal cats. Pearson correlation coefficients (R values) and corresponding Benjamini-Hochberg (BH) critical p values were determined as a threshold for significance to correct for multiple comparisons. Those associations for which p values reached the threshold for significance are shaded.
| Parameter 1 | Parameter 2 | R-Value | P-Value | Rank | BH Critical Value |
|---|---|---|---|---|---|
| IOP | CD | 0.78 | 0.0007 | 1 | 0.005 |
| CD | Age | 0.73 | 0.0013 | 2 | 0.01 |
| CT | Age | 0.68 | 0.0034 | 3 | 0.015 |
| IOP | Age | 0.67 | 0.0042 | 4 | 0.02 |
| CD | CT | 0.63 | 0.0094 | 5 | 0.025 |
| CTT | Age | −0.63 | 0.0094 | 6 | 0.025 |
| IOP | CT | 0.57 | 0.219 | 7 | 0.035 |
| CT | CTT | −0.035 | 0.1814 | 8 | 0.04 |
| IOP | CTT | −0.30 | 0.2528 | 9 | 0.045 |
| CD | CTT | −0.272 | 0.308 | 10 | 0.05 |
Table 3.
Correlation between age, corneal touch threshold (CTT), corneal thickness (CT), intraocular pressure (IOP), and corneal diameter (CD) in FCG cats. Pearson correlation coefficients (R values) and corresponding Benjamini-Hochberg (BH) critical p values were determined as a threshold for significance to correct for multiple comparisons. Those associations for which p values reached the threshold for significance are shaded.
| Parameter 1 | Parameter 2 | R-Value | P-Value | Rank | BH Critical Value |
|---|---|---|---|---|---|
| CD | CTT | −0.86 | 0.0001 | 1 | 0.005 |
| CD | Age | 0.78 | 0.0011 | 2 | 0.01 |
| CTT | Age | −0.74 | 0.0024 | 3 | 0.015 |
| CT | Age | 0.61 | 0.0202 | 4 | 0.02 |
| CD | CT | 0.49 | 0.0739 | 5 | 0.025 |
| IOP | CD | 0.31 | 0.2827 | 6 | 0.03 |
| CT | CTT | −0.30 | 0.3050 | 7 | 0.035 |
| IOP | Age | 0.29 | 0.3131 | 8 | 0.04 |
| IOP | CTT | −0.27 | 0.3556 | 9 | 0.045 |
| IOP | CT | −0.04 | 0.8817 | 10 | 0.05 |
Figure 2A–B.
Scatter plots with linear regression showing relationship between corneal sensitivity (mean corneal touch threshold, CTT) and corneal diameter (CD) in [A] FCG cats and in [B] normal cats.
To further evaluate the associations outlined above, linear mixed effects models (LMMs) were used. First, LMMs were fitted for all pairs of the variables age, CTT, CT, IOP, and CD for each study population. The only included terms in these models were the two variables, one as explanatory and one as the outcome. The results are reported as the estimated effects with 99% confidence intervals for normal cats and FCG cats (Table 4). From this table, it can be seen that there are significant relationships between CT and age for normal cats, CD and age for FCG cats, CTT and age for both normal and FCG cats, and finally between CTT and CD for both normal and FCG cats.
Table 4.
Linear mixed effects models in cats with FCG and normal cats. Each row represents the results of a model, each column either the outcome or an explanatory. Estimates of effect sizes are provided with 99% confidence intervals in parentheses. Only those explanatory variables for which confidence intervals do not contain zero (shaded) are considered significant associations in these analyses. Corneal touch threshold=CTT, corneal thickness=CT, intraocular pressure =IOP, and corneal diameter=CD.
| Linear mixed effects models in normal cats and FCG cats | |||
|---|---|---|---|
| Outcome | Explanatory | Estimate (99% CI) Normal Cats | Estimate (99% CI) FCG Cats |
| CT | Age | 1.44 (0.49; 2.39) | 1.8 (−0.01; 3.62) |
| CT | CD | 5.12 (−7.48; 17.7) | 9.05 (−2.67; 20.9) |
| CT | IOP | −0.01 (−0.95; 0.95) | 0.1 (−0.63; 0.82) |
| CD | Age | 0.02 (−0.01; 0.06) | 0.09 (0.03; 0.15) |
| CD | IOP | 0.02 (0; 0.05) | 0.01 (−0.01; 0.05) |
| CTT | Age | −0.44 (−0.7; −0.18) | −0.73 (−1.24; −0.22) |
| CTT | CT | −0.05 (−0.16; 0.07) | −0.05 (−0.27; 0.18) |
| CTT | CD | −4.22 (−7.71; −0.71) | −6.68 (−9.72; −3.42) |
| CTT | IOP | −0.23 (−0.57; 0.1) | −0.2 (−0.59; 0.19) |
| IOP | Age | 0.15 (−0.17; 0.47) | 0.35 (−0.55; 1.24) |
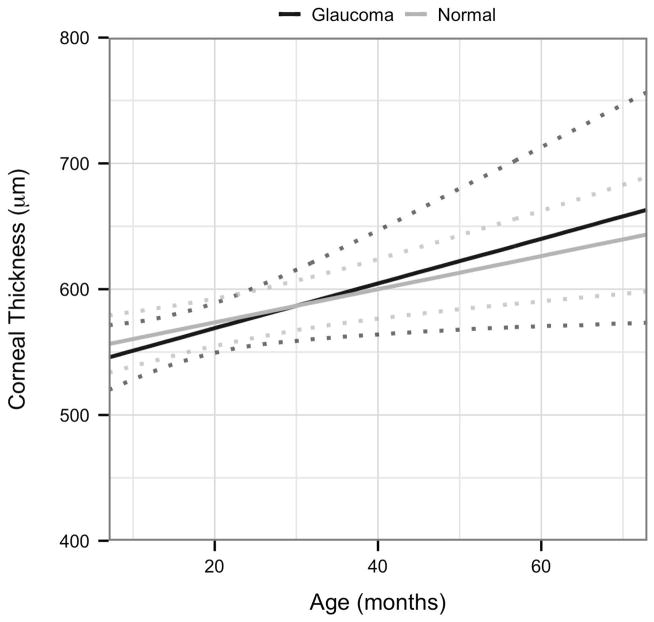
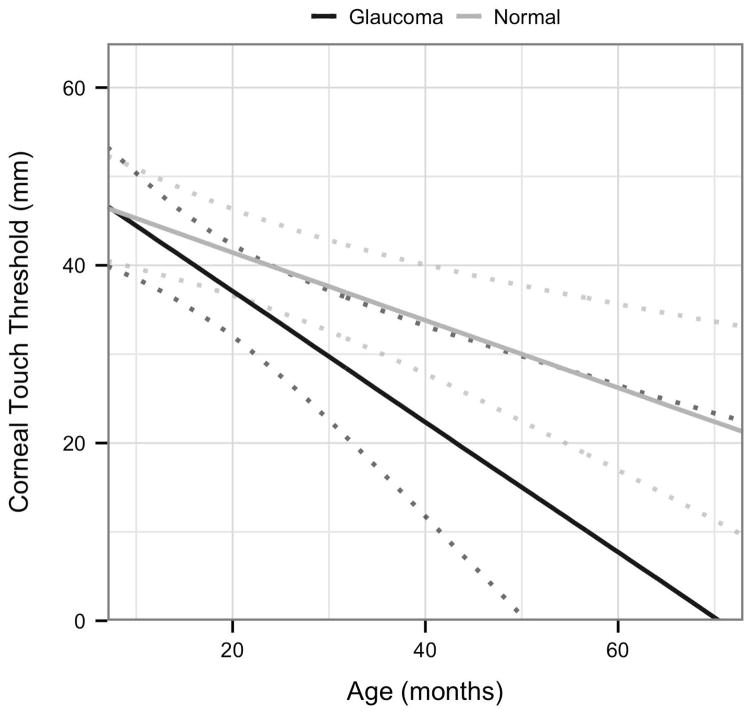
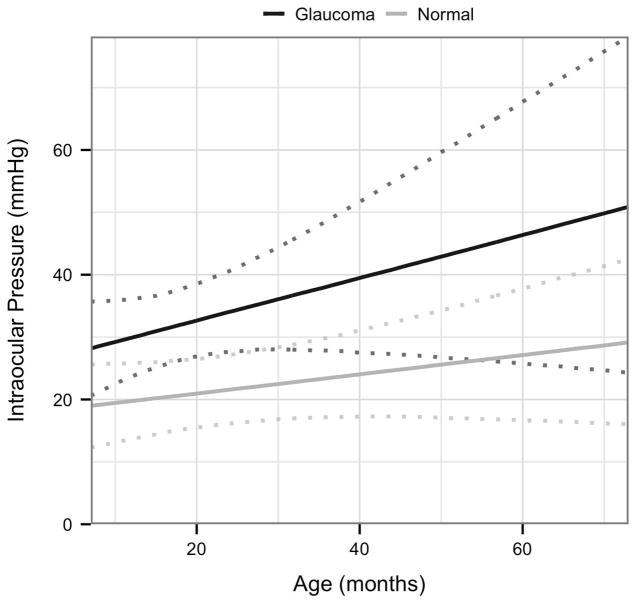
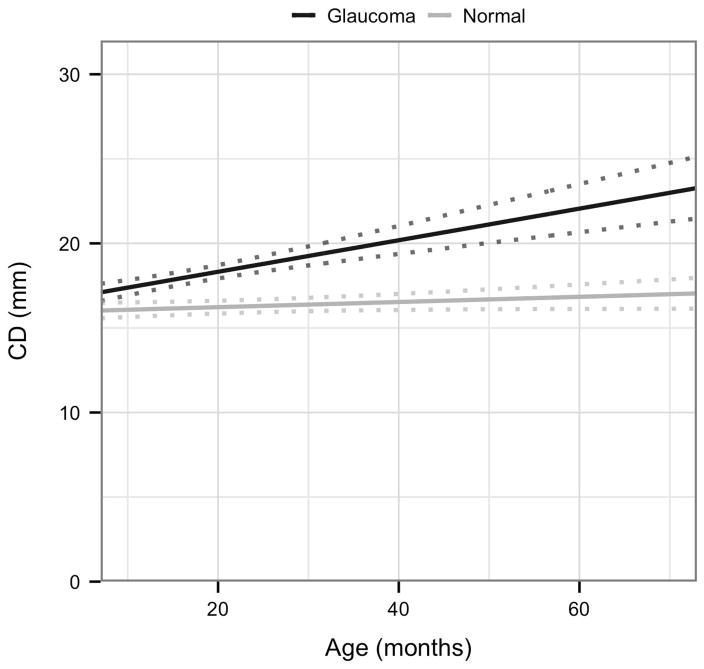
Next, to rigorously assess strength of association between disease status and the four variables CT, CTT, CD, and IOP, a more complex LMM was fitted for each of these with disease status (Normal or FCG) and age as explanatory variables. Based on scatter plots, a first order interaction term between age and disease status was included. The results are shown in Table 5 and Figs. 3A–D. As can be seen from confidence intervals, age is significantly associated with CT and negatively associated with CTT. The only other effect that is significant is the interaction effect between age and disease status in the CD model. This indicates that for this model, the effect of age is significantly greater in FCG cats than in normal cats.
Table 5.
Linear mixed effects models: Each row represents the results of a model, each column provides either the intercept or an explanatory variable. Estimates of effect sizes are provided with 95% confidence intervals in parentheses. Only those explanatory variables for which confidence intervals do not contain zero (shaded) are considered significant associations in these analyses. Corneal touch threshold=CTT, central corneal thickness=CT, intraocular pressure=IOP, and corneal diameter=CD.
| (Intercept) | Age | Glaucoma | Age:Glaucoma | |
|---|---|---|---|---|
| CT | 547.397 (522.024; 572.771) | 1.32 (0.525; 2.115) | −14.305 (−55.638; 27.028) | 0.482 (−1.261; 2.225) |
| CD | 15.927 (15.419; 16.435) | 0.015 (−0.001; 0.031) | 0.519 (−0.308; 1.346) | 0.079 (0.044; 0.113) |
| CTT | 49.132 (42.552; 55.712) | −0.381 (−0.588; −0.175) | 2.625 (−8.093; 13.344) | −0.349 (−0.801; 0.103) |
| IOP | 17.9 (10.5; 25.4) | 0.154 (−0.078; 0.386) | 7.783 (−4.281; 19.847) | 0.195 (−0.314; 0.704) |
Figure 3A–D.
Visualization of the linear mixed effects model fitting:[A] central corneal thickness as a response to age and disease status; [B] corneal touch threshold as a response to age and disease status; [C] intraocular pressure as a response to age and disease status, and [D] corneal diameter as a response to age and disease status; each plot indicates 95% confidence intervals.
Discussion
The current study was designed to determine the effect of congenital glaucoma on corneal sensitivity in cats. In addition, we examined relationships between corneal sensitivity, age, IOP and other ocular parameters in normal cats and cats with FCG. Corneal sensitivity can be quantified in a clinical research setting by corneal esthesiometry, although the technique is considered both subjective and operator dependent, [11, 13, 14, 21–26] and filament properties may be influenced by factors including temperature and humidity[27, 28]. We attempted to address these limitations in our study by having a single observer obtain all CTT measurements and conducting all tests at the same time of day, over just two days, within a climate-controlled vivarium in which environmental temperature and humidity were regulated and recorded.
As expected in this form of early onset glaucoma, mean corneal diameter was significantly larger in glaucomatous cats relative to normal cats, and in cats with FCG, larger corneal diameters correlated with decreased corneal sensitivity. Mean CTT was slightly lower in glaucomatous eyes relative to normal eyes, however the difference was not statistically significant and the values obtained in our study are generally similar to previously published ranges in normal cats. [14, 29–31] Similar findings were reported in prior studies involving human PCG patients.[10] Prior studies in human patients have shown that buphthalmos at an early age may be associated with significant reduction in corneal sensitivity, though in these patients increased CD, is also associated with increased CT and corneal edema. [8, 9] In 1988, Patel and others noted that persistent corneal edema led to decreased corneal sensitivity, even after normalization of IOP, in patients that had prolonged elevation of IOP. [8] In the veterinary literature, one prospective clinical study evaluating dogs with buphthalmos and/or chronically elevated intraocular pressure prior evisceration with intraocular prosthesis placement determined that corneal sensitivity in eyes with chronically elevated IOP was significantly reduced relative contralateral control eyes. [13] These reports support our finding that larger corneal diameter correlated with decreased corneal sensitivity in FCG cats. Despite reported associations between decreased corneal sensitivity in the presence of corneal edema, corneal edema is not a prominent feature in cats with FCG. Indeed a few cats with clinically appreciable corneal edema (secondary to anterior lens subluxation) were excluded from our esthesiometry study and FCG cats in our study tended to have marginally thinner central corneas than age-matched control cats. Therefore, confounding effects of underlying diseases (such as inflammation, corneal edema or lens luxation) are less of a consideration in our study population than in more heterogeneous groups of human and canine glaucoma patients reported previously.
Although our findings suggest that larger corneal diameters may be associated with lower corneal sensitivity, corneal sensitivity was not significantly different between FCG and normal cats. This finding could be due to several limitations of Cochet Bonnet esthesiometry, as outlined above. Secondly, large standard deviations were noted in values for most of the parameters measured in FCG cats in the study. Thus, pronounced inter-individual variability and relatively small sample sizes within our study population, reduced our statistical power to detect small differences with the number of subjects available. Corneal innervation and density has previously been reported using in vivo confocal microscopy in mesocephalic and brachycephalic dogs and cats, birds and horses at other institutions. [15, 32, 33] Future studies using in vivo confocal microscopy to evaluate sub-basal nerve architecture and density could be used to limit subjectivity and validate indirect esthesiometry values.
In conclusion, we demonstrated a negative correlation between CD and corneal sensitivity in cats with congenital glaucoma. While this might indicate a potential loss of corneal sensitivity, perhaps attributable to a reduction corneal nerve fiber density in buphthalmos, this was not definitively established in our study. As previously discussed, corneal nerves and sensation play a vital role in maintaining the normal protective mechanisms and functions of the cornea. [13, 15, 16] As in brachycephalic breeds, lower corneal sensitivity identified in FCG could conceivably predispose glaucomatous cats to corneal disease, particularly in those animals that develop lagophthalmos and corneal exposure due pronounced globe enlargement. However, caution should be exercised in extrapolating the findings of this study to cats with other forms of glaucoma, as our study population was comprised of relatively young cats with a single, specific form of glaucoma. Thus, our findings may not be representative of a wider population of older cats with glaucoma secondary to other ocular disorders. Further studies are warranted to explore the effects of buphthalmos and corneal enlargement on corneal sensitivity and innervation in FCG and in other forms of feline glaucoma.
Acknowledgments
This study was supported in part by an Unrestricted Grant to the Department of Ophthalmology and Visual Sciences from Research to Prevent Blindness; NIH Core grant for Vision Research, P30 EY016665; Lions Eye Bank of Wisconsin Gift of Sight Discovery Fund; UW-Madison Companion Animal Fund, and a National Glaucoma Research Grant from the Bright Focus Foundation. The authors also thank Ocular Services on Demand LLC for loan of the ultrasonic pachymeter used in this study, and Dr. Ellison Bentley for her helpful comments on the manuscript.
References
- 1.McLellan GJ, Teixeira LB. Feline glaucoma. Veterinary Clinics of North America Small Animal Practice. 2015;45:1307–1333. vii. doi: 10.1016/j.cvsm.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Kuehn MH, Lipsett KA, Menotti-Raymond M, et al. A mutation in LTBP2 causes congenital glaucoma in domestic cats (Felis catus) PLoS One. 2016;11:e0154412. doi: 10.1371/journal.pone.0154412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rutz-Mendicino MM, Snella EM, Jens JK, et al. Removal of potentially confounding phenotypes from a Siamese-derived feline glaucoma breeding colony. Comparative Medicine. 2011;61:251–257. [PMC free article] [PubMed] [Google Scholar]
- 4.Alodhayb S, Morales J, Edward DP, et al. Update on pediatric glaucoma. Seminars in Ophthalmology. 2013;28:131–143. doi: 10.3109/08820538.2013.771196. [DOI] [PubMed] [Google Scholar]
- 5.Doozandeh A, Yazdani S, Ansari S, et al. Corneal profile in primary congenital glaucoma. Acta Ophthalmologica. 2017 doi: 10.1111/aos.13357. [DOI] [PubMed] [Google Scholar]
- 6.Sarfarazi M, Akarsu AN, Hossain A, et al. Assignment of a locus (GLC3A) for primary congenital glaucoma (buphthalmos) to 2p21 and evidence for genetic heterogeneity. Genomics. 1995;30:171–177. doi: 10.1006/geno.1995.9888. [DOI] [PubMed] [Google Scholar]
- 7.Stoilov I, Akarsu AN, Sarfarazi M. Identification of three different truncating mutations in cytochrome P4501B1 (CYP1B1) as the principal cause of primary congenital glaucoma (Buphthalmos) in families linked to the GLC3A locus on chromosome 2p21. Human Molecular Genetics. 1997;6:641–647. doi: 10.1093/hmg/6.4.641. [DOI] [PubMed] [Google Scholar]
- 8.Patel BC, Tullo AB. Corneal sensation in acute angle closure glaucoma. Acta Ophthalmologica (Copenhagen) 1988;66:44–46. doi: 10.1111/j.1755-3768.1988.tb08532.x. [DOI] [PubMed] [Google Scholar]
- 9.Riss B, Drobec P. Corneal sensitivity in chronic open-angle glaucoma (author’s transl) Kliniche Monatsblatter Fur Augenheilkunde. 1981;179:467–469. doi: 10.1055/s-2008-1057365. [DOI] [PubMed] [Google Scholar]
- 10.Gatzioufas Z, Labiris G, Hafezi F, et al. Corneal sensitivity and morphology of the corneal subbasal nerve plexus in primary congenital glaucoma. Eye (London) 2014;28:466–471. doi: 10.1038/eye.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brooks DE, Clark CK, Lester GD. Cochet-Bonnet aesthesiometer-determined corneal sensitivity in neonatal foals and adult horses. Veterinary Ophthalmology. 2000;3:133–137. doi: 10.1046/j.1463-5224.2000.00125.x. [DOI] [PubMed] [Google Scholar]
- 12.Miller C, Utter ML, Beech J. Evaluation of the effects of age and pituitary pars intermedia dysfunction on corneal sensitivity in horses. American Journal of Veterinary Research. 2013;74:1030–1035. doi: 10.2460/ajvr.74.7.1030. [DOI] [PubMed] [Google Scholar]
- 13.Blocker T, Hoffman A, Schaeffer DJ, et al. Corneal sensitivity and aqueous tear production in dogs undergoing evisceration with intraocular prosthesis placement. Veterinary Ophthalmology. 2007;10:147–154. doi: 10.1111/j.1463-5224.2007.00524.x. [DOI] [PubMed] [Google Scholar]
- 14.Blocker T, Van Der Woerdt A. A comparison of corneal sensitivity between brachycephalic and Domestic Short-haired cats. Veterinary Ophthalmology. 2001;4:127–130. doi: 10.1046/j.1463-5224.2001.00189.x. [DOI] [PubMed] [Google Scholar]
- 15.Kafarnik C, Fritsche J, Reese S. Corneal innervation in mesocephalic and brachycephalic dogs and cats: assessment using in vivo confocal microscopy. Veterinary Ophthalmology. 2008;11:363–367. doi: 10.1111/j.1463-5224.2008.00659.x. [DOI] [PubMed] [Google Scholar]
- 16.Müller LJ, Marfurt CF, Kruse F, et al. Corneal nerves: structure, contents and function. Experimental Eye Research. 2003;76:521–542. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 17.Tripathi RC. Ultrastructure of the exit pathway of the aqueous in lower mammals. (A preliminary report on the “angular aqueous plexus”) Experimental Eye Research. 1971;12:311–314. doi: 10.1016/0014-4835(71)90155-2. [DOI] [PubMed] [Google Scholar]
- 18.McLellan GJ, Kemmerling JP, Kiland JA. Validation of the TonoVet® rebound tonometer in normal and glaucomatous cats. Veterinary Ophthalmology. 2013;16:111–118. doi: 10.1111/j.1463-5224.2012.01038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan-Ling T. Sensitivity and neural organization of the cat cornea. Investigative Ophthalmology & Visual Science. 1989;30:1075–1082. [PubMed] [Google Scholar]
- 20.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. Journal of Statistical Software. 2014;67(1) [Google Scholar]
- 21.Stapleton F, Tan ME, Papas EB, et al. Corneal and conjunctival sensitivity to air stimuli. British Journal of Ophthalmology. 2004;88:1547–1551. doi: 10.1136/bjo.2004.044024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy PJ, Patel S, Marshall J. A new non-contact corneal aesthesiometer (NCCA) Ophthalmic & Physiological Optics. 1996;16:101–107. [PubMed] [Google Scholar]
- 23.Good KL, Maggs DJ, Hollingsworth SR, Scagliotti RH, et al. Corneal sensitivity in dogs with diabetes mellitus. American Journal of Veterinary Research. 2003;64:7–11. doi: 10.2460/ajvr.2003.64.7. [DOI] [PubMed] [Google Scholar]
- 24.Kaps S, Richter M, Spiess BM. Corneal esthesiometry in the healthy horse. Veterinary Ophthalmology. 2003;6:151–155. doi: 10.1046/j.1463-5224.2003.00283.x. [DOI] [PubMed] [Google Scholar]
- 25.Kalf KL, Utter ME, Wotman KL. Evaluation of duration of corneal anesthesia induced with ophthalmic 0. 5% proparacaine hydrochloride by use of a Cochet-Bonnet aesthesiometer in clinically normal horses. American Journal of Veterinary Research. 2008;69:1655–1658. doi: 10.2460/ajvr.69.12.1655. [DOI] [PubMed] [Google Scholar]
- 26.Knickelbein KE, Scherrer NM, Lassaline M. Corneal sensitivity and tear production in 108 horses with ocular disease. Veterinary Ophthalmology. 2017 doi: 10.1111/vop.12481. [DOI] [PubMed] [Google Scholar]
- 27.Millodot M, Larson W. Effect of bending the nylon thread of the Cochet Bonnet aesthesiometer upon the recorded pressure. Contact Lens. 1967;1:5–7. [Google Scholar]
- 28.Kolstrad A. Corneal sensitivity by low temperatures. Acta Ophthalmologica. 1970;48:789–793. [PubMed] [Google Scholar]
- 29.Wieser B, Tichy A, Nell B. Correlation between corneal sensitivity and quantity of reflex tearing in cows, horses, goats, sheep, dogs, cats, rabbits, and guinea pigs. Veterinary Ophthalmology. 2013;16:251–262. doi: 10.1111/j.1463-5224.2012.01069.x. [DOI] [PubMed] [Google Scholar]
- 30.Binder DR, Herring IP. Duration of corneal anesthesia following topical administration of 0. 5% proparacaine hydrochloride solution in clinically normal cats. American Journal of Veterinary Research. 2006;67:1780–1782. doi: 10.2460/ajvr.67.10.1780. [DOI] [PubMed] [Google Scholar]
- 31.Giudici V, Baeza S, Douet JY, et al. Corneal anesthesia following application of 0. 4% oxybuprocaine hydrochloride ophthalmic solution to normal feline eyes. Veterinary Ophthalmology. 2015;18:141–146. doi: 10.1111/vop.12179. [DOI] [PubMed] [Google Scholar]
- 32.Kafarnik C, Fritsche J, Reese S. In vivo confocal microscopy in the normal corneas of cats, dogs and birds. Veterinary Ophthalmology. 2007;10:222–230. doi: 10.1111/j.1463-5224.2007.00543.x. [DOI] [PubMed] [Google Scholar]
- 33.Ledbetter EC, Scarlett JM. In vivo confocal microscopy of the normal equine cornea and limbus. Veterinary Ophthalmology. 2009;12(Suppl 1):57–64. doi: 10.1111/j.1463-5224.2009.00730.x. [DOI] [PubMed] [Google Scholar]