Abstract
Objective:
To compare the effects of laboratory-based training in implementation intentions (II; experimental strategy) and verbal rehearsal (VR; control strategy) on self-reported everyday prospective memory among people with Parkinson disease (PD) and to investigate potential correlates of change in self-reported everyday prospective memory in response to this training.
Method:
This was a randomized-controlled trial. Participants with mild to moderate PD without dementia underwent one session of training in either II (n = 25) or VR (n = 27). Then they were instructed to use their strategy as much as possible in their everyday lives to help them remember to do things. The Prospective and Retrospective Memory Questionnaire Prospective Scale (PRMQ-Pro) administered at baseline and one month after training assessed training-related change in self-reported everyday prospective memory. Baseline depressive symptoms, perceptions of the strategy (credibility, expectancy), prospective memory-related awareness, global cognition, and disease severity were correlated to PRMQ-Pro Change scores (post minus pre) to determine their association with response to training.
Results:
The VR group’s PRMQ-Pro scores declined from pre to post training, while the II group’s remained stable (p = 0.03). This effect was driven by change in self-cued everyday prospective memory tasks. Higher baseline depressive symptoms, treatment expectancy, and global cognition related to better response to training in the II group (rs ≤ −0.40, ps ≤ 0.05).
Conclusions:
II training may prevent everyday prospective memory decline among people with PD. In addition, people with higher depression, stronger expectations of improvement from strategy training, or better global cognition may benefit the most from II training.
Keywords: Parkinson disease, memory, executive function, randomized controlled trial, cognitive rehabilitation
Introduction
Parkinson disease (PD) is the second most common neurodegenerative disorder, affecting approximately 1–2% of the population over the age of 65 (Alves, Forsaa, Pedersen, Dreetz Gjerstad, & Larsen, 2008). It is classified as a movement disorder, and clinical diagnosis is based on the presence of bradykinesia, rigidity, and/or resting tremor (Postuma et al., 2015). However, about one third of people in the earliest stages of PD have mild cognitive deficits, typically in memory, executive and attentional control functions (Foltynie, Brayne, Robbins, & Barker, 2004; Muslimovic, Post, Speelman, & Schmand, 2005). These deficits are attributed to frontostriatal circuitry dysfunction due to dopamine depletion in the basal ganglia and prefrontal cortex (Cools, 2006; Owen, 2004). Importantly, they relate to disability, reduced quality of life, and restricted participation early in the course of PD, potentially to a larger extent than motor impairment (Cahn et al., 1998; Foster, 2014; Foster & Hershey, 2011; Klepac, Trkulja, Relja, & Babic, 2008; Rosenthal et al., 2010). Pharmacologic and surgical treatments for PD do not prevent or treat cognitive impairment and may even exacerbate the problem (Burn, Weintraub, Ravina, & Litvan, 2014; Cools, 2006; Leroi, Collins, & Marsh, 2006; Xie, Meng, Xiao, Zhang, & Zhang, 2016). As such, interventions that mitigate the negative functional consequences of cognitive impairment in people with PD are a top research priority (Burn et al., 2014; Calleo et al., 2012; Deane et al., 2014; Hindle, Petrelli, Clare, & Kalbe, 2013; Leung et al., 2015; Walton, Naismith, Lampit, Mowszowski, & Lewis, 2017).
Due to its high functional and clinical relevance, PD-related prospective memory impairment is a prime target for cognitive intervention (Costa, Carlesimo, & Caltagirone, 2012; Kliegel & Martin, 2003). Good prospective memory, or the ability to remember to execute delayed intentions at the appropriate moment in the future (McDaniel & Einstein, 2007), is essential for independent living (e.g. paying bills on time, turning the stove off after using it) and adherence to important PD-related health behaviors (e.g. taking medications, doing home exercises). People with PD consistently demonstrate prospective memory deficits in laboratory studies (Ramanan & Kumar, 2013) and report more everyday prospective memory failures compared to healthy older adults (Foster, McDaniel, Repovs, & Hershey, 2009; Pirogovsky, Woods, Vincent Filoteo, & Gilbert, 2012). Further, prospective memory problems in people with PD relate to activity limitations and reduced health-related quality of life (Costa, Peppe, et al., 2015; Costa, Zabberoni, et al., 2015; Pirogovsky et al., 2012). Interventions that improve prospective memory in people with PD could positively impact daily function and clinical care for this population.
In their conceptual model, Kliegel, Altgassen, Hering, and Rose (2011) describe the process of prospective memory as encompassing in four phases: (1) intention formation – the intention to execute an action at a particular moment in the future is formed and encoded; (2) intention retention – the intention is retained in memory over a delay period that involves unrelated tasks (i.e. ongoing activity); (3) intention retrieval – the appropriate moment (i.e. cue) occurs and the intended action is retrieved from memory; (4) intention execution – the intention is successfully carried out. Each of these phases requires distinct underlying cognitive resources, the extent to which depends on characteristics of the particular prospective memory task. Following this model, prospective memory impairment is conceptualized as a mismatch between the cognitive resources required by the particular task and the individual’s available cognitive resources.
In relation to PD, prospective memory impairment is thought to stem from deficits in executive control processes that can underlie intention formation and intention retrieval (Foster, Rose, McDaniel, & Rendell, 2013; Kliegel et al., 2011). For example, tasks with complex intentions may require strategic encoding or planning during intention formation. Studies show that people with PD fail to self-initiate these processes, which then relates to subsequent failures in intention retrieval and execution (Altgassen, Zollig, Kopp, Mackinlay, & Kliegel, 2007; Foster et al., 2013; Kliegel, Phillips, Lemke, & Kopp, 2005). Regarding intention retrieval, tasks with cues that are perceptually salient or are processed as a part of the ongoing activity (i.e. focal cues) can be retrieved relatively automatically and thus do not require much executive control, whereas those with cues that are not processed as a part of the ongoing activity (i.e. non-focal and time-based cues) require strategic attentional control – namely, monitoring and shifting – to be retrieved (McDaniel & Einstein, 2000). People with PD are impaired on prospective memory tasks with non-focal and time-based cues relative to those with salient or focal cues (Costa, Peppe, Caltagirone, & Carlesimo, 2008; Foster et al., 2009; Foster et al., 2013; Raskin et al., 2010). Thus, PD-related prospective memory impairment is most apparent when intention formation or intention retrieval require the self-initiation of executive control processes such as planning, strategic encoding, and attentional control.
In light of the view that prospective memory impairment in PD stems primarily from executive dysfunction, two general approaches to improving prospective memory in PD can be pursued. The first is direct training to augment or restore the deficient executive control processes that underlie prospective memory impairment (i.e. process training), and the second is training in strategies to compensate for or circumvent deficits in the executive control processes that underlie prospective memory impairment (i.e. strategy training) (Brom & Kliegel, 2014; Hering, Rendell, Rose, Schnitzspahn, & Kliegel, 2014). In terms of the first approach, direct training of shifting ability (an executive control process) significantly improved PD participants’ performance on a laboratory prospective memory task (Costa et al., 2014). This finding is consistent with the bulk of the cognitive rehabilitation research in PD, which has shown that process training produces improved performance on neuropsychological tests that assess the cognitive processes that are trained (e.g. working memory, processing speed) (Leung et al., 2015). However, the process training approach has had limited effect on daily function in PD (e.g. Disbrow et al., 2012; Leung et al., 2015; Paris et al., 2011; Sammer, Reuter, Hullmann, Kaps, & Vaitl, 2006). In contrast, the few cognitive rehabilitation studies that have incorporated strategy training show promise for improving daily function in PD (Foster, Spence, & Toglia, 2017; Pena et al., 2014; Reuter, Mehnert, Sammer, Oechsner, & Engelhardt, 2012). This pattern of results dovetails with a study of prospective memory in healthy older adults, which found that strategy training was better than process training (shifting ability) for improving everyday prospective memory performance (Brom & Kliegel, 2014). Given the above evidence and the need for interventions that mitigate the impact of PD-related prospective memory impairment on daily function, we pursued a prospective memory strategy training intervention for people with PD.
A strategy that circumvents the executive control demands of tasks and improves prospective memory performance across a variety of populations is the implementation intentions (II) strategy (Chen et al., 2015; Wieber, Thurmer, & Gollwitzer, 2015). This associative encoding and planning strategy involves specifying the intended action (Y) and the appropriate moment or cue for action (X) and creating a “When X, I will do Y” statement (e.g. “When I eat breakfast, I will take my medication”) during intention formation (Gollwitzer, 1999). Full use of II requires the person to repeat the statement aloud several times and visualize him or herself encountering the future moment or cue and executing the intended action. The elaborate, specific, and dual verbal/visual encoding that occurs with forming II is hypothesized to increase the accessibility of the cue and strengthen the association between the cue and intended action and thus facilitate automatic cue detection and intended action retrieval when the cue is encountered (Gollwitzer, 1999; McDaniel, Howard, & Butler, 2008; Rummel, Einstein, & Rampey, 2012; Webb & Sheeran, 2007; Wieber et al., 2015)1. Therefore, II target both aspects of prospective memory tasks that can be challenging for people with PD due to executive dysfunction: intention formation and intention retrieval (Foster et al., 2013; Kliegel et al., 2011). II facilitate strategic encoding of intentions during the intention formation phase, which should then reduce the attentional monitoring demands of intention retrieval. In line with this proposed mechanism of action, II have been found to improve prospective memory in populations with subtle frontal-executive decline similar to that experienced by non-demented people with PD, such as healthy older adults, multiple sclerosis, and very mild Alzheimer’s disease (Chen et al., 2015; Kardiasmenos, Clawson, Wilken, & Wallin, 2008; Shelton et al., 2016), whereas they appear to be less effective in the context of concomitant retrospective memory impairment that may interfere with intention retention, such as that which occurs with traumatic brain injury (Mioni, Rendell, Terrett, & Stablum, 2015).
Following this reasoning, we conducted a randomized controlled trial comparing the effects of II and verbal rehearsal (VR) on prospective memory in PD (Foster, McDaniel, & Rendell, 2017). In line with previous studies (e.g. Brom & Kliegel, 2014; Chasteen, Park, & Schwarz, 2001; Kardiasmenos et al., 2008; Liu & Park, 2004), we selected VR as an active control condition to ensure equal exposure to the prospective memory tasks (in terms of time spent attending to the tasks and verbalization) without explicit facilitation of strategic or elaborate associative encoding (C. P. McFarland & Glisky, 2011). We used a single session of training, which has been shown to improve both laboratory and real-world prospective memory in healthy older adults (e.g. Brom & Kliegel, 2014; Liu & Park, 2004; C. P. McFarland & Glisky, 2011; Umanath, Toglia, & McDaniel, 2016) and neuroclinical populations (Kardiasmenos et al., 2008; O’Carroll, Chambers, Dennis, Sudlow, & Johnston, 2013; Shelton et al., 2016). We found that training in both encoding strategies improved non-demented PD participants’ performance on the Virtual Week (Rendell & Henry, 2009), a life-like laboratory prospective memory test. Whereas both strategies produced greater gains in focal compared to non-focal tasks, II tended to be more effective than VR for nonrepeated and non-focal tasks. These results show that people with PD can use intention formation strategies to improve their performance on a variety of prospective memory tasks and that II may be particularly effective for tasks with challenging encoding and retrieval conditions (nonrepeated and non-focal tasks, respectively). However, just because people with PD can successfully apply strategies in the controlled environment in which they were learned, we cannot assume they will spontaneously transfer the use of those strategies to everyday prospective memory challenges (McDaniel & Bugg, 2012). Therefore, the purpose of this study was to determine whether the encoding strategy training provided during the above-described study may enhance everyday prospective memory in people with PD. After receiving laboratory-based training and practice in either II or VR, participants were instructed to use their respective strategy as much as possible in their daily lives for the next month. We hypothesized that the II group would report greater improvements in everyday prospective memory after one month than the VR group.
Although we predicted significant group-related effects of strategy training on self-reported everyday prospective memory, we also anticipated that there would be considerable variation within groups in terms of this effect. As discussed by Kliegel and colleagues (Kliegel et al., 2011), individual characteristics such as motivation and metacognitive awareness may influence the tendency to use prospective memory strategies in daily life. For example, limited awareness of prospective memory abilities could reduce recognition of situations in which to use strategies and result in limited or inconsistent use (Toglia & Kirk, 2000). Similarly, one’s perceptions of the validity of a strategy or its likelihood of producing benefits may determine whether he or she chooses to adopt the strategy at all (Devilly & Borkovec, 2000). In addition, PD in particular is associated with features such as depression, global cognitive decline, and motor and non-motor dysfunction that may impact a person’s motivation or ability to learn and apply strategies in daily life. Therefore, our second objective was to investigate potential correlates of change in self-reported everyday prospective memory in response to training. We hypothesized that individual differences in certain cognitive, motivational and disease-related characteristics would be associated with the direction and magnitude of change in everyday prospective memory from before to after training. Finally, to gain additional insight into real-world strategy use after training, we conducted an exploratory interview with participants about their strategy use during the one-month follow-up period.
Methods
This study was approved by the Human Research Protection Office at Washington University in St. Louis (WU). All participants gave written informed consent before testing.
Participants
Participants were community-dwelling volunteers with PD recruited from the WU Movement Disorders Center. Inclusion criteria were as follows: at least 50 years of age, diagnosed with idiopathic PD based on UK Brain Bank Criteria (Hughes, Daniel, Kilford, & Lees, 1992), and classified as Hoehn & Yahr disease stage I-III (mild to moderate disease) (Hoehn & Yahr, 1967). Exclusion criteria were as follows: suspected dementia or global cognitive impairment determined by Movement Disorders Society diagnostic criteria (Emre et al., 2007) or Mini Mental Status Examination score < 27 (Folstein, Folstein, & McHugh, 1975), currently taking medications that interfere with cognitive function (e.g., anticholinergics), change in medication over the course of the study, other neurological disorders (e.g., stroke), history of brain surgery (e.g., deep brain stimulation), history of or current psychotic disorder, current psychiatric conditions that could interfere with study participation (e.g., severe depressive symptoms, major depressive episode), or any other features that would interfere with study participation (e.g., non-English speaking).
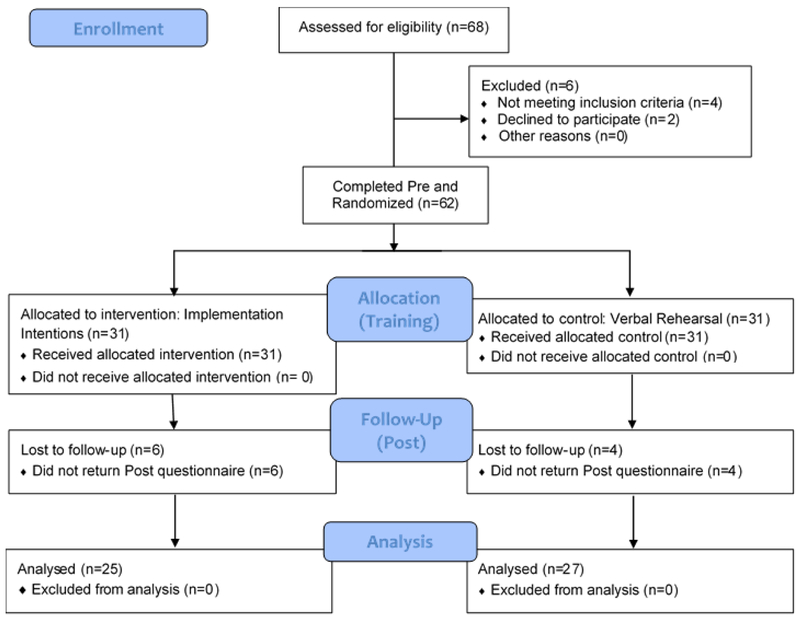
The final sample consisted of 52 participants (25 II, 27 VR) (Figure 1). There were no significant differences between included participants and those lost to follow-up in any demographic, clinical, primary or secondary variables; however, there MoCA scores were slightly lower (although not significantly) in the group lost to follow-up, t(60) = 1.81, p = 0.10. Demographic and clinical characteristics of the analyzed sample are presented in Table 1. There were no group differences in any of these characteristics. Using a MoCA cutoff score of 25/26 (Dalrymple-Alford et al., 2010), 3 II and 4 VR participants met criteria for possible mild cognitive impairment in PD (PD-MCI) (Litvan et al., 2012), χ2 = 0.09, p = 0.77. According to BDI-II criteria, 19 II and 19 VR had no or minimal depressive symptoms, 3 II and 6 VR participants had mild depressive symptoms, and 3 II and 2 VR had moderate depressive symptoms, χ2 = 1.13, p = 0.57. Antiparkinsonian medication regimens included levodopa-carbidopa only (14 II, 15 VR), levodopa-carbidopa with a dopamine agonist, COMT inhibitor, or both (8 II, 10 VR), dopamine agonist only (1 II, 0 VR), MAO inhibitor only (1 II, 0 VR), and no antiparkinsonian medications (1 II, 2 VR) and did not differ between groups, χ2 = 4.71, p = 0.58.
Figure 1.
Consolidated Standards of Reporting Trials flow diagram illustrating participant recruitment, randomization, attrition and analysis of final sample.
Table 1.
Demographic and clinical characteristics of the sample (N = 52).
| Implementation | Verbal | Effect | ||
|---|---|---|---|---|
| Variable | Intentions | Rehearsal | Statistics | size* |
| (n = 25) | (n = 27) | |||
| Male/female ratio | 12/13 | 13/14 | χ2<0.01, p=0.99 | −0.001 |
| Age (years) | 63.8 (4.6) | 62.7 (5.5) | t=0.78, p=0.44 | 0.22 |
| Education (years) | 16.4 (2.6) | 16.0 (2.3) | t=0.64, p=0.52 | 0.16 |
| Race | χ2=2.28, p=0.32 | 0.21 | ||
| White | 23 | 24 | ||
| Other | 2 | 3 | ||
| Age at diagnosis (years) | 60.2 (5.4) | 58.7 (6.1) | t=0.91, p=0.37 | 0.26 |
| Duration of diagnosis (years) | 4.2 (3.6) | 5.0 (3.0) | t=0.83, p=0.41 | −0.24 |
| Primary motor sign | χ2=1.65, p=0.44 | 0.18 | ||
| Tremor | 16 | 15 | ||
| Bradykinesia/rigidity | 6 | 5 | ||
| Mixed | 3 | 7 | ||
| Hoehn & Yahr Stage | χ2=2.49, p=0.47 | 0.22 | ||
| 1 | 2 | 3 | ||
| 2 | 18 | 21 | ||
| 2.5 | 4 | 1 | ||
| 3 | 1 | 2 | ||
| UPDRS (on medications) | 17.2 (10.0) | 15.3 (6.9) | t=0.79, p=0.43 | 0.22 |
| BDI-II | 11.0 (8.3) | 10.4 (5.3) | t=0.32, p=0.75 | 0.09 |
| MoCA | 26.9 (1.8) | 26.4 (2.0) | t=0.95, p=0.35 | 0.26 |
Note. Numbers represent means (standard deviation) or number of participants. UPDRS = Unified Parkinson’s Disease Rating Scale, Motor subscale; BDI-II = Beck Depression Inventory, Second Edition; MoCA = Montreal Cognitive Assessment.
Phi (r) for χ2 tests or Cohen’s d for t-tests.
Design
This was a single-blind randomized controlled trial (NCT01469741) with an in-person baseline testing session, an in-person training session, and mailed or in-person post-training data collection (Figure 1). All data were collected while participants were on their regular antiparkinsonian medications.
Baseline Testing Session (Pre)
Demographic information was collected through interview. Clinical characteristics related to PD were collected from clinical records (e.g., Hoehn & Yahr stage, disease duration, medications). The primary outcome measure, the Prospective and Retrospective Memory Questionnaire Prospective Scale (PRMQ-Pro) (Crawford, Smith, Maylor, Della, & Logie, 2003), was administered at this time (described below). In addition, we measured a number of characteristics that we hypothesized might influence a participant’s response to prospective memory strategy training (i.e., the direction and magnitude of change in reported everyday prospective memory). General constructs relevant to PD included motor dysfunction severity (Unified Parkinson’s Disease Rating Scale Motor Examination, UPDRS) (Fahn et al., 1987), global cognitive function (Montreal Cognitive Assessment, MoCA) (Nasreddine et al., 2005), and depressive symptoms (Beck Depression Inventory, Second Edition, BDI-II) (Beck, Steer, & Brown, 1996). Constructs more specifically related to prospective memory or the strategy training itself included prospective memory-related awareness and perceived credibility and expectancy of the strategy, respectively (described below).
Training Session
One week after the baseline testing session, participants returned to the laboratory for the training session. They were randomly assigned to the experimental (implementation intentions [II]) or control (verbal rehearsal [VR]) encoding strategy group and completed laboratory-based strategy training. Training occurred in the context of the computerized Virtual Week prospective memory test by instructions from the examiner and automated messages from the Virtual Week (for full description and screen shots of the specific version used in this study, see Foster, McDaniel, et al., 2017; for overview, see also Rendell & Henry, 2009). The Virtual Week takes the form of a board game, with one circuit of the board representing one day. Participants use the mouse to interact with the game (e.g. roll the die, move their token around the board, perform prospective memory tasks). As they progress through each day, they encounter time-appropriate activities displayed in boxes on the screen for which they make decisions (i.e. the ongoing activity of this prospective memory paradigm). They also encounter prospective memory tasks (8 tasks per day) that they have to remember to “perform” sometime later that day by clicking a box on the screen and selecting the task from a list. In this study, participants played 3 days of the Virtual Week, which involved 24 total prospective memory tasks. II group participants were taught to form a “When X, I will do Y” statement when they encounter prospective memory tasks during the Virtual Week, recite the statement aloud three times, and imaging themselves performing the prospective memory task during the Virtual Week in accordance with the statement for 30 seconds. For example, when they encountered the prospective memory task, “Drop in dry cleaning when you go shopping,” they were to form the statement “When I go shopping, I will drop in my dry cleaning,” say it out loud three times, and imagine themselves reaching the shopping activity and performing the dry cleaning task. In contrast, VR group participants were simply told to recite the prospective memory tasks they encounter aloud at least three times and study them for 30 seconds. After this instruction, participants used their respective strategy during a practice day and three test days of the computerized Virtual Week, with the test days alone providing over 30 minutes (M = 33.9, SD = 11.5) of strategy practice. Automated messages (and the examiner, if necessary) prompted participants to use their strategy when prospective memory tasks were administered, thus ensuring that participants were at least completing the verbal recitation portion of the strategies. Additionally, in both conditions the prospective memory tasks remained on the screen for 30 seconds to prevent participants from moving ahead too quickly. Upon completion of the Virtual Week, participants in both groups were instructed to use their respective strategy as much as possible in their everyday lives to help them remember to do things. They were given a handout with strategy instructions as reference, and the examiner answered questions and provided clarification if necessary.
Post-training Data Collection (Post)
One month after the training session, Post data were collected. Participants either came to the laboratory to complete the PRMQ-Pro and a follow-up interview (described below) or they completed the PRMQ-Pro by mail and the follow-up interview by phone.
Measures
Primary Outcome: Reported Everyday Prospective Memory
We administered the self-report Prospective and Retrospective Memory Questionnaire Prospective scale (PRMQ-Pro) (Crawford et al., 2003) at Pre and Post to measure reported everyday prospective memory. It consists of eight items describing everyday prospective memory failures that participants rate according to the frequency with which they occur. The scale can be divided into self-cued (Pro-Self; 4 items) and environment-cued (Pro-Env; 4 items) subscales. For example, the item “If you tried to contact a friend or relative who was out, would you forget to try again later?” measures self-cued prospective memory. The item “Do you forget to buy something you planned to buy, like a birthday card, even when you see the shop?” measures environment-cued prospective memory. Each item is rated on a five-point scale (1 = Never; 5 = Very Often), with higher scores indicating more frequent failures or worse everyday prospective memory. This study used the PRMQ-Pro (range 8–40), Pro-Self (range 4–20), and Pro-Env (range 4–20) scores as outcome variables.
Secondary Variables: Characteristics Associated with Everyday Prospective Memory Change
We used the Credibility and Expectancy Questionnaire (CEQ) (Devilly & Borkovec, 2000) to measure how convincing and logical participants found the strategy (Credibility; 3 items) and how strongly participants felt their everyday prospective memory would improve as a result of strategy use (Expectancy; 3 items). Items had 0–10 response scales. Item scores were averaged within each construct to yield separate Credibility and Expectancy scores, with higher scores indicating higher credibility or expectancy.
To measure prospective memory-related awareness, we asked participants to predict and “postdict” their prospective memory performance on the computerized Virtual Week (Foster, McDaniel, et al., 2017; Rendell & Henry, 2009). After completing the Virtual Week practice day but before the test days, participants predicted how many of the 24 prospective memory tasks they would execute accurately during the test. Then after completing the test days, participants postdicted how many of the 24 prospective memory tasks they executed accurately. The difference between their prediction and actual performance is an indicator of their “metacognitive knowledge” (i.e. existing knowledge or beliefs of their prospective memory abilities), while the difference between their postdiction and actual performance is an indicator of their “on-line awareness” (i.e. ability to monitor and appraise their prospective memory performance in real time) (S. J. Smith, Souchay, & Moulin, 2011; Toglia & Kirk, 2000). We used the absolute difference for both components, so larger values corresponded to poorer prospective memory-related awareness.
Exploratory Follow-up Interview about Everyday Prospective Memory Strategy Use
At Post, we asked the participants several questions about their strategy use in everyday life during the month following training. First, we asked if they remembered the strategy they learned and, if so, asked them to state or describe it. Answers were written down verbatim and later coded into the following categories: No memory/accuracy, Partially correct, Correct. The remaining questions and their response options were as follows: Did you use the strategy? (No, Yes); How often/much did you use the strategy? (Never, 1x/week or 1–5 times total, 2–5x/week or 6–20 times total, 1x/day, More than 1x/day); Do you think the strategy worked? (No, Not sure, Yes).
Statistical Analysis
Study data were stored and managed using REDCap electronic data capture tools hosted at WU (Harris et al., 2009) and analyzed with IBM SPSS Statistics 22. Descriptive statistics were calculated for all variables. Independent samples t-tests and Chi-squared tests were used for group comparisons of demographic and clinical characteristics, secondary variables, and follow-up interview data. Mixed general linear models (GLM) with planned pairwise comparisons were used to determine strategy training effects on reported everyday prospective memory (separate models for PRMQ-Pro, Pro-Self, and Pro-Env) with group (II, VR) as the between-subjects factor and time (Pre, Post) as the within-subjects factor. PRMQ-Pro Change scores (Post minus Pre) were calculated and then correlated (partial correlations controlling for Pre PRMQ-Pro) with potential influential variables (e.g., depression, global cognitive function, credibility) to investigate possible effect modifiers of prospective memory strategy training. All statistical tests were two tailed, and an alpha level of p < 0.05 was considered significant.
Results
Effect of Implementation Intentions and Verbal Rehearsal Training on Self-reported Everyday Prospective Memory
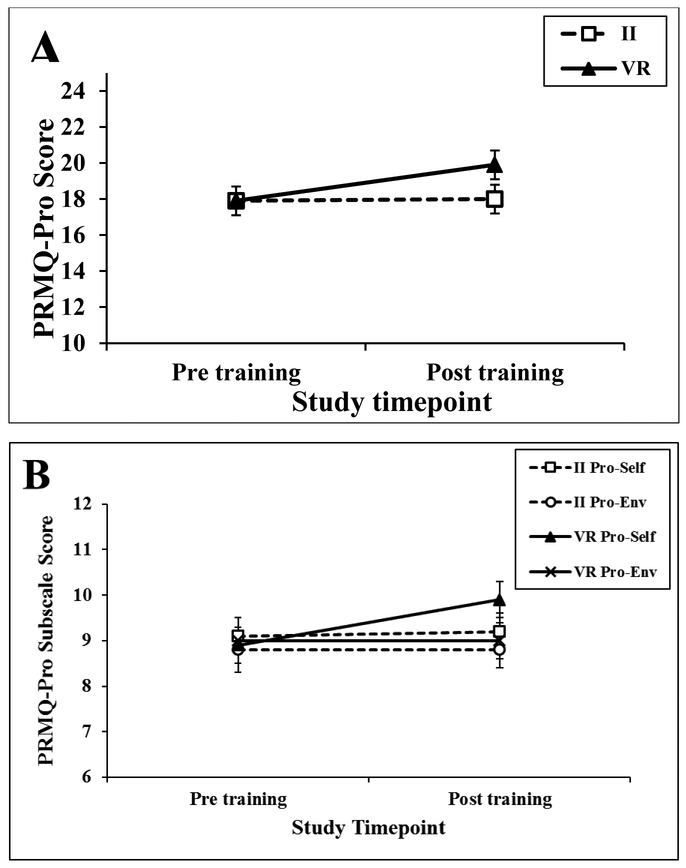
For PRMQ-Pro, there was a time X group interaction, F(1, 50) = 4.98, p = 0.03. The VR group reported worse everyday prospective memory from Pre to Post, F(1, 50) = 8.15, p = 0.006, while the II group had no change, F(1, 50) = 0.01, p = 0.92 (Figure 2A). There were no main effects of time or group for PRMQ-Pro (Fs ≤ 2.99, ps ≥ 0.09). For Pro-Self, there was a main effect of time, F(1, 50) = 7.35, p = 0.009, that was qualified by a time X group interaction, F(1, 50) = 4.45, p = 0.04. The VR group reported worse self-cued everyday prospective memory from Pre to Post, F(1, 50) = 12.08, p = 0.001, while the II had no change, F(1, 50) = 0.17, p = 0.68 (Figure 2B). There were no effects for the Pro-Env scale (Fs ≤ 0.15, ps ≥ 0.70) (Figure 2B).
Figure 2.
Group Pre and Post strategy training Prospective and Retrospective Memory Questionnaire scores for the (A) Prospective scale and (B) Prospective Self-cued and Prospective Environment-cued subscales. Error bars depict standard error of the mean.
Note. PRMQ-Pro = Prospective and Retrospective Memory Questionnaire Prospective scale
Characteristics Associated with Self-reported Everyday Prospective Memory Change
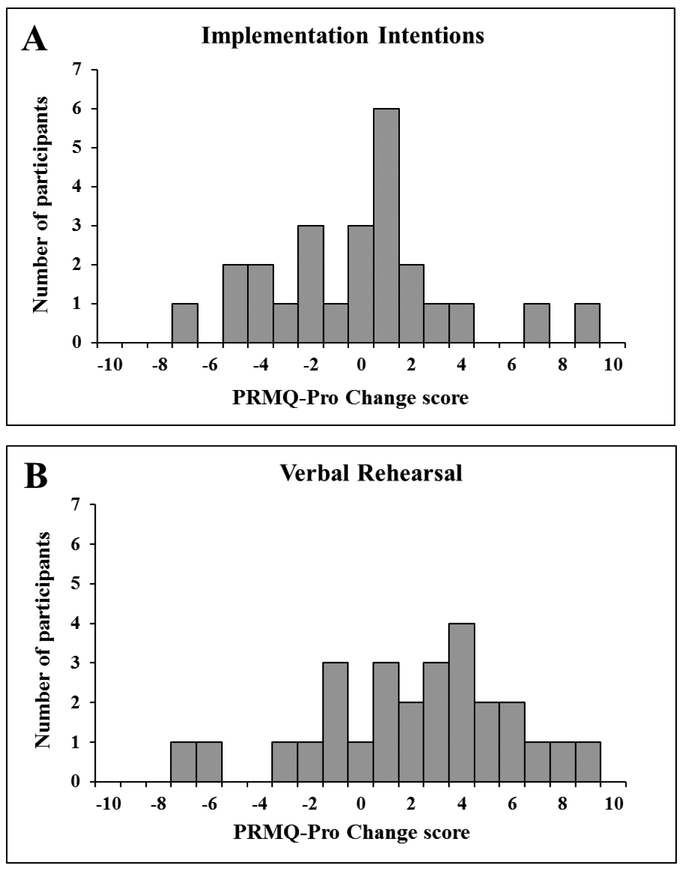
PRMQ-Pro Change is presented in Table 2, and data for the variables assessed as potential correlates of reported everyday prospective memory change are in Table 1 (UPDRS, MoCA, BDI-II) and Table 2 (CEQ, prospective memory-related awareness). There were no group differences in CEQ or prospective memory-related awareness (ps ≥ 0.13). The VR group had higher PRMQ-Pro Change (i.e., greater decline) than the II group, t(50) = 2.23, p = 0.03. As illustrated in Figure 3, there was substantial variation in the magnitude and direction of PRMQ-Pro Change scores in both groups. Within the II group, PRMQ-Pro Change correlated with MoCA (r = −0.46, p = 0.02), BDI-II (r = −0.40, p = 0.05), and CEQ Expectancy (r = −0.46, p = 0.02), such that higher cognition, depressive symptoms and expectancy were associated with greater improvement in reported everyday prospective memory from Pre to Post. There were no significant correlations between PRMQ-Pro Change and UPDRS, CEQ Credibility, and prospective memory-related awareness within the II group (rs ≤ 0.18, ps ≥ 0.39) or between PRMQ-Pro Change and any variables within the VR group (rs ≤ 0.27, ps ≥ 0.19).
Table 2.
Change in reported everyday prospective memory, perceptions of prospective memory strategy training, and prospective memory-related awareness.
| Variable | Implementation | Verbal | Statistics | Effect |
|---|---|---|---|---|
| Intentions | Rehearsal | size (d) | ||
| (n = 25) |
(n = 27) |
|||
| PRMQ-Pro change a | −0.08 (3.66) | 2.22 (3.92) | t=2.23, p=0.03 | −0.61 |
| CEQ Credibility | 6.52 (1.52) | 7.14 (1.49) | t=1.54, p=0.13 | −0.41 |
| CEQ Expectancy | 4.21 (1.24) | 4.78 (1.95) | t=1.44, p=0.19 | −0.34 |
| Prospective memory-related awareness | ||||
| Metacognitive knowledge b | 5.16 (3.46) | 5.59 (3.46) | t=0.45, p=0.65 | −0.12 |
| Online awareness c | 4.64 (3.92) | 4.42 (4.37) | t=0.19, p=0.85 | 0.05 |
Note. Numbers represent means (standard deviation). CEQ = Credibility Expectancy Questionnaire; PRMQ-Pro = Prospective and Retrospective Memory Questionnaire Prospective Scale
Calculated as Post minus Pre; higher scores indicate more reported everyday prospective memory problems at Post compared to Pre.
Absolute difference between prediction of Virtual Week score and actual Virtual Week score; higher scores indicate less accurate predictions (poorer metacognitive knowledge).
Absolute difference between postdiction of Virtual Week score and actual Virtual Week score; higher scores indicate less accurate postdictions (poorer online awareness).
Figure 3.
Distribution of Prospective and Retrospective Memory Questionnaire Prospective scale Change scores for the (A) implementation intentions and (B) verbal rehearsal groups. Higher scores indicate more reported everyday prospective memory problems at Post compared to Pre strategy training.
Note. II = implementation intentions; VR = verbal rehearsal; PRMQ-Pro = Prospective and Retrospective Memory Questionnaire Prospective Scale; Pro-Self = Prospective and Retrospective Memory Questionnaire Prospective Self-cued subcale; Pro-Env = Prospective and Retrospective Memory Questionnaire Prospective Environment-cued subscale
Exploratory Follow-up Interview Data
Descriptive data for the follow-up interview are in Table 3. There were no group differences in the distribution of answers for any of the questions, χ2s ≤ 2.07, ps ≥ 0.36.
Table 3.
Numbers and percentages (in parentheses) of participants responding to each response option for each question of exploratory follow-up interview about everyday prospective memory strategy use.
| Question and response option | Implementation | Verbal | Statistics | Effect |
|---|---|---|---|---|
| Intentions | Rehearsal | size | ||
| (n =25) | (n = 27) | (r) | ||
| 1. Do you remember the strategy? Describe. | χ2=2.07, p=0.36 | 0.20 | ||
| No memory/accuracy | 5 (20) | 2 (7) | ||
| Partially correct | 12 (48) | 17 (63) | ||
| Correct | 8 (32) | 8 (30) | ||
| 2. Did you use the strategy? | χ2<0.01, p=0.94 | 0.01 | ||
| No | 2 (8) | 2 (7) | ||
| Yes | 23 (92) | 25 (93) | ||
| 3. How often/much did you use the strategy? | χ2=1.32, p=0.86 | 0.16 | ||
| Never | 2 (8) | 2 (7) | ||
| 1x/week; 1-5 times | 9 (36) | 6 (22) | ||
| 2-5x/week; 6-20 times | 7 (28) | 9 (33) | ||
| 1x/day | 3 (12) | 4 (15) | ||
| > 1x/day | 4 (16) | 6 (22) | ||
| 4. Do you think the strategy worked? | χ2=0.27, p=0.88 | 0.07 | ||
| No | 2 (8) | 2 (7) | ||
| Not sure | 5 (20) | 4 (15) | ||
| Yes | 18 (72) | 21 (78) |
Discussion
This study tested the effect of laboratory-based encoding strategy training on self-reported everyday prospective memory in people with PD without dementia. Specifically, we aimed to determine whether the associative encoding strategy of II would produce greater improvements than the less elaborate encoding strategy of VR. We also investigated potential correlates of change in self-reported everyday prospective memory in response to training. Specifically, whether individual differences in several cognitive, motivational, and disease-related characteristics related to the direction and magnitude of change in everyday prospective memory from before to after training. After a single session of instruction and practice in either II or VR using the Virtual Week prospective memory test, participants were instructed to use their respective strategy as much as possible to accomplish their real-life prospective memory tasks over the following month. The self-report PRMQ Prospective scale administered before and one month after training showed significant decline in self-reported everyday prospective memory in the VR group but not in the II group. In addition, better global cognition, higher expectancy of improvement, and more severe depressive symptoms related to a more positive response to II training.
Our data are consistent with the notion that II is a more robust prospective memory strategy than VR and may help to compensate for PD-related deficits in executive control processes that underlie intention formation and retrieval (Foster, McDaniel, et al., 2017; Kliegel et al., 2011). Previously, we found that although both strategies improved laboratory prospective memory performance among people with PD, II produced larger effects for tasks with higher strategic encoding and attentional monitoring demands (nonrepeated and non-focal tasks, respectively) (Foster, McDaniel, et al., 2017). This study expands on our previous work to show that training in II may also benefit everyday prospective memory among people with PD.
Our primary results are somewhat surprising for a number of reasons. First is the finding that the group-related post-training difference in self-reported everyday prospective memory was due to decline in the VR group rather than improvement in the II group. This pattern contrasts with laboratory performance from the same sample, which improved in both groups after training and to a larger extent in the II group (Foster, McDaniel, et al., 2017). However, it is consistent with a recently-proposed function of cognitive intervention in PD as something which may mediate cognitive decline rather than improve cognition (Walton et al., 2017). Specifically, our results are in line with the notion that cognitive intervention may briefly prevent or delay PD-related cognitive decline (Walton et al., 2017). However, evidence on the trajectory of cognitive decline in early, non-demented PD and time-course effects of cognitive intervention in PD is limited (Leung et al., 2015; Walton et al., 2017), so it is not entirely clear how to interpret the VR group’s self-reported decline over the relatively short one-month follow-up period used in this study.
The second counterintuitive finding is that the training effects were driven by changes in self-cued rather than environment-cued prospective memory. II are typically thought to support intention retrieval in part by facilitating detection of environmental cues (Gollwitzer, 1999; Wieber et al., 2015). However, everyday prospective memory tasks with environmental cues showed no change in response to II training in this study. In contrast, II appeared to maintain PD participants’ self-reported everyday prospective memory on tasks for which there are no environmental cues. There is evidence that II can enhance performance on non-focal tasks (which are similar to the self-cued PRMQ tasks, see Foster et al., 2009) by increasing attentional monitoring (R. E. Smith, McConnell Rogers, McVay, Lopez, & Loft, 2014), so perhaps this is what occurred in the current study. Alternatively, it may be that the formation of II forced people to define environmental cues for previously self-cued tasks, thereby reducing their attentional monitoring demands and allowing for more automatic cue detection and intention retrieval. The current study design did not allow for the examination of such mechanisms.
As anticipated, there was variability within both groups in terms of the direction and magnitude of improvement reported after strategy training. Our correlational data suggest that treatment expectancy, global cognitive function and level of depression may contribute to these individual differences in response to II training. Evidence from physical and cognitive-behavioral intervention studies supports the finding that higher treatment expectancy is a positive predictor of outcomes, likely because it motivates engagement in treatment and application of treatment techniques (Devilly & Borkovec, 2000; Newman & Fisher, 2010; Smeets et al., 2008). This finding has important clinical implications because expectancy can be increased before treatment through the use of a strong therapeutic rationale and motivational interviewing (Newman & Fisher, 2010; Smeets et al., 2008).
The finding that better MoCA scores were associated with a better response to training likely reflects the general cognitive demands of learning something new and transferring or generalizing it across situations. None of our participants had dementia, but several in each group met screening criteria for possible PD-MCI (MoCA score ≤ 25), which could have been a determining factor in their level of improvement from II training. Although studies show that people with MCI can benefit from strategy-based interventions (Chandler, Parks, Marsiske, Rotblatt, & Smith, 2016; Rodakowski, Saghafi, Butters, & Skidmore, 2015), external strategies or environmental approaches that require less self-initiation (e.g. setting alarms, visual reminders, care partner support) may be more appropriate for them. Alternatively, a small study conducted by Costa et al. (2014) suggests that shifting training may improve prospective memory in PD participants with MCI.
We initially expected that higher depression would relate to poorer response to training through its negative effects on motivation and engagement in training (Lenze et al., 2004; Skidmore et al., 2010), but we found the opposite. This may be explained in relation to a cognitive initiative framework, whereby people with depression do not necessarily lack cognitive resources but instead fail to strategically engage their cognitive resources in tasks naturally (Hertel, 1994; Hertel & Hardin, 1990; Hertel & Rude, 1991). However, when their attention is directed toward key features of cognitive task or a useful strategy (as occurred with II training in the current study), they can make use of such information to improve their performance, potentially to a greater extent than people without depression (for evidence to support this notion in prospective memory, see Albinski, Kliegel, Sedek, & Kleszczewska-Albinska, 2012; Hertel, 1994; Hertel & Hardin, 1990; Hertel & Rude, 1991). Another potential explanation for our finding is the empowering nature of strategy training in general. Strategy use enables people to have better control over their functioning and provides mastery experiences through which to develop self-efficacy (Bandura, 1977). These effects may have been particularly salient for people with initially higher levels of depressive symptoms.
Knowing who responds to certain treatments can aid in the tailoring of interventions and guide clinicians in selecting appropriate clients to whom they should administer said treatments (i.e. people who are likely to benefit). Alternatively, it can reveal potentially modifiable characteristics (e.g. expectancy) to address before beginning the treatment to maximize the likelihood that the person will engage at a level necessary to derive benefit. Ultimately, these practices will result in more effective and cost-effective intervention delivery. Continued and more thorough examination of heterogeneity in response to treatment and treatment effect modifiers will be critical to the successful translation of findings from strategy training research to clinical practice.
Although there were group differences in the laboratory and self-reported everyday effects of prospective memory strategy training, the follow-up interview results showed no differences in terms of participants’ accuracy of strategy recall, reported daily life strategy use, or perceptions of strategy effectiveness. Given that the training itself required minimal time and resources, it is encouraging that almost all participants reported using their strategy at least once per week and a majority thought that it worked. However, about two-thirds of participants in both groups did not have fully accurate memory for their strategy, so it is unclear how effectively or appropriately they were using it in daily life. This may help to explain the relatively small self-reported everyday effects and suggests that a more rigorous training program may have produced more robust effects.
This study has some design-related issues that limit our conclusions. The sample size was relatively small and, in light of the finding that global cognition was related to response to training, inclusion of data from the participants who were lost to follow up could have influenced our group-related findings. Furthermore, we did not conduct a comprehensive neuropsychological assessment, so we do not know the cognitive status of our sample and our ability to interpret results related to potential PD-MCI and the influence of other cognitive processes on response to prospective memory strategy training is limited. In addition, the one month follow-up period was likely too short to provide information on any sustainable effects of training.
Another potentially problematic feature is that our primary outcome measure and follow-up interview were self-reported, so we do not have objective evidence of prospective memory performance or strategy use in daily life. In particular, the validity of the PRMQ as an indicator of prospective memory ability in PD is inconclusive. In some studies it discriminated between PD and healthy participants (specifically the Pro-Self scale; Foster et al., 2009; Pirogovsky et al., 2012), whereas other studies found no differences (S. J. Smith et al., 2011). Similarly, in some studies it correlated with objective prospective memory test scores (Costa, Peppe, et al., 2015; S. J. Smith et al., 2011), whereas in other studies it did not (Foster et al., 2009; Pirogovsky et al., 2012). This may explain the different pattern of training-related findings across the laboratory (reported in Foster, McDaniel, et al., 2017) and self-reported everyday prospective memory measures in the current sample. Lack of association between self-reported and objectively-measured prospective memory could be due to issues such as depressive symptoms, limited insight, and reporter bias. However, it is likely also due to a number of important aspects of “reality” that are not captured by many objective prospective memory tests, such as variation in real-world prospective memory challenge, additional daily demands, compensatory strategy use, task importance, and motivation (Cuttler, Graf, Pawluski, & Galea, 2011; Ihle, Schnitzspahn, Rendell, Luong, & Kliegel, 2012; Niedzwienska & Barzykowski, 2012; Phillips, Henry, & Martin, 2008; Rabbitt, Maylor, Mcinnes, Bent, & Moore, 1995; Uttl & Kibreab, 2011; Verhaeghen, Martin, & Sedek, 2012). This is especially true of laboratory-based tests, but even so-called “naturalistic” paradigms are artificial in that they use experimenter-generated tasks and thus may not tap into personal and motivational aspects of real-life prospective memory (Phillips et al., 2008). Thus, self-report measures of cognition can be informative in the absence of agreement with objective measures of cognitive ability (Rabbitt et al., 1995; Vlagsma et al., 2017). Furthermore, because they incorporate the individual’s experience and perspective, they are critical for delivering patient-centered care (Wiklund, 2004). We were interested in understanding these real-life and clinically-relevant issues, so we selected self-report over an objective measure of everyday prospective memory for this study.
This study revealed a number of issues for further investigation. In terms of intervention development, a more intense multi-session training program that incorporates methods to explicitly “train for transfer” (e.g., variable training tasks, spacing, homework, metacognitive framework) (Umanath et al., 2016) may produce more conclusive findings related to meaningful real-world change. Future studies should include comprehensive neuropsychological assessment to fully characterize participants’ cognition, informant-report and/or naturalistic performance-based outcome measures to help corroborate self-report or at least provide more complete information about a person’s prospective memory and strategy use outside of the laboratory or clinic, and longer term tracking of prospective memory after strategy training. In addition, research should aim to gain a better understanding of the potential effect of II on everyday self-cued prospective memory tasks.
In summary, our results suggest that the use of II may prevent decline in everyday prospective memory among non-demented people with PD. Furthermore, training in this strategy may be particularly beneficial for those with better global cognition, worse depressive symptoms, or higher expectations of improvement from strategy-use. Although there were statistically significant findings, the degree of change on the PRMQ that should be considered clinically significant is unclear. Regardless, this study has provided information to contribute to the development of future strategy training interventions for people with PD that take into consideration not only what to train, but also who to train and how. Further, it provides support for the value of strategy training for prospective memory impairment in PD.
Acknowledgements
This research was supported by the NIH K23HD071059 and UL1TR000448 (Washington University Institute of Clinical and Translational Sciences) and the Advanced Research Center of the Greater St. Louis Chapter of the American Parkinson Disease Association.
Footnotes
It is worth noting that evidence for the added value of visualization (versus simply creating the “When X, I will do Y” statement) is inconsistent in the existing literature on II (Chen et al., 2015; McDaniel et al., 2008; C. McFarland & Glisky, 2012).
References
- Albinski R, Kliegel M, Sedek G, & Kleszczewska-Albinska A (2012). Positive effects of subclinical depression in prospective memory and ongoing tasks in young and old adults. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn, 19(1–2), 35–57. doi: 10.1080/13825585.2011.628377 [DOI] [PubMed] [Google Scholar]
- Altgassen M, Zollig J, Kopp U, Mackinlay R, & Kliegel M (2007). Patients with Parkinson’s disease can successfully remember to execute delayed intentions. Journal of the International Neuropsychological Society, 13(5), 888–892 [DOI] [PubMed] [Google Scholar]
- Alves G, Forsaa EB, Pedersen KF, Dreetz Gjerstad M, & Larsen JP (2008). Epidemiology of Parkinson’s disease. J Neurol, 255 Suppl 5, 18–32. doi: 10.1007/s00415-008-5004-3 [DOI] [PubMed] [Google Scholar]
- Bandura A (1977). Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev, 84(2), 191–215 [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, & Brown GK (1996). Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Brom SS, & Kliegel M (2014). Improving everyday prospective memory performance in older adults: comparing cognitive process and strategy training. Psychol Aging, 29(3), 744–755. doi: 10.1037/a0037181 [DOI] [PubMed] [Google Scholar]
- Burn D, Weintraub D, Ravina B, & Litvan I (2014). Cognition in movement disorders: where can we hope to be in ten years? Mov Disord, 29(5), 704–711. doi: 10.1002/mds.25850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahn DA, Sullivan EV, Shear PK, Pfefferbaum A, Heit G, & Silverberg G (1998). Differential contributions of cognitive and motor component processes to physical and instrumental activities of daily living in Parkinson’s disease. Arch. Clin. Neuropsychol., 13(7), 575–583 [PubMed] [Google Scholar]
- Calleo J, Burrows C, Levin H, Marsh L, Lai E, & York MK (2012). Cognitive rehabilitation for executive dysfunction in Parkinson’s disease: application and current directions. Parkinsons Dis, 2012, 512892. doi: 10.1155/2012/512892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler MJ, Parks AC, Marsiske M, Rotblatt LJ, & Smith GE (2016). Everyday Impact of Cognitive Interventions in Mild Cognitive Impairment: a Systematic Review and Meta-Analysis. Neuropsychol Rev, 26(3), 225–251. doi: 10.1007/s11065-016-9330-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasteen AL, Park DC, & Schwarz N (2001). Implementation intentions and facilitation of prospective memory. Psychol Sci, 12(6), 457–461. doi: 10.1111/1467-9280.00385 [DOI] [PubMed] [Google Scholar]
- Chen XJ, Wang Y, Liu LL, Cui JF, Gan MY, Shum DH, & Chan RC (2015). The effect of implementation intention on prospective memory: a systematic and meta-analytic review. Psychiatry Res, 226(1), 14–22. doi: 10.1016/j.psychres.2015.01.011 [DOI] [PubMed] [Google Scholar]
- Cools R (2006). Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson’s disease. Neuroscience and Biobehavioral Reviews, 30(1), 1–23 [DOI] [PubMed] [Google Scholar]
- Costa A, Carlesimo GA, & Caltagirone C (2012). Prospective memory functioning: a new area of investigation in the clinical neuropsychology and rehabilitation of Parkinson’s disease and mild cognitive impairment. Review of evidence. Neurol Sci. doi: 10.1007/s10072-012-0935-y [DOI] [PubMed] [Google Scholar]
- Costa A, Peppe A, Caltagirone C, & Carlesimo GA (2008). Prospective memory impairment in individuals with Parkinson’s disease. Neuropsychology, 22(3), 283–292 [DOI] [PubMed] [Google Scholar]
- Costa A, Peppe A, Serafini F, Zabberoni S, Barban F, Caltagirone C, & Carlesimo GA (2014). Prospective memory performance of patients with Parkinson’s disease depends on shifting aptitude: evidence from cognitive rehabilitation. J Int Neuropsychol Soc, 20(7), 717–726. doi: 10.1017/S1355617714000563 [DOI] [PubMed] [Google Scholar]
- Costa A, Peppe A, Zabberoni S, Serafini F, Barban F, Scalici F, . . . Carlesimo GA (2015). Prospective memory performance in individuals with Parkinson’s disease who have mild cognitive impairment. Neuropsychology, 29(5), 782–791. doi: 10.1037/neu0000184 [DOI] [PubMed] [Google Scholar]
- Costa A, Zabberoni S, Peppe A, Serafini F, Scalici F, Caltagirone C, & Carlesimo GA (2015). Time-based prospective memory functioning in mild cognitive impairment associated with Parkinson’s disease: relationship with autonomous management of daily living commitments. Front Hum Neurosci, 9, 333. doi: 10.3389/fnhum.2015.00333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford JR, Smith G, Maylor EA, Della SS, & Logie RH (2003). The Prospective and Retrospective Memory Questionnaire (PRMQ): Normative data and latent structure in a large non-clinical sample. Memory, 11(3), 261–275 [DOI] [PubMed] [Google Scholar]
- Cuttler C, Graf P, Pawluski JL, & Galea LA (2011). Everyday life memory deficits in pregnant women. Can J Exp Psychol, 65(1), 27–37. doi: 10.1037/a0022844 [DOI] [PubMed] [Google Scholar]
- Dalrymple-Alford JC, MacAskill MR, Nakas CT, Livingston L, Graham C, Crucian GP, . . . Anderson TJ (2010). The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology, 75(19), 1717–1725. doi: 10.1212/WNL.0b013e3181fc29c9 [DOI] [PubMed] [Google Scholar]
- Deane KH, Flaherty H, Daley DJ, Pascoe R, Penhale B, Clarke CE, . . . Storey S (2014). Priority setting partnership to identify the top 10 research priorities for the management of Parkinson’s disease. BMJ Open, 4(12), e006434. doi: 10.1136/bmjopen-2014-006434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devilly GJ, & Borkovec TD (2000). Psychometric properties of the credibility/expectancy questionnaire. J Behav Ther Exp Psychiatry, 31(2), 73–86 [DOI] [PubMed] [Google Scholar]
- Disbrow EA, Russo KA, Higginson CI, Yund EW, Ventura MI, Zhang L, . . . Sigvardt KA (2012). Efficacy of tailored computer-based neurorehabilitation for improvement of movement initiation in Parkinson’s disease. Brain Res, 1452, 151–164. doi: 10.1016/j.brainres.2012.02.073 [DOI] [PubMed] [Google Scholar]
- Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, . . . Dubois B (2007). Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord, 22(12), 1689–1707; quiz 1837. doi: 10.1002/mds.21507 [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton RL, Members of the UDC, Marsden CD, Goldstein M, & Calne DB (1987). Unified Parkinson’s disease rating scale Recent developments in Parkinson’s disease (pp. 153–163). New York: Macmillan. [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatry Research, 12(3), 189–198 [DOI] [PubMed] [Google Scholar]
- Foltynie T, Brayne CE, Robbins TW, & Barker RA (2004). The cognitive ability of an incident cohort of Parkinson’s patients in the UK. The CamPaIGN study. Brain, 127(Pt 3), 550–560 [DOI] [PubMed] [Google Scholar]
- Foster ER (2014). Instrumental activities of daily living performance among people with Parkinson’s disease without dementia. Am J Occup Ther, 68(3), 353–362. doi: 10.5014/ajot.2014.010330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster ER, & Hershey T (2011). Everyday executive function is associated with activity participation in Parkinson disease without dementia. OTJR: Occupation, Participation and Health, 31(1), 16–22. doi: 10.3928/15394492-20101108-04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster ER, McDaniel MA, & Rendell PG (2017). Improving Prospective Memory in Persons With Parkinson Disease: A Randomized Controlled Trial. Neurorehabil Neural Repair, 31(5), 451–461. doi: 10.1177/1545968317690832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster ER, McDaniel MA, Repovs G, & Hershey T (2009). Prospective memory in Parkinson disease across laboratory and self-reported everyday performance. Neuropsychology, 23(3), 347–358. doi: 10.1037/a0014692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster ER, Rose NS, McDaniel MA, & Rendell PG (2013). Prospective memory in Parkinson disease during a virtual week: Effects of both prospective and retrospective demands. Neuropsychology, 27(2), 170–181. doi: 10.1037/a0031946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster ER, Spence D, & Toglia J (2017). Feasibility of a cognitive strategy training intervention for people with Parkinson’s disease. Disabil Rehabil, 1–8. doi: 10.1080/09638288.2017.1288275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollwitzer PM (1999). Implementation intentions: strong effects of simple plans. American Psychologist, 54, 493–503 [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde JG (2009). Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform, 42(2), 377–381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hering A, Rendell PG, Rose NS, Schnitzspahn KM, & Kliegel M (2014). Prospective memory training in older adults and its relevance for successful aging. Psychol Res, 78(6), 892–904. doi: 10.1007/s00426-014-0566-4 [DOI] [PubMed] [Google Scholar]
- Hertel PT (1994). Depressive deficits in memory: implications for memory improvement following traumatic brain injury. NeuroRehabilitation, 4(3), 143–150. doi: 10.3233/NRE-1994-4304 [DOI] [PubMed] [Google Scholar]
- Hertel PT, & Hardin TS (1990). Remembering with and without awareness in a depressed mood: evidence of deficits in initiative. J Exp Psychol Gen, 119(1), 45–59 [DOI] [PubMed] [Google Scholar]
- Hertel PT, & Rude SS (1991). Depressive deficits in memory: focusing attention improves subsequent recall. J Exp Psychol Gen, 120(3), 301–309 [DOI] [PubMed] [Google Scholar]
- Hindle JV, Petrelli A, Clare L, & Kalbe E (2013). Nonpharmacological enhancement of cognitive function in Parkinson’s disease: a systematic review. Mov Disord, 28(8), 1034–1049. doi: 10.1002/mds.25377 [DOI] [PubMed] [Google Scholar]
- Hoehn MM, & Yahr MD (1967). Parkinsonism: onset, progression and mortality. Neurology, 17(5), 427–442 [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, & Lees AJ (1992). Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry, 55(3), 181–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle A, Schnitzspahn K, Rendell PG, Luong C, & Kliegel M (2012). Age benefits in everyday prospective memory: the influence of personal task importance, use of reminders and everyday stress. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn, 19(1–2), 84–101. doi: 10.1080/13825585.2011.629288 [DOI] [PubMed] [Google Scholar]
- Kardiasmenos KS, Clawson DM, Wilken JA, & Wallin MT (2008). Prospective memory and the efficacy of a memory strategy in multiple sclerosis. Neuropsychology, 22(6), 746–754. doi: 2008–15268-008 [pii] 10.1037/a0013211 [DOI] [PubMed] [Google Scholar]
- Klepac N, Trkulja V, Relja M, & Babic T (2008). Is quality of life in non-demented Parkinson’s disease patients related to cognitive performance? A clinic-based cross-sectional study. European Journal of Neurology, 15(2), 128–133. doi: 10.1111/j.1468-1331.2007.02011.x [DOI] [PubMed] [Google Scholar]
- Kliegel M, Altgassen M, Hering A, & Rose NS (2011). A process-model based approach to prospective memory impairment in Parkinson’s disease. Neuropsychologia, 49(8), 2166–2177. doi: 10.1016/j.neuropsychologia.2011.01.024 [DOI] [PubMed] [Google Scholar]
- Kliegel M, & Martin M (2003). Prospective memory research: Why is it relevant? International Journal of Psychology, 38(4), 193–194. doi: Doi 10.1080/00207590344000114 [DOI] [Google Scholar]
- Kliegel M, Phillips LH, Lemke U, & Kopp UA (2005). Planning and realisation of complex intentions in patients with Parkinson’s disease. Journal of Neurology, Neurosurgery, and Psychiatry, 76(11), 1501–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenze EJ, Munin MC, Dew MA, Rogers JC, Seligman K, Mulsant BH, & Reynolds CF 3rd. (2004). Adverse effects of depression and cognitive impairment on rehabilitation participation and recovery from hip fracture. Int J Geriatr Psychiatry, 19(5), 472–478. doi: 10.1002/gps.1116 [DOI] [PubMed] [Google Scholar]
- Leroi I, Collins D, & Marsh L (2006). Non-dopaminergic treatment of cognitive impairment and dementia in Parkinson’s disease: a review. J Neurol Sci, 248(1–2), 104–114. doi: S0022–510X(06)00217–6 [pii] 10.1016/j.jns.2006.05.021 [DOI] [PubMed] [Google Scholar]
- Leung IH, Walton CC, Hallock H, Lewis SJ, Valenzuela M, & Lampit A (2015). Cognitive training in Parkinson disease: A systematic review and meta-analysis. Neurology, 85(21), 1843–1851. doi: 10.1212/WNL.0000000000002145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvan I, Goldman JG, Troster AI, Schmand BA, Weintraub D, Petersen RC, . . . Emre M (2012). Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov Disord, 27(3), 349–356. doi: 10.1002/mds.24893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LL, & Park DC (2004). Aging and medical adherence: the use of automatic processes to achieve effortful things. Psychol Aging, 19(2), 318–325. doi: 10.1037/0882-7974.19.2.318 2004–14948-008 [pii] [DOI] [PubMed] [Google Scholar]
- McDaniel MA, & Bugg JM (2012). Memory Training Interventions: What has been forgotten? J Appl Res Mem Cogn, 1(1), 58–60. doi: 10.1016/j.jarmac.2011.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel MA, & Einstein GO (2000). Strategic and automatic processes in prospective memory retrieval: A multiprocess framework. Applied Cognitive Psychology, 14, S127–S144 [Google Scholar]
- McDaniel MA, & Einstein GO (2007). Prospective memory: An overview and synthesis of an emerging field. Thousand Oaks, CA: Sage Publications. [Google Scholar]
- McDaniel MA, Howard DC, & Butler KM (2008). Implementation intentions facilitate prospective memory under high attention demands. Memory and Cognition, 36(4), 716–724 [DOI] [PubMed] [Google Scholar]
- McFarland C, & Glisky E (2012). Implementation intentions and imagery: individual and combined effects on prospective memory among young adults. Mem Cognit, 40(1), 62–69. doi: 10.3758/s13421-011-0126-8 [DOI] [PubMed] [Google Scholar]
- McFarland CP, & Glisky EL (2011). Implementation intentions and prospective memory among older adults: an investigation of the role of frontal lobe function. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn, 18(6), 633–652. doi: 10.1080/13825585.2011.613449 [DOI] [PubMed] [Google Scholar]
- Mioni G, Rendell PG, Terrett G, & Stablum F (2015). Prospective memory performance in traumatic brain injury patients: a study of implementation intentions. J Int Neuropsychol Soc, 21(4), 305–313. doi: 10.1017/S1355617715000211 [DOI] [PubMed] [Google Scholar]
- Muslimovic D, Post B, Speelman JD, & Schmand B (2005). Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology, 65(8), 1239–1245. doi: 10.1212/01.wnl.0000180516.69442.95 [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, . . . Chertkow H (2005). The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc, 53(4), 695–699. doi: JGS53221 [pii] 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- Newman MG, & Fisher AJ (2010). Expectancy/Credibility Change as a Mediator of Cognitive Behavioral Therapy for Generalized Anxiety Disorder: Mechanism of Action or Proxy for Symptom Change? Int J Cogn Ther, 3, 245–261. doi: 10.1521/ijct.2010.3.3.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedzwienska A, & Barzykowski K (2012). The age prospective memory paradox within the same sample in time-based and event-based tasks. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn, 19(1–2), 58–83. doi: 10.1080/13825585.2011.628374 [DOI] [PubMed] [Google Scholar]
- O’Carroll RE, Chambers JA, Dennis M, Sudlow C, & Johnston M (2013). Improving adherence to medication in stroke survivors: a pilot randomised controlled trial. Ann Behav Med, 46(3), 358–368. doi: 10.1007/s12160-013-9515-5 [DOI] [PubMed] [Google Scholar]
- Owen AM (2004). Cognitive dysfunction in Parkinson’s disease: the role of frontostriatal circuitry. Neuroscientist, 10(6), 525–537. doi: 10.1177/1073858404266776 [DOI] [PubMed] [Google Scholar]
- Paris AP, Saleta HG, de la Cruz Crespo Maraver M, Silvestre E, Freixa MG, Torrellas CP, . . . Bayes AR (2011). Blind randomized controlled study of the efficacy of cognitive training in Parkinson’s disease. Mov Disord, 26(7), 1251–1258. doi: 10.1002/mds.23688 [DOI] [PubMed] [Google Scholar]
- Pena J, Ibarretxe-Bilbao N, Garcia-Gorostiaga I, Gomez-Beldarrain MA, Diez-Cirarda M, & Ojeda N (2014). Improving functional disability and cognition in Parkinson disease: randomized controlled trial. Neurology, 83(23), 2167–2174. doi: 10.1212/WNL.0000000000001043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips LH, Henry JD, & Martin M (2008). Adult aging and prospective memory: The importance of ecological validity In Kliegel M, McDaniel MA & Einstein GO (Eds.), Prospective memory: Cognitive, neuroscience, developmental, and applied perspectives. (pp. 161–185). London, UK: Lawrence Erlbaum. [Google Scholar]
- Pirogovsky E, Woods SP, Vincent Filoteo J, & Gilbert PE (2012). Prospective Memory Deficits are Associated with Poorer Everyday Functioning in Parkinson’s Disease. J Int Neuropsychol Soc, 1–10. doi: S1355617712000781 [pii] 10.1017/S1355617712000781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, . . . Deuschl G (2015). MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord, 30(12), 1591–1601. doi: 10.1002/mds.26424 [DOI] [PubMed] [Google Scholar]
- Rabbitt P, Maylor E, Mcinnes L, Bent N, & Moore B (1995). What Goods Can Self-Assessment Questionnaires Deliver for Cognitive Gerontology. Applied Cognitive Psychology, 9, S127–S152. doi: DOI 10.1002/acp.2350090709 [DOI] [Google Scholar]
- Ramanan S, & Kumar D (2013). Prospective memory in Parkinson’s disease: a meta-analysis. J Int Neuropsychol Soc, 19(10), 1109–1118. doi: 10.1017/S1355617713001045 [DOI] [PubMed] [Google Scholar]
- Raskin SA, Woods SP, Poquette AJ, McTaggart AB, Sethna J, Williams RC, & Troster AI (2010). A differential deficit in time- versus event-based prospective memory in Parkinson’s disease. Neuropsychology, 25(2), 201–209. doi: 10.1037/a0020999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendell PG, & Henry JD (2009). A review of Virtual Week for prospective memory assessment: Clinical implications. Brain Impairment, 10(1), 14–22 [Google Scholar]
- Reuter I, Mehnert S, Sammer G, Oechsner M, & Engelhardt M (2012). Efficacy of a multimodal cognitive rehabilitation including psychomotor and endurance training in Parkinson’s disease. J Aging Res, 2012, 235765. doi: 10.1155/2012/235765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodakowski J, Saghafi E, Butters MA, & Skidmore ER (2015). Non-pharmacological interventions for adults with mild cognitive impairment and early stage dementia: An updated scoping review. Molecular aspects of medicine, 43–44, 38–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal E, Brennan L, Xie S, Hurtig H, Milber J, Weintraub D, . . . Siderowf A (2010). Association between cognition and function in patients with Parkinson disease with and without dementia. Mov Disord, 25(9), 1170–1176. doi: 10.1002/mds.23073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rummel J, Einstein GO, & Rampey H (2012). Implementation-intention encoding in a prospective memory task enhances spontaneous retrieval of intentions. Memory, 20(8), 803–817. doi: 10.1080/09658211.2012.707214 [DOI] [PubMed] [Google Scholar]
- Sammer G, Reuter I, Hullmann K, Kaps M, & Vaitl D (2006). Training of executive functions in Parkinson’s disease. J.Neurol.Sci, 248(1–2), 115–119 [DOI] [PubMed] [Google Scholar]
- Shelton JT, Lee JH, Scullin MK, Rose NS, Rendell PG, & McDaniel MA (2016). Improving Prospective Memory in Healthy Older Adults and Individuals with Very Mild Alzheimer’s Disease. J Am Geriatr Soc, 64(6), 1307–1312. doi: 10.1111/jgs.14134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skidmore ER, Whyte EM, Holm MB, Becker JT, Butters MA, Dew MA, . . . Lenze EJ (2010). Cognitive and affective predictors of rehabilitation participation after stroke. Arch Phys Med Rehabil, 91(2), 203–207. doi: 10.1016/j.apmr.2009.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets RJ, Beelen S, Goossens ME, Schouten EG, Knottnerus JA, & Vlaeyen JW (2008). Treatment expectancy and credibility are associated with the outcome of both physical and cognitive-behavioral treatment in chronic low back pain. Clin J Pain, 24(4), 305–315. doi: 10.1097/AJP.0b013e318164aa75 [DOI] [PubMed] [Google Scholar]
- Smith RE, McConnell Rogers MD, McVay JC, Lopez JA, & Loft S (2014). Investigating how implementation intentions improve non-focal prospective memory tasks. Conscious Cogn, 27, 213–230. doi: 10.1016/j.concog.2014.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SJ, Souchay C, & Moulin CJ (2011). Metamemory and prospective memory in Parkinson’s disease. Neuropsychology, 25(6), 734–740. doi: 10.1037/a0025475 [DOI] [PubMed] [Google Scholar]
- Toglia J, & Kirk U (2000). Understanding awareness deficits following brain injury. NeuroRehabilitation, 15(1), 57–70 [PubMed] [Google Scholar]
- Umanath S, Toglia J, & McDaniel MA (2016). Training prospective memory for transfer In Strobach T & Karbach J (Eds.), Cognitive training: An overview of features and applications. (pp. 81–91). Switzerland: Springer. [Google Scholar]
- Uttl B, & Kibreab M (2011). Self-report measures of prospective memory are reliable but not valid. Can J Exp Psychol, 65(1), 57–68. doi: 10.1037/a0022843 [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Martin M, & Sedek G (2012). Reconnecting cognition in the lab and cognition in real life: The role of compensatory social and motivational factors in explaining how cognition ages in the wild. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn, 19(1–2), 1–12. doi: 10.1080/13825585.2011.645009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlagsma TT, Koerts J, Tucha O, Dijkstra HT, Duits AA, van Laar T, & Spikman JM (2017). Objective Versus Subjective Measures of Executive Functions: Predictors of Participation and Quality of Life in Parkinson Disease? Arch Phys Med Rehabil. doi: 10.1016/j.apmr.2017.03.016 [DOI] [PubMed] [Google Scholar]
- Walton CC, Naismith SL, Lampit A, Mowszowski L, & Lewis SJ (2017). Cognitive Training in Parkinson’s Disease. Neurorehabil Neural Repair, 31(3), 207–216. doi: 10.1177/1545968316680489 [DOI] [PubMed] [Google Scholar]
- Webb TL, & Sheeran P (2007). How do implementation intentions promote goal attainment? A test of component processes. Journal of Experimental Social Psychology, 43(2), 295–302. doi: 10.1016/j.jesp.2006.02.001 [DOI] [Google Scholar]
- Wieber F, Thurmer JL, & Gollwitzer PM (2015). Promoting the translation of intentions into action by implementation intentions: behavioral effects and physiological correlates. Frontiers in Human Neuroscience, 9. doi: Artn 395 10.3339/Fnhum.2015.00395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiklund I (2004). Assessment of patient-reported outcomes in clinical trials: the example of health-related quality of life. Fundam Clin Pharmacol, 18(3), 351–363. doi: 10.1111/j.1472-8206.2004.00234.x [DOI] [PubMed] [Google Scholar]
- Xie Y, Meng X, Xiao J, Zhang J, & Zhang J (2016). Cognitive Changes following Bilateral Deep Brain Stimulation of Subthalamic Nucleus in Parkinson’s Disease: A Meta-Analysis. Biomed Res Int, 2016, 3596415. doi: 10.1155/2016/3596415 [DOI] [PMC free article] [PubMed] [Google Scholar]