Abstract
Background
In response to the opioid epidemic and new guidelines, many patients on high-dose long term opioid therapy (LTOT) for chronic pain are getting tapered off opioids. As a result, a unique clinical challenge is emerging: while many on LTOT have poor pain control, functional decline, psychiatric instability, aberrancies and misuse, these issues may often worsen with opioid tapering. Currently, a clear explanation and practical guidance on how to manage this perplexing clinical scenario is lacking.
Methods
We offer a commentary with our perspective on possible mechanisms involved in this clinical phenomena and offer practical management guidance, supported by available evidence.
Results
It is not well recognized that allostatic opponent process involved in development of opioid dependence can cause worsening pain, functional status, sleep and psychiatric symptoms over time, and significant fluctuation of pain and other affective symptoms due to their bidirectional dynamic interaction with opioid dependence (‘affective dynamism’). These elements of complex persistent dependence (CPD), the grey area between simple dependence and addiction, can lead to escalating and labile opioid need, often generating aberrant behaviors. Opioid tapering, a seemingly logical intervention in this situation, may lead to worsening of pain, function and psychiatric symptoms due to development of protracted abstinence syndrome. We offer practicing clinicians management principles and practical guidance focused on management of CPD in addition to chronic pain in these difficult clinical scenarios.
Conclusion
Awareness of the science of the neuroplasticity effects of repeated use of opioids is necessary to better manage these patients with complex challenges.
INTRODUCTION
In response to the role of excess prescription opioid use in the opioid epidemic and emerging data regarding excess risks associated with long term opioid therapy (LTOT) for pain, the new CDC Guideline for Prescribing Opioids for Chronic Pain proposed an upper safe limit of 90 milligram (mg) morphine equivalent daily (MMED), and a recommendation for opioid tapering and eventual cessation among those above safe limits if the risk benefit balance is not favorable.1 An estimated 20% of patients on LTOT for non-cancer pain in primary care report severe pain-related problems, high psychiatric illness load and addictive behaviors including aberrancies that significantly limited their life, often with high opioid doses,2 i.e. perceived safety risk may appear to outweigh benefit.3 Adhering to the recommendation of opioid taper among these patients, especially those with psychiatric comorbidity will be particularly challenging. A recent report of a system wide opioid tapering efforts in this population in primary care settings suggests limited success, with only 35% of the high dose patients with high psychiatric comorbidity were successfully brought down below the safe limit of 120 MME/day over a year and the success was mostly limited to lower dose levels of the high dose group.4
The conundrum of opioid tapering
With increasing clinical experience of opioid tapering, a challenging therapeutic and clinical phenomena is emerging: two clinical interventions exactly opposite in nature, continuation and discontinuation/taper of LTOT for pain, can often result in the same set of persistent symptoms. While LTOT can lead to poorly controlled pain, poor psychosocial and functional status, psychiatric instability, aberrancies and misuse among a proportion of patients, the logical therapeutic intervention of opioid tapering and discontinuation, on the other hand can cause persistent worsening of these same issues (archetypal patient story in box 1), leading to confusing clinical scenarios and sometimes disastrous consequences including death.5 Such challenging clinical scenarios will likely be more common in the coming years with mounting pressure to adhere to safe upper dose limits. Clinicians and patients facing this challenge need better understanding of the underlying phenomena and practical guidance to manage these patients.
Box 1. Archetypal patient story.
A 61-year-old patient with post-traumatic stress disorder (PTSD) and chronic pain due to degenerative spine disease was able to maintain a business and provide for his family with fentanyl patches (>400 MMED) to control his debilitating pain for over a decade. Over time, pain and function worsened; insomnia, anger and depression slowly emerged, and PTSD worsened. He sought more opioids from physicians for better pain control and to maintain his functional life. He interpreted multiple failed attempts by himself to stop opioids as evidence that they were helping to manage the pain driven by advancing spine disease, which in turn was driving his psychiatric worsening. However, radiographic investigations revealed stable spine disease. He got no clear answers from physicians why his pain was increasing despite this, and wondered if they missed something.
On one of the visits with his primary care provider (PCP), he was told about the new CDC Guideline and the concerns about safety and inefficacy of high opioid doses and an opioid taper was offered. He was assured that the pain would be stable with dose reduction, and he might actually do better. He reluctantly agreed, and the fentanyl dose was slowly tapered in half over next 3 months. However, his pain, function, mood, anger, insomnia, anxiety, and PTSD all worsened. His PCP advised him to stay the course and he was offered additional support including referral to substance abuse treatment. Neither the patient nor the substance abuse treatment program felt he was addicted to opioids.
MMED: Milligram morphine equivalent daily
Neuroplastic mechanisms behind the clinical conundrum
The explanations for this phenomenon lie in a deeper understanding of how opioid tolerance and dependence interact with pain, analgesia, relief and other related psychological symptoms through reward mechanisms and drive patients’ opioid need. In this manuscript, (1) we first provide a commentary supported by available evidence on how the complex neuroplastic and behavioral effects associated with opioid dependence and tolerance could modulate pain and other clinical symptoms among patients on LTOT and undergoing taper, and (2) then describe management principles that offer practical guidance to clinicians based on the above and offer some recommendations regarding opioid taper and management.
Pain and relief, rewarding affective experiences
Although most patients and providers focus only on the intensity of the sensory perception of pain (nociception or the physical pain), the associated affective experiences, immediate unpleasantness and an extended pain affect (suffering), and the resulting overt behavioral response (moaning, altered activity, medication need and use, etc) are essential to the overall experience of pain. The immediate unpleasantness involves very little cognitive processes, whereas the other extended affective experiences of pain (extended pain affect) are driven by complex cognitive processes involving memory, appraisals and judgements that generates the meanings or the implications that pain holds for the patient’s life and their future, which in turn fuels the pain related suffering involving depression, frustration, anxiety and anger (negative affective state) experienced by the patient.6
Once considered in this light, pain relief amounts to more than a reduction in physical sensation of pain (analgesia) that is often measured clinically using pain scales and mediated by nociceptive neural pathways, but also involves a relief in the affective components of pain experience.7 Newer neurobiological understanding posit that pain relief involves a significant measure of affective “rewarding” experience (see box 1 for definition) mediated through mesolimbic reward and learning pathways involving endogenous opioid system, separate from pain pathways. The same relief-reward pathways are also shared by the processes that drive the experiences of relief from other distressing psychological symptoms like depression, anger, frustration or anxiety (negative affective states) evoked by various psychiatric disorders like depression, insomnia and PTSD, medical diseases and external stress that plays important role in further shaping the overall clinical experience of pain.7–15 Also, other addictive substances like cannabis which do not have a notable analgesia effect, but has direct effects on relief and reward pathways can on the other hand potentially provide pain relief as evidenced recent popularity of “medical marijuana” for treatment of chronic pain (see Box 3, patient story 1).
Box 3. Patient stories of complex persistent dependence and protracted abstinence syndrome.
Patient story 1
A 45-year-old patient with PTSD developed chronic neck pain at the site of biopsy for a Hodgkins lymphoma diagnosis that is under remission for over 5 years now. The patient was on LTOT for past 5 years with oxycodone 20 milligrams (mg) 4 times a day. However, the patient had significant volatility of pain and associated anger, depression and anxiety requiring escalation of opioids for relief intermittently. Patients’ PCP started a dose reduction stating safety concerns based on CDC guidelines. The patient developed uncontrollable pain, anger and anxiety with depressed mood and sense of worthlessness. The PTSD symptoms also worsened. He started using marijuana to control his symptoms. Patient expressed that although pain score was not reduced much, marijuana was giving relief from pain and other symptoms allowing to have some quality of life. However, marijuana use was not allowed by the clinic resulting in administrative cessation of opioids. This led to angry confrontations with PCP and other providers resulting in loss of healthcare provider.
The patient was diagnosed with complex persistent opioid dependence while on LTOT and protracted abstinence syndrome after dose reduction that escalated with cessation. Patient was restarted back on oxycodone at prior dose while engaged in stress management and psychoeducation regarding pain and dependence. He stabilized within a month regarding pain, other negative affective symptoms and PTSD. The patient is trying to stop marijuana use and thinking over a switch to buprenorphine based treatment of complex persistent dependence.
Patient story 2
A 55-year-old patient with discoid lupus and painful non-healing ulcer of the lower extremity is maintained on high dose LTOT for over 5 years. The patients’ opioid dose steadily escalated to fentanyl patch 200 mcg/hour every 72 hours and oxycodone 10 mg every 6 hours because of pain that steadily worsened during the years of LTOT despite the wound staying stable. The pain relief from fentanyl patch reapplication was minimal and consistently wearing off after one day and the oxycodone gave minimal relief for about an hour. Patient was spending the other two days in bed or in chair with legs up, unable to do even minimally physically challenging activities. Patient was despondent as a big family event was coming up in 3 weeks and the patient would not be able to perform duties as the head of the family because of the physical limitations.
The patient was diagnosed with complex persistent opioid dependence and was initiated on buprenorphine/naloxone 8/2 milligram sublingually twice a day. Pain stabilized and physical activity improved within 2 weeks. Patient was happily able to fulfil duties in the family event. Buprenorphine/naloxone dose was increased to 24/6 milligram daily after 6 months when patient developed aseptic necrosis of the femoral head due to prolonged steroid use related to lupus. Patient remains stable a year after entering treatment and enjoys life to the fullest.
Patient story 3
A 53-year old patient with multiple shoulder surgeries and chronic pain who was managed with high dose opioid therapy (180 MMED) presented 1 year after his opioids being tapered off with a blood pressure (BP) of 245/128, severe chest pain and diffuse body pain. Patient also reports severe depression, anxiety, insomnia, restless legs at night and severe loss of functional status after opioid taper, and gives history of over 15 emergency room visits and few hospitalizations for high BP, stroke like symptoms, chest pain to rule out myocardial infarction. Patients’ BP and other symptoms would come under control with nitroglycerine, multiple anti-hypertensives and intravenous opioids while in the hospital and each time the patient would be discharged with multiple antihypertensives, but no pain medications. All work up was negative.
In the clinic, this was recognized as severe complex persistent opioid dependence with protracted abstinence syndrome and patient was induced on buprenorphine/naloxone and stabilized in a day on 8/2 mg twice a day. BP immediately came down and pains resolved within an hour. By 48 hours, the patient was back to normal clinically and fully functional as 2 years back. However, patient missed appointments and forgot to refill buprenorphine after a month, and was readmitted to the hospital for a day with chest pain and high blood pressure again. Patient was reinitiated on buprenorphine/naloxone at prior dose with stabilization. A close case management plan was also instituted to help the patient with buprenorphine adherence.
Thus, even if purely physical nociception is one part of pain, the affective experiences are critical to the patient experience of both pain and its relief. The affective balance between pain and relief involves reward system, making them susceptible to neuroadaptive modulation of learning, memory and behaviors. Repeated exposure to addictive substances like opioids that provide pain relief and have direct effects on reward systems can lead to a particular type of such neuromodulation.
Opioids, pain relief and reward: Boon and the curse
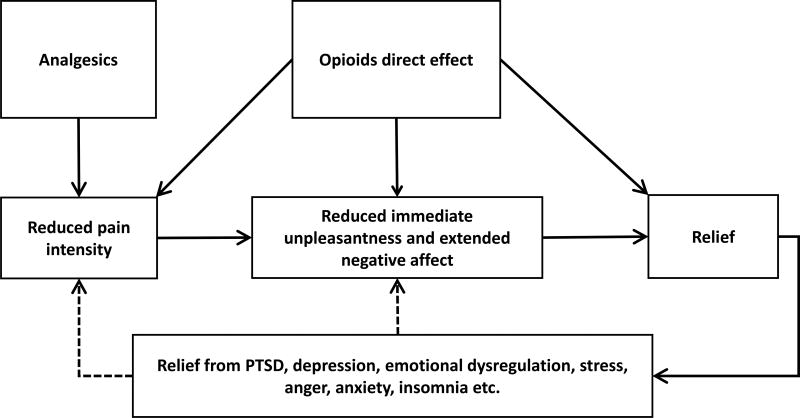
Analgesics such as non-steroidal anti-inflammatory agents are thought to have specific effects mostly confined to the nociceptive pathways providing analgesia, whereas, opioids have additional effects on reward pathways that mediate relief, thus directly alleviating immediate and extended negative affective states associated with pain.7–16 Thus, opioids’ mechanism of action putatively involves both direct analgesic effect (analgesic relief) and direct effect on relief (affective relief), making them much more appealing pain medications than non-opioids to many suffering from pain (see figure 1).17 On the other hand, repeated use of opioids coupled with a highly salient negative reinforcing reward (pain relief) can set off a chain of neuroplastic changes in reward based learning and memory pathways and behavioral changes that lead to tolerance and dependence in many, and eventually addiction in a small proportion, similar to that seen with pleasure-seeking (hedonic) use, a positive reinforcing reward (see box 2 for definitions18).19–23
Figure 1.
Multi modal action of opioids in pain relief
Box 2. Definitions.
Reinforcing
Behaviors associated with the stimulus tend to be repeated
Reward
A stimulus interpreted by brain as Positive or beneficial (Positive reinforcing- e.g. hedonic effect), or avoiding negative outcome/injury or restoring normal affective tone (Negative reinforcement- pain relief, avoiding withdrawals)
Tolerance
A decrease in the effect of the drug despite a constant dose, or a need for increased dose to maintain a stable effect.
Dependence
An adapted state due to excessive substance stimulation that can cause cognitive, emotional, or physical withdrawal symptoms when substance use is ceased.
Physical withdrawal symptoms do not develop with every substance (e.g.: cocaine), or in every one using a substance, and do not always indicate compulsive use/addiction. Physical dependence mechanisms are different from psychological dependence.
Addiction
Compulsive self-use despite negative consequences.
DSM and ICD criteria for Opioid use disorder/dependence are methods used to diagnose various levels of addiction. In practice, clinicians mostly use clinical gestalt based on their understanding of addiction.
While the clinical picture of the progression from dependence to addiction is rather clearly discernable in hedonic use where opioid is a drug procured by the individual themselves, the picture is a bit murky in LTOT for pain where it is a medication offered or administered in relation to a clinician-identified pain care need, often if not exclusively based on a therapeutic relationship.24 A more nuanced neurobehavioral understanding is required to interpret the clinical picture associated with increasing tolerance and dependence in patients with chronic pain and prescribed LTOT.
Opioid dependence and modulation of pain
Opioid tolerance (definition in box 2), although well recognized, is often described just as an expected pharmacologic effect mediated by molecular mechanisms and receptor adaptations involving the dose, frequency and duration of opioid administration, that can be overcome by increasing opioid dose or opioid rotation, unless there is clear opioid addiction.15,25–34 Similarly, the clinical effect of physiological dependence (definition in box 2) is seen within the narrow confines of well recognized acute opioid withdrawal symptoms that last for a short interval of about 4–10 days and are medically manageable.35,36 However, there are several additional powerful effects of neuroplastic behavioral changes with repeated use of opioids associated with opioid dependence and tolerance that do not get enough attention from either physicians or patients. This includes: (1) opponent effect, (2) allostatic reset, (3) affective dynamism and (4) protracted abstinence syndrome. These effects develop at varying levels in different individuals, and in a proportion of patients on LTOT for chronic pain (not in every one), the clinical sequalae of these effects can potentially cause dramatic changes of the clinical scenario in following ways:
Repeated use of opioids for pain can worsen pain and associated psychological symptoms experienced by the patient over time. But, each dose of opioids will still provide salient relief to the patient, albeit, at a lower level.
Dependence (not necessarily addiction), when well established, interacts bidirectionally and dynamically with pain, other symptoms, stress, sleep and psychological distress causing significant lability of all these, driving up the perceived need for opioids and other medications, especially psychoactive ones, to control various symptoms.
Although an appealing option in many with above problems, a dose reduction or opioid cessation in those with well-established opioid dependence (not necessarily addiction), can often result in significantly worsened pain, psychiatric status and medical condition that persist for months or weeks beyond acute withdrawals. This persistent state of “protracted abstinence syndrome” can often be relieved by reinstatement or substitution of opioids, and might be resistant to other non-opioid and non-medication treatments.
Although not well recognized in relation to therapeutic opioid use in pain, these ideas are fundamental to our current understanding of the development of dependence and addiction. It is not necessary for a patient to have a full blown addictive disorder in order to develop the protracted abstinence syndrome from opioids; LTOT as a part of legitimate treatment is sufficient cause. A brief mechanistic insight into these elements is provided in the following sections.
Opioid dependence and allostatic opponent effect
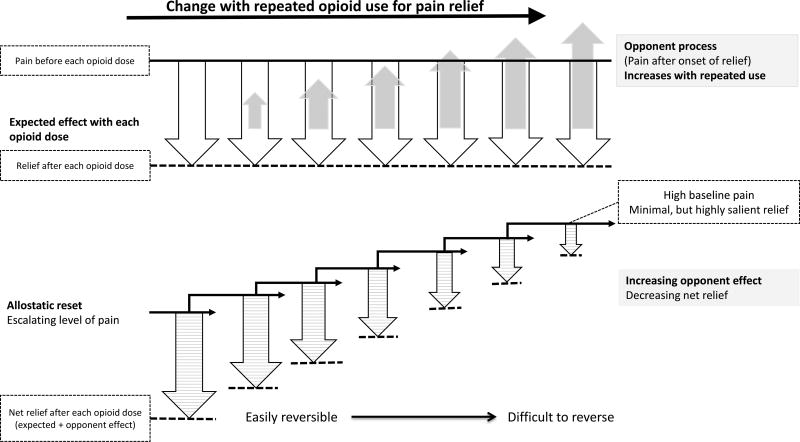
Richard Solomon introduced the concept of opponent process in 1970s to explain motivational behavioral changes in development of addiction. Evocation of behavioral processes that changes the affective balance (unpleasant to unpleasant or negative to positive valence), as in opioid use for pain relief, results in a secondary “opponent effect” shortly after the primary effect. I.e., pain after initial relief or distress after initial pleasure. The opponent effect that is insignificant in the beginning, grows in magnitude with repeated behaviors resulting in declining magnitude and shorter duration of the primary effect.7,15,33,37 In the case of repeated use of opioids for pain, the growing opponent effect of pain after initial relief results in reduction in quantity and duration of the net relief after each opioid administration (figure 2).8,37–39 This is a behavioral and experiential effect separate from or in addition to the withdrawal hyperalgesia and opioid induced hyperalgesia, a noxious sensory phenomenon.7,15,37,39,40 A similar behavioral effect can be expected with other negative affective states like depression, anger and anxiety that are often relieved by opioid administration, whereby these symptoms worsen and the relief after each opioid administration diminishes with repeated opioid exposure.39 All these together may tend to increase the patients’ perceived opioid need (Box 3, patient story1).
Figure 2.
Graphical representation of the mechanism of worsening pain and decreasing relief with long term use of opioids for pain: Allostatic opponent process in Complex Persistent Dependence
Cessation of opioids, the apparent logical intervention that can relieve the opponent effect,15,37 often becomes impossible in a proportion of patients due to another concomitant change, “allostatic reset” a physiological process fundamental to the understanding of the progression of dependence that contributes to the increasing opioid need experienced by the patient.21,23,37 Allostasis can be defined as the response of organisms to persistent external and internal demands, by which stability is maintained through change, achieving a state of chronic deviation of the regulatory system outside of the normal parameters (allostatic state) with establishment of a new set point (allostatic reset). The brain introduces experiences, memories, anticipation and re-evaluation of anticipation of needs to meet the physiological requirements of this new allostatic state.23 With regards to pain and repeated opioid use, the baseline level of pain, suffering and opioid need to maintain a new balance gets reset to higher points (see figure 2). The allostatic reset together with the opponent process establishes a state of persistent pain and suffering interspersed with short-lived relief after each opioid administration (Box 3, patient story 2).8,37–39 Reversibility to lower levels often becomes difficult as the accompanying behavioral modifications that sustain this allostatic state gets hardwired. Opioid cessation or dose decrease can often lead to induction of behavioral changes (opioid seeking) driven by the automatic physiological need to reestablish prior allostatic state and avoid withdrawals.23
Taken together, allostatic opponent process provide a plausible explanation of worsening pain, function and psychiatric instability, and increasing opioid need associated with LTOT for pain, as in the clinical cases presented (Box 1, 3, and 4).
Box 4. Patient stories of challenges with management of complex persistent dependence.
Patient story 4
A patient in 40s with borderline personality disorder (BPD), PTSD and frequent exacerbations of chronic back pain continued to have chronic abdominal pain with frequent exacerbations associated with severe anxiety, panic, PTSD symptom exacerbations, and uncontrollable nausea, vomiting and diarrhea, many years after curative ileal resection for Crohns disease. Despite high dose LTOT (>500 MMED) using a combination of fentanyl patch, hydromorphone and oxycodone, patient required two or three emergency room (ER) visits during most weeks. The patient was usually treated with intravenous hydromorphone, fluids and bowel rest, and discharged home in a day or two.
A diagnosis of complex persistent dependence was made as a unifying explanation for the exacerbation of pain, anxiety, PTSD and GI symptoms. Patient was started on Buprenorphine/naloxone 8/2 mg three times daily, and all the symptoms settled down quickly. Slowly the patient engaged in treatment for PTSD and BPD. Patient says, “I am a new person. I still get pains, but it is not so bad as it was, and I don’t feel the necessity to visit ER.” Patient had to visit ER only once in the past year after being started on Buprenorphine.
Patient story 5
A 62-year-old patient with multifocal chronic pain syndrome and brittle diabetes with peripheral neuropathy following complications of liver transplant over a decade back for liver failure from for transfusion acquired hepatitis C, was on oxycodone 10 mg 4 times a day and gabapentin for over a decade. The pain started getting worse a year back and patient used some extra oxycodone and started drinking alcohol to treat pain. PCP tapered patient off opioids because of aberrancy. The diabetes got worse and immunosuppressive therapy became inconsistent, and patient also lost PCP in the process. As pain and mood got dramatically worse within a few months, patient started snorting heroin for pain relief, which progressed within a few months to intravenous heroin use using his insulin needles. Patient overdosed 6 times in a few weeks and the police directed patient to care.
Patient was diagnosed as complex persistent dependence and protracted abstinence syndrome after opioid cessation which then progressed to opioid use disorder (intravenous heroin). Patient was reluctant to pursue OUD care as local clinic was able to provide buprenorphine only if patient was willing to participate in onerous intensive outpatient program (IOP) requiring daily visits and they were explicit that pain will not be and cannot be addressed by buprenorphine (a common misconception in addiction world). Because of this experience, the patient was resistant to buprenorphine and methadone, and methadone was too risky considering his medical state. The pain clinic did not have buprenorphine availability at that time.
Based on a harm reduction approach, patient was started back on oxycodone under close supervision (Weekly physician visits for prescription, urine toxicology and counselling, and close family supervision) with intention of keeping the patient engaged in treatment and see if heroin use would stop once pain is controlled (as patient claimed it would). Pain was dramatically better, but oxycodone was wearing off too soon. Patient stopped using heroin and drinking alcohol for a few weeks. However, patient started using heroin again for pain control, but at much lower frequency and dose. After a few weeks, the patient came to self-realization that there was a heroin problem that needed to be addressed urgently, and voluntarily entered buprenorphine IOP program, this time with assurance from current provider that buprenorphine treatment will also address pain. After a bit of struggle on lower doses, patient stabilized on 16 mg daily dose of buprenorphine. Patient now has manageable pains, and diabetes and transplant care is back on track.
Patient story 6
A 43-year-old patient with chronic foot pain from work related stress fractures was requiring 50 mg of methadone daily for pain control. Patients’ PCP reduced methadone to 30 MG daily in 8 weeks, and pain, mood and functionality worsened and patient experienced withdrawals frequently compromising ability to work and take care of family. Patient reported no psychiatric disease other than difficulty in managing family stress. A diagnosis of complex persistent dependence was made and patient was reinitiated on prior dose. Patient regained excellent pain control and functionality. After extensive psychoeducation, patient decided to pursue slow opioid taper under her control with physician support. Methadone was slowly tapered off completely in a year, and pains persisted, but not distressful as before. Over next 2 months, patient started experiencing withdrawals like symptoms with exertional fatigue or towards night, and these were severely distressful. This was diagnosed as protracted abstinence syndrome and patient was started on buprenorphine/naloxone 2/0.5 mg daily with a goal of slow taper over next 6–12 months (first to extend the dosage duration, i.e. 2 MG every other day after 2 months, then every 3 days and 4 days and then reducing the dose before stopping). Patients’ symptoms and discomfort resolved and the patient is committed to opioid taper.
Affective dynamism
Tolerance and dependence are not static phenomena with stable levels of severity, but, rather, dynamic processes that interact bi-directionally with the associated symptoms and internal and external environments of the individual. Stress, anxiety, depression, anger, insomnia, irritability and expressions of psychiatric disorders like PTSD can alter moment to moment the level of tolerance and dependence and opioid need experienced by the patient, and vice versa both during opioid maintenance and protracted withdrawal state. This sets up a state of lability/fluctuation of psychiatric symptoms and associated affective states like anger, frustration, distress, depression and anxiety (“affective dynamism”) and emotional dysregulation in people on LTOT, which in part explains erratic behavior including threatened and actual violent behavior and suicides among patients.20,41,42 This ‘affective dynamism’ often imposes escalation and lability of opioid need while the patient is on steady opioid dose or during taper (Box 3, patient stories 1,2 and 3; Box 4 patient story 4).
Protracted abstinence syndrome
With regards to withdrawals from opioid or any substance, there is scientific evidence of presence of both acute and protracted phases of withdrawal, but, acute withdrawal gets the most attention of patients and providers, probably because of its dramatic physical presentation over a short interval of about 4–10 days.35,36 Varying degrees of protracted withdrawal emerge following acute withdrawal, a condition referred to as “protracted abstinence syndrome” that can last for months or years in people with long-standing opioid dependence.36,43 This is presumed to be due to the hard-to-reverse allostatic changes associated with progression of tolerance and dependence.23 Extended withdrawal symptoms specific to protracted abstinence in opioid dependence include anxiety, depression, sleep disturbances, fatigue, dysphoria (i.e., feeling down or emotionally blunted), irritability, decreased ability to focus, and deficits in executive control that can last for months beyond the period of acute withdrawal. The larger phenomena of protracted opioid abstinence syndrome involves varying levels of rebound and reemergence of original symptoms (pain and disability in this case) and comorbid psychiatric disorders (like PTSD) and medical comorbidities, in addition to opioid-specific protracted abstinence symptoms (e.g. Box 4, patient story 4).43 The original symptoms and comorbid disorders may be experienced at higher levels of distress than before opioid initiation due to allostatic changes. Severe protracted abstinence syndrome after opioid cessation among LTOT patients can possibly lead to illicit prescription opioid or heroin use with rapid development of opioid use disorder (Box 4, patient story 5). Protracted abstinence syndrome offers a plausible explanation for persistent suffering with opioid dose reduction and cessation as seen with the archetypal patient and other patient stories described (Box 3, Box 4).
When tapering opioids among those on LTOT, especially those with comorbid psychiatric disease, the clinician has to be aware that protracted abstinence syndrome phenomena can potentially expose patients to substantial risk of physical, functional, medical and psychiatric instability along with harmful behaviors like suicide and violence, and relapse of SUD including OUD (Box 1,3 and 4).5,43,44
Management Principles
Complex persistent dependence, the grey area between dependence and addiction
A clear diagnostic dichotomy of OUD Vs. no OUD dictating discrete management pathways would be optimal, especially for primary care physicians trying to triage care in patients with complex pain on LTOT. However, as elegantly pointed out by Ballantyne et. al, a diagnostic distinction between dependence and addiction is nearly impossible in many patients on LTOT with the available criteria,20 creating a diagnostic and therapeutic orphan status for these patients, somewhere in the grey area between the clear demarcations of simple dependence and frank addiction.24 Ballantyne et al20,24 put forth the term “Complex Persistent Dependence” (CPD) to describe the physiological and clinical state that exists in this grey area.
Clinically significant CPD can be recognized as a patient’s desire to continue or increase the dose of LTOT, or inability to discontinue LTOT despite a prescriber’s recommendation to discontinue it. The symptoms of CPD include worsening pain, function, affective symptoms and sleep disturbance, affective dynamism with escalating opioid need while maintained on LTOT, and protracted withdrawal syndrome on opioid dose reduction or cessation.
Based on typological classification and description of primary care patients with chronic pain on LTOT,2 it is reasonable to hypothesize that having ≥100 MMED opioid dose and/or significant pain dysfunction, aberrancies and misuse, psychiatric burden and prior history of or active SUD offers an easy cut off for PCPs to identify these difficult to manage patients with high likelihood of CPD that may cause significant persistent adverse effects with opioid dose tapering. Real life experiences suggested that attempt at opioid taper is difficult in patients with chronic pain and high opioid doses.4 These patients may have little insight into the role opioids are playing in their current state and thus may have little motivation and significant fear related to making a change.
Treatment approach in complex persistent dependence
Among those who develop significant CPD on LTOT, escalation of opioid doses for better pain control can often paradoxically result in worsening pain and poor functionality. At this stage, pain, insomnia and affective instabilities are largely the symptomatic expressions of CPD (Box 4, patient story 4). A therapeutic focus on these peripheral symptoms without adequate management of dependence is unlikely to yield clinical success and often leads to potentially dangerous psychoactive polypharmacy including anti-depressants, antipsychotics, benzodiazepines, muscle relaxants, z-drugs and stimulants.
Buprenorphine, a useful tool in Complex Persistent Dependence
Buprenorphine, a partial mu opioid agonist with a ceiling effect on side effects like sedation, constipation and hedonic properties, but no clinically-relevant ceiling effect on analgesia, is emerging as a helpful analgesic agent in patients with poorly controlled chronic pain with full agonist opioids like morphine, oxycodone, fentanyl and hydromorphone. It offers good analgesia and effective treatment of dependence through its long half-life.45–48 These properties can allow the patient to stop the full agonist opioid therapy that is potentially worsening the pain and function through CPD, and switch to buprenorphine, which is associated with lower levels of dependency and comparatively higher levels of safety. Once transitioned to buprenorphine, it can either be continued or tapered in a slow fashion that is often more comfortable to the patient.
We have found buprenorphine dosed multiple times a day (aka, split dosing, e.g.: 8 MG two to four times a day) to be effective for many patients with chronic pain and CPD. Patients have to discontinue other opioids at least 8–12 hours before initiating buprenorphine to avoid induced withdrawals. Stopping the opioids in evening and initiating buprenorphine next morning is an easy strategy. A switch from methadone is often better tolerated when it is 40 mg or below daily dose. Home based induction is convenient, patient friendly and less resource intense when compared to office based induction, and is safe when deployed with proper education and support.
Close patient centered engagement with their providers is an integral part of their effective treatment. Both patients and providers need education regarding chronic pain and opioid dependence/tolerance. Psychotherapies focused on chronic pain and opioid dependence can be effective. Other multimodal therapies for chronic pain may be more acceptable to treatment resistant patients with chronic pain after the affective dynamism or protracted abstinence are ameliorated with adequate treatment of CPD with buprenorphine. Details of the progress of archetypal patient and other patients with treatment of CPD is provided in boxes 1, 3 and 4.
Methadone also can be helpful,49,50 especially when buprenorphine is not tolerated by patient or available. But, full agonist properties raise the problem of worsening CPD with time, which is less of a problem with buprenorphine. Unlike the general assumption, a special × license is not required for use of sub lingual formulations of buprenorphine for pain and the Drug Enforcement Agency (DEA) does not prohibit the use of sub lingual buprenorphine formulations for treatment of pain,51 and in fact, the SAMHSA guidelines on buprenorphine in opioid addiction (TIP 40, page 76) endorses that OUD patients with uncontrolled pain can be treated with split doses of buprenorphine in settings outside of substance abuse treatment program like primary care clinics or specialty clinics if indicated.52 However, misinformed local insurance and pharmacy formulary restrictions may often disallow such use of buprenorphine for pain. In that case, we recommend making a clinical diagnosis of opioid dependence collaboratively with the patient and then starting the buprenorphine substitution when indicated. More recently transdermal and buccal formulations of buprenorphine have been approved by FDA for pain management and clinical experience is growing with these medications.
A proportion of patients with CPD may not tolerate buprenorphine or methadone and will not be a safe candidate for methadone treatment. In these patients, providers and patients are often left with the hard choice of continuing full agonist opioids acknowledging the risks involved or choosing the difficult task of slow opioid tapering. If opioids are continued, we recommend managing pain exclusively with scheduled opioid doses, preferably long acting ones, avoiding as needed doses for breakthrough pain.
A patient centered opioid taper plan
Many with simple dependence or CPD, especially those on low daily dose and low psychiatric comorbidity may tolerate opioid taper (Box 4, patient story 5). When starting an opioid taper plan, it is particularly important to define what “success” in an opioid taper means. It should be much more than a simple reduction in dose. An opioid taper can be considered successful only if the probable risk improvement with dose reduction can be balanced with the degree of achievement of goals that are important to patient, namely stability or improvement in pain and function, avoiding instability and harm related to medical, psychiatric and psychological conditions and avoiding significant protracted abstinence syndrome. The process should also assure that patents feels that they are treated with dignity and respect, are involved in decision process and remains engaged in continued treatment.53 Patient involvement in decision and taper plan with support and psychoeducation is critical to its success (box 4: patient story 6). Forced involuntary tapers can result in poor outcomes and patients feeling abandoned (Box 1, Box 3, Box 4).5
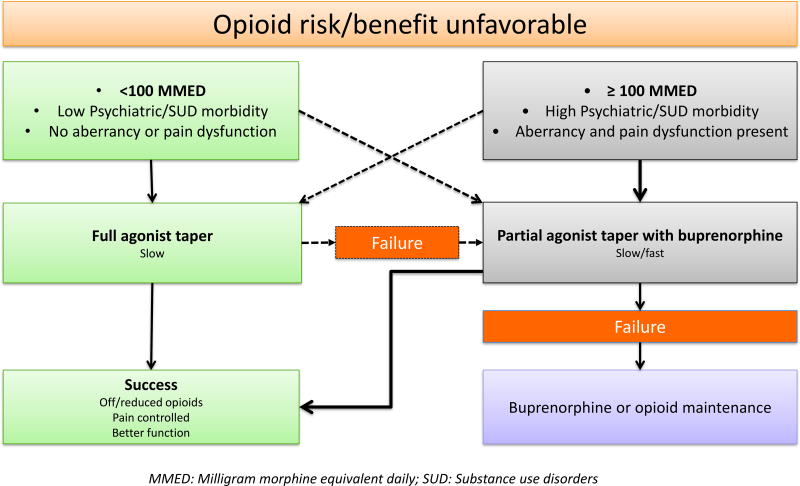
If an opioid taper is considered in patients maintained on LTOT for many years, based on our clinical experience, we propose an opioid taper plan as illustrated in figure 3 that offers two pathways based on the patient’s current daily opioid dose. As stated above, ≥100 MMED opioid dose and/or significant pain dysfunction, aberrancies and misuse, psychiatric burden and prior history of or active SUD offers an easy cut off to identify high likelihood of CPD that may cause significant persistent adverse effects with opioid dose tapering. Among those with opioid dose of ≥100 MMED and/or significant psychiatric comorbidity, pain dysfunction and opioid aberrancy, a rotation to the partial agonist buprenorphine, followed by a taper is the preferred way, whereas a full agonist opioid taper can be tried among those on less than 100 MMED and/or with low psychiatric comorbidity, pain dysfunction and aberrant behavior. If the full mu agonist taper fails, the patient can be rotated to buprenorphine and tapered (Box 4, patient story 6). If both taper attempts fail, we recommend pain treatment maintenance with buprenorphine (e.g.: archetypal case). Although often stated as easy and straightforward, opioid tapers can often become challenging. Attempts at opioid taper have to be realistically tempered by the evidence that small studies have reported high failure rates with both full agonist and buprenorphine based opioid tapers.54,55 Clinical trials are needed to further develop and test these approaches.
Figure 3.
A patient centered opioid tapering plan
In some patients on LTOT, an opioid taper is much more a complicated medical intervention than, for example, discontinuing a blood pressure medication because of the possibility of significant protracted withdrawal symptoms developing in a proportion of patients. So, we recommend primary care physicians embarking on tapering plan to be cognizant of this serious adverse effect of opioid tapering and prepare contingency plans if required. These real issues need to be discussed with patient before starting opioid taper.
Conclusions
Many of the patients with chronic pain on LTOT exist between the grey area between simple dependence and addiction. The patients in this grey area probably have Complex Persistent Dependence with allostatic opponent effect causing worsening pain and function, sleep disturbance and psychiatric symptoms, and affective dynamism causing fluctuation of these symptoms that drive opioid need of the patient leading to aberrant behaviors. Opioid dose reduction or cessation may lead to worsening of these symptoms and pain and function due to development of protracted abstinence syndrome. This makes continuation and withdrawal of LTOT infinitely complex and difficult therapeutic maneuvers for the patients and providers. A management plan focused on the syndrome of Complex Persistent Dependence in addition to chronic pain would be more successful in these patients. Awareness of the science of neuroplastic changes associated with opioid dependence and addiction and its interaction with psychiatric illness is necessary for the good management of these patients. Theory based clinical research focused on opioid dependence/tolerance rather than pain alone is lacking in this field and much needed.
Acknowledgments
We would like to thank William C. Becker for extensive review and edits that greatly enhanced this article.
FUNDING
Ajay Manhapra was supported by the Research in Addiction Medicine Scholars (RAMS) Program, Grant number R25DA033211 from the National Institute on Drug Abuse, and VA/OAA Interprofessional Advanced Fellowship in Addiction Treatment. The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
The authors declare they have no conflicts of interest.
References
- 1.Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain - United States, 2016. MMWR Recomm Rep. 2016;65(1):1–49. doi: 10.15585/mmwr.rr6501e1. [DOI] [PubMed] [Google Scholar]
- 2.Banta-Green CJ, Merrill JO, Doyle SR, Boudreau DM, Calsyn DA. Opioid use behaviors, mental health and pain--development of a typology of chronic pain patients. Drug and alcohol dependence. 2009;104(1–2):34–42. doi: 10.1016/j.drugalcdep.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Von Korff MR, Franklin G. Responding to America's Iatrogenic Epidemic of Prescription Opioid Addiction and Overdose. Med Care. 2016;54(5):426–429. doi: 10.1097/MLR.0000000000000537. [DOI] [PubMed] [Google Scholar]
- 4.Weimer MB, Hartung DM, Ahmed S, Nicolaidis C. A chronic opioid therapy dose reduction policy in primary care. Substance abuse : official publication of the Association for Medical Education and Research in Substance Abuse. 2016;37(1):141–147. doi: 10.1080/08897077.2015.1129526. [DOI] [PubMed] [Google Scholar]
- 5.Weeks WB. Hailey. JAMA. 2016;316(19):1975–1976. doi: 10.1001/jama.2016.10251. [DOI] [PubMed] [Google Scholar]
- 6.Riley JL, Wade JB. Psychological and Demographic Factors that Modulate the Different Stages and Dimensions of Pain. In: Price DD, Bushnell MC, editors. Psychological methods of pain control: basic science and clinical perspectives. Seattle, WA: IASP Press; 2004. [Google Scholar]
- 7.Leknes S, Brooks JC, Wiech K, Tracey I. Pain relief as an opponent process: a psychophysical investigation. Eur J Neurosci. 2008;28(4):794–801. doi: 10.1111/j.1460-9568.2008.06380.x. [DOI] [PubMed] [Google Scholar]
- 8.Seymour B, O'Doherty JP, Koltzenburg M, et al. Opponent appetitive-aversive neural processes underlie predictive learning of pain relief. Nature neuroscience. 2005;8(9):1234–1240. doi: 10.1038/nn1527. [DOI] [PubMed] [Google Scholar]
- 9.Leknes S, Berna C, Lee MC, Snyder GD, Biele G, Tracey I. The importance of context: when relative relief renders pain pleasant. Pain. 2013;154(3):402–410. doi: 10.1016/j.pain.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franklin JC, Puzia ME, Lee KM, et al. The Nature of Pain Offset Relief in Nonsuicidal Self-Injury: A Laboratory Study. Clinical Psychological Science. 2013;1(2):110–119. [Google Scholar]
- 11.Navratilova E, Morimura K, Xie JY, Atcherley CW, Ossipov MH, Porreca F. Positive emotions and brain reward circuits in chronic pain. J Comp Neurol. 2016 doi: 10.1002/cne.23968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zubieta JK. Pain signal as threat and reward. Neuron. 2010;66(1):6–7. doi: 10.1016/j.neuron.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Fields HL. Understanding how opioids contribute to reward and analgesia. Reg Anesth Pain Med. 2007;32(3):242–246. doi: 10.1016/j.rapm.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Navratilova E, Porreca F. Reward and motivation in pain and pain relief. Nat Neurosci. 2014;17(10):1304–1312. doi: 10.1038/nn.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baker TB, Tiffany ST. Morphine-Tolerance as Habituation. Psychol Rev. 1985;92(1):78–108. [PubMed] [Google Scholar]
- 16.Howe CQ, Sullivan MD. The missing 'P' in pain management: how the current opioid epidemic highlights the need for psychiatric services in chronic pain care. Gen Hosp Psychiatry. 2014;36(1):99–104. doi: 10.1016/j.genhosppsych.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Lee MC, Wanigasekera V, Tracey I. Imaging opioid analgesia in the human brain and its potential relevance for understanding opioid use in chronic pain. Neuropharmacology. 2014;84:123–130. doi: 10.1016/j.neuropharm.2013.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nature reviews Neuroscience. 2001;2(10):695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- 19.Meyer PJ, King CP, Ferrario CR. Motivational Processes Underlying Substance Abuse Disorder. Current topics in behavioral neurosciences. 2015 doi: 10.1007/7854_2015_391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ballantyne JC, Sullivan MD, Kolodny A. Opioid Dependence vs Addiction: A Distinction Without a Difference? Archives of internal medicine. 2012;172(17):1342–1343. doi: 10.1001/archinternmed.2012.3212. [DOI] [PubMed] [Google Scholar]
- 21.Koob GF, Le Moal M. Review. Neurobiological mechanisms for opponent motivational processes in addiction. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2008;363(1507):3113–3123. doi: 10.1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koob GF, Stinus L, Le Moal M, Bloom FE. Opponent process theory of motivation: neurobiological evidence from studies of opiate dependence. Neuroscience and biobehavioral reviews. 1989;13(2–3):135–140. doi: 10.1016/s0149-7634(89)80022-3. [DOI] [PubMed] [Google Scholar]
- 23.Le Moal M, Koob GF. Drug addiction: pathways to the disease and pathophysiological perspectives. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2007;17(6–7):377–393. doi: 10.1016/j.euroneuro.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Ballantyne JC. Assessing the prevalence of opioid misuse, abuse, and addiction in chronic pain. Pain. 2015;156(4):567–568. doi: 10.1097/j.pain.0000000000000105. [DOI] [PubMed] [Google Scholar]
- 25.Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain — United States, 2016. MMWR Recommendations and Reports. 2016;65(1):1–49. doi: 10.15585/mmwr.rr6501e1. [DOI] [PubMed] [Google Scholar]
- 26.Lembke A. Why doctors prescribe opioids to known opioid abusers. The New England journal of medicine. 2012;367(17):1580–1581. doi: 10.1056/NEJMp1208498. [DOI] [PubMed] [Google Scholar]
- 27.Eisenberg E, Suzan E, Pud D. Opioid-induced hyperalgesia (OIH): a real clinical problem or just an experimental phenomenon? J Pain Symptom Manage. 2015;49(3):632–636. doi: 10.1016/j.jpainsymman.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Yi P, Pryzbylkowski P. Opioid Induced Hyperalgesia. Pain medicine (Malden, Mass) 2015;16(Suppl 1):S32–36. doi: 10.1111/pme.12914. [DOI] [PubMed] [Google Scholar]
- 29.Bespalov A, Muller R, Relo AL, Hudzik T. Drug Tolerance: A Known Unknown in Translational Neuroscience. Trends in pharmacological sciences. 2016 doi: 10.1016/j.tips.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Ballantyne JC, Shin NS. Efficacy of opioids for chronic pain: a review of the evidence. The Clinical journal of pain. 2008;24(6):469–478. doi: 10.1097/AJP.0b013e31816b2f26. [DOI] [PubMed] [Google Scholar]
- 31.Raffa RB, Pergolizzi JV., Jr Opioid-induced hyperalgesia: is it clinically relevant for the treatment of pain patients? Pain Manag Nurs. 2013;14(3):e67–83. doi: 10.1016/j.pmn.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Volkow ND, McLellan AT. Opioid Abuse in Chronic Pain--Misconceptions and Mitigation Strategies. The New England journal of medicine. 2016;374(13):1253–1263. doi: 10.1056/NEJMra1507771. [DOI] [PubMed] [Google Scholar]
- 33.Miguez G, Laborda MA, Miller RR. Classical conditioning and pain: conditioned analgesia and hyperalgesia. Acta Psychol (Amst) 2014;145:10–20. doi: 10.1016/j.actpsy.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalant H. Research on tolerance: What can we learn from history? Alcoholism-Clinical and Experimental Research. 1998;22(1):67–76. doi: 10.1111/j.1530-0277.1998.tb03618.x. [DOI] [PubMed] [Google Scholar]
- 35.Satel SL, Kosten TR, Schuckit MA, Fischman MW. Should protracted withdrawal from drugs be included in DSM-IV? The American journal of psychiatry. 1993;150(5):695–704. doi: 10.1176/ajp.150.5.695. [DOI] [PubMed] [Google Scholar]
- 36.Heilig M, Egli M, Crabbe JC, Becker HC. Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addiction biology. 2010;15(2):169–184. doi: 10.1111/j.1369-1600.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Solomon RL. The opponent-process theory of acquired motivation: the costs of pleasure and the benefits of pain. Am Psychol. 1980;35(8):691–712. doi: 10.1037//0003-066x.35.8.691. [DOI] [PubMed] [Google Scholar]
- 38.Shurman J, Koob GF, Gutstein HB. Opioids, Pain, the Brain, and Hyperkatifeia: A Framework for the Rational Use of Opioids for Pain. Pain Medicine. 2010;11(7):1092–1098. doi: 10.1111/j.1526-4637.2010.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White JM. Pleasure into pain: the consequences of long-term opioid use. Addictive behaviors. 2004;29(7):1311–1324. doi: 10.1016/j.addbeh.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 40.Arout CA, Edens E, Petrakis IL, Sofuoglu M. Targeting Opioid-Induced Hyperalgesia in Clinical Treatment: Neurobiological Considerations. CNS drugs. 2015;29(6):465–486. doi: 10.1007/s40263-015-0255-x. [DOI] [PubMed] [Google Scholar]
- 41.Koob GF, Buck CL, Cohen A, et al. Addiction as a stress surfeit disorder. Neuropharmacology. 2014;76(Pt B):370–382. doi: 10.1016/j.neuropharm.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ballantyne JC, Stannard C. New addiction criteria: diagnostic challenges persist in treating pain with opioids. Pain. 2013;1 [Google Scholar]
- 43.SAMHSA. Protracted withdrawal. Substance abuse treatment advisory. 2010;9(1):1–8. [Google Scholar]
- 44.Kertesz SG. Turning the tide or riptide? The changing opioid epidemic. Substance abuse : official publication of the Association for Medical Education and Research in Substance Abuse. 2017;38(1):3–8. doi: 10.1080/08897077.2016.1261070. [DOI] [PubMed] [Google Scholar]
- 45.Berland DW, Malinoff HL, Weiner MA, Przybylski R. When Opioids Fail in Chronic Pain Management: The Role for Buprenorphine and Hospitalization. American Journal of Therapeutics. 2013;20(4):316–321. doi: 10.1097/MJT.0b013e31827ab599. [DOI] [PubMed] [Google Scholar]
- 46.Butler S. Buprenorphine—Clinically useful but often misunderstood. Scandinavian Journal of Pain. 2013;4(3):148–152. doi: 10.1016/j.sjpain.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 47.Foster B, Twycross R, Mihalyo M, Wilcock A. Buprenorphine. J Pain Symptom Manage. 2013;45(5):939–949. doi: 10.1016/j.jpainsymman.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 48.Johnson RE, Fudala PJ, Payne R. Buprenorphine: considerations for pain management. J Pain Symptom Manage. 2005;29(3):297–326. doi: 10.1016/j.jpainsymman.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 49.Rhodin A, Gronbladh L, Nilsson LH, Gordh T. Methadone treatment of chronic non-malignant pain and opioid dependence--a long-term follow-up. Eur J Pain. 2006;10(3):271–278. doi: 10.1016/j.ejpain.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 50.Neumann AM, Blondell RD, Jaanimagi U, et al. A preliminary study comparing methadone and buprenorphine in patients with chronic pain and coexistent opioid addiction. J Addict Dis. 2013;32(1):68–78. doi: 10.1080/10550887.2012.759872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heit HA, Covington E, Good PM. Dear DEA. Pain medicine (Malden, Mass) 2004;5(3):303–308. doi: 10.1111/j.1526-4637.2004.04044.x. [DOI] [PubMed] [Google Scholar]
- 52.Center for Substance Abuse T. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction. Rockville (MD): Substance Abuse and Mental Health Services Administration (US); 2004. SAMHSA/CSAT Treatment Improvement Protocols. [PubMed] [Google Scholar]
- 53.VA/DoD Clinical Practive Guideleine Management of Opioid Therapy for Chronic pain. The Management of Opioid Therapy for Chronic Pain Working Group; 2010. [Google Scholar]
- 54.Blondell RD, Ashrafioun L, Dambra CM, Foschio EM, Zielinski AL, Salcedo DM. A Clinical Trial Comparing Tapering Doses of Buprenorphine with Steady Doses for Chronic Pain and Co-existent Opioid Addiction. Journal of addiction medicine. 2010;4(3):140–146. doi: 10.1097/ADM.0b013e3181ba895d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berna C, Kulich RJ, Rathmell JP. Tapering Long-term Opioid Therapy in Chronic Noncancer Pain: Evidence and Recommendations for Everyday Practice. Mayo Clin Proc. 2015;90(6):828–842. doi: 10.1016/j.mayocp.2015.04.003. [DOI] [PubMed] [Google Scholar]